Submitted:
18 December 2023
Posted:
19 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Protocol
Eligibility Criteria
Search Strategy
Study Screening Process
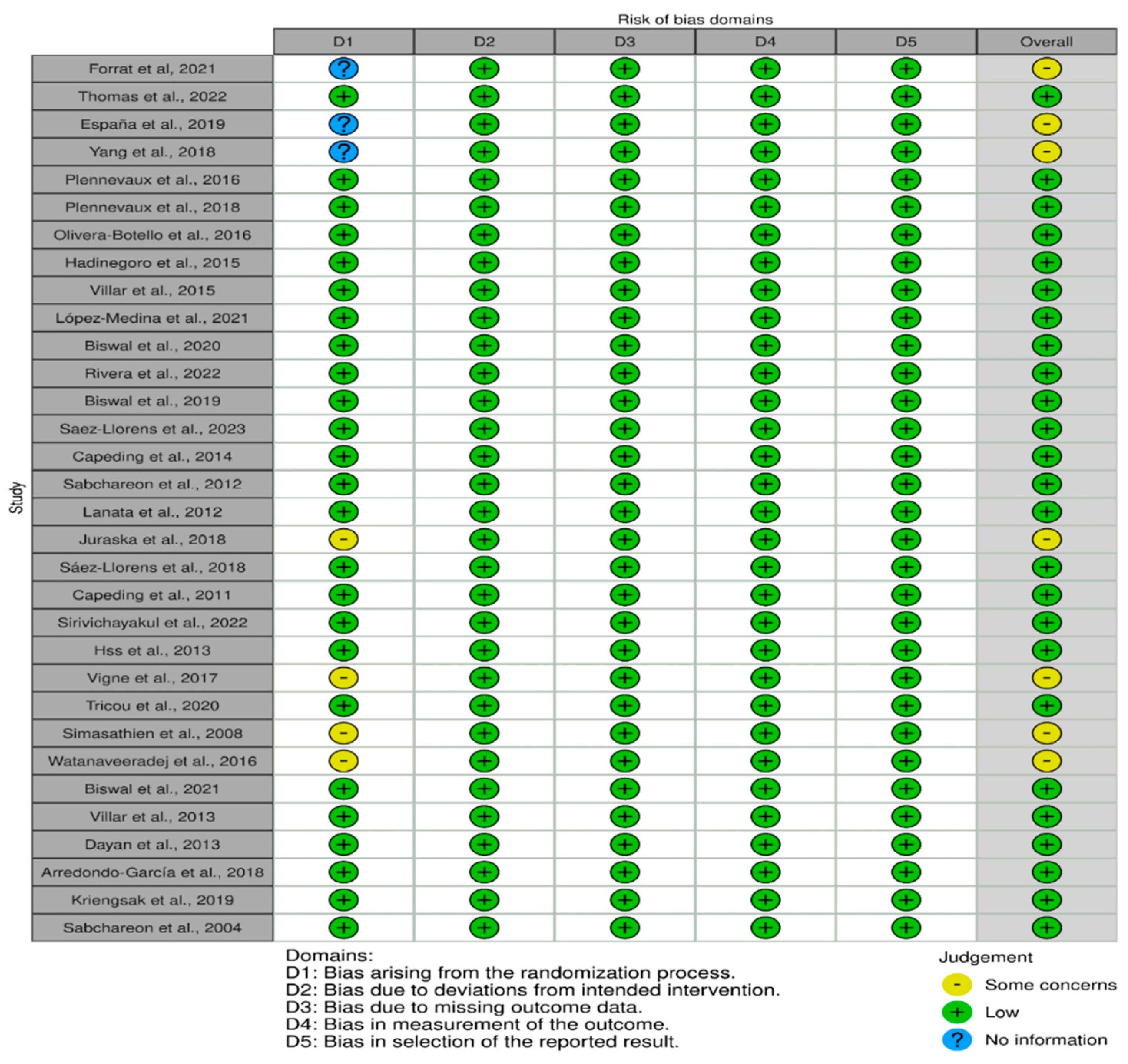
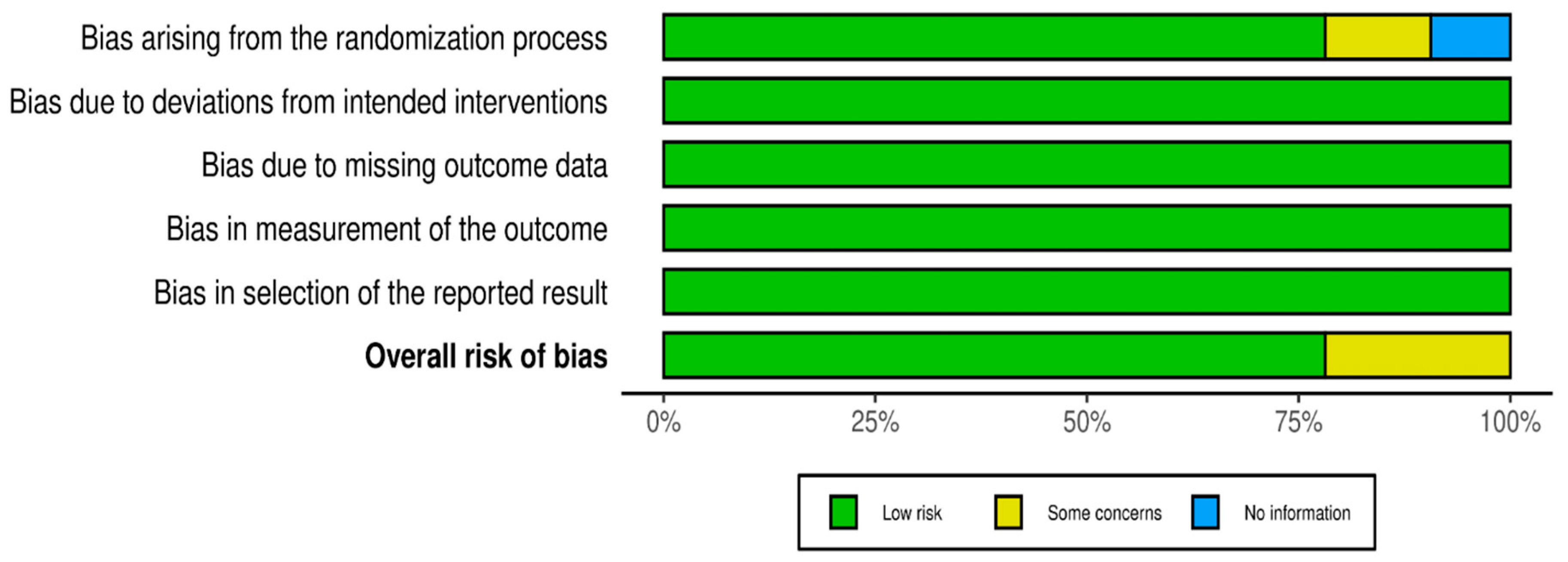
Quality Assessment
Data Extraction
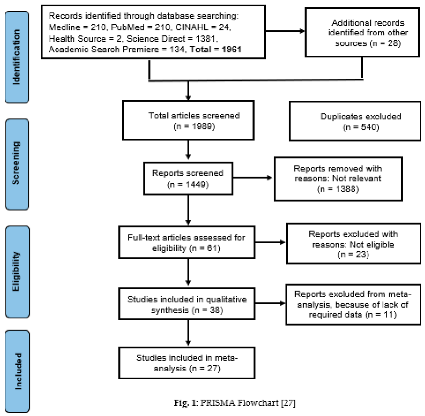
3. Results
3.1. Distribution of 5 Domains Risk of Bias among 32 RCTs
3.2. Summary Findings of Risk of Bias of 32 RCTs
3.3. Evaluating Systematic Review Findings
3.3.1. Efficacy of Dengue Vaccine
3.3.2. Immunogenicity of Dengue Vaccine Candidates
3.3.3. Safety of Dengue Vaccine
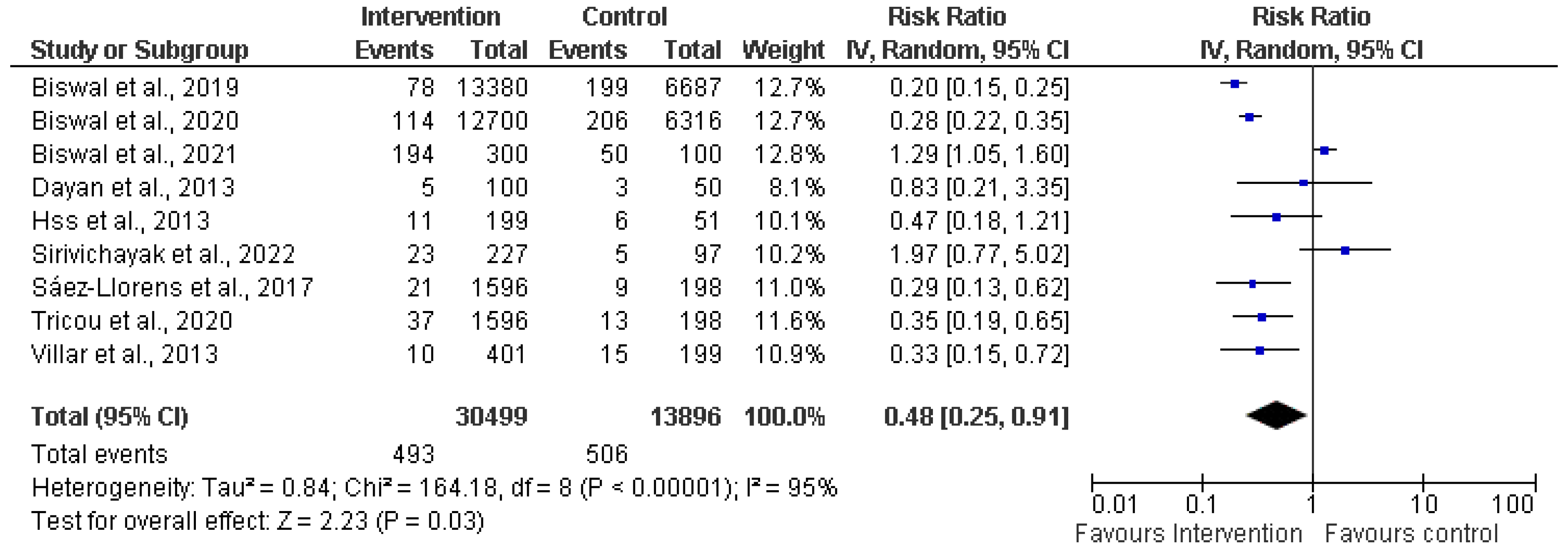
3.4. Meta-analysis
3.4.1. Efficacy of Dengue Vaccine
3.4.2. Immunogenicity of Dengue Vaccine
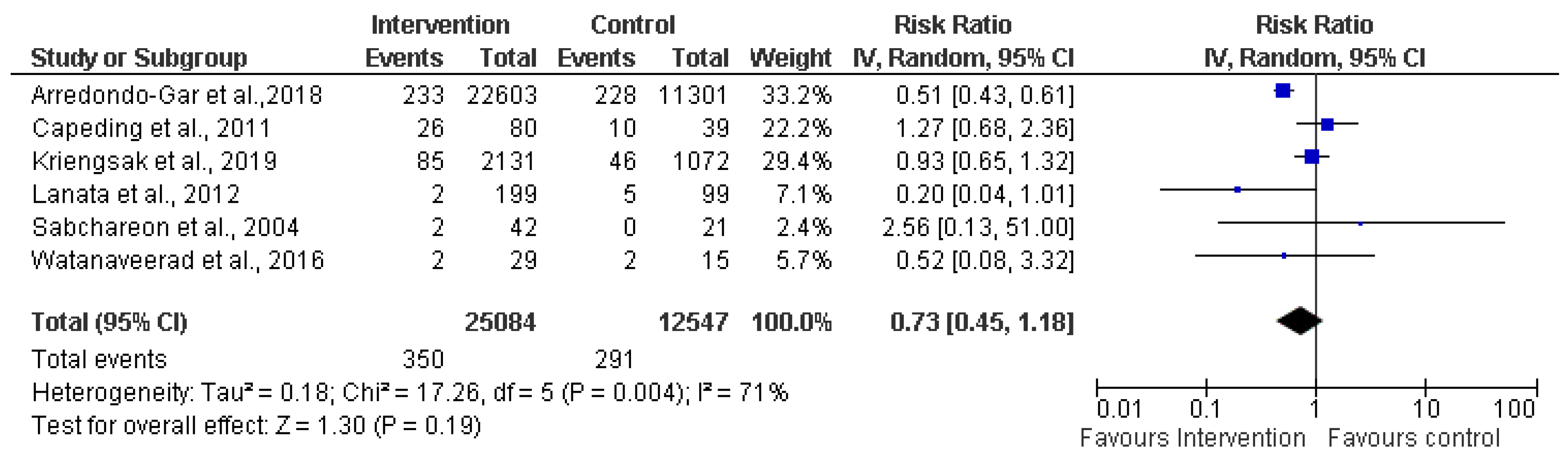
3.4.3. Safety of Dengue Vaccine
4. Discussion
4.1. Efficacy
4.2. Immunogenicity
4.3. Safety
4.4. Meta-Analysis Findings
4.5. Vaccine Efficacy
4.6. Vaccine Immunogenicity
4.7. Vaccine Safety
4.8. Study limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clements, A.N. Arboviruses: Characteristics and concepts. In: Clements AN, editor. The biology of mosquitoes: Transmission of viruses and interactions with bacteria. Wallingford: CAB International 2012, 125. [Google Scholar]
- Joshi, V.; Mourya, D.T.; Sharma, R.C. Persistence of dengue-3 virus through transovarial transmission passage in successive generations of Aedes aegypti mosquitoes. Am J Trop Med Hyg. 2002, 67, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Kyle, J.L.; Harris, E. Global spread, and persistence of dengue. Annu Rev Microbiol. 2008, 62, 71–92. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Dengue and severe dengue. 2021. Available online: https://www.who.int/en/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 18 December 2023).
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Dengue. Lancet 2007, 370, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Nguyen, T.L.; Lei, H.Y.; Lin, Y.S.; Le, B.L.; Huang, K.J.; Lin, C.F.; et al. Volume replacement in infants with dengue hemorrhagic fever/dengue shock syndrome. Am J Trop Med Hyg. 2006, 74, 684–691. [Google Scholar] [PubMed]
