Submitted:
18 December 2023
Posted:
19 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Section
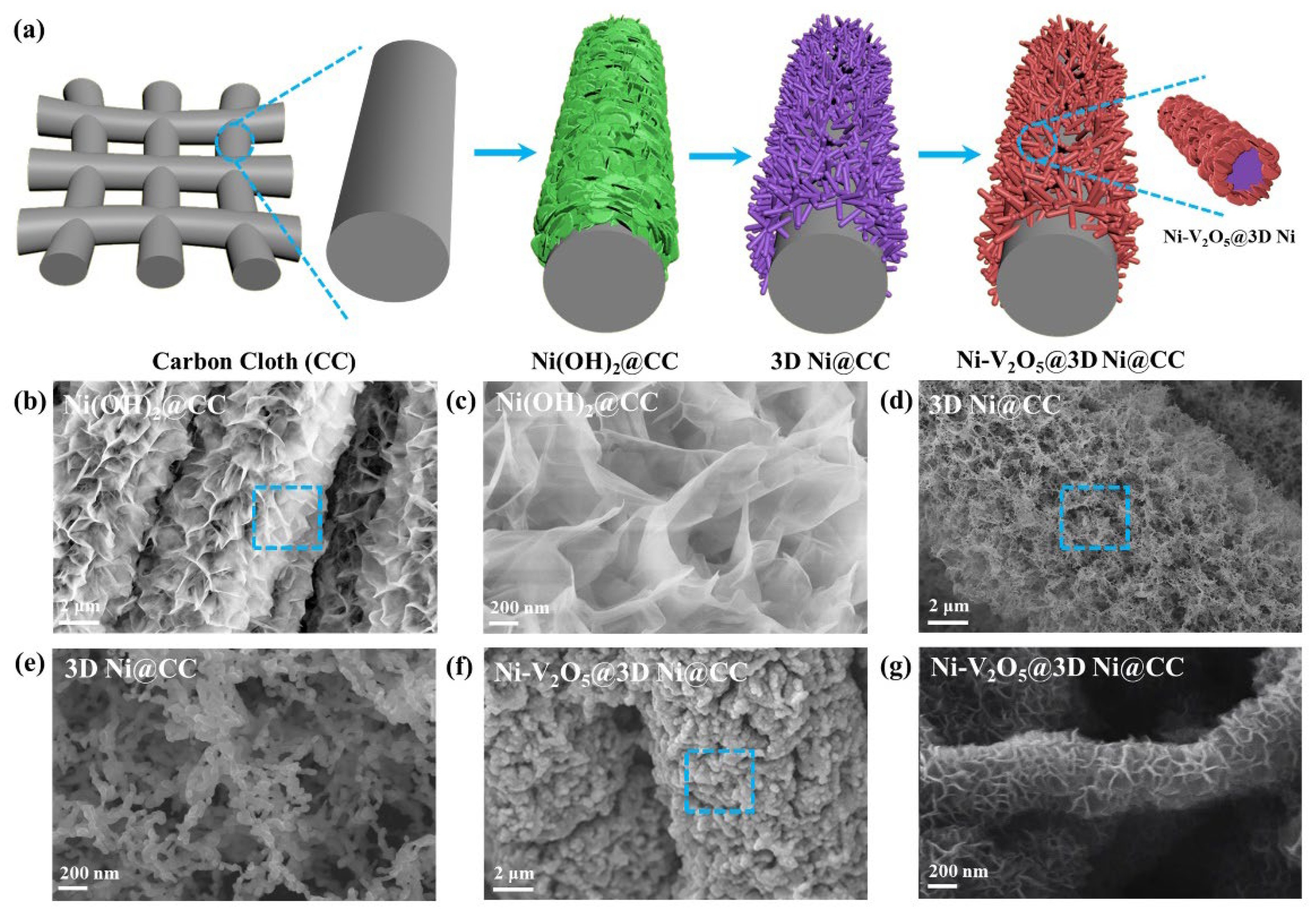
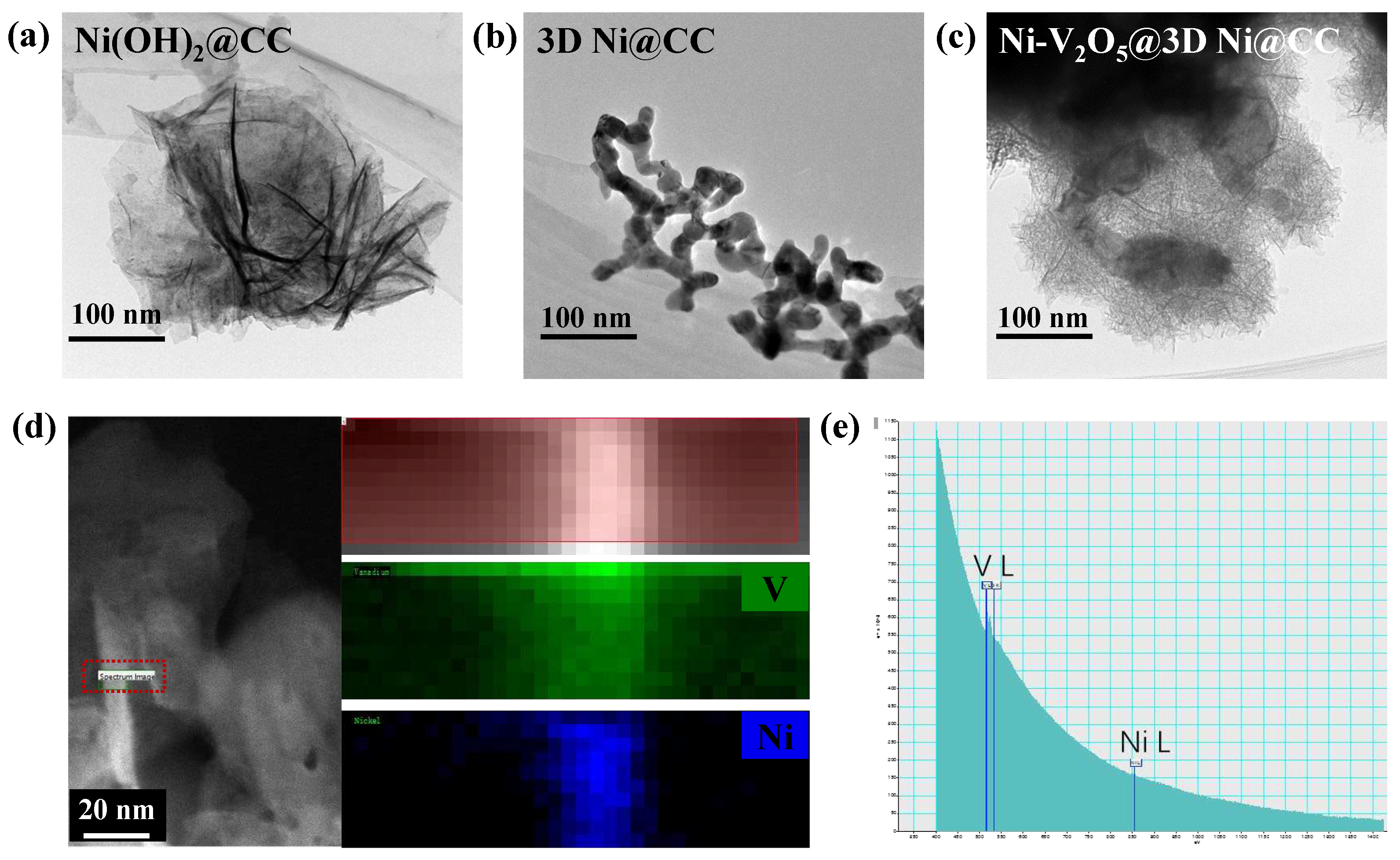
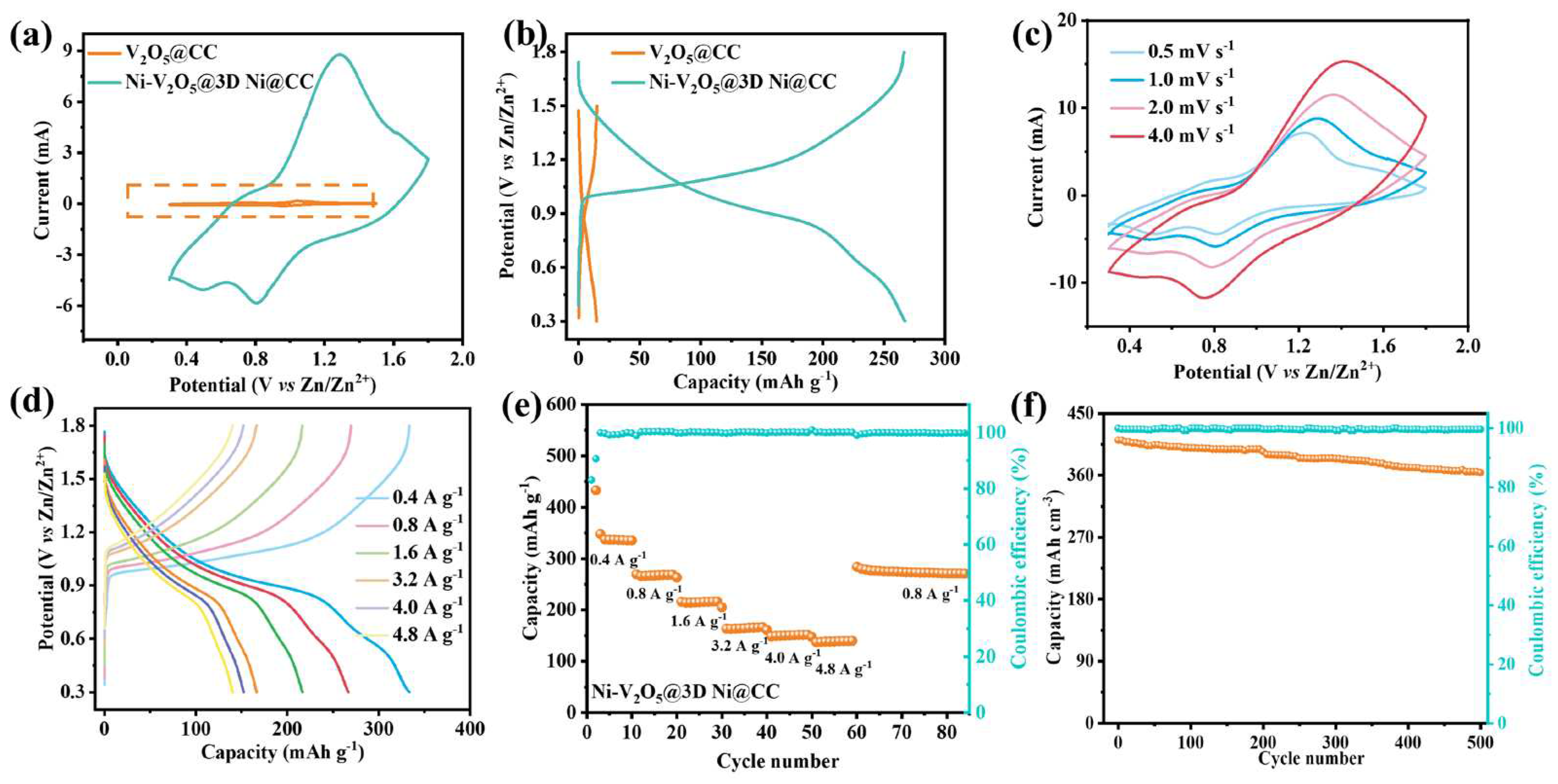
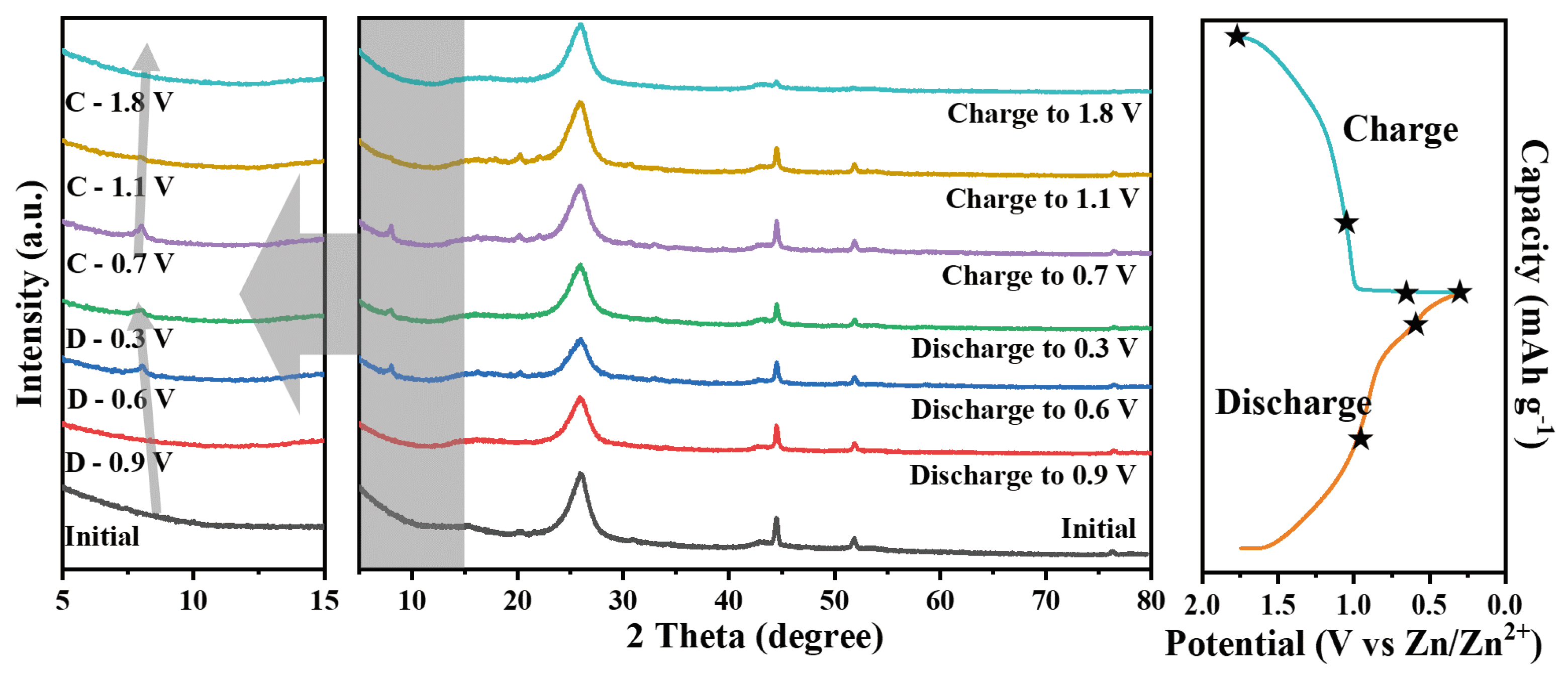
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Wang, J.-G.; You, Z.; Wei, C.; Kang, F.; Wei, B., Rechargeable aqueous zinc-ion batteries: Mechanism, design strategies and future perspectives. Materials Today 2021, 42, 73-98.
- Huang, M.; Wang, X.; Liu, X.; Mai, L., Fast Ionic Storage in Aqueous Rechargeable Batteries: From Fundamentals to Applications. Advanced Materials 2022, 34, (9).
- Yan, J.; Ang, E. H.; Yang, Y.; Zhang, Y.; Ye, M.; Du, W.; Li, C. C. , High-Voltage Zinc-Ion Batteries: Design Strategies and Challenges. Advanced Functional Materials 2021, 31, (22).
- Song, J.; Xu, K.; Liu, N.; Reed, D.; Li, X., Crossroads in the renaissance of rechargeable aqueous zinc batteries. Materials Today 2021, 45, 191-212.
