Submitted:
26 December 2023
Posted:
27 December 2023
You are already at the latest version
Abstract
Keywords:
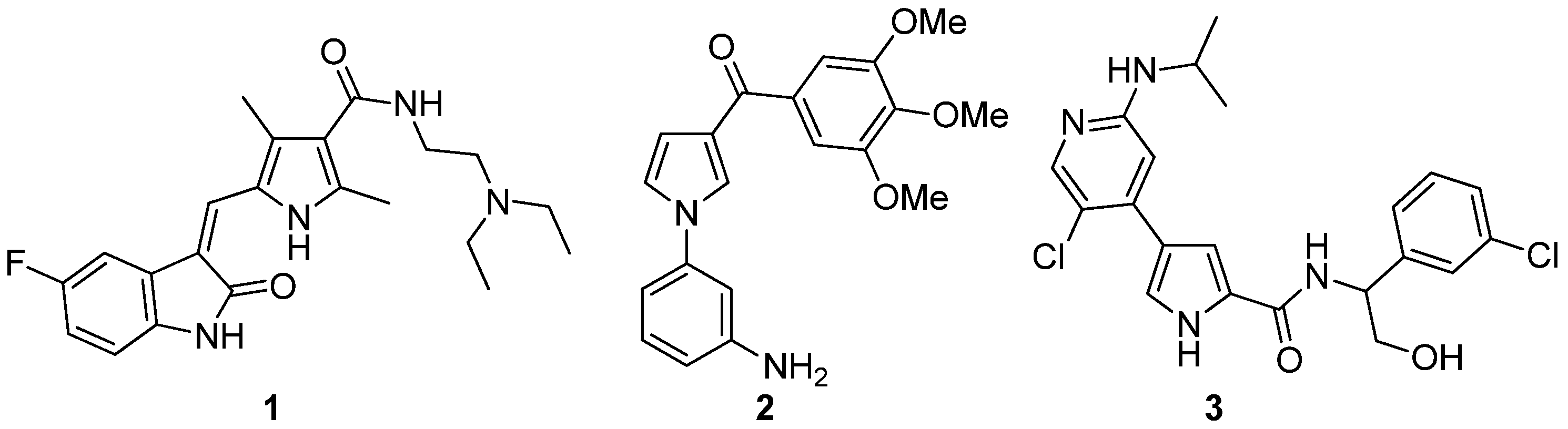
1. Introduction
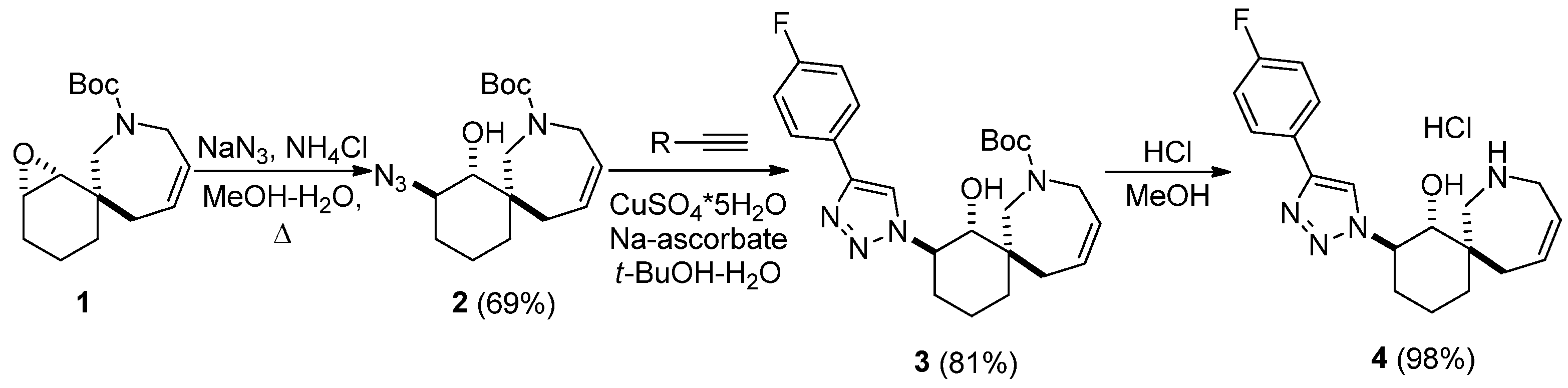
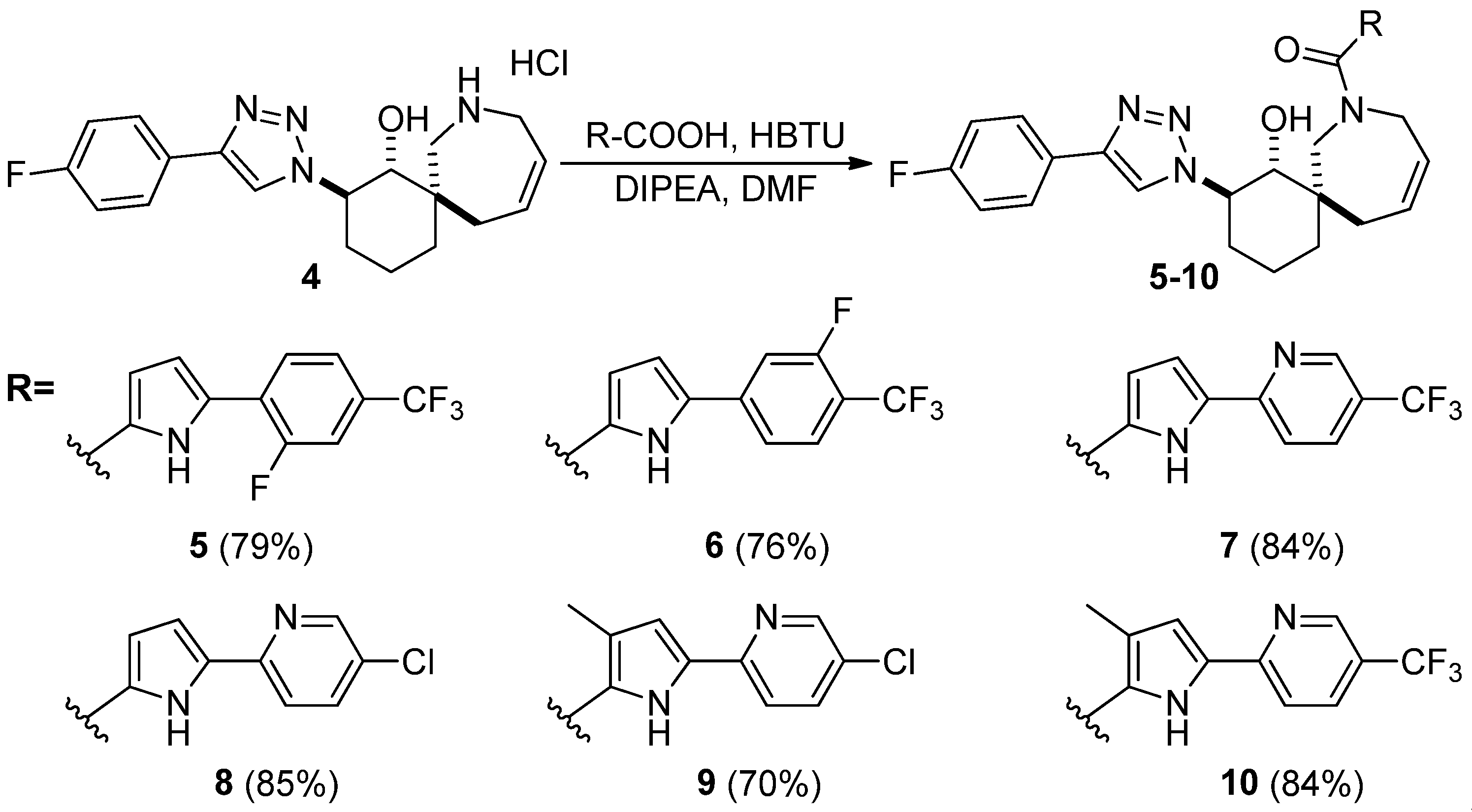
2. Results and Discussion
4. Materials and Methods
General procedure for synthesis of compounds 5-10:
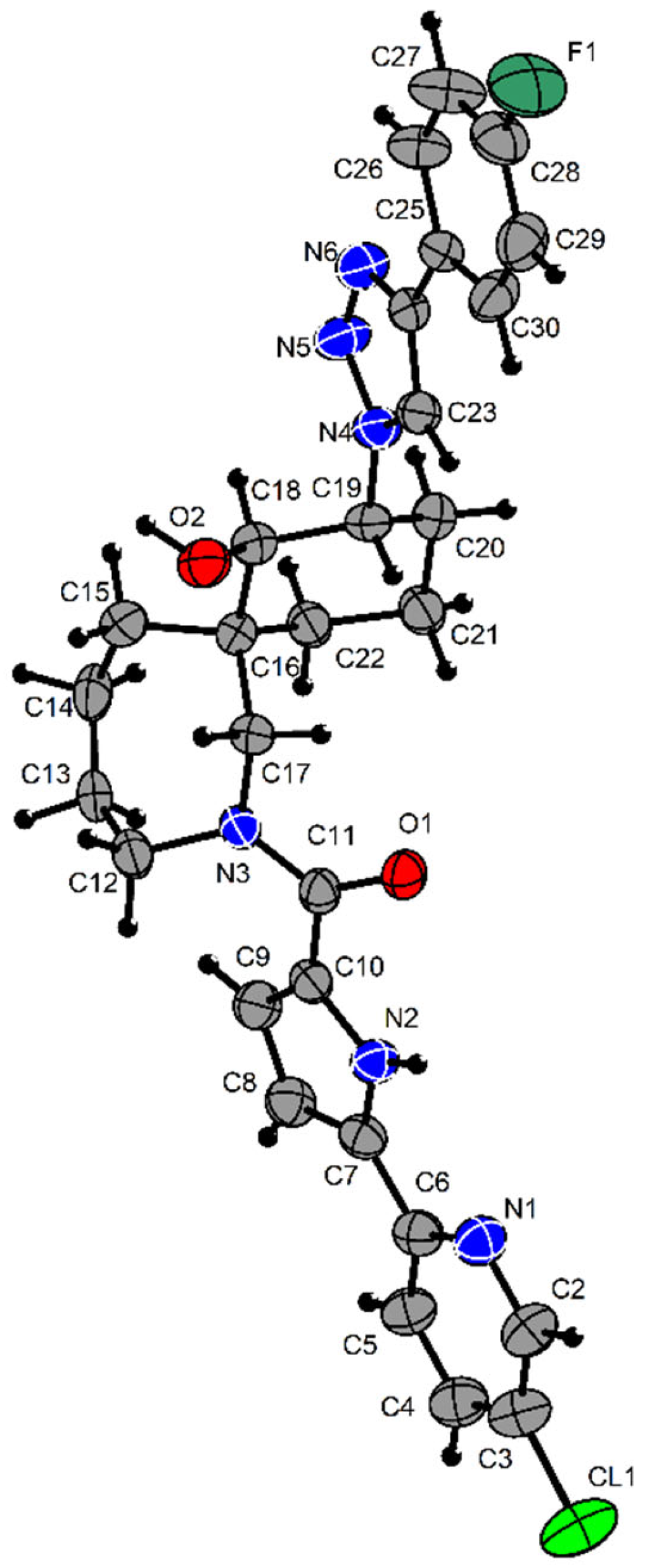
Crystallography Details
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chhikara, B.S.; Parang, K. Global Cancer Statistics 2022: The Trends Projection Analysis. Chem Biol Lett 2023, 10, 451. [Google Scholar]
- Barghi Lish, A.; Foroumadi, A.; Kolvari, E.; Safari, F. Synthesis and Biological Evaluation of 12-Aryl-11-Hydroxy-5,6-Dihydropyrrolo[2″,1″:3′,4′]Pyrazino[1′,2′:1,5]Pyrrolo[2,3- d ]Pyridazine-8(9 H )-One Derivatives as Potential Cytotoxic Agents. ACS Omega 2023, acsomega.3c04167. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, E.S.; Mireskandari, K.; Abdel-Jalil, R.; Amini, M. An Approach to Pharmacological Targets of Pyrrole Family From Medicinal Chemistry Viewpoint. MRMC 2022, 22, 2486–2561. [Google Scholar] [CrossRef]
