Submitted:
05 December 2023
Posted:
07 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
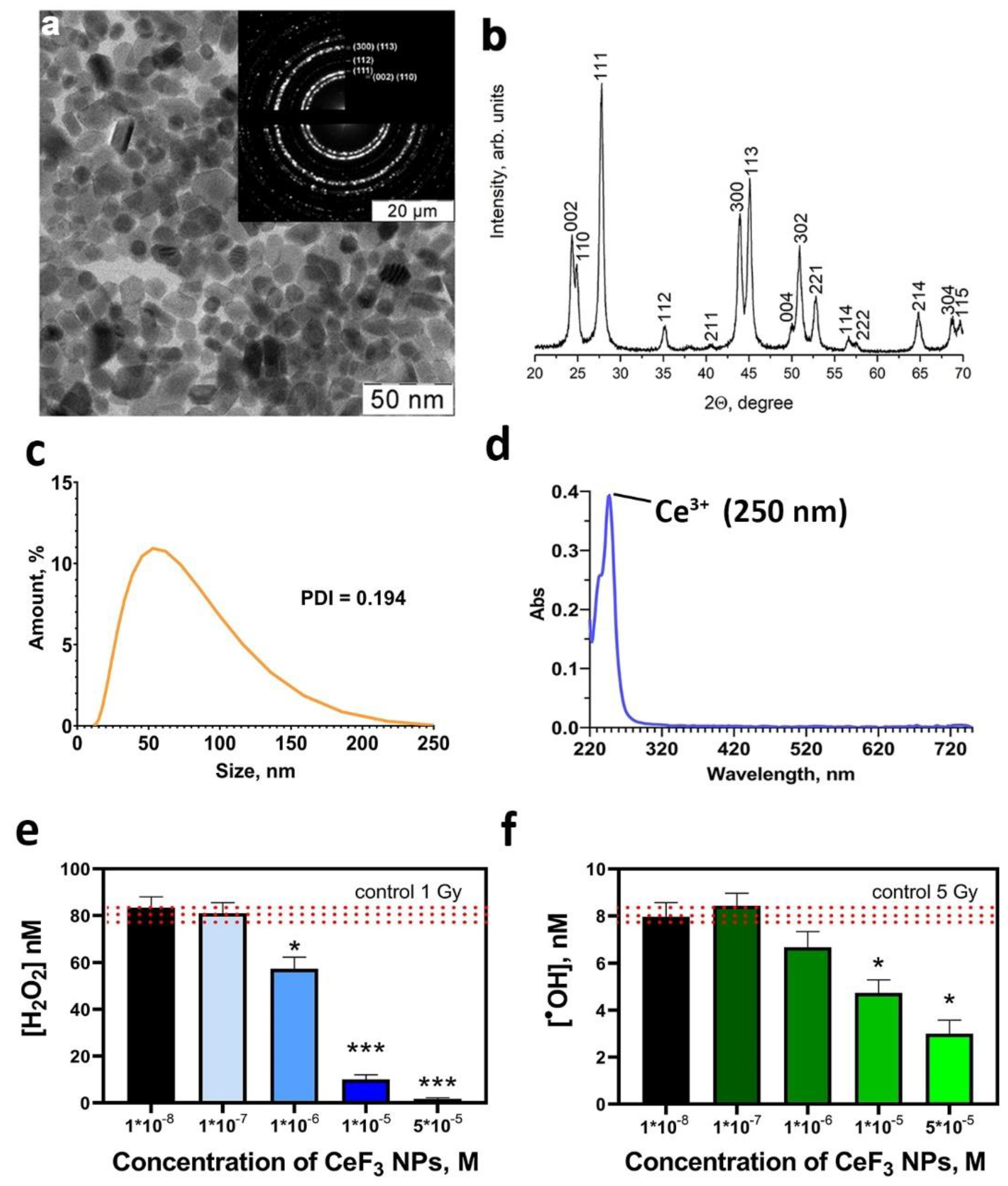
2.1. Synthesis and Characterization of CeF3 NPs
2.2. Cell Cultures
2.3. X-ray Irradiation
2.4. Assessment of CeF3 NPs Antioxidant Activity
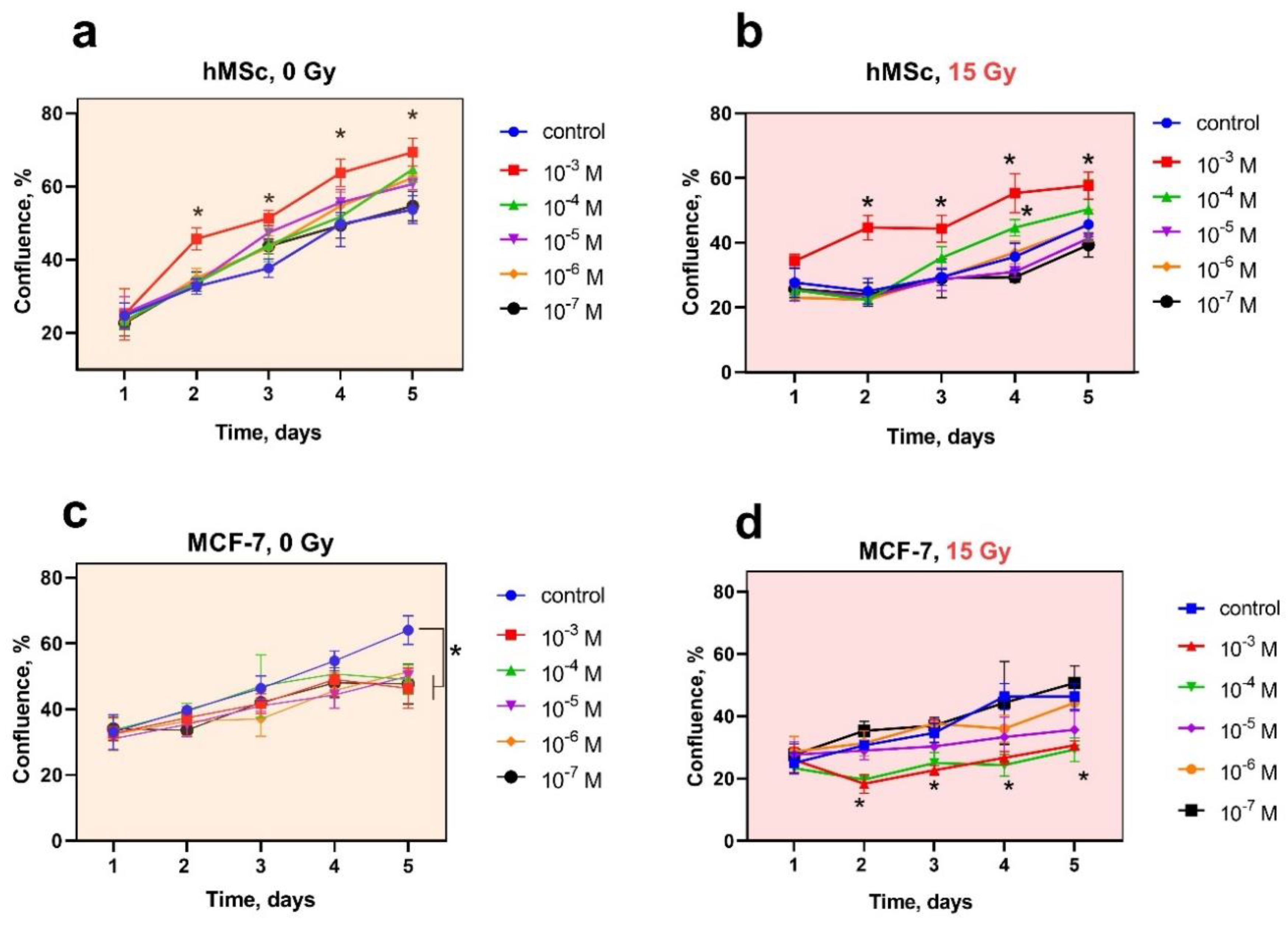
2.5. Cell Proliferation Analysis
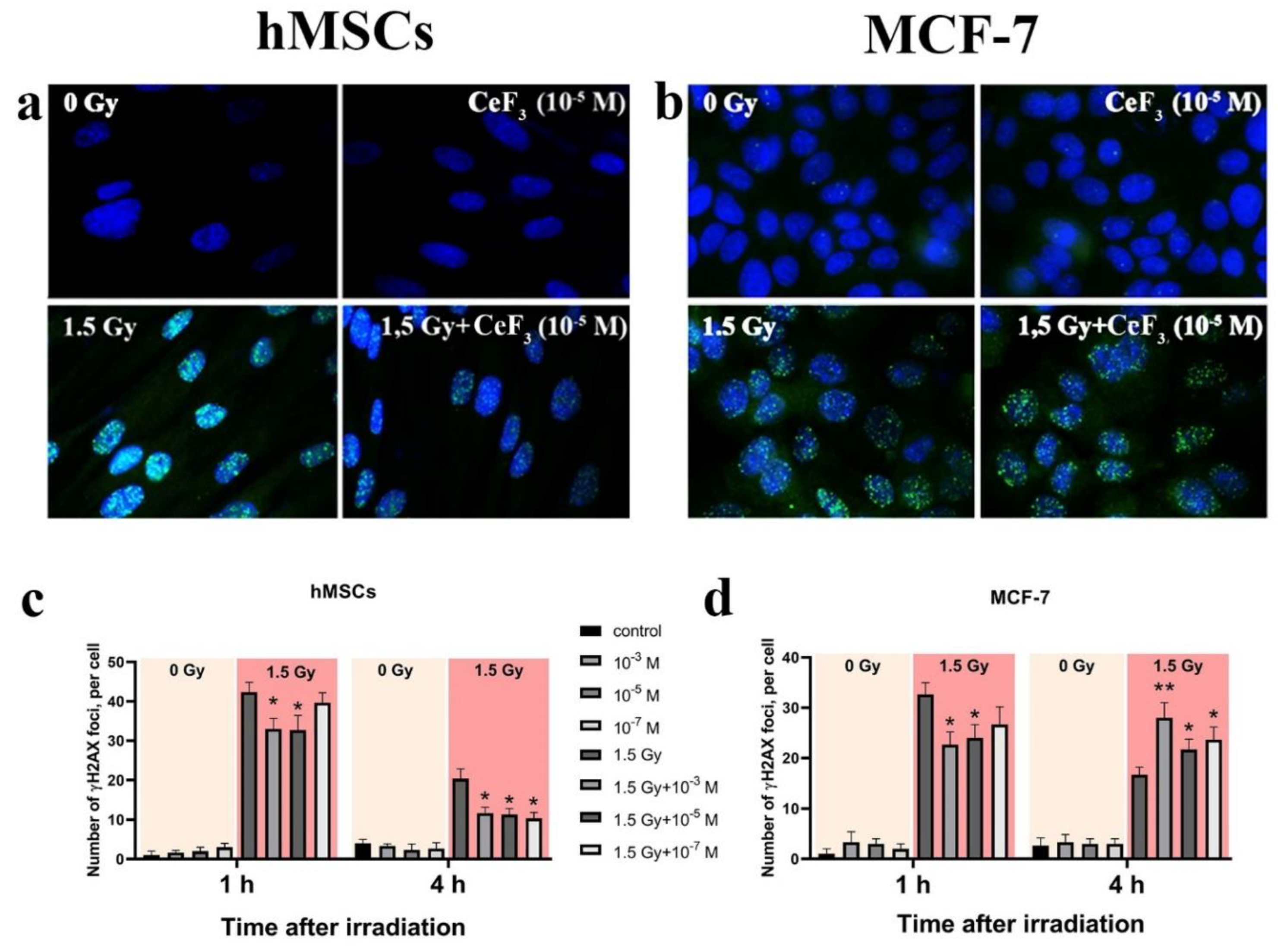
2.6. Analysis of DNA Double-Strand Breaks by γ-H2AX Foci
2.7. RT-PCR (Genes Expression Analysis)
2.8. Statistical Analysis
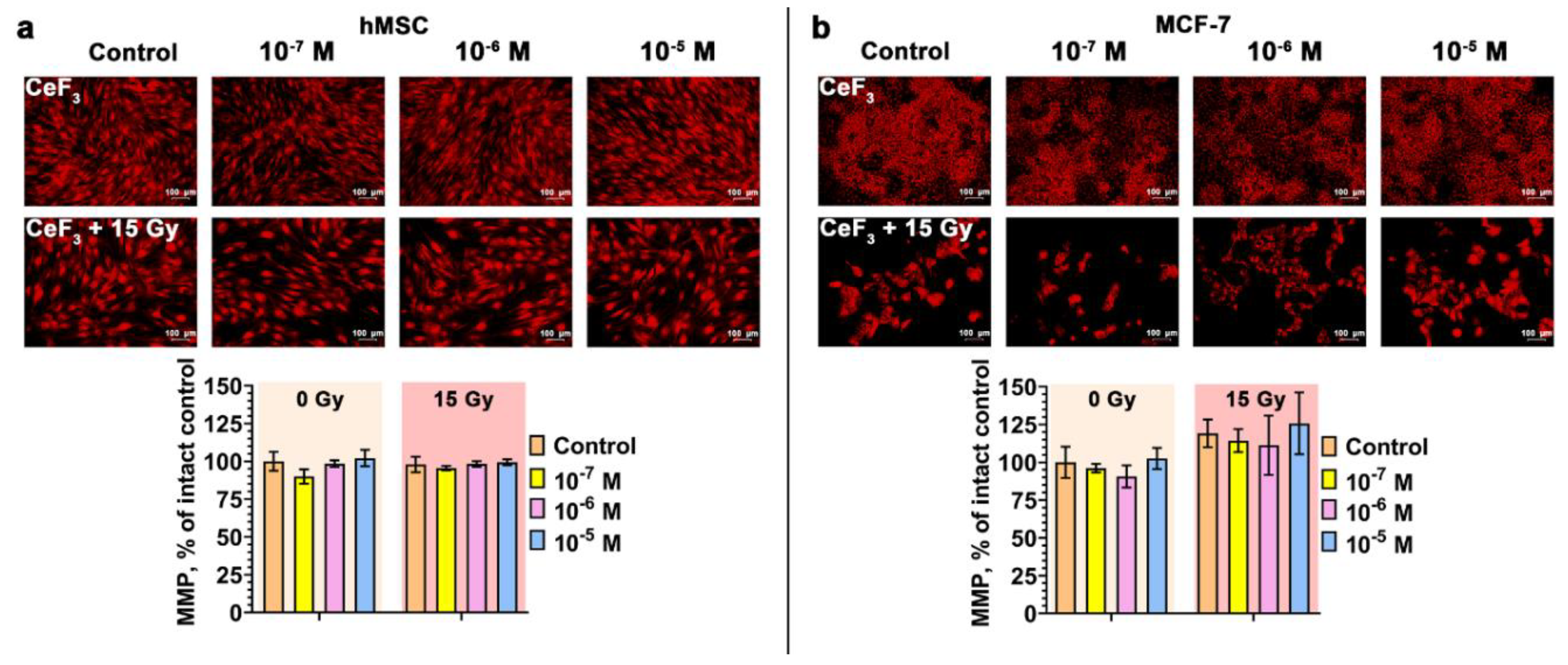
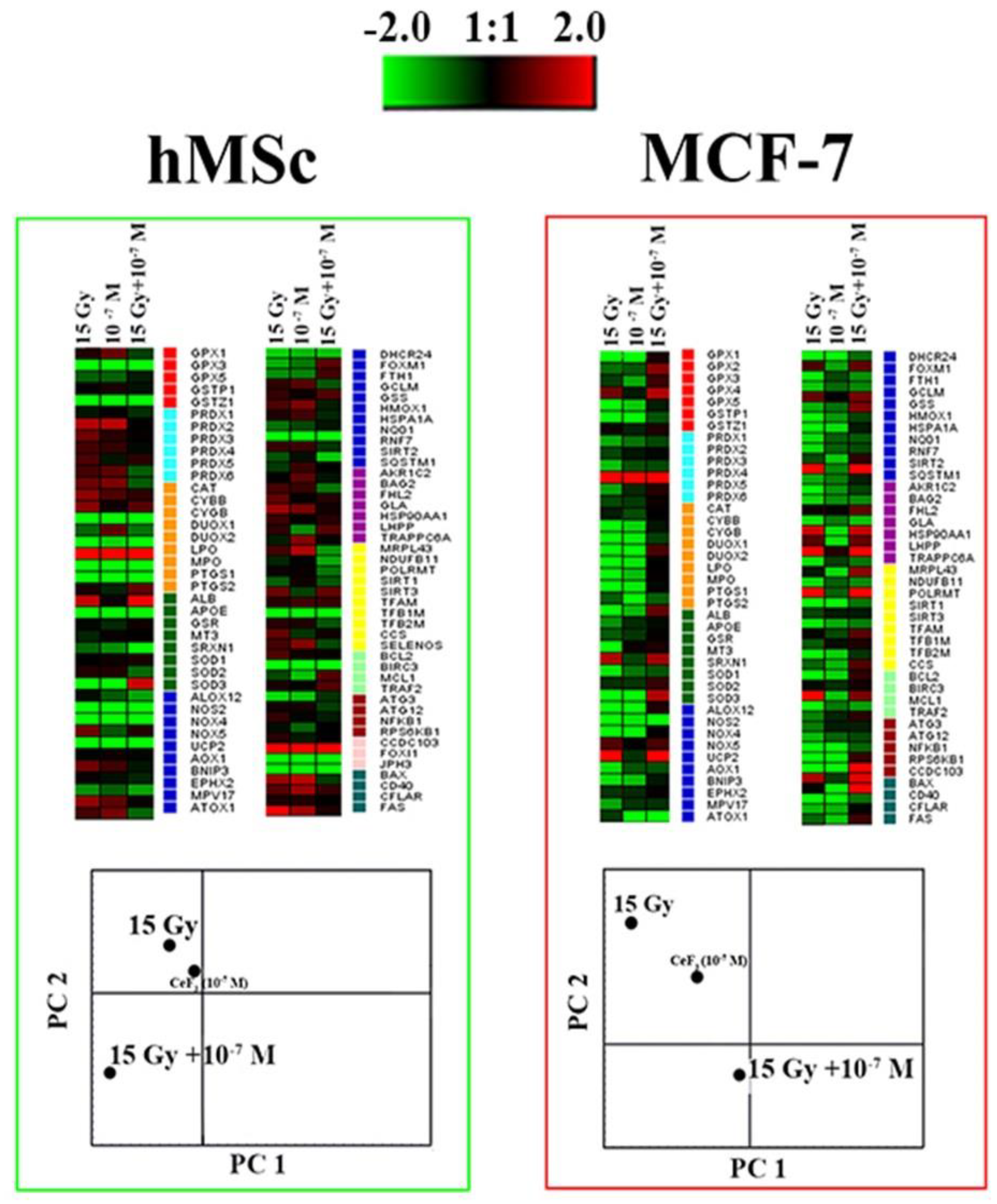
3. Results and Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int J Med Sci 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J Clin Biochem 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Soto, G.; Ortiz-López, R.; Rojas-Martínez, A. Ionizing Radiation-Induced DNA Injury and Damage Detection in Patients with Breast Cancer. Genet Mol Biol 2015, 38, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tang, B.; Xie, X.; Xiao, Y.-F.; Yang, S.-M.; Zhang, J.-W. The Interplay between DNA Repair and Autophagy in Cancer Therapy. Cancer Biol Ther 2015, 16, 1005–1013. [Google Scholar] [CrossRef]
- Elsaid, M.Y.; Shahi, A.; Wang, A.R.; Baiu, D.C.; Li, C.; Werner, L.R.; Singhal, S.; Hall, L.T.; Weichert, J.P.; Armstrong, E.A.; et al. Enhanced Radiosensitivity in Solid Tumors Using a Tumor-Selective Alkyl Phospholipid Ether Analog. Molecular Cancer Therapeutics 2018, 17, 2320–2328. [Google Scholar] [CrossRef] [PubMed]
