1. Introduction
In recent decades, Europe has experienced a significant increase in the number of geriatric individuals. Population projections of European Union, estimate that the old-age dependency ratio will increase from 33.0 % in 2022 to 59.7 % in 2100 [
1].
Demographic changes are transforming the healthcare landscape. Older adults represent a larger proportion of the population, increasing the demand for long-term care facilities. Nursing homes (NHs) are intended to mimic the home environment of residents, but often become spaces where people with significant underlying conditions live in limited spaces, sharing caregivers in a communal setting [
2]. Despite providing less intensive medical care, long-term care facilities have not been immune to healthcare-associated infections, affecting the vulnerable NH population [
3,
4].
Infections represent a major challenge for older people living in NH, as their compromised immune system known as immunosenescence and underlying health problems make them more susceptible to these conditions [
5].
Urinary tract infections (UTIs) are common infections among elderly people living in NHs in Europe [
6]. This pathology ranges from asymptomatic bacteriuria to sepsis associated with UTI, requiring hospitalization. The diagnosis of symptomatic UTI in older adults usually requires the presence of localized genitourinary symptoms, fever, pyuria, and a urine culture confirming the presence of a uropathogen [
7]. In cases of symptomatic UTI, antimicrobial therapy is considered appropriate [
8].
Given the potential for UTIs to lead to severe infections, healthcare professionals may diagnose UTIs relying solely on vague symptoms, such as changes in behavior or alterations in the appearance of urine.
Recognizing the importance that antibiotics are often prescribed for this infection, even though a considerable portion of these prescriptions are considered inappropriate [
9,
10,
11]. This practice contributes significantly to increasing antimicrobial resistance (AMR), which poses a threat to public health, healthcare systems, and social well-being [
12]. Infections caused by antibiotic-resistant bacteria are associated with increased morbidity and mortality, as well as increased treatment costs due to the high risk of complications and hospital admissions [
13,
14].
AMR is linked to significant economic costs. In Europe, for instance, AMR is estimated to result in an annual economic burden surpassing nine billion euros [
15,
16]. In NHs this concern is exacerbated by the problem of suboptimal antimicrobial prescribing due to lack of diagnostic capacity [
17]. The aging of the population in NHs has presented a representative change with an increase in complexity, including higher rates of multimorbidity and frailty [
18]. This demographic change leads to increased susceptibility to serious complications, especially infectious diseases, highlighting the need for improved antimicrobial stewardship programs and prevention measures for healthcare-associated infections [
2].
Therefore, the aim of our study is to evaluate the clinical characteristics and prevalence of antimicrobial prescriptions for UTIs in geriatric residents residing in NHs throughout Spain.
2. Results
2.1. Number of Registrations
Between February and April 2023, our study included 1505 infection registries in 34 NHs across Spain in five different nodes. During this period, healthcare workers in these facilities documented a total of 719 suspected cases of UTIs in elderly patients. The mean age was 85.5 (SD: 7.9) years, 74,5% were women. Additionally, 42 (5.8%) patients had urinary catheters.
The most common symptoms reported included confusion, foul-smelling and cloudy urine. The most common diagnosis was cystitis. A significant percentage of the patients, specifically 83.6%, underwent a urine dipstick. Among those tested, 40% were prescribed fosfomycin as part of their treatment (
Table 1).
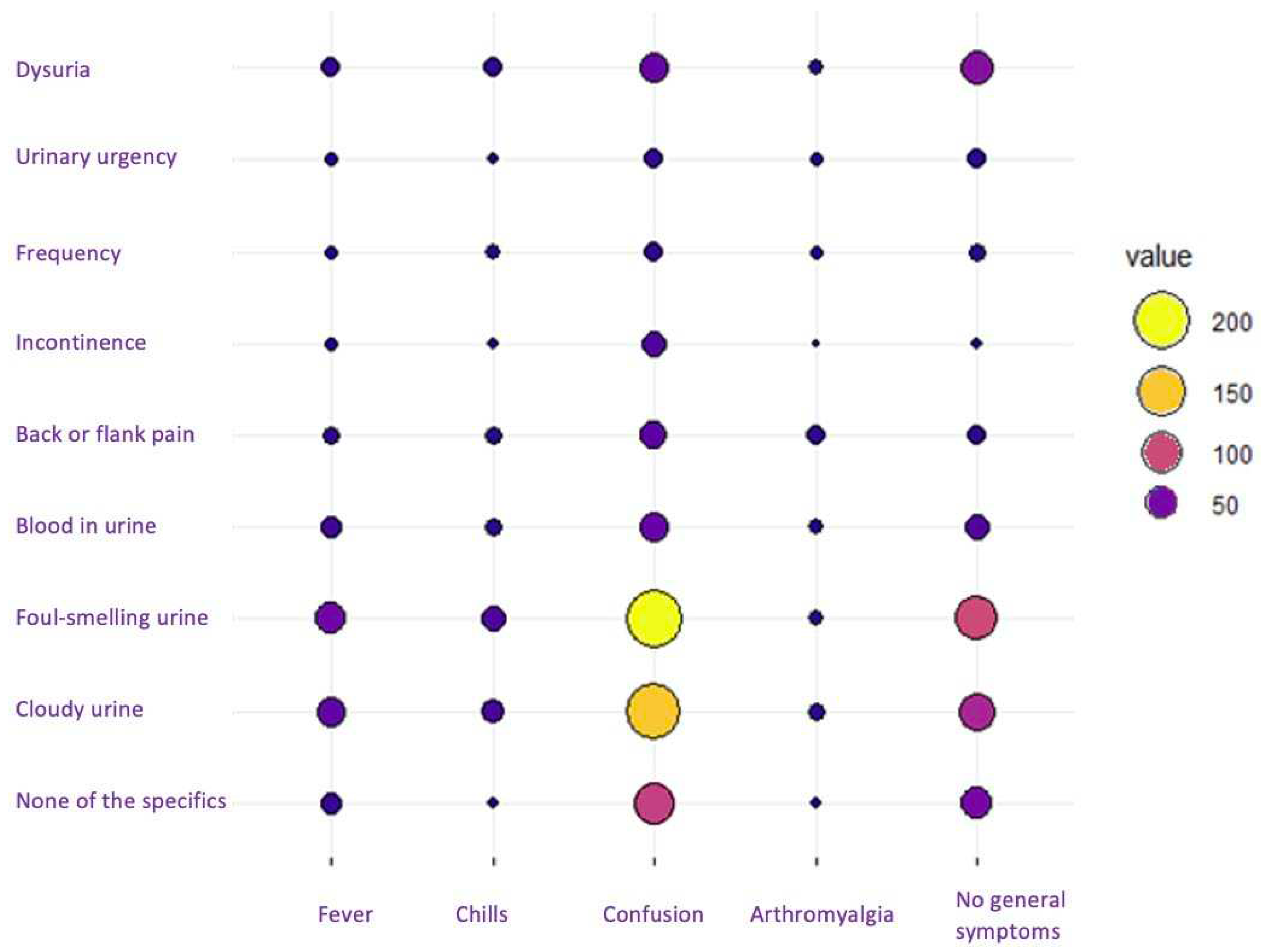
As shown in
Figure 1, when cross-referencing variables between general symptoms and those specific to UTIs, the most prominent circles converged at the intersection of confusion with foul-smelling and cloudy urine, indicating the highest frequency of recorded symptoms.
