1. Introduction
Fire blight, caused by
Erwinia amylovora, is a devastating bacterial disease that threatens apple and pear production worldwide [
1]. The infection of the host by
E. amylovora occurs during open bloom, when bacterial populations increase on the stigma. In the presence of water, the bacterial cells flow down into the hypanthium and reach the natural flower openings. Bacterial cells then invade the plant tissue, resulting in the infection of blossoms and shoots [
2]. Antibiotics, such as streptomycin and kasugamycin, have been noted to have superior blossom blight control efficacy, with significant population reductions in
E. amylovora on apple flower stigmas [
3]. In Canada and the US, streptomycin and kasugamycin are the most commonly used pesticides against fire blight [
4]. However, streptomycin resistant strains of
E. amylovora have been identified in different countries around the world [
5,
6,
7,
8]. The importance of this is underscored by the fact that excessive use of antibiotics in agriculture has led to increasing awareness of the possibility of antibiotic resistance gene transfer to human and animal pathogenic microbes [
9]. Currently, the European Union regulatory agencies have restricted the use of streptomycin in agriculture, while Canada and the USA have only prohibited its use for organic farms [
10].
Commercial biocontrol agents (BCAs) using antagonistic bacteria or lytic bacteriophages are available [
9,
11,
12]. The BCA mode of action relies on the antagonistic interactions that result in competition for niches and nutrients and/or induction of plant defenses [
13]. Commercially available bacterial-dependent BCAs against fire blight are available, such as BlightBan
TM A506, Bloomtime Biological
TM [
14] and lytic phage-dependent BCAs such as, AgriPhage–Fireblight (OmniLytics, USA), have been authorized by the US Environmental Protection Agency (EPA) for fire blight treatment [
15]. The phage-carrier system (PCS) is another form of BCA that combines antagonistic bacterial carriers with phages as a double-pronged treatment that was developed for fire blight control when the plants are blooming [
16,
17,
18,
19]. A study using PCS with
Pantoea agglomerans and
Erwinia amylovora phages showed a 56% reduced infection on flowers of potted apple trees, and this treatment compared well with the application of the antibiotic, streptomycin [
20].
Spray drying and freeze-drying are the most widely used methods for producing BCA powders. The spray drying approach is preferable, as it is characterized by a high production rate and open production mode, as well as being easily scaled to industrial levels. Furthermore, the spray drying operation time and production costs are 30–50 fold lower when compared to the costly and time-consuming freeze-drying approach [
21,
22,
23]. However, a significant drawback of spray drying is the loss of cell viability due to the use of high temperature and rapid dehydration during the spray drying process.
Previous spray dry trials with
P. agglomerans showed low viability (3-29% viability) after spray drying due to the heat sensitivity of the cells [
24]. The research presented herein describes an optimized formulation and protocol that leads to improved viability of spray dried
P. agglomerans. Notably, the protocol demonstrated greater than 90% viability for both the
P. agglomerans cells alone, as well as for this bacterium infected with
Erwinia phages as a PCS. In follow-up experiments, a green fruit disc assay confirmed high efficacy of the spray dried
P. agglomerans to control
E. amylovora growth. Thus, the spray drying protocol developed herein provides a viable avenue to make industrial scale production of
P. agglomerans BCA/PCS possible. Moreover, this protocol has broader implications for other antagonistic bacteria that are known to be sensitive to spray drying, given the easily adaptable formulation and protocol.
2. Materials and Methods
2.1. Bacterial Isolates
All bacterial strains used in this study are listed in
Table 1. Cultures were stored at -80 °C in Microbank cryobeads (Pro-Bank Diagnostics, Richmond Hill, ON, Canada). To prepare the working culture stock, one Microbank cryobead was mixed with one drop of PBS buffer (10 mM, pH 7.2) and plated on 2.3% (w/v) Difco
TM nutrient agar (NA) plates (BD, MD, USA). The plates were incubated for 16-18 h at 27 °C and then stored at 4 °C for 1 to 2 wks. Working cultures were obtained from the initial cultures by streaking single colonies onto NA and incubated at 27 °C for 16-18 h.
