Submitted:
20 December 2023
Posted:
21 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Synthesis of selenium substituted (hetero)aryl hybrids:
2.2. Mice and parasites
2.3. Anti-promastigotes assays:
2.4. Peritoneal macrophages
2.5. Treatment of infected macrophages
2.6. Cytotoxicity assays:
3. Results
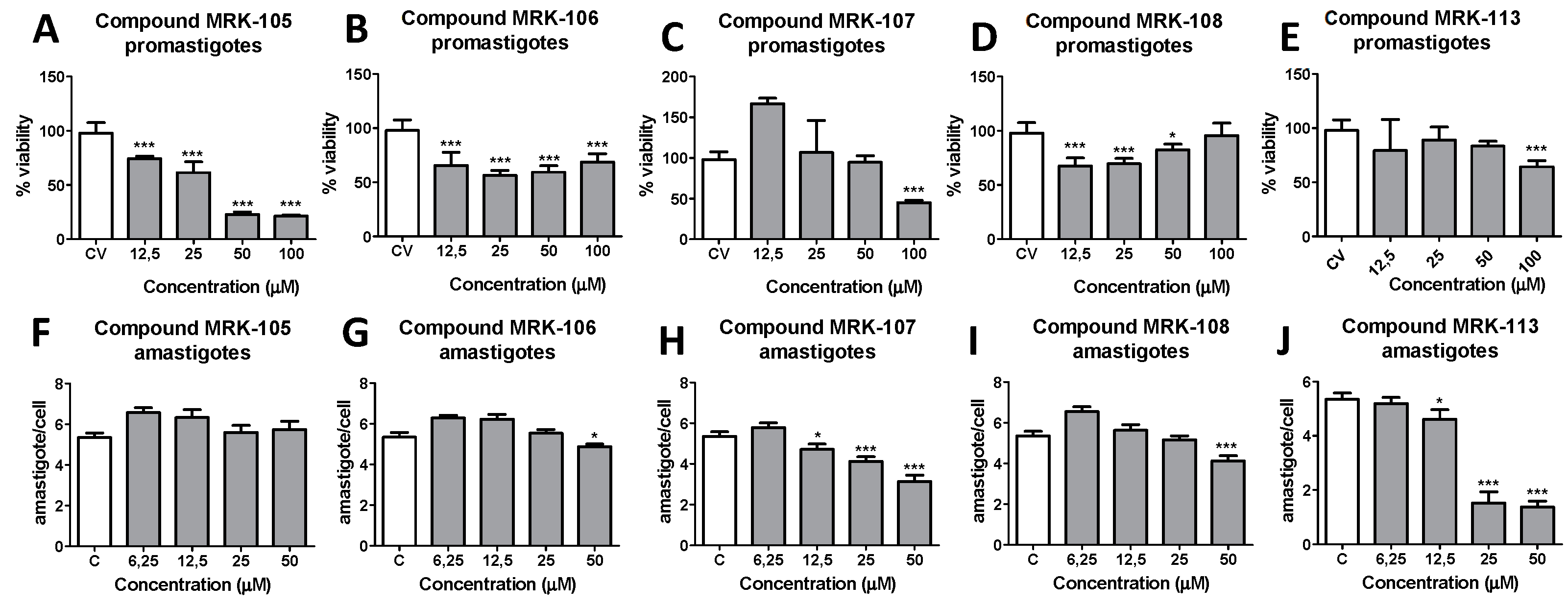