A total of 46 residents who exhibited neither general nor specific symptoms indicative of a UTI were reported. Within this cohort, 43 persons (93.5%) received antibiotic treatment, predominantly with fosfomycin for 1 or 2 days. Out of the 43 treated cases, urine dipstick diagnostic tests were conducted in 65% of instances, while urine cultures were carried out in 35%.
2.2. Diagnoses
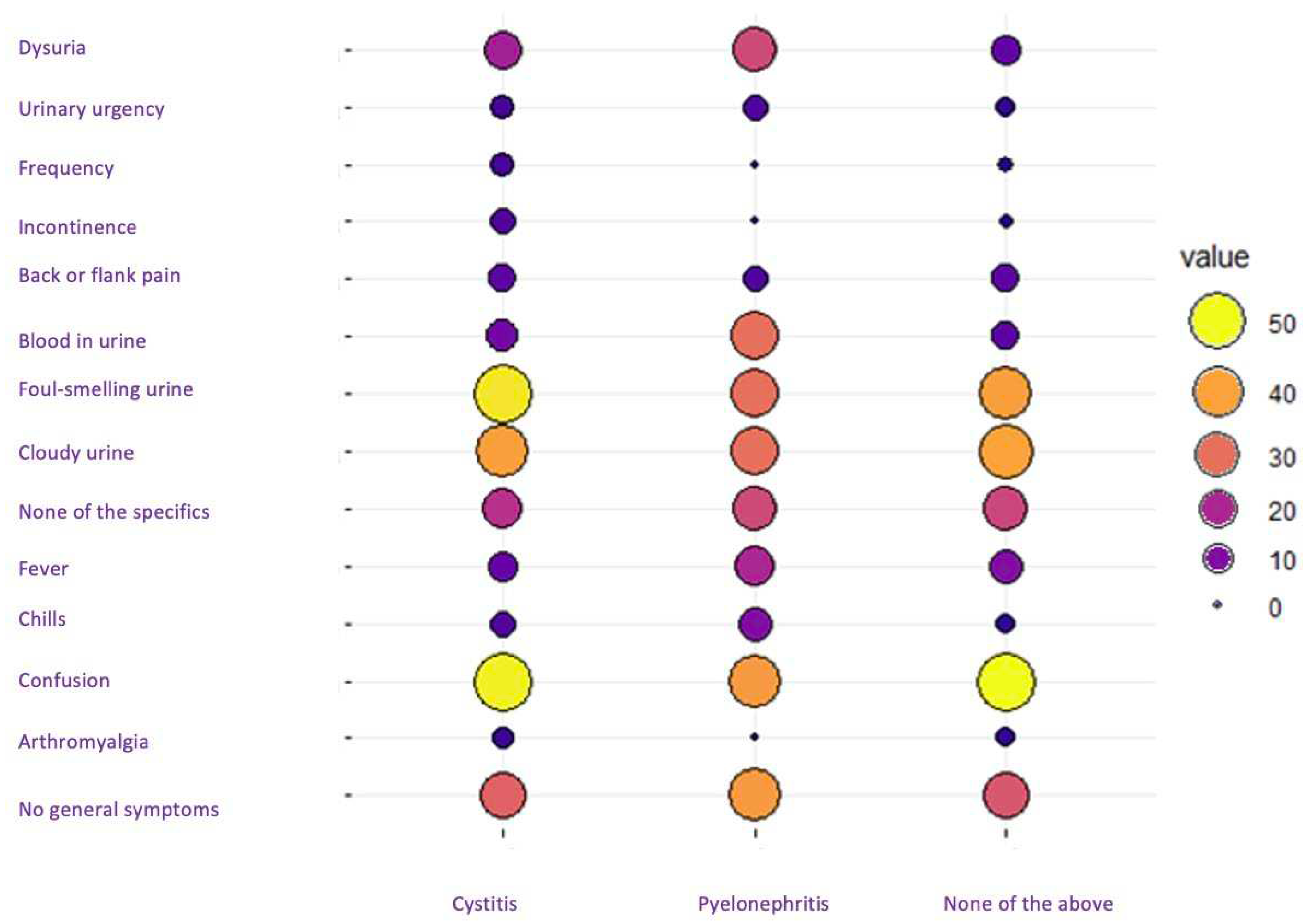
The two primary diagnoses of UTIs presented distinct symptom patterns. Notably, there was a similarity in the percentage of symptoms between cystitis and undetermined infection, suggesting that the cases in which the diagnosis "none of the above" was selected probably corresponded to cases of cystitis. In contrast, the symptomatic pattern of patients diagnosed with pyelonephritis diverged from the other two groups. Patients with pyelonephritis had a higher prevalence of dysuria and gross hematuria and, surprisingly, a lower presence of confusion compared to cases of cystitis and undetermined infection (
Figure 2).