2.2. Bacteriophages
The
E. amylovora bacteriophages used in this study are ϕEa21-4 (Myovirus) and ϕEa46-1-A1 (Podovirus) as listed in
Table 2. To propagate each phage, a bacterial host (
Table 2) suspension was prepared by suspending 5-6 colonies in 3 mL of 0.8% (w/v) nutrient broth (NB) (BD, MD, USA) to obtain an OD
600 of ~ 0.6. Using a 250 mL baffled Erlenmeyer flask, 100 µL of the bacterial suspension was added to 75 mL of NB, which was incubated at 27 °C with 150 rpm shaking (New Brunswick Innova
® 42, Eppendorf, Hamburg, Germany) for 3 -4 h. A 100 µL aliquot of phage stock (5.0 x 10
9 PFU/mL) was added, and the mixture was incubated for 16-18 h at 27 °C with 150 rpm shaking (New Brunswick Innova
® 42, Eppendorf, Hamburg, Germany). Following incubation, 1 mL of chloroform was added to the culture and incubated with shaking for 5 min. The bacterial culture was centrifuged at 8500 x
g at 4 °C for 15 min, the pellet discarded, and the supernatant filtered through a 0.22 µm Steriflip filter (Millipore, Billerica, MA, USA). The working phage stocks were stored at 4 °C in dark amber glass vials until needed and the titers were determined by using qPCR (
Section 2.3) [
19].
2.3. Quantitative PCR (qPCR)
Phage titers and
P. agglomerans cell numbers were determined using qPCR using a protocol described by Gayder and coworkers (2019). Phage working solutions (or lysate) were treated with DNase I to remove non-encapsulated phage DNA prior to qPCR. DNase I treatments contained 10 µL of phage lysate, 1 µL of 2000 U/mL DNase I (NEB, MA, USA), 2 µL of 10X DNase I buffer (NEB, MA, USA) and 7 µL of water. The solution was incubated for 40 min at 37 °C and then 20 min at 80 °C. The qPCR 20 µl reactions consisted of 4 µL of 5X EVOlution Probe qPCR Mix (Montreal Biotech Inc., QC, Canada), 200 nM of each primer [
19], 100 mM of the probe and 2 µL of the template. The reaction conditions began with 10 min at 95 °C, followed by 40 cycles of 10s at 95 °C and 45s at 54 °C. Dilutions of standard plasmid, pTotalStdA (10
11, 10
8, and 10
5 copies/mL), which contains amplicons for
E. amylovora and
P. agglomerans, and phages ϕEa21-4 and ϕEa46-1-A1 were included in every run to generate a standard curve. A linearly fit standard curve of Ct value vs copies/mL of the pTotalStdA was used to calculate the sample population quantities (genomes/mL). Populations of bacteria and phage were directly interpreted from this curve assuming that each cell or phage capsid contained a single genome. All qPCR quantification was performed with a Bio-Rad CFX96 qPCR System (Bio-Rad, CA, USA).
2.4. Bacterial Counting by Platting
Bacterial cell cultures or reconstituted suspensions were serially diluted in PBS and 100 µL of these dilutions was spread on NA and then incubated for 16-18 h at 27 °C shaking (New Brunswick Innova® 42, Eppendorf, Hamburg, Germany). Bacterial colonies were enumerated, and the final CFU/mL was calculated accordingly by taking into account the dilution and plating factors. Plating was carried out in triplicate.
2.5. Carrier Preparation
P. agglomerans was subcultured weekly on NA and, after 20 h at 27 °C, the plates were stored at 4 °C until needed that week.
P. agglomerans cells were grown in NB as an initial rich-nutrient growth condition. For growth under stress conditions (
i.e., osmotic-adaptation),
P. agglomerans was grown on medium that contains 5.0 g/L yeast extract, 10.0 g/L sucrose and 35.5 g/L NaCl (YSN media) or in NB with the same amount of NaCl to reach a water activity (
aw) in the medium of 0.98
aw; where
aw is the ratio between the vapour pressure of a sample compared to a control [
26]. Cells were harvested by centrifugation (8500 x
g for 10 min at 4 °C), resuspended in 10 mM PBS at pH 7.2 and enumerated using the serial dilution and spread plate technique on NA (
Section 2.4). The colonies on the plates were counted after incubation for 16-18 h at 27 °C shaking (New Brunswick Innova
® 42, Eppendorf, Hamburg, Germany).
