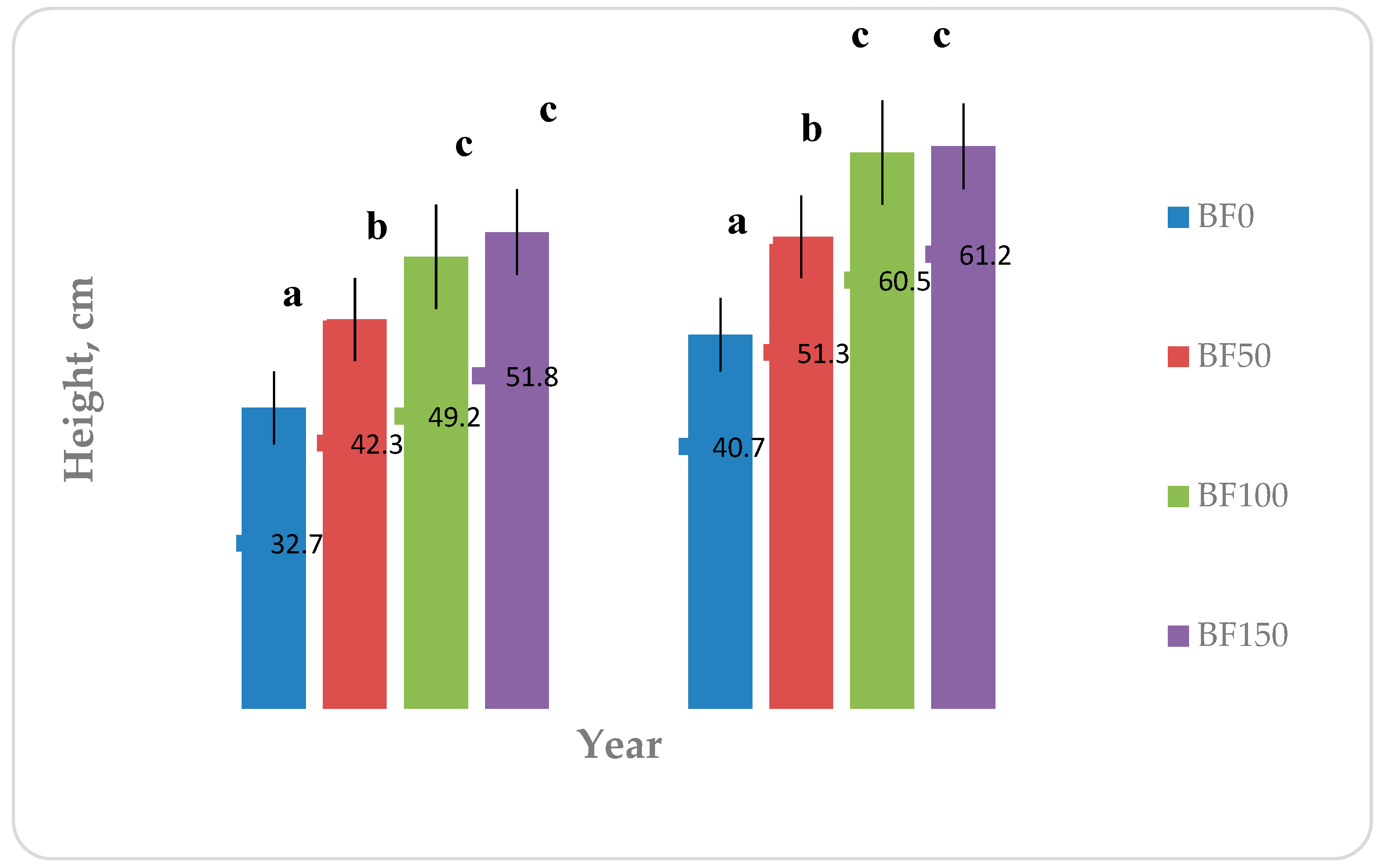1. Introduction
Greece is considered one of the most biologically diverse countries of the European continent with high plant diversity and endemism. Greece accounts for 6% of the Mediterranean area and 26% of its flora. Medicinal and aromatic plants play a crucial role in sustainable development, environmental protection, and human health [
1,
2]. There's a plant called
A. citrodora Paláu. with important health benefits and ecosystem services.
A. citrodora, more specifically, originated in South America [
3] and was introduced to Europe by the Spaniards. Today, it is cultivated in many Latin and Central American countries, southern European countries, such as Greece and France, northern Africa (Algeria and Morocco), China, and Iran. It is used as both a culinary and a medicinal herb. Alloysia is a genus in the
Verbenaceae family, which contains many species and genera. Botanical synonyms include
Aloysia triphylla (L'Hér.) Britton,
Lippia citriodora Kunth,
Lippia triphylla (L'Hér.) Kuntze,
Verbena triphylla L'Hér. and
Zappania citrodora Lam [
4]. There are many medicinal and aromatic properties associated with this shrub. Alloysia is an evergreen perennial shrub, a deciduous sub-shrub, and there are about 2300 species in the genus Alloysia. A warm, moist environment with plenty of sunlight is ideal for it. It becomes deciduous when exposed to frost.
A. citrodora prefers light, sandy, medium loamy, well-drained acid, neutral, and basic alkaline (pH of 4.5–7.8.) soils [
5,
6] and has an annual water requirement of 500 to 1.300 mm. The lemon-scented essential oil from the
A. citrodora have been widely studied for its calming, digestive, abdominal-discomfort, lemony flavour properties [
5,
6,
7].
A. citrodora traditionally has been utilized as a remedy for gastrointestinal and respiratory disorders. Besides antimalarial properties, some species also possess antiviral, antispasmodic, antibacterial, antioxidant, and cytostatic properties [
8,
9,
10,
11].