In the examination and diagnosis of residents with and without urinary catheterization, both groups shown a similar distribution of genders. Interestingly, the prevalence of undetermined infections was notably higher among residents with urinary catheterization. Despite the similarity in the most frequent diagnosis between the two groups, this disparity in the incidence of undetermined infections suggests a possible association with the presence of urinary catheters. No statistically significant differences were observed: in patients without catheterization, the p value was 0.1, and with urinary catheterization, the p value was 0.3. (
Table 2).
2.3. Antibiotic Therapy
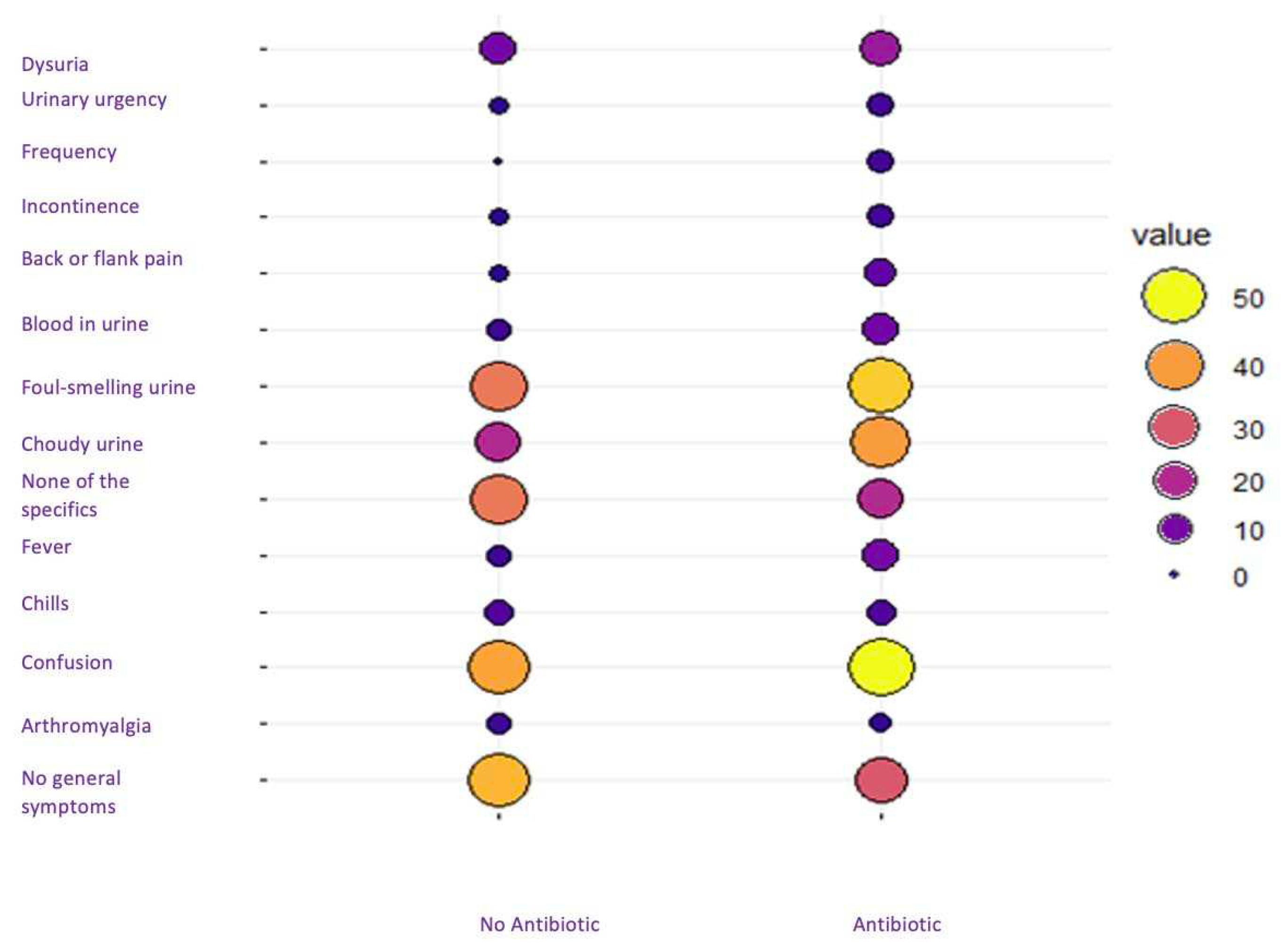
A total of 40 residents (5.5%) were not treated with antibiotics. Interestingly, the untreated group had slightly fewer cases of confusion, cloudy urine, and foul-smelling urine compared to the treated residents. In addition, there was a slightly increased prevalence of no symptoms in the untreated group, although the differences between the two groups was minimal (
Figure 3).
Concerning the diagnosis, cystitis was the most frequently recorded, accounting for almost 70% of the patients; however, only 15% of the patients undertook a urine culture (
Table 3).