2.6. Phage-Carrier Formulation
2.6.1. Polymer screening and Formulation Optimization
Different concentrations and combinations of polymers (all from Sigma-Aldrich, CA, USA) were used for formulations to explore with spray drying (
Table 3). These polymers include D(+)-trehalose dihydrate, maltodextrin (dextrose equivalents 4.0-7.0 and 16.5-19.5), gum arabic (from acacia tree) and talc with carboxymethylcellulose sodium salt (CMC).
P. agglomerans cell pellets were resuspended in a formulation mixture for 30 min to produce the formulated cells (f
Pa).
The effect of formulation chemicals on the carrier infectivity by
Erwinia phages was tested before selecting the suitable formulation for spray drying. Different concentrations of formulation chemicals were prepared in NB, as outlined in
Table 3, and inoculated with
P. agglomerans Pa39-7 (10
3 CFU/mL) together with the ϕEa21-4 phage (10
5 PFU/mL) in 96 well plates prior to incubation at 27 °C in a Synergy H1 microplate reader (BioTeK, Agilent, CA, USA) for 24 h. Readings of the plates at an OD
600 were taken every 15 min and compared with the control sample (no phage added) in order to monitor growth/infectivity. After the initial trials, further optimization was carried out using different concentrations and combinations of the selected formulation chemicals as shown in
Table 4.
Table 3.
Polymers used for screening.
Table 3.
Polymers used for screening.
| Chemicals |
Final Concentration (%, w/v) |
| D(+)-Trehalose |
0.05, 0.50, 5.00 |
| Maltodextrin* |
0.05, 0.50, 5.00 |
| Maltodextrin* |
0.05, 0.50, 5.00 |
| Talc/CMC** |
0.01, 0.10, 1.00 |
Table 4.
Concentration ranges used for optimization of the carrier formulation.
Table 4.
Concentration ranges used for optimization of the carrier formulation.
| Chemicals |
Final Concentration (%, w/v) |
| D(+)-Trehalose |
13-15 |
| Maltodextrin* |
5-15 |
| Talc |
0-2 |
| CMC** |
0-1 |
2.6.2. Spray drying of phage infected and non-infected carrier cells
Osmotically-stressed or non-stressed
P. agglomerans cells (s
Pa or ns
Pa, respectively), were prepared by mixing cell pellets with a formulation solution (1g/100 mL) for 30 min before spray drying to form formulated stressed (sf
Pa) or formulated non-stressed (nsf
Pa). For phage infected cells (
i.e., the phage-carrier system
), P. agglomerans cell pellets were resuspended in PBS and mixed with the phage (ϕEa21-4 or ϕEa46-1-A1) at an MOI of 1.0 for 30 min. The cells were then harvested by centrifugation (8500 x
g for 10 min at 4 °C) and resuspended in the formulation mixture (1 g/100 mL) for spray drying. The spray drying process was carried out using a Yamato ADL311S model (Yamato Scientific Co., Tokyo, Japan) with a feeding rate of 11-14 mL/min, inlet temperature of 160 °C and an outlet temperature of 60 °C. The spray dried powder was collected and stored in white plastic polyethylene high density tubes [
27] at 4 °C or room temperature (RT) with activated Silica gel packets (5 g each) (Millipore Sigma, CA, USA).
2.7. Storage Stability of the Spray Dried Preparations
Spray dried powder of phage infected, and non-infected carrier cells was collected and stored in polyethylene tubes (as mentioned above), then enclosed in Statshield® Moisture Barrier Al-bags (DESCO, CA, USA) and sealed with an Impulse Sealer, model AIE 300 (American international electric, CA, USA). Spray dried samples in sealed tubes were stored at RT or 4 °C for 16 wks (~ 4 months). At the time of sampling (biweekly), one tube was taken, and three technical replicates were prepared by reconstitution of the powder in sterile distilled water (0.9 g/ 3 mL). P. agglomerans cells were enumerated by plating on NA, and the logarithmic cell counts were plotted versus time. For phage enumerations in PCS powder, 100 µL of reconstituted powder was used to inoculate 10 mL of NB and incubated at 27 °C with shaking at 180 rpm shaking (New Brunswick Innova® 42, Eppendorf, Hamburg, Germany). The phages were enumerated by qPCR at time intervals of 0, 2, 4 and 18 h (T0, T2, T4 and TO/N). To test the viability of the phage in the reconstituted PCS powder, 100 µL volume of a 1/10 dilution of the reconstituted powder was platted on NA plates and incubated for 16-18 h at 27 °C. The plaques formed by active phages were counted and PFU/mL was assessed after taking into account the dilution and plating factors.
