Introduction
Pharmacovigilance is defined as the science that aims to monitor the safety and take measures to reduce the risks and increase the benefits of pharmaceutical products, including drugs and vaccines. Thus, its actions are aimed at monitoring the risk/benefit ratio, as well as improving patient safety and quality of life [
1,
2].
The onset of disease new coronavirus of 2019 (COVID-19) surprised the world in an unprecedented and negative way, exposing human frailty [
3,
4]. The work of the scientific community in the world stunned the world for its efficiency and effectiveness when mapping the virus genome and the creation of immunizing, in record-breaking time, due to the need imposed by the aggressiveness of the virus. The urgency of vaccines also scared the population who was unaware of both the virus and its immunizers [
5,
6], leading people to mistrust and rejection by the reactions that the vaccine could provoke [
7].
In Brazil, four immunizers were approved to be used without restrictions for people over eighteen years old, whose manifestations of adverse reactions were emerging and worrying the scientific community, leading managers to create the pharmacovigilance protocols, in order to map and minimize the most serious collateral damages.
The pharmacovigilance supports science and the citizen who holds so many doubts about the use of the vaccine, enhanced by unsubstantiated information, which spreads widely and rapidly thanks to the ease with which people connect to the internet. The objective of this study was to evaluate government pharmacovigilance actions as a resource for controlling and understanding the adverse effects caused by vaccines in use in Brazil for COVID-19.
Methods
The present study is a narrative literature review carried out by searching documentaries, newsletters in government websites and article. The databases used to search for articles were such as PubMed, LILACS, MedScape and Scielo, The following descriptors were used for selection identified in Medical Subject Headings and Descriptors in Health Sciences: “Pharmacovigilance AND COVID-19 in Brazil”, “Vaccine Development AND COVID-19 in Brazil”, “Vaccination Hesitancy AND COVID-19 in Brazil”, “Pharmacovigilance AND COVID-19”, “Vaccine Development AND COVID-19”, “Vaccination Hesitancy AND COVID-19”, Pharmacovigilance AND COVID-19, Vaccine Development AND COVID-19, Vaccination Hesitancy AND COVID-19, “Public Health Surveillance AND COVID-19”. The search was performed using Portuguese, English and Spanish from May 2021 to June 2022
