1. Introduction
Panax quinquefolium L., also known as American ginseng, is a perennial herb from the family Araliaceae [
1]. The beneficial effects of American ginseng products, such as antitumor and anti-inflammatory activity, improving immunity, and lowering blood sugar, are mainly attributed to bioactive ingredients such as ginsenosides, polyphenols, and polysaccharides [
2,
3,
4,
5]. The cultivation period for American ginseng is long (harvesting is generally conducted after 4–5 years [
6]), with the ginsenoside content in the roots being dependent on the cultivation period [
7]. However, the leaves can be harvested every year since the ginsenoside content therein is less dependent on the age of the plant [
8]. The activity of ginsenoside is closely related to the type, quantity, and positions of the sugar groups it carries: the fewer sugar groups in the ginsenoside, the higher its physiological activity [
9,
10]. It has been proved that the ginsenoside content in the leaves of American ginseng is significantly higher than that in the roots [
11]. In our previous research, we showed that heat treatment transforms the constant ginsenosides in ginseng leaves into rare ginsenosides with higher physiological activity and significantly increases the total polyphenolic content (TPC) [
12]. Moreover, American ginseng leaves are a high-quality source of ginsenosides, polyphenols, and other bioactive ingredients that are not only underused but also often discarded.
The most commonly used industrial methods for American ginseng processing involve physical, chemical, or biological treatment [
13], with the physical method involving heat treatment being the most commonly used. The outcomes from several studies have proved that heat treatment increases the amounts of highly bioactive components, such as ginsenosides, polyphenols [
15], and acidic polysaccharides [
16] in American ginseng. Specifically, Zhang et al. [
14] reported that heat treatment decreased the amounts of constant ginsenosides and increased those of rare ginsenosides.
Far-infrared irradiation (FIR) is a heat treatment method frequently used in the food industry that can penetrate plant materials, generate heat on the surface and the inside, and induce the decomposition of multi-chain molecular groups [
17,
18]. In our previous research, we showed that FIR can significantly increase the TPC in ginseng leaves and promote the transformation of ginsenosides [
12]. Moreover, FIR treatment of
Lycium barbarum [
19], rice [
20], black rice [
21], and mango [
22] can significantly increase the amounts of bioactive components such as polyphenols, flavonoids, and anthocyanins. However, few studies have been conducted on the effect of FIR on the bioactive components in American ginseng leaves.
Since American ginseng leaves provide a high-quality source of polyphenols, ginsenosides, and other bioactive components, exploiting this resource is of utmost importance. The purpose of the present study is to determine the effects of FIR treatment on the bioactive components (polyphenols, flavonoids, and ginsenosides) and the antioxidant activity of American ginseng leaves. Our approach could pave the way for the utilization of American ginseng leaves for the production of biomedicines and other health-related benefits.
2. Materials and Methods
2.1. Chemicals
An organic solvent (high-performance liquid chromatography (HPLC) grade) was purchased from Merck KGaA (Darmstadt, Germany). Folin phenol reagent was purchased from Wako Pure Chemicals (Osaka, Japan). Kaempferol and panasenoside standards were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Ginsenosides were purchased from ChromaDex (Santa Ana, CA, USA California, USA) and Ambo Institute (Daejeon, Korea).
2.2. Sample Collection and FIR Treatment
Four-year-old American ginseng leaves were collected from Wendeng, Weihai, Shandong Province. The intact and undamaged leaves were washed with distilled water and then wiped with gauze. Fresh American ginseng leaves were fully dried in an oven at 50 °C for 24 hours, and then ground with a grinder. The powder was sieved through a 200-micron sieve to obtain a uniform particle size. The powder was divided into two parts: (1) the non-FIR-treated control (Con; 0) and (2) FIR treatments in an FIR dryer (HKD-10; Korea Energy Technology, Seoul, Korea) for 30 min at 160 °C (FIR-160), 170 °C (FIR-170), 180 °C (FIR-180), 190 °C (FIR-190), or 200 °C (FIR-200).
2.3. Bioactive Compound Analysis
2.3.1. Sample Extraction
First, 2 g of sample was added to 100 mL of 80% (v/v) methanol. Sample extraction was carried out in an oscillating incubator at 30 °C for 24 hours. The mixture was centrifuged at 500×g for 10 minutes at room temperature (05pr-22 centrifuge, Hitachi, Tokyo, Japan). Afterward, the supernatant was collected and then filtered using Whatman No. 42 filter paper (Whatman Inc., Clifton, NJ, USA). The filtrate was concentrated using a vacuum rotary evaporator at 40 °C (Eyela Co., Tokyo, Japan). The sample was then freeze-dried using a vacuum freeze dryer (Christ Alpaha 1-4, Germany). The sample was subsequently stored in a refrigerator at 20 °C before being used in subsequent experiments.
2.3.2. TPC Determination
TPC was determined by using the Folin–Ciocalteu method as follows. First, 1.9 mL of distilled water and 1.0 mL of Folin–Ciocalteu reagent were mixed in a tube, after which 0.1 mL of the sample solution (2 mg/mL dissolved in 80% (v/v) methanol) was added. Subsequently, 1.0 mL of 20% Na2CO3 was added, followed by incubation for 2 hours at 25 °C. Afterward, the absorbance of the sample was recorded at 765 nm. The results are expressed as milligrams of gallic acid equivalent (mgGAE) per gram of dry weight.
2.3.3. HPLC Analysis of the Amounts of Panasenoside and Kaempferol
The amounts of panasenoside and kaempferol in American ginseng leaves were determined by using HPLC. The equipment was an HPLC system (CBM-20A; Shimadzu Co, Ltd., Kyoto, Japan) with two gradient pump systems (LC-20AT; Shimadzu, Japan), an auto sample injector (SIL20A; Shimadzu), a UV detector (SPD-20A; Shimadzu), and a column oven (CTO-20A; Shimadzu). Ground leaves (0.1 mg) were suspended in 1 mL of 80% (v/v) methanol and filtered through a 0.22 μm membrane filter before being injected onto the HPLC system. HPLC separation was performed on an Inertsil ODS-SP C18 column (250 mm × 4.6 mm, 5 µm; GL Sciences, Tokyo, Japan). The injection volume was 10 μL. The gradient running phase was programmed as a combination of solvent A (water with 0.1% trifluoroacetic acid) and solvent B (acetonitrile), during which solvent B was sequentially increased from 14% to 18% (0 to 10 min), 18% to 30% (10 to 20 min), 30% to 60% (20 to 30 min), 60% to 65% (30 to 33 min), 65% to 100% (33 to 40 min), 100% to 100% (40 to 50 min), and then finally adjusted from 100% to 14% (50 to 65 min). The operating temperature was set at 35 °C. The flow rate of the mobile phase was kept at 1.0 mL per minute. The detector was set at 355 nm for monitoring panasenoside and kaempferol.