A. citrodora leaves can be used as an ingredient in stews and soups as well as fresh leaves [
12]. Several studies have demonstrated that lemon verbena's related curative properties are due to essential oils and flavonoids [
13]. Approximately 0.22% to 1.00% of
A. citrodora leaves are hydrodistilled to yield the essential oil. Volatiles are influenced by harvesting season, time of day, and particle size [
14]. The literature indicates that in Greece more attention is paid to the essential oil of
A. citrodora, while their effects on parameters influencing ecosystem structure (such as biodiversity, soil properties, etc.) are neglected.
Nitrogen is an essential element which applied to the agricultural fields to increase the total crop biomass [
15]. Synthenic fertilizers can provoke pollution and contamination of soil and waters [
16,
17]. At present there is the trend of using biological fertilizer instead of chemical ones. The organic fertilizers contains bacteria and fungus that can help to nutrients’ uptake by plants and can also, provoke a significant quantitative and qualitative crop production increase [
18].
Consumers’ concern about food security has led to be prompted chemical free products by the farmers. Biofertilizers are able to motivate the useful elements for the plants, improve the nutrients’ availability supply and enhance the nutrient process in soil through biological actions. The mechanisms of bioferitizers’ fuction are direct or indirect. The fisrt ones affect the growth of plants directly by nitrogen fixation, phosphate solibilazation etc. The second ones protect the plants from the harmful effects of pathogens [
16,
19,
20,
21].
According to literature the knowledge of the application of a biological fertilizer recommendation program in
A. citrodora cultivation is lacking [
22]. Most of the published papers are mentioned to the determination of its chemical oil composition [
23,
24,
25,
26,
27].
For the above purpose the aim of this study was to evaluate the use of a biofertilizer in different doses on agronomic characteristics and herbaceous plant diversity of A. citrodora in central Greece.
2. Materials and Methods
2.1. Study area
The field experiments were established in 2014 using
A. citrodora cuttings which were obtained from a local nursery. Two growing periods (2014 and 2015) of
A. citrodora plants were studied. The experiments conducted in the Velestino city (Volos, Magnesia). The studied area is located at an altitude of 120 m above sea level (
Figure 1). The area has a Mediterranean climate with hot, dry summers and cool, humid winters. There is a high amount of calcium in this clay loam soil as well as good drainage [
28].
2.2. Soil analysis
The 1st year two soil samples, from two depths (0-30 and 30-60 cm) were collected using the proper soil sampler. The 2nd year one soil sample was collected from one depth (0 – 40 cm). Each soil sample was consisted from five soil subsamples. The soil samples were transported to the Soil laboratory of the Institute of Industrial and Forage Crops (Larissa), air-dried and sieved using a 2-mm sieve. Soil samples were analyzed for pH (1:2.5 d. H
2O), electrical conductivity (1:5 d. H
2O), calcium carbonate (CaCO
3) using a calcimeter, the percentage (%) of sand, clay and silt using the Bouyoukos method and organic matter with Walkley – Black method , Available P (Olsen method, analyzed with ammonium vanadomolybdate / ascorbic blue and measured in a UV spectrophotometer at 882 nm) and Exchangeable Κ (1:10 at 1M CH
3COONH
4 pH 7, analyzed in a flame photometer) according to Rowell (1994) [
29].
The soil was loam with pH 7.6, 1.8% organic matter and 11.5 – 12% CaCO
3. The physicochemical properties of the soil are presented in
Table 1.
2.3. Meteological data
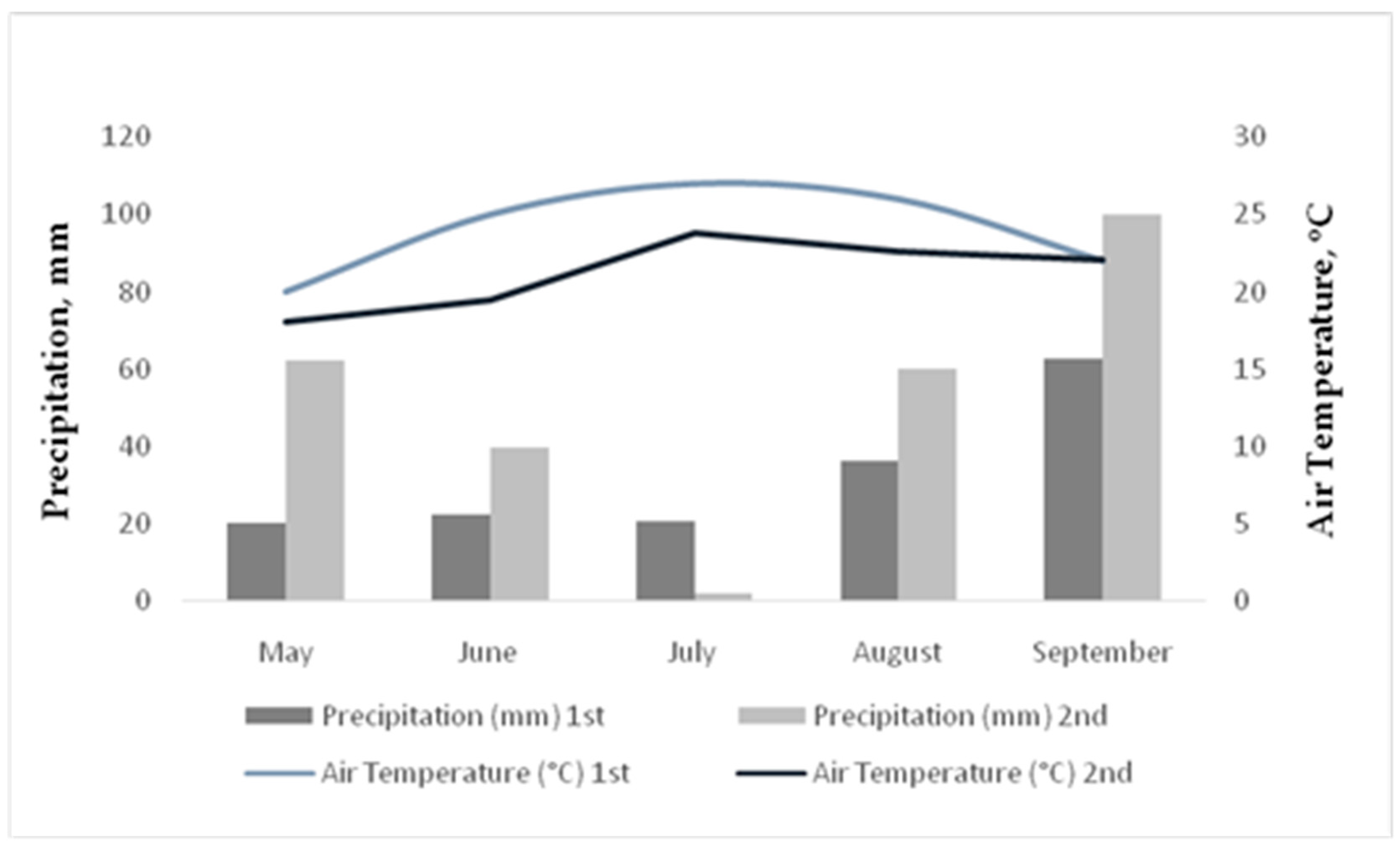
The meteorological data are presented in
Figure 2. Total precipitation levels were 162.3 mm and 264.5 mm from May until September the 1st and 2nd year, respectively. The higher rainfall events were on May 2015 (62.5 mm) and on September 2015 (100 mm). The average temperature was at least 3
oC higher the 1nd year compared to 2st year.
2.4. Field experiment
