1. Introduction
For the preservation of endodontically treated teeth, timely and adequate post-endodontic restoration is crucial [
1]. In the case of extensive loss of crown structure, glass fiber posts cemented with composite cements are a common therapeutic choice because of their satisfactory survival rates and biomimetic behavior [
2,
3,
4,
5,
6]. Optimal post placement together with reliable adhesion is the fundamental requirement for effective sealing and prevention of micro and nanoleakage [
2,
7]. Factors influencing the adhesion of glass fiber-reinforced composite posts to radicular dentin are associated with substrate (dentin), post and luting agent (cement) [
2,
3,
4,
5,
6]. The curing of cement and its interaction with dentin and post play a significant role [
2,
3,
4,
5,
6]. Despite the advancements in the polymerization and chemistry of resin cements, achieving proper adhesion to radicular dentin remains a significant clinical challenge [
4,
8]. The primary cause of failure in glass fiber-reinforced composite posts is debonding [
2,
3,
5,
6,
9,
10]. This can be attributed to cementation technique sensitivity, which involves the removal of sealer and gutta-percha, dentin disinfection, and smear layer removal [
2,
3,
4,
8,
10,
11]. Further challenges in post cementing are associated with unfavorable configuration factor and achieving adequate degree of conversion in the radicular space [
8]. Controlled environment of the post space during cementation procedure (primarily moisture control) ensures adequate conversion of dual-cure composites [
3,
4,
8,
11]. Overall adhesion of glass fiber post to dentin is determined by post–cement interface and the cement–dentin interface [
5,
8,
9,
12]. According to some studies, adhesion failures predominantly occur at the dentin-cement interface, rendering it the weakest link in terms of bond strength [
5,
9,
12].
Simplifying cementing techniques by using self-adhesive resin cements (SARC) offers advantages due to a reduced number of phases and technical sensitivity [
10,
11,
13,
14]. This type of cement eliminates the need for prior use of adhesion systems and includes multipurpose acids and methacrylate monomers containing a phosphate group. These components react with hydroxyapatite, providing both a micromechanical and chemical bond [
9,
10,
13,
14]. One SARC available on the market is G-CEM One (GC, Tokyo, Japan). It comprises of self-adhesive cement and a primer. The primer contains functional monomers and chemical initiator accelerating chemical cure of the cement from the tooth surface, which all enhances bond between the cement and dentin [
14,
15].
Dentin surface cleanliness is important for adequate interaction with luting cement and the success of therapy involving glass fiber posts [
8,
9]. Cleaning and disinfection of the post space, with or without the removal of the smear layer, may also have an impact on the bond strength. The most common solutions used for the post space cleaning and disinfection are sodium hypochlorite, chlorhexidine, ethylenediaminetetraacetic acid, and a combination of these liquids [
9,
10,
11,
16]. The most frequently employed root canal rinsing fluid is sodium hypochlorite (NaOCl), typically utilized in concentrations ranging from 0.5 to 6%. Functioning as a solvent for organic substances and antiseptic, it does, however, come with drawbacks such as toxicity and an inability to eliminate the inorganic elements of the smear layer [
11,
16,
17]. Chlorhexidine (CHX), categorized as a biguanide, is applied at concentrations of 0.2%, 1%, and 2%. It exhibits effective antimicrobial activity without causing toxicity to surrounding tissues. However, chlorhexidine does not dissolve either organic or inorganic content [
9,
11]. Ethylenediaminetetraacetic acid (EDTA) is a chelator agent and successfully removes inorganic components [
9,
11,
16,
17]. However, it should be noted that EDTA has limitations, as it does not eliminate organic content and lacks antiseptic properties. There is no unanimous position in what order should NaOCl and EDTA be used during cleaning and disinfection. According to some authors, it is not advisable to alternate the use of NaOCl and EDTA during instrumentation, as it may compromise the primary actions of both rinsing fluids and weaken the integrity of intraradicular dentin [
16,
17,
18]. Others suggest that NaOCl be used first followed by EDTA to avoid erosion, but potential beneficial effects of NaOCl irrigation after EDTA, with its deproteinizing action on exposed collagen fibrils, has been reported, as well [
19,
20,
21,
22]. Recently, 1-Hydroxy ethylidene-l, l-diphosphonic acid (HEDP), also recognized as etidronic acid or etidronate, has entered the market and found application in endodontics. HEDP functions as a mild chelator and shows promise as a potential substitute for EDTA. One of its noteworthy properties is its compatibility with sodium hypochlorite, ensuring it can be mixed without diminishing its antimicrobial efficacy. Typically utilized at a concentration of 9% with 2.5% NaOCl, HEDP exhibits a demineralization effect less potent than EDTA. For this reason, during final rinsing, it requires an extended period to adequately remove the smear layer [
16,
17,
18].