2.3.4. HPLC Analysis of the Amounts of Ginsenosides
The ginsenoside amounts were determined by using the same HPLC equipment described in Section 3.3.3. The prepared sample solution was filtered through a 0.22 μm membrane filter. The injection volume onto a Kinetex C18 column (100 mm × 4.6 mm, 2.6 µm; Torrance, CA, USA) was 10 μL. The gradient running phase was programmed as a combination of solvent A (water) and solvent B (acetonitrile), during which solvent B was sequentially increased from 17% to 23% (0 to 30 min), 23% to 24% (30 to 35 min), 24% to 32% (35 to 45 min), 32% to 44% (45 to 48 min), 44% to 44% (48 to 52 min), 44% to 55% (52 to 65 min), 55% to 100% (65 to 85 min), 100% to 100% (85 to 95 min), and finally adjusted from 100% to 17% (95 to 105 min). The operating temperature and flow rate were the same as those in the procedure outlined in Section 3.3.3. The detector was set at 203 nm for monitoring ginsenosides.
2.4. Determination of the Free Radical Scavenging and Antioxidant Activities
2.4.1. Determination of the Free Radical Scavenging Activity
American ginseng leaf powder (90 mg) was added to 30 mL of 80% (v/v) methanol in a 50 mL tube, and processed at 30 °C for 30 min via sonication. Subsequently, the sample was centrifuged at 3500 revolutions/min for 15 min. The supernatant was collected and filtered through a 0.22 μm membrane filter.
The DPPH free radical scavenging activity was measured using the procedure described by Kossah et al. [
23] with some modifications. Briefly, 1 ml of sample solution at varying concentrations was mixed with 3 ml of DPPH solution. After reacting in the dark at room temperature for 30 minutes, the absorbance was measured at 517 nm.
The radical scavenging activity of ABTS was measured using the procedure described by Lim et al. [
24] with some modifications. Briefly, 0.5 ml of sample solution at varying concentrations was mixed with 5 ml of ABTS solution. After reacting in the dark at room temperature for 10 minutes, the absorbance was measured at 734 nm.
2.4.2. Determination of the Reducing Power
Sample processing was the same as that mentioned in Section 3.4.1. Measurement of the reducing power was conducted using the procedure described by Oyaizu et al. [
25] with some modifications. Briefly, 0.4 ml of sample solution at varying concentrations was mixed with 1 ml of 0.2 mol/l phosphate buffer (pH 6.6) and 1 ml of 1% potassium ferrocyanide solution. The mixture was then incubated in a 50 °C water bath for 30 minutes, after which 1 ml of 10% trichloroacetic acid was added, followed by thorough mixing and centrifugation at 3000 revolutions/min for 10 minutes. After centrifugation, take 2ml of the supernatant and add 2ml of distilled water and 0.4ml of 0.1% ferric chloride. Next, vortex the mixture. Measure the absorbance of the mixture at 700 nm.