- Li Petri, G.; Spanò, V.; Spatola, R.; Holl, R.; Raimondi, M.V.; Barraja, P.; Montalbano, A. Bioactive Pyrrole-Based Compounds with Target Selectivity. European Journal of Medicinal Chemistry 2020, 208, 112783. [Google Scholar] [CrossRef] [PubMed]
- Kilic-Kurt, Z.; Bakar-Ates, F.; Aka, Y.; Kutuk, O. Design, Synthesis and in Vitro Apoptotic Mechanism of Novel Pyrrolopyrimidine Derivatives. Bioorganic Chemistry 2019, 83, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Curreli, F.; Ahmed, S.; Benedict Victor, S.M.; Iusupov, I.R.; Belov, D.S.; Markov, P.O.; Kurkin, A.V.; Altieri, A.; Debnath, A.K. Preclinical Optimization of Gp120 Entry Antagonists as Anti-HIV-1 Agents with Improved Cytotoxicity and ADME Properties through Rational Design, Synthesis, and Antiviral Evaluation. J. Med. Chem. 2020, 63, 1724–1749. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Pan, M.; Su, X.; Dai, Y.; Fu, M.; Cai, X.; Shi, W.; Huang, W.; Qian, H. Discovery of Novel Pyrrole-Based Scaffold as Potent and Orally Bioavailable Free Fatty Acid Receptor 1 Agonists for the Treatment of Type 2 Diabetes. Bioorganic & Medicinal Chemistry 2016, 24, 1981–1987. [Google Scholar] [CrossRef]