2.8. Efficacy Testing of Phage-Carrier with a Green Pear Disc Assay
Green pears were purchased from a local grocery store, washed, rinsed, surface sterilized with 70% (v/v) ethanol and rinsed twice with sterile distilled water. Under sterile conditions, a copper borer was used to cut into the fruit and produce pear plugs from which discs were cut measuring 1.5 mm in diameter and 1 mm in depth. The discs were placed into 2 mL sterile Eppendorf tubes using sterile forceps. Treatments consisted of formulated P. agglomerans (Pa39-7, formulated P. agglomerans plus phages (Pa39-7 + phage фEa21-4), a positive control (1:1 ratio of E. amylovora strain Ea6-4 and D7) and non-infected formulated P. agglomerans (negative control). Two biological replicates, containing eight technical replicates were set up for each treatment. To each pear plug, 10µl of treatment was added and sample tubes were collected at 0, 24 and 48 h. A 10 µL volume of pathogen was added at 106 CFU/mL at 24 h and allowed to incubate for an additional 24 h. Following this time period, 500 µL of 0.01 M phosphate buffer pH 7.0 was added to the tubes containing the disc and sonicated for 3 min at high power for 3 min. A 200 µL volume of the sonicated supernatant was removed into 2 mL sterile Eppendorf tubes and the cells were heat killed at 80 °C for 10 min. The heat-treated supernatant was frozen at - 20 °C prior to evaluation by qPCR. A Student’s t-test was performed comparing the means between the 24 and 48 h experimental data. Statistical analyses were performed using the JMP programme (JMP Statistical Discovery LLC, Cary, NC, USA). A three-way random analysis of variance (ANOVA) was performed on natural log (ln) + 1 transformed data comparing the effects of two formulated products at suppressing the population of E. amylovora in vitro on green pear discs over time.
2.9. Powder Physical Properties
2.9.1. Water activity (aw)
Carrier and PCS powder water activities were measured by using an Aqualab 4TE water activity meter according to the manufacturer’s instructions. Three technical replicates of powders were prepared and tested for the shelf-life experiment.
2.9.2. Transmission Electron Microscopy (TEM)
Copper–rhodium (150-mesh) grids were covered with a thin layer of amorphous carbon made hydrophilic by a 45 s vacuum glow-discharge. PCS powder was rehydrated in filtered MilliQ water and then 4–6 μL were placed on individual grids and left for 2 min to adsorb. Excess sample was gently removed by filter paper. The grid was then washed three times with water. Sample on the grids were stained with 2% (w/v) uranyl acetate. A Tecnai G2 transmission electron microscope (FEI Company, Hillsboro, OR, USA) at the University of Guelph Electron Microscopy Unit (Guelph, ON, Canada) was used for visualization, operating at 200 kV under variable magnification (x 50,000). Images were acquired using the Gatan Ultascan 4K CCD (Pleasanton, CA, United States) and Gatan Digital Micrograph software imaging system.
4. Discussion
In fire blight management, BCAs and phages (registered in the USA) are commercially available as alternatives to antibiotic utilization [
9,
14,
15]. Subsequently, PCS was developed as an approach that combines the biological activity of antagonistic bacteria,
P. agglomerans, and
Erwinia phages that specifically target and kill the fire blight pathogen [
16,
17,
28]. An optimized and economical production protocol is required for industrial scale production of the PCS and spray drying or freeze-drying are the most widely used methods for production [
21]. However, freeze-drying is costly, time consuming and operates as a closed system, which means that it cannot be used in a continuous largescale production mode. In contrast, spray drying has a higher powder production rate, continuous production mode, is easily scalable to industrial levels and is 30-50 fold more cost effective compared to freeze-drying [
21,
22,
23]. The major drawback of spray drying is the loss of cell/phage viability due to exposure to high temperatures during preparation. For example,
P. agglomerans carrier cells are thermally sensitive and have exhibited a significant reduction in cell viability (only 3 - 30% cell viability following spray drying) [
24,
29,
30]. Bacterial growth under osmotically-adapted conditions is known to increase tolerance to desiccation. More specifically, osmotically-adapted
P. agglomerans can accumulate glycine-betaine, ectoine and D(+)-trehalose [
26,
30,
31]. Under this premise, osmotically-adapted (
i.e., grown in a standard medium with NaCl to a water activity of 0.97-0.98) and spray-dried
Pantoea were able to consistently display cell viabilities around 30% [
24]. However, these low viability numbers remain a hindrance to industrial scale production.