2.5. Statistical Analysis
The data were analyzed statistically using SAS software (Enterprise Guide version 7.1; SAS Institute Inc., Cary, NC, USA). The data between the non-FIR-treated control and FIR treatment groups were analyzed by using one-way analysis of variance. Differences between the experimental groups were evaluated by using Tukey’s honestly significant difference (HSD) test at the p < 0.05 significance level.
3. Results and Discussion
3.1. The Effect of FIR Heat Treatment on the TPC in American Ginseng Leaves
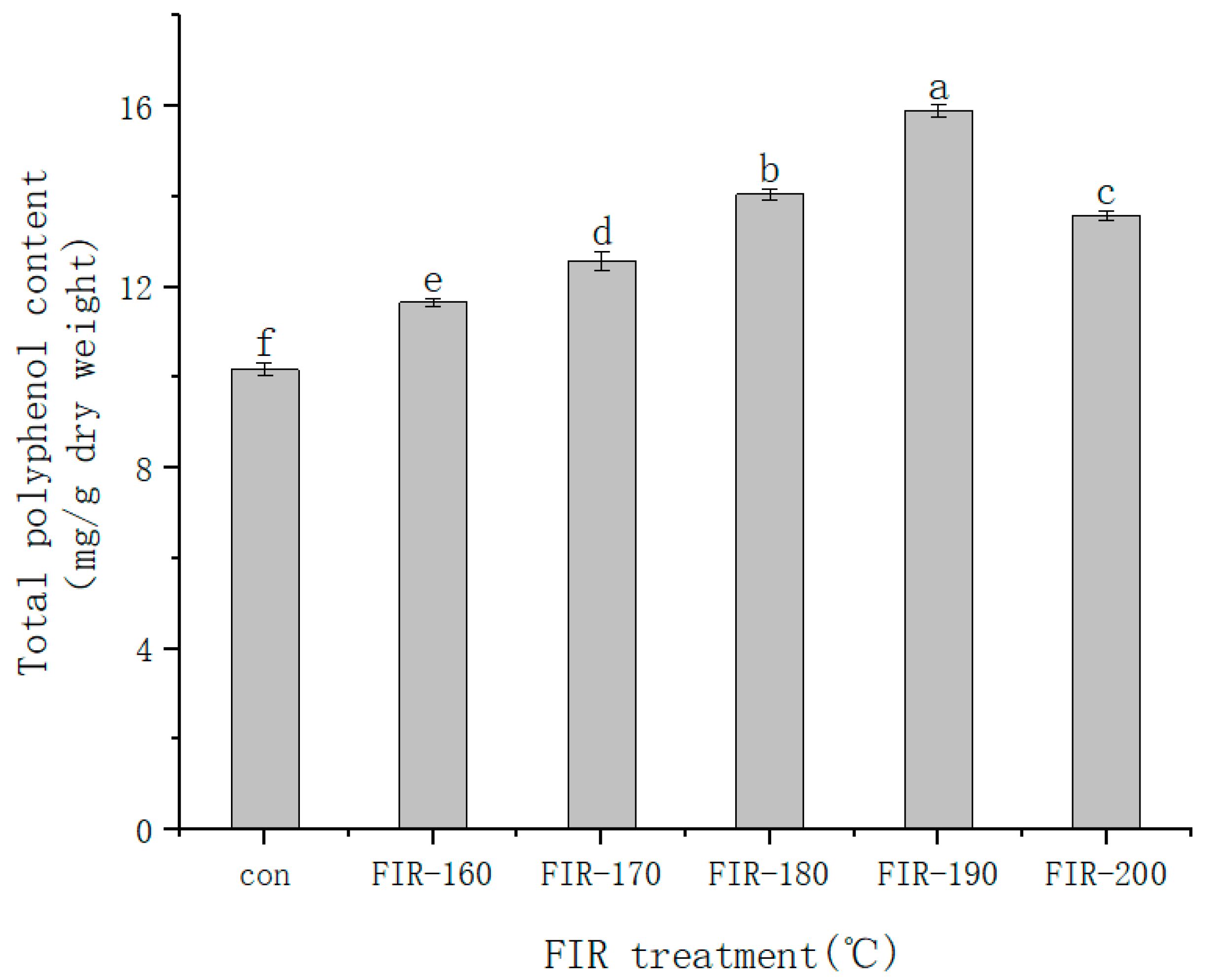
The change in TPC in American ginseng leaves after FIR heat treatment is shown in
Figure 1. The TPC gradually increased up to 190 °C heat treatment and then declined. At 190 °C, the highest TPC was 15.89 mg/g, which was 1.56 times higher than without treatment. According to Hwang et al. [
26], phenolic compounds can scavenge free radicals by providing hydrogen, so promoting the generation of polyphenols in American ginseng leaves via FIR heat treatment improves the antioxidant activity of the extract. Our results are similar to those reported before. For example, Kim et al. [
15] heated American ginseng at 100 °C for 120 minutes, which increased the TPC by 30% compared to untreated American ginseng. Lee et al. [
27] used subcritical water at 150–200 °C to produce a red ginseng extract in which the TPC increased with increasing temperature up to 190 °C for 20 minutes, after which it declined. In the present study, the TPC in American ginseng leaves increased after FIR heat treatment. We speculate that there are two reasons. First, most phenolic compounds in plants are covalently bonded to insoluble polymers and are released by heat treatment [
28,
29]. The second is that heating can promote the hydrolysis of macromolecular polyphenols, thereby producing smaller molecular polyphenols [
30]. From the above experimental results, it can be concluded that the highest TPC content in American ginseng leaves was produced via FIR heat treatment at 190 °C.
3.2. The Effect of FIR Heat Treatment on the Amounts of Panasenoside and Kaempferol in American Ginseng Leaves
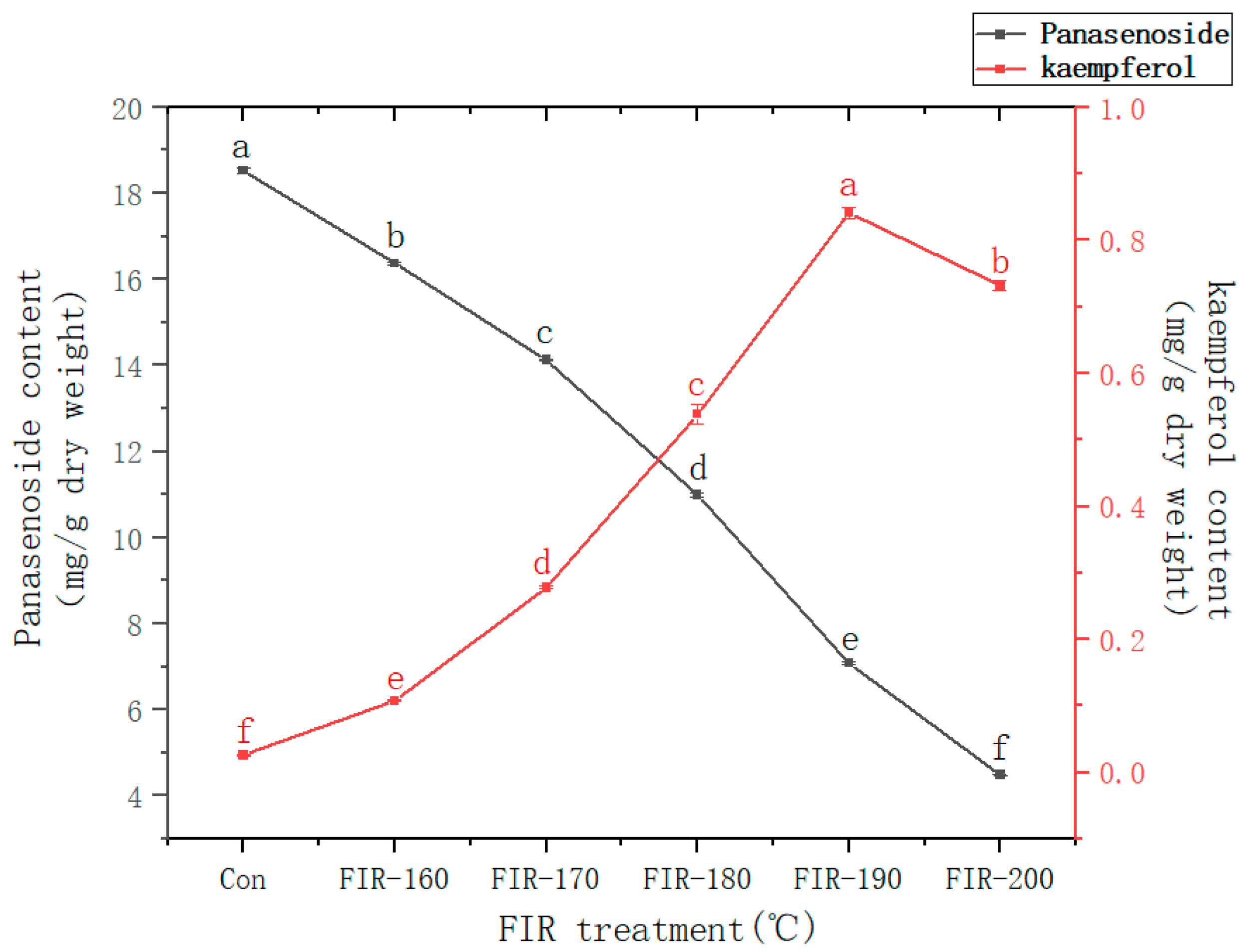
Figure 2 shows the kaempferol and panasenoside content in American ginseng leaves before and after FIR treatment at various temperatures. The panasenoside content in the leaves before FIR treatment was 18.52 mg/g. Increasing the FIR treatment temperature decreased the panasenoside content until its lowest value after treatment at 200 °C (4.49 mg/g), which was 76% lower than that without treatment. Contrary to the change in panasenoside content, that of kaempferol increased from 0.026 mg/g without treatment up to 0.841 mg/g (FIR-190) at 190 °C, after which it decreased. The kaempferol content after FIR treatment at 190 °C was 32 times higher than that without treatment.