Duplicates and incomplete studies that did not have the necessary information to address the topic proposed in the study were excluded. Subsequently, a critical reading was performed, and the information was collected using an Excel spreadsheet with the following information: title, year of publication and authors. Then, the content was analyzed and used for the development of the study.
Results
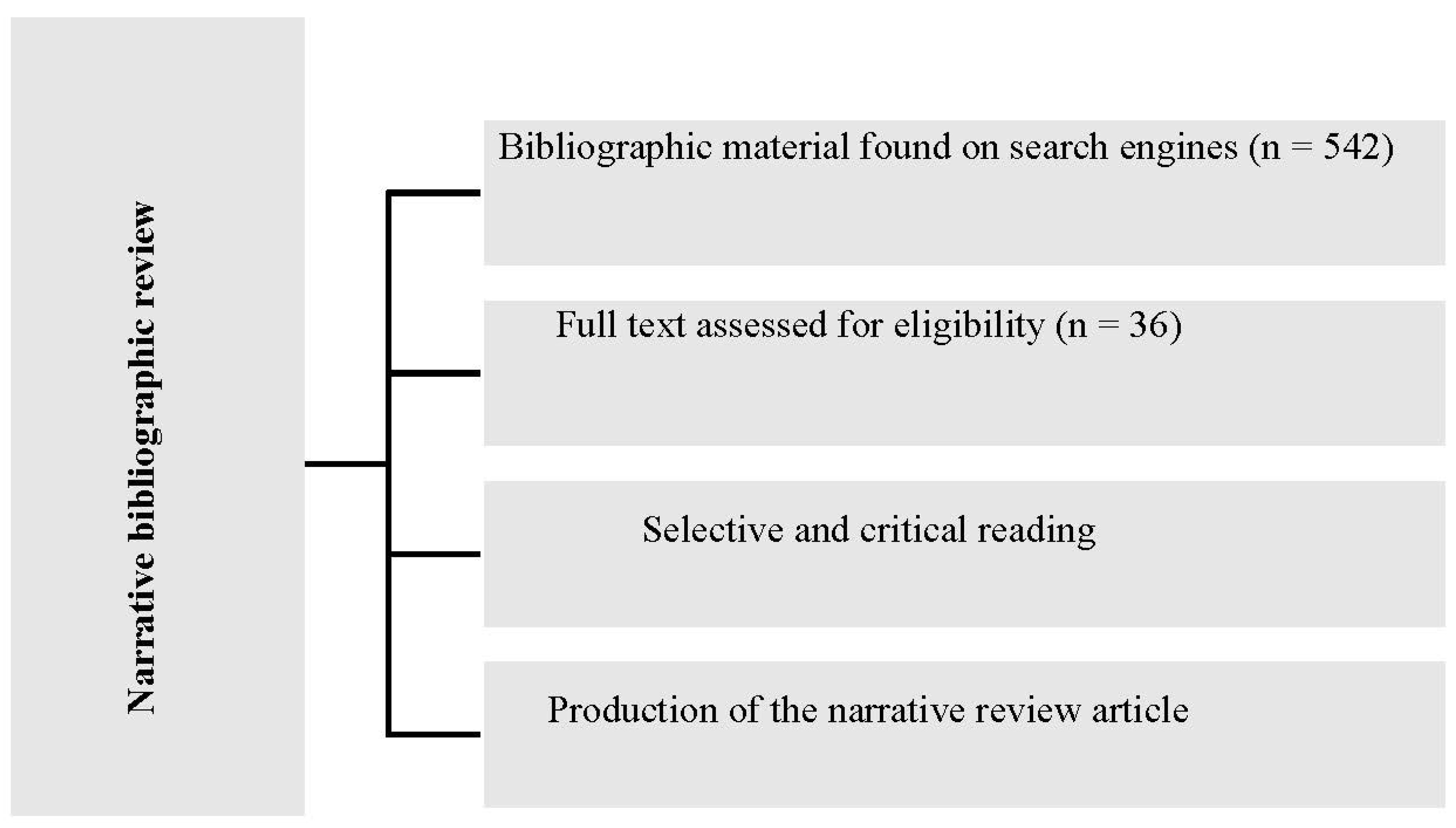
From the database search, 3 journal articles, 4 government bulletins and 535 scientific articles were identified (
Table 1). Of the total of 542 bibliographic materials, 36 were selected according to the selection criteria and objectives of the study, as shown in
Figure 1.
Pharmacovigilance
According to of the World Health Organization (WHO), pharmacovigilance is defined as “the science and activities related to the identification, evaluation, understanding and prevention of adverse effects or any other problems related to the use of medicines and vaccines” [
8].
Analyzing the timeline there are cases on record related to the use of medicines since the end of the 19th century, by way of illustration sudden death cause using chloroform in anesthesia and jaundice caused using arsenic in treating syphilis [
9]. However, the historic milestone of pharmacovigilance in the world is due to the so-called Thalidomide-disaster—thousands of cases of phocomelia (rare congenital malformation) cause using thalidomide for to treat or prevent nausea in pregnant women in the 50s and 60s. An increase from 1.5% to 20% in the incidence of cases of congenital malformations was observed in women who took thalidomide during pregnancy. A review of the studies carried out in the pre-commercial phase indicated that the data had been misinterpreted [
2,
10,
11].
After the world tragedy cause by the thalidomide, in 1961, a need was found to make international efforts on safety issues relating to medicinal products. The sixteenth World Health Event (1963) adopted a resolution reaffirming the need for immediate action in relation to the rapid dissemination of information on adverse reactions to medicines and which, subsequently, led to the creation of the Pilot Research Project for the international monitoring of medicines, since 1978, that is operationalized by Uppsala Monitoring Centre (UMC)
, in Sweden, through the pharmacovigilance network around the world. Nowadays, the program brings together more than 140 countries [
12].