3.1. Anti-promastigotes and anti-amastigotes assays:
3.2. Cytotoxicity and selectivity index:
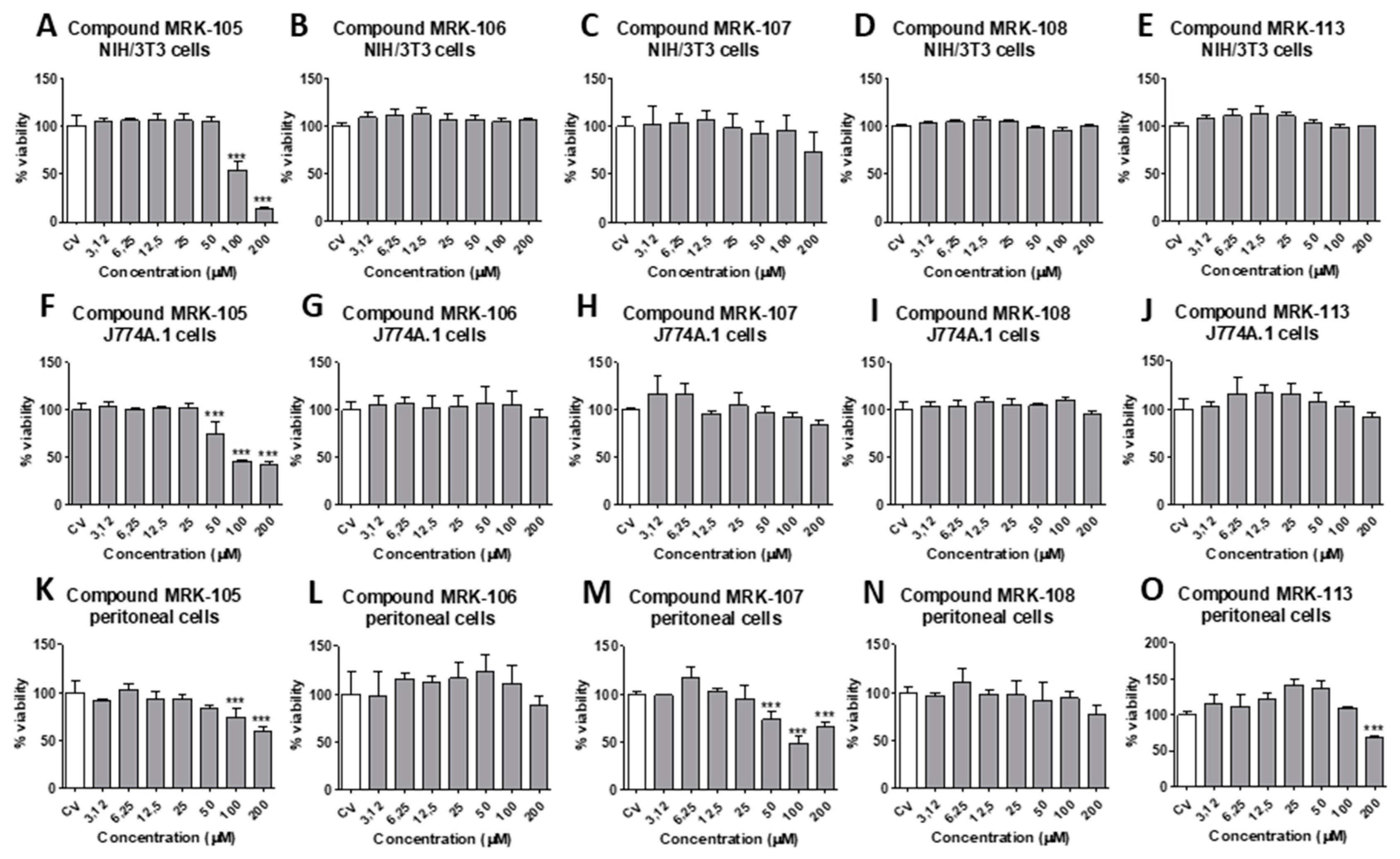
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leishmaniasis. (n.d.). Available online: https://www.who.int/health-topics/leishmaniasis (accessed on 16 December 2023).