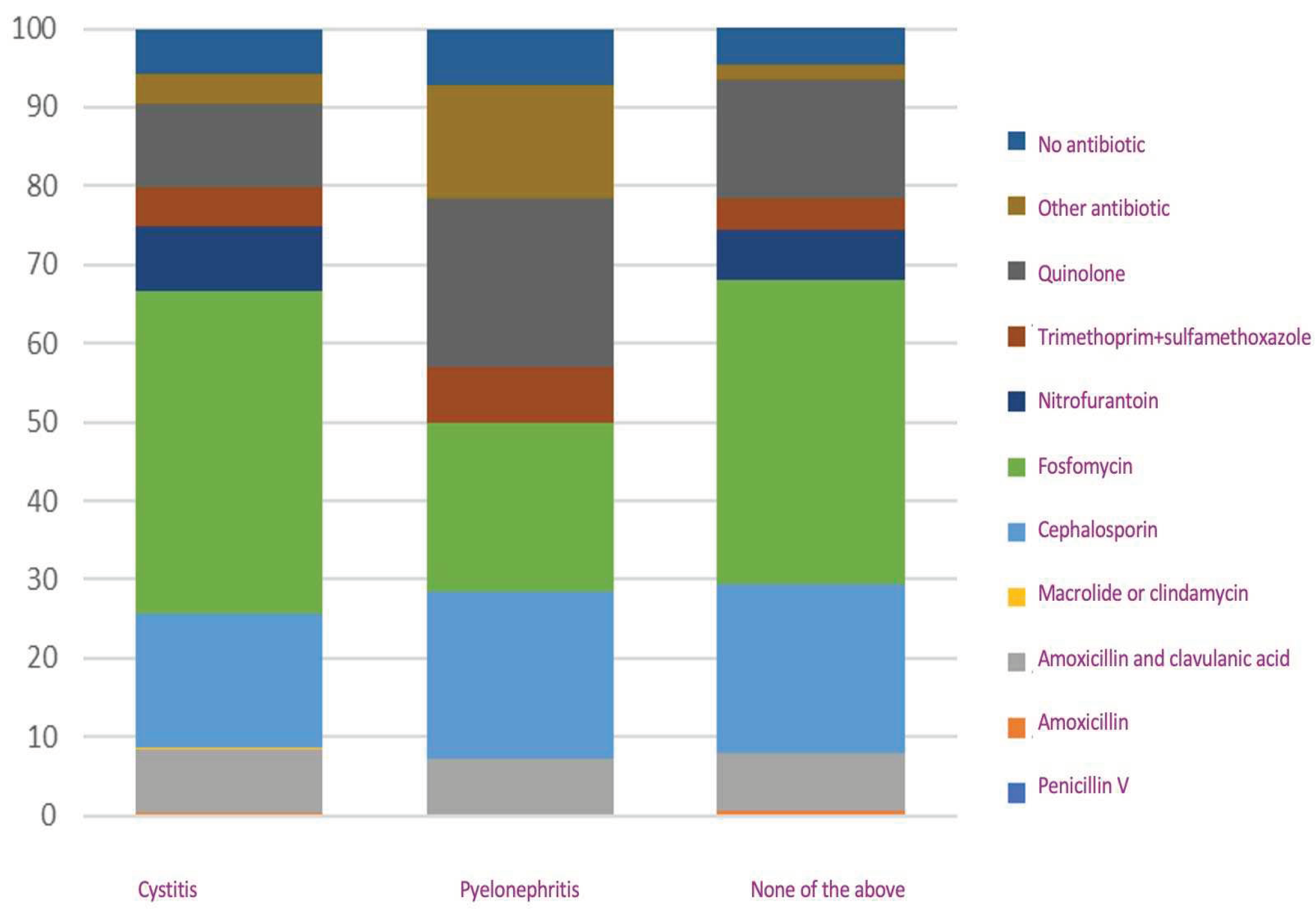
In analyzing antibiotic prescriptions, we identified a similarity in the treatment pattern when the diagnosis "none of the above" was recorded, as opposed to the pattern observed in cases diagnosed as cystitis. However, there was a variation in antibiotic prescribing practices for pyelonephritis when compared with acute cystitis. In cases of pyelonephritis, there was a higher prevalence of quinolones and cephalosporins, whereas fosfomycin was prescribed less frequently compared with acute cystitis. When cross-referencing the variables diagnosis and antibiotic prescription, we found all variables had p= >0.05, except for amoxicillin and clavulanic acid, where we found a p= 0.002 (
Figure 4).
A total of 353 women without an indwelling catheter were diagnosed with uncomplicated cystitis and treated with antibiotics. Nearly half of these treatments corresponded to fosfomycin, with cephalosporins, quinolones, and amoxicillin and clavulanic acid following in frequency. The median treatment duration was observed to be 7 days. For instance, the two-day fosfomycin regimen was more commonly used than the single-dose regimen, and nitrofurantoin was predominantly given for 7 days. Similarly, a substantial number of quinolones and cephalosporins were prescribed for a duration of 7 days. Furthermore, a notable percentage of residents received antibiotic regimens lasting 10 days or more (
Table 4).
3. Discussion
We found a prevalent pattern in which general symptoms, especially confusion, converged with specific symptoms, such as foul-smelling urine and cloudy urine as the most frequently recorded manifestations in cases with a diagnosis of cystitis. In contrast, patients diagnosed with pyelonephritis presented a distinct symptom profile, characterized by a higher prevalence of dysuria and gross hematuria and a lower presence of confusion compared to cases of cystitis and indeterminate infection. However, 6% of residents showed no general or specific symptoms, most of which were treated with antibiotics. Another remarkable finding was the high prescription of cephalosporins and fluoroquinolones and the high use of long therapies, which are not in line with the current clinical guidelines.
3.1. Limitations
The study has several limitations. Participants participated on a voluntary basis and as shown in some studies volunteer professionals might have greater interest in quality improvement programmes and research than the general population of professionals [
19]. From a theoretical perspective, the treatment decision ideally follows the diagnosis decision-making. Diagnostic procedures and treatment decisions are closely intertwined. Healthcare professionals may determine antibiotic prescription concurrently with or even prior to definitively diagnosing the patient’s condition. Subsequently, participants might adapt the diagnosis to align with the treatment decision, potentially introducing a diagnostic misclassification bias [
20].
3.2. Interpretation of Results and Comparison with Literature
The most prevalent symptoms in the current study were confusion, foul-smelling urine and cloudy urine. This contrasts with a cohort study, which identified dysuria, change in urine characteristics, and change in mental status as significantly associated with the combined outcome of bacteriuria plus pyuria [
21]. Yet, in another study, the most common presentation of symptomatic infection in patients was noted as fever without localizing genitourinary symptoms or signs [
22]. Nevertheless, a prevalent challenge is identified in some residents since they may present with altered mental status, falls, lack of appetite, or compromised mobility, coupled with a positive urine culture, were often diagnosed with UTI, leading to concerns of overdiagnosis and inappropriate antibiotic use. This trend, observed in various care centers, highlights the importance of refining diagnostic criteria to ensure accurate identification of UTIs and mitigate the risk of unnecessary antibiotic treatment [
23]. Hence, McGeer’s criteria provide a systematic framework for diagnosing UTIs in both non-catheterized and catheterized residents. These criteria offer a comprehensive and standardized approach to guide healthcare professionals in accurately diagnosing UTIs in diverse clinical scenarios, promoting consistency in evaluation and management [
24]. However, the utilization of these criteria in patients with advanced dementia continues to be a subject of debate, with antibiotic overuse being particularly prevalent within this subgroup. A descriptive study focusing on patients with advanced dementia residing in NHs revealed that merely 19% of probable UTIs, treated with antibiotics, aligned with these diagnostic criteria [
25]. Subsequent research further indicated that despite the widespread administration of antibiotics for suspected UTIs in institutionalized patients with severe dementia, such treatment did not correlate with any noticeable improvement in survival outcomes [
26,
27].
