Submitted:
29 December 2023
Posted:
03 January 2024
You are already at the latest version
Abstract
Keywords:
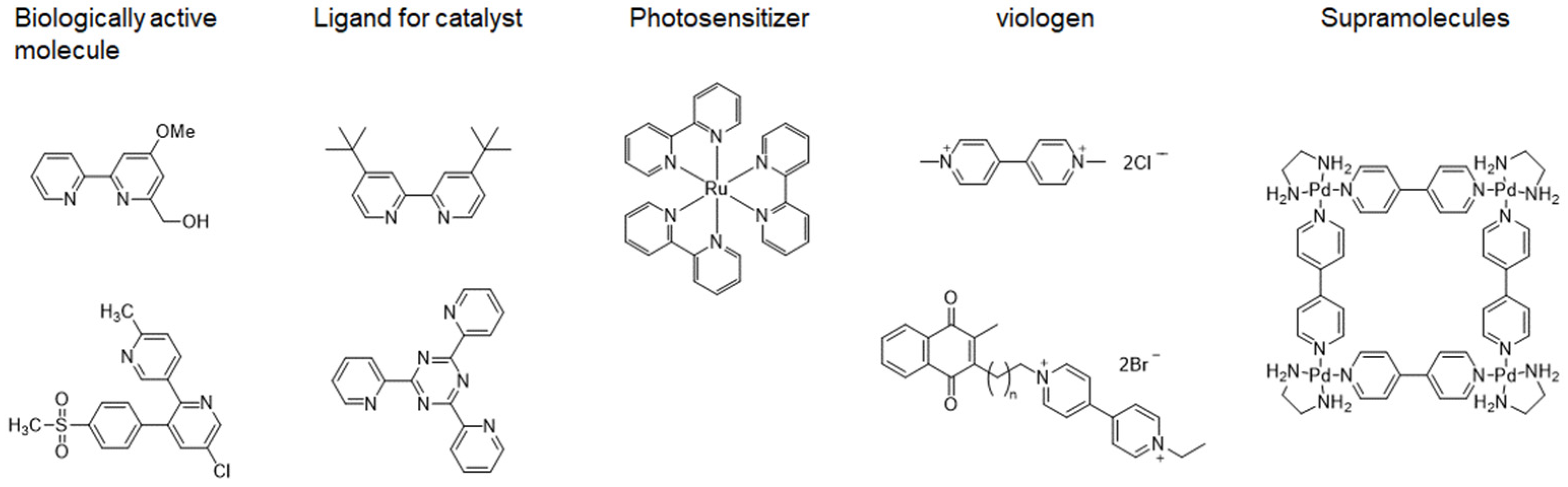
1. Introduction
2. Metal-catalyzed cross-coupling (Suzuki, Negishi, and Stille coupling reactions)
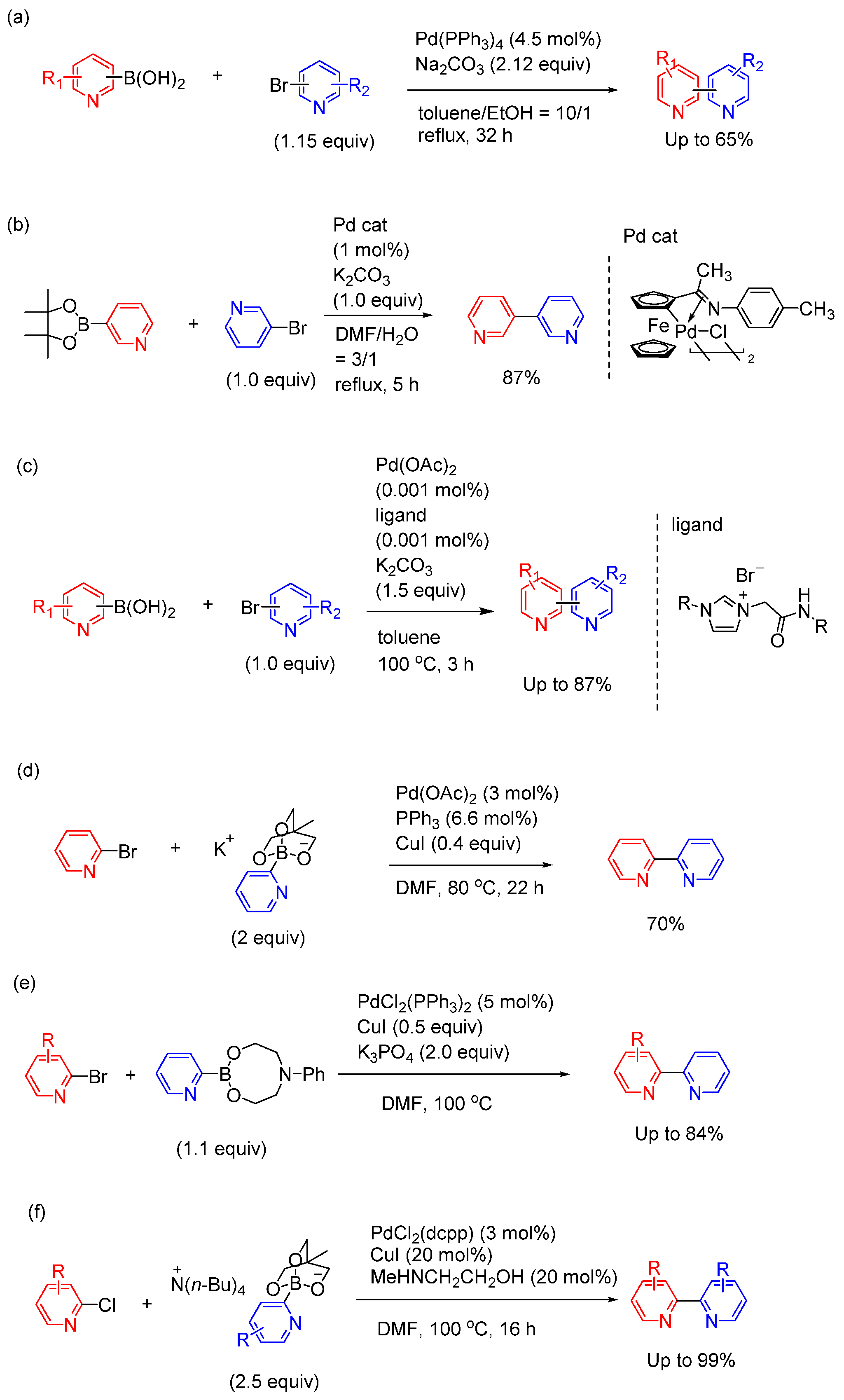
2.1. Suzuki coupling reaction in homogeneous catalytic system
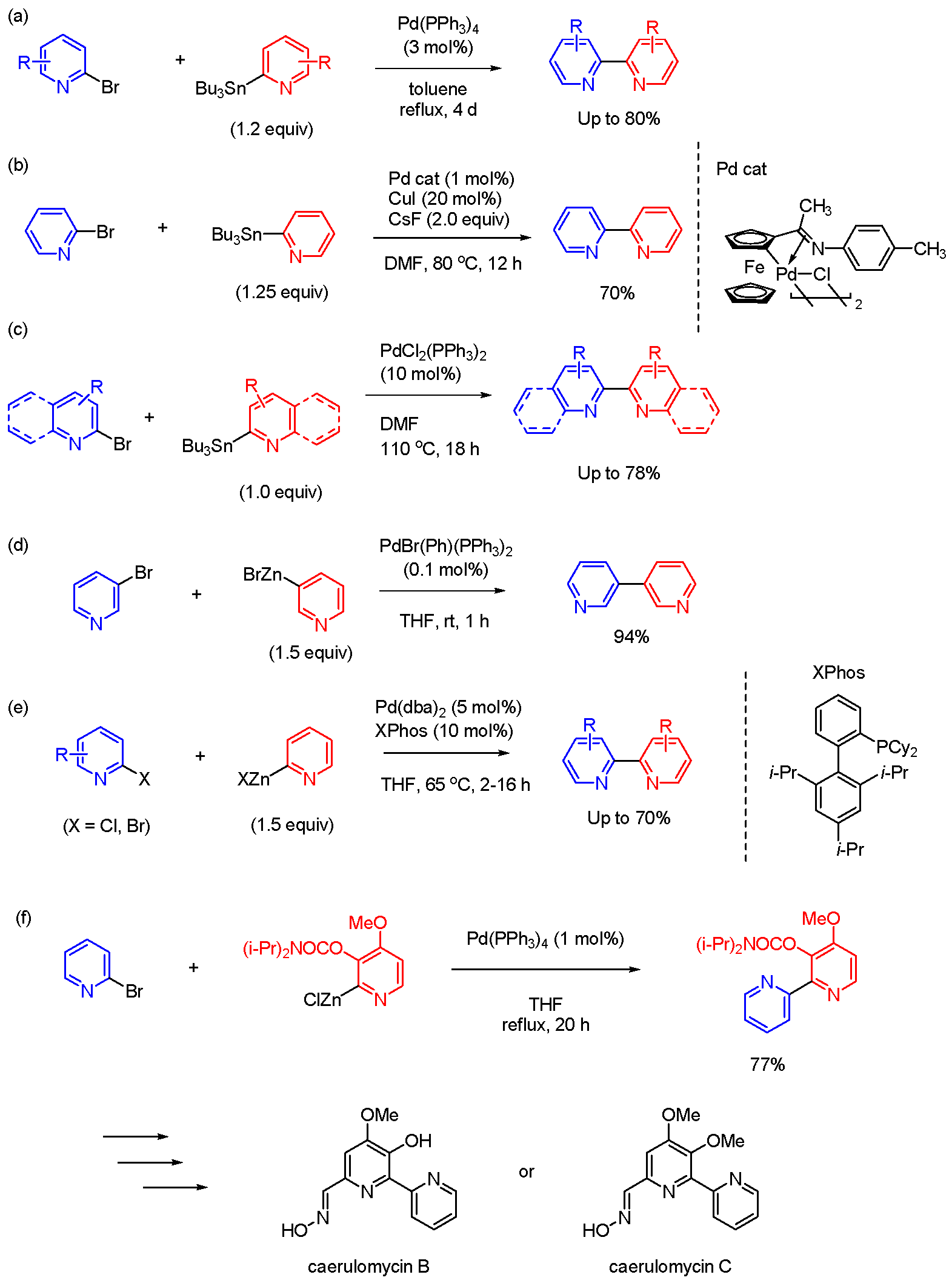
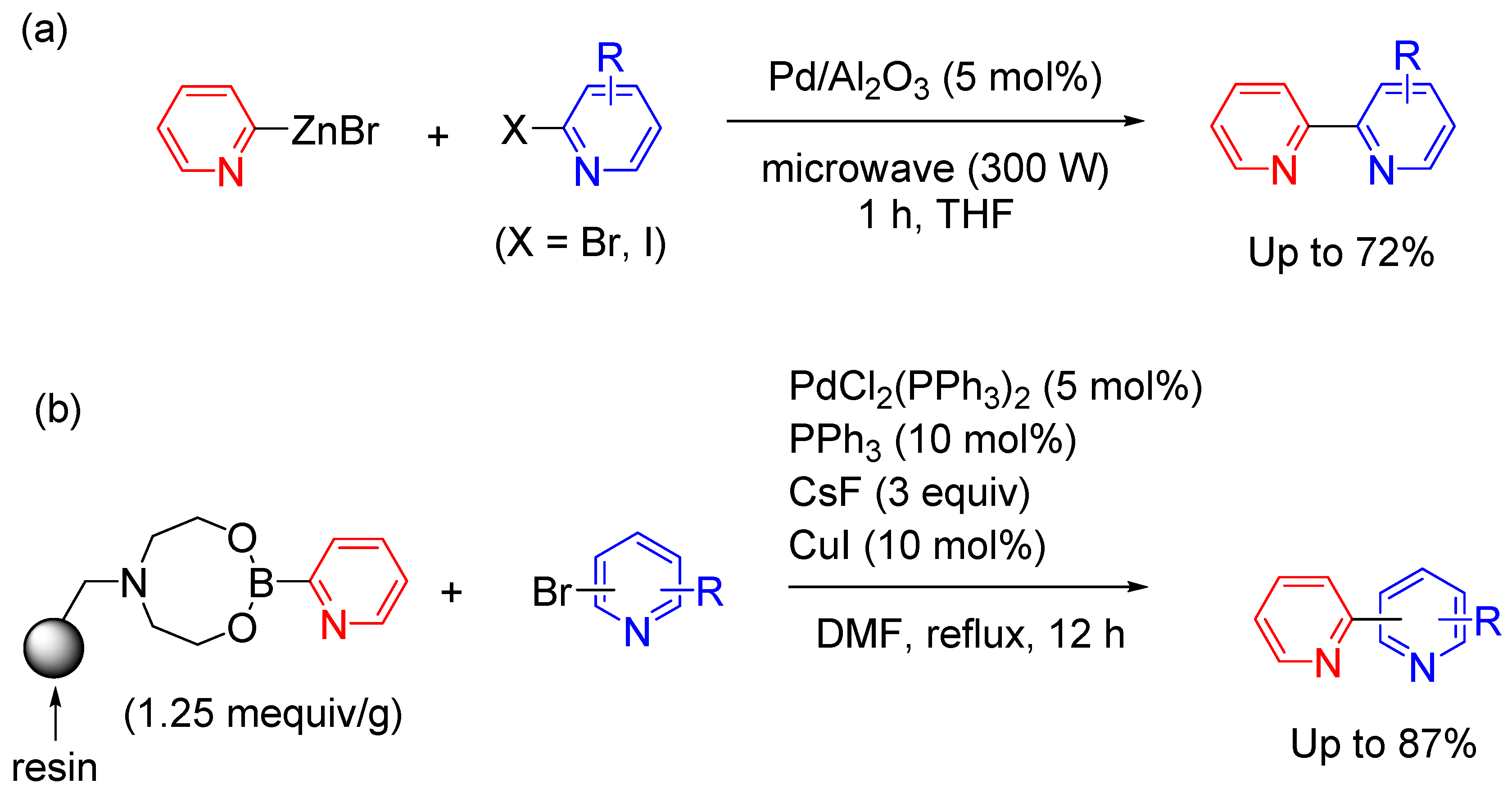
2.2. Negishi and Stille coupling reactions in homogeneous catalytic system
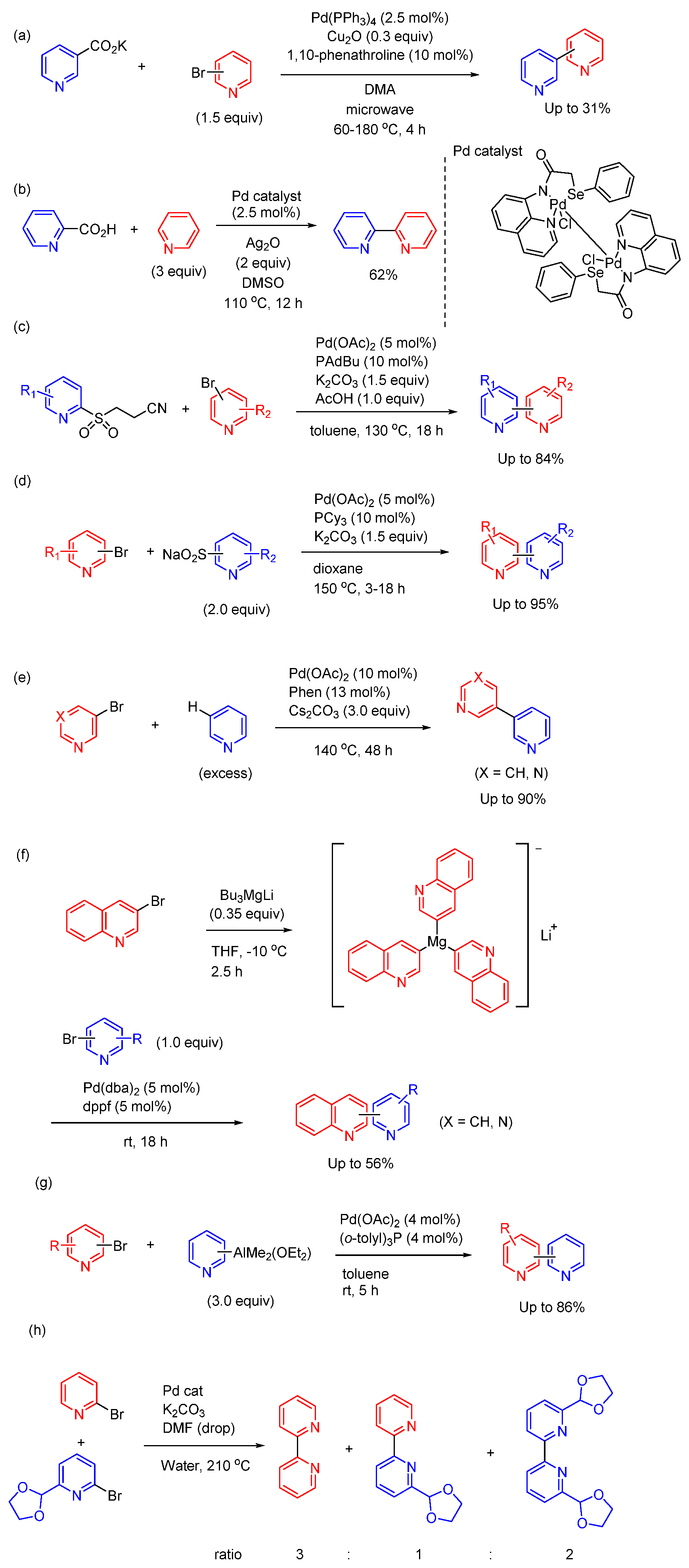
2.3. Other cross-coupling reaction in homogeneous catalytic system
2.4. Cross-coupling reaction in heterogeneous catalytic system
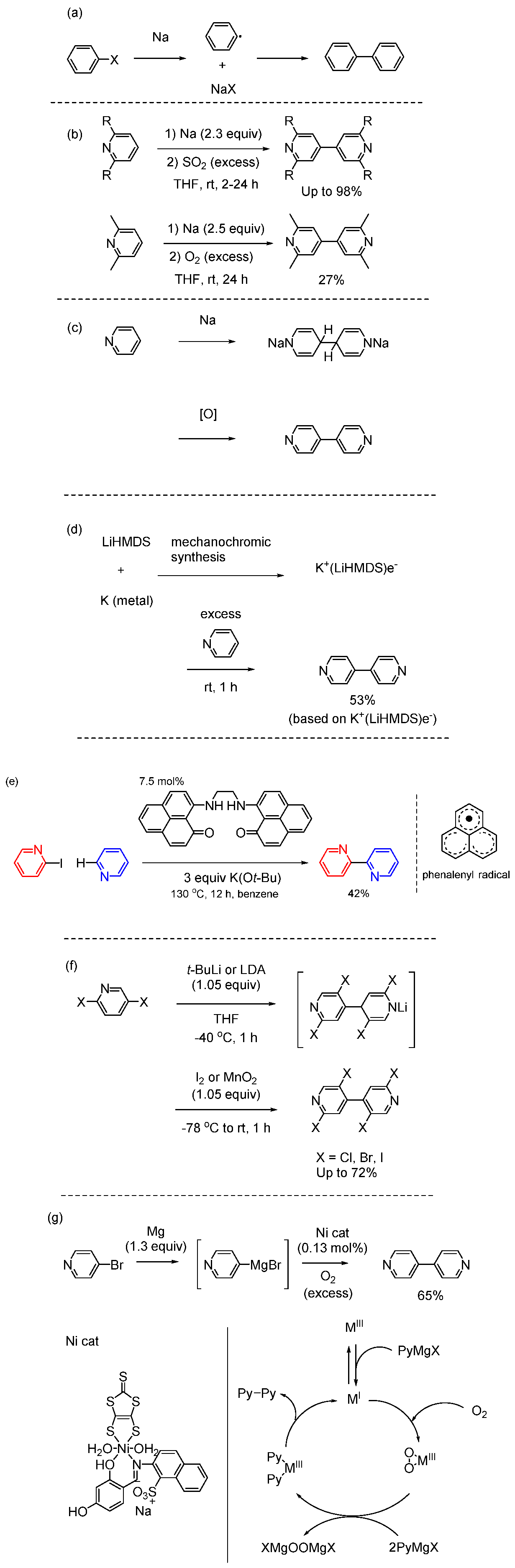