This study established an improved
P. agglomerans viability protocol by optimizing the formulation mixtures, spray drying temperatures, nozzle clogging and reconstitution times. The culmination of this work indicates that
P. agglomerans (either under non-stressed or osmotically non-adapted conditions) treated with formula II have approximately 70% survival after spray drying. Further optimization in the introduced protocol was carried out by growing
P. agglomerans on NB with NaCl at an a
w of 0.98 for 18-20 h. This osmotic-adaptation further improved cell viability to more than 95% for
P. agglomerans alone (BCA) or more than 90% for PCS, which far exceeds the previously published results of ~30% for osmotically adapted [
30] and 3% for non-adapted [
24]. Thus, this enhanced protocol makes it a very promising approach for industrial scale production of
P. agglomerans as BCA or PCS applications to control fire-blight, but also more widely as an avenue to explore for other bacteria recalcitrant to these processes.
For the PCS, the modified protocol was also assessed for survival and infectivity of the phage in the carrier bacteria. In the presence of D(+)-trehalose and maltodextrin the survival of
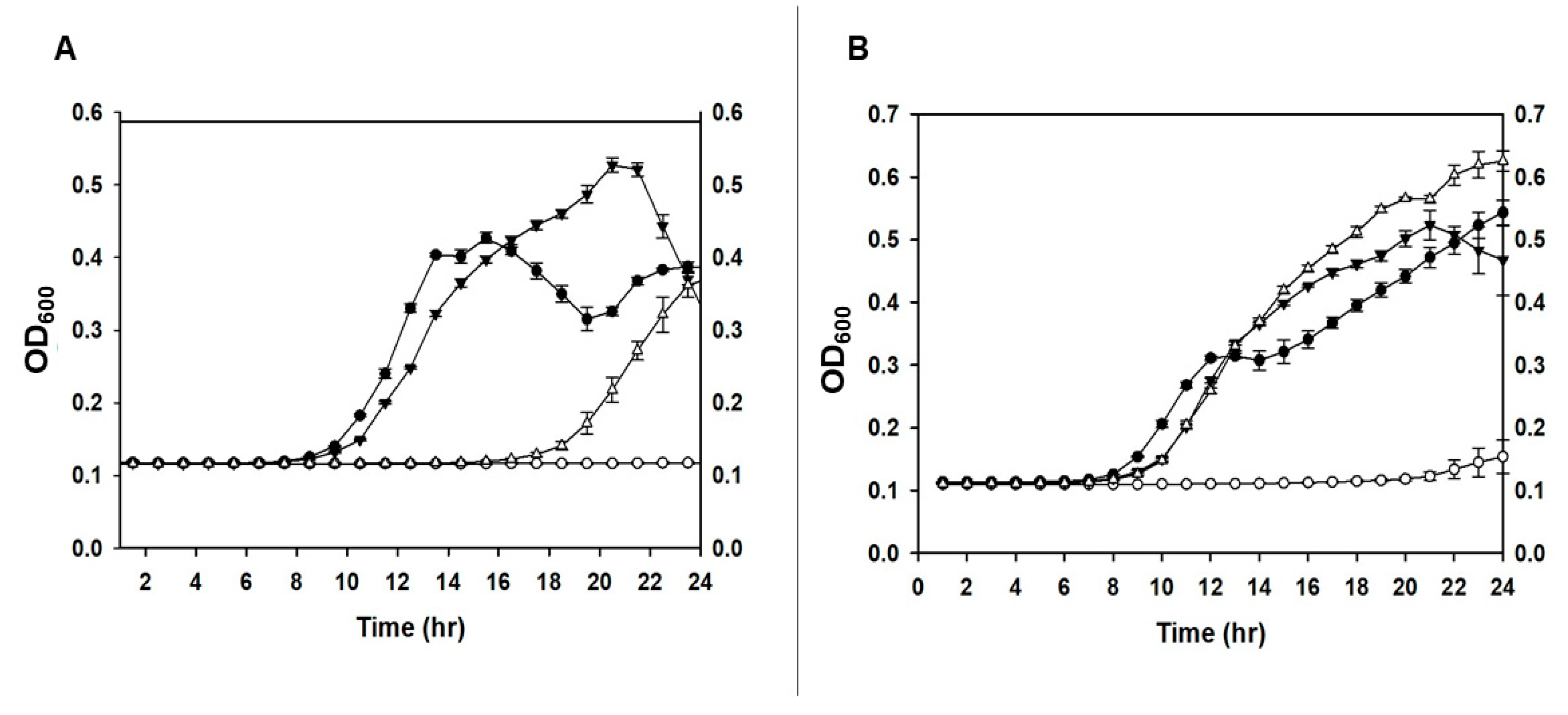
P. agglomerans with the phage was markedly lower; thereby suggesting that not only had phage ϕEa21-4 survived the spray drying process, but that the presence of formula II components enhanced its infectivity (
Figure 1A&B). The presence of formulation compounds has been suggested in the literature to increase the viscosity and hence phage adsorption and/or can lead to changes in the bacterial metabolism [
32,
33]. While plausible as a reason for the observed increase, further research would be needed to confirm these possibilities in our studies.