- Moding, E.J.; Kastan, M.B.; Kirsch, D.G. Strategies for Optimizing the Response of Cancer and Normal Tissues to Radiation. Nat Rev Drug Discov 2013, 12, 526–542. [Google Scholar] [CrossRef]
- Hu, Y.; Paris, S.; Barsoumian, H.; Abana, C.O.; He, K.; Wasley, M.; Younes, A.I.; Masrorpour, F.; Chen, D.; Yang, L.; et al. Radiation Therapy Enhanced by NBTXR3 Nanoparticles Overcomes Anti-PD1 Resistance and Evokes Abscopal Effects. Int J Radiat Oncol Biol Phys 2021, 111, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Bagley, A.F.; Ludmir, E.B.; Maitra, A.; Minsky, B.D.; Li Smith, G.; Das, P.; Koong, A.C.; Holliday, E.B.; Taniguchi, C.M.; Katz, M.H.G.; et al. NBTXR3, a First-in-Class Radioenhancer for Pancreatic Ductal Adenocarcinoma: Report of First Patient Experience. Clin Transl Radiat Oncol 2022, 33, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Verry, C.; Dufort, S.; Villa, J.; Gavard, M.; Iriart, C.; Grand, S.; Charles, J.; Chovelon, B.; Cracowski, J.-L.; Quesada, J.-L.; et al. Theranostic AGuIX Nanoparticles as Radiosensitizer: A Phase I, Dose-Escalation Study in Patients with Multiple Brain Metastases (NANO-RAD Trial). Radiother Oncol 2021, 160, 159–165. [Google Scholar] [CrossRef]