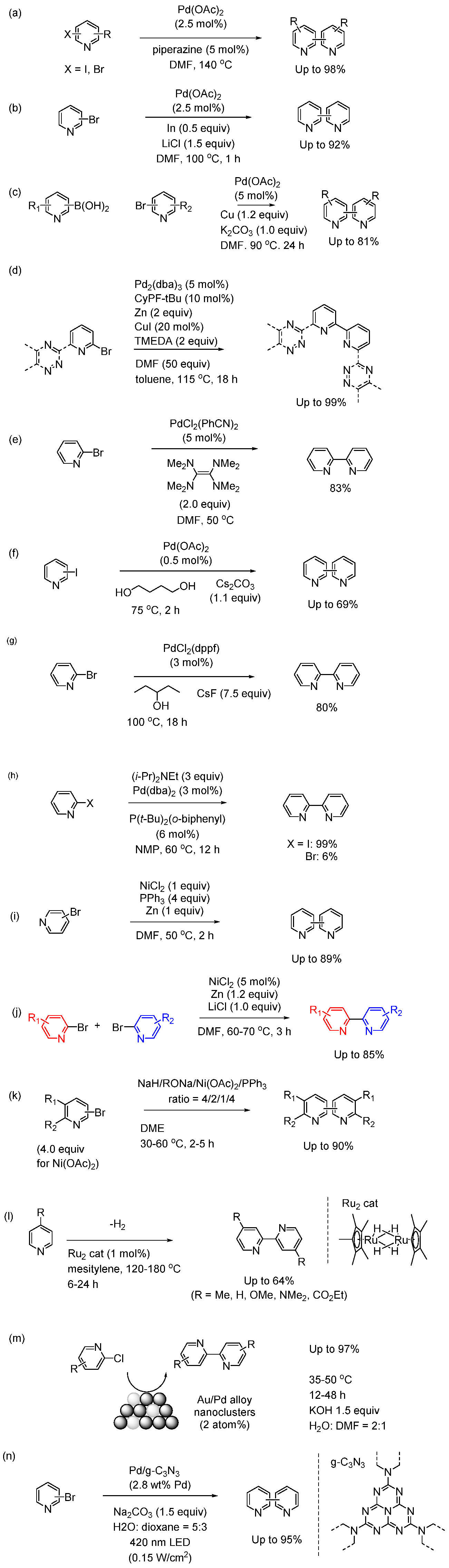
3. Metal-catalyzed homo-coupling reaction (Wurtz coupling and Ullmann coupling)
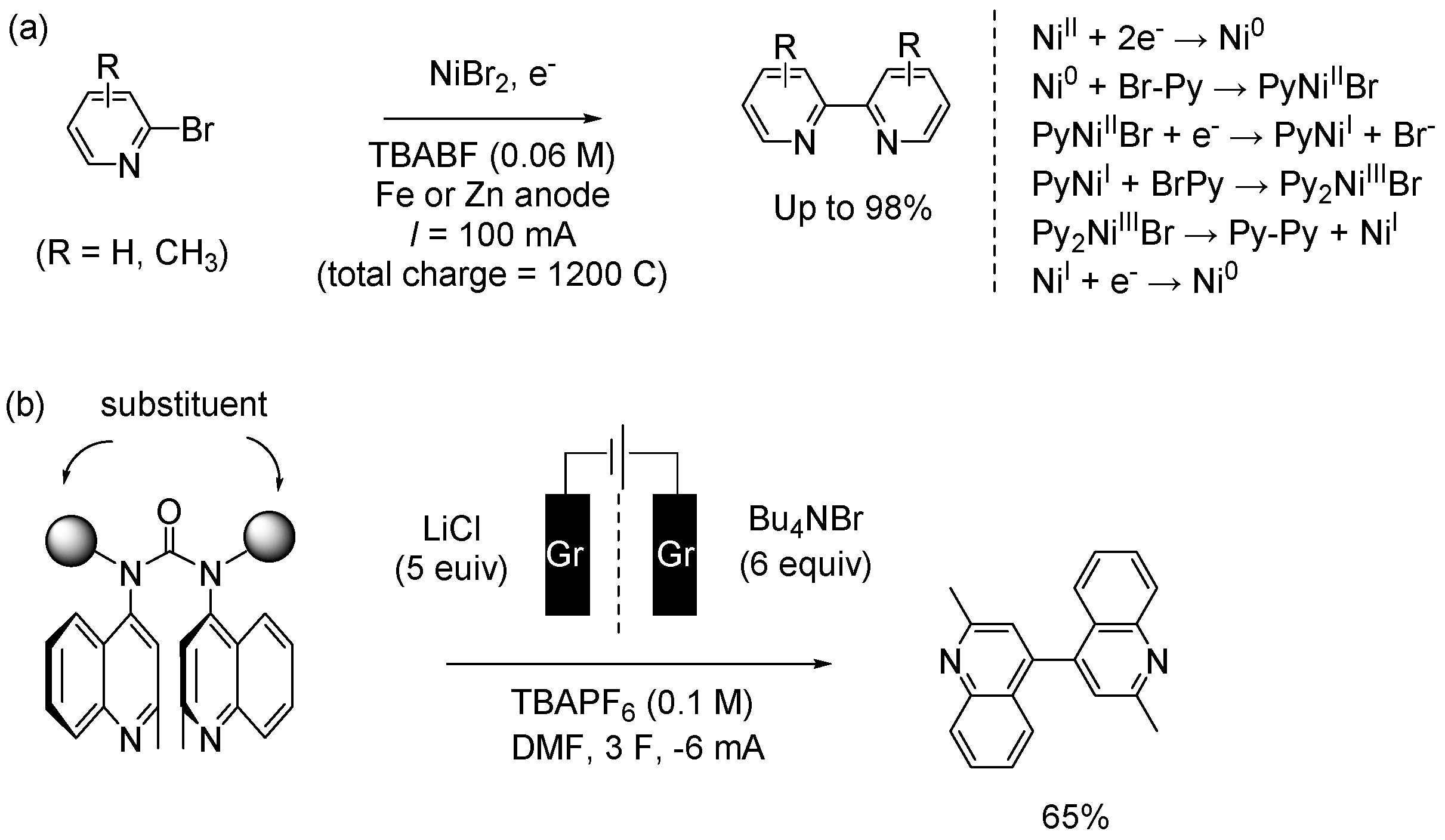
4. Electrochemical methods
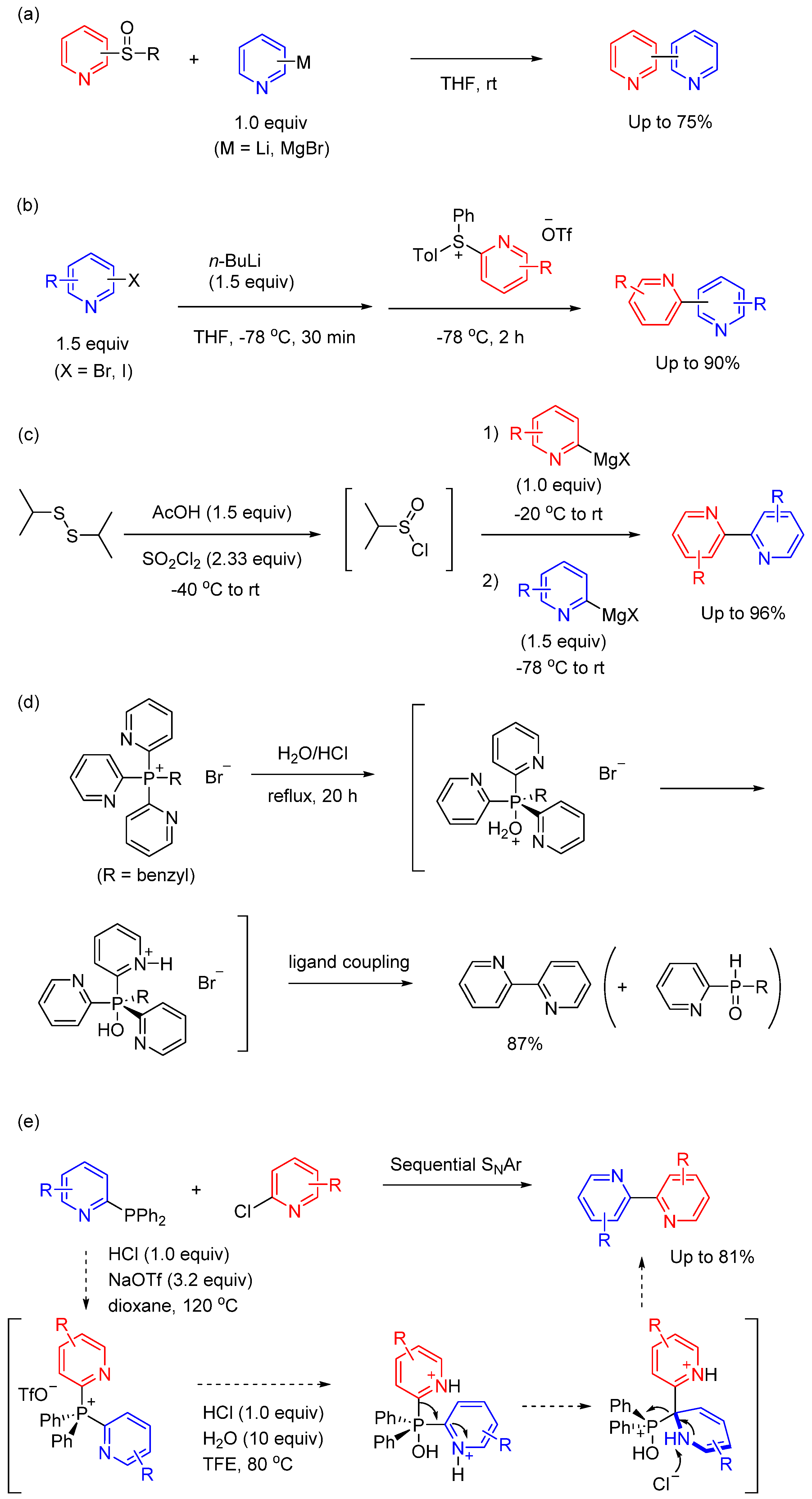
5. Other methods
6. Summary
Funding
Acknowledgments
Conflicts of Interest
References
- Kaes, C.; Katz, A.; Hosseini, M. W. Bipyridine: The Most Widely Used Ligand. A Review of Molecules Comprising at Least Two 2,2‘-Bipyridine Units. Chem. Rev. 2000, 100, 3553–3590. [Google Scholar] [CrossRef] [PubMed]
- Hassan, J.; Sevignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl−Aryl Bond Formation One Century after the Discovery of the Ullmann Reaction. Chem. Rev. 2002, 102, 1359–1470. [Google Scholar] [CrossRef] [PubMed]