Previous studies investigating the influence of irrigants on the fiber-post bond strength, mainly focused on the application of a single solution in the post-space [
9,
23]. The aim of the present study was to implement recommended final irrigation protocols in endodontics [
22,
24,
25,
26] and assess their impact on the bond strength between SARC and dentin treated with several irrigation solutions (NaOCl, CHX, EDTA, saline and etidronic acid). According to the available literature, etidronic acid has not been investigated in this specific context. The null hypothesis was that different irrigantion protocols would not influence the bond strength of SARC to radicular dentin.
2. Materials and Methods
Radicular Dentin Samples Preparation
The study was approved by a local Ethical committee, approval number 05-PA-30-16-3/2023.
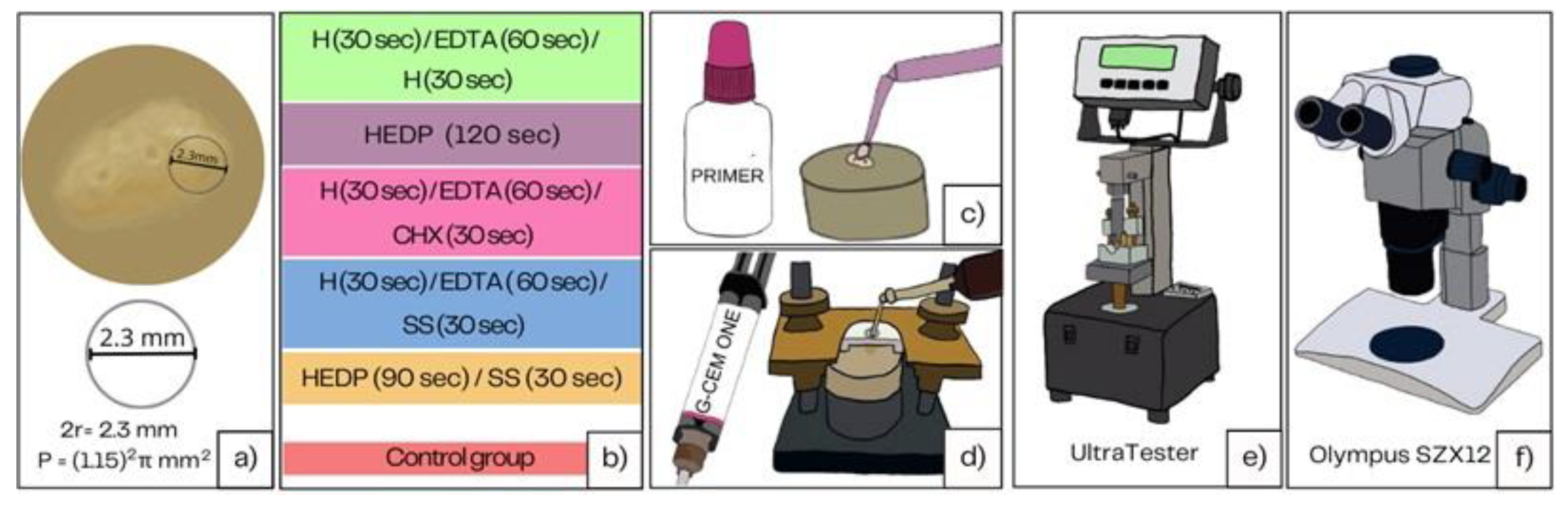
Forty healthy human third molars with fully developed roots were collected and preserved in 1% chloramine solution after extraction, over a period of one month. The crowns of the teeth are cut with a fissure diamond bur at the enamel-cement junction. From the coronary third of the roots, 2.2 mm thick dentin slabs were cut using a low-speed saw (IsoMet, Buehler, Duesseldorf, Germany). The saw was directed perpendicularly to the longitudinal axis of the roots at 200 rpm with continuous water cooling. Radicular dentin slabs were then stored in saline until mounting in cold-curing methacrylate resin (Technovit 4004, Kulzer, Germany). The samples with insufficient dentin width were excluded. An Ultradent mold (Ultradent Products, South Jordan, UT, USA) was used to embed radicular dentin samples in the methacrylate resin. To create a flat bonding area, dentin surface was polished with 600-grit silicon carbide (SiC) paper (PRESI, Eybenes, France), rinsed thoroughly and stored in distilled water until further testing (
Figure 1).
Irrigation and Bonding Procedure