- Gourley, S. W.; Brown, R.; Adams, B. D.; Higgins, D. J. J., Zinc-ion batteries for stationary energy storage. Joule 2023, 7, (7), 1415-1436.
- Yang, X.; Gao, T.; Zhao, R.; Wu, Q.; Shi, K.; She, Z.; Lu, S.; Xie, Q.; Ruan, Y. J. E. T., Expanding Layer Spacing of Carbon-Coated Vanadium Oxide via Ammonium Ions for Fast Electrochemical Kinetics in Aqueous Zinc-Ion Batteries. Energy Technology 2023, 11, (2), 2200990.
- Li, Z.; Tan, J.; Wang, Y.; Gao, C.; Wang, Y.; Ye, M.; Shen, J., Building better aqueous Zn-organic batteries. Energy & Environmental Science 2023, 16, (6), 2398-2431.
- Zhao, Z.; Zhang, H.; Shi, X.; Zhang, Y.; Tang, C.; Zhao, H.; Liu, J.; Wang, G.; Li, L. J. S., Zincophilic Metal-Organic-Framework Interface Mitigating Dendrite Growth for Highly Reversible Zinc Metal Batteries. Small 2023, 2304723.
- Li, M.; Li, Z.; Wang, X.; Meng, J.; Liu, X.; Wu, B.; Han, C.; Mai, L., Comprehensive understanding of the roles of water molecules in aqueous Zn-ion batteries: from electrolytes to electrode materials. Energy & Environmental Science 2021, 14, (7), 3796-3839.
- Dong, H.; Li, J.; Guo, J.; Lai, F.; Zhao, F.; Jiao, Y.; Brett, D. J. L.; Liu, T.; He, G.; Parkin, I. P., Insights on Flexible Zinc-Ion Batteries from Lab Research to Commercialization. Advanced Materials 2021, 33, (20).
- Lin, Z.; Shi, H.-Y.; Lin, L.; Yang, X.; Wu, W.; Sun, X., A high capacity small molecule quinone cathode for rechargeable aqueous zinc-organic batteries. Nature Communications 2021, 12, (1).
- Zhao, J.; Xu, Z.; Zhou, Z.; Xi, S.; Xia, Y.; Zhang, Q.; Huang, L.; Mei, L.; Jiang, Y.; Gao, J.; Zeng, Z.; Tan, C., A Safe Flexible Self-Powered Wristband System by Integrating Defective MnO2–x Nanosheet-Based Zinc-Ion Batteries with Perovskite Solar Cells. ACS Nano 2021, 15, (6), 10597-10608.
- Zhu, X.; Cao, Z.; Wang, W.; Li, H.; Dong, J.; Gao, S.; Xu, D.; Li, L.; Shen, J.; Ye, M., Superior-Performance Aqueous Zinc-Ion Batteries Based on the In Situ Growth of MnO2 Nanosheets on V2CTX MXene. ACS Nano 2021, 15, (2), 2971-2983.
- Cui, G.; Zeng, Y.; Wu, J.; Guo, Y.; Gu, X.; Lou, X. W., Synthesis of Nitrogen-Doped KMn8O16 with Oxygen Vacancy for Stable Zinc-Ion Batteries. Advanced Science 2022, 9, (10).
- Li, Y.; Li, X.; Duan, H.; Xie, S.; Dai, R.; Rong, J.; Kang, F.; Dong, L., Aerogel-structured MnO2 cathode assembled by defect-rich ultrathin nanosheets for zinc-ion batteries. Chemical Engineering Journal 2022, 441.
- Ma, Y.; Xu, M.; Liu, R.; Xiao, H.; Liu, Y.; Wang, X.; Huang, Y.; Yuan, G., Molecular tailoring of MnO2 by bismuth doping to achieve aqueous zinc-ion battery with capacitor-level durability. Energy Storage Materials 2022, 48, 212-222.
- Zeng, Y.; Lu, X. F.; Zhang, S. L.; Luan, D.; Li, S.; Lou, X. W., Construction of Co-Mn Prussian Blue Analog Hollow Spheres for Efficient Aqueous Zn-ion Batteries. Angewandte Chemie International Edition 2021, 60, (41), 22189-22194.