- Newkome, G. R.; Patri, A. K.; Holder, E.; Schubert, U. S. Synthesis of 2,2’-Bipyridines: Versatile Building Blocks for Sexy Architectures and Functional Nanomaterials. Eur. J. Org. Chem. 2004, 235–254. [Google Scholar] [CrossRef]
- Schubert, U. S.; Kersten, J. L.; Pemp, A. E.; Eisenbach, C. D.; Newkome, G. R. A New Generation of 6,6′-Disubstituted 2,2′-Bipyridines: Towards Novel Oligo(bipyridine) Building Blocks for Potential Applications in Materials Science and Supramolecular Chemistry. Eur. J. Org. Chem. 1998, 2573–2581. [Google Scholar]
- Mathieu, J.; Fraisse, B.; Lacour, D.; Ghermani, N.; Montaigne, F.; Marsura, A. An Original Supramolecular Helicate from a Bipyridine–Bipyrazine Ligand Strand and NiII by Self-Assembly. Eur. J. Inorg. Chem. 2006, 133–136. [Google Scholar]
- Liu, B.; Yu, W. L.; Pei, J.; Liu, S. Y.; Lai, Y. H.; Huang, W. Design and Synthesis of Bipyridyl-Containing Conjugated Polymers: Effects of Polymer Rigidity on Metal Ion Sensing. Macromolecules 2001, 34, 7932–7940. [Google Scholar] [CrossRef]
- Balzani, V.; Juris, A. Photochemistry and photophysics of Ru(II) polypyridine complexes in the Bologna group. From early studies to recent developments. Coord. Chem. Rev. 2001, 211, 97–115. [Google Scholar] [CrossRef]
- Blakemore, D. “Synthetic Methods in Drug Discovery”, The Royal Society of Chemistry, 2016, vol. 1, pp. 1‒69.