From 1962 on, continuing the storyline, the North American regulatory agency (Food and Drug Administration) then began to require more rigorous non-clinical and clinical studies of drug manufacturers. In 1968, the WHO started the pilot phase of International Drug Adverse Reaction Monitoring Program, in which 10 countries were involved. Since 1978, the Program is implemented by UMC, in Sweden, and today brings together more than 130 countries.
In the late 90s, with the approval of the National Medicines and the establishment of the National Health Surveillance Agency (ANVISA), it was institutionalized the responsibility for establishing, coordinate and monitor the toxicological and pharmacological surveillance systems, in addition to regulating, controlling, and inspecting medicinal products for human use which pose a risk to public health in Brazil, a fact that enabled the implementation of a national pharmacovigilance program.
In Brazil, the Ordinance MS no. 696, from May 7th, 2001, established the National Medicines Monitoring Centre (CNMM), responsible for the implementation and coordination of the national pharmacovigilance system, based in pharmacovigilance of ANVISA, he was accepted into the International Program of the World Health Organization, as its 62nd effective member.
COVID-19 vaccines
The high morbidity and mortality rate of the COVID-19 pandemic has led to the largest and most diverse effort in vaccine development ever. Approximately 7 months after characterizing the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral genome, around 200 vaccines with different production platforms were at different stages of development, including at least five in phase III trials.
Although no vaccine is 100% effective, immunization has been instrumental in preventing deaths, severe cases of COVID-19, and containing the pandemic [
13].
Vaccination against COVID-19 in Brazil began in the second half of January 2021 with, two vaccines from separate laboratories: ChAdOx1 nCoV-19 developed by the British pharmaceutical group AstraZeneca, in partnership with the University of Oxford and technology transfer to the Oswaldo Cruz Foundation (FIOCRUZ), in Rio de Janeiro and CoronaVac, Butantan vaccine produced in partnership with the Chinese biopharmaceutical Sinovac. In May, there was the inclusion of a third BNT162b2 mRNA COVID-19 vaccine (Pfizer–BioNTech) and the fourth vaccine Ad26.COV2. S COVID-19 vaccine (Janssen/Johnson & Johnson) was acquired for use in Brazilian territory in June 2021.
As informed by the Butantan Institute, at the beginning of the vaccination program, these four vaccines against the disease were authorized by ANVISA for use in Brazil, being used in the National Immunization Program (PNI), of the Ministry of Health.
Epidemiologists point out that only rapid vaccination, combined with restrictive measures, can control COVID-19 transmission. “So far, vaccines have shown good results even with new variants”, they emphasized [
14]. And scientists point out that vaccination should be as rapid as possible, being a race against time, as the virus is constantly changing. The intention, with the speed of the vaccination process, is to prevent the virus from continuing to evolve and, eventually, new variants become even more threatening than previous variants.
In Brazil, according to research conducted in Londrina, Paraná, showed that 75% of deaths from COVID-19 recorded in the first ten months of 2021 occurred in individuals who were not immunized against the disease. The unvaccinated elderly died nearly three times as often as the immunized. Among people under 60 years of age, the number of deaths among unvaccinated individuals was 83 times higher than among immunized individuals [
15].
Adverse reaction
The systematic observation of adverse events to immunobiological medicinal products marketed by established practices is essential for the periodic evaluation of the benefit-risk ratio compared to known adverse events, as well as for the knowledge of rare and unspecified adverse events [
16].
Adverse events are coded according to the Medical Dictionary for Regulatory Activities—Meddra (Medical Dictionary for Regulatory Activities), a highly specific and standardized medical terminology, to facilitate the international exchange of regulatory information on medical products used by humans [
17].
Leonardi [
4] points out that the concept of pharmacovigilance established by the WHO has four major pillars related to adverse effects and problems related to the use of medicines: identification, evaluation, understanding and prevention.