- Chen, C.; Shi, M.; Zhao, Y.; Yang, C.; Zhao, L.; Yan, C., Al-Intercalated MnO2 cathode with reversible phase transition for aqueous Zn-Ion batteries. Chemical Engineering Journal 2021, 422.
- Cui, H.; Wang, T.; Huang, Z.; Liang, G.; Chen, Z.; Chen, A.; Wang, D.; Yang, Q.; Hong, H.; Fan, J.; Zhi, C., High-Voltage Organic Cathodes for Zinc-Ion Batteries through Electron Cloud and Solvation Structure Regulation. Angewandte Chemie International Edition 2022, 61, (30).
- Liu, L.; Wu, Y. C.; Huang, L.; Liu, K.; Duployer, B.; Rozier, P.; Taberna, P. L.; Simon, P., Alkali Ions Pre-Intercalated Layered MnO2 Nanosheet for Zinc-Ions Storage. Advanced Energy Materials 2021, 11, (31).
- Islam, S.; Alfaruqi, M. H.; Putro, D. Y.; Park, S.; Kim, S.; Lee, S.; Ahmed, M. S.; Mathew, V.; Sun, Y. K.; Hwang, J. Y.; Kim, J., In Situ Oriented Mn Deficient ZnMn2O4@C Nanoarchitecture for Durable Rechargeable Aqueous Zinc-Ion Batteries. Advanced Science 2021, 8, (4).
- Deng, S.; Tie, Z.; Yue, F.; Cao, H.; Yao, M.; Niu, Z., Rational Design of ZnMn2O4 Quantum Dots in a Carbon Framework for Durable Aqueous Zinc-Ion Batteries. Angewandte Chemie International Edition 2022, 61, (12).
- Tang, H.; Chen, W.; Li, N.; Hu, Z.; Xiao, L.; Xie, Y.; Xi, L.; Ni, L.; Zhu, Y., Layered MnO2 nanodots as high-rate and stable cathode materials for aqueous zinc-ion storage. Energy Storage Materials 2022, 48, 335-343.
- Liu, S.; Mao, J.; Pang, W. K.; Vongsvivut, J.; Zeng, X.; Thomsen, L.; Wang, Y.; Liu, J.; Li, D.; Guo, Z., Tuning the Electrolyte Solvation Structure to Suppress Cathode Dissolution, Water Reactivity, and Zn Dendrite Growth in Zinc-Ion Batteries. Advanced Functional Materials 2021, 31, (38).
- Ma, X.; Cao, X.; Yao, M.; Shan, L.; Shi, X.; Fang, G.; Pan, A.; Lu, B.; Zhou, J.; Liang, S., Organic-Inorganic Hybrid Cathode with Dual Energy-Storage Mechanism for Ultrahigh-Rate and Ultralong-Life Aqueous Zinc-Ion Batteries. Advanced Materials 2021, 34, (6).
- Moon, H.; Ha, K. H.; Park, Y.; Lee, J.; Kwon, M. S.; Lim, J.; Lee, M. H.; Kim, D. H.; Choi, J. H.; Choi, J. H.; Lee, K. T., Direct Proof of the Reversible Dissolution/Deposition of Mn2+/Mn4+ for Mild-Acid Zn-MnO2 Batteries with Porous Carbon Interlayers. Advanced Science 2021, 8, (6).
- Wang, W.; Kale, V. S.; Cao, Z.; Lei, Y.; Kandambeth, S.; Zou, G.; Zhu, Y.; Abouhamad, E.; Shekhah, O.; Cavallo, L.; Eddaoudi, M.; Alshareef, H. N., Molecular Engineering of Covalent Organic Framework Cathodes for Enhanced Zinc-Ion Batteries. Advanced Materials 2021, 33, (39).
- Sambandam, B.; Mathew, V.; Kim, S.; Lee, S.; Kim, S.; Hwang, J. Y.; Fan, H. J.; Kim, J., An analysis of the electrochemical mechanism of manganese oxides in aqueous zinc batteries. Chem 2022, 8, (4), 924-946.
