Introduction
Degenerative myxomatous mitral valve disease (DMVM) is the most common heart disease in small animals, accounting for around 75% of cases of heart disease in dogs [
9]. The prevalence of MVD increases markedly with age in small dogs, with up to 85% showing evidence of valve damage by 13 years of age [
3].
According to Petrus Gimenes and Mantovani, (2019), the proper functioning of the mitral valve is based on the structural and functional performance of six components: the posterior wall of the left atrium, the valve ring, the valve leaflets or cusps, the tendon chordae, the papillary muscles of the left ventricle and the left ventricular wall, each of these components plays an independent and synergistic role, contributing to complex functions that maintain valve competence. Any structural changes in the components of the mitral apparatus affect valve mechanics, compromising its efficiency.
The disease involves a gradual myxomatous degeneration over time with the disorganization of collagen bundles and a reduction in their content, excess production of glycosaminoglycan that results in a change in the valve structure leading to poor coaptation of the leaflets. Poor coaptation of the leaflets allows regurgitation of the mitral valve resulting in the murmur that is characteristic of the disease [
7].
According to the latest consensus published by ACVIM in 2019, for the diagnosis of the disease, an echocardiogram (ECHO) and chest x-ray in the absence of ECHO are recommended, however, caution should be taken due to the marked variation in thoracic conformation and racial differences in the vertebral cardiac scales. normal, the disease can also be recognized during a screening or routine examination by auscultation of a typical heart murmur when there is regurgitation of the mitral valve.
The main symptoms that the animal presents when it reaches the advanced stage of the disease are: Exercise intolerance, presence of dry cough, decreased appetite, difficulty breathing, and syncope are findings that have been associated with a worse prognosis in affected dogs [
6].
There are four basic stages of the disease, according to the classification system for treating dogs with DMVM published by Atkins et al. 2019, described below (
Table 1) [
9].
This work aims to report the case of an animal that was referred to a cardiologist in 2021, being diagnosed with Myxomatous Mitral Valve Disease in stage B2 and demonstrating the evolution of the disease to date.
Case Report
A Fox Terrier dog, male, 13 years old, neutered, weighing 12 kg, was treated at the Veterinary Clinic Animal Medical Center (AMC), located in Pouso Alegre - MG, Brazil, and was sent for cardiological evaluation. He consulted a general practitioner due to complaints of intense tremors, where a chest x-ray was requested. During this examination, an increase in the cardiac silhouette was observed in a topography corresponding to the left atrium, resulting in the patient being referred to a specialist.
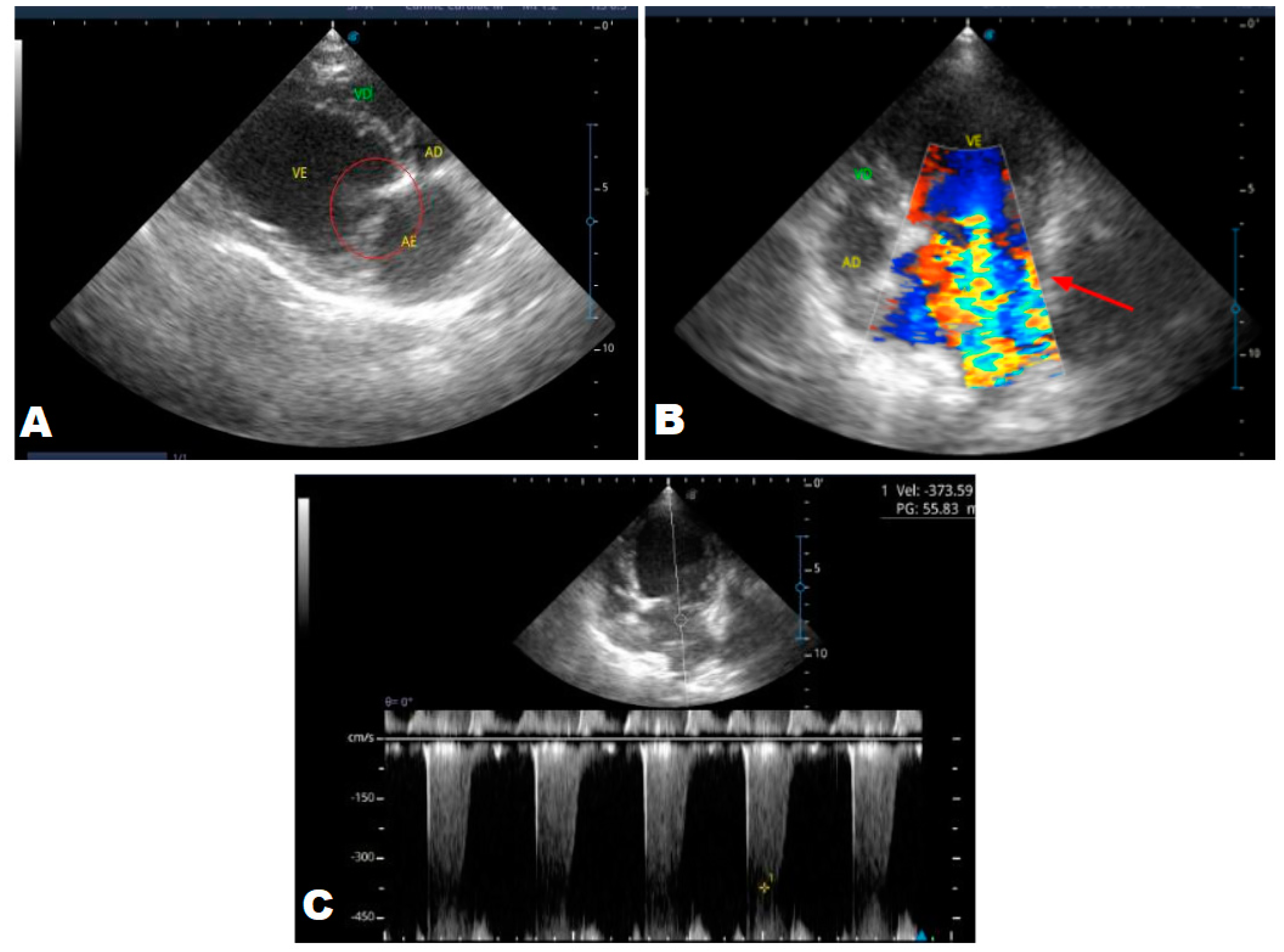
In the anamnesis, the owners reported that the animal never had coughs, syncope, or convulsions, just tremors since it was a puppy. On physical examination, a grade 3/6 murmur was heard in the mitral focus, normal heart and respiratory rates and systemic arterial hypertension (180mmHg), and normal-colored mucous membranes. An echocardiogram (ECHO) was performed (
Figure 1), which showed a thickened mitral valve and enlarged heart chambers. At first was prescribed Pimobendan PO at a dose of 0.25 mg/kg BID, continuous use, and requested to return in 30 days.