- Pudovkin, M.S.; Zelenikhin, P.V.; Shtyreva, V.; Morozov, O.A.; Koryakovtseva, D.A.; Pavlov, V.V.; Osin, Y.N.; Evtugyn, V.G.; Akhmadeev, A.A.; Nizamutdinov, A.S.; et al. Coprecipitation Method of Synthesis, Characterization, and Cytotoxicity of Pr3+:LaF3 (CPr = 3, 7, 12, 20, 30%) Nanoparticles. Journal of Nanotechnology 2018, 2018, e8516498. [Google Scholar] [CrossRef]
- Shcherbakov, A.B.; Zholobak, N.M.; Baranchikov, A.E.; Ryabova, A.V.; Ivanov, V.K. Cerium Fluoride Nanoparticles Protect Cells against Oxidative Stress. Materials Science and Engineering: C 2015, 50, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, A.; Popov, A.; Ermakova, O.; Ivanova, O.; Baranchikov, A.; Kamenskikh, K.; Shekunova, T.; Shcherbakov, A.; Popova, N.; Ivanov, V. The First Inorganic Mitogens: Cerium Oxide and Cerium Fluoride Nanoparticles Stimulate Planarian Regeneration via Neoblastic Activation. Materials Science and Engineering: C 2019, 104, 109924. [Google Scholar] [CrossRef] [PubMed]
- Clement, S.; Deng, W.; Camilleri, E.; Wilson, B.C.; Goldys, E.M. X-Ray Induced Singlet Oxygen Generation by Nanoparticle-Photosensitizer Conjugates for Photodynamic Therapy: Determination of Singlet Oxygen Quantum Yield. Sci Rep 2016, 6, 19954. [Google Scholar] [CrossRef] [PubMed]
