Submitted:
06 December 2023
Posted:
07 December 2023
You are already at the latest version
Abstract
Keywords:
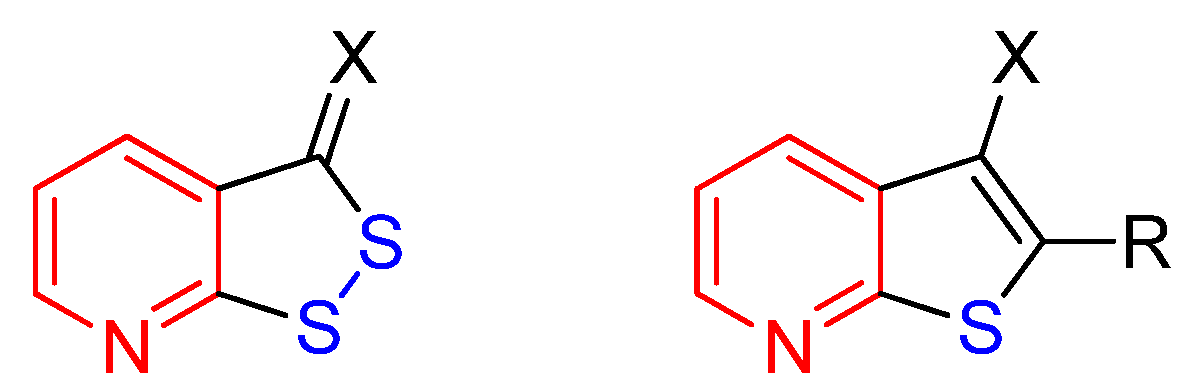
1. Introduction
2. Results and Discussion
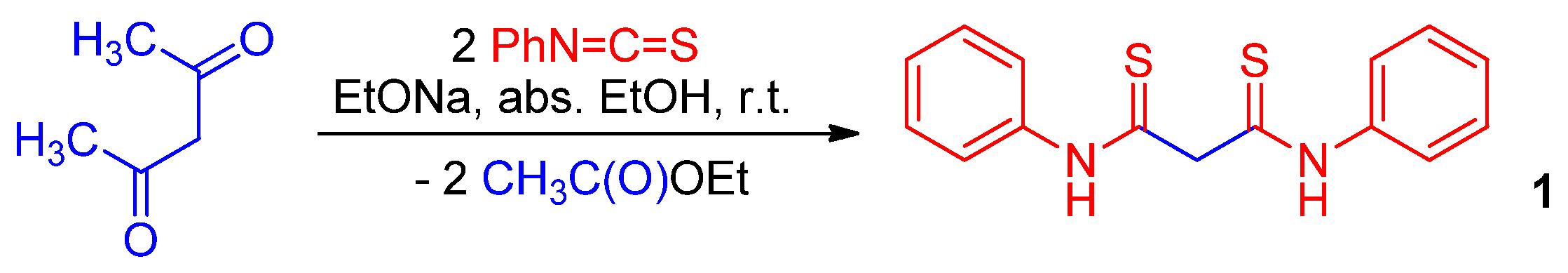