- Deka Boruah, B.; Mathieson, A.; Park, S. K.; Zhang, X.; Wen, B.; Tan, L.; Boies, A.; De Volder, M., Vanadium Dioxide Cathodes for High-Rate Photo-Rechargeable Zinc-Ion Batteries. Advanced Energy Materials 2021, 11, (13).
- Hu, L.; Wu, Z.; Lu, C.; Ye, F.; Liu, Q.; Sun, Z., Principles of interlayer-spacing regulation of layered vanadium phosphates for superior zinc-ion batteries. Energy & Environmental Science 2021, 14, (7), 4095-4106.
- Zhang, Z.; Xi, B.; Wang, X.; Ma, X.; Chen, W.; Feng, J.; Xiong, S., Oxygen Defects Engineering of VO2·xH2O Nanosheets via In Situ Polypyrrole Polymerization for Efficient Aqueous Zinc Ion Storage. Advanced Functional Materials 2021, 31, (34).
- Wang, X.; Zhang, Z.; Xi, B.; Chen, W.; Jia, Y.; Feng, J.; Xiong, S., Advances and Perspectives of Cathode Storage Chemistry in Aqueous Zinc-Ion Batteries. ACS Nano 2021, 15, (6), 9244-9272.
- Cao, J.; Zhang, D.; Yue, Y.; Wang, X.; Pakornchote, T.; Bovornratanaraks, T.; Zhang, X.; Wu, Z.-S.; Qin, J., Oxygen defect enriched (NH4)2V10O25·8H2O nanosheets for superior aqueous zinc-ion batteries. Nano Energy 2021, 84.
- Li, R.; Xing, F.; Li, T.; Zhang, H.; Yan, J.; Zheng, Q.; Li, X., Intercalated polyaniline in V2O5 as a unique vanadium oxide bronze cathode for highly stable aqueous zinc ion battery. Energy Storage Materials 2021, 38, 590-598.
- Pan, Z.; Liu, X.; Yang, J.; Li, X.; Liu, Z.; Loh, X. J.; Wang, J. , Aqueous Rechargeable Multivalent Metal-Ion Batteries: Advances and Challenges. Advanced Energy Materials 2021, 11, (24).
- Du, Y.; Wang, X.; Zhang, Y.; Zhang, H.; Man, J.; Liu, K.; Sun, J., High mass loading CaV4O9 microflowers with amorphous phase transformation as cathode for aqueous zinc-ion battery. Chemical Engineering Journal 2022, 434.
- Feng, Z.; Zhang, Y.; Sun, J.; Liu, Y.; Jiang, H.; Cui, M.; Hu, T.; Meng, C., Dual ions enable vanadium oxide hydration with superior Zn2+ storage for aqueous zinc-ion batteries. Chemical Engineering Journal 2022, 433.
- Wang, X.; Naveed, A.; Zeng, T.; Wan, T.; Zhang, H.; Zhou, Y.; Dou, A.; Su, M.; Liu, Y.; Chu, D., Sodium ion stabilized ammonium vanadate as a high-performance aqueous zinc-ion battery cathode. Chemical Engineering Journal 2022, 446.
- Yang, W.; Yang, Y.; Yang, H.; Zhou, H., Regulating Water Activity for Rechargeable Zinc-Ion Batteries: Progress and Perspective. ACS Energy Letters 2022, 7, (8), 2515-2530.
- Zhang, R.; Liang, P.; Yang, H.; Min, H.; Niu, M.; Jin, S.; Jiang, Y.; Pan, Z.; Yan, J.; Shen, X.; Wang, J., Manipulating intercalation-extraction mechanisms in structurally modulated δ-MnO2 nanowires for high-performance aqueous zinc-ion batteries. Chemical Engineering Journal 2022, 433.
- Peng, Y.; Xu, J.; Xu, J.; Ma, J.; Bai, Y.; Cao, S.; Zhang, S.; Pang, H., Metal-organic framework (MOF) composites as promising materials for energy storage applications. Advances in Colloid and Interface Science 2022, 307.