Upon return, the owners reported that the animal was more active and was not easily tired. His pressure had already decreased from 180 mmHg to 160 mmHg, he continued using pimobendan.
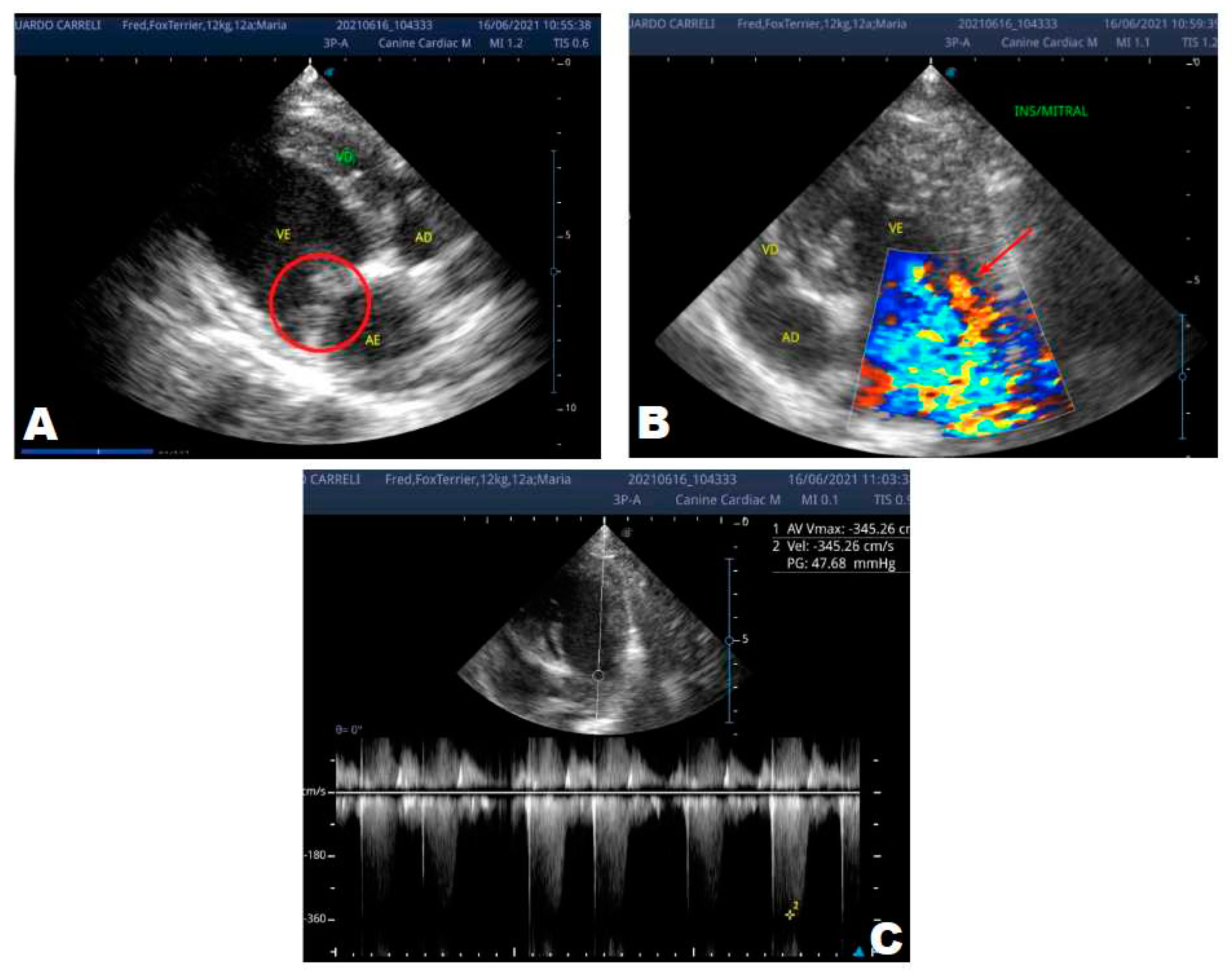
One year later, the animal returned for a new evaluation, repeating the ECHO (
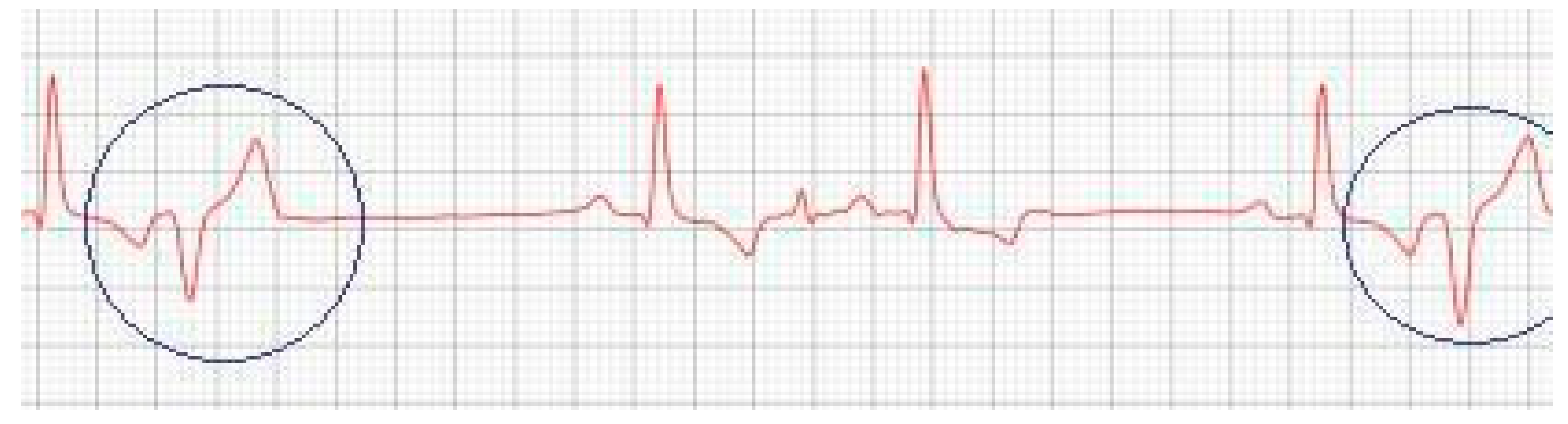
Figure 2), which showed the progression of the increase in the left atrium. The electrocardiogram (ECG) (
Figure 3) shows baseline sinus arrhythmia with the presence of a premature ventricular complex (
Figure 4). Owners reporting drowsiness, hyporexia, and syncope, however, did not present tiredness or cough. Blood pressure was 80 mmHg.
Due to the arrhythmia, Sotalol was prescribed at a dose of 25 mg/kg PO, BID for continuous use and Omega 3 PO at a dose of 600 mg/kg SID became necessary, and pimobendan continued.
Table 2.
Electrocardiographic report (2022).
Table 2.
Electrocardiographic report (2022).
| Observed parameters |
Observed parameters |
Observed parameters |
| QRS axis: 67.07 ° |
QT Interval: 210 ms |
Duration of T: 54 ms |
| P axis: 58.01 ° |
PR Interval: 108 ms |
QRS duration: 72 ms |
| Amplitude of S: -0.06 mV |
R amplitude: 2.26 mV |
Minimum HR: 49 bpm |
| PR Segment: 52 ms |
P amplitude: 0.22 mV |
Average HR: 95 bpm |
| ST segment: 84 ms |
T amplitude: -0.74 mV |
Maximum HR: 297 bpm |
| Duration of P: 56 ms |
|
|
Comments
Baseline sinus arrhythmia.
QRS axis within normal limits for the species. Episodes of the premature ventricular complex were observed.
An increase in the duration of the P wave and QRS complex was observed, suggestive of atrial and left ventricular overload.
Ventricular repolarization disorder was observed due to an increase in the amplitude of the T wave (> 25% of the R wave) being compatible with electrolyte changes and/or myocardial hypoxia.
Conclusions: Baseline sinus arrhythmia with the presence of premature ventricular complex |
At the beginning of 2023, the patient returned to repeat the cardiological evaluation. During the anamnesis, the Guardian reported that the patient did not present episodes of syncope and remained stable during this period, continuing the previously adopted treatment.