- Kmetiuk, L.B.; Tirado, T.C.; Bondo, L.M.; Biondo, A.W.; Figueirdo, F.B. Leishmania spp. in indigenous populations: A mini-review. Front. Public Health. 2022, 10, 1033803. [Google Scholar] [CrossRef] [PubMed]
- Altamura, F.; Rajesh, R.; Catta-Preta, M.C.; Moretti, N.S.; Cestari, I. The current drug discovery landscape for trypanosomiasis and leishmaniasis: Challenges and strategies to identify drug targets. Drug Dev. Res. 2020, 83, 225–252. [Google Scholar] [CrossRef] [PubMed]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, J.; Sundar, S. Current and emerging medications for the treatment of leishmaniasis. Expert Opin. Pharmacother. 2019, 20, 1251–1265. [Google Scholar] [CrossRef]
- Mazire, P.H.; Saha, B.; Roy, A. Immunotherapy for visceral leishmaniasis: A trapeze of balancing counteractive forces. Int. Immunopharmacol. 2022, 110, 108969. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Chakravarty, J. An update on pharmacotherapy for leishmaniasis. Expert Opin. Pharmacother. 2015, 16, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Verdan, M.; Taveira, I.; Lima, F.; Abreu, F.; Nico, D. Drugs and nanoformulations for the management of Leishmania infection: a patent and literature review (2015-2022). Expert Opin Ther Pat 2023, 33, 137–150. [Google Scholar] [CrossRef]
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawski, K. Selenium Compounds as Novel Potential Anticancer Agents. Int. J. Mol. Sci. 2021, 22, 1009. [Google Scholar] [CrossRef]
- Indira Priyadarsini, K.; G. Singh, B.; Kunwar, A. Current Developments on Synthesis, Redox Reactions and Biochemical Studies of Selenium Antioxidants. Curr. Chem. Biol. 2013, 7, 37–46. [Google Scholar] [CrossRef]
- Hoque, E.; Tran, P.; Jacobo, U.; Bergfeld, N.; Achary, S.; Shamshina, J.L.; Reid, T.W.; Abidi, N. Antimicrobial Coatings for Medical Textiles via Reactive Organo-Selenium Compounds. Molecules 2023, 28, 6381. [Google Scholar] [CrossRef]
- Hassan, A.A.; Kalinina, E.; Tatarskiy, V.; Shtil, A. The Thioredoxin System of Mammalian Cells and Its Modulators. Biomedicines 2022, 10, 1757. [Google Scholar] [CrossRef]
- Begines, P.; Martos, S.; Laguens, I.; Maya, I.; Padron, J.M.; Lopez, O.; Fernandes-Bolanos, J.G. Chemoselective Preparation of New Families of Phenolic-Organoselenium Hybrids—A Biological Assessment. Molecules 2022, 27, 1315. [Google Scholar] [CrossRef] [PubMed]
- da Costa, N.S.; Lima, L.S.; Oliveira, F.A.M.; Galiciolli, M.E.A.; Manzono, M.I.; Garlet, Q.I.; Irioda, A.C.; Oliveira, C.S. Antiproliferative Effect of Inorganic and Organic Selenium Compounds in Breast Cell Lines. Biomedicines 2023, 11, 1346. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Xu, H. Incorporating Selenium into Heterocycles and Natural Products─From Chemical Properties to Pharmacological Activities. J. Med. Chem. 2022, 65, 4436–4456. [Google Scholar] [CrossRef] [PubMed]
- Sari, M.H.M.; Ferreira, L.M.; Prado, V.C.; Nogueira, C.W.; Cruz, L. Nano-based formulations as an approach for providing a novel identity for organoselenium compounds. Eur. J. Pharm. Biopharm. 2022, 178, 69. [Google Scholar] [CrossRef] [PubMed]