A two – year field experiment was established and the experimental design was a split-plot based on randomized complete blocks. Before the transplant the plants was raised for three months in polyethylene bags. The transplant was carried out on 3rd May 2014 in rows with spacing of 60 cm and 60 cm and each plot had 18 plants. The experiments included four nitrogen treatments of a biological fertilizer (6-0.5-0.3), BF0: 0 kg ha-1, BF50: 50 kg ha-1, BF100: 100 kg ha-1 and BF150: 150 kg ha-1. The fertilizer was manually applied at the base of plants. The composition of the biological fertilizer was: 85% organic matter, 6% total N, 0.5% P2O5 and 0.3% K2O (named Biosol). Also, during the experiments the plants were watered according to their needs so that the plots maintain their moisture level constant, using the drip irrigation system.
In total, the treatments were 12 with three replicates each and a total of 216 plants were transplanted in field.
Plant height, dry stem weight, dry leaves weight, dry total yield and Leaf Area Index were measured during the two growing years. The plant height of each plot was calculated from the average of five randomly plants. Then, these five plants were harvested by hand at 10 cm above the soil surface and immediately were weighted to record the fresh total weight, using a mobile balance. Furthermore, the plants were separated into leaves, flowers and stems and each edible part was weighted. Then, the plants were transported to the Lab for further measurements. Plant tissue of each plot was dried at 40oC until constant weight. Each plant part and the total biomass were weighted so that the dry weight to be calculated.
LAI measurements were performed during three different cutting periods from two plants of every different plot. Leaf area index was determined using an automatic LI-COR (model LI-3000A). The measured agronomic data are summarized in
Table 2.
2.5. Sampling of herbaceous plants
A sampling of herbaceous plants was conducted during May – June in 12 plots of 0.25 m
2, every year. In each plot, herbaceous plant species richness and density in an organic
Aloysia citrodora were recorded [
30]. Also, 12 soil samples, were collected using the proper soil sampler. The physicochemical properties of the studied soil samples for the two years are presented in
Table 3 (2014) and
Table 4 (2015).
2.6. Statistical analysis
Species Diversity and Richness IV software was used to calculate the Shannon plant diversity index [
30]. The Shannon index (SH) index takes into account both species abundance and species richness and it is the most commonly used index [
31,
32]. The SH is calculated for any sample population as follows:
where, s equals the number of species and pi is the relative cover of its species [
33,
34] (for a detailed description for this index, see Seaby and Henderson [
30]). Correlations between the measured variables (soil organic matter (%), pH, CaCO
3, texture-clay, silt, sand, P, K and Mg) and SH in an organic
L. citriodora were analyzed using Pearson correlation coefficients. Before the calculation of the Pearson correlation matrix, the data had to be transformed to logarithms to conform to the normal distribution criterion.
All statistical analyses for the data were performed using the software package Statgraphics plus 18 to the LSD test about the level of significance 95% (p<0.05).
3. Results
3.1. Plant height
Figure 3 shows the results regarding the height of the plants as they were measured during the harvest on 29/09/2014 and the second final harvest on 29/08/2015. As shown in
Figure 3, the maximum height of the plants during the 1st year and 2nd year reached a value of 51.8 and 61.2 cm in BF150 plots. Fertilization seems to have had a statistically significant effect in the 1st and 2nd year of establishment. Specifically, both in two cultivation years between the BF0 and the other treatments there was statistically significant difference. Furthermore, statistically significant difference was observed among BF50 and BF100 and BF150 fertilization. Furthermore, the 2nd year was noticed an increase in plant height of BF150 compared to BF100 (1.09%).
