Submitted:
15 December 2023
Posted:
18 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Correlation Analyses
2.3. Linear Regression Analyses
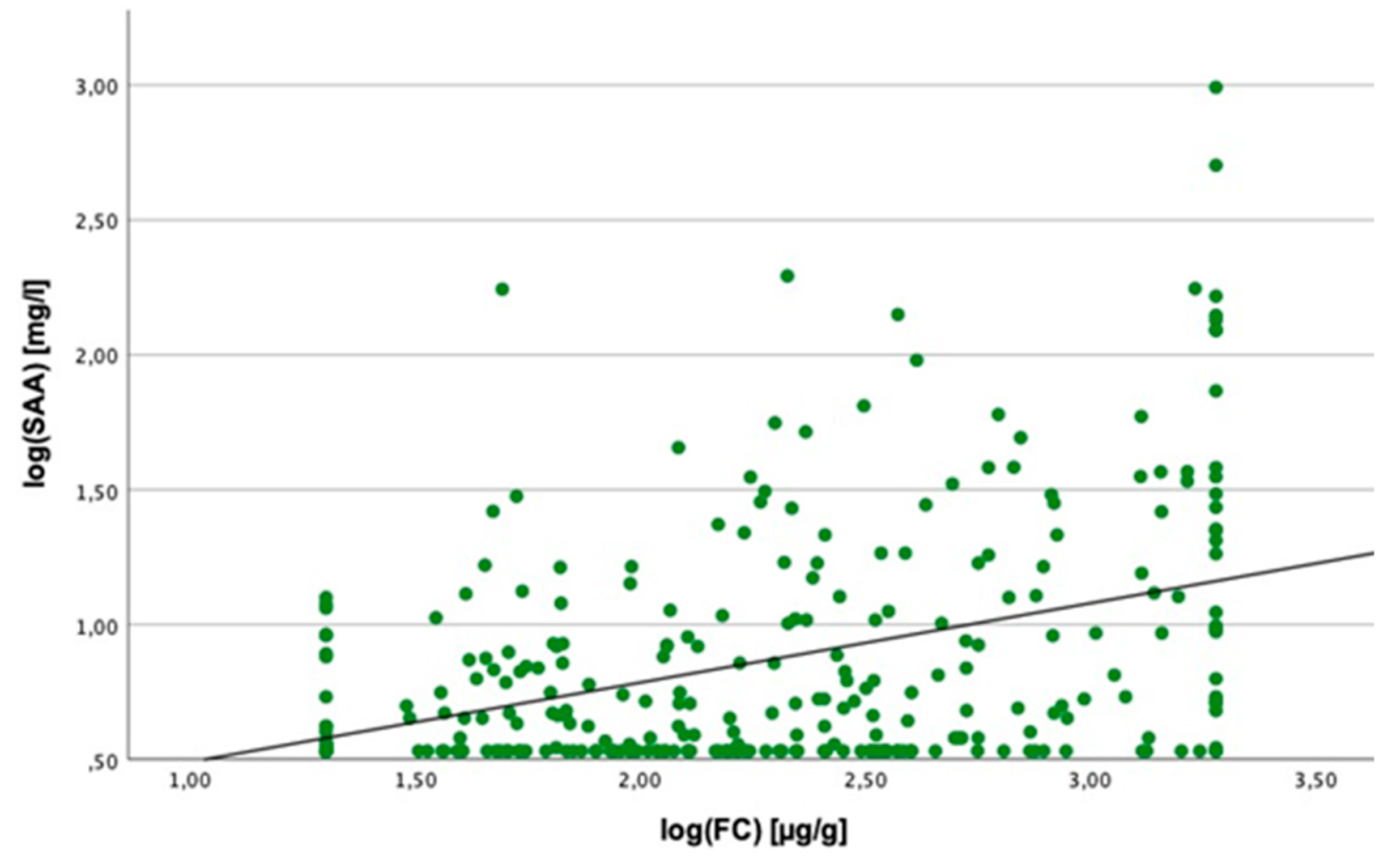
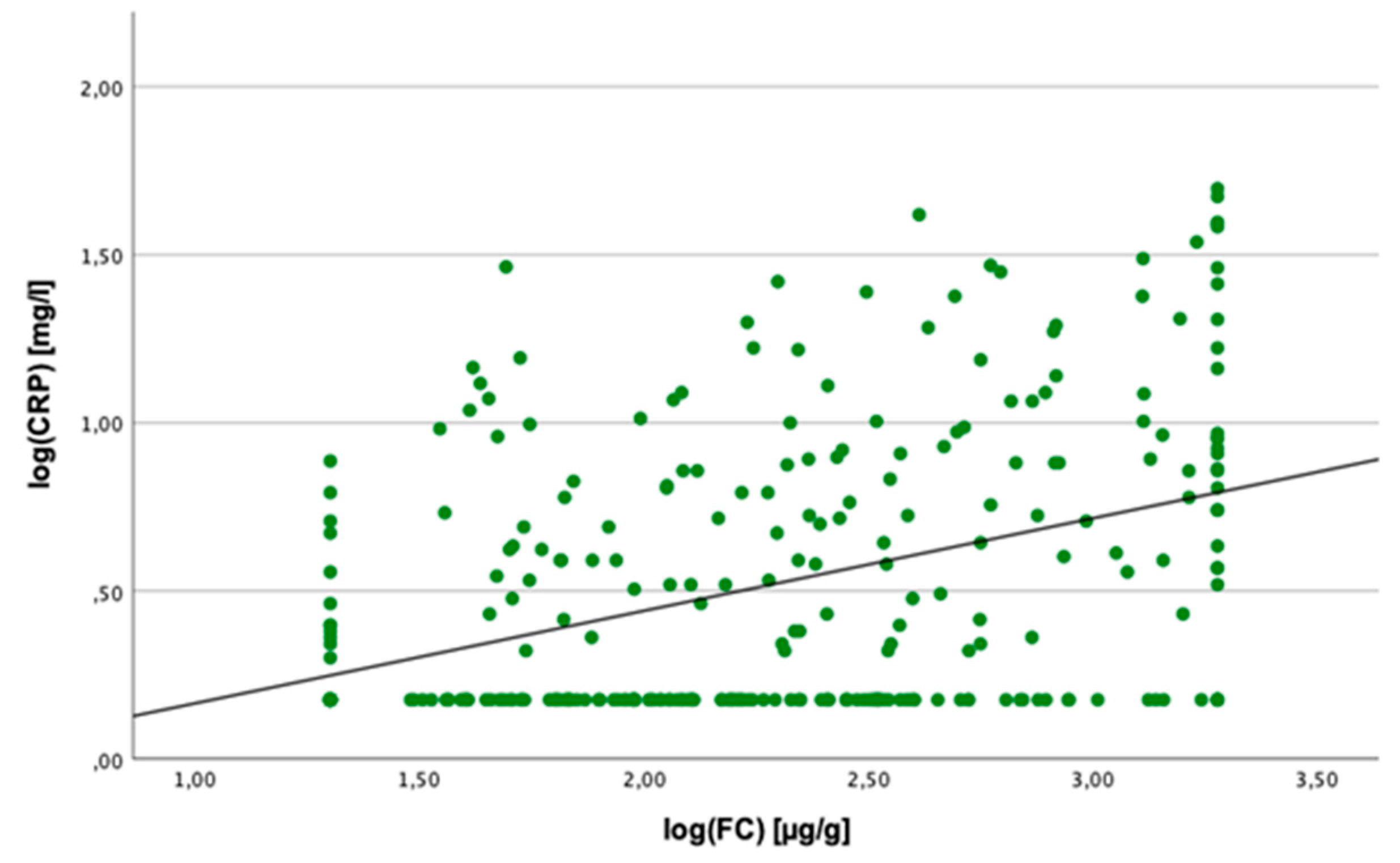
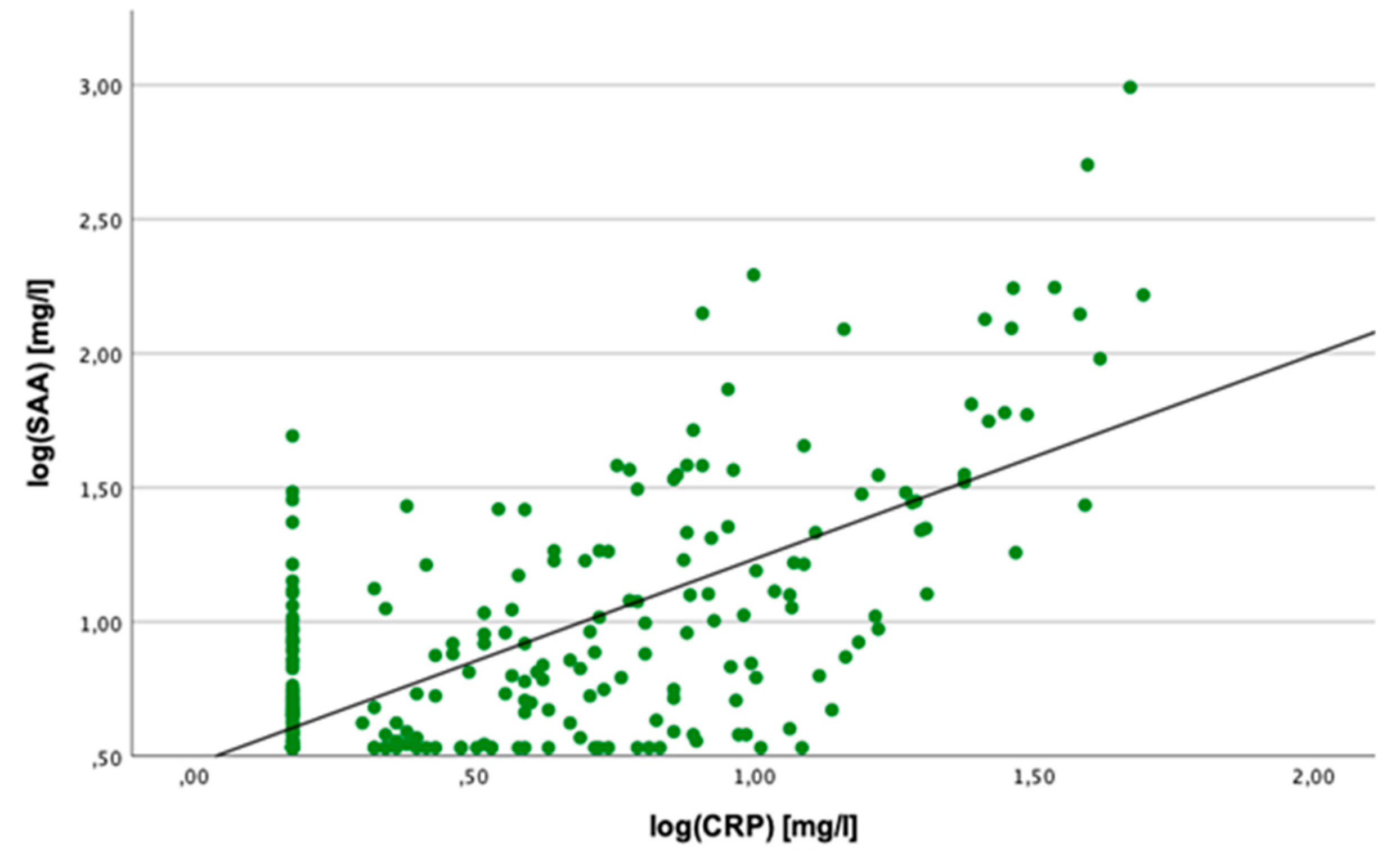
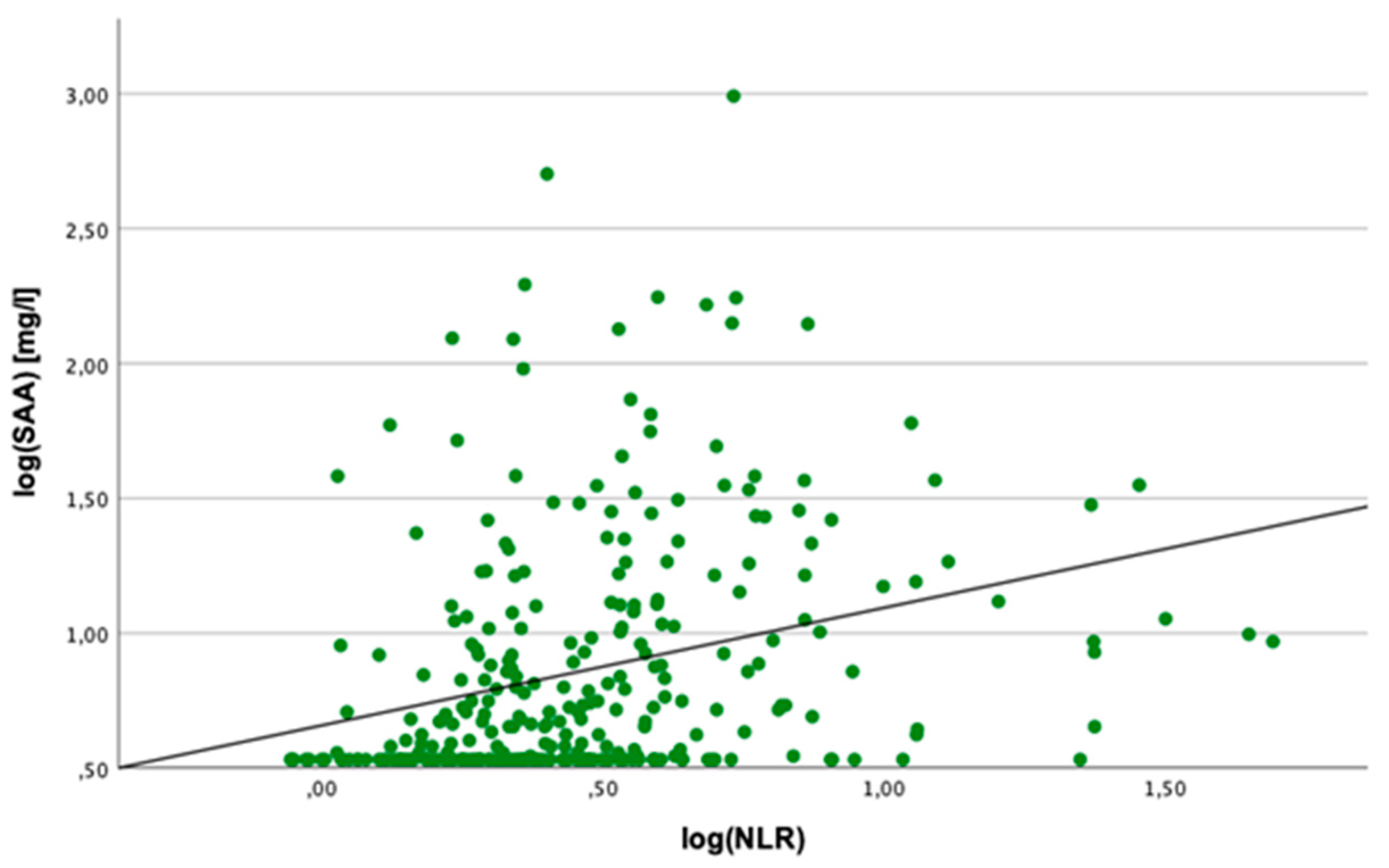
2.4. Cut-Off Value for SAA Concentration
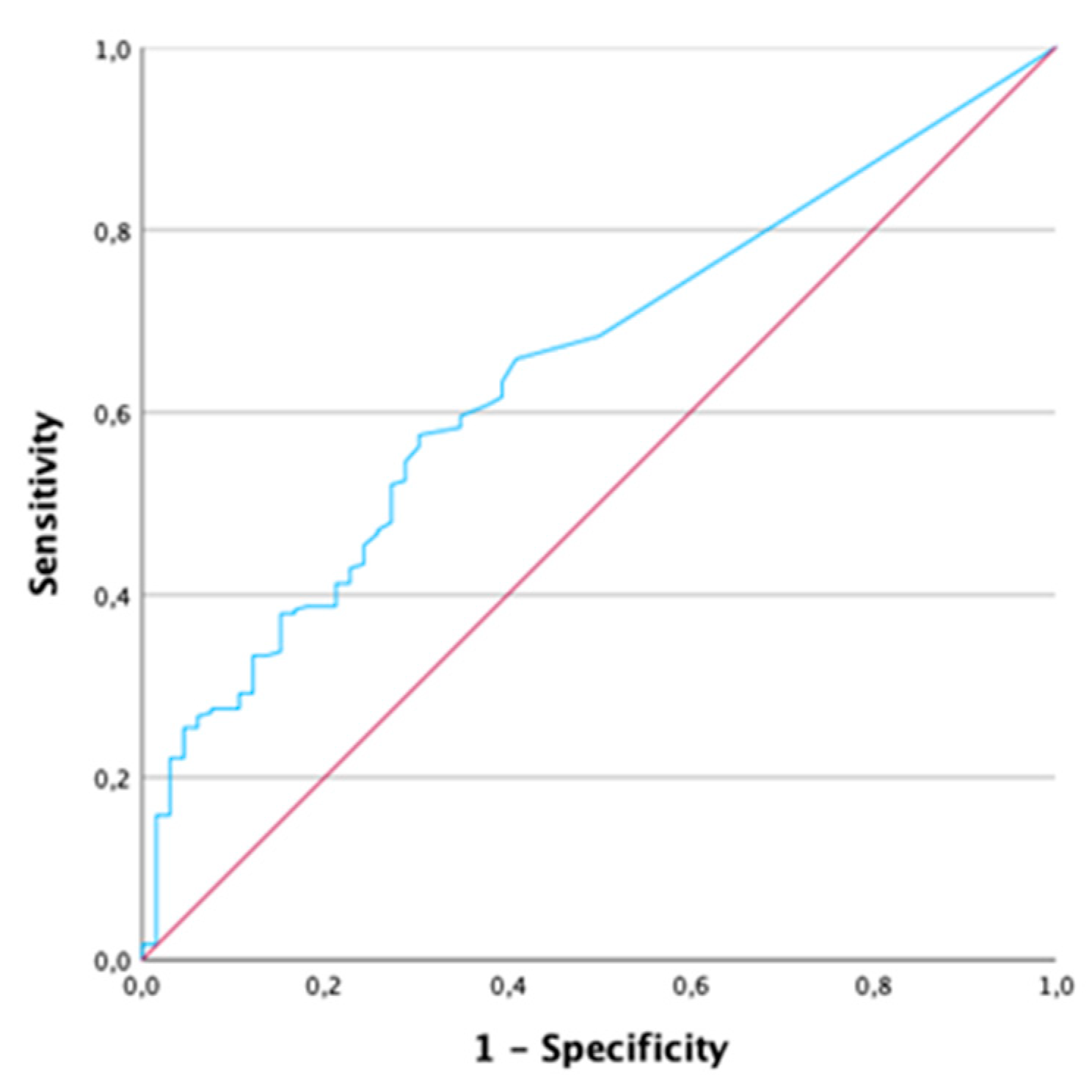
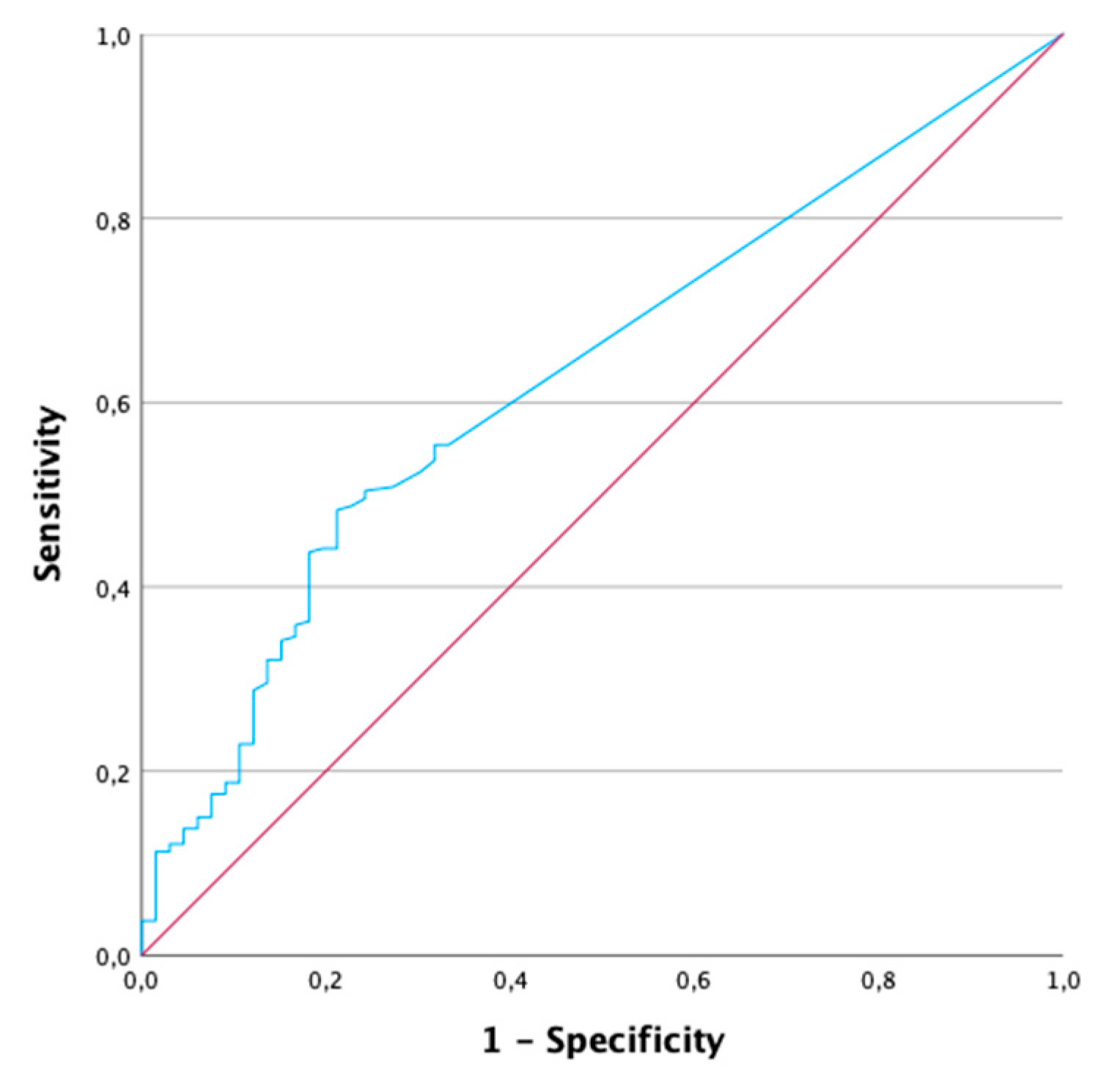
2.5. Patients with CRP < 5 mg/l
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Measurement of SAA and CRP Concentrations
4.3. Measurement of FC Concentrations
4.4. Assessment of Clinical Disease Activity
4.5. Montreal Classification
| Age at diagnosis | A1: < 17 years A2: 17 – 40 years A3: > 40 years |
| Location | L1: ileal L2: colonic L3: ileocolonic L4: isolated upper disease |
| Behaviour | B1: non-stricturing, non-penetrating B2: stricturing B3: penetrating |
| Extent | E1: Ulcerative proctitis E2: Left sided UC (distal UC) E3: Extensive UC (pancolitis) |