- Veloso, I.C.; Delanogare, E.; Machado, A.E.; Braga, S.P.; Rosa, G.K.; de Bem, A.F.; Rafique, J.; Saba, S.; da Trindade, R.N.; Galetto, F.Z.; Moreira, E.L.G. A selanylimidazopyridine (3-SePh-IP) reverses the prodepressant- and anxiogenic-like effects of a high-fat/high-fructose diet in mice. J. Pharm. Pharmacol. 2021, 73, 673. [Google Scholar] [CrossRef]
- Nie, Y.; Li, S.; Lu, Y.; Zhong, M.; Li, X.; Zhang, Y.; He, X. New Organoselenium (NSAIDs-Selenourea and Isoselenocyanate) Derivatives as Potential Antiproliferative Agents: Synthesis, Biological Evaluation and in Silico Calculations. Molecules 2022, 27, 4328. [Google Scholar] [CrossRef] [PubMed]
- Rafique, J.; Farias, G.; Saba, S.; Zapp, E.; Bellettini, I.C.; Momoli Salla, C.A.; Bechtold, I.H.; Scheide, M.R.; Santos Neto, J.S.; Souza Jr., D.M.; Braga, H.C.; Ribeiro, L.F.B.; Gastaldon, F.; Pich, C.T.; Frizon, T.E.A. Selenylated-oxadiazoles as promising DNA intercalators: Synthesis, electronic structure, DNA interaction and cleavage. Dyes Pigm. 2020, 180, 108519. [Google Scholar] [CrossRef] [PubMed]
- Begines, P.; Martos, S.; Lagunes, I.; Maya, I.; Padron, J.M.; Lopez, O.; Fernández-Bolaños, J.G. Chemoselective Preparation of New Families of Phenolic-Organoselenium Hybrids—A Biological Assessment. Molecules 2022, 27, 1315. [Google Scholar] [CrossRef]
- Chuai, H.; Zhang, S.-Q.; Bai, H.; Li, J.; Wang, Y.; Sun, J.; Wen, E.; Zhang, J.; Xin, M. Small molecule selenium-containing compounds: Recent development and therapeutic applications. Eur. J. Med. Chem. 2021, 223, 113621. [Google Scholar] [CrossRef]
- Alcolea, V.; Pérez-Silanes, S. Selenium as an interesting option for the treatment of Chagas disease: A review. Eur. J. Med. Chem. 2020, 206, 112673. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, S.; Fernández-Rubio, C.; Mansouri, R.; Ali-Hassanzadeh, M.; Ghani, E.; Karimazar, M.; Manzano-Román, R.; Nguewa, P. Selenium and protozoan parasitic infections: Selenocompounds and selenoproteins potential. Parasitol. Res. 2022, 121, 49. [Google Scholar] [CrossRef] [PubMed]
- Tieknik, E.R.T. Therapeutic potential of selenium and tellurium compounds: Opportunities yet unrealized. Dalton Trans. 2012, 41, 6390. [Google Scholar] [CrossRef] [PubMed]
- Aatif, M.; Raza, M.A.; Javed, K.; Nashre-ul-Islam, S.M.; Farhan, M.; Alam, M.W. Potential Nitrogen-Based Heterocyclic Compounds for Treating Infectious Diseases: A Literature Review. Antibiotics 2022, 11, 1750. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R. Introduction: Heterocycles. Chem. Rev. 2004, 104, 2125. [Google Scholar] [CrossRef]
- Franco, M.S.; Saba, S.; Rafique, J.; Braga, A.L. KIO4-mediated Selective Hydroxymethylation/Methylenation of Imidazo-Heteroarenes: A Greener Approach. Angew. Chem., Int. Ed. Engl. 2021, 60, 18454. [Google Scholar] [CrossRef]
- Ye, Z.; Adhikari, S.; Xia, Y.; Dai, M. Expedient syntheses of N-heterocycles via intermolecular amphoteric diamination of allenes. Nat. Commun. 2018, 9, 721. [Google Scholar] [CrossRef] [PubMed]
- Saba, S.; dos Santos, C.R.; Zavarise, B.R.; Naujorks, A.A.S.; Franco, M.S.; Schneider, A.R.; Scheide, M.R.; Affeldt, R.F.; Rafique, J.; Braga, A.L. Photoinduced, Direct C(sp2)−H Bond Azo Coupling of Imidazoheteroarenes and Imidazoanilines with Aryl Diazonium Salts Catalyzed by Eosin Y. Chem. Euro. J. 2020, 26, 4461. [Google Scholar] [CrossRef]