3.2. Dry plant biomass
Table 5 shows the dry weight results of the steams, leaf and the total dry biomass, as measured in 2014 and 2015. In 2014 three cuttings of plants took place on 30/06/2014, 28/07/2014 and 29/09/2014, specifically 57, 85 and 146 days after planting. In 2015 plants harvested three times on 20/05/2015, 2/07/2015 and 29/08/2015, 382, 424 and 511 days after planting. In 2014 the maximum amount of dry stem (955 kg ha
-1), dry leaf (990 kg ha
-1) and total dry weight (1945 kg ha
-1) was observed in the BF150 fertilization. In 2015, the fertilization that gave the largest amount of dry total weight was the BF150 treatment, with yield of 3935.83 kg ha
-1, followed by BF100 and BF50 with 3233.83 and 2797.50 kg ha
-1, respectively. Furthermore, in two studied years statistically difference was noticed between the BF150 level and other treatments.
3.3. Leaf area index
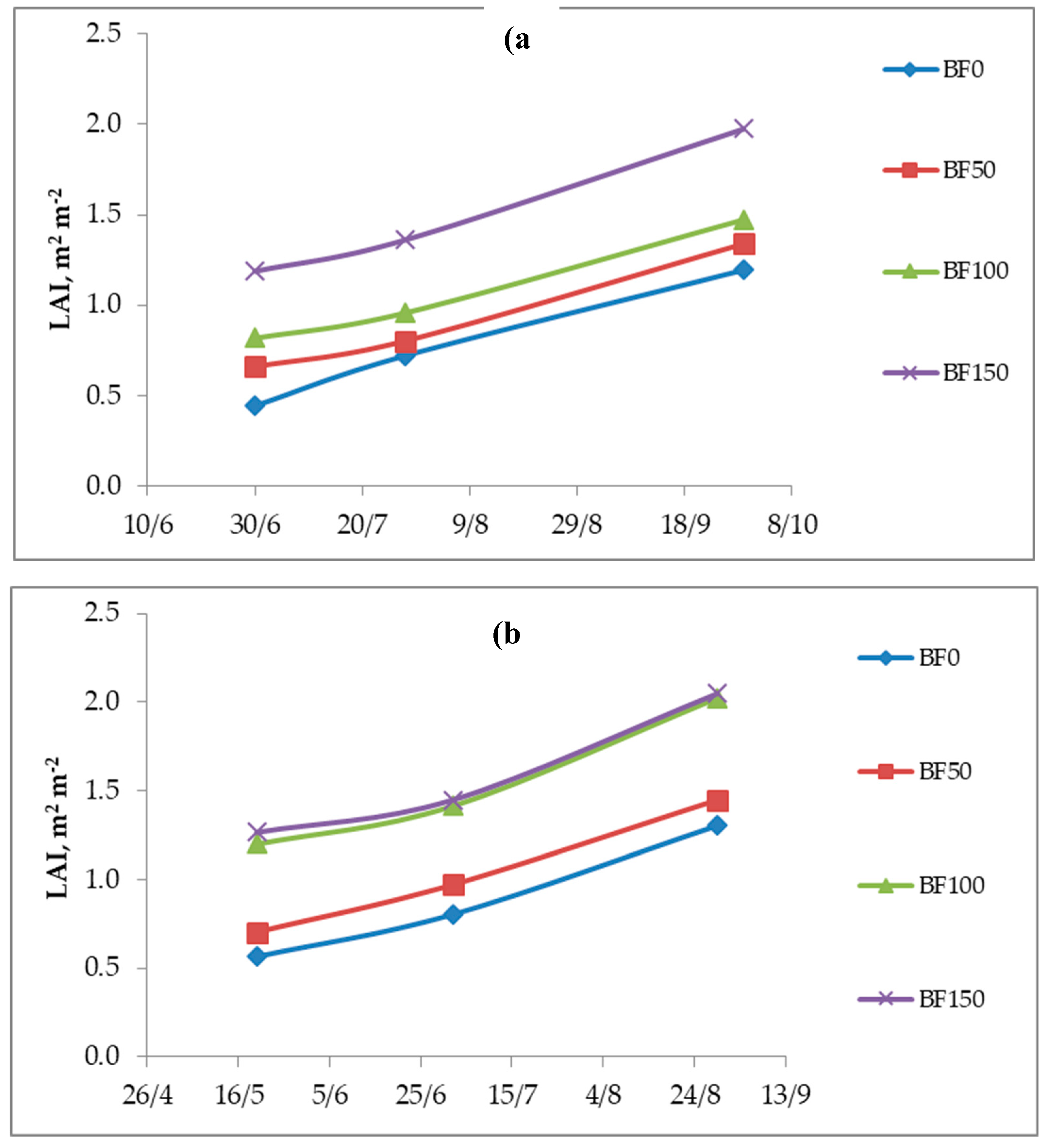
The results of LAI are illustrated in
Figure 4. Three measurements of LAI were conducted, in the 1st year on 30/06/2014, 28/07/2014 and 29/09/2014 and the in 2nd year on 20/05/2015, 2/07/2015 and 29/08/2015. The 1st growing year the higher LAI values observed in the BF150 application and a significant increase in LAI values of BF150 plots was noticed which were 62.55%, 47% and 39.49% in 1st, 2nd and 3rd measurements, respectively, compared to BF0. In 2nd growing year the BF100 and BF150 treatments provoked the highest values of LAI and between these fertilizations there was no statistically difference. Moreover, in 2nd year the LAI value raised by 27.18% in BF100 treatment.