When administering a drug or drug under study in addition to the useful therapeutic effects, in some people there are certain unwanted effects. Researchers are emphatic in reporting that there is no drug without risk of adverse reaction. The probability of occurrence may vary, the reaction may be mild or severe, it may be predictable or not, but the doctor/researcher and the patient/subject of the research should always be attentive to the possibility of its appearance [
18].
As a result of the pandemic caused by COVID-19, vaccines produced in record time, already in application, also cause adverse effects, as any drug in use. The Health Units that administer immunobiological (vaccines, serums, and immunoglobulins) must notify and investigate these occurrences and register them in the PNI Information System-Post- Vaccination Adverse Event (SIPNI-EAPV) to be analyzed by the state and national level [
19].
Since the beginning of the application of vaccines in Brazil, in early 2021, there was already the concern of the population as to the effects of the vaccines that would be applied; even so, Henze [
20] argues that “the observed side effects of the vaccine last little and the benefits of the vaccine outweigh”.
Health control
Although the speed in developing an effective and safe vaccine is essential in the face of a pandemic scenario, clinical development for authorization and licensing of emergency use is a major challenge. In this context, pharmacovigilance of vaccine safety and surveillance of virus variants are essential to ensure the well-being of the population. Thus, one of the most important responsibilities of the Surveillance Plan is to ensure the protection of human subjects participating in clinical trials [
21].
The Brazilian government, through the National Health Surveillance Agency, controls the use of medicines and their effects on citizens, whose negative reactions need to meet the provisions of RCB no 406/2020 on Good Pharmacovigilance Practices for Drug Registrants of Human Use [
22].
For the appropriate management of post-vaccine adverse events (EAPV) of a new vaccine, ANVISA says it is essential to have a sensitive surveillance system to assess the safety of the product and to respond quickly to all the concerns of the population related to vaccines. These activities require early notification and investigation of the event [
16].
According to the Agency, the EAPV Surveillance cycle consists of detection of suspected cases of adverse events following immunization (AEFI), notification, registration in information system, investigation (clinical examinations, laboratory tests, etc.) and active search for new events, evaluation of information, classification of causality, feedback, or timely feedback.
Thus, adverse events detected by health services are reported by health professionals in the e-SUS online system notifies (
https://notifica.saude.gov.br/), these records are investigated and closed by post-adverse events surveillance vaccination of the Municipal and State Immunization Coordination, with subsequent review and support by the Ministry of Health [
17]. In the case of living organisms and because it is a new disease, with characteristics still under constant research, taking into account its variants, this cycle will be repeated continuously, being up to health professionals and citizens, the responsibility to inform and notify related bodies about adverse events.
Vigimed, according to Leonardi [
23], was the tool created to report adverse events related to the use of medicines and vaccines, in an agreement signed between ANVISA and the WHO collaborating center, the UMC, a pharmacovigilance software for International Drug Monitoring.
According to ANVISA, after recording adverse effects in VigiMed, notifications are analyzed according to severity, the risk associated with the adverse event, predictability, that is, whether the event was expected or not, and the causal link between the event and the medicinal product or vaccine used [
24].
Depending on the case, the agency explains, the notification may lead to the opening of an investigation process and, therefore, several measures can be taken. These measures include from the communication of the health risk, from the elaboration and subsequent dissemination of alerts and reports, as well as changes to the package leaflet, restriction of use or marketing, prohibition of lots and even the cancellation of the health record of the medicine or vaccine.
Recent data
Rare adverse events have also been described following immunization with approved COVID-19 vaccines. In April 2021, five patients who had venous thrombosis and thrombocytopenia after receiving the first dose of the adenoviral vector vaccine ChAdOx1nCoV-19. These five cases occurred in a population of over 130,000 vaccinated persons, thus representing a rare condition defined as vaccine-induced immune thrombotic thrombocytopenia [
25,
26,
27].
In the United States, the Ad26.COV2. S COVID-19 vaccine (Janssen/Johnson & Johnson) was approved for emergency use on February 27, 2021. As of April 12, 2021, approximately 7 million doses of the Ad26 vaccine. COV2.S were administered in the US and 6 cases of cerebral venous sinus thrombosis with thrombocytopenia were identified among recipients, resulting in a temporary national pause in vaccination with this product on April 13, 2021 [
28,
29,
30].