- Rizzo, C.; Amata, S.; Pibiri, I.; Pace, A.; Buscemi, S.; Piccionello, A.P. FDA-Approved Fluorinated Heterocyclic Drugs from 2016 to 2022. Int. J. Mol. Sci. 2023, 24, 7728. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V. Prescribed drugs containing nitrogen heterocycles: an overview. RSC Adv. 2020, 10, 44247. [Google Scholar] [CrossRef]
- Taylor, A.P.; Robinson, R.P.; Fobian, Y.M.; Blackemore, D.C.; Jones, L.H.; Fadeyi, O. Modern advances in heterocyclic chemistry in drug discovery. Org. Biomol. Chem. 2016, 14, 6611. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.N.; Jena, S.; Mukerjee, M.; Maiti, B.; Chanda, K. Green synthesis of biologically active heterocycles of medicinal importance: a review. Environ. Chem. Lett. 2021, 19, 3315. [Google Scholar]
- Scheide, M.R.; Peterle, M.M.; Saba, S.; Neto, J.S.S.; Lenze, G.F.; Cezar, R.D.; Felix, J.F.; Botteselle, G.V.; Schnedier, R.; Rafique, J.; Braga, A.L. Borophosphate glass as an active media for CuO nanoparticle growth: An efficient catalyst for selenylation of oxadiazoles and application in redox reactions. Sci. Rep. 2020, 10, 15233. [Google Scholar] [CrossRef]
- Neto, J.S.S.; Grangja, I.J.A.; Scheide, M.R.; France, M.S.; Moraes, C.A.O.; Beatriz, A.; de Lima, D.P.; Botteselle, G.V.; Frizon, T.E.A.; Saba, S.; Rafique, J.; Braga, A.L. Catalyst- and metal-free C(sp2)–H bond selenylation of (N-hetero)-arenes using diselenides and trichloroisocyanuric acid at room temperature. Sci. Rep. 2023, 13, 14251. [Google Scholar] [CrossRef]
- Doerner, C.V.; Neto, J.S.S.; Cabreira, C.R.; Saba, S.; Sandjo, L.P.; Rafique, J.; Braga, A.L.; de Assis, F.F. Synthesis of 3-selanyl-isoflavones from 2-hydroxyphenyl enaminones using trichloroisocyanuric acid (TCCA): a sustainable approach. New J. Chem. 2023, 47, 5598. [Google Scholar] [CrossRef]
- Peterle, M.M.; Scheide, M.R.; Silva, L.T.; Saba, S.; Rafique, J.; Braga, A.L. Copper-Catalyzed Three-Component Reaction of Oxadiazoles, Elemental Se/S and Aryl Iodides: Synthesis of Chalcogenyl (Se/S)-Oxadiazoles. ChemistrySelect 2019, 3, 13191. [Google Scholar] [CrossRef]
- Moraes, C.A.O.; Santos, R.B.C.; Cavalcante, M.F.O.; Guilhermi, J.S.; Ali, M.A.; Botteselle, G.V.; Frizon, T.E.A.; Shah, M.I.A.; Liao, L.M.; Beatriz, A.; Saba, S.; Rafique, J. Urea hydrogen peroxide (UHP) and Ethyl Lactate, an eco-friendly combo system in the direct C(sp2)-H bond selenylation of imidazo[2,1-b]thiazole and related structures. ACS Omega 2023. [Google Scholar] [CrossRef]
- Jacques, M.T.; de Souza, M.; Brabosa, F.A.R.; Canto, R.F.S.; Lopes, S.C.; Prediger, R.D.; Braga, A.L.; Aschner, M.; Farina, M. Novel Probucol Analogue, 4,4′-Diselanediylbis (2,6-Di-tert-Butylphenol), Prevents Oxidative Glutamate Neurotoxicity In Vitro and Confers Neuroprotection in a Rodent Model of Ischemic Stroke. ACS Chem. Neurosci. 2023, 14, 16–2857. [Google Scholar] [CrossRef]
- Corrêa, B. A., Síntese e avaliação do potencial antioxidante de cumarinas funcionalizadas com selênio, TCC Universidade Federal de Santa Catarina, Florianópolis-Brazil, 11/2015.