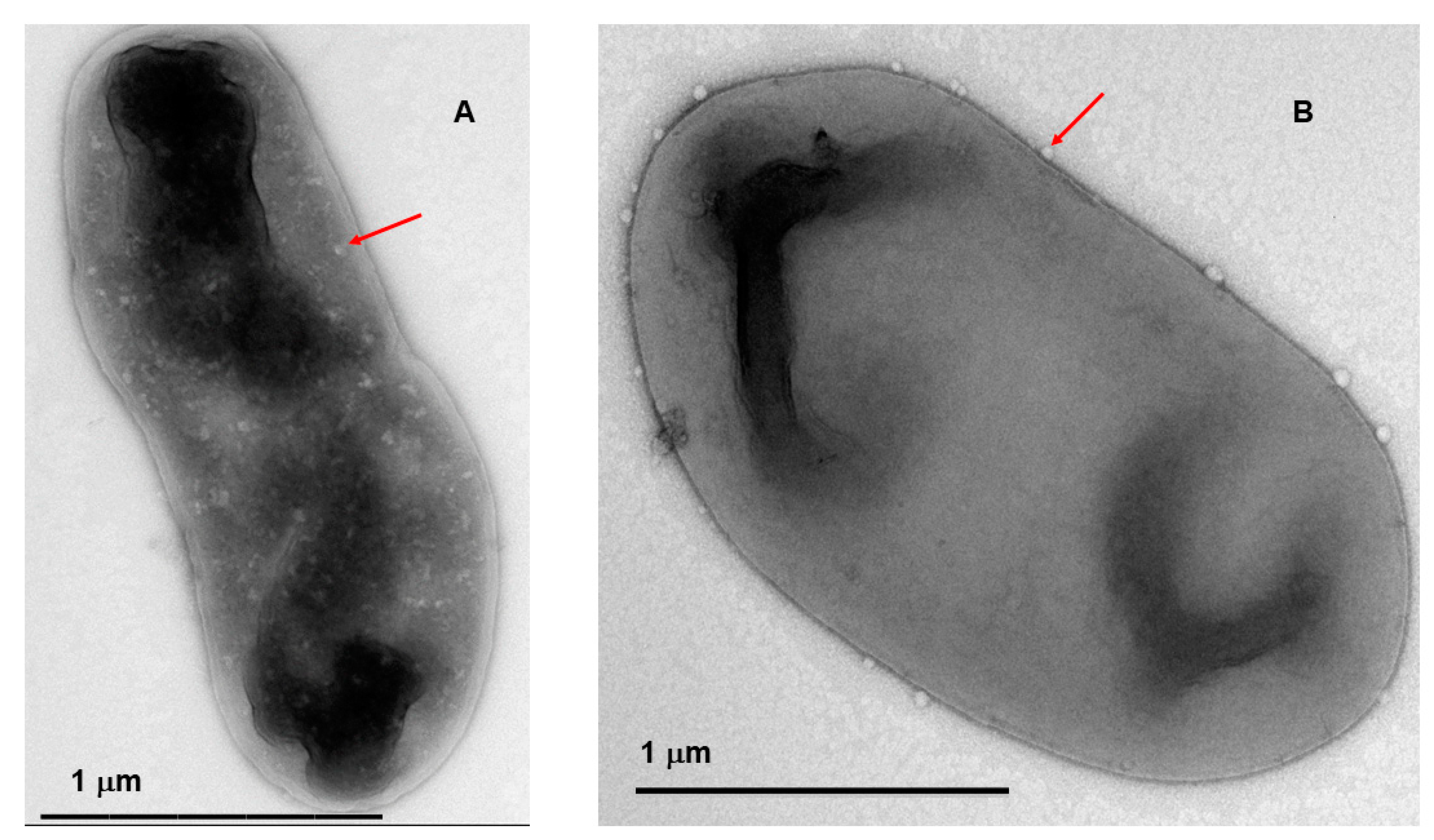
Maintaining the viability of the carrier and the ability of these cells to be infected with
E. amylovora phages are important factors for a suitable PCS. In this work, formula II was noted to protect both phage-infected and non-infected cells, as shown by a combination of plaque assays, NB inoculation with reconstituted powder, as well as electron microscopy imaging results (
Figure 3,
Figure 4). In addition, the powder storage protocol used in this study utilized low O
2-permeability tubes, silica gel packets to control humidity, and enclosure of the bottle in Statshield® Moisture Barrier Al-bags, along with different storage temperatures. Combing these storage conditions led to high cell viability of both carrier and PCS powders for almost 4 months (
i.e., only 1-2 log reductions in viability over this period). By this process, high humidity content in the powder, which can cause the crystallization of the amorphous trehalose matrix and decrease the cell viability [
34,
35], appears to have been mitigated. Our low temperature results are also consistent with other studies that demonstrated that powder storage temperature has previously been noted to be an important factor that can affect the viability of the cells during storage due to membrane lipid oxidation [
36,
37]. Thus, by combining all of these factors we have been able to generate a PCS that has proven longevity that may be amenable to production and delivery for field applications.
As an initial
in vitro test of the efficacy of the BCA and PCS powders in mitigating