- Szczukowski, Ł.; Redzicka, A.; Wiatrak, B.; Krzyżak, E.; Marciniak, A.; Gębczak, K.; Gębarowski, T.; Świątek, P. Design, Synthesis, Biological Evaluation and in Silico Studies of Novel Pyrrolo[3,4-d]Pyridazinone Derivatives with Promising Anti-Inflammatory and Antioxidant Activity. Bioorganic Chemistry 2020, 102, 104035. [Google Scholar] [CrossRef]
- Motzer, R.J.; Hutson, T.E.; Tomczak, P.; Michaelson, M.D.; Bukowski, R.M.; Oudard, S.; Negrier, S.; Szczylik, C.; Pili, R.; Bjarnason, G.A.; et al. Overall Survival and Updated Results for Sunitinib Compared With Interferon Alfa in Patients With Metastatic Renal Cell Carcinoma. JCO 2009, 27, 3584–3590. [Google Scholar] [CrossRef]
- Demetri, G.D.; Van Oosterom, A.T.; Garrett, C.R.; Blackstein, M.E.; Shah, M.H.; Verweij, J.; McArthur, G.; Judson, I.R.; Heinrich, M.C.; Morgan, J.A.; et al. Efficacy and Safety of Sunitinib in Patients with Advanced Gastrointestinal Stromal Tumour after Failure of Imatinib: A Randomised Controlled Trial. The Lancet 2006, 368, 1329–1338. [Google Scholar] [CrossRef]
- La Regina, G.; Bai, R.; Coluccia, A.; Famiglini, V.; Pelliccia, S.; Passacantilli, S.; Mazzoccoli, C.; Ruggieri, V.; Sisinni, L.; Bolognesi, A.; et al. New Pyrrole Derivatives with Potent Tubulin Polymerization Inhibiting Activity As Anticancer Agents Including Hedgehog-Dependent Cancer. J. Med. Chem. 2014, 57, 6531–6552. [Google Scholar] [CrossRef]