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wakai, M.; Hayashi, R.; Tanaka, S.; Naito, T.; Kumada, J.; Nomura, M.; Takigawa, H.; Oka, S.; Ueno, Y.; Ito, M.; et al. Serum amyloid A is a better predictive biomarker of mucosal healing than C-reactive protein in ulcerative colitis in clinical remission. BMC Gastroenterol. 2020, 20, 85. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Ferrante, M.; Magro, F.; Campbell, S.; Franchimont, D.; Fidder, H.; Strid, H.; Ardizzone, S.; Veereman-Wauters, G.; Chevaux, J.-B.; et al. Results from the 2nd Scientific Workshop of the ECCO (I): Impact of mucosal healing on the course of inflammatory bowel disease. J. Crohn’s Colitis 2011, 5, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Malter, L.; Hudesman, D. Disease monitoring in inflammatory bowel disease. World J. Gastroenterol. 2015, 21, 11246–11259. [Google Scholar] [CrossRef] [PubMed]
- Plevris, N.; Lees, C.W. Disease Monitoring in Inflammatory Bowel Disease: Evolving Principles and Possibilities. Gastroenterology 2022, 162, 1456–1475.e1. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhou, G.; Lin, J.; Li, L.; Zeng, Z.; Chen, M.; Zhang, S. Serum Biomarkers for Inflammatory Bowel Disease. Front. Med. 2020, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E. Biomarkers of Inflammation in Inflammatory Bowel Disease. Gastroenterology 2015, 149, 1275–1285.e2. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Yarur, A.J.; Quintero, M.A.; Jain, A.; Czul, F.; Barkin, J.S.; Abreu, M.T. Serum Amyloid A as a Surrogate Marker for Mucosal and Histologic Inflammation in Patients with Crohnʼs Disease. Inflamm. Bowel Dis. 2017, 23, 158–164. [Google Scholar] [CrossRef]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef]
- Ishihara, S.; Tada, Y.; Kawashima, K.; Kataoka, M.; Sonoyama, H.; Yamashita, N.; Oka, A.; Kusunoki, R.; Fukuba, N.; Mishima, Y.; et al. Serum amyloid A level correlated with endoscopic findings in patients with Crohn’s disease—Possible biomarker for evaluating mucosal healing. Dig. Liver Dis. 2018, 50, 553–558. [Google Scholar] [CrossRef]
- Singh, S.; Ananthakrishnan, A.N.; Nguyen, N.H.; Cohen, B.L.; Velayos, F.S.; Weiss, J.M.; Sultan, S.; Siddique, S.M.; Adler, J.; Chachu, K.A. ; AGA Clinical Guidelines Committee. Electronic address: clinicalpractice@gastro.org. AGA Clinical Practice Guideline on the Role of Biomarkers for the Management of Ulcerative Colitis. Gastroenterology 2023, 164, 344–372. [Google Scholar] [CrossRef]