Panasenoside (kaempferol 3-o-glucosyl-(1→2)-galactoside) is a kaempferol glycoside [
31]. According to the findings of our previous research on ginseng leaves, we speculate that during FIR treatment, energy can make panasenoside deglycosylate break the bond between kaempferol aglycone and the first glycosyl, resulting in a significant increase in kaempferol content. However, astragalin (kaempferol 3-β-D-glucopyranoside) was not detected. The deglycosylation of panasenoside is in concert with the degradation of kaempferol; increasing the FIR temperature gradually caused the deglycosylation rate of panasenoside to become lower than the degradation rate of kaempferol. This explains why the panasenoside content in American ginseng leaves decreased rapidly while the kaempferol content increased relatively slowly, reaching the highest value after FIR treatment at 190 °C [
12]. Although the kaempferol content in American ginseng leaves reached the highest value after FIR treatment at 190 °C, that in ginseng leaves was different and it stopped increasing after FIR treatment at 180 °C. This could have been caused by the different compositions and amount of panasenoside in the raw material used. Kaempferol (a deglycosylation product) has high anti-inflammatory [
2], antitumor [
3], and antioxidant activities [
32]. According to the above experimental results, it can be concluded that the kaempferol content in American ginseng leaves was markedly increased by FIR treatment at 190 °C.
3.3. The Effect of FIR Treatment on the Ginsenoside Content in American Ginseng Leaves
3.3.1. Protopanaxadiol (PPD)-Type Ginsenosides
The change in PPD-type ginsenoside content in American ginseng leaves after FIR heat treatment at various temperatures is shown in
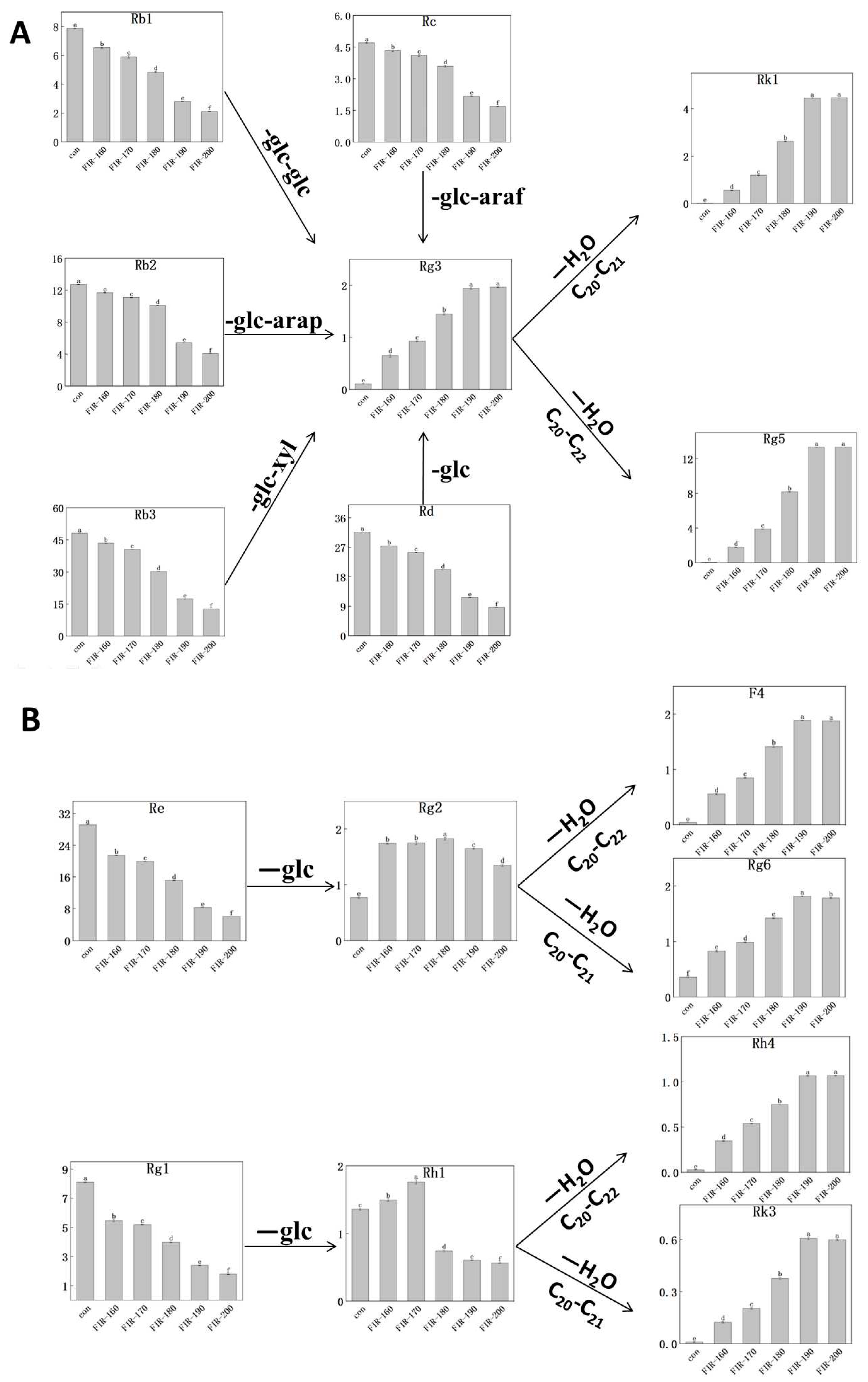
Figure 3A. The amounts of main ginsenosides Rb1, Rb2, Rb3, Rc, and Rd are negatively correlated with the FIR treatment temperature. When the FIR temperature was 200 °C, their amounts (2.12, 4.10, 12.70, 1.70, and 8.66 mg/g, respectively) were even lower than in the untreated leaves (equating to reductions of 73%, 68%, 74%, 64%, and 73%, respectively). On the contrary, the amounts of rare PPD-type ginsenosides Rg5, Rk1, and Rg3 showed an upward trend with increasing FIR temperature; their amounts were the highest after FIR treatment at 190 °C: 13.37, 4.46, and 1.94 mg/g compared to 0.05, 0.02, and 0.11 mg/g in untreated leaves, equating to increases of 266, 222, and 17 times, respectively.