- Sullivan, R.J.; Infante, J.R.; Janku, F.; Wong, D.J.L.; Sosman, J.A.; Keedy, V.; Patel, M.R.; Shapiro, G.I.; Mier, J.W.; Tolcher, A.W.; et al. First-in-Class ERK1/2 Inhibitor Ulixertinib (BVD-523) in Patients with MAPK Mutant Advanced Solid Tumors: Results of a Phase I Dose-Escalation and Expansion Study. Cancer Discovery 2018, 8, 184–195. [Google Scholar] [CrossRef]
- Bianco, M.D.C.A.D.; Marinho, D.I.L.F.; Hoelz, L.V.B.; Bastos, M.M.; Boechat, N. Pyrroles as Privileged Scaffolds in the Search for New Potential HIV Inhibitors. Pharmaceuticals 2021, 14, 893. [Google Scholar] [CrossRef]
- Kombarov, R.; Altieri, A.; Genis, D.; Kirpichenok, M.; Kochubey, V.; Rakitina, N.; Titarenko, Z. BioCores: Identification of a Drug/Natural Product-Based Privileged Structural Motif for Small-Molecule Lead Discovery. Mol Divers 2010, 14, 193–200. [Google Scholar] [CrossRef]
- Zheng, Y.-J.; Tice, C.M. The Utilization of Spirocyclic Scaffolds in Novel Drug Discovery. Expert Opinion on Drug Discovery 2016, 11, 831–834. [Google Scholar] [CrossRef]
- Ritchie, T.J.; Macdonald, S.J.F. The Impact of Aromatic Ring Count on Compound Developability – Are Too Many Aromatic Rings a Liability in Drug Design? Drug Discovery Today 2009, 14, 1011–1020. [Google Scholar] [CrossRef]