- Constable, E. C.; Housecroft, C. E. The Early Years of 2,2′-Bipyridine—A Ligand in Its Own Lifetime. Molecules 2019, 24, 3951. [Google Scholar] [CrossRef] [PubMed]
- Chelucci, G.; Thummel, R. P. Chiral 2,2′-Bipyridines, 1,10-Phenanthrolines, and 2,2′:6′,2′′-Terpyridines: Syntheses and Applications in Asymmetric Homogeneous Catalysis. Chem. Rev. 2002, 102, 3129–3170. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, N. C. Chiral 2,2’-bipyridines: ligands for asymmetric induction. J. Chem. Soc. Perkin Trans. 1 2002, 1831–1842. [Google Scholar] [CrossRef]
- Campeau, L.-C. Fagnou, K. Applications of and alternatives to π-electron-deficient azine organometallics in metal catalyzed cross-coupling reactions. Chem. Soc. Rev. 2007, 36, 1058–1068. [Google Scholar] [CrossRef]
- Hapke, M.; Brandt, L.; Lützen, A. Versatile tools in the construction of substituted 2,2’-bipyridines—cross-coupling reactions with tin, zinc and boron compounds. Chem. Soc. Rev. 2008, 37, 2782–2797. [Google Scholar] [CrossRef]
- Miyamura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Metal-catalyzed cross-coupling reactions, Diederich, F. Stang, P. J. Eds. Wiley-VCH, Weinheim, Germany, 1998.
- Stanforth, S. P. Catalytic cross-coupling reactions in biaryl synthesis. Tetrahedron 1998, 54, 263–303. [Google Scholar] [CrossRef]
- Suzuki, A. Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles, 1995–1998. J. Organomet. Chem. 1999, 576, 147–168. [Google Scholar] [CrossRef]
- Kotha, S.; Lahiri, K.; Kashinath, D. Recent applications of the Suzuki–Miyaura cross-coupling reaction in organic synthesis. Tetrahedron 2002, 58, 9633–9695. [Google Scholar] [CrossRef]
- Suzuki, A.; Yamamoto, Y. Cross-coupling Reactions of Organoboranes: An Easy Method for C‒C Bonding. Chem. Lett. 2011, 40, 894–901. [Google Scholar] [CrossRef]
- Miyaura, N. Organoboron Compounds. Top Curr. Chem. 2002, 219, 11–59. [Google Scholar]
- Matondo, H.; Ouhaja, N.; Souirti, S.; Baboulene, M. Oxidation and Suzuki Cross-Coupling Reaction from Azaheteroarylboronic Acids. Main Group Met. Chem. 2002, 25, 163–167. [Google Scholar] [CrossRef]
- Denton, T. T.; Zhang, X.; Cashman, J. R. 5-Substituted, 6-Substituted, and Unsubstituted 3-Heteroaromatic Pyridine Analogues of Nicotine as Selective Inhibitors of Cytochrome P-450 2A6. J. Med. Chem. 2005, 48, 224–239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, L.; Song, M.; Mak, T. C. W.; Wu, Y. Highly efficient cyclopalladated ferrocenylimine catalyst for Suzuki cross-coupling reaction of 3-pyridylboronic pinacol ester with aryl halides. J. Organomet. Chem. 2006, 691, 1301–1306. [Google Scholar] [CrossRef]
- Kumar, M. R.; Park, K.; Lee, S. Synthesis of Amido-N-imidazolium Salts and their Applications as Ligands in Suzuki–Miyaura Reactions: Coupling of Hetero- aromatic Halides and the Synthesis of Milrinone and Irbesartan. Adv. Synth. Catal. 2010, 352, 3255–3266. [Google Scholar] [CrossRef]
- Matondo et al. reported 2-pyridylboronic acid by the hydrolysis of 2-pyridyl(tristrimethylsilyl)borate. See: Motondo, H.; Souirti, S.; Baboulène, M. Improved Synthesis of Azaheteroarylboronic Acids Using tris-Trimethylsilylborate Under Mild Conditions. Synth. Commun. 2003, 33, 795–800. [CrossRef]
- Cool, X. A. F.; de Gombert, A.; McKnight, J.; Pantaine, L. R. E.; Wills, M. C. The 2-Pyridyl Problem: Challenging Nucleophiles in Cross-Coupling Arylations. Angew. Chem. Int. Ed. 2021, 60, 11068–11091. [Google Scholar]
- Yamamoto, Y.; Takizawa, M.; Yu, X.-Q.; Miyaura, N. Palladium-Catalyzed Cross-Coupling Reaction of Heteroaryltriolborates with Aryl Halides for Synthesis of Biaryls. Heterocycles 2010, 80, 359–368. [Google Scholar] [CrossRef]
- Jones, N. A.; Antoon, J. W.; Bowie, Jr. A. L.; Borak, J. B.; Stevens, E. P. Synthesis of 2,2′-bipyridyl-type compounds via the Suzuki-Miyaura cross-coupling reaction. J. Heterocyclic Chem. 2007, 44, 363–367. [Google Scholar] [CrossRef]
- Sakashita, S.; Takizawa, M.; Sugai, J.; Ito, H.; Yamamoto, Y. Tetrabutylammonium 2-Pyridyltriolborate Salts for Suzuki-Miyaura Cross-Coupling Reactions with Aryl Chlorides. Org. Lett. 2013, 15, 4308–4311. [Google Scholar] [CrossRef]
- Stille, J. A. Palladium-katalysierte Kupplungsreaktionen organischer Elektrophile mit Organozinn-Verbindungen. Angew. Chem. 1986, 98, 504–519. [Google Scholar] [CrossRef]
- Negishi, E.; King, A. O.; Okukado, N. Selective carbon-carbon bond formation via transition metal catalysis. 3. A highly selective synthesis of unsymmetrical biaryls and diarylmethanes by the nickel- or palladium-catalyzed reaction of aryl- and benzylzinc derivatives with aryl halides. J. Org. Chem. 1977, 42, 1821–1823. [Google Scholar] [CrossRef]
- For example, see: Kumazawa, K. ; Yamanoi, Y.; Yoshizawa, M.; Kusukawa, T.; Fujita, M. A palladium(II)-clipped aromatic sandwich. Angew. Chem. Int. Ed. 2004, 43, 5936–5940. [Google Scholar] [CrossRef] [PubMed]
- Heller, M.; Schubert, U. S. Functionalized 2,2‘-Bipyridines and 2,2‘:6‘,2‘ ‘-Terpyridines via Stille-Type Cross-Coupling Procedures. J. Org. Chem. 2002, 67, 8269–8272. [Google Scholar] [CrossRef]
- Ma, G.; Leng, Y.; Wu, Y.; Wu, Y. Facile synthesis of 3-arylpyridine derivatives by palladacycle-catalyzed Stille cross-coupling reaction. Tetrahedron 2013, 69, 902–909. [Google Scholar] [CrossRef]
- G. Verniest, X. Wang, N. De Kimpe, A. Padwa, Heteroaryl Cross-Coupling as an Entry toward the Synthesis of Lavendamycin Analogues: A Model Study. J. Org. Chem. 2010, 75, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-Q.; Schmitt, M.; Bihel, F. POxAP Precatalysts and the Negishi Cross-Coupling Reaction. Synthesis 2020, 52, 51–59. [Google Scholar] [CrossRef]
- Tang, S. Q.; Bricard, J.; Schmitt, N.; Bihel, F. Fukuyama Cross-Coupling Approach to Isoprekinamycin: Discovery of the Highly Active and Bench-Stable Palladium Precatalyst POxAP. Org. Lett. 2019, 21, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Luzung, M. R.; Patel, J. S.; Yin, J. A Mild Negishi Cross-Coupling of 2-Heterocyclic Organozinc Reagents and Aryl Chlorides. J. Org. Chem. 2010, 75, 8330–8332. [Google Scholar] [CrossRef]
- Downs, R. P.; Chin, A.-L.; Dean, K. M.; Carrick, J. D. Synthesis of Functionalized Hemi-1,2,4-triazinyl-[2,2′]-bipyridines via Telescoped Condensation of [2,2′]-bipyridinyl-6-carbonitrile. J. Heterocycl. Chem. 2017, 54, 3008–3014. [Google Scholar] [CrossRef]