- Simmons, C.P.; Farrar, J.J.; Nguyen, V.; Wills, B. Dengue. N Engl J Med. 2012, 366, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, A.; Ooi, E.-E.; Horstick, O.; Wills, B. Dengue. Lancet 2019, 393, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Brady, O.J.; Gething, P.W.; Bhatt, S.; Messina, J.P.; Brownstein, J.S.; Hoen, A.G.; et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012, 6, e1760. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef]
- Gubler, D.J. Dengue, urbanization, and globalization: The Unholy trinity of the 21st century. Trop Med Health 2011, 39 (Suppl. 4), 3–11. [Google Scholar] [CrossRef]
- Murray, N.E.; Quam, M.B.; Wilder-Smith, A. Epidemiology of dengue: past, present, and future prospects. Clin Epidemiol. 2013, 5, 299–309. [Google Scholar] [CrossRef]
- Messina, J.P.; Brady, O.J.; Golding, N.; Kraemer, M.U.G.; Wint, G.R.W.; Ray, S.E.; et al. The current and future global distribution and population at risk of dengue. Nat Microbiol. 2019, 4, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Quam, M.B.M.; Zhang, T.; Sang, S. Global burden for dengue and the evolving pattern in the past 30 years. J Travel Med. 2021, 28, 146. [Google Scholar] [CrossRef]
- Silva, N.M.; Santos, N.C.; Martins, I.C. Dengue and Zika Viruses: Epidemiological History, Potential Therapies, and Promising Vaccines. Trop Med Infect Dis. 2020, 5, 150. [Google Scholar] [CrossRef]
- Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A.; Stanaway, J.D. The global economic burden of dengue: a systematic analysis. Lancet Infect Dis. 2016, 16, 935–941. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Disease Outbreak News; Dengue in Bangladesh. 2023. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON481 (accessed on 14 September 2023).