- Lovering, F. Escape from Flatland 2: Complexity and Promiscuity. Med. Chem. Commun. 2013, 4, 515. [Google Scholar] [CrossRef]
- Iusupov, I.R.; Lukyanenko, E.R.; Altieri, A.; Kurkin, A.V. Design and Synthesis of Fsp3-Enriched Spirocyclic-Based Biological Screening Compound Arrays via DOS Strategies and Their NNMT Inhibition Profiling. ChemMedChem 2022, 17, e202200394. [Google Scholar] [CrossRef]
- Iusupov, I.R.; Lyssenko, K.A.; Altieri, A.; Kurkin, A.V. (1RS,2RS,6RS)-2-(6-Amino-9H-Purin-9-Yl)-8-Azaspiro[5.6]Dodec-10-En-1-Ol Dihydrochloride. Molbank 2022, 2022, M1495. [Google Scholar] [CrossRef]
- Curreli, F.; Ahmed, S.; Benedict Victor, S.M.; Iusupov, I.R.; Spiridonov, E.A.; Belov, D.S.; Altieri, A.; Kurkin, A.V.; Debnath, A.K. Design, Synthesis, and Antiviral Activity of a Series of CD4-Mimetic Small-Molecule HIV-1 Entry Inhibitors. Bioorganic & Medicinal Chemistry 2021, 32, 116000. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr A Found Crystallogr 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Brandenburg, K.; Berndt, M. DIAMOND, Version 2.1 e. Crystal Impact GbR, Bonn, Germany, 1999. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