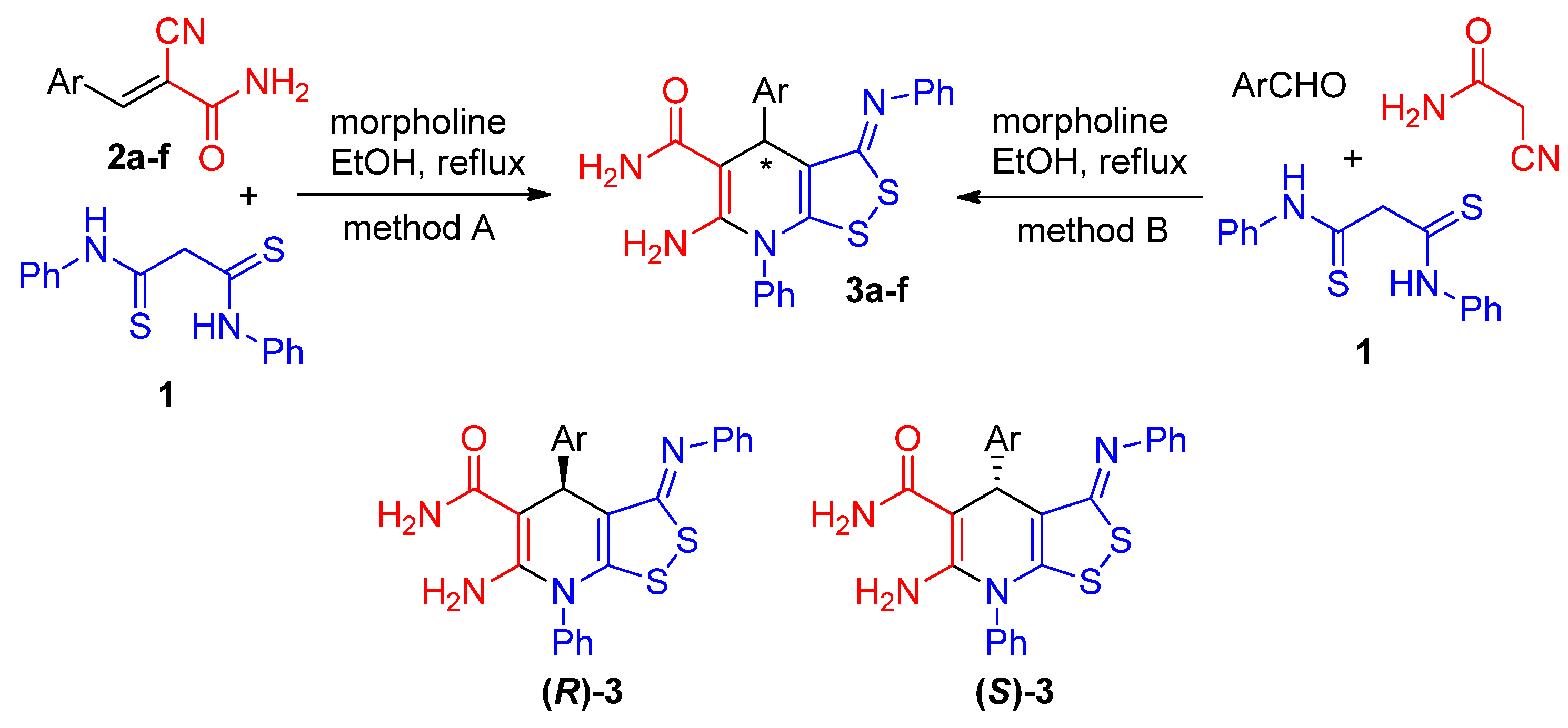
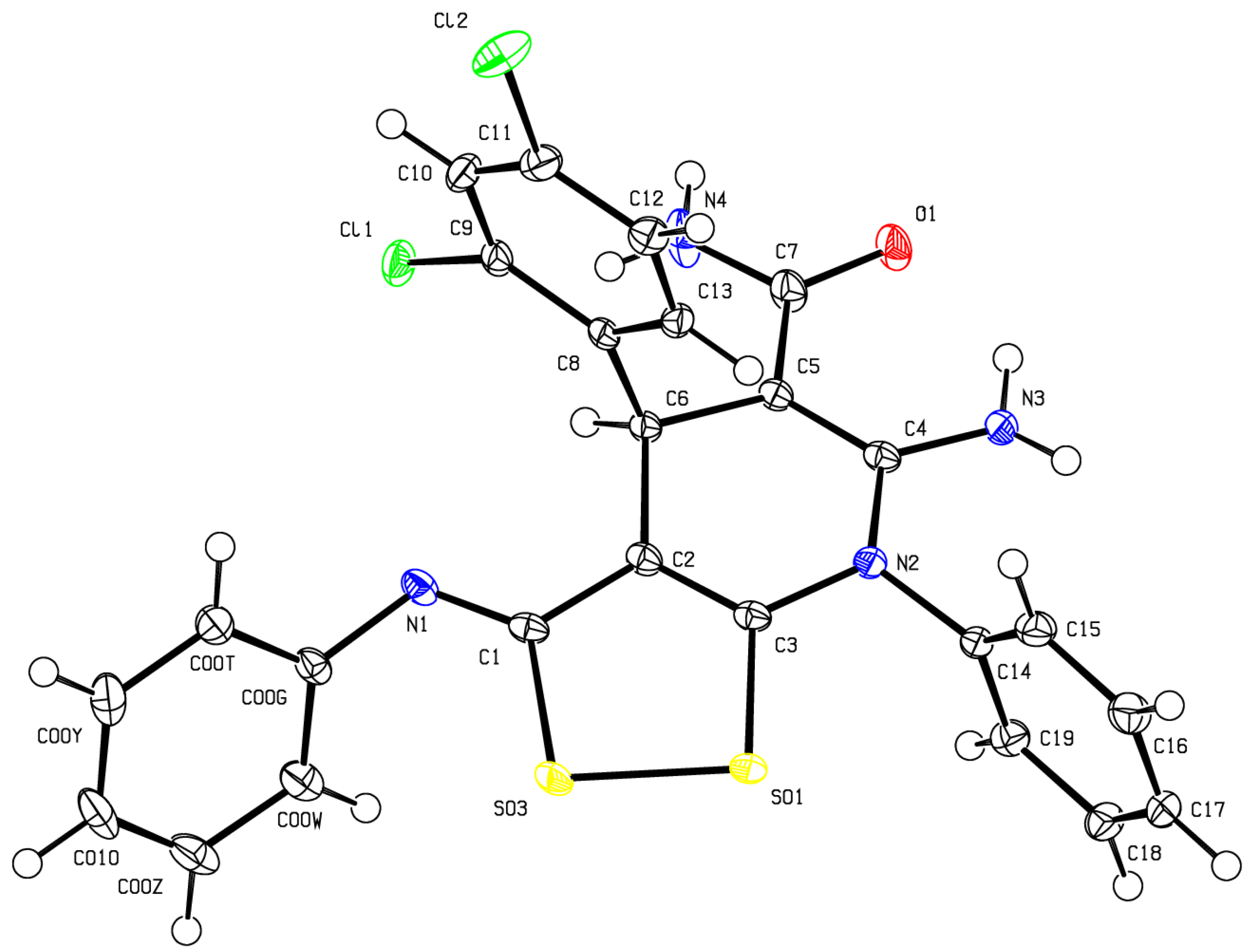
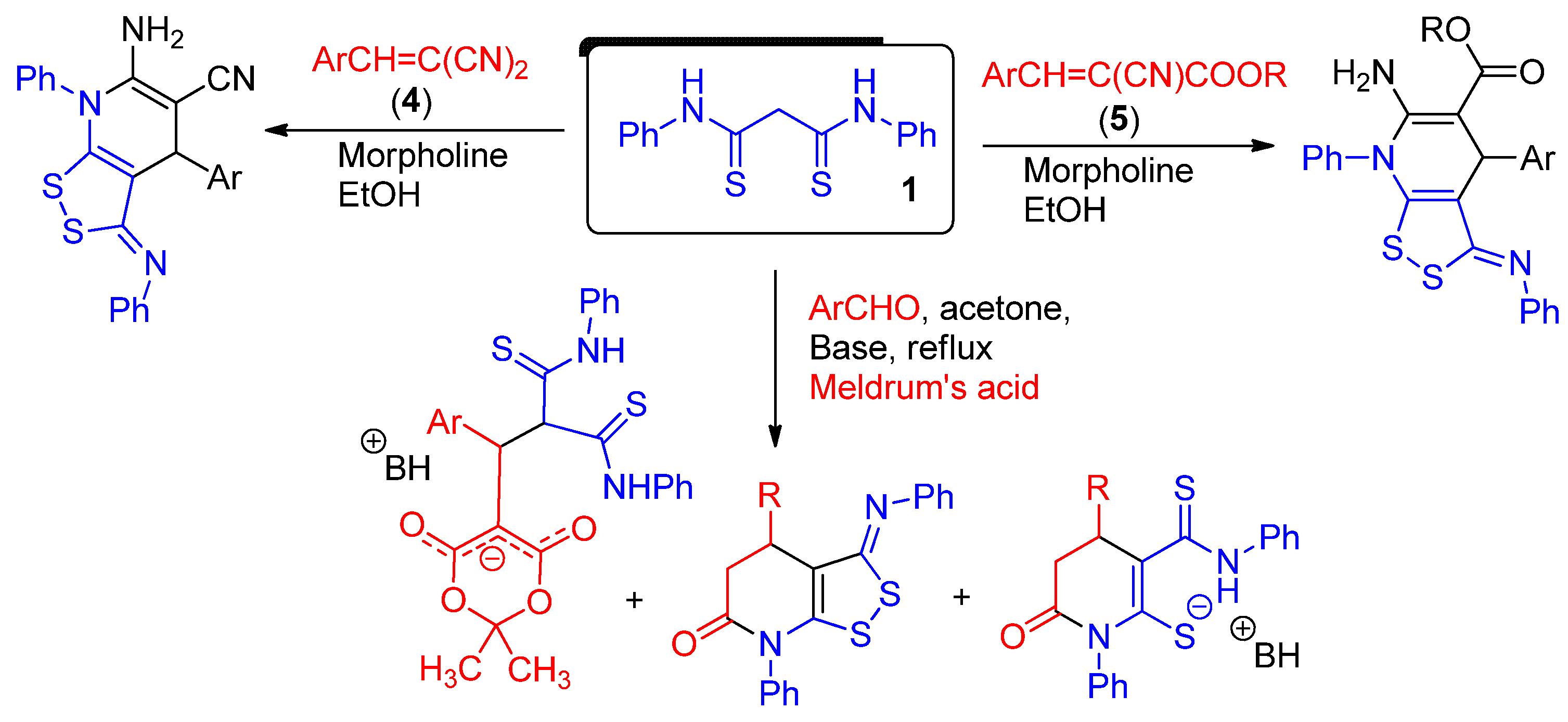