Recognizing that the diagnosis of UTIs cannot rely solely on signs and symptoms in geriatric population; thus, the necessity for additional diagnostic testing becomes evident, particularly to enhance antimicrobial prescribing in NH residents suspected of having a UTI. Bacteriological urine culture has emerged as a standard diagnostic test for UTIs, especially crucial for older individuals given the microbiological differences in this demographic [
26]. Another frequently employed diagnostic tool is the urine dipstick. According to a meta-analysis, urine dipstick tests prove beneficial primarily in ruling out the presence of an infection when both nitrites and leukocyte-esterase yield negative results [
28]. Furthermore, it is noteworthy that a substantial proportion of older adults show bacteria in their urine, a condition known as asymptomatic bacteriuria, i.e. >100,000 colony forming units on urine culture, without urinary tract specific symptoms [
29]. The prevalence of bacteriuria varies significantly, ranging from 25% to 50% in institutionalized women and from 15% to 40% in men [
8,
30]. A Belgian study further supported this observation, revealing an exceptionally high prevalence, reaching 80–90%, of asymptomatic bacteriuria in a specific subpopulation of female NH residents characterized by urinary incontinence or a high degree of dependence and disorientation [
31]. Although asymptomatic bacteriuria may act as a protective barrier to symptomatic UTI, antimicrobial therapy is generally not indicated as it is unnecessary and even harmful [
7,
8,
32].
In our study, we observed that urine culture was conducted in a relatively low proportion, accounting for only 15% of the patients, while the dipstick method was used in 85% of the reported cases. This distribution prompts a discussion on UTI diagnostics in NHs. The predominant use of dipstick testing may be influenced by factors such as ease of implementation, rapid results, and practical considerations, especially in a setting like long-term care facilities where efficient and feasible diagnostic approaches are crucial. The utilization of urine dipstick testing is recommended when symptoms indicative of UTI is present, aiming to either confirm or rule out the diagnosis based on the results, particularly in cases where the test yields negative outcomes [
33]. Consideration should be given to elderly residents with dementia because this population group is more prone to colonization with antibiotic-resistant pathogens than other residents [
34]. This cautious approach emphasizes the importance of personalized and thoughtful diagnostic strategies, especially in vulnerable populations within NH settings. It is remarkable that only 15% of the records requested urine cultures with antibiograms, indicating a relatively low rate of thorough microbiological investigation. However, despite this, there was substantial empirical antibiotic use, underscoring a notable discrepancy between diagnostic testing practices and the prevalence of antibiotic prescribing. This raises concerns about the potential for indiscriminate antibiotic use without a clear understanding of the microbial landscape, emphasizing the importance of aligning therapeutic decisions with comprehensive diagnostic information to optimize patient care and address antibiotic resistance.
A total of 94.4% of the records involved the prescription of antibiotics. The inappropriate use of antibiotics in healthcare settings poses a significant public health threat, primarily due to the rise of antibiotic-resistant bacteria. NHs are particularly notable for their elevated rates of antibiotic use, with estimates suggesting that as much as 75% of prescribed antibiotics may be inappropriate [
35]. The most prevalent antibiotics prescribed in our study were fosfomycin, accounting for 40% of all the cases, followed by cephalosporins and fluoroquinolones. In this line Pulia, et al. found that fluoroquinolones constituted the most prescribed class of antibiotics for UTIs, with a frequency of 35.6% [
35]. While nitrofurantoin and trimethoprim took precedence in a study with a prevalence of 39% and 41%, respectively [
36], fluoroquinolones, sulfonamides, and first-generation cephalosporins were noted as the most prescribed antibiotics in another study [
37]. Moreover, the use of specific antibiotics like levofloxacin, ciprofloxacin, and sulfamethoxazole-trimethoprim was highlighted in different study [
3].
We analyzed the time of antibiotic treatment given in women with simple cystitis without catheterization. The median treatment duration was observed to be 7 days. For instance, the two-day fosfomycin regimen was more commonly used than the single-dose regimen. A substantial number of quinolones, nitrofurantoin and cephalosporins were prescribed for a duration of 7 days. Furthermore, a notable percentage of residents received antibiotic regimens lasting 10 days or more. In this line, a meta-analysis, including 9605 adult non-pregnant women with uncomplicated cystitis, compared the efficacy of 3-day oral antibiotic therapy with prolonged therapy lasting 5 days or more. The findings indicated no significant difference in symptomatic failure rates between the 3-day and prolonged antibiotic regimens, both in the short term (RR 1.16, 95% CI: 0.96-1.41) and long-term follow-up (RR 1.17, 95% CI: 0.99-1.38). However, the 3-day treatment was less effective than prolonged therapy in preventing bacteriological failure, with a relative risk of 1.37 (95% CI: 1.07-1.74) for short-term follow-up and 1.47 (95% CI: 1.22-1.77) for long-term follow-up. More importantly adverse effects were more common in the prolonged therapy group, indicating a relative risk of 0.83 (95% CI: 0.79-0.91) [
38]. In a systematic review encompassing 61 randomized clinical trials and involving 20,780 patients with acute uncomplicated cystitis in adult women, the efficacy of various antibiotic regimens for treating UTIs concluded that treatment durations with third generation and fourth-generation quinolones and pivmecillinam could be shorter than the currently recommended regimens for acute uncomplicated cystitis. Even though there is a suggestion for shorter regimens with other antibiotics, it is emphasized that further research is essential given the currently low quality of supporting evidence [
39].