A total of 72 samples were prepared and randomly assigned to 6 groups (five experimental and one control group). Some specimens were lost during bonding procedure resulting in final number of samples per group ranging from N=8-12, Ntotal=52. Irrigation solutions used were 2.5% sodium hypochlorite solution (H), Ethylene-diamine-tetra-acetic acid (EDTA), 2% chlorhexidine solution (CHX), etidronic acid (or 1-Hydroxyethylidene-1,1-diphosphonic acid, HEDP) and saline solution (SS). HEDP solution was prepared by adding 1.8 g of etidronic acid to 20 mL of 2,5 % NaOCl. The samples were exposed to different irrigation protocols during two minutes in five experimental and one control group: 1)H 30s/EDTA 60s/H 30s, 2) HEDP 120s, 3) HIPO 30s/EDTA 60s/CHX 30s, 4) HIPO 30s /EDTA 60 s/SS 30 s, and 5) HEDP 90s/SS 30 s 6) no irrigation- Control Group (CG).
After irrigation, the dentin surface was gently dried with air and cotton rolls until there was no longer any discernible wetness, and bonding procedure was performed immediately. The bonding location was marked with a polymer adhesive strip that had a 2.5 mm-diameter hole and a 0.2 mm thickness. The GC G-CEM ONE Adhesive Ehancing Primer (GC, Tokyo, Japan) was applied to the dentin surface according to manufacturer’s instruction (left for 10 s, dried, no polymerization) and afterwards, composite cement cylinders (GC G-CEM LinkForce, GC Tokyo, Japan LOT 2206131, LOT 2109111) (2.3 mm internal diameter and 3.0 mm height) were created on the adhering surface using a bonding clamp and plastic mold inserts (Ultradent Products, South Jordan, UT, USA). Bluephase Style LED curing unit (Ivoclar Vivadent, Lichtenstein, Schaan) was used to light cure the resin composite cement for 20 s with 1000 mW/cm2. The samples were left in plastic molds for another four minutes to allow dark polymerization to finish. Samples were kept in distilled water at 37 °C and 100% moisture (NUVE ES 120, NÜVE, Ankara, Turkey) for 10 days before being broken in shear mode.
Shear Bond Strength Testing
For testing shear bond strength of composite resin cement to radicular dentin, ISO standard 29022 was applied. UltraTester (Ultradent Products, SAS Institute Inc., Cary, NC, USA) was used at constant speed of 1 mm/min until fracture of specimens.