Immediately after the granting of the authorization in the United Kingdom (United Kingdom) and the United States (US) in early/mid-December 2020, there were unique reports of reactions from hypersensitivity in a very small number of patients, possibly due to a component in the Pfizer/BioNTech BNT162B2 or Moderna mRNA-1273 vaccines Both, so there is a consensus that these vaccines are contraindicated only when there are allergy to preparations in similar new mRNA technologies. These reactions were resolved after treatment is one of the components of the vaccine or in cases of severe allergic reaction to the first dose [
31]. Rare cases of myocarditis and pericarditis have been reported especially ins, both, so there is a consensus that these vaccines are contraindicated only when there are allergy to preparations in similar new mRNA technologies. These reactions were resolved after treatment is one of the components of the vaccine or in cases of severe allergic reaction to the first dose [
31]. Rare cases of myocarditis and pericarditis have been reported especially in in male adolescents and young adults several days after COVID-19 mRNA vaccination (Pfizer- BioNTech or Moderna) [
32].
The CoronaVac vaccine in phase 1 and 2 trials showed good safety, tolerability, and immunogenicity in healthy adults 18 years of age and older. However, there are reports of pityriasis rosea that developed 4 days after the first dose of the vaccine and were present for 1 week in a phase 3 clinical trial conducted in Turkey [
33]. Acute rheumatoid arthritis is also reported for 18 days with the presence of swelling and pain in the left knee joint after CoronaVac vaccination.
According to the International Council for Harmonization of Technical Requirements for Pharmaceuticals [
34], systematic observation of adverse events to immunobiological medicinal products marketed by established practices is essential for periodic risk-benefit assessment in comparison with known adverse events, as well as for the knowledge of rare adverse events and not described in drug leaflet that, perhaps, may arise.
ANVISA, considering international specifications, performed its control of AEFI considering the Organ Class System and Term Preference and calculated its incidence per 1,000 doses applied for the non-serious events, and a hundred thousand doses applied to severe and rare events [
12].
In periodic bulletins, the agency reports that very rare, serious events and deaths are still discussed weekly in the Inter-institutional Committee for Pharmacovigilance of Vaccines and other Immunobiological—formed by the PNI/SVS, Pharmacovigilance Management, National Health Surveillance Agency, National Institute of Health Quality Control, in addition to specialists with expertise in vaccinations and pharmacovigilance of vaccines, including immunologists, infectiologists, neurologists, cardiologists, rheumatologists and pediatricians.
In the first four months of the campaign (January 18th to May 23rd, 2021) 74,563 cases of AEFI were reported. The incidence of AEFI is observed per 1,000 doses administered each day of vaccination. Of the reported AEFI, 70,110 were classified as EANG and 4,453 events were classified as EAG, of which 2,277 were related to deaths [
17].
Table 2 shows the incidence of AEFI by type of vaccine, corresponding to the first five months of the campaign, still without the use of Janssen’s immunization.
It is noteworthy, however, according to the Ministry of Health, that direct comparisons of the incidence of these events between the different vaccines should take into account the vaccinated population with each immunobiological, that vaccination was initiated by the most vulnerable groups, such as the elderly—who present a higher risk of occurrence of serious coincident adverse events (adverse events caused by other conditions and not by vaccines)—and health professionals—which are more sensitive to notification and adverse events, and the incorporation of the different vaccines has been done sequentially [
7]. Thus, according to the data presented, pharmacological surveillance has been performed, responding to vaccination cycles, as groups become eligible for receiving immunization.
Discussion
It is worth remembering that Brazil is internationally recognized by its National Vaccination Plan. Created in 1973, the program makes vaccines available to the population through the Brazil’s Unified Health System, one of the largest free health service delivery programs in the world. Through massive vaccination, Brazil has already eradicated diseases such as smallpox and polio (childhood paralysis), offering to its population all vaccines recommended by the World Health Organization [
35].