- Dia, I.; Diagne, C.T.; Ba, Y.; Diallo, D.; Konate, L.; Diallo, M. Insecticide susceptibility of Aedes aegypti populations from Senegal and Cape Verde Archipelago. Parasites Vectors 2012, 5, 238. [Google Scholar] [CrossRef]
- Mulderij-Jansen, V.; Pundir, P.; Grillet, M.E.; Lakiang, T.; Gerstenbluth, I.; Duits, A.; Tami, A.; Bailey, A. Effectiveness of Aedes-borne infectious disease control in Latin America and the Caribbean region: A scoping review. PLoS ONE 2022, 17, e0277038. [Google Scholar] [CrossRef]
- Weeratunga, P.; Rodrigo, C.; Fernando, S.D.; Rajapakse, S. Control methods for Aedes albopictus and Aedes aegypti. Cochrane Database Syst Rev. 2017, 2017, CD012759. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.N.; Shearer, F.M.; Barker, C.M.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 2015, 4, e08347. [Google Scholar] [CrossRef]
- Guy, B.; Barrere, B.; Malinowski, C.; Saville, M.; Teyssou, R.; Lang, J. From research to phase III: preclinical, industrial, and clinical development of the Sanofi Pasteur tetravalent dengue vaccine. Vaccine 2011, 29, 7229–7241. [Google Scholar] [CrossRef]
- Sabchareon, A.; Wallace, D.; Sirivichayakul, C.; Limkittikul, K.; Chanthavanich, P.; Suvannadabba, S.; et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomized, controlled phase 2b trial. Lancet 2012, 380, 1559–1567. [Google Scholar] [CrossRef]
- Osorio, J.E.; Velez, I.D.; Thomson, C.; Lopez, L.; Jimenez, A.; Haller, A.A.; Silengo, S.; et al. Safety and immunogenicity of a recombinant live attenuated tetravalent dengue vaccine (DENVax) in flavivirus-naive healthy adults in Colombia: a randomized, placebo-controlled, phase 1 study. Lancet Infect Dis. 2014, 14, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Yoon, I.K. A review of Dengvaxia: development to deployment. Hum Vaccin Immunother. 2019, 15, 2295–2314. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Medicine 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane. 2023. Available online: www.training.cochrane.org/handbook (accessed on 13 September 2023).