The conditions applied can be dry heat, wet heat, etc. The main transformation pathways for PPD-type ginsenoside conversion during dry heat treatment are as follows: Rb1, Rb2, Rb3, Rc, Rd→Rg3→Rk1, Rg5 [
33]. Increasing the FIR temperature significantly increased the amounts of Rk1, Rg5, and the other ginsenoside but not Rg3. This could be because Rg3, which is an intermediate produced by the deglycosylation of Rb1, Rb2, Rb3, Rc, Rd, and other ginsenosides, can also undergo dehydration and deglycosylation at similar reaction rates. Although ginsenosides have great potential, the bioavailability of the main ginsenosides is low because their absorption rates from the circulatory system are very low. On the contrary, the rare ginsenosides have shown good bioavailability because they can more easily permeate through cell membranes due to their small molecular weight [
34]. In the present study, increasing the FIR treatment temperature decreased the main PPD-type ginsenoside content by 72% and increased the rare ginsenoside content to hundreds of times higher than without treatment. Although many researchers have reported that heat treatment can promote the transformation of ginsenosides, different heat treatment methods and conditions affect their transformation rate. For example, Park et al. [
35] reported that after heat treatment at 120 °C, the main ginsenosides were converted into rare ginsenosides Rg3, Rg5, and Rk1 with lower polarity; They were difficult to detect in untreated samples and increased to 15.2, 3.6, and 2.9 µg/mg, respectively, afterward. We found that the amounts of rare ginsenosides Rg5 and Rk1 were higher while the Rg3 content was lower than they reported. This could be because the reaction rate of deglycosylation of Rd→Rg3 was different from that of dehydration of Rg3→Rg5 and Rk1 due to the differences in heat treatment method and temperature. Steam and drying treatment can increase the amounts of Rg3, Rg5, and Rk1 and decrease that of Rb1 [
36]. The findings from the above research are similar to ours using FIR treatment of American ginseng leaves. When increasing the FIR treatment temperature from 160–190 °C, the amounts of PPD-type rare ginsenosides increased significantly. Especially, the amounts of rare ginsenoside Rg5 increased by 1.76 mg/g (con→FIR-160), 2.10 mg/g (FIR-160→FIR-170), 4.31 mg/g(FIR-170→FIR-180) and 5.16 mg/g (FIR-180→FIR-190). Heat treatment can transform main PPD-type ginsenosides into rare ones with higher physiological activity and clinical value.
3.3.2. Protopanaxatriol (PPT)-Type Ginsenosides
Changes in the amounts of PPT-type ginsenosides in American ginseng leaves according to the FIR treatment temperature shown in
Figure 3B are similar to those for the PPD-type ginsenosides; i.e., the main ginsenoside content decreased and the corresponding rare ginsenoside content increased with increasing temperature. When increasing the FIR treatment temperature, the amounts of main ginsenosides Re and Rg1 decreased while those of Rg2 and Rh1 increased first and then decreased. Meanwhile, after FIR treatment up to 190 °C, the amounts of rare ginsenosides F4, Rg6, Rh4, and Rk3 did not increase (1.89, 1.82, 1.07, and 0.61 mg/g, respectively). Compared with untreated leaves, the amounts of main ginsenosides Re and Rg1 decreased by 79.27% and 77.96%, respectively, while those of rare ginsenoside Rk3, F4, Rh4, and Rg6 were 64, 41, 37, and 5 times higher, respectively.
Under FIR heat treatment, the main transformation paths for the PPT-type ginsenosides are as follows: Re→Rg2→Rg6 and F4; Rg1→Rh1→Rh4 and Rk3 [
33]. Our experimental results show that FIR treatment increased the amounts of rare ginsenosides Rg6, F4, Rh4, and Rk3 by 4, 40, 36, and 63 times, respectively. Xue et al. [
8] used an autoclave to heat American ginseng to a high temperature and obtained similar results. With increasing temperature and time, the PPT-type rare ginsenoside content in American ginseng also showed an increasing trend. Ji et al. [
37] reported that processing a ginseng extract at 90 °C greatly increased the amounts of rare ginsenosides F4, Rk3, and Rh4 compared to the untreated ginseng extract. Their results are similar to ours in that PPT-type main ginsenosides are transformed into rare ginsenosides via heat treatment. Rg2 and Rh1 are intermediate products of the transformation of Re and Rg1, respectively. Increasing the FIR treatment temperature caused the amounts of ginsenoside Rg2 and Rh1 to first increase and then decrease, which could be because the deglycosylation rate of Re and Rg1 transformation into Rg2 and Rh1, respectively, is lower than the dehydration rate of the latter two products [
38]. As shown in
Figure 3A,B, the highest amounts of rare PPD- and PPT-type ginsenosides were obtained at an FIR treatment temperature of 190 °C, and decreased when the temperature was increased to 200 °C; this could have been caused by the degradation of the ginsenosides due to the higher FIR energy [
12]. However, the decrease in the amounts of rare ginsenoside content at 200 °C is slightly different from that after FIR treatment of ginseng leaves [
12], which could have been caused by the different composition and amounts of ginsenosides in the raw materials used [
39]. Although increasing the FIR treatment temperature from 160–190 °C incremented the rare PPT-type and PPD-type ginsenoside by different amounts, the change of content increment was not significant. This could be due to the different structures of PPD-type and PPT-type ginsenoside aglycones resulting in different dehydration rates.