Failure Mode Analysis
After shear bond strength recordings, the samples were observed under a stereomicroscope (Olympus SZX12) to determine the fracture mode. Fractures were categorized as follows: AD (adhesive failure), MFc (more than 50% of the surface cohesive failure in cement), MFa (more than 50% of the surface adhesive, less than 50% cohesive in cement).
Statistical Analysis
The obtained results were analyzed using one way ANOVA for the assessment of the differences between arithmetic means of independent groups. Post-hoc Games-Howell test was used to determine which groups differ significantly.
3. Results
The highest mean shear bond strength (SBS) was recorded for the control group (M = 22.23 MPa), while the lowest mean SBS was recorded for H/EDTA/SS (M = 8.8 MPa) (
Table 1).
The homogeneity of variances was tested and presented in
Table 2.
The Kolmogorov-Smirnov normality test (
Table 2) indicated that the assumption of normality was satisfied at all levels. However, the Levene test (F = 3.262, df1 = 5, df2 = 52, p < 0.05) revealed that variances are heterogeneous, indicating a statistically significant difference. Therefore, before conducting the ANOVA analysis, logarithmic data transformation was carried out.
After the transformation, it became evident that the standard deviations were more uniform (
Table 3), and homogeneity was confirmed by the Levene homogeneity test for variances (F = 1.163, df1 = 5, df2 = 52, p = 0.340). Therefore, it was possible to implement ANOVA. The ANOVA results revealed a statistically significant difference among six groups of root dentin samples treated with different irrigation protocols (F = 1.163, df1 = 5, df2 = 52, p = 0.340) (
Table 4). The post-hoc Games-Howell test identified significant differences between several pairs of groups: the control group and H/EDTA/H (p < 0.05), the control group and H/EDTA/CHX (p < 0.05), and the control group and H/EDTA/SS (p < 0.05). No statistically significant differences were observed among the other groups (
Table 5, Figure 2).
Figure 2 Presentation of differences in arithmetic means of SBS in groups treated with different irrigation protocols.
Considering the type of fracture, adhesive fractures were predominantly observed, followed mixed adhesive and cohesive fractures in the material, and mixed cohesive in the material and adhesive. Cohesive fractures in dentin were not observed (
Table 6).
4. Discussion
Irrigation solutions used in post-space cleaning and disinfection can affect dentin structural changes and surface characteristics, potentially influencing the adhesion of resin cement to the radicular dentin surface [
19,
27]
. The bond strength of G-cem One self-adhesive resin cement to radicular dentin has not been studied in this context, nor has the use of etidronic acid. The results of the present study indicate that combined irrigation protocols with disinfecting and chelating solutions significantly affected bond strength, with the highest strength observed in the control group. Among the experimental groups, the group irrigated with etidronic acid exhibited the highest bond strength. The null hypothesis, stating that different irrigation protocols would have no influence on bond strength, was rejected.
Although the push-out test would be more relevant in estimating the bond strength of the post to dentin, the shear bond strength used in this study holds clinical relevance, as the irrigation solutions affect dentin. Therefore, the irrigation protocols reflect on the dentin-cement interface, not on the cement-post interface. Furthermore, in previous studies associated with the bond of fiber posts to dentin, it was shown that failures predominantly occur at the cement-dentin interface due to difficulties in hybrid layer formation. [
20,
28,
29].
Instead of evaluating the impact of individual solutions on the bond strength to dentin, as observed in most previous studies, this research focused on assessing the influence of final irrigation protocols involving multiple solutions. According to the available literature, there is no universally accepted intra-radicular cleaning protocol for self-adhesive cements that would enhance the retention of fiber posts and adequately clean the space. [
10]. In the available literature, the most commonly applied final irrigation protocols were NaOCl/EDTA/NaOCl, followed by NaOCl/EDTA/CHX, lasting for 90-120 seconds, with or without agitation or ultrasonic activation [
30,
31]. Additional protocols in the present study included irrigation with etidronic acid and NaOCl/EDTA/SS.