- Thomas, J.; Harden, A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Medical Research Methodology 2008, 8, 45. [Google Scholar] [CrossRef]
- Forrat, R.; Dayan, G.H.; DiazGranados, C.A.; Bonaparte, M.; Laot, T.; Capeding, M.R.; et al. Analysis of hospitalized and severe dengue cases over the 6 years of follow-up of the tetravalent dengue vaccine (CYD-TDV) efficacy trials in Asia and Latin America. Clin Infect Dis. 2021, 73, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Chansinghakul, D.; Limkittikul, K.; Gilbert, P.B.; Hattasingh, W.; Moodie, Z.; et al. Associations of human leukocyte antigen with neutralizing antibody titers in a tetravalent dengue vaccine phase 2 efficacy trial in Thailand. Hum Immunol. 2022, 83, 53–60. [Google Scholar] [CrossRef] [PubMed]
- España, G.; Hogea, C.; Guignard, A.; ten Bosch, Q.A.; Morrison, A.C.; Smith, D.L.; et al. Biased efficacy estimates in phase-III dengue vaccine trials due to heterogeneous exposure and differential detectability of primary infections across trial arms. PLoS ONE 2019, 14, e0210041. [Google Scholar] [CrossRef]
- Yang, Y.; Meng, Y.; Halloran, M.E.; Longini, I.M., Jr. Dependency of vaccine efficacy on preexposure and age: A closer look at a tetravalent dengue vaccine. Clin Infect Dis. 2018, 66, 178–184. [Google Scholar] [CrossRef]
- Plennevaux, E.; Sabchareon, A.; Limkittikul, K.; Chanthavanich, P.; Sirivichayakul, C.; Moureau, A. Detection of dengue cases by serological testing in a dengue vaccine efficacy trial: Utility for efficacy evaluation and impact of future vaccine introduction. Vaccine 2016, 34, 2707–2712. [Google Scholar] [CrossRef]
- Sridhar, S.; Luedtke, A.; Langevin, E.; Zhu, M.; Bonaparte, M.; Machabert, T.; Savarino, S.; et al. Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. New England Journal of Medicine 2018, 379, 327–340. [Google Scholar] [CrossRef]
- Plennevaux, E.; Moureau, A.; Arredondo-García, J.L.; Villar, L.; Pitisuttithum, P.; Tran, N.H.; et al. Impact of dengue vaccination on serological diagnosis: Insights from phase III dengue vaccine efficacy trials. Clin Infect Dis. 2018, 66, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Moodie, Z.; Juraska, M.; Huang, Y.; Zhuang, Y.; Fong, Y.; Carpp, L.N.; Self, S.G.; et al. Neutralizing antibody correlates analysis of tetravalent dengue vaccine efficacy trials in Asia and Latin America. J Infect Dis. 2018, 217, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Olivera-Botello, G.; Coudeville, L.; Fanouillere, K.; Guy, B.; Chambonneau, L.; Noriega, F.; Jackson, N. CYD-TDV Vaccine Trial Group. Tetravalent dengue vaccine reduces symptomatic and asymptomatic dengue virus infections in healthy children and adolescents Aged 2-16 years in Asia and Latin America. J Infect Dis. 2016, 214, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Hadinegoro, S.R.; Arredondo-García, J.L.; Capeding, M.R.; Deseda, C.; Chotpitayasunondh, T.; Dietze, R.; et al. CYD-TDV dengue vaccine working group. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med. 2015, 373, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Villar, L.; Dayan, G.H.; Arredondo-García, J.L.; Rivera, D.M.; Cunha, R.; Deseda, C.; et al. CYD15 study group. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med. 2015, 372, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Dayan, G.; Arredondo, J.L.; Carrasquilla, G.; Deseda, C.C.; Dietze, R.; Luz, K.; Costa, M.S.; et al. Prospective cohort study with active surveillance for fever in four dengue-endemic countries in Latin America. American Journal of Tropical Medicine and Hygiene 2015, 93, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Dayan, G.H.; Langevin, E.; Gilbert, P.B.; Wu, Y.; Moodie, Z.; Forrat, R.; Price, B.; Frago, C.; et al. Assessment of the long-term efficacy of a dengue vaccine against symptomatic, virologically-confirmed dengue disease by baseline dengue serostatus. Vaccine 2020, 38, 3531–3536. [Google Scholar] [CrossRef] [PubMed]
- López-Medina, E.; Biswal, S.; Saez-Llorens, X.; Borja-Tabora, C.; Bravo, L.; Sirivichayakul, C.; Vargas, L.M.; et al. Efficacy of a Dengue Vaccine Candidate (TAK-003) in Healthy Children and Adolescents 2 Years after Vaccination. J Infect Dis. 2022, 225, 1521–1532. [Google Scholar] [CrossRef]
- Biswal, S.; Borja-Tabora, C.; Martinez Vargas, L.; Velásquez, H.; Theresa Alera, M.; Sierra, V.; et al. TIDES study group. Efficacy of a tetravalent dengue vaccine in healthy children aged 4-16 years: a randomized, placebo-controlled, phase 3 trial. Lancet 2020, 395, 1423–1433. [Google Scholar] [CrossRef]
- Rivera, L.; Biswal, S.; Sáez-Llorens, X.; Reynales, H.; López-Medina, E.; Borja-Tabora, C.; et al. Three-year Efficacy and Safety of Takeda’s Dengue Vaccine Candidate (TAK-003). Clin Infect Dis. 2022, 75, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Biswal, S.; Reynales, H.; Saez-Llorens, X.; Lopez, P.; Borja-Tabora, C.; Kosalaraksa, P.; et al. TIDES Study Group. Efficacy of a Tetravalent Dengue Vaccine in Healthy Children and Adolescents. N Engl J Med. 2019, 381, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Llorens, X.; Biswal, S.; Borja-Tabora, C.; Fernando, L.; Liu, M.; Wallace, D.; et al. TIDES Study Group. Effect of the tetravalent dengue vaccine TAK-003 on sequential episodes of symptomatic dengue. Am J Trop Med Hyg. 2023, 108, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Reynales, H.; Carrasquilla, G.; Zambrano, B.; Cortes, S.M.; Machabert, T.; Jing, J.; et al. Secondary analysis of the efficacy and safety trial data of the tetravalent dengue vaccine in children and adolescents in Colombia. The Pediatric Infectious Disease Journal 2020, 39, e30–e36. [Google Scholar] [CrossRef] [PubMed]