During the year 2020, the race for the creation and effectiveness of the immunizing, raised ethical debates that continue to be discussed, which implies the acceptance of the population to take the vaccines applied in Brazil, especially when one thinks about their side effects, which are their side effects.
In this sense, Colgrove [
17] already recalled that, among the risks related to vaccines, non- vaccination is considered the most important. The deleterious effects associated with the use of vaccines, when present and scientifically proven, occur at a very low frequency and are insignificant when compared to the risks related to non-vaccination.
Strategies to encourage the use of vaccines, the author continues, “are traditionally adopted in public health, but may be insufficient to ensure an increase in vaccine coveragel” [
17]. In this context, it is necessary to maintain a clear understanding of the value of vaccines.
Both in the population and among health professionals, who demonstrate resistance to immunization in all parts of the world, either by personal beliefs, or by the influence of information which has been misinterpreted and widely disseminated over the Internet.
The importance of vaccination at the present, while there are available immunizers, provides for the non-proliferation of the disease, since the mutating virus has been kept moving, reaching vaccinated and immunized, which does not interrupt the disease cycle.
Corroborating this statement, the reports reported by Carbinatto [
18] point out that different sequences indicate that SARS-Cov-2 mutates, forming “sub-groups” of the same virus. According to estimates, the study points out, this has been happening at a frequency of about one mutation per month. But these changes also reveal which viruses are closest to each other and which are more distant—by allowing the construction of a “family tree”.
Variants of the disease have already been introduced worldwide and, in several countries with high levels of vaccinations, restrictive measures have again been imposed, with a view to increasing the contamination of even those who have chosen to receive the available immunizer, with the aggravation of variants carrying more aggressive and lethal strains, still under study for the adaptation of new immunizing. Adverse reactions are common to any medicine, they are frightening due to the unprecedented nature of COVID-19, but they are like known effects, which does not discredit the effectiveness of vaccination.
Conclusion
The Brazilian data for adverse events, although the country is continental in its size, are acceptable given the number of deaths recorded. It is known that this investigative process must be permanent, including new immunized groups, new immunizers approved and acquired by governments, the cooperation of health workers belonging to the immunization program, throughout the network involved in combating coronavirus.
Pharmacovigilance is effective, and it is necessary, as in so many other public services, to be shared for the knowledge of the population that ignores such salutary and indispensable information in times of false information, of disinformation and resistance to free medical treatments available in Brazil.
Lastly, it is necessary to give credit to science and trust in public measures to control this disease and all others that require the use of drugs, because there is a commitment of the agency responsible for this health control, there is transparency in the policy used and there is availability of all the inherent information, allowing the Brazilian population, in particular, to credit the Unified Health System and its assistance programs employed, with emphasis on the National Vaccination Program, in evidence at the moment.
Author Contributions
Moraes MC: conceptualization data curation, analysis, methodology, investigation; Duarte M: data curation, visualization, writing-original draft and writing-review and editing; Nunes R: project administration, resources and supervision. All authors reviewed and approved the final version submitted for publication.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pharmacovigilance Risk Assessment Committee. PRAC Strategy on Measuring the Impact of Pharmacovigilance Activities (Revision 2). London: European Medicine Agency; 2022. Available from: https://www.ema.europa.eu/en/documents/other/prac-strategy-measuring-impact-pharmacovigilance-activities_en.pdf. Accessed in 2023 (Aug. 25).