Previous investigations conducted in long-term care facilities have disclosed that, generally, the decision to initiate antibiotic therapy was deemed appropriate in 49% to 63% of cases [
40,
41,
42]. The Antimicrobial consumption in the EU/EEA (ESAC-Net) report provided insights into the prevalence of antimicrobial agents in long-term care facilities across 24 European Union countries during 2016–2017. Among 181,462 eligible residents in 3052 long-term care facilities, 5035 individuals had received at least one antimicrobial agent on the survey day. These results accounted for an overall prevalence of antimicrobial use at 5.8%, with Spain recording the highest consumption rate at 11.7% [
43]. Another study carried out in Canada found that 2190 (5.9%) long-term care residents were receiving antibiotic prescriptions on the study date [
11]. The Centers for Disease Control and Prevention highlighted a parallel impact in the United States, contributing an additional 20 billion dollars to direct healthcare costs, with an associated annual loss of productivity totaling about 35 billion dollars [
44]. Given the high incidence of antibiotic prescription in this population, which can be both ineffective and potentially harmful, the research group recommended a treatment protocol tailored to the diagnosis of UTIs and highlighting the no need to treat asymptomatic bacteriurias [
20,
45,
46].
The treatment of 43 out of the 46 cases with asymptomatic bacteriuria using antibiotics, and the observation that a majority of these patients underwent dipstick analysis, emphasize the tendency among professionals to treat patients based on urinary dipstick results even in the absence of specific symptoms. This indicates that NH professionals might rely on diagnostics too much, while not paying enough attention to patients’ symptoms [
47].
4. Materials and Methods
4.1. Design
This is a cross-sectional report and is part of a before/after intervention study. We performed a quality control methodology, which is an evidence-based multifaceted intervention to improve quality of care by implementing guidelines. The proposed multifaceted intervention seeks to address all the dimensions of the normalization process theory by facilitating the open discussion of variation in the behavior of the different NHs and strengthening the communication between the nursing staff and the residents. To evaluate the results of the intervention, two point-prevalence audits are performed. These audits are carried out before and after the multifaceted intervention, where the participants register in two charts anonymized data of the consultations they have regarding UTI, in two short periods of time: before the intervention and after the intervention. In this study we describe the characteristics of the registrations of UTIs reported in the first audit in the period from February to April 2023. Thirty-four NHs across Spain were recruited. The participants who documented the infections were either a doctor, a nurse, a nurse assistant, or a nurse helper in each nursing home.
4.2. Audit Registrations
The first day the participants registered the infection audit and counted the percentage of residents on antibiotic use. The chart included cases of UTI, their general symptoms (fever, chills, confusion, arthromyalgias or no general symptoms), specific symptoms (dysuria, urinary urgency, frequency, urinary incontinence, back or flank pain, blood in urine, foul-smelling urine, cloudy urine and no symptoms), diagnosis (cystitis, pyelonephritis, none), and antibiotic treatment penicillin V, amoxicillin, amoxicillin and clavulanic acid, macrolide or clindamycin, cephalosporin, fosfomycin, nitrofurantoin, trimethoprim +sulfamethoxazole, quinolone, another antibiotic, or none.
4.3. Statistical Analysis
We used the Fisher exact test to the frequencies of prescriptions after and before the audit for each group of professionals and setting/country, to test the null hypothesis of no effect of the audit. Antibiotic prescription was considered as the dependent variable (yes/no). Those models control for symptoms and duration, as well as for the demand for antibiotics by the resident. The goodness of fit was assessed using the Wald test of the model, with the deviance test to compare alternative models. Statistical significance was considered with P<0.05. The data were analyzed with the Stata v17 statistical program.
4.4. Informed Consent
All professionals that were willing to participate in the study were given written and verbal information (Participant Information Sheet) and had time to consider the information and asked questions. If they accepted to participate, they were asked to complete and signed 2 copies of the Participant Consent Form on paper, one copy was securely stored in the coordinator site and a copy was given to the participant. Each professional was identified by a code. The audit templates identified the professional by this code.
4.5. Ethical Considerations
The study was reviewed and approved by the Ethics Committee Board of the Primary Care Research Institute IDIAP Jordi Gol, Barcelona, Spain (reference number 22/119-P).
5. Conclusions
The study highlights the prevalence and challenges of urinary tract infections in NH, emphasizing the need for refined diagnostic criteria to avoid overdiagnosis and inappropriate antibiotic use. McGeer’s criteria are suggested for systematic UTI diagnosis, although challenges persist in advanced dementia cases. We also discuss the diagnostic tools employed, such as urine culture and dipstick tests, emphasizing the importance of tailored and thoughtful diagnostic strategies, particularly in vulnerable populations. The discrepancy between diagnostic testing practices and empirical antibiotic prescribing raises concerns about potential indiscriminate antibiotic use. The study also reports a high rate of antibiotic prescription, primarily involving fosfomycin, cephalosporins, and fluoroquinolones, echoing concerns about antibiotic resistance. The analysis of antibiotic treatment duration reveals variability, with a substantial number of residents receiving regimens lasting 10 days or more. We conclude by recommending a tailored treatment protocol for UTIs in long-term care, considering factors such as gender, comorbidities, and infection type, while discouraging unnecessary antibiotic use for asymptomatic conditions.
Author Contributions
Conceptualization, P.M., B.O-B., R.M-B,, A,G-S., R.Mon., R.Mor., A.N.S., J.M-N., C.S.B., C.R.J., C.L.; methodology, B.O-B, A.G-S., R.Mon., R.Mor., C.L.; validation, B.O-B, R.M-B., A.G-S., R.Mon., R.Mor., C.L.; formal analysis, B.O-B., R.Mon., C,L.; investigation, P.M.; resources, A.G-S., R.Mon., R.Mor., C.L.; data curation, A.G-S., R.Mon., C.L.; writing—original draft preparation, P.M., B-O-B., R.M-B, C.L.; writing—review and editing, B.O-B., R.Mon.; supervision, B.O-B., R.M-B., A.G-S., R.Mon., R.Mor., A.N.S., J.M-N., C.S.B., C.R.J., E.L.P., C.L.; project administration, A.G-S., R.Mon., C.L.; funding acquisition, C.L. All authors have read and agreed to the published version of the manuscript.