3.4. The Effects of FIR Treatment on the Free Radical Scavenging and Antioxidant Activities of American Ginseng Leaves
2,2-Diphenyl-1-picrylhydrazyl (DPPH) and 2,2’-azino-bis(3-ethylbenzothiazoline-6-
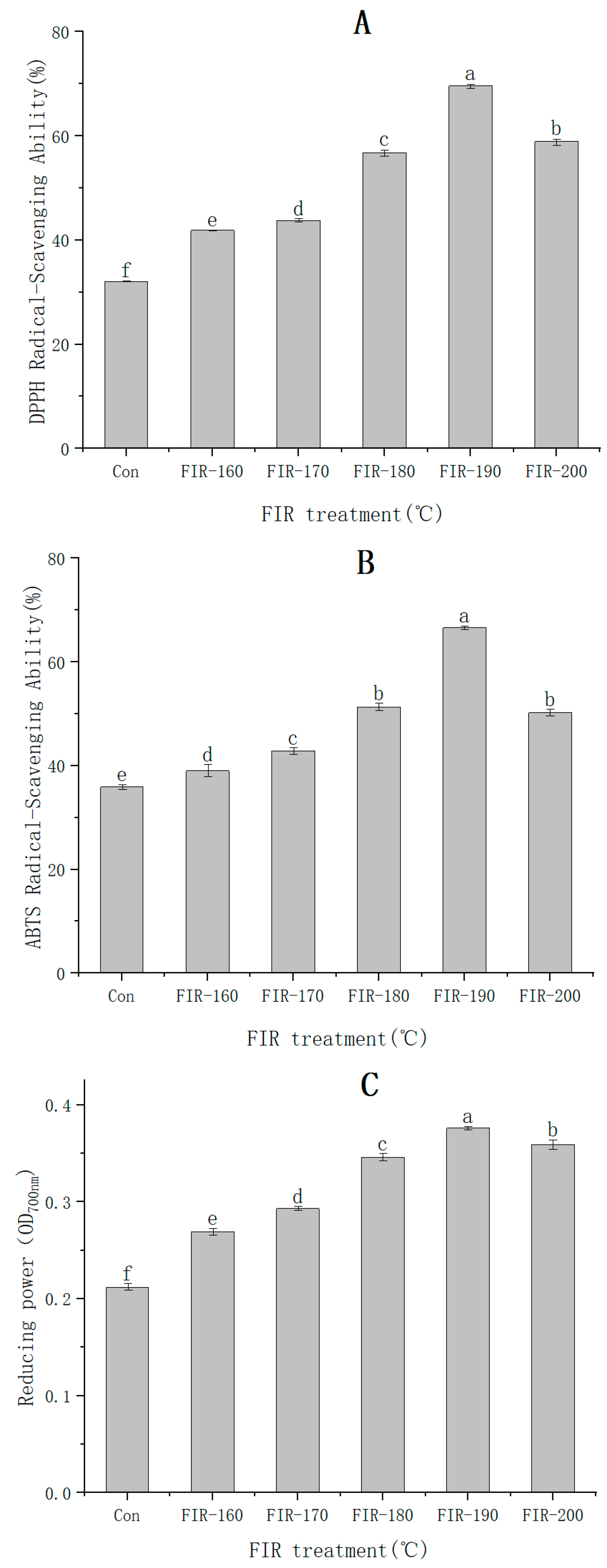
sulfonic acid) (ABTS) scavenging assays, and the ferric reducing antioxidant power (FRAP) assay were used to determine the effect of FIR treatment on the antioxidant capacity of American ginseng leaf extract. The results shown in
Figure 4A–C indicate that FIR treatment significantly improved the antioxidant capacity of American ginseng leaf extract. The DPPH and ABTS scavenging activities of American ginseng leaves treated using FIR at 190 °C were 2.17 times (32.08% vs. 69.62%) and 1.86 times (35.88% vs. 66.61%) higher than that in untreated leaves. The absorbance measured by using the FRAP method increased by 1.77 times (0.212 vs. 0.374) after FIR treatment at 190 °C. When the temperature of FIR treatment was raised to 200 °C, the DPPH and ABTS scavenging rates and the reducing power all showed a downward trend.
Many antioxidants naturally covalently bind to insoluble polymers. If the bonds are weak, FIR can release and activate natural low-molecular-weight antioxidants such as phenolic acids, flavonoids, and carotenes [
40]. The results in
Figure 1 and
Figure 4 indicate that FIR treatment up to 190 °C increases the TPC and antioxidant activity in American ginseng leaves, respectively, after which they decrease. This is similar to our previous research results in ginseng leaves. We speculate that FIR treatment can improve the antioxidant activity of ginseng leaves by releasing bound phenols. When the FIR treatment temperature is too high, the phenolic degradation is accelerated, resulting in decreases in TPC and antioxidant activity [
12]. Similar results have also been reported for the FIR treatment of rice, peanut shells, and
Angelica sinensis [
20,
40,
41]. Although many studies have been conducted on American ginseng leaves, this is the first time that the antioxidant activity of American ginseng leaf extract has been assessed after FIR treatment. The rational use of FIR treatment could improve the antioxidant activity of medicinal plants and increase their medicinal value. From the above experimental results, it can be concluded that 190 °C is the optimal FIR heating temperature for processing American ginseng leaves to obtain higher antioxidant activity.
4. Conclusions
In this study, the effects of FIR on bioactive components and antioxidant activity of American ginseng leaves were investigated in detail. Our research results show that FIR treatment is an efficient processing method to produce beneficial bioactive components in American ginseng leaves. Higher TPC and amounts of kaempferol and rare ginsenosides F4, Rg6, Rh4, Rk3, Rk1, Rg3, and Rg5 were obtained via FIR treatment at 190 °C. Moreover, the antioxidant activity was significantly improved by the increase in TPC. The results of this study could provide a reference for the rationalization and utilization of high-value biomedicines from American ginseng leaves.
Author Contributions
Collected documents, drafted manuscripts, and organized data into tables, X.W.; software and validation, R.H. and J.L.; investigation, X.S.; data curation, S.S.; writing—original draft preparation, J.L.; conceptualization, supervision, funding acquisition, writing—review and editing, C.J. and D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Technological innovation and development planning of Yantai (2023YD080), National Natural Science Foundation of China (General program, No. 32272281) and Youth Innovation Technology Project of Higher School in Shandong Province (Food Nanotechnology innovation team).