- Losytskyy, M.; Kuzmenko, L.; Shcherbakov, O.; Gamaleia, N.; Marynin, A.; Yashchuk, V.; Shcherbakov, A. Energy Transfer in Ce0.85Tb0.15F3 Nanoparticles-CTAB Shell-Chlorin E6 System. Nanoscale Research Letters 2017, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.H.; Hwang, M.P.; Kim, S.Y.; Jang, H.S.; Lee, K.H. A Systematic In-Vivo Toxicity Evaluation of Nanophosphor Particles via Zebrafish Models. Biomaterials 2014, 35, 440–449. [Google Scholar] [CrossRef] [PubMed]
- McCormack, R.N.; Mendez, P.; Barkam, S.; Neal, C.J.; Das, S.; Seal, S. Inhibition of Nanoceria’s Catalytic Activity Due to Ce3+ Site-Specific Interaction with Phosphate Ions. J. Phys. Chem. C 2014, 118, 18992–19006. [Google Scholar] [CrossRef]
- Kim, C.K.; Kim, T.; Choi, I.-Y.; Soh, M.; Kim, D.; Kim, Y.-J.; Jang, H.; Yang, H.-S.; Kim, J.Y.; Park, H.-K.; et al. Ceria Nanoparticles That Can Protect against Ischemic Stroke. Angewandte Chemie International Edition 2012, 51, 11039–11043. [Google Scholar] [CrossRef] [PubMed]
- Soh, M.; Kang, D.-W.; Jeong, H.-G.; Kim, D.; Kim, D.Y.; Yang, W.; Song, C.; Baik, S.; Choi, I.-Y.; Ki, S.-K.; et al. Ceria–Zirconia Nanoparticles as an Enhanced Multi-Antioxidant for Sepsis Treatment. Angewandte Chemie 2017, 129, 11557–11561. [Google Scholar] [CrossRef]
- Szczeszak, A.; Ekner-Grzyb, A.; Runowski, M.; Szutkowski, K.; Mrówczyńska, L.; Kaźmierczak, Z.; Grzyb, T.; Dąbrowska, K.; Giersig, M.; Lis, S. Spectroscopic, Structural and in Vitro Cytotoxicity Evaluation of Luminescent, Lanthanide Doped Core@shell Nanomaterials GdVO4:Eu3+5%@SiO2@NH2. Journal of Colloid and Interface Science 2016, 481, 245–255. [Google Scholar] [CrossRef]