- Bio-Rad. Measuring cytotoxicity or proliferation - alamarBlue Assay Protocol. Bio-Rad [Internet]. Bio-Rad. Available online: https://www.bio-rad-antibodies.com/measuring-cytotoxicity-proliferation-spectrophotometry-fluorescence-alamarblue.html.
- Brioschi, M.B.C.; Coser, E.M.; Coelho, A.C.; Gadelha, F.R.; Miguel, D.C. Models for cytotoxicity screening of antileishmanial drugs: what has been done so far? Int. J. Antimicrob. Agents 2022, 60, 106612. [Google Scholar] [CrossRef]
- Álvarez-Bardón M, Pérez-Pertejo Y, Collazos A, Sepúlveda-Crespo D, Carballeira NM, Tekwani BL, et al. Screening Marine Natural Products for New Drug Leads against Trypanosomatids and Malaria. Mar. Drugs, 2020, 18, 187. [CrossRef]
- Kakkar, S.; Narasimhan, B. A comprehensive review on biological activities of oxazole derivatives. BMC Chem. 2019, 13, 16. [Google Scholar] [CrossRef]
- Moraski, G.C.; Chang, M.; Villegas-Estrada, A.; Franzblau, S.G.; Möllmann, U.; Miller, M.J. Structure–activity relationship of new anti-tuberculosis agents derived from oxazoline and oxazole benzyl esters. Euro. J. Med. Chem. 2020, 45, 1703. [Google Scholar] [CrossRef] [PubMed]
- Moraski, G.C.; Markley, L.D.; Chang, M.; Cho, S.; Franzblau, S.G.; Hwang, C.H.; Boshoff, H.; Miller, M.J. Generation and exploration of new classes of antitubercular agents: The optimization of oxazolines, oxazoles, thiazolines, thiazoles to imidazo[1,2-a]pyridines and isomeric 5,6-fused scaffolds. Bioorg. Med. Chem., 2012, 20, 2214. [Google Scholar] [CrossRef]
- Baréa, P.; de Paula, J.C.; Alonso, L.; de Oliveira, A.R.; da Costa, W.F.; Alonso, A.; Nakamura, C.V.; Sarragiotto, M.H. Synthesis, Antileishmanial Activity and Spin Labeling EPR Studies of Novel β-Carboline-Oxazoline and β-Carboline-Dihydrooxazine Derivatives. J. Braz. Chem. Soc. 2020, 31, 1170. [Google Scholar] [CrossRef]