Funding
Pfizer inc. managed by Global Medical Grants & Global Bridges Mayo Clinic Quality Improvement, grant ID 71388143.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was reviewed and approved by the Ethics Committee Board of the Primary Care Research Institute IDIAP Jordi Gol, Barcelona, Spain (reference number 22/119-P, date of approval 26 October 2022).
Informed Consent Statement
Informed consent was obtained from all the participating nursing home staff involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank all participants who have voluntarily implemented the audit registrations.
Conflicts of Interest
C.L. reports grants from Abbott Diagnostics not related to this study. All other authors declare no competing interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- European Union (EU) Eurostat. Eurostat – Population projections 2023 based. Population Projections in the EU - Statistics Explained.
- Bouza, E.; Navarro, J.A.G.; Alonso, S.; Alonso, J.C.D.; Escobar, C.; Gómez, B.J.F.; Borrás, M.I.G.; Rojas, A.J.G.; Pavón, F.J.G.; Gracia, D.; et al. Infection control in long term care institutions for the elderly: A reflection document on the situation in Spain. Rev. Espanola De Quimioter. 2023, 36, 346–379. [Google Scholar] [CrossRef] [PubMed]
- Alberg, T.; Holen, Ø.; Blix, H.S.; Lindbæk, M.; Bentele, H.; Eriksen, H.M. Antibiotic Use and Infections in Nursing Homes. Tidsskr. Den Nor. legeforening 2017, 137, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Cotter, M.; Donlon, S.; Roche, F.; Byrne, H.; Fitzpatrick, F. Healthcare-associated infection in Irish long-term care facilities: results from the First National Prevalence Study. J. Hosp. Infect. 2012, 80, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Abizanda Soler, P.; R.M.L; et al. Tratado de Medicina Geriátrica. Fundamentos de La Atención Sanitaria a Los Mayores; Elsevier: Spain, 2015. [Google Scholar]
- Ricchizzi, E.; Latour, K.; Kärki, T.; Buttazzi, R.; Jans, B.; Moro, M.L.; Nakitanda, O.A.; Plachouras, D.; Monnet, D.L.; Suetens, C.; et al. Antimicrobial use in European long-term care facilities: results from the third point prevalence survey of healthcare-associated infections and antimicrobial use, 2016 to 2017. Eurosurveillance 2018, 23, 1800394. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B. The epidemiology of urinary tract infection. Nat. Rev. Urol. 2010, 7, 653–660. [Google Scholar] [CrossRef]
- Nicolle, L.E. ASYMPTOMATIC BACTERIURIA IN THE ELDERLY. Infect. Dis. Clin. North Am. 1997, 11, 647–662. [Google Scholar] [CrossRef]
- Arnold, S.H.; Jensen, J.N.; Kousgaard, M.B.; Siersma, V.; Bjerrum, L.; Holm, A. Reducing Antibiotic Prescriptions for Urinary Tract Infection in Nursing Homes Using a Complex Tailored Intervention Targeting Nursing Home Staff: Protocol for a Cluster Randomized Controlled Trial. JMIR Res. Protoc. 2020, 9, e17710. [Google Scholar] [CrossRef] [PubMed]
- van Buul, L.W.; Veenhuizen, R.B.; Achterberg, W.P.; Schellevis, F.G.; Essink, R.T.G.M.; de Greeff, S.C.; Natsch, S.; van der Steen, J.T.; Hertogh, C.M.P.M. Antibiotic Prescribing In Dutch Nursing Homes: How Appropriate Is It? J. Am. Med. Dir. Assoc. 2015, 16, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Daneman, N.; Gruneir, A.; Newman, A.; Fischer, H.D.; Bronskill, S.E.; Rochon, P.A.; Anderson, G.M.; Bell, C.M. Antibiotic use in long-term care facilities. J. Antimicrob. Chemother. 2011, 66, 2856–2863. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development - European Centre for Disease Prevention and Control. Antimicrobial Resistance Tackling the Burden in the European Union Briefing Note for EU/EEA Countries Contents; 2019.
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Cassone, M.; Mody, L. Colonization with Multidrug-Resistant Organisms in Nursing Homes: Scope, Importance, and Management. Curr. Geriatr. Rep. 2015, 4, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.; Lane, N.; Tanuseputro, P.; Mojaverian, N.; Talarico, R.; Wodchis, W.P.; Bronskill, S.E.; Hsu, A.T. Increasing Complexity of New Nursing Home Residents in Ontario, Canada: A Serial Cross-Sectional Study. J. Am. Geriatr. Soc. 2020, 68, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.; Robertson, N.; Farooqi, A. Audit in general practice: factors influencing participation. BMJ 1995, 311, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Advani, S.D.; Claeys, K. Behavioral Strategies in Diagnostic Stewardship. Infect. Dis. Clin. North Am. 2023, 37, 729–747. [Google Scholar] [CrossRef] [PubMed]
- Juthani-Mehta, M.; Quagliarello, V.; Perrelli, E.; Towle, V.; Van Ness, P.H.; Tinetti, M. Clinical Features to Identify Urinary Tract Infection in Nursing Home Residents: A Cohort Study. J. Am. Geriatr. Soc. 2009, 57, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, L.E. Urinary Tract Infections in the Older Adult. Clin. Geriatr. Med. 2016, 32, 523–538. [Google Scholar] [CrossRef]
- Juthani-Mehta, M.; Quagliarello, V.; Perrelli, E.; Towle, V.; Van Ness, P.H.; Tinetti, M. Clinical Features to Identify Urinary Tract Infection in Nursing Home Residents: A Cohort Study. J. Am. Geriatr. Soc. 2009, 57, 963–970. [Google Scholar] [CrossRef]