The failure of the bond between self-adhesive resin cement (SARC) and radicular dentin could be attributed to incomplete or inadequate infiltration of the material into the partly or fully exposed collagen matrix, depending on the solutions used. The results of the present study indicated that protocols involving NaOCl and EDTA resulted in the weakest bond strengths, irrespective of the final flush. This can be attributed to several effects of these solutions on dentin. EDTA (17%) possesses significant etching/demineralizing potential, which can lead to the selective removal of hydroxyapatite (HAp), following a decalcification route rather than an adhesion route, as defined by Yoshioka et al. [
32]. Due to the excessive removal of calcium hydroxyapatite and full collagen exposure, the salts between calcium ions and functional monomers in the primer or self-adhesive cement become unstable. In this context, the results of this study align with Barreto et al.'s findings, which reported the unsuitability of chelating solutions, including EDTA, for root canal cleaning before the application of self-adhesive resin cement [
20].
There are several approaches to mitigate the negative impact of excessive exposure of dentin matrix. One suggested approach is the application of chlorhexidine (CHX) after the use of the decalcifying agent. This is recommended to inhibit matrix metalloproteinases, as the durability of the bond can be further compromised by the proteolytic action of these enzymes [
33].
The reported effects of chlorhexidine (CHX) pretreatment of dentin on the longevity of the bond between a fiber post and dentin are controversial, ranging from positive and non-significant to detrimental [
21,
33,
34]
. In the present study, the application of chlorhexidine (CHX) after EDTA did not significantly improve bond strength. However, it's important to note that the bond strength was assessed after 10 days, preventing definitive conclusions about the long-term effect of CHX application.
Furthermore, self-adhesive cements follow a modified adhesion route in the adhesion-decalcification concept (without a previous chelator), similar to self-etch adhesives, where collagen fibers should not be exposed and devoid of hydroxyapatite (HAp). In such cases, the inhibiting effect of CHX may not be relevant. In fact, it has been reported that with self-etch adhesives, the addition of CHX did not influence the bond strength results. [
35].
It has also been suggested that collagen fibrils, exposed due to the action of EDTA, can be removed by sodium hypochlorite (NaOCl), which has strong proteolytic activity. This suggestion, contrary to erosion reports[
22], supports the use of NaOCl as the final irrigant for resin-based sealers [
24]. However, in the present study, the application of neither CHX nor NaOCl after EDTA did not result in improved bond strength. Contrary to the results of this study, Barreto et al. [
20] reported higher bond strength after applying 2.5% NaOCl for 60 seconds after EDTA. In the present study, NaOCl of the same concentration was applied for only 30 seconds, which might have been too short to reverse the negative effect of the chelating solution on bond strength. Scientific confirmation for this and other collagen-depletion strategies in improving hybridization is still lacking. Delgado et al. [
25]
concluded in their systematic review that there are no differences in the bond strength of adhesive materials to dentin when collagen-depletion was carried out after acid-etching, compared to a conventional hybrid layer. Moreover, the possibly beneficial effect of the deproteinizing activity of NaOCl on exposed dentinal fibrils is not significant in the context of self-adhesive cement bonding.
NaOCl itself could be the sole cause of the decrease in bond strength. NaOCl fragments collagen fibers and chlorinates terminal groups, generating chloramine-derived radicals. These radicals could potentially interfere with the free radical polymerization of the resin material (cement in this case) at the material-dentin interface [
26]
. On the other hand, some components of G-cem primer could contribute to bond strength by enhancing polymerization, even on etched dentin. The chemical initiator of polymerization in the G-cem primer (adhesive-enhancing primer) accelerates the curing of the cement from the dentin surface and contributes to a higher degree of conversion. However, the beneficial effect of chemical interaction, such as ionic bonding between calcium in the hydroxyapatite-rich submicronic hybrid layer and functional monomers (10-MDP, 4-MET), is not expected because collagen fibers are denuded from HAp after EDTA [
36]
.