- Gaudino, E.C.; Tagliapietra, S.; Martina, K.; Palmisano, G.; Cravotto, G. Recent advances and perspectives in the synthesis of bioactive coumarins. RSC Advances 2016, 6, 46394. [Google Scholar] [CrossRef]
- Gupta, O.; Pradhan, T.; Bhatia, R.; Monga, V. Recent advancements in anti-leishmanial research: Synthetic strategies and structural activity relationships. Euro. J. Med. Chem. 2021, 223, 113606. [Google Scholar] [CrossRef] [PubMed]
- Fourmigué, M.; Dhaka, A. Chalcogen bonding in crystalline diselenides and selenocyanates: From molecules of pharmaceutical interest to conducting materials. Coord. Chem. Rev. 2020, 403, 213084. [Google Scholar] [CrossRef]
- Jeannin, O.; Huynh, H.-T.; Riel, A.M.S.; Fourmigué, M. Chalcogen bonding interactions in organic selenocyanates: from cooperativity to chelation. New J. Chem. 2018, 42, 10502. [Google Scholar] [CrossRef]
- Huang, M.-F.N.; Luis, J.A.S.; da Silva, A.P.; Rocha, J.C.; Lima, T.K.S.; Scotti, M.T.; Scotti, L.; de Oliveira, R.F.; Souza, H.D.S.; de Athayde-Filho, P.F.; Barbosa-Filho, J.M. Synthesis, in silico Study and Antileishmanial Evaluation of New Selenides Derived from 7-Chloro-quinoline and N-Phenylacetamides. J. Braz. Chem. Soc. 2021, 32, 712–721. [Google Scholar] [CrossRef]
- Khatun, S.; Singh, A.; Bader, G.N.; Sofi, F.A. Imidazopyridine, a Promising Scaffold with Potential Medicinal Applications and Structural Activity Relationship (SAR): Recent Advances. J. Biomol. Struct. Dyn. 2021, 40, 14279. [Google Scholar] [CrossRef]
- Fersing, C.; Boudot, C.; Pedron, J.; Hutter, S.; Primas, N.; Castera-Ducros, C.; Bourgeade-Delmas, S.; Sournia-Saquet, A.; Moreau, A.; Cohen, A.; Stigliani, J.; Pratviel, G.; Crozet, M.D.; Wyllie, S.; Fairlamb, A.H.; Valentin, A.; Rathelot, P.; Azas, N.; Castro, B.; Verhaeghe, P. 8-Aryl-6-Chloro-3-Nitro-2-(Phenylsulfonylmethyl)Imidazo[1,2-a]Pyridines as Potent Antitrypanosomatid Molecules Bioactivated by Type 1 Nitroreductases. Eur. J. Med. Chem. 2018, 157, 115. [Google Scholar] [CrossRef]