- Fornasier G, Francescon S, Leone R, Baldo P. An historical overview over pharmacovigilance. Int J Clin Pharm. 2018;40(4):744-7. PMID: 29948743. [CrossRef]
- Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-33. PMID: 31978945. [CrossRef]
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. PMID: 31986264. [CrossRef]
- Friedler A. Sociocultural, behavioural and political factors shaping the COVID-19 pandemic: the need for a biocultural approach to understanding pandemics and (re)emerging pathogens. Glob Public Health. 2020;16(1):17-35. PMID: 33019889. [CrossRef]
- Gully PR. Pandemics, regional outbreaks, and sudden-onset disasters. Healthc Manage Forum. 2020;33(4):164-9. PMID: 32022584. [CrossRef]
- Hadj Hassine I. Covid-19 vaccines and variants of concern: a review. Rev Med Virol. 2021;32(4):e2313. PMID: 34755408. [CrossRef]
- World Health Organization. Diagnostics laboratory emergency use listing. 2023. Available from: https://cdn.who.int/media/docs/default-source/in-vitro-diagnostics/200703-pqt-ivd-352-v2-eul-immunoassay-requirements-ncov.pdf?sfvrsn=bdf71066_2&download=true. Accessed in 2023 (Aug. 28).
- Routledge P. 150 years of pharmacovigilance. Lancet. 1998;351(9110):1200-1. PMID: 9643710. [CrossRef]
- Mcbride WG. Thalidomide and congenital abnormalities. Lancet. 1961;278(7216):1358. [CrossRef]
- Ridings JE. The thalidomide disaster, lessons from the past. Methods Mol Biol. 2013; 947:575-86. https://link.springer.com/protocol/10.1007/978-1-62703-131-8_36.
- Instituto Butantan. Quais são as diferenças entre as vacinas contra Covid-19 que estão sendo aplicadas no Brasil? Portal do Butantan. 2021. Available from: https://butantan.gov.br/covid/butantan-tira-duvida/tira-duvida-noticias/quais-sao-as-diferencas-entre-as-vacinas-contra-covid-19-que-estao-sendo-aplicadas-no-brasil. Accessed in 2023 (Aug. 28).
- Alegretti L. Vacina contra Covid-19: quais os efeitos colaterais mais comuns e por que não há motivo para se preocupar. BBC News Brasil. 19 jun 2021. Available from: https://www.bbc.com/portuguese/geral-57435543. Accessed in 2022 (Nov. 28).
- Instituto Butantan. Não vacinados representam 75% das mortes por Covid-19, diz estudo brasileiro. Portal do Butantan. 2022. Available from: https://butantan.gov.br/noticias/nao-vacinados-representam-75-das-mortes-por-covid-19-diz-estudo-brasileiro?fbclid=IwAR1yIBgJK5vtpLpkFq1eTq36mxMAz%20SD2F82hVDu56DDHAHtw7DJdLO10Ovk. Accessed in 2022 (Nov. 28).
- Brasil. Protocolo de vigilância epidemiológica de eventos adversos pós-vacinação. Estratégia de vacinação contra o vírus SARSCoV-2 (Covid19). Brasília-DF: Ministério da Saúde; 2020. Disponível em: https://www.gov.br/saude/pt-br/coronavirus/vacinas/pni. Acesso em: 02/08/2021.
- Brasil. Boletim Epidemiológico Especial, no. 68. Doença pelo Coronavírus COVID-19. Semana Epidemiológica 24 (13/6 a 19/6/2021). Brasília-DF: Ministério da Saúde; 2021. Available from: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/covid-19/2021/68_boletim_epidemiologico_covid.pdf. Accessed in 2022 (Nov. 28).
- Brasil. Boletim Epidemiológico. Situação epidemiológica dos eventos adversos pós-vacinação contra a covid-19, Brasília-DF: Ministério da Saúde; Volume 52 | Nº 9 | Mar. 2021. Available from: https://sbim.org.br/images/files/notas-tecnicas/boletim-epidemio-09v52-eapv-covid19-210315.pdf/. Accessed in 2023 (Set 02).
- Brasil. Secretaria de Saúde da Bahia. Eventos Adversos Pós-Vacinação. 2022. Available from: http://www.saude.ba.gov.br/suvisa/vigilancia-epidemiologica/%20eventos-adversos-pos-vacinacao/. Accessed in 2023 (Aug. 28).