3.4. Herbaceous plant composition
In total, 20 plant species in the
A. citrodora ecosystem were recorded in the study area (
Table 4). The most frequently occurring plant was
Avena sterilis L. (Family:
Poaceae) (Status: Native, Chorology: Mediterranean-SW Asian, Life-form: Therophyte, Habitat: Agricultural and Ruderal habitats) and
Chenopodium album (Family:
Chenopodiaceae) (Status: Native, Chorology: Cosmopolitan, Life-form: Therophyte, Habitat: Agricultural and Ruderal habitats) in
Aloysia triphylla L. ecosystem (
Figure 5).
Table 6.
Herbaceous plant species in Aloysia citrodora.
Table 6.
Herbaceous plant species in Aloysia citrodora.
Herbaceous
plant species |
Family
|
Aloysia citrodora Ecosystem |
|
Aegilops geniculata Roth. |
Poaceae |
+ |
| Amaranthus deflexus L. |
Amaranthaceae |
+ |
|
Anthemis arvensis L. |
Asteraceae |
+ |
|
Arctium lappa L. |
Asteraceae |
+ |
|
Avena sterilis L. |
Poaceae |
+ |
|
Bellis perennis L. |
Asteraceae |
+ |
|
Calystegia sepium (L.) R. Br. |
Convolvulaceae |
+ |
|
Capsella bursa-pastoris (L.) Medik. |
Brassicaceae |
+ |
|
Chenopodium album L. |
Chenopodiaceae |
+ |
|
Fumaria officinalis L. |
Fumariaceae |
+ |
|
Glaucium flavum Crantz |
Papaveraceae |
+ |
|
Heliotropium europaeum L. |
Boraginaceae |
+ |
|
Lamium amplexicaule L. |
Lamiaceae |
+ |
|
Lolium perenne L. |
Poaceae |
+ |
|
Polygonum aviculare L. |
Polygonaceae |
+ |
|
Sinapis arvensis L. |
Brassicaceae |
+ |
|
Sonchus arvensis L. |
Asteraceae |
+ |
|
Sorghum halepense (L.) Pers. |
Poaceae |
+ |
|
Stellaria media (L.) Vill. |
Caryophyllaceae |
+ |
|
Veronica persica Poir. |
Veronicaceae |
+ |
| Total |
|
20 |
3.5. Environmental Factors affecting the Shannon plant diversity index
In our study, the Pearson correlation coefficients (
Table 7) showed that there were soil parameters such as the soil organic matter (SOM), the Phosphorus (P) and the Potassium (K), on which the SH plant diversity index depended significantly for organic A. citrodora.
4. Discussion
4.1. Plant height
The 1st year the plant height was ranged from 32.7 cm (control) to 51.8 cm (BF150), while the 2nd year the height was between 40.7 (control) – 61.2 cm (BF150). The BF100 level had a significant increase on plant height (18.73%) the 2nd year while BF150 fertilization increased the plant height 15.26% compared to the 1st year. Kassahun et al. [
36] found that the
A. citrodora plants’ height varied from 61.67-87.14 cm, results that are in disagreement with our study. According to the literature biological fertilizers can improve the plant growth by improving the soil fertility [
37]. The beneficial effects of bio-fertilizers have been investigated in many crops (38-39).
4.2. A. citrodora total dry yield
The total dry production (stem, leaf) was increased after the use of nitrogen via the biological fertilizer. The 2nd year an average increase of 49.8% in total biomass was observed in all the treatments. That increase was expected because A. citrodora is a perennial scrub which come to full production after the 2nd year of cultivation. It is remarkable that the BF150 treatment provoked the most effectively raise in 2nd year (50.58%) compared to 1st. Until now, only few investigations have studied the use of organic fertilization in A. citrodora and its positive effects in dry biomass [
18,
22]. Afonso et al. [
22] mention that the application of a biological fertilizer in A. citrodora cultivation from 0 to 100 kg N ha-1 increased the leaf and total yield, while between 100 to 150 kg N ha-1 there was a stabilization in yield. Studies showed that the use of bio-fertilizer (Azospirilum and Azotobacter) increased the Salvia plants’ dry weight [
40].
4.3. LAI
The BF100 and BF150 biological fertilizer levels have a significantly positive effect in Leaf Area Index, in second studied year. Specifically, in 2nd year the increase of LAI using the BF100 fertilizer reached an average value of 27.18%. No publications are found to have studied the impact of bio-fertilization (chemical or organic) in leaf Area Index of
A. citrodora. The bio-fertilizer application plays an important role in the photosynthesis and in the process of the green surface production [
41,
42].
4.4. Herbaceous plant composition
The agricultural landscape is a cultural landscape. Agroecosystems are the basic components of rural landscapes. There are large numbers of flora in Agroecosystems, and those systems are considered agricultural systems possessed of a high ecological value for biodiversity (high-nature-value farming systems). The most frequently occurring plant was
Avena sterilis L. and
Chenopodium album in
A. citrodora ecosystem. These plant species are characteristic of agroecosystems, according to Dimopoulos et al. [
35]. Several suitable regions of Greece have already been invaded by
Chenopodium album and
Avena sterilis. The most important factors explaining herbaceous plant species composition, based on literature [
43] are management practices and environmental factors. It is noteworthy, plants are an important indicator of environmental health since they connect the ground to the air. Particularly, they draw most of the data they need from the ground, but their peaks are also directly related to the air because their components make up gas collectors. As a result, comparing plant chemical composition with that of plants that grow in a healthy environment is an important sign of contamination in plant growth areas. According to the literature
Chenopodium album is an indicator plant in the
A. citrodora ecosystem, as it indicates soil nitrogen and humus levels [
44] which is very important for farmers regarding crop management decision making.