- Ylade, M.; Agrupis, K.A.; Daag, J.V.; Crisostomo, M.V.; Tabuco, M.O.; Sy, A.K.; Nealon, J.; et al. Effectiveness of a single-dose mass dengue vaccination in Cebu, Philippines: A case-control study. Vaccine 2021, 39, 5318–5325. [Google Scholar] [CrossRef] [PubMed]
- Capeding, M.R.; Tran, N.H.; Hadinegoro, S.R.; Ismail, H.I.; Chotpitayasunondh, T.; Chua, M.N.; et al. CYD14 Study Group. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomized, observer-masked, placebo-controlled trial. Lancet 2014, 384, 1358–1365. [Google Scholar] [CrossRef]
- Sabchareon, A.; Wallace, D.; Sirivichayakul, C.; Limkittikul, K.; Chanthavanich, P.; Suvannadabba, S.; et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomized, controlled phase 2b trial. Lancet 2012, 380, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Juraska, M.; Magaret, C.A.; Shao, J.; Carpp, L.N.; Fiore-Gartland, A.J.; Benkeser, D.; et al. Viral genetic diversity and protective efficacy of a tetravalent dengue vaccine in two phase 3 trials. Proc Natl Acad Sci U.S.A. 2018, 115, E8378–E8387. [Google Scholar] [CrossRef]
- Sáez-Llorens, X.; Tricou, V.; Yu, D.; Rivera, L.; Jimeno, J.; Villarreal, A.C.; Dato, E.; et al. Immunogenicity and safety of one versus two doses of tetravalent dengue vaccine in healthy children aged 2-17 years in Asia and Latin America: 18-month interim data from a phase 2, randomized, placebo-controlled study. Lancet Infect Dis. 2018, 18, 162–170. [Google Scholar] [CrossRef]
- Capeding, R.Z.; Luna, I.A.; Bomasang, E.; Lupisan, S.; Lang, J.; Forrat, R.; Wartel, A.; et al. Live-attenuated, tetravalent dengue vaccine in children, adolescents, and adults in a dengue-endemic country: randomized controlled phase I trial in the Philippines. Vaccine 2011, 29, 3863–3872. [Google Scholar] [CrossRef]
- Sirivichayakul, C.; Barranco-Santana, E.A.; Rivera, I.E.; Kilbury, J.; Raanan, M.; Borkowski, A.; et al. Long-term safety and immunogenicity of a tetravalent dengue vaccine candidate in children and adults: A randomized, placebo-controlled, phase 2 study. J Infect Dis. 2022, 225, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Hss, A.S.; Koh, M.T.; Tan, K.K.; Chan, L.G.; Zhou, L.; Bouckenooghe, A.; Crevat, D.; et al. Safety and immunogenicity of a tetravalent dengue vaccine in healthy children aged 2-11 years in Malaysia: a randomized, placebo-controlled, Phase III study. Vaccine 2013, 31, 5814–5821. [Google Scholar] [CrossRef]
- Vigne, C.; Dupuy, M.; Richetin, A.; Guy, B.; Jackson, N.; Bonaparte, M.; Hu, B.; Saville, M.; et al. Integrated immunogenicity analysis of a tetravalent dengue vaccine up to 4 y after vaccination. Hum Vaccin Immunother. 2017, 13, 2004–2016. [Google Scholar] [CrossRef] [PubMed]
- Tricou, V.; Sáez-Llorens, X.; Yu, D.; Rivera, L.; Jimeno, J.; Villarreal, A.C.; Dato, E.; et al. Safety and immunogenicity of a tetravalent dengue vaccine in children aged 2-17 years: a randomized, placebo-controlled, phase 2 trial. Lancet. 2020, 395, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Simasathien, S.; Thomas, S.J.; Watanaveeradej, V.; Nisalak, A.; Barberousse, C.; Innis, B.; et al. Safety and immunogenicity of a tetravalent live-attenuated dengue vaccine in flavivirus naive children. Am J Trop Med Hyg. 2008, 78, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Watanaveeradej, V.; Simasathien, S.; Mammen, M.P.; Nisalak, A.; Tournay, E.; Kerdpanich, P.; Samakoses, R.; et al. Long-Term safety and immunogenicity of a tetravalent live-attenuated dengue vaccine and evaluation of a booster dose administered to healthy Thai children. Am J Trop Med Hyg. 2016, 94, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Biswal, S.; Mendez Galvan, J.F.; Macias Parra, M.; Galan-Herrera, J.F.; Carrascal Rodriguez, M.B.; Rodriguez Bueno, E.P.; et al. Immunogenicity, and safety of a tetravalent dengue vaccine in dengue-naïve adolescents in Mexico City. Rev Panam Salud Publica. 2021, 45, e67. [Google Scholar] [CrossRef] [PubMed]
- Villar, L.Á.; Rivera-Medina, D.M.; Arredondo-García, J.L.; Boaz, M.; Starr-Spires, L.; Thakur, M.; Zambrano, B.; et al. Dayan, G.H. Safety, and immunogenicity of a recombinant tetravalent dengue vaccine in 9-16-year-olds: a randomized, controlled, phase II trial in Latin America. Pediatr Infect Dis J. 2013, 32, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Dayan, G.H.; Thakur, M.; Boaz, M.; Johnson, C. Safety, and immunogenicity of three tetravalent dengue vaccine formulations in healthy adults in the USA. Vaccine 2013, 31, 5047–5054. [Google Scholar] [CrossRef]
- Arredondo-García, J.L.; Hadinegoro, S.R.; Reynales, H.; Chua, M.N.; Rivera Medina, D.M.; Chotpitayasunondh, T.; et al. CYD-TDV Dengue vaccine study group. Four-year safety follow-up of the tetravalent dengue vaccine efficacy randomized controlled trials in Asia and Latin America. Clin Microbiol Infect. 2018, 24, 755–763. [Google Scholar] [CrossRef]
- Kriengsak, L.; Chanthavanich, P.; Lee, K.S.; Lee, J-S.; Chatchen, S.; Lim, S-K.; et al. Dengue virus seroprevalence study in Bangphae district, Ratchaburi, Thailand: A cohort study in 2012-2015. PLoS Negl Trop Dis. 2022, 16, e0010021. [Google Scholar]
- Sabchareon, A.; Lang, J.; Chanthavanich, P.; Yoksan, S.; Forrat, R.; Attanath, P.; Sirivichayakul, C.; et al. Safety and immunogenicity of a three-dose regimen of two tetravalent live-attenuated dengue vaccines in five- to twelve-year-old Thai children. Pediatr Infect Dis J. 2004, 23, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Lanata, C.F.; Andrade, T.; Gil, A.I.; Terrones, C.; Valladolid, O.; Zambrano, B.; Saville, M.; et al. Immunogenicity and safety of tetravalent dengue vaccine in 2–11-year-olds previously vaccinated against yellow fever: randomized, controlled, phase II study in Piura, Peru. Vaccine 2012, 30, 5935–5941. [Google Scholar] [CrossRef] [PubMed]