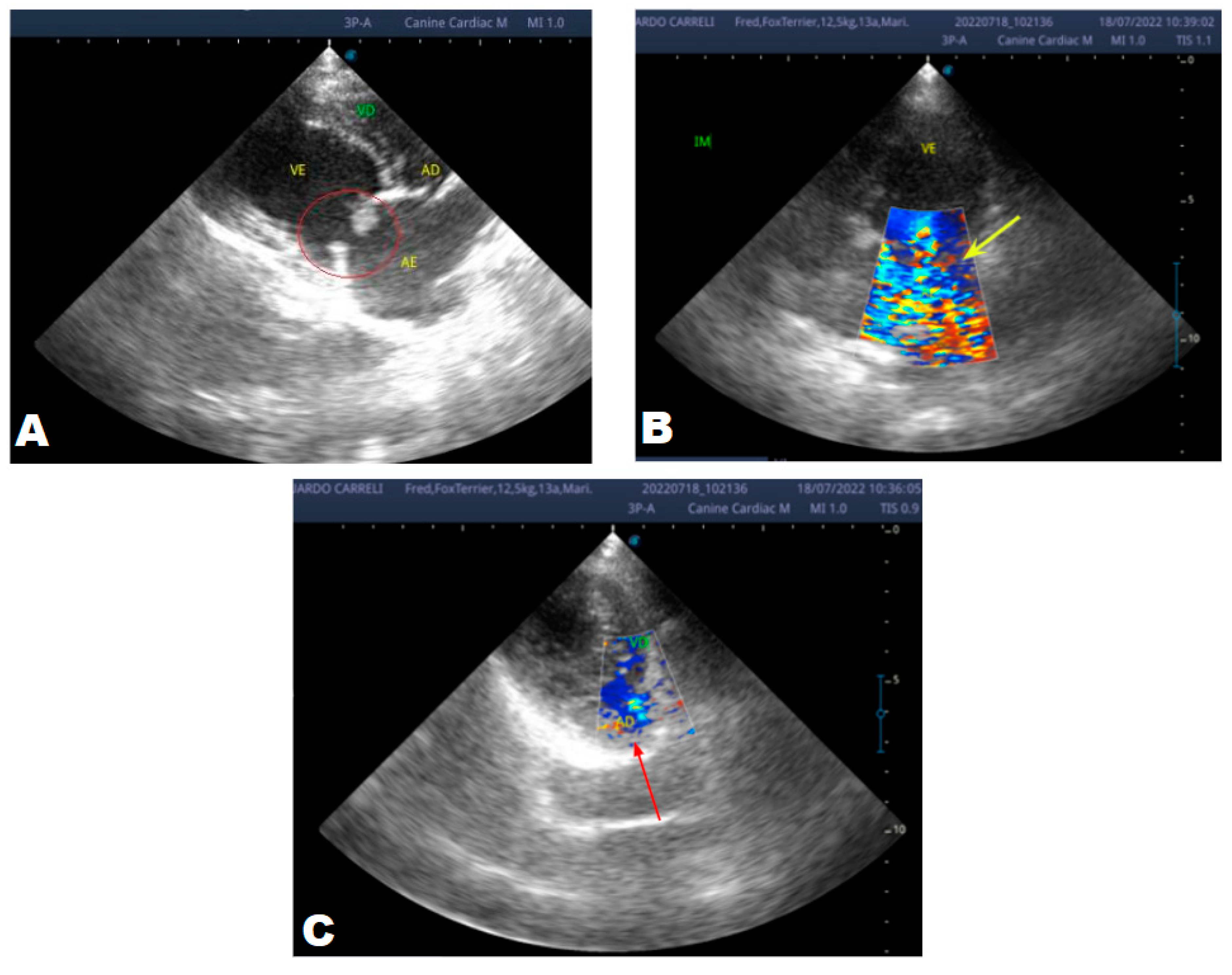
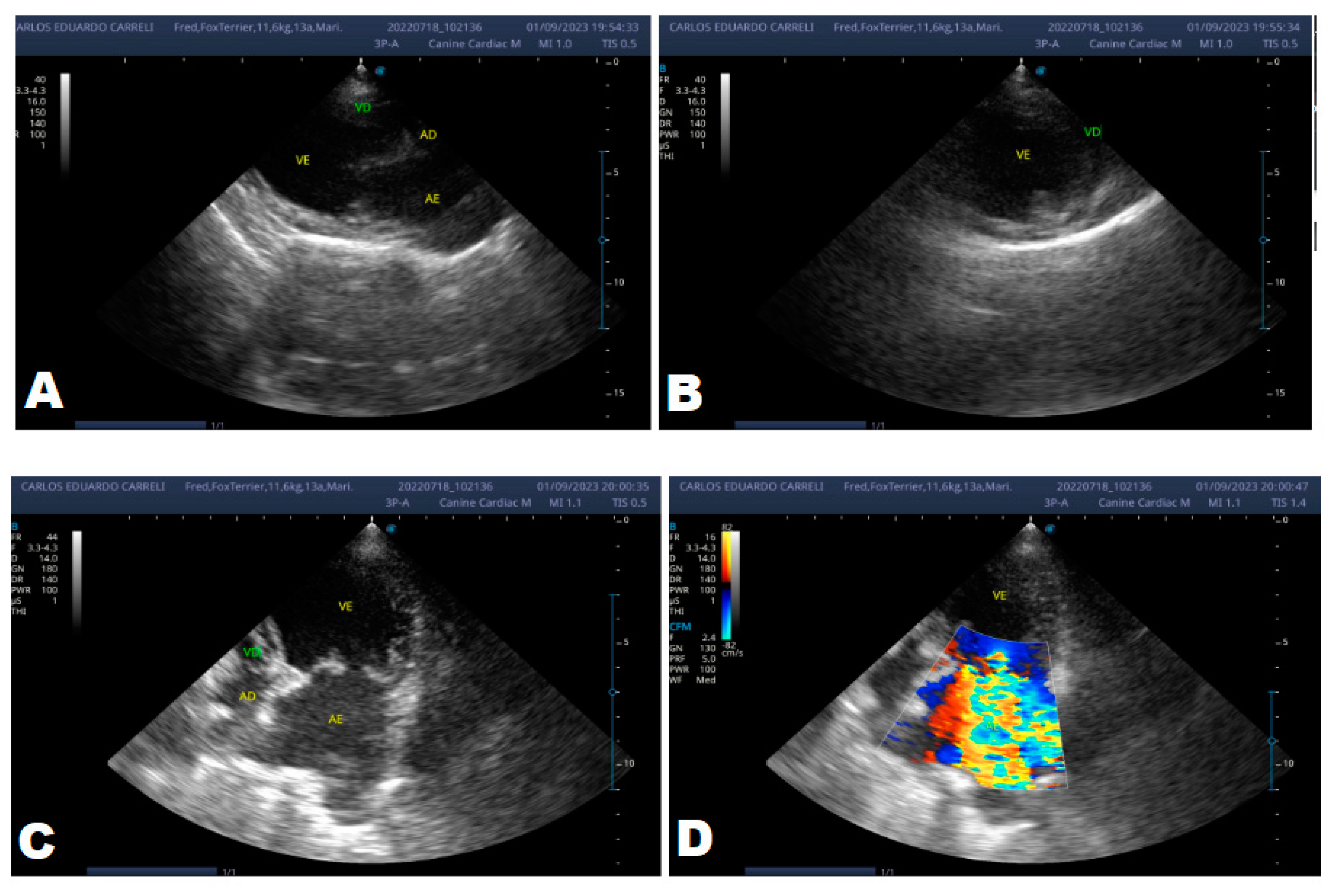
Figure 5.