The present study also demonstrated that in the NaOCl/EDTA protocols, the final flush with saline did not result in a change in bond strength (the NaOCl/EDTA/SS protocol was not significantly different from NaOCl/EDTA/NaOCl and NaOCl/EDTA/CHX). This may suggest that the negative oxidizing effect of NaOCl on polymerization is not as significant as the negative effect of the demineralizing action of EDTA on overall SBS. However, the oxidizing effect of NaOCl on polymerization could explain why cohesive fractures within the cement were more observed in the groups irrigated with NaOCl. Nevertheless, adhesive fractures were most observed in all groups indicating that the interaction between the luting agent and dentin and formation of hybrid layer is the most common failure point.
Another proposed approach to reduce overexposure of dental fibrils during root canal cleaning before cementation includes the use of weaker chelators, such as etidronate (HEDP). This allows for more effective material infiltration of the exposed dentin matrix, as the depth of demineralization is lower [
19]. The results of this study support this approach, as the best outcomes among the experimental groups were observed for the HEDP and HEDP/SS group. This can be attributed to the weaker decalcifying action of HEDP compared to EDTA, and it aligns with the findings of Tartari et al. [
19] who demonstrated that the mixture of NaOCl and HEDP resulted in more mineral than collagen on the surface of the substrate compared to other decalcifying agents. Furthermore, HEDP, belonging to bisphosphonates, exhibits a high affinity for HAp and increases dentin's surface free energy as it adsorbs to its surface [
24]
. This increase in surface free energy enables better surface wetting (of low viscosity materials sech as G-cem primer). Surface wetting belongs to primary mechanisms of adhesion of any dental material, together with micromechanical interlocking and chemical bond [
37].
The results of the present study reveal superior outcomes in the control group where dentin was not treated with either disinfecting or chelating solutions. The composition of G-Cem primer and cement itself theoretically allows for the omission of smear layer removal using chelators. However, caution is needed when drawing such a conclusion. It's crucial to emphasize that the acidic G-Cem primer was applied, whose low viscosity could compensate for the low surface free energy of the smear layer-covered dentin, enabling acceptable wetting. On the other hand, adequate surface wetting is challenging in the case of self-adhesive resin cement (SARC) due to its viscosity, further impeded by the low surface energy of dentin with the smear layer. If the cement is applied without the primer, theoretically, it might be beneficial to increase the surface free energy by removing the smear layer to improve surface wetting. Additionally, the removal of the smear layer enables cement penetration into dentinal tubules, thereby increasing bond strength [
23]. The mechanism of adhesion for self-adhesive resin cement (SARC) is based on micromechanical retention and chemical bonding between the material and dentin. The high acidity of the cement promotes hybridization without the need for smear layer removal (34). Functional monomers within the primer, such as 10-MDP and 4-MET, or the self-adhesive composite cement, can penetrate the smear layer, integrating it into a new interfacial layer[
15]. This results in increased surface energy and wetting, enabling microretention (interlocking). Moreover, dentin treated with functional acidic monomers like MDP and 4-MET leaves only partially demineralized dentin up to 1 micrometer, forming ionic bonds with Ca2+ released from hydroxyapatite crystals within the thin hybrid layer, further contributing to bond strength. Ca-10-MDP salts are resistant to hydrolytic degradation and create a few nanometers thick (4 nm) layer (nano-layering) [
37].
Regarding bond strength, the present study suggests that disinfecting and smear layer removal protocols may be avoided before cementation using G-Cem One SARC. The manufacturer does not recommend any specific irrigation, but it advises against using EDTA before fiber post cementation with G-CEM One. This recommendation aligns with the study's results. Disinfecting and smear layer removal using a mixture of HEDP and NaOCl resulted in significantly higher bond strength of G-Cem One SARC to dentin compared to other irrigation protocols involving NaOCl and EDTA. Disinfecting and cleaning of the post space are essential parts of the cementation protocol and should be adapted to the type of cement used.