Data Availability Statement
The data presented in this study are available in article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cai, J.; Zhang, Q.; Huang, H. Ginseng extract and its constituents alleviate cisplatin toxicity and reverse cisplatin resistance. Integr. Cancer Sci. Therap. 2019, 6, 1–7. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Liu, H.; Bao, L. Advances in research on the effects of natural drugs with immune-promoting effects on immune function. Eur. J. Inflamm. 2020, 18, 2058739220926878. [Google Scholar] [CrossRef]
- He, S.; Lyu, F.; Lou, L.; Liu, L.; Li, S.; Jakowitsch, J.; Ma, Y. Anti-tumor activities of Panax quinquefolius saponins and potential biomarkers in prostate cancer. J. Ginseng Res. 2021, 45, 273–286. [Google Scholar] [CrossRef]
- Im, D.S. Pro-Resolving Effect of Ginsenosides as an Anti-Inflammatory Mechanism of Panax ginseng. Biomolecules 2020, 10, 444. [Google Scholar] [CrossRef]
- Zhi, L.; ChunYuan, Q.; JiaXin, L.; YanFang, W.; Wei, L.; ChongZhi, W.; DongSheng, W.; Jia, S.; GuangZhi, S.; ChunSu, Y. Hypoglycemic and Hypolipidemic Effects of Malonyl Ginsenosides from American Ginseng (Panax quinquefolius L.) on Type 2 Diabetic Mice. ACS omega 2021, 6, 33652–33664. [Google Scholar] [CrossRef]
- Walsh, J.P.; Garnham, C.P.; Yeung, K.K.C.; Sumarah, M.W. Ilyonectria Root Rot of Ginseng Is Attenuated via Enzymatic Degradation of the Extracellular Fe3+-Bound Siderophore N,N′,N″-Triacetylfusarinine C. ACS Agric. Sci. Technol. 2022, 2, 402–408. [Google Scholar] [CrossRef]
- Fang, X.; Wang, M.; Zhou, X.; Wang, H.; Wang, H.; Xiao, H. Effects of growth years on ginsenoside biosynthesis of wild ginseng and cultivated ginseng. BMC Genomics 2022, 23, 325. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Yao, Y.; Yang, X.S.; Feng, J.; Ren, G.X. Improved antimicrobial effect of ginseng extract by heat transformation. J. Ginseng Res. 2017, 41, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.W.; Wong, A.S.T. Pharmacology of ginsenosides: A literature review. Chin. Med. 2010, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Yoo, M.H.; Noh, K.H.; Oh, D.K. Biotransformation of ginsenosides by hydrolyzing the sugar moieties of ginsenosides using microbial glycosidases. Appl. Microbiol. Biot. 2010, 87, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Kang, O.J.; Kim, J.S. Comparison of Ginsenoside Contents in Different Parts of Korean Ginseng (Panax ginseng C.A. Meyer). Prev. Nutr. Food Sci. 2016, 21, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Liu, J.R.; Wang, X.; Sun, X.M.; Gong, H.S.; Jin, C.W.; Eom, S.H. Thermal Control Using Far-Infrared Irradiation for Producing Deglycosylated Bioactive Compounds from Korean Ginseng Leaves. Molecules 2022, 27, 4782. [Google Scholar] [CrossRef]
- Jang, G.Y.; Kim, M.Y.; Lee, Y.J.; Li, M.; Shin, Y.S.; Lee, J.; Jeong, H.S. Influence of organic acids and heat treatment on ginsenoside conversion. J. Ginseng Res. 2018, 42, 532–539. [Google Scholar] [CrossRef]
- Zhang, F.; Tang, S.; Zhao, L.; Yang, X.; Yao, Y.; Hou, Z.; Xue, P. Stem-leaves of Panax as a rich and sustainable source of less-polar ginsenosides: Comparison of ginsenosides from Panax ginseng, American ginseng and Panax notoginseng prepared by heating and acid treatment. J. Ginseng Res. 2021, 45, 163–175. [Google Scholar] [CrossRef]
- Kim, K.T.; Yoo, K.M.; Lee, J.W.; Eom, S.H.; Hwang, I.K.; Lee, C.Y. Protective effect of steamed American ginseng (Panax quinquefolius L.) on V79-4 cells induced by oxidative stress. J. Ethnopharmacol. 2007, 111, 443–450. [Google Scholar] [CrossRef]
- Kim, K.T.; Yoo, K.M. Effect of hot water boiling and autoclaving on physicochemical properties of American ginseng (Panax quinquefolium L.). J. Ginseng Res. 2009, 33, 40–47. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, Y.; Zhang, J.; Xi, H.; Duan, X. Effects of far-infrared radiation temperature on drying characteristics, water status, microstructure and quality of kiwifruit slices. J. Food Meas. Charact. 2019, 13, 3086–3096. [Google Scholar] [CrossRef]
- Aboud, S.A.; Altemimi, A.B.; RS Al-HiIphy, A.; Yi-Chen, L.; Cacciola, F. A Comprehensive Review on Infrared Heating Applications in Food Processing. Molecules 2019, 24, 4125. [Google Scholar] [CrossRef]
- Zhang, Q.; Wan, F.; Yue, Y.; Zang, Z.; Xu, Y.; Jiang, C.; Shang, J.; Wang, T.; Huang, X. Study on Ultrasonic Far-Infrared Radiation Drying and Quality Characteristics of Wolfberry (Lycium barbarum L.) under Different Pretreatments. Molecules 2023, 28, 1732. [Google Scholar] [CrossRef]
- Ratseewo, J.; Meeso, N.; Siriamornpun, S. Changes in amino acids and bioactive compounds of pigmented rice as affected by far-infrared radiation and hot air drying. Food Chem. 2020, 306, 125644. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zhang, Y.; Zhu, Z.; Wang, Y.; Zeng, Z.; Liu, C. Comparative study on physicochemical and nutritional properties of black rice influenced by superheated steam, far infrared radiation, and microwave treatment. Innov. Food Sci. Emerg. 2023, 84, 103282. [Google Scholar] [CrossRef]