- Runowski, M.; Ekner-Grzyb, A.; Mrówczyńska, L.; Balabhadra, S.; Grzyb, T.; Paczesny, J.; Zep, A.; Lis, S. Synthesis and Organic Surface Modification of Luminescent, Lanthanide-Doped Core/Shell Nanomaterials (LnF3@SiO2@NH2@Organic Acid) for Potential Bioapplications: Spectroscopic, Structural, and in Vitro Cytotoxicity Evaluation. Langmuir 2014, 30, 9533–9543. [Google Scholar] [CrossRef]
- Ansari, A.A.; Parchur, A.K.; Kumar, B.; Rai, S.B. Highly Aqueous Soluble CaF2:Ce/Tb Nanocrystals: Effect of Surface Functionalization on Structural, Optical Band Gap, and Photoluminescence Properties. J Mater Sci: Mater Med 2016, 27, 178. [Google Scholar] [CrossRef] [PubMed]
- Grzyb, T.; Runowski, M.; Szczeszak, A.; Lis, S. Influence of Matrix on the Luminescent and Structural Properties of Glycerine-Capped, Tb3+-Doped Fluoride Nanocrystals. J. Phys. Chem. C 2012, 116, 17188–17196. [Google Scholar] [CrossRef]
- Ansari, A.A. Influence of Surface Functionalization on Structural and Photo-Luminescence Properties of CeF3:Tb Nanoparticles. Applied Surface Science 2017, 409, 285–290. [Google Scholar] [CrossRef]
- Samanta, T.; Sarkar, S.; Adusumalli, V.N.K.B.; Praveen, A.E.; Mahalingam, V. Enhanced Visible and near Infrared Emissions via Ce3+ to Ln3+ Energy Transfer in Ln3+-Doped CeF3 Nanocrystals (Ln = Nd and Sm). Dalton Trans. 2015, 45, 78–84. [Google Scholar] [CrossRef]