- Hartigh, L.J.D.; May, K.S.; Zhang, X.-S.; Chait, A.; Blaser, M.J. Serum amyloid A and metabolic disease: evidence for a critical role in chronic inflammatory conditions. Front. Cardiovasc. Med. 2023, 10, 1197432. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; von Martels, J.Z.H.; de Vos, P.; Faber, K.N.; Dijkstra, G. Increased fecal calprotectin levels in Crohn’s disease correlate with elevated serum Th1- and Th17-associated cytokines. PLOS ONE 2018, 13, e0193202. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; von Martels, J.Z.H.; Gabriëls, R.Y.; Blokzijl, T.; Buist-Homan, M.; Heegsma, J.; Jansen, B.H.; van Dullemen, H.M.; Festen, E.A.M.; ter Steege, R.W.F.; et al. A Combined Set of Four Serum Inflammatory Biomarkers Reliably Predicts Endoscopic Disease Activity in Inflammatory Bowel Disease. Front. Med. 2019, 6, 251. [Google Scholar] [CrossRef]
- Colombel, J.-F.; Panaccione, R.; Bossuyt, P.; Lukas, M.; Baert, F.; Vaňásek, T.; Danalioglu, A.; Novacek, G.; Armuzzi, A.; Hébuterne, X.; et al. Effect of tight control management on Crohn's disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet 2017, 390, 2779–2789. [Google Scholar] [CrossRef]
- Roblin, X.; Serone, A.; Yoon, O.K.; Zhuo, L.; Grant, E.; Woo, J.; Liu, J.; Galien, R.; D’haens, G. Effects of JAK1-Preferential Inhibitor Filgotinib on Circulating Biomarkers and Whole Blood Genes/Pathways of Patients With Moderately to Severely Active Crohn’s Disease. Inflamm. Bowel Dis. 2021, 28, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Lobatón, T.; López-García, A.; Rodríguez-Moranta, F.; Ruiz, A.; Rodríguez, L.; Guardiola, J. A new rapid test for fecal calprotectin predicts endoscopic remission and postoperative recurrence in Crohn's disease. J. Crohn’s Colitis 2013, 7, e641–e651. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Liu, Y.; Zhu, L.; Xu, L.; Shen, H. Evaluation of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as potential markers for ulcerative colitis: a retrospective study. BMC Gastroenterol. 2022, 22, 485. [Google Scholar] [CrossRef]