4.5. Environmental Factors affecting the Shannon plant diversity index
The physical characteristics of soil are a key factor, which defines its own physical consistency, a physical consistency that is actively influenced by biological processes, while chemical elements are used to specify soil properties [
45,
46,
47]. In our study, SH plant diversity correlated positively with the soil organic matter (SOM), the Phosphorus (P) and the Potassium (K). This is probably due to that organic matter in soils preserves nutrients, upgrades the nutrient circle, constructs soil composition, improves water permeability, decreases soil density, resists rapid changes in soil pH, serves as a power source for microorganisms and increases the rate of assimilating copper, magnesium, and zinc into the soil, therefore all of the above promotes the composition and plant diversity [
48]. It is noteworthy that organic soil provides important nutrients such as phosphorus and potassium used by plants in large quantities for their growth and survival. Soil Phosphorus (P) being an organic and inorganic substance, it is found in soils, water, and most living organisms. Many P chemical mixtures are in harmony and extend from solution P (absorbed by plants) to stable, unstable or even unobtainable compounds (the most common). As the most important nutrient for plants, phosphorus plays a crucial role. Phosphorus plays many roles in plants, but the biggest one is for storing and transferring energy [
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50]. Furthermore, potassium (K) is an important element in determining soil fertility and plant diversity. The accessibility of plants in soil solution is separated into interchangeables and irreplaceables and exists in soil crystal lattices [
51,
52]. Among its many functions, it maintains plant turgor, stomatal movement, cell expansion, pH, phloem transport and protein synthesis. According to Maestre et al. [
53] and Korol et al. [
54] stressed the importance of P cycling with regard to plant diversity. Potassium increases the resistance of plants to dry climates such as in the Mediterranean zones and P gives the energy to all biological reactions and therefore both elements contribute to the increase of biodiversity [
55].
5. Conclusions
Generally, the BF100 application provoked almost the same results in plant height and in dry weight of the edible parts of the cultivation compared to BF150. This means that the best bio-fertilizer for the A. citrodora cultivation is the BF100 nitrogen level instead of BF150, although the results of the BF150 was a little bit better. The use of BF100 fertilizer can help positively the agronomic characteristics of A. citrodora with the minimum cost not only for the producers but also for the environment generally.
A. citrodora ecosystems provide a variety of functions and services that are beneficial to humans and animals. There is a significant finding in this study that soil factors (soil organic matter, P and K) promote positive herbaceous plant diversity within the A. citrodora ecosystem. These results are crucial for understanding the ecology and dynamics of this ecosystem. As aromatic and medicinal plants have beneficial effects, as well as their contributions to the development of several sectors like medicine, there is a need to continue the study in the future. It is notable that the data from this study is relevant to health-care programme development, aromatherapy, phytotherapy, economic agricultural policy development, alternative food program, and ethnobotany.
The climate and physicochemical properties of the soils in Greece favour the growth of aromatic, medicinal plants that can produce products of excellent quality, even if cultivated in mountainous and semi-mountainous areas. There are many such lands in our country and the cultivation of these plants can provide a serious income to rural residents.
Author Contributions
Conceptualization, A.M, A.S. and E.S; methodology, A.M., A.S. and A.L.; validation, A.M., A.S. and A.L; software, A.L.; investigation, A.Μ, A.S., M.T, A.L, and E.S..; data curation, A.Μ, A.S. and M.T. and A.L.; writing—original draft preparation, A.M., A.S., M.T., A.L. and E.S.; writing—review and editing, A.M., A.S., M.T., A.L. and E.S. ; supervision, A.M A.S., A.L. and E.S.; project administration, A.M., A.S. and E.S.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Solomou, A.; Martinos, K.; Skoufogianni, E.; Danalatos, N.G. Medicinal and Aromatic Plants Diversity in Greece and Their Future Prospects: A Review. Agricultural Science. 2016, 4, 9-20. [CrossRef]
- Solomou, A. D.; Sfougaris, A. Contribution of agro-environmental factors to yield and plant diversity of olive grove ecosystems (Oleaeuropaea L.) in the Mediterranean landscape. Agronomy. 2021, 11(1), 161. [CrossRef]
- Duarte, M. C.T.; Leme, E.E.; Delarmelina, C.; Soares, A.A.; Figueira, G.M.; Sartorato, A. Activity of Essential Oils from Brazi ian Medicinal Plants on Escherichia coli. J. Ethnol. Pharmacol. 2007, 111, 197–201. [CrossRef]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular plants of Greece: An annotated checklist. Sup. Willdenowia, 2016, 46, 301–347.
- Duarte, M.C.T.; Figueira, G.M.; Sartoratto, A.; Rehder, V.L.G.; Delarmelina, C.; Marta, C. Anti-candida activity of Brazilian medicinal plants. J. Ethnoph. 2005, 97, 305–311. [CrossRef]
- Vogel, H.; Silva M.L.; Razmilic, I. Seasonal fluctuation of essential oil in lemon verbena (Aloysia triphylla). Acta Horticulture. 2002, 500, 75-80. [CrossRef]
- Argyropoulou, C.; Daferera, D.; Tarantilis, P.; Fasseas, C.; Polissiou, M.(2007). Chemical composition of the essential oil from leaves of Lippia Citriodora H. B.K. (Verbenaceae) at two developmental stages. J. Biochem. System. Ecol. 2007, 35, 831–837. [CrossRef]
- Ragone, M.I.΄Sella,M. Conforti΄, P.΄Volonti, M.G.΄Consolini, A.E. The spasmolytic effect of Aloysia citriodora, Palau (South American cedrοn) is partially due to its vitexin but not isovitexin on rat duodenums. J. Ethnopharmacol. 2007, 113, 258-266. [CrossRef]
- Bilia, A.R.; Giomi,M.; Innocenti, M.; Gallori, S.; Vincieri, F.F. HPLC–DAD– ESI–MS analysis of the constituents of aqueous preparations of verbena and lemon verbena and evaluation of the antioxidant activity. J. Pharmaceut. Biomed. 2008, 46, 463-470. [CrossRef]
- Funes, L.; Fernandez-Arroyo, S.; Laporta, O.; Pons, A., Roche, E.; Segura-Carretero, A.; Fernαndez-Gutiιrrez, A.; Micol, V. Correlation between plasma antioxidant capacity and verbascoside levels in rats after oral administration of lemon verbena extract. Food Chem. 2009, 117, 589-598.