Echocardiogram (January/2023): observed increase in the left atrium and ventricle; thickened/degenerated/prolapsed mitral valve (A); observed in Doppler study, systolic turbulent flow within the left atrium characterizing severe mitral valve insufficiency (B). Hemodynamic assessment - Maximum velocity gradient mitral regurgitation: 3.74 m/s / 55.83 mmHg: Observed left ventricular diastolic dimension above normal limits, with normal systolic function parameters, characterizing systolic dysfunction; preserved diastolic function.
Figure 5.
Echocardiogram (January/2023): observed increase in the left atrium and ventricle; thickened/degenerated/prolapsed mitral valve (A); observed in Doppler study, systolic turbulent flow within the left atrium characterizing severe mitral valve insufficiency (B). Hemodynamic assessment - Maximum velocity gradient mitral regurgitation: 3.74 m/s / 55.83 mmHg: Observed left ventricular diastolic dimension above normal limits, with normal systolic function parameters, characterizing systolic dysfunction; preserved diastolic function.
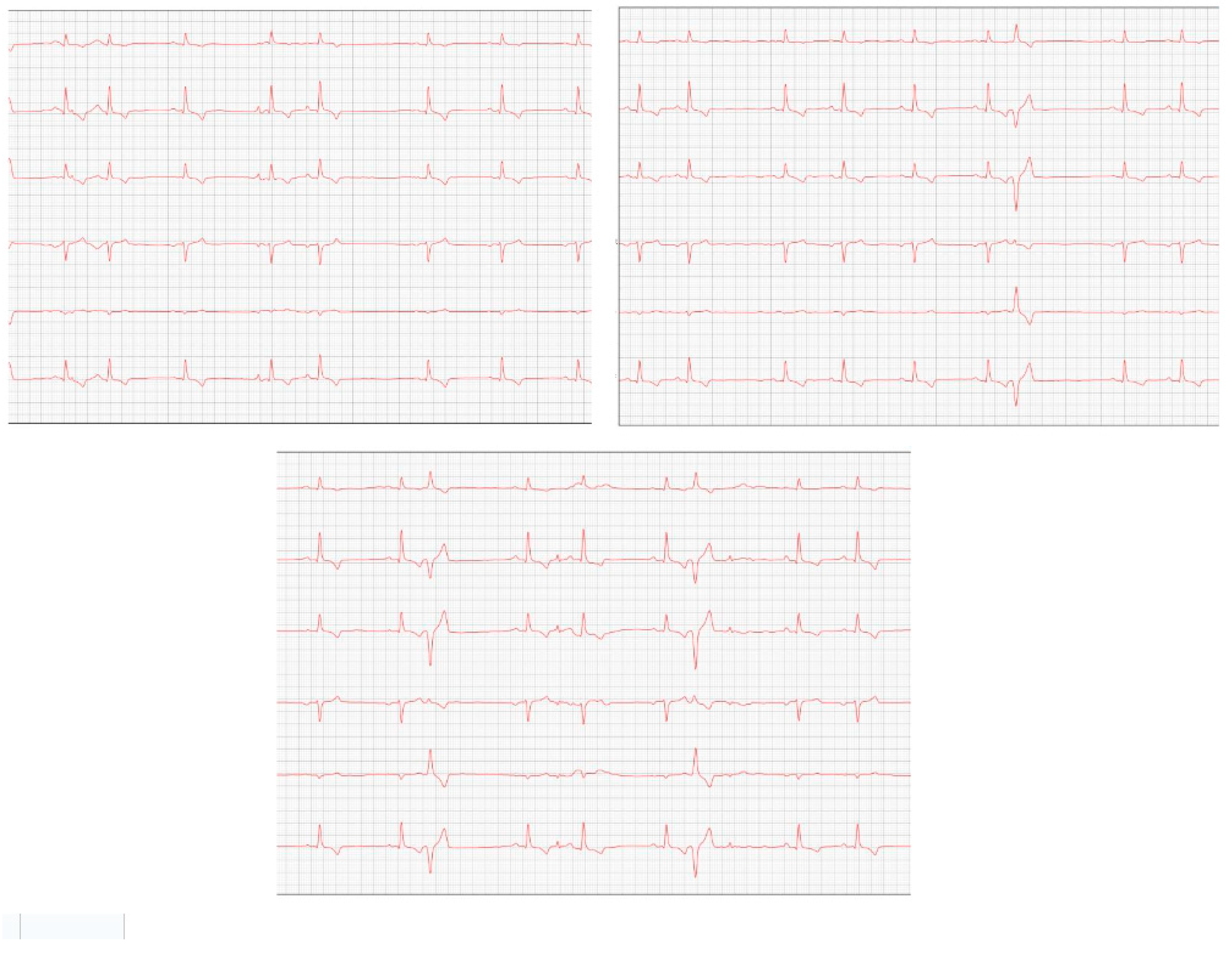
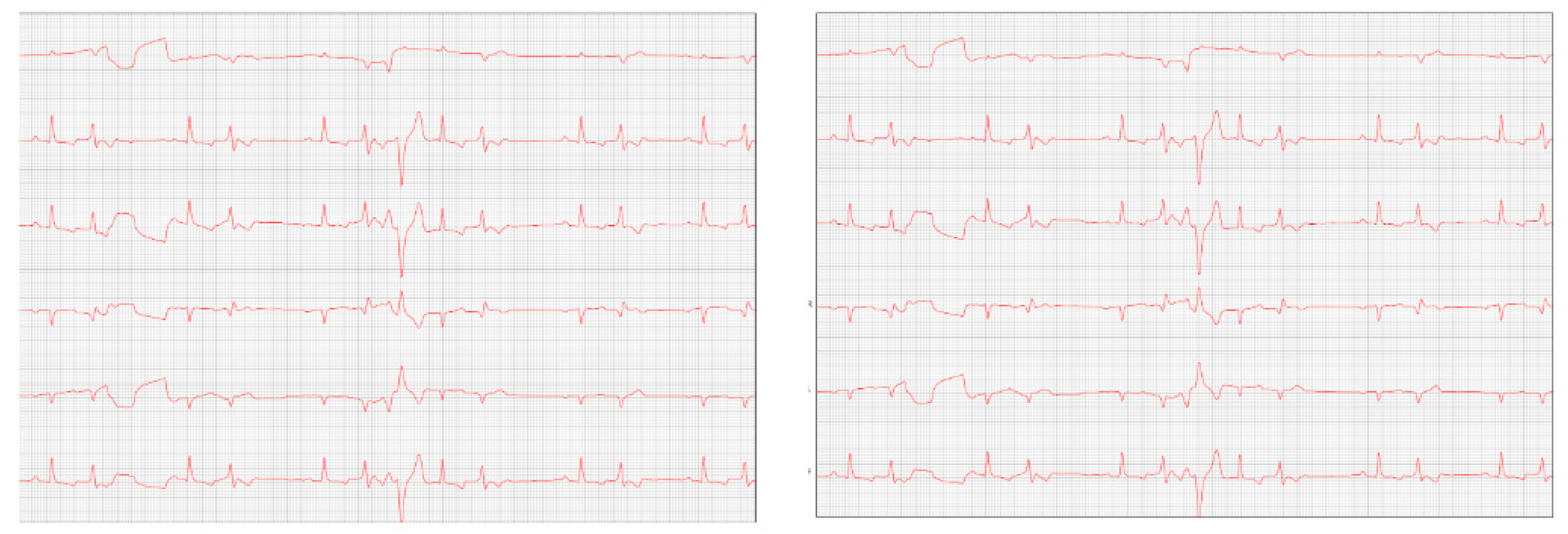
After eight months, the patient returned with a worsening condition, repeated ECHO (
Figure 6) and ECG (
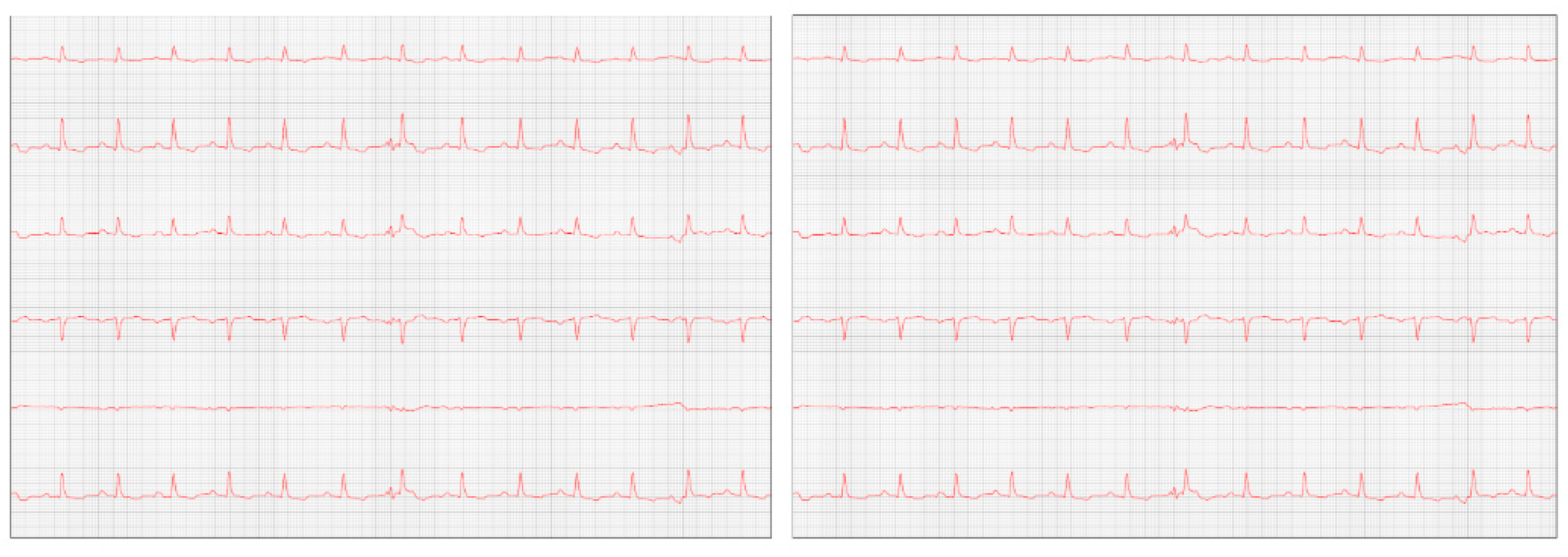
Figure 7) where diastolic dysfunction and worsening of the arrhythmia were observed. Amiodarone 8.7 mg/kg orally was prescribed, replacing sotalol, 1 tablet BID, and in addition, Furosemide (Lasix) 1.7 mg/kg orally was prescribed every 12 hours. Pimobendan was continued. After a week, a reassessment was carried out and the ECG demonstrated stability in the arrhythmia (
Figure 8).