- Cherfane, C.E.; Gessel, L.; Cirillo, D.; Zimmerman, M.B.; Polyak, S. Monocytosis and a Low Lymphocyte to Monocyte Ratio Are Effective Biomarkers of Ulcerative Colitis Disease Activity. Inflamm. Bowel Dis. 2015, 21, 1769–1775. [Google Scholar] [CrossRef]
- Kwon, J.H.; Im, J.P.; Ye, B.D.; Cheon, J.H.; Jang, H.J.; Lee, K.M.; Kim, Y.S.; Kim, S.W.; Kim, Y.H.; Song, G.A.; et al. Disease Phenotype, Activity and Clinical Course Prediction Based on C-Reactive Protein Levels at Diagnosis in Patients with Crohn’s Disease: Results from the CONNECT Study. Gut Liver 2016, 10, 595–603. [Google Scholar] [CrossRef]
- MacGregor, A.J.; Gallimore, J.R.; Spector, T.D.; Pepys, M.B. Genetic Effects on Baseline Values of C-Reactive Protein and Serum Amyloid A Protein: A Comparison of Monozygotic and Dizygotic Twins. Clin. Chem. 2004, 50, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A.; Maaser, C.; Calabrese, E.; Annese, V.; Fiorino, G.; Kucharzik, T.; Vavricka, S.R.; Verstockt, B.; Van Rheenen, P.; Tolan, D.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspects. J. Crohn’s Colitis 2019, 13, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohn's Colitis 2019, 13, 144–164K. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Spencer, E.A.; Colombel, J.-F.; Ungaro, R.C. Approach to the Management of Recently Diagnosed Inflammatory Bowel Disease Patients: A User’s Guide for Adult and Pediatric Gastroenterologists. Gastroenterology 2021, 161, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.; Bradshaw, J. A SIMPLE INDEX OF CROHN'S-DISEASE ACTIVITY. Lancet 1980, 315, 514–514. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, R.S.; Ayres, R.C.S.; E Pounder, R.; Allan, R.N. A simple clinical colitis activity index. Gut 1998, 43, 29–32. [Google Scholar] [CrossRef]
- Satsangi, J.; Silverberg, M.S.; Vermeire, S.; Colombel, J.F. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006, 55, 749–753. [Google Scholar] [CrossRef]
| Parameter | Cases (306) |
|---|---|
| Age (years), Median (Range) | 44 (18 - 77) |
| Female, n (%) | 159 (52.0) |
| Male, n (%) | 147 (48.0) |
| Crohn’s disease, n (%) | 182 (59.5) |
| Ulcerative Colitis, n (%) | 116 (37.9) |
| IBD unclassified, n (%) | 8 (2.61) |
| Disease duration at serum sample collection (years), Median (IQR) | 10 (16) |
| Montreal Classification (n,%) | |