- Wang, J.; Ansari, A.A.; Malik, A.; Syed, R.; Ola, M.S.; Kumar, A.; AlGhamdi, K.M.; Khan, S. Highly Water-Soluble Luminescent Silica-Coated Cerium Fluoride Nanoparticles Synthesis, Characterizations, and In Vitro Evaluation of Possible Cytotoxicity. ACS Omega 2020, 5, 19174–19180. [Google Scholar] [CrossRef]
- Dong, H.; Du, S.-R.; Zheng, X.-Y.; Lyu, G.-M.; Sun, L.-D.; Li, L.-D.; Zhang, P.-Z.; Zhang, C.; Yan, C.-H. Lanthanide Nanoparticles: From Design toward Bioimaging and Therapy. Chem. Rev. 2015, 115, 10725–10815. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Li, T.; Chen, Z.; Geng, Y.; Xie, X.; Li, S.; Yang, H.; Wu, C.; Liu, Y. Luminescent/Magnetic PLGA-Based Hybrid Nanocomposites: A Smart Nanocarrier System for Targeted Codelivery and Dual-Modality Imaging in Cancer Theranostics. IJN 2017, 12, 4299–4322. [Google Scholar] [CrossRef] [PubMed]
- Asadullina, N.R.; Usacheva, A.M.; Smirnova, V.S.; Gudkov, S.V. Antioxidative and Radiation Modulating Properties of Guanosine-5′-Monophosphate. Nucleosides, Nucleotides & Nucleic Acids 2010, 29, 786–799. [Google Scholar] [CrossRef]
- Manevich, Y.; Held, K.D.; Biaglow, J.E. Coumarin-3-Carboxylic Acid as a Detector for Hydroxyl Radicals Generated Chemically and by Gamma Radiation. Radiat Res 1997, 148, 580–591. [Google Scholar]