- Leroux-Roels, G.; Bonanni, P.; Tantawichien, T.; Zepp, F. Vaccine development. Perspectives in Vaccinology 2011, 1, 115–150. [Google Scholar] [CrossRef]
- Ghosh, A.; Dar, L. Dengue vaccines: challenges, development, current status, and prospects. Indian J Med Microbiol. 2015, 33, 3–15. [Google Scholar] [CrossRef]

| Databases | Search keywords | Number of Articles Found |
|---|---|---|
| PubMed (1) | "Dengue fever" OR "Dengue epidemics" OR "Dengue vaccine" OR "Dengue Vaccine prospects" | 129 |
| PubMed (2) | "Dengue fever" OR "Dengue epidemics" AND "Dengue vaccine" OR "Dengue vaccine development" OR "Dengue vaccine prospects" AND "Dengue vaccine efficacy" OR "Dengue vaccine safety" OR "Dengue serotypes" | 81 |
| CINAHL | "Dengue fever" OR "Dengue epidemics" AND "Dengue vaccine" OR "Dengue vaccine development" OR "Dengue vaccine prospects" AND "Dengue vaccine efficacy" OR "Dengue vaccine safety" OR "Dengue serotypes" OR "Clinical trials" OR "Epidemiological studies" | 24 |
| Medline | Dengue fever" OR "Dengue epidemics" AND "Dengue vaccine" OR "Dengue vaccine development" OR "Dengue vaccine prospects" AND "Dengue vaccine efficacy" OR "Dengue vaccine safety" OR "Dengue serotypes" OR "Clinical trials" OR "Epidemiological studies | 210 |
| Health Source | "Dengue fever" OR "Dengue epidemics" AND "Dengue vaccine" OR "Dengue vaccine development" OR "Dengue vaccine prospects" AND "Dengue vaccine efficacy" OR "Dengue vaccine safety" OR "Dengue serotypes" | 2 |
| Science Direct | "Dengue fever" OR "Dengue epidemics" AND "Dengue vaccine" OR "Dengue vaccine development" AND "Dengue vaccine efficacy" OR "Dengue vaccine safety" OR "Dengue serotypes" OR "Clinical trials" OR "Epidemiological studies" | 1,381 |
| Academic Search Premiere | "Dengue fever" OR "Dengue epidemics" AND "Dengue vaccine" OR "Dengue vaccine development" OR "Dengue vaccine prospects" AND "Dengue vaccine efficacy" OR "Dengue vaccine safety" OR "Dengue serotypes" OR "Clinical trials" OR "Epidemiological studies" | 134 |
| Author & year of publication | Purpose | Study Design | Sample size | Age of participants | Main findings | Country |
|---|---|---|---|---|---|---|
| Forrat et al., 2021 [30] | Assessed hospitalized and severe virologically confirmed dengue (VCD) over the complete 6-year follow-up of 3 CYD-TDV efficacy studies (CYD14, CYD15, and CYD23/CYD57). | RCT | 29,229 | 2-16 years | CYD-TDV demonstrated robust protection against hospitalized and severe VCD over the entire 6-year follow-up in participants who were seropositive and ≥9 years old. Protection was also observed in seropositive 6–8 year-olds. | Asia and Latin America |
| Thomas et al., 2022 [31] | Evaluated potential associations of host human leukocyte antigen (HLA) alleles with dengue antibody responses, CYD-TDV vaccine efficacy, and virologically confirmed dengue (VCD) cases. | RCT | 334 | 4-11 years | Specific HLA alleles that are significantly associated with dengue NAb titers were identified. | Thailand |
| España et al., 2019 [32] | Evaluated the vaccine efficacy for susceptibility (VES) as a measure of the protective effects of vaccination against the first symptomatic, virologically confirmed case of dengue. | RCT | 51,253 | 2-16 years | Discovered a distinct bias in VE estimates away from the null due to lower detectability of primary DENV infections among seronegative individuals in the vaccinated group. | Peru |
| Yang et al., 2018 [33] | Evaluated the dependence of Tetravalent Dengue Vaccine efficacy on baseline immunity status and age groups (children) | RCT | 31,125 | 5 – 11 years | The CYD-TDV vaccine was highly efficacious for all dengue serotypes among children aged >5 years who have acquired baseline immunity from previous exposure. | USA |
| Plennevaux et al., 2016 [34] | Detected dengue cases by serological testing in a dengue vaccine efficacy trial | RCT | 2266 | 4-11 years | Reliance on serological assessments would lead to a significant number of false positives during routine clinical practice and surveillance following the introduction of the dengue vaccine | Thailand |
| Sridhar et al., 2018 [35] | Assessed the risk of hospitalization for VCD in seronegative vaccine recipients who were 9 years of age or older at enrollment (the primary endpoint). | Case–cohort study | 3578 | 2-16 years | CYD-TDV protected against severe VCD and hospitalization for VCD for 5 years in persons who had exposure to dengue before vaccination. | Asia-Pacific region, Latin America, and Thailand |
| Plennevaux et al., 2018 [36] | Assessed the impact of dengue vaccination on the serological diagnosis of dengue in larger and more diverse epidemiological settings of 2 phase III CYD-TDV efficacy studies. | RCT | 31,000 | 2-16 years | Results showed that baseline dengue serostatus (as defined by the PRNT50) had an impact on the IgM and IgG levels observed in VCD and other febrile episodes among CYD-TDV recipients and controls. | Asia and Latin America |
| Moodie et al., 2018 [37] | Investigated the association of neutralizing antibody titers with dengue occurrence with the level of vaccine efficacy to prevent dengue | Case-Cohort study | 31,144 | 2-16 years | Neutralizing antibody titers postdose 3 correlates with CYD-TDV vaccine efficacy to prevent dengue. High titers are associated with high VE for all serotypes, baseline serostatus groups, age groups, and both trials. | Asia and Latin America |