E. amylovora infections, reconstituted powders were used on pear slices as a model system of infection. Both the BCA and PCS preparations were able to decrease
E. amylovora growth by 3 log reduction compared to untreated samples after 24 h of infection time with
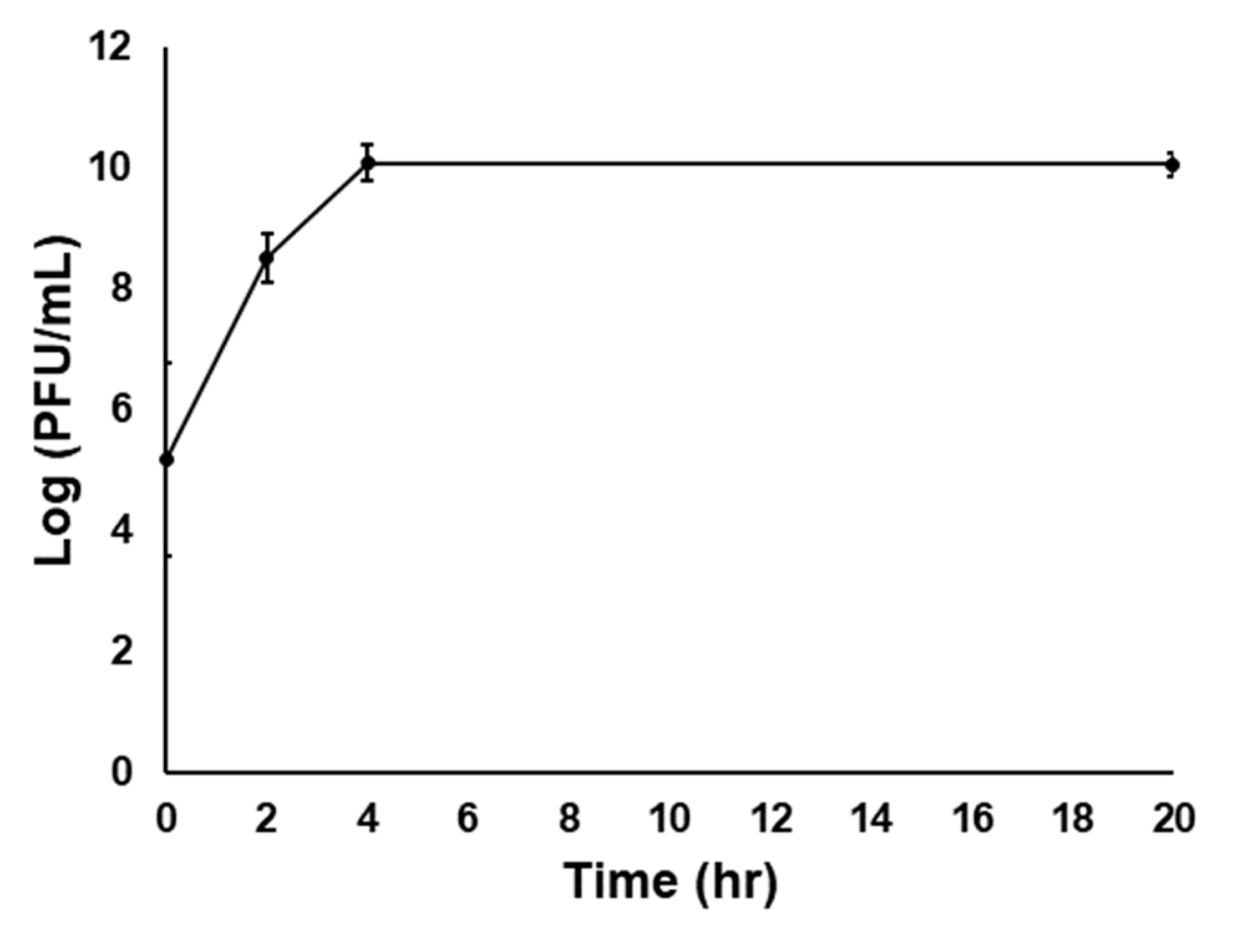
E. amylovora. Interestingly, the populations of carrier and phage (in the case of PCS) were not significantly changed over the entire 48 h experiment. These results are in contrast to our separate experiment where reconstituted PCS in NB led to a doubling of the phage counts after 24 h with shaking at 27 °C (
Figure 3). It is plausible that there are alterations or delays in the infection process for the phage in the pear disc assay given that these environmental conditions are likely to be different than the rich NB media. Indeed, published data has noted that there are several factors that can contribute to discrepancies in phage counts between assays. For example, the bacterial carrier metabolic state can play a vital role in phage adsorption/infectivity and consequently phage propagation. For instance, the burst size of T4 phage is dependent on the cell state of
E. coli strain B23 [40]. T4 burst size can vary between 1-40 progeny/cell depending on whether they are in the mid-log or stationary phase of growth, where the latter may produce only one phage per cell since the cells have entered a hibernation of scavenger response mode[41]. The cell’s metabolic state can also influence the sigma factors present within cells, which can differentially orchestrate the activity of the DNA-dependent RNA polymerase (RNAP) holoenzyme (Eσ) during the transcription process [42]. For example,