- Beninger P. Pharmacovigilance: An Overview. Clin Ther. 2018;40(12):1991-2004. PMID: 30126707. [CrossRef]
- Henze R. Quais são os efeitos colaterais mais comuns das vacinas contra a Covid-19? 2021. Available from: https://www.selecoes.com.br/coronavirus/vacinas-contra-a-covid-19-quais-sao-os-efeitos-colaterais-mais-comuns/. Acessed in 2023 (Aug. 30).
- Brasil. Agência Nacional de Vigilância Sanitária. Farmacovigilância. 2020. Available from: https://www.gov.br/anvisa/pt-br/assuntos/noticias-anvisa/2020/confira-o-painel-de-notificacoes-de-farmacovigilancia. Accessed in 2023 (Aug. 30).
- Leonardi E. Farmacovigilância - ANVISA e oms inovam na notificação de eventos adversos. ICTQ. 2018. Available from: https://ictq.com.br/industria-farmaceutica/821-farmacovigilancia-anvisa-e-oms-inovam-na-notificacao-de-eventos-adversos. Accessed in 2023 (Aug. 30).
- Brasil. Evento adverso: o que a Anvisa faz com a sua notificação. ANVISA; 2021. Available from: https://www.gov.br/anvisa/pt-br/assuntos/noticias-anvisa/2021/evento-adverso-o-que-a-anvisa-faz-com-a-sua-notificacao. Accessed in 2023 (Aug. 30).
- Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. 2021;384(22):2124-30. [CrossRef]
- Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med. 2021;384(22):2092-101. [CrossRef]
- Scully M, Singh D, Lown R, et al. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. 2021;384(23):2202-11. [CrossRef]
- See I, Su JR, Lale A, et al. US Case Reports of Cerebral Venous Sinus Thrombosis With Thrombocytopenia After Ad26.COV2.S Vaccination, March 2 to April 21, 2021. JAMA. 2021;325(24):2448-56. PMID: 33929487; [CrossRef]
- Centers for Disease Control and Prevention. Joint CDC and FDA Statement on Johnson & Johnson COVID-19 Vaccine. CDC Newsroom; 2021. Available from: https://www.cdc.gov/media/releases/2021/s0413-JJ-vaccine.html. Accessed in 2023 (Aug. 30).
- Shay DK, Gee J, Su JR, et al. Safety Monitoring of the Janssen (Johnson & Johnson) COVID-19 Vaccine — United States, march–april 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):680-4. PMID: 33956784; [CrossRef]
- Sokolowska M, Eiwegger T, Ollert M, et al. EAACI statement on the diagnosis, management and prevention of severe allergic reactions to COVID-19 vaccines. Allergy. 2021;76(6):1629-39. PMID: 33452689; [CrossRef]
- Centers for Disease Control and Prevention. Myocarditis and Pericarditis After mRNA COVID-19 Vaccination. CDC; 2022. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html. Accessed in 2023 (Aug. 30).
- Akdaş E, İlter N, Öğüt B, Erdem Ö. Pityriasis rosea following CoronaVac COVID-19 vaccination: a case report. J Eur Acad Dermatol Venereol. 2021;35(8):e491-3. PMID: 33914958; [CrossRef]
- International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use. Pharmacovigilance Planning: E2E – Current Step 4 version. 2004. Available from: https://www.ich.org/page/efficacy-guidelines. Acessed in 2023 (Aug. 30).
- Jaguraba M. Vacinação: PNI do Brasil é um dos melhores do mundo. Vatican News; 2018. Available from: https://www.vaticannews.va/pt/mundo/news/2018-07/vacinacao-pni-do-brasil-um-dos-melhores-do-mundo.html. Accessed in 2023 (Aug. 30).
- Colgrove J. Vaccine refusal revisited – the limits of public health persuasion and coercion. N Engl J Med. 2016;375(14):1316-7. PMID: 27705252; [CrossRef]
- Carbinatto B. Por que sequenciar o genoma do novo coronavírus é importante. Super Interessante; 2020. Available from: https://super.abril.com.br/ciencia/por-que-sequenciar-o-genoma-do-novo-coronavirus-e-importante/. Accessed in 2023 (Aug. 30).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).