- Mongin, F.; Trécourt, F.; Gervais, B.; Mongin, O.; Quéguiner, G. First Synthesis of Caerulomycin B. A New Synthesis of Caerulomycin C. J. Org. Chem. 2002, 67, 3272–3276. [Google Scholar] [CrossRef]
- Rodriguez, N.; Gooβen, L. J. Decarboxylative coupling reactions: a modern strategy for C–C-bond formation. Chem. Soc. Rev. 2011, 40, 5030–5048. [Google Scholar] [CrossRef] [PubMed]
- Cornella, J.; Larrosa, I. Decarboxylative Carbon–Carbon Bond-Forming Transformations of (Hetero)aromatic Carboxylic Acids. Synthesis 2012, 44, 653–676. [Google Scholar] [CrossRef]
- Chennamanneni, L. R.; William, A.D.; Johannes, C. W. Palladium-catalyzed decarboxylative cross-coupling of 3-pyridyl and 4-pyridyl carboxylates with aryl bromides. Tetrahedron Lett. 2015, 56, 1293–1296. [Google Scholar] [CrossRef]
- Singh, S.; Shinde, V. N.; Kumar, S.; Meena, N.; Bhuvanesh, N.; Rangan, K.; Kumar, A.; Joshi, H. Mono and Dinuclear Palladium Pincer Complexes of NNSe Ligand as a Catalyst for Decarboxylative Direct C‒H Hetroarylation of (Hetero)arenes. Chem Asian J. 2023, 18, e202300628. [Google Scholar] [CrossRef]
- Cook, X. A. F.; Pantaine, L. R. E.; Blakemore, D. C.; Moses, I. B.; Sach, N. W.; Shavnya, A.; Willis, M. C. Base-Activated Latent Heteroaromatic Sulfinates as Nucleophilic Coupling Partners in Palladium-Catalyzed Cross-Coupling Reactions. Angew. Chem. Int. Ed. 2021, 60, 22461–22468. [Google Scholar] [CrossRef] [PubMed]
- Markovic, T.; Rocke, B. N.; Blakemore, D. C.; Mascitti, V.; Willis, M. C. Pyridine sulfinates as general nucleophilic coupling partners in palladium-catalyzed cross-coupling reactions with aryl halides. Chem. Sci. 2017, 8, 4437–4442. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Gao, G.-L.; Edmunds, A. J. F.; Worthington, P. A.; Morris, J. A.; Yu, J.-Q. Ligand-Promoted C3-Selective Arylation of Pyridines with Pd Catalysts: Gram-Scale Synthesis of (±)-Preclamol. J. Am. Chem. Soc. 2011, 133, 19090–19093. [Google Scholar] [CrossRef]
- Dumouchel, S.; Mongin, F.; Trécourt, F.; Quéguiner, G. Synthesis and reactivity of lithium tri(quinolinyl)magnesates. Tetrahedron 2003, 59, 8629–8640. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, L.; Li, Y.; Xie, T.; Zhou, S. Synthesis of Heteroaryl Compounds through Cross-Coupling Reaction of Aryl Bromides or Benzyl Halides with Thienyl and Pyridyl Aluminum Reagents. J. Org. Chem. 2014, 79, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Nicasio-Collazo, J.; Wrobel, K.; Wrobel, K.; Serrano, O. Csp2–Br bond activation of Br-pyridine by neophylpalladacycle: formation of binuclear seven-membered palladacycle and bipyridine species. New J. Chem. 2017, 41, 8729–8733. [Google Scholar] [CrossRef]
- Moore, L. R.; Vicic, D. A. A. Heterogeneous-Catalyst-Based, Microwave-Assisted Protocol for the Synthesis of 2,2’-Bipyridines. Chem. Asian J. 2008, 3, 1046–1049. [Google Scholar] [CrossRef] [PubMed]
- Gros, P.; Doudouh, A.; Fort, Y. New polystyrene-supported stable source of 2-pyridylboron reagent for Suzuki couplings in combinatorial chemistry. Tetrahedron Lett. 2004, 45, 6239–6241. [Google Scholar] [CrossRef]
- Wurtz, A. Sur Une Nouvelle Classes de Radicaux rganiques. Ann. Chem. Phys. 1855, 44, 275–313. [Google Scholar]
- Hunig, S.; Wehner, I. A Convenient Synthesis of 2,2′,6,6′-Tetramethyl-4,4′-bipyridine and Its Oxidation to 2,2′,6,6′-Tetracarboxy-4,4′-bipyridine. Synthesis 1989, 552–554. [Google Scholar] [CrossRef]
- Yamanoi, Y.; Terasaki, N.; Miyachi, M.; Inoue, Y.; Nishihara, H. Enhanced photocurrent production by photosystem I with modified viologen derivatives. Thin Solid Films, 2012, 520, 5123–5127. [Google Scholar] [CrossRef]
- Davison, N.; Quirk, J. A.; Tuna, F.; Collison, D.; McMullin, C. L.; Michaels, H.; Morritt, G. H.; Waddell, P. G.; Gould, J. A.; Freitag, M.; Dawson, J. A.; Lu, E. A room-temperature-stable electride and its reactivity: Reductive benzene/pyridine couplings and solvent-free Birch reductions. Chem 2023, 9, 576–591. [Google Scholar] [CrossRef]
- Banik, A.; Paira, R.; Shaw, B. K.; Vijaykumar, G.; Mandal, S. K. Open-Shell Phenalenyl in Transition Metal-Free Catalytic C–H Functionalization. J. Org. Chem. 2018, 83, 3236–3244. [Google Scholar] [CrossRef] [PubMed]
- Abboud, M.; Mamane, V.; Aubert, E.; Lecomte, C.; Fort, Y. Synthesis of Polyhalogenated 4,4’-Bipyridines via a Simple Dimerization Procedure. J. Org. Chem. 2010, 75, 3224–3231. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, H.; Shi, W.; Lei, A. Bond Formations between Two Nucleophiles: Transition Metal Catalyzed Oxidative Cross-Coupling Reactions. Chem. Rev. 2011, 111, 1780–1824. [Google Scholar] [CrossRef]
- Bhat, A. P. I.; Bhat, B. R. Single-step oxidative homocoupling of aryl Grignard reagents via Co(II), Ni(II) and Cu(II) Complexes under air. Appl. Organometal. Chem. 2014, 28, 383–388. [Google Scholar] [CrossRef]
- Ramnial, T.; Taylor, S. A.; Clyburne, J. A. C.; Walsby, C. J. Grignard reagents in ionic solvents: electron transfer reactions and evidence for facile Br–Mg exchange. Chem. Commun. 2007, 2066–2068. [Google Scholar] [CrossRef]
- Ullmann, F.; Bielecki, J. Ueber Synthesen in der Biphenylreihe. Chem. Ber. 1901, 34, 2174–2185. [Google Scholar] [CrossRef]
- Ullmann, F.; Meyer, G. M.; Loewenthal, O.; Gilli, E. Ueber symmetrische Biphenylderivate. Liebigs Ann. Chem. 1904, 332, 38–81. [Google Scholar] [CrossRef]
- Newkome, G. R.; Patri, A. K.; Holder, E.; Schubert, U. S. Synthesis of 2,2’-Bipyridines: Versatile Building Blocks for Sexy Architectures and Functional Nanomaterials. Eur. J. Org. Chem. 2004, 235–254. [Google Scholar] [CrossRef]
- Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl−Aryl Bond Formation One Century after the Discovery of the Ullmann Reaction. Chem. Rev. 2002, 102, 1359–1470. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T. D.; Crouch, R. D. Cu, Ni, and Pd Mediated Homocoupling Reactions in Biaryl Syntheses: The Ullmann Reaction. Org. React. 2004, 63, 265–555. [Google Scholar] [CrossRef]
- Fanta, P. E. The Ullmann Synthesis of Biaryls, 1945-1963. Chem. Rev. 1964, 64, 613–632. (d) Fanta, P. E. The Ullmann Synthesis of Biaryls. Synthesis 1974, 9–21. [CrossRef]
- Larock, R. C. Comprehensive Organic Transformations: A Guide to Functional Group Preparations, 2nd ed.; Wiley-VCH, New York, 1999.