Table 3.
Electrocardiographic report (September/2023).
Table 3.
Electrocardiographic report (September/2023).
| Observed parameters |
Observed parameters |
Observed parameters |
| Minimum HR: 71 bpm |
T duration: 68 ms |
S amplitude:: -0.01 mV |
| Average HR: 125 bpm |
QT interval: 196 ms |
T amplitude: -0.32 mV |
| Maximum HR: 400 bpm |
ST segment: 56 ms |
P axis: 83.89 º |
| P duration:: 48 ms |
P amplitude: 0.29 mV |
QRS axis: 81.32 º |
| QRS duration:: 72 ms |
R amplitude: 1.69 mV |
PR Interval: 104 ms |
Comments: Sustained ventricular bigeminy with clusters of ventricular trigeminy and sinus arrhythmia.
Duration and amplitude of the P wave and QRS complex with values within normal limits for the species, size, and age of the patient.
Normal electric axis.
Normal T wave.
Presence of polymorphic premature ventricular extrasystoles, isolated in pairs, in triplets, and at times organized into ventricular bigeminy and trigeminy.
Nothing else worth noting during the 4 minutes and 41 seconds of monitoring. |
|
Conclusions: Sustained ventricular bigeminy with clusters of ventricular trigeminy and sinus arrhythmia. |
Discussion
In the reported case, the patient arrived at the clinic in stage B2, where he was asymptomatic and the echocardiographic examination showed enlargement of the left heart chambers, thickened and degenerated mitral valve (
Figure 1), left ventricular diastolic dimension above normal limits. , with normal systolic function parameters, characterizing systolic dysfunction. The thickening and degeneration of the mitral valve indicates its insufficiency and the other changes characterize diastolic dysfunction.
Diagnosed with degenerative myxomatous mitral valve disease, treatment with pimobendan began. According to Boswood et. al. (2016), its mechanism of action includes a positive inotropic combination and balanced vasodilation, caused by calcium sensitization and phosphodiesterase inhibition, as a result, the effects of pimobendan may include increased cardiac output (CO), myocardial contractility and decreased preload and afterload.
In 2022, one year after the diagnosis, the animal returned for annual control exams. The ECHO showed an increase in the left atrium and ventricle, thickened/degenerated mitral valve (
Figure 2), preserved diastolic function, and low probability of pulmonary hypertension; the ECG detected an increase in the duration of the P wave and the QRS complex (
Figure 3), suggestive of atrial and left ventricular overload, baseline sinus arrhythmia with the presence of a premature ventricular complex.
In most cases, cardiac arrhythmias are not clinically relevant unless they are associated with heart disease or cause an extreme change in heart rate, being very slow, fast, or irregular. [
10]. Choosing a medication for treatment must consider some things, such as clinical status, associated cardiac or systemic comorbidities, and drug association [
13]. Class III antiarrhythmics block potassium channels, preventing a new action potential from occurring before complete repolarization, causing a prolongation of this potential and the refractory period [
5].
To control the arrhythmia presented by the animal with DMVM, sotalol was prescribed. According to Treseder et al. (2019), sotalol is an antiarrhythmic agent commonly used in human and veterinary medicine to control ventricular and supraventricular arrhythmias. Its antiarrhythmic properties are well established and are largely attributed to the blockade of potassium channels with concomitant non-selective β-adrenergic blockade.
In 2023, the animal returned and repeated only the ECHO (
Figure 5), where no significant changes were observed during this period, except for a structural change in the mitral valve, identifying a prolapse and increase in the left ventricular diastolic dimension, with systolic function parameters. normal, characterizing systolic dysfunction; diastolic function remained preserved.
Petrus, Gimenes, and Mantovani (2019, p. 156), report that mitral valve prolapse is a common complication that generally occurs with the progression of MVD. As MVD progresses, mitral valve regurgitant flow increases, promoting volume overload in the left atrium, with an increase in filling pressures in the left heart chambers. There is also progressive cardiac remodeling, characterized by eccentric hypertrophy, initially in the left atrium and, later, in the left ventricle. Systolic dysfunction occurs in the advanced stages of MVD, which culminates in hemodynamic changes and progressive diastolic dysfunction of the left ventricle, affecting general cardiac performance, and determining the appearance of congestive heart failure syndrome.
In the same year, eight months later, the patient returned with a worsening condition, presenting pulmonary edema. With these changes, the animal's condition progressed to stage C, requiring repeat echocardiographic and electrocardiographic examinations.
The ECHO (
Figure 6) showed an increase in the cardiac chambers as well as diastolic dysfunction when compared to the previous exam. The ECG (
Figure 7) revealed polymorphic premature ventricular extrasystoles. Due to this change, sotalol was replaced by amiodarone.