- Xing, Z.; Xu, G.; Han, J.; Chen, G.; Lu, B.; Liang, S.; Zhou, J., Facing the capacity fading of vanadium-based zinc-ion batteries. Trends in Chemistry 2023, 5, (5), 380-392.
- Chen, Z.; Cui, H.; Hou, Y.; Wang, X.; Jin, X.; Chen, A.; Yang, Q.; Wang, D.; Huang, Z.; Zhi, C., Anion chemistry enabled positive valence conversion to achieve a record high-voltage organic cathode for zinc batteries. Chem 2022, 8, (8), 2204-2216.
- Xiao, X.; Zheng, Z.; Zhong, X.; Gao, R.; Piao, Z.; Jiao, M.; Zhou, G., Rational Design of Flexible Zn-Based Batteries for Wearable Electronic Devices. ACS Nano 2023, 17, (3), 1764-1802.
- Paul, R.; Zhai, Q.; Roy, A. K.; Dai, L. J. I. M., Charge transfer of carbon nanomaterials for efficient metal-free electrocatalysis. 2022, 1, (1), 28-50.
- Wang, Y.; Song, J.; Wong, W. Y., Constructing 2D Sandwich-like MOF/MXene Heterostructures for Durable and Fast Aqueous Zinc-Ion Batteries. Angewandte Chemie International Edition 2023, 62, (8).
- Zong, Q.; Wang, Q.; Liu, C.; Tao, D.; Wang, J.; Zhang, J.; Du, H.; Chen, J.; Zhang, Q.; Cao, G., Potassium Ammonium Vanadate with Rich Oxygen Vacancies for Fast and Highly Stable Zn-Ion Storage. ACS Nano 2022, 16, (3), 4588-4598.
- Liu, Y.; Liu, Y.; Wu, X., Defect engineering of vanadium-based electrode materials for zinc ion battery. Chinese Chemical Letters 2023, 34, (7).
- Gao, F.; Mei, B.; Xu, X.; Ren, J.; Zhao, D.; Zhang, Z.; Wang, Z.; Wu, Y.; Liu, X.; Zhang, Y., Rational design of ZnMn2O4 nanoparticles on carbon nanotubes for high-rate and durable aqueous zinc-ion batteries. Chemical Engineering Journal 2022, 448.
- Li, X.; Ma, Y.; Yue, Y.; Li, G.; Zhang, C.; Cao, M.; Xiong, Y.; Zou, J.; Zhou, Y.; Gao, Y., A flexible Zn-ion hybrid micro-supercapacitor based on MXene anode and V2O5 cathode with high capacitance. Chemical Engineering Journal 2022, 428.
- Sun, R.; Guo, X.; Dong, S.; Wang, C.; Zeng, L.; Lu, S.; Zhang, Y.; Fan, H., Zn3V3O8@ZnO@NC heterostructure for stable zinc ion storage from assembling nanodisks into cross-stacked architecture. Journal of power sources 2023, 567.
- Wang, T.; Li, S.; Weng, X.; Gao, L.; Yan, Y.; Zhang, N.; Qu, X.; Jiao, L.; Liu, Y., Ultrafast 3D Hybrid-Ion Transport in Porous V2O5 Cathodes for Superior-Rate Rechargeable Aqueous Zinc Batteries. Advanced Energy Materials 2023, 13, (18).
- Zhao, D.; Wang, X.; Zhang, W.; Zhang, Y.; Lei, Y.; Huang, X.; Zhu, Q.; Liu, J., Unlocking the Capacity of Vanadium Oxide by Atomically Thin Graphene-Analogous V2O5·nH2O in Aqueous Zinc-Ion Batteries. Advanced Functional Materials 2023, 33, (13).
- Zhang, L.; Zhu, J.; Li, X.; Mu, S.; Verpoort, F.; Xue, J.; Kou, Z.; Wang, J. J. I. M., Nurturing the marriages of single atoms with atomic clusters and nanoparticles for better heterogeneous electrocatalysis. 2022, 1, (1), 51-87.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