- Nwaka, S.; Ramirez, B.; Brun, R.; Maes, L.; Douglas, F.; Ridley, R. Advancing Drug Innovation for Neglected Diseases—Criteria for Lead Progression. PLoS Negl. Trop. Dis. 2009, 3, e440. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, M.V.; Bloomer, W.D.; Rosenzweig, H.S.; O’Shea, I.P.; Wilkinson, S.R.; Kaiser, M.; Chatelain, E.; Ioset, J.-R. Discovery of potent nitrotriazole-based antitrypanosomal agents: In vitro and in vivo evaluation. Bioorg. Med. Chem. 2015, 23, 6467. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.A.; Spillere, A.R.; Neves, G.M.; Kagami, L.P.; von Poser, G.L.; Canto, R.F.S.; Eifler-Lima, V.R. Natural and Synthetic Coumarins as Antileishmanial Agents: A Review. Eur. J. Med. Chem. 2020, 203, 112514. [Google Scholar] [CrossRef] [PubMed]
- Faheem, F.; Dey, S.; Johri, S.; Abirami, M.; Kumar, B.K.; Taramelli, D.; Basilico, N.; Balaña-Fouce, R.; Sekhar, K.V.G.C.; Murugesan, S. Search for Structurally Diverse Heterocyclic Analogs as Dual-Acting Antimalarial and Antileishmanial Agents: An Overview. Eur. J. Med. Chem. Rep. 2022, 4, 100031. [Google Scholar] [CrossRef]
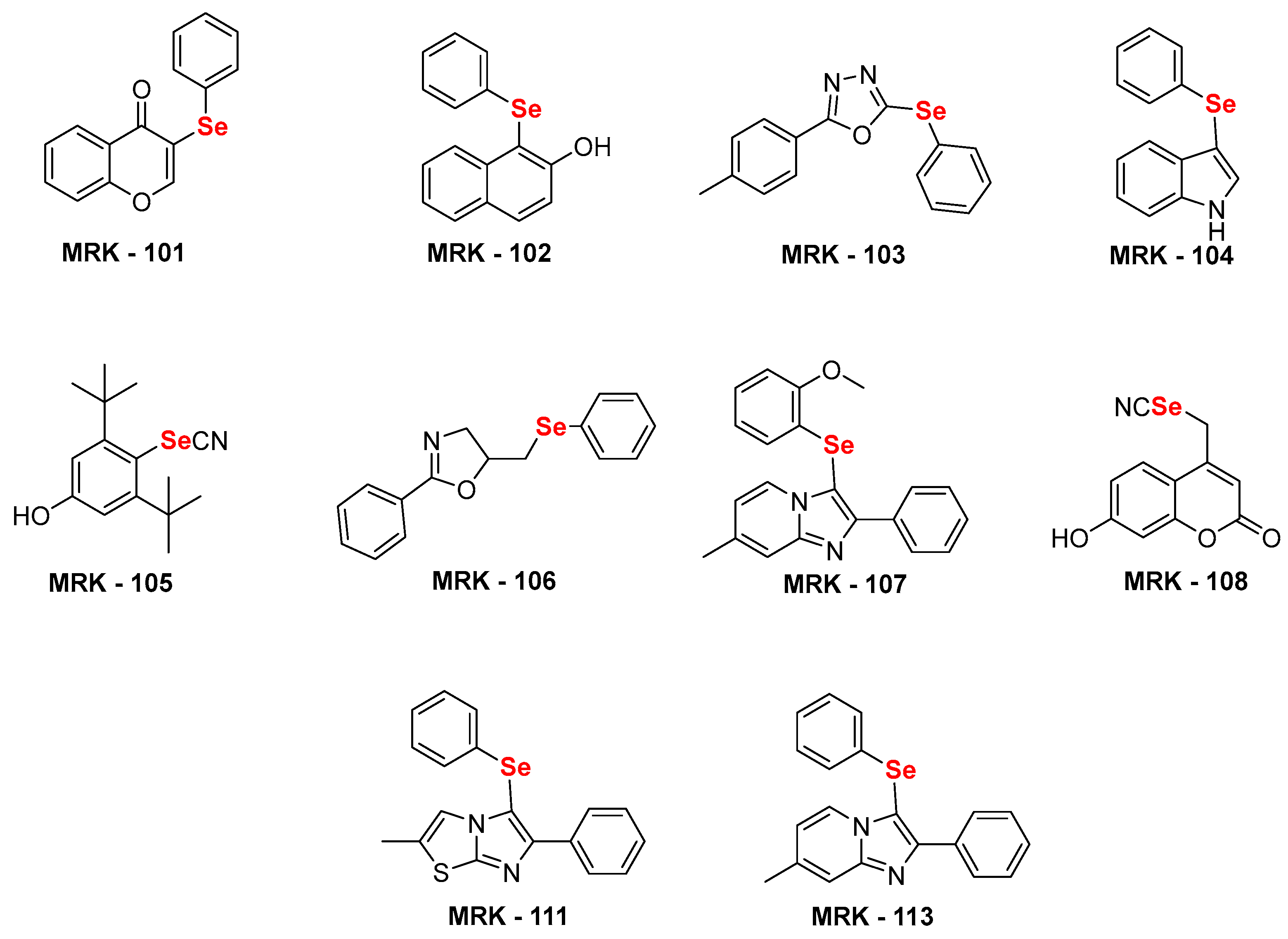
| Compound | CC50 (µM) peritoneal macrophages |
IC50 (µM) L. amazonensis promastigotes |
SIa | IC50 (µM) L. amazonensis amastigotes |
SIb |
|---|---|---|---|---|---|
| 101 | > 200 | 30.46 | 6.57 | > 50 | ind |
| 102 | > 200 | 15.15 | 13.20 | > 50 | ind |
| 103 | > 200 | 15.48 | 12.92 | > 50 | ind |
| 104 | > 200 | 16.17 | 12.37 | > 50 | ind |
| 105 | > 200 | 12.17 | 16.43 | > 50 | ind |
| 106 | > 200 | 3.95 | 50.53 | > 50 | ind |
| 107 | > 200 | 27.37 | 7.31 | 18.31 | 10.92 |
| 108 | > 200 | 4.22 | 47.31 | > 50 | ind |
| 111 | > 200 | 17.55 | 11.40 | > 50 | ind |
| 113 | > 200 | 40.98 | 4.88 | 15.93 | 12.55 |
| ANFB | 25.15 | 9.40 | 2.67 | 0.97 | 25.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