- Wang, Z.L.; Quan, Z.W.; Jia, P.Y.; Lin, C.K.; Luo, Y.; Chen, Y.; Fang, J.; Zhou, W.; O’Connor, C.J.; Lin, J. A Facile Synthesis and Photoluminescent Properties of Redispersible CeF3, CeF3:Tb3+, and CeF3:Tb3+/LaF3 (Core/Shell) Nanoparticles. Chem. Mater. 2006, 18, 2030–2037. [Google Scholar] [CrossRef]
- Gai, S.; Yang, P.; Li, X.; Li, C.; Wang, D.; Dai, Y.; Lin, J. Monodisperse CeF3, CeF3:Tb3+, and CeF3:Tb3+@LaF3 Core/Shell Nanocrystals: Synthesis and Luminescent Properties. J. Mater. Chem. 2011, 21, 14610–14615. [Google Scholar] [CrossRef]
- Greenhaus, H.L.; Feibush, A.M.; Gordon, L. Ultraviolet Spectrophotometric Determination of Cerium(III). Anal. Chem. 1957, 29, 1531–1534. [Google Scholar] [CrossRef]
- Malyukin, Y.; Maksimchuk, P.; Seminko, V.; Okrushko, E.; Spivak, N. Limitations of Self-Regenerative Antioxidant Ability of Nanoceria Imposed by Oxygen Diffusion. J. Phys. Chem. C 2018, 122, 16406–16411. [Google Scholar] [CrossRef]
- Dutta, P.; Pal, S.; Seehra, M.S.; Shi, Y.; Eyring, E.M.; Ernst, R.D. Concentration of Ce3+ and Oxygen Vacancies in Cerium Oxide Nanoparticles. Chem. Mater. 2006, 18, 5144–5146. [Google Scholar] [CrossRef]
- Kobashigawa, S.; Kashino, G.; Suzuki, K.; Yamashita, S.; Mori, H. Ionizing Radiation-Induced Cell Death Is Partly Caused by Increase of Mitochondrial Reactive Oxygen Species in Normal Human Fibroblast Cells. Radiat Res 2015, 183, 455–464. [Google Scholar] [CrossRef]