- Regnier, T.; Combrinck, S. In vitro and in vivo screening of essential oils for the control of wet bubble disease of Agaricus bisporus. S.Afr. J. Bot. 2010, 76, 681-685. [CrossRef]
- Van Wyk, B.E. Food Plants of the World: Identification, Culinary Uses and Nutritional Value, Publisher: CABIT, Briza, Pretoria, 2005; p.p.2459.
- Rosa, P.T.V.; Meireles, M.A.M. Rapid estimation of the manufacturing cost of extracts obtained by super critical fluid extraction. J. Food Eng. 2005, 67, 235-240. [CrossRef]
- Martinez, J.; Rosa, P.T.V.; Meireles, M.A.A. Extraction of clove and vetiver oils with super critical carbondioxide: Modeling and simulation. The Open Chemical Engineering Journal. 2007, 1, 1-7.
- Sharma, L.K.; Bali, S.K. A Review of Methods to Improve Nitrogen Use Efficiency in Agriculture. Sustainability. 2018, 10(1), 51. [CrossRef]
- Kumar, R.; Kumawat, N.; Y. K. Sahu, Y.K. (2017). Role of biofertilizers in agriculture. Popular kheti. 2017, 5(4), 63-66.
- Mishra, D.; Rajvir, S.; Mishra, U.; Kumar, S.S. Role of bio-fertilizer in organic agriculture: a review. Research Journal of Recent Sciences. 2013, 2, 39-41.
- Mohammadi, M.; Ahmad Tobeh, A.; Vahidipour, H.R.; Fakhari, R.. Effects of biological fertilizers on essential oil components and quantitative and qualitative yield of lemon verbena (Lippia citriodora). International Journal of Agriculture and Crop Sciences. 2013, 5(12), 1374-1380.
- Dawood, R. A. E.; Abd El-Azeem, A.M.S.; Gohar, I. M. A. (2023). Effects of nitrogen fertilizers, bio-fertilizer and molasses on yield quality of sugar beet plants (Beta vulgaris L.). Egyptian Sugar Journal. 2023, 20, 53-62.
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; P. Tribedi, P. Biofertilizers: a potential approach for sustainable agriculture development. Environmental Science and Pollution Research. 2017, 24(4), 3315-3335.
- Bhattacharjee, R.; U. Dey, U. Biofertilizer, a way towards organic agriculture: A review. African Journal of Microbiology Research. 2014, 8(24), 2332-2343.
- Afonso, S.; Arrobas, M.; Ferreira, I.Q.; Rodrigues, M.R. Leaf nutrient concentration standards for lemon verbena (Aloysia citrodora Paláu) obtained from field and pot fertilization experiments. Journal of Applied Research on Medicinal and Aromatic Plants. 2018, 8, 33-40. [CrossRef]
- Ebadi, M. T.; Azizi, M.; Sefidkon, F.; Ahmadi, N. Influence of different drying methods on drying period, essential oil content and composition of Lippia citriodora Kunth. Journal of Applied Research on Medicinal and Aromatic Plants . 2015, 2(4), 182-187. [CrossRef]
- Elechosa, M. A.; Di Leo Lira, P.; Viturro, C.I.; Heit, C.; Molina, A.C.; Martinez, A.J.; Lopez, S.; Molina, A.M.; van Baren, C.M.; Bandoni, A.L. Essential oil chemotypes of Aloysia citrodora (Verbenaceae) in Northwestern Argentina. Biochemical Systematics and Ecology. 2017, 74, 19-29. [CrossRef]
- Bahramsoltani, R.; Rostamiasrabadi, P.; Shahpiri, Z.; Marques, A. M.; Rahimi, R.; Farzaei, M.. "Aloysia citrodora Paláu (Lemon verbena): A review of phytochemistry and pharmacology. J Ethnopharmacol. 2018, 222, 34-51. [CrossRef]
- Majewska, E.; Kozłowska, M.; Majewska, E.; Tarnowska, K.; Gruczyńska-Sękowska, E.; Kowalska, D. Chemical Composition and Biological Activity of Lemon verbena (Lippia citriodora) Essential Oil – A Review. Journal of Essential Oil Bearing Plants. 2022,25(4), 796-810. [CrossRef]
- Shahhoseini, R.; Hosseini, N.; Ghorbanpour, M. Study of Essential Oil Content and Composition of Different Parts of Lemon verbena (Lippia citriodora) Grown in Iran. Journal of Essential Oil Bearing Plants. 2014, 17(1), 120-125. [CrossRef]
- Mitsios, J.; Toulios, M.; Charoulis, A.; Gatsios, F.; Floras, S. Soil study and soil Chart of the Experimental field of the University of Thessaly in Velestino area. Publisher: Zymel, Athens, 2000.