| Olivera-Botello et al., 2016 [38] | Investigated whether vaccination with CYD-TDV protected individuals from asymptomatic infection, using a commonly used surrogate measure, primary, secondary, or other seroconversion | RCT | 31,126 | 2-16 years | Vaccine efficacy was marginally higher in subjects aged 9–16 years (38.6%). | Asia and Latin America (Colombia, Brazil, Mexico, Puerto Rico, and Honduras) |
| Hadinegoro et al., 2015 [39] | Reported long-term safety phase and integrated analyses of data from the efficacy surveillance phase to provide a global view of the clinical profile of the CYD-TDV dengue vaccine. | RCT | 33,266 | 2-16 years | The risk among children 2 to 16 years of age was lower in the vaccine group than in the control group. | Asia–Pacific countries, and Latin American countries |
| Villar et al., 2015 [40] | Investigated the efficacy of a Tetravalent Dengue Vaccine in Children in Latin America | RCT | 20,869 | 9-16 years | The CYD-TDV dengue vaccine was efficacious against VCD and severe VCD and led to fewer hospitalizations for VCD in five Latin American countries where dengue is endemic. | Colombia, Brazil, Mexico, Puerto Rico, and Honduras |
| Dayan et al., 2015 [41] | Vaccine efficacy against symptomatic virologically confirmed dengue (VCD) was assessed by age group and baseline dengue serostatus. | Case cohort study |
436 | 3-9 years | CYD-TDV provided long-term efficacy against symptomatic VCD in seropositive participants with evidence of persistent protection up to six years after the first dose. | Asia-Pacific and Latin America |
| Dayan et al., 2020 [42] | Investigated the effectiveness of a single-dose mass dengue vaccination in Cebu, Philippines | Case-cohort study | 31,126 | 9-14 years | A single dose of CYD-TDV protected children from severe dengue and dengue with warning signs. | Philippines |
| López-Medina et al., 2021 [43] | Investigated the efficacy of a TAK-003 in healthy children 2 years after vaccination | RCT | 20,099 | 4–16 years | TAK-003 demonstrated continued benefit independent of baseline serostatus in reducing dengue with some decline in efficacy during the second year | Latin America (Brazil, Colombia, Dominican Republic, Panama & Nicaragua), Sri Lanka, Thailand, Philippines |
| Biswal et al., 2020 [44] | Assess the efficacy, safety, and immunogenicity of a live attenuated tetravalent dengue vaccine (TAK-003) in healthy children | RCT | 20,099 | 4-16 years | TAK-003 was well tolerated and efficacious against symptomatic dengue in children regardless of serostatus before immunization. | Asia and Latin America |
| Rivera et al., 2022 [45] | Investigated a three-year efficacy and safety of Takeda’s Dengue Vaccine Candidate | RCT | 20,099 | 4-16 years | TAK-003 was efficacious against symptomatic dengue over 3 years. There were no safety risks. | Latin America (Panama, Nicaragua) and Asia (Philippines, Sri Lanka) |
| Biswal et al., 2019 [46] | Investigated the efficacy of a Tetravalent Dengue Vaccine in healthy children | RCT | 20,071 | 4-16 years | TAK-003 was efficacious against virologically confirmed dengue fever among healthy children, irrespective of previous dengue exposure. | Brazil, Colombia, Dominican Republic, Nicaragua, Panama, Philippines, Sri Lanka, and Thailand |
| Saez-Llorens et al., 2023 [47] | Investigated the effect of the Tetravalent Dengue Vaccine TAK-003 on Sequential Episodes of Symptomatic Dengue | RCT | 13,380 | 4-16 years | TAK-003 vaccination resulted in a reduced risk of experiencing sequential episodes of symptomatic dengue in children | Latin America (Columbia) and Asia (Philippines, Sri Lanka, Thailand) |
| Reynales et al., 2020 [48] | Analyzed the efficacy and safety Trial Data of the Tetravalent Dengue Vaccine in children in Colombia. | Case-cohort study |
9740 | 9–16 years | CYD-TDV protected against severe VCD and hospitalization for VCD among individuals previously exposed to dengue before vaccination. | Colombia |
| Ylade et al., 2021 [49] | Conducted a case-control study in Cebu province following the dengue mass vaccination. | Case-control study |
490 | 9-14 years | A single dose of CYD-TDV given to nine to fourteen-year-old children through a community-based mass vaccination program conferred protection against dengue with warning signs. | Philippines |
| Capeding et al., 2014 [50] Sabchareon et al., 2012 [51] |
Assessed the efficacy of the CYD dengue vaccine against symptomatic, virologically confirmed dengue in children. Investigated the efficacy and safety of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai school children |
RCT RCT |
10,275 4002 |
2-14 years 4–11 years |
Findings showed that the dengue vaccine is efficacious when given as three injections at months 0, 6, and 12 to children aged 2–14 years in endemic areas in Asia, and has a good safety profile. Efficacious but differed by serotype. The dengue vaccine was well tolerated, with no safety signals after 2 years of follow-up after the first dose. |
Asia-Pacific countries (Indonesia, Malaysia, Philippines, Thailand, and Vietnam Thailand |
| Juraska et al., 2018 [52] | Evaluated the efficacy of a tetravalent dengue vaccine in two phase 3 trials | RCT | 563 | 2-16 years | Greater estimated vaccine efficacy of CYD-TDV against serotypes was recorded. | Brazil and Thailand |
| Sáez-Llorens et al., 2018 [53] | Assessed the immunogenicity and safety of Takeda’s tetravalent dengue vaccine (TDV) candidate over 48 months in children living in dengue-endemic countries. | RCT | 1800 | 2-17 years | Takeda vaccine was well tolerated and immunogenic against all four dengue serotypes, irrespective of baseline dengue serostatus. | Dominican Republic, Panama, and the Philippines. |