- Zhang, B.; Davidson, M.M.; Zhou, H.; Wang, C.; Walker, W.F.; Hei, T.K. Cytoplasmic Irradiation Results in Mitochondrial Dysfunction and DRP1-Dependent Mitochondrial Fission. Cancer Res 2013, 73, 6700–6710. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Qi, F.; Kobayashi, J. Potential Relationship between the Biological Effects of Low-Dose Irradiation and Mitochondrial ROS Production. J Radiat Res 2018, 59, ii91–ii97. [Google Scholar] [CrossRef] [PubMed]
- Waterman, D.P.; Haber, J.E.; Smolka, M.B. Checkpoint Responses to DNA Double-Strand Breaks. Annu Rev Biochem 2020, 89, 103–133. [Google Scholar] [CrossRef] [PubMed]
- Pires, B.R.B.; Silva, R.C.M.C.; Ferreira, G.M.; Abdelhay, E. NF-kappaB: Two Sides of the Same Coin. Genes 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Wang, K.; Kolattukudy, P.E. Cerium Oxide Nanoparticles Inhibits Oxidative Stress and Nuclear Factor-κB Activation in H9c2 Cardiomyocytes Exposed to Cigarette Smoke Extract. J Pharmacol Exp Ther 2011, 338, 53–61. [Google Scholar] [CrossRef]
- Singh, V.; Gupta, D.; Arora, R. NF-kB as a Key Player in Regulation of Cellular Radiation Responses and Identification of Radiation Countermeasures. Discoveries (Craiova) 3, e35. [CrossRef]
- Pesaraklou, A.; Matin, M.M. Cerium Oxide Nanoparticles and Their Importance in Cell Signaling Pathways for Predicting Cellular Behavior. Nanomedicine 2020, 15, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