- McGeer, A.; Campbell, B.; Emori, T.; Hierholzer, W.J.; Jackson, M.M.; Nicolle, L.E.; Peppier, C.; Rivera, A.; Schollenberger, D.G.; Simor, A.E.; et al. Definitions of infection for surveillance in long-term care facilities. Am. J. Infect. Control. 1991, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.L.; Shaffer, M.L.; Loeb, M.B.; Givens, J.L.; Habtemariam, D.; Kiely, D.K.; D’agata, E. Infection Management and Multidrug-Resistant Organisms in Nursing Home Residents With Advanced Dementia. JAMA Intern. Med. 2014, 174, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Monette, J.; Miller, M.A.; Monette, M.; Laurier, C.; Boivin, J.; Sourial, N.; Le Cruguel, J.; Vandal, A.; Cotton-Montpetit, M. Effect of an Educational Intervention on Optimizing Antibiotic Prescribing in Long-Term Care Facilities. J. Am. Geriatr. Soc. 2007, 55, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Dufour, A.B.; Shaffer, M.L.; D’Agata, E.M.; Habtemariam, D.; Mitchell, S.L. Survival After Suspected Urinary Tract Infection in Individuals with Advanced Dementia. J. Am. Geriatr. Soc. 2015, 63, 2472–2477. [Google Scholar] [CrossRef] [PubMed]
- Devillé, W.L.J.M.; Yzermans, J.C.; Van Duijn, N.P.; Bezemer, P.D.; Van Der Windt, D.A.W.M.; Bouter, L.M. The urine dipstick test useful to rule out infections. A meta-analysis of the accuracy. BMC Urol. 2004, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.T.; Nicolle, L.E.; Norman, D.C. Management of Complicated Urinary Tract Infection in Older Patients. J. Am. Geriatr. Soc. 1996, 44, 1235–1241. [Google Scholar] [CrossRef]
- Nicolle, L.E. Urinary Tract Infections in Long-Term–Care Facilities. Infect. Control Hosp. Epidemiol. 2001, 22, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Biggel, M.; Heytens, S.; Latour, K.; Bruyndonckx, R.; Goossens, H.; Moons, P. Asymptomatic bacteriuria in older adults: the most fragile women are prone to long-term colonization. BMC Geriatr. 2019, 19, 170. [Google Scholar] [CrossRef]
- Trautner, B.W. Asymptomatic bacteriuria: when the treatment is worse than the disease. Nat. Rev. Urol. 2011, 9, 85–93. [Google Scholar] [CrossRef]
- Juthani-Mehta, M.; Tinetti, M.; Perrelli, E.; Towle, V.; Quagliarello, V. Role of Dipstick Testing in the Evaluation of Urinary Tract Infection in Nursing Home Residents. Infect. Control. Hosp. Epidemiology 2007, 28, 889–891. [Google Scholar] [CrossRef]
- Pop-Vicas, A.; Mitchell, S.L.; Kandel, R.; Schreiber, R.; D’Agata, E.M.C. Multidrug-Resistant Gram-Negative Bacteria in a Long-Term Care Facility: Prevalence and Risk Factors. J. Am. Geriatr. Soc. 2008, 56, 1276–1280. [Google Scholar] [CrossRef] [PubMed]
- Pulia, M.; Kern, M.; Schwei, R.J.; Shah, M.N.; Sampene, E.; Crnich, C.J. Comparing appropriateness of antibiotics for nursing home residents by setting of prescription initiation: a cross-sectional analysis. Antimicrob. Resist. Infect. Control. 2018, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Tandan, M.; O’connor, R.; Burns, K.; Murphy, H.; Hennessy, S.; Roche, F.; Donlon, S.; Cormican, M.; Vellinga, A. A comparative analysis of prophylactic antimicrobial use in long-term care facilities in Ireland, 2013 and 2016. Eurosurveillance 2019, 24, 1800102. [Google Scholar] [CrossRef] [PubMed]
- Riester, M.R.; Deng, Y.; Zullo, A.R. Antibiotic Prescribing in United States Nursing Homes, 2013–2017. J. Infect. Dis. 2023, 228, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Katchman, E.A.; Milo, G.; Paul, M.; Christiaens, T.; Baerheim, A.; Leibovici, L. Three-day vs longer duration of antibiotic treatment for cystitis in women: Systematic review and meta-analysis. Am. J. Med. 2005, 118, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Kim, J.H.; Lee, J.Y.; Ku, N.S.; Lee, H.S.; Park, J.-Y.; Kim, J.W.; Kim, K.J.; Cho, K.S. Reappraisal of the treatment duration of antibiotic regimens for acute uncomplicated cystitis in adult women: a systematic review and network meta-analysis of 61 randomised clinical trials. Lancet Infect. Dis. 2020, 20, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.J.; McLellan, S.C.; Cheng, A.C.; Culton, J.M.; Parikh, S.N.; Peleg, A.Y.; Kong, D.C.M. Surveillance of infection burden in residential aged care facilities. The Medical Journal of Australia 2012, 196, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Stuart, R.L.; Wilson, J.; Bellaard-Smith, E.; Brown, R.; Wright, L.; Vandergraaf, S.; Gillespie, E.E. Antibiotic use and misuse in residential aged care facilities. Intern. Med. J. 2012, 42, 1145–1149. [Google Scholar] [CrossRef]
- Zimmer, J.G.; Bentley, D.W.; Valenti, W.M.; Watson, N.M. Systemic Antibiotic Use in Nursing Homes: A Quality Assessment. J. Am. Geriatr. Soc. 1986, 34, 703–710. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control European Centre for Disease Prevention and Control, 2020.
- Antibiotic Resistance Threats in the United States, 2019; Atlanta, Georgia, 2019.
- Abascal, N.P.; Catalá, J.C.; Cruz-Jentoft, A. Protocolo diagnóstico y terapéutico de la infección urinaria en el paciente mayor. Medicine - Form Med Cont 2022, 13, 3682–3685. [Google Scholar] [CrossRef]
- Rowe, T.A.; Juthani-Mehta, M. Diagnosis and Management of Urinary Tract Infection in Older Adults. Infect. Dis. Clin. North Am. 2013, 28, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.; Vleeming, M.; Giorgi, W.; Dinant, G.-J.; Cals, J.; de Bont, E. Patients’ Experiences, Expectations, Motivations, and Perspectives around Urinary Tract Infection Care in General Practice: A Qualitative Interview Study. Antibiotics 2023, 12, 241. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).