- Yao, L.; Fan, L.; Duan, Z. Effect of different pretreatments followed by hot-air and far-infrared drying on the bioactive compounds, physicochemical property and microstructure of mango slices. Food Chem. 2020, 305, 125477. [Google Scholar] [CrossRef] [PubMed]
- Kossah, R.; Zhang, H.; Chen, W. Antimicrobial and antioxidant activities of Chinese sumac (Rhus typhina L.) fruit extract. Food Control 2011, 22, 128–132. [Google Scholar] [CrossRef]
- Lim, Y.J.; Kwon, S.J.; Qu, S.; Kim, D.G.; Eom, S.H. Antioxidant contributors in seed, seed coat, and cotyledon of γ-ray-induced soybean mutant lines with different seed coat colors. Antioxidants 2021, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Hwang, I.G.; Kim, H.Y.; Joung, E.M.; Woo, K.S.; Jeong, J.H.; Yu, K.W.; Lee, J.; Jeong, H.S. Changes in ginsenosides and antioxidant activity of Korean ginseng (Panax ginseng C.A. Meyer) with Heating Temperature and Pressure. Food Sci. Biotechnol. 2010, 19, 941–949. [Google Scholar] [CrossRef]
- Lee, J.H.; Ko, M.J.; Chung, M.S. Subcritical water extraction of bioactive components from red ginseng (Panax ginseng C.A. Meyer). J. Supercrit. Fluid. 2018, 133, 177–183. [Google Scholar] [CrossRef]
- Yoo, K.M.; Lee, C.; Lo, Y.M.; Moon, B. The Hypoglycemic Effects of American Red Ginseng (Panax quinquefolius L.) on a Diabetic Mouse Model. J. Food Sci. 2012, 77, 147–152. [Google Scholar] [CrossRef]
- Musilova, J.; Lidikova, J.; Vollmannova, A.; Frankova, H.; Urminska, D.; Bojnanska, T.; Toth, T. Influence of Heat Treatments on the Content of Bioactive Substances and Antioxidant Properties of Sweet Potato (Ipomoea batatas L.) Tubers. J. Food Quality 2020, 2020, 8856260. [Google Scholar] [CrossRef]
- Escobedo, R.; Miranda, R.; Martínez, J. Infrared irradiation: Toward green chemistry, a review. Int. J. Mol. Sci. 2016, 17, 453. [Google Scholar] [CrossRef]
- Qian, Z.; Lu, J.; Gao, Q.; Li, S. Rapid method for simultaneous determination of flavonoid, saponins and polyacetylenes in Folium Ginseng and Radix Ginseng by pressurized liquid extraction and high-performance liquid chromatography coupled with diode array detection and mass spectrometry. J. Chromatogr. A 2009, 1216, 3825–3830. [Google Scholar] [CrossRef]
- Nasanbat, B.; Uchiyama, A.; Amalia, S.N.; Inoue, Y.; Yokoyama, Y.; Ogino, S.; Torii, R.; Hosoi, M.; Motegi, S.I. Kaempferol therapy improved MC903 induced-atopic dermatitis in a mouse by suppressing TSLP, oxidative stress, and type 2 inflammation. J. Dermatol. Sci. 2023, 111, 93–100. [Google Scholar] [CrossRef]
- Wang, L.; Yang, X.; Yu, X.; Yao, Y.; Ren, G. Evaluation of antibacterial and anti-inflammatory activities of less polar ginsenosides produced from polar ginsenosides by heat-transformation. J. Agric. Food Chem. 2013, 61, 12274–12282. [Google Scholar] [CrossRef]
- Murugesan, M.; Mathiyalagan, R.; Boopathi, V.; Kong, B.M.; Choi, S.K.; Lee, C.S.; Yang, D.C.; Kang, S.C.; Thambi, T. Production of minor ginsenoside CK from major ginsenosides by biotransformation and its advances in targeted delivery to tumor tissues using nanoformulations. Nanomaterials-Basel 2022, 12, 3427. [Google Scholar] [CrossRef]
- Park, E.H.; Kim, Y.J.; Yamabe, N.; Park, S.H.; Kim, H.K.; Jang, H.J.; Kim, J.H.; Cheon, G.J.; Ham, J.; Kang, K.S. Stereospecific anticancer effects of ginsenoside Rg3 epimers isolated from heat-processed American ginseng on human gastric cancer cell. J. Ginseng Res. 2014, 38, 22–27. [Google Scholar] [CrossRef]
- Yoo, S.; Park, B.I.; Kim, D.H.; Lee, S.; Lee, S.H.; Shim, W.S.; Seo, Y.K.; Kang, K.; Lee, K.T.; Yim, S.V. Ginsenoside Absorption rate and extent enhancement of black ginseng (CJ EnerG) over red ginseng in healthy adults. Pharmaceutics 2021, 13, 487. [Google Scholar] [CrossRef]
- Ji, Y.J.; Kim, H.D.; Lee, E.S.; Jang, G.Y.; Seong, H.A. Heat Treatment Enhances the Neuroprotective Effects of Crude Ginseng Saponin by Increasing Minor Ginsenosides. Int. J. Mol. Sci. 2023, 24, 7223. [Google Scholar] [CrossRef]
- Park, S.E.; Na, C.S.; Yoo, S.A.; Seo, S.H.; Son, H.S. Biotransformation of major ginsenosides in ginsenoside model culture by lactic acid bacteria. J. Ginseng Res. 2017, 41, 36–42. [Google Scholar] [CrossRef]
- Kim, M.S.; Jeon, S.J.; Youn, S.J.; Lee, H.; Park, Y.J.; Kim, D.O.; Kim, B.Y.; Kim, W.; Baik, M.Y. Enhancement of minor ginsenosides contents and antioxidant capacity of american and canadian ginsengs (Panax quinquefolius) by puffing. Antioxidants 2019, 8, 527. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Jeong, S.M.; Kim, S.Y.; Park, H.R.; Nam, K.; Ahn, D. Effect of far-infrared radiation and heat treatment on the antioxidant activity of water extracts from peanut hulls. Food Chem. 2006, 94, 489–493. [Google Scholar] [CrossRef]
- Azad, M.O.K.; Piao, J.P.; Park, C.H.; Cho, D.H. Far infrared irradiation enhances nutraceutical compounds and antioxidant properties in Angelica gigas Nakai powder. Antioxidants 2018, 7, 189. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).