- Sambiagio, C.; Marsden, S. P.; Blacker, A. J.; McGowan P., C. Copper catalysed Ullmann type chemistry: from mechanistic aspects to modern development. Chem. Soc. Rev. 2014, 43, 3525–3550. [Google Scholar] [CrossRef] [PubMed]
- Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl−Aryl Bond Formation One Century after the Discovery of the Ullmann Reaction. Chem. Rev. 2002, 102, 1359–1470. [Google Scholar] [CrossRef] [PubMed]
- Iyoda, M.; Otsuka, H.; Sato, K.; Nisato, N.; Oda, M. Homocoupling of Aryl Halides Using Nickel(II) Complex and Zinc in the Presence of Et4NI. An Efficient Method for the Synthesis of Biaryls and Bipyridines. Bull. Chem. Soc. Jpn. 1990, 63, 80–87. [Google Scholar] [CrossRef]
- Chen, M.; Hu, J.; Tang, X.; Zhu, Q. Piperazine as an Inexpensive and Efficient Ligand for Pd-Catalyzed Homocoupling Reactions to Synthesize Bipyridines and Their Analogues. Curr. Org. Synth. 2019, 16, 173–180. [Google Scholar] [CrossRef]
- Lee, K.; Lee, P. H. Efficient homo-coupling reactions of heterocyclic aromatic bromides catalyzed by Pd(OAc)2 using indium. Tetrahedron Lett. 2008, 49, 4302–4305. [Google Scholar] [CrossRef]
- Li, C.; Shi, Y.; Chen, Q.; Zhang, K.; Yang, G. Copper Powder and Pd(II) Salts Triggered One-Pot Aromatic Halide Homocoupling via a Radical Pathway. J. Org. Chem. 2023, 88, 2306–2313. [Google Scholar] [CrossRef]
- Waters, G. D.; Carrick, J. D. Convergent access to bis-1,2,4-triazinyl-2,2’-bipyridines (BTBPs) and 2,2’-bipyridines via a Pd catalyzed Ullmann-type reaction. RSC Adv. 2020, 10, 10807–10815. [Google Scholar] [CrossRef]
- Kuroboshi, M.; Waki, Y.; Tanaka, H. Palladium-Catalyzed Tetrakis(dimethylamino)ethylene-Promoted Reductive Coupling of Aryl Halides. J. Org. Chem. 2003, 68, 3938–3942. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, L.; Feng, W. Facile palladium–catalyzed homocoupling of aryl halides using 1,4-butanediol as solvent, reductant and O,O-ligand. ChemistrySelect 2016, 1, 630–634. [Google Scholar] [CrossRef]
- Zeng, M.; Du, Y.; Shao, L.; Qi, C.; Zhang, X.-M. Palladium-Catalyzed Reductive Homocoupling of Aromatic Halides and Oxidation of Alcohols. J. Org. Chem. 2010, 75, 2556–2563. [Google Scholar] [CrossRef] [PubMed]
- Penalva, V.; Hassan, J.; Lavenot, L.; Gozzi, C.; Lemaire, M. Direct homocoupling of aryl halides catalyzed by palladium. Tetrahedron Lett. 1998, 39, 2559–2560. [Google Scholar] [CrossRef]
- Hassan, J.; Penalva, V.; Lavenot, L.; Gozzi, C.; Lemaire, M. Catalytic alternative of the Ullmann reaction. Tetrahedron 1998, 54, 13793–13804. [Google Scholar] [CrossRef]
- Torii, S.; Tanaka, H.; Morisaki, K. Pd(0)-catalyzed electro-reductive coupling of aryl halides. Tetrahedron Lett. 1985, 26, 1655–1658. [Google Scholar] [CrossRef]
- Manoso, A. S.; DeShong, P. Improved Synthesis of Aryltriethoxysilanes via Palladium(0)-Catalyzed Silylation of Aryl Iodides and Bromides with Triethoxysilane. J. Org. Chem. 2001, 66, 7449–7455. [Google Scholar] [CrossRef]
- Tiecco, M.; Testaferri, L.; Tingoli, M.; Chianelli, D.; Montanucci, M. A Convenient Synthesis of Bipyridines by Nickel-Phosphine Complex-Mediated Homo Coupling of Halopyridines. Synthesis 1984, 736–738. [Google Scholar] [CrossRef]
- Liao, L.-Y.; Kong, X.-R.; Duan, X.-F. Reductive Couplings of 2-Halopyridines without External Ligand: Phosphine-Free Nickel-Catalyzed Synthesis of Symmetrical and Unsymmetrical 2,2’-Bipyridines. J. Org. Chem. 2014, 79, 777–782. [Google Scholar] [CrossRef]
- Li, H.; Fan, H.; Hu, B.; Hu, L.; Chang, G.; Song, J. Spatial Structure Regulation: A Rod-Shaped Viologen Enables Long Lifetimein Aqueous Redox Flow Batteries. Angew. Chem. Int. Ed. 2021, 60, 26971–26977. [Google Scholar] [CrossRef]
- Vanderesse, R.; Lourak, M.; Fort, Y.; Caubere, P. Activation of reducing agents. Sodium hydride containing complex reducing agents 23. Symmetrical coupling of nitrogen-containing heterocyclic halides. Tetrahedron Lett. 1986, 27, 5483–5486. [Google Scholar] [CrossRef]
- Dhital, R. N.; Kamonsatikul, C.; Somsook, E.; Sakurai, H. Bimetallic gold–palladium alloy nanoclusters: an effective catalyst for Ullmann coupling of chloropyridines under ambient conditions. Catal. Sci. Technol. 2013, 3, 3030–3035. [Google Scholar] [CrossRef]