Amiodarone is also a class III antiarrhythmic, being the most indicated in these cases as it is considered broad spectrum, as it can be used in supraventricular and ventricular arrhythmias [
5]. However, its use should be reserved for cases where the animal no longer responds to sotalol treatment, due to its various side effects [
10]. Its most common adverse effects are liver changes, causing an increase in the concentration of gastrointestinal and enzymes, such as vomiting, diarrhea, and anorexia [
11]. Normally, these effects are dose-dependent and reversible if their supply is interrupted at the beginning of clinical manifestations [
10].
For pulmonary edema, furosemide (Lasix) was prescribed. Furosemide is a loop diuretic widely used in veterinary clinics. The Food and Drug Administration (FDA) has approved furosemide to treat conditions with volume overload and edema secondary to exacerbation of congestive heart failure, liver failure, or renal failure, including nephrotic syndrome [
4]. Its mechanism of action is to inhibit the tubular reabsorption of sodium and chloride in the proximal and distal tubules and in the thick ascending loop of Henle, inhibiting the sodium chloride cotransport system, resulting in excessive excretion of water along with sodium, chloride, magnesium, and calcium [
4].
One week later, the patient returned for a reevaluation of the ECG (
Figure 8), which demonstrated stability in the arrhythmia.
In the year 2023, there were some complications regarding the patient's treatment. The owner reported that he was no longer able to administer the medications as recommended, providing pimobendan every 24 hours or at a longer interval. Regarding other medications, it was said that the animal accepted it more easily, but was unable to administer it at the correct time, which may justify the worsening of the condition in a short period. The animal is currently stable and continues to receive monitoring.
Conclusion
Myxomatous mitral valve degeneration is a progressive disease and responsible for the largest number of heart diseases in small animals, especially in small dogs. We observed that the time taken for the disease to progress from stage B2 to C was two years, with correct monitoring and treatment reducing its progression over a while. In this case, medications were extremely important to keep the animal's condition stable.
Author Contributions
Investigation: All authors; Data curation: K.S., M.S. and C.C.; Writing—original draft: K.S. and M.S.; Writing—review and editing: All authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Written informed consent was obtained from the owner of the animal.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Al-Mosawi, A. Pharmacological Treatment of Chronic Symptomatic Premature Ventricular Contractions: An Educational Article and Expert Opinion. Journal of Medical and Clinical Studies.; 6; 2582-0869, 2023. [CrossRef]
- Boswood, A, et al. Effect of Pimobendan in Dogs with Preclinical Myxomatous Mitral Valve Disease and Cardiomegaly: The EPIC Study—A Randomized Clinical Trial. Journal of Veterinary Internal Medicine, 30, 1765-1779, 2016. [CrossRef]
- Keene, BW, Atkins, CE, Bonagura, JD, et al. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J Vet Intern Med.; 33: 1127–1140, 2019. [CrossRef]
- Khan, Tahir; Patel, Roshan; Siddiqui, Abdul. Furosemide. Study Guide from StatPearls Publishing, Treasure Island, 2018.
- Larsson, Maria Helena Matiko Akao. Medicamentos antiarrítmicos. In: SPINOSA, Helenice de Souza; Górniak, Silvana Lima; Bernardi, Maria Martha. Farmacologia: Aplicada à medicina veterinária. 7. ed. Rio de Janeiro: Guanabara Koogan Ltda, 6, 382-388, 2023.
- Menciotti, Giulio; Borgarelli, Michele. Review of diagnostic and therapeutic approach to canine myxomatous mitral valve disease. Journals Veterinary Sciences, 4, 2017. [CrossRef]
- McNair AJ, Markby GR, Tang Q, MacRae VE, Corcoran BM. TGF-β phospho antibody array identifies altered SMAD2, PI3K/AKT/SMAD, and RAC signaling contribute to the pathogenesis of myxomatous mitral valve disease. Front Vet Sci. Oct 16;10:1202001, 2023. [CrossRef]
- Pinkos A, Stauthammer C. Degenerative Valve Disease: Classification, Diagnosis, and Treatment of Mitral Regurgitation. Today's Veterinary Practice, 2021.
- Petrus, Lilian; Gimenes, André; Mantovani, Matheus. Degeneração mixomatosa Valvar. In: LARSSON, Maria. Tratado de cardiologia de cães e gatos. 9, 155-170, 2019.
- Riviere, Jim E.; Papich, Mark G. Adams Booth. Farmacologia e Terapêutica Veterinária. Rio de Janeiro, Grupo GEN, 2021.
- Tárraga, Kátia Mitsube. Medicamentos antiarrítmicos. In: SPINOSA, Helenice de Souza; Góniak, Silvana Lima; Bernardi, Maria Martha. Farmacologia: Aplicada à medicina veterinária. 6. ed. Rio de Janeiro: Grupo GEN. 6, 503-512, 2017.
- Treseder, J.R.; LeBlanc, N.L. Scollan, K.F. Inotropic and chronotropic effects of sotalol in healthy dogs. Journal of Veterinary Cardiology. 25,14-24. 2019. [CrossRef]
- Yamaki, Fernanda Lie; Pessoa, Rebecca Bastos; Larsson, Maria Helena Matiko Akao. Sistema cardiovascular: Arritmias cardíacas. In: Jericó, Márcia Marques; Neto, João Pedro de Andrade; Kogika, Márcia Mery. Tratado de medicina interna de cães e gatos. 2. ed. Grupo GEN. 14, 1212-1237, 2023.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).