| Age at diagnosis (MC and UC) (A1, A2, A3) | 25 (8.2), 212 (69.3), 69 (22.5) |
| Location (MC) (L1, L2, L3, L4) | 68 (37.4), 21 (11.5), 83 (45.6), 11 (6.0) |
| Behaviour (MC) (B1, B2, B3) | 69 (37.9), 64 (35.2), 63 (34.6) |
| Location (CU) (E1, E2, E3) | 12 (10.3), 44 (37.9), 64 (55.2) |
| History of resecting surgery for IBD, n (%) | 85 (27.8) |
| Extraintestinal manifestation(s), n (%) | 102 (33.3) |
| HBI/SCCAI (Mean) (SD) | 3.2 (3.6)/2.9 (3.0) |
| Biologic therapy at the time of sample collection, n (%) | 152 (49.7) |
| Number of days between FC and SAA determination, Mean (SD) | 3.7 (4.4) |
| N | Mean | Standard deviation | Minimum | Maximum | Percentile | |||
|---|---|---|---|---|---|---|---|---|
| 25 | 50 (Median) | 75 | ||||||
| FC concentration [µg/g] | 306 | 446.1 | 591.1 | 20.0 | 1900.0 | 54.5 | 170.5 | 529.8 |
| SAA concentration [mg/l] | 306 | 18.7 | 67.6 | 3.40 | 980.0 | 3.40 | 4.70 | 10.9 |
| CRP concentration [mg/l] | 306 | 5.69 | 7.98 | 1.50 | 49.7 | 1.50 | 2.10 | 6.40 |
| NLR | 303 | 4.07 | 5.33 | 0.88 | 49.2 | 1.89 | 2.70 | 3.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