- Kawashima, T.; Takao, T.; Suzuki, H. Dehydrogenative Coupling of 4-Substituted Pyridines Catalyzed by Diruthenium Complexes. J. Am. Chem. Soc. 2007, 129, 11006–11007. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Guo, Y.; An, W.; Ren, Y.-L.; Qin, Y.; Niu, C.; Zheng, X. Coupling photocatalytic water oxidation with reductive transformations of organic molecules. Nat. Commun. 2022, 13, 6186. [Google Scholar] [CrossRef] [PubMed]
- Cassol, T. M.; Demnitz,F. W. J.; Navarro, M.; Neves, Eduardo A. d. Two convenient and high-yielding preparations of 6,6’-dimethyl-2,2’-bipyridine by homocoupling of 6-bromopicoline. Tetrahedron Lett. 2000, 41, 8203–8206. [Google Scholar] [CrossRef]
- Oliveira, J. L.; Silva, M. J.; Florêncio, T.; Urgin, K.; Sengmany, S.; Léonel, E.; Nédélec, J.-Y.; Navarro, M. Electrochemical coupling of mono and dihalopyridines catalyzed by nickel complex in undivided cell. Tetrahedron 2012, 68, 2383–2390. [Google Scholar] [CrossRef]
- de Franḉa, K. W. R.; Marcelo Navarro, M.; Léonel, É.; Durandetti, M.; Nédélec. J.-Y. Electrochemical Homocoupling of 2-Bromomethylpyridines Catalyzed by Nickel Complexes. J. Org. Chem. 2002, 67, 1838–1842. [Google Scholar] [CrossRef] [PubMed]
- Stammers, E.; Parsons, C. D.; Clayden, J.; Lennox, A. J. J. Electrochemical synthesis of biaryls by reductive extrusion from N,N’-diarylureas. Nat. Commun. 2023, 14, 4561. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, D.; Klose, I.; Oost, R.; Neuhaus, J.; Maulide, N. Bond-Forming and -Breaking Reactions at Sulfur(IV): Sulfoxides, Sulfonium Salts, Sulfur Ylides, and Sulfinate Salts. Chem. Rev. 2019, 119, 8701–8780. [Google Scholar] [CrossRef]
- Foubelo, F.; Yus, M. Functionalised Organolithium Compounds by Sulfur-Lithium Exchange. Chem. Soc. Rev. 2008, 37, 2620–2633. [Google Scholar] [CrossRef]
- Furukawa, N.; Shibutani, T.; Fujihara, H. Preparation of pyridyl Grignard reagents and cross coupling reactions with sulfoxides bearing azaheterocycles. Tetrahedron Lett. 1987, 28, 5845–5848. [Google Scholar] [CrossRef]
- Kawai, T.; Furukawa, N.; Oae, S. A Convenient Preparation of Bipyridines through Ligand Coupling Reaction with σ-Sulfurane Formed by Treatment of Methyl 2-Pyridyl Sulfoxide with Grignard Reagents. Tetrahedron Lett. 1984, 25, 2549–2552. [Google Scholar] [CrossRef]
- Oae, S.; Uchida, Y. Ligand-Coupling Reactions of Hypervalent Species. Acc. Chem. Res. 1991, 24, 202–208. [Google Scholar] [CrossRef]
- Oae, S. Ligand coupling reactions of hypervalent species. Pure Appl. Chem. 1996, 68, 805–812. [Google Scholar] [CrossRef]
- Oae, S.; Inubushi, Y.; Yoshihara, M. Thionyl Chloride—A Good Ligand Coupling Reagent. Phosphorous Sulfur Silicon Relat. Elem. 1995, 103, 101–110. [Google Scholar] [CrossRef]
- Duong, V. K.; Horan, A. M.; McGarrigle, E. M. Synthesis of Pyridylsulfonium Salts and Their Application in the Formation of Functionalized Bipyridines. Org. Lett. 2020, 22, 8451–8457. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Tsien, J.; Qin, T. Sulfur(IV)-Mediated Unsymmetrical Heterocycle Cross-Couplings. Angew. Chem. Int. Ed. 2020, 59, 7372–7376. [Google Scholar] [CrossRef] [PubMed]
- Mann, F. G.; Watson, J. Conditions of Salt Formation in Polyamines and Kindred Compounds. Salt Formation in the Tertiary 2-Pyridylamines, Phosphines, and Arsines. J. Org. Chem. 1948, 13, 502–531. [Google Scholar] [CrossRef]
- Newkome, G. R.; Hager, D. C. A New Contractive Coupling Procedure. Convenient Phosphorus Expulsion Reaction. J. Am. Chem. Soc. 1978, 100, 5567–5568. [Google Scholar] [CrossRef]
- Uchida, Y.; Kozawa, H.; Oae, S. Formation of 2,2’-bipyridyl by ligand coupling on the phosphorous atom. Tetrahedron Lett. 1989, 30, 6365–6368. [Google Scholar] [CrossRef]
- Boyle, B. T.; Hilton, M. C.; McNally, A. Nonsymmetrical Bis-Azine Biaryls from Chloroazines: A Strategy Using Phosphorus Ligand-Coupling. J. Am. Chem. Soc. 2019, 141, 15441–15449. [Google Scholar] [CrossRef] [PubMed]
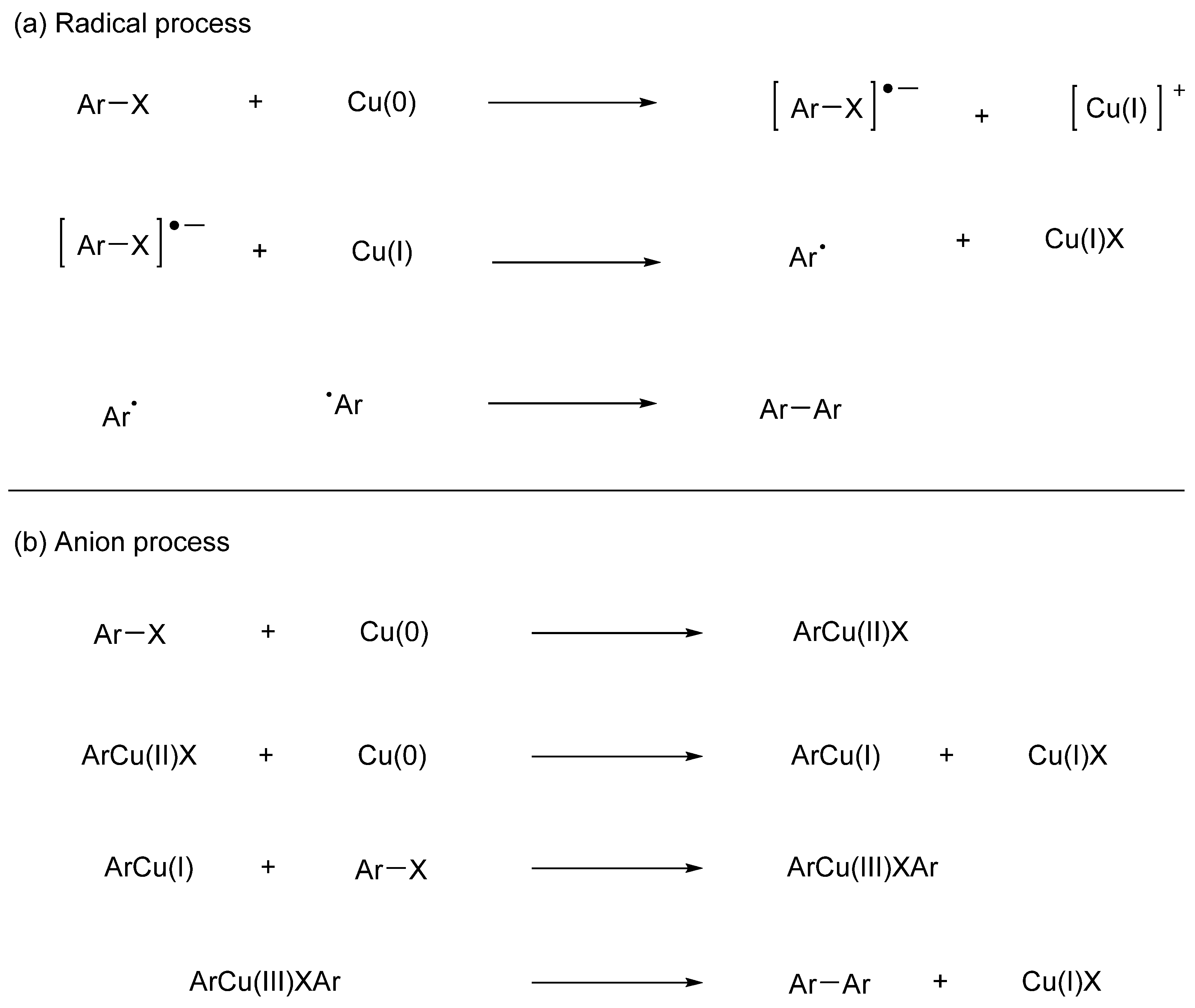
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