| Capeding et al., 2011 [54] | Assessed the safety and immunogenicity of the vaccine among children in a flavivirus-endemic region. | RCT | 126 | 2-17 years | This phase I study of a live attenuated, tetravalent recombinant dengue vaccine in children supports its safety and tolerability in a flavivirus-endemic population. | Philippines |
| Sirivichayakul et al., 2022 [55] | Reported long-term safety and immunogenicity of Takeda’s tetravalent dengue vaccine candidate (TAK-003) in healthy children and adults living in dengue-endemic areas | RCT | 212 | 1-11 years | The trial demonstrated the persistence of neutralizing antibody titers against TAK-003 over 3 years in children living in dengue-endemic countries, with limited contribution from natural infection. TAK-003 was well tolerated. | Puerto Rico, Columbia, Singapore, and Thailand. |
| Hss et al., 2013 [56] | Evaluated the safety and immunogenicity of Phase III lots of a candidate vaccine (CYD-TDV) in children in Malaysia. | RCT | 250 | 2-11 years | This study demonstrated a satisfactory safety profile and a balanced humoral immune response against all four DENV serotypes for CYD-TDV administered via a three-dose regimen to children in Malaysia. | Malaysia |
| Vigne et al., 2017 [57] | Investigated an unprecedented integrated summary of the immunogenicity of CYD-TDV to identify the parameters driving the neutralizing humoral immune response and evolution over time. | RCT | 5,780 | 9-17 years | CYD-TDV elicits neutralizing antibody responses against all dengue serotypes, with differences by age and endemicity, which persist above baseline levels in endemic countries. | Asia Pacific (including Australia), Latin America, and USA |
| Tricou et al., 2020 [58] | Assessed the immunogenicity and safety of three different dose schedules of a tetravalent dengue vaccine (TAK-003) over 48 months in children living in dengue-endemic countries | RCT | 1800 | 2-17 years | TAK-003 elicited antibody responses against all four serotypes, which persisted to 48 months postvaccination, regardless of baseline serostatus. No important safety risks were identified. | Dominican Republic, Panama, and the Philippines |
| Simasathien et al., 2008 [59] | Conducted a pilot, safety, and immunogenicity trial of the vaccine candidate in healthy Thai children to prepare for its eventual evaluation in Thai infants. | RCT | 89 | 6–7 years | The vaccine was well tolerated with no serious adverse events or alert laboratory values. | Thailand |
| Watanaveeradej et al., 2016 [60] | Evaluated the safety and immunogenicity of two doses of a live-attenuated, tetravalent dengue virus vaccine (F17/Pre formulation) and a booster dose in a dengue-endemic setting in two studies | RCT | 56 | 2-8 years | The results of these two follow-up studies indicate that the live-attenuated DENV candidate vaccine jointly developed by the WRAIR and GSK did not elicit a durable primary humoral immune response. | Thailand |
| Biswal et al., 2021 [61] | Assessed the immunogenicity and safety of a tetravalent dengue vaccine in dengue-naïve children | RCT | 400 | 12–17 years | TAK-003 was immunogenic against all four serotypes and was well tolerated in dengue-naïve adolescents living in Mexico City. No safety risk either. | Mexico |
| Villar et al., 2013 [62] | Evaluated the safety and immunogenicity of a candidate recombinant, live-attenuated, tetravalent dengue vaccine (CYD-TDV) on Latin American children | RCT | 600 | 2-16 years | CYD-TDV had a favorable safety profile and elicited antibody responses against all 4 dengue virus serotypes in 9–16-year-olds in Latin America. | Colombia, Honduras, Mexico and Puerto Rico |
| Dayan et al., 2013[63] | Evaluated the immunogenicity and Safety of a Recombinant Tetravalent Dengue Vaccine in Children. | RCT | 150 | 9-16 | CYD-TDV vaccination elicited a neutralizing antibody response against serotypes 1–4 and was well tolerated in children/adolescents in a dengue-endemic region. | Brazil |
| Arredondo-García et al., 2018 [64] | The study compared the tetravalent dengue vaccine to placebo in 3 clinical trials & examined the risk of hospital admission due to confirmed dengue. | RCT | 23,429 | 2-16 years | The overall Relative Risk in those aged <9 years for Year 1 to Year 4 was 0.786 (95% CI 0.60e1.03), with a higher protective effect in the 6-8 year olds than in the 2-5 year olds. | 5-Asian–Pacific countries and 5- Latin American countries and Thailand |
| Kriengsak et al., 2019 [65] | Investigated the long-term safety of a tetravalent dengue vaccine (CYD-TDV) in children in a phase Π b follow-up study in Thailand | RCT | 3,997 | 4-11 years | The risk of hospitalized VCD among children in Thailand vaccinated with CYD-TDV is reduced in those aged ≥9 years over six years of follow-up. | Thailand |
| Sabchareon et al., 2004[66] Lanata et al., 2012[67] |
Evaluated the safety and immunogenicity of tetravalent live-attenuated dengue vaccines after a three-dose vaccination series in Thai children. Assessed the safety and immunogenicity of a recombinant, live, attenuated, tetravalent dengue vaccine candidate (CYD-TDV). |
RCT RCT |
1587 300 |
5-12years 2-11 years |
No serious adverse event related to the vaccines occurred. Most children experienced mild to moderate fever, rash, headache, and myalgia occurring within 12 days after Dose 1 and generally lasting 3 days or less. There were no vaccine-related SAEs, no withdrawals for adverse events after dengue vaccination, and no immediate adverse events. |
Thailand Peru |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