E. coli contains six σ factors, including σ
70 that is present during exponential growth and σ
S that is found during the stationary phase [42,45] and activated by various stress signals, including hyperosmolarity [46]. In the present spray drying protocol,
P. agglomerans was exposed to salt stress during osmotic adaptation and stationary phase growth, which combined with further phyllosphere pressures/responses led to slower/lower release of phages and concomitantly higher numbers of
P. agglomerans. While the PCS and BCA both showed antagonistic behaviour towards
E. amylovora, the pear disc assay highlights the importance of assaying beyond the typical
in vitro rich culture scenarios and provides a future avenue to explore observations over longer time periods, MOI concentrations, reconstitution variables and more.
Author Contributions
Conceptualization, Nassereldin Ibrahim, Joel Weadge, Qi Wang, Antonet Svircev and Hany Anany; methodology, Nassereldin Ibrahim, Tracy Quo, Janet Lin, Darlene Nesbitt, Qi Wang and Hany Anany; validation, Qi Wang, Antonet Svircev and Hany Anany; formal analysis Nassereldin Ibrahim, Tracy Quo , Janet Lin, Darlene Nesbitt; writing—original draft preparation, Nassereldin Ibrahim, Joel Weadge and Hany Anany; writing—review and editing, Qi Wang, Antonet Svircev, Joel Weadge and Hany Anany; supervision, Qi Wang, Joel Weadge and Hany Anany; funding acquisition, Qi Wang, Antonet Svircev, and Hany Anany. All authors have read and agreed to the published version of the manuscript.