- Rowell, D.L. Soil Science: Methods and Applications; Longman Group UK Ltd.: London, UK, 1994.
- Cook C.W.; Stubbendieck, J. Range Research: Basic Problems and Techniques. Publisher: Society for Range Management, Denver Colorado, 1986.
- Seaby, R. M.; Henderson, P. A. Species diversity and richness. Version 4.1.2. Publisher: Pisces Conservation, Lymington, UK, 2007.
- Heuserr, M. J. J. Putting diversity indices into practice. Some considerations for forest management in TheNetherlands. In Proceedings of the Conference on assessment of biodiversity for improved forest planning, MonteVerita, Switzerland, October 7-11, 171–80.
- Kent, M.; Coker. P. Vegetation description and analysis: A practical approach, Publisher: John Wiley and Sons, Inc, New York, 1992; p.p.623.
- Whittaker, R. H. Evolution and measurement of species diversity. Taxon. 1972, 21, 213-251. [CrossRef]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular plants of Greece: An annotated checklist. Willdenowia. 2013, 46(3), 301-347. [CrossRef]
- Kassahun, B.M.; Wondu Bekele Yosef, W.Y.; Mekonnen, S.A.Performance of Lemon Verbena (Aloysia triphylla L.) for Morphological, Economic and Chemical Traits in Ethiopia. American-Eurasian J. Agric. & Environ. Sci. 2013, 13 (11), 1576-1581.
- Itelima, J. U.; Bang, W. J.; Onyimba, I. A.; Sila, M. D.; Egbere. O. J. Bio-fertilizers as key player in enhancing soil fertility and crop productivity: (A Review). Direct Research Journal of Agriculture and Food Science. 2018, 6(3), 73–83. [CrossRef]
- Kalra A. Organic cultivation of Medicinal and aromatic plants. A hope for sustainability and quality enhancement. Journal of Organic Production of Medicinal, Aromatic and Dye- Yielding Plants (MADPs). 2003. FAO.p 198.
- Copetta, A.; Lingua, G.; Berta, G. Effects of three AM fungi on growth, distribution of glandular hairs, and essential oil production in Ocimum basilicum L. var. Genovese . Mycorrhiza. 2006, 16, 485–494. [CrossRef]
- Youssef, A. A.; Edris, A. E.; Gomaa, A. M. A comparative study between some plant growth regulators and certain growth hormones producing microorganisms on growth and essential oil composition of Salvia officinalis L. Plant Annual. Agricultural Science. 2004, 49, 299–311.
- Han, H. S.; K. D. Lee. Effect of inoculation with phosphate and potassium co-insolubilizing bacteria on mineral uptake and growth of pepper and cucumber. Plant, Soil and Environment. 2006, 52, 130–6. [CrossRef]
- Kader, M. A. Effects of Azotobacter inoculant on the yield and nitrogen uptake by wheat. Journal of Biological Sciences. 2002, 2, 259–61. [CrossRef]
- Cirujeda, A.; Aibar, J.; Zaragoza, C. Remarkable changes of weed species in Spanish cereal fields from 1976 to 2007. Agronomy Sust. Developm. 2011, 31, 675– 688. [CrossRef]
- Wildwaterwall. Available online: https://sites.google.com/site/wildwaterwall/biokalliergeies/phyta-deiktes-edaphous (accessed on 16/01/2023).
- De Vos, J.A.; Raats, P.A.C.; Vos, E.C. Microscopic soil physical processes considered within an agronomical and a soil biological context. Agric. Ecosyst. Environment. 1994, 5, 43-73. [CrossRef]
- Hassink, J.; Whitmore, A.P. A model of the physical protection of organic matter in soils. Soil Sci. Soc. American J., 1997, 61, 131-139. [CrossRef]
- Arunachalam, K.; Arunachalam, A.; Tripathi, R.S.; Pandey, H.N. Dynamics of microbial population during the aggradation phase of a selectively logged subtropical humid forest in Northeast India. Trop. Ecol., 1997, 38, 333-341.
- Borah, D.K.; Rattan, R.K.; Banerjee, N.K. Effect of soil organic matter on the adsorption of Zn, Cu and Mn in soils. J. fnd. SOC. Soil Sci. 1992, 40, 277-282. [CrossRef]
- Khan, M.S.; Zaidi, A.; Ahemad, M.; Oves, M.; Wani, P.A. Plant growth promotion by phosphate solubilizing fungi – current perspective. Arch Agron Soil Sci. 2010, 56, 73–98. [CrossRef]
- Sharma, S.; Sayyed, R.; Trivedi, M.; Gobi, T. Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus. 2013, 2, 587. [CrossRef]
- Barber, S.A. Soil nutrient bioavailability: A mechanistic approach, 2nd Edition; Publisher: John Wiley & Sons, New York, 1984; p. 398.
- Mengel, K.; Kirkby, E.A. A Principles of Plant Nutrition, 4th Edition; Publisher: I PI, Basel, Switzerland; 1987; p.p. 849.
- Maestre, F. T.; Quero, J. L.; Gotelli, N. J.; Escudero, A.; Ochoa, V.; Delgado-Baquerizo, M.; Garcıa-Gomez, M., Bowker, M. A.; Soliveres, S.; Escolar, C. Plant species richness and ecosystem multifunctionality in global drylands. Science. 2012, 335 (6065), 214–8. [CrossRef]
- Korol, A. R.; Ahn, C.; Noe, G. B. Richness, biomass, and nutrient content of a wetland macrophyte community affect soil nitrogen cycling in a diversity-ecosystem functioning experiment. Ecological Engineering. 2016, 95, 252–65. [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. International Journal of Molecular Sciences. 2013, 14 (4), 7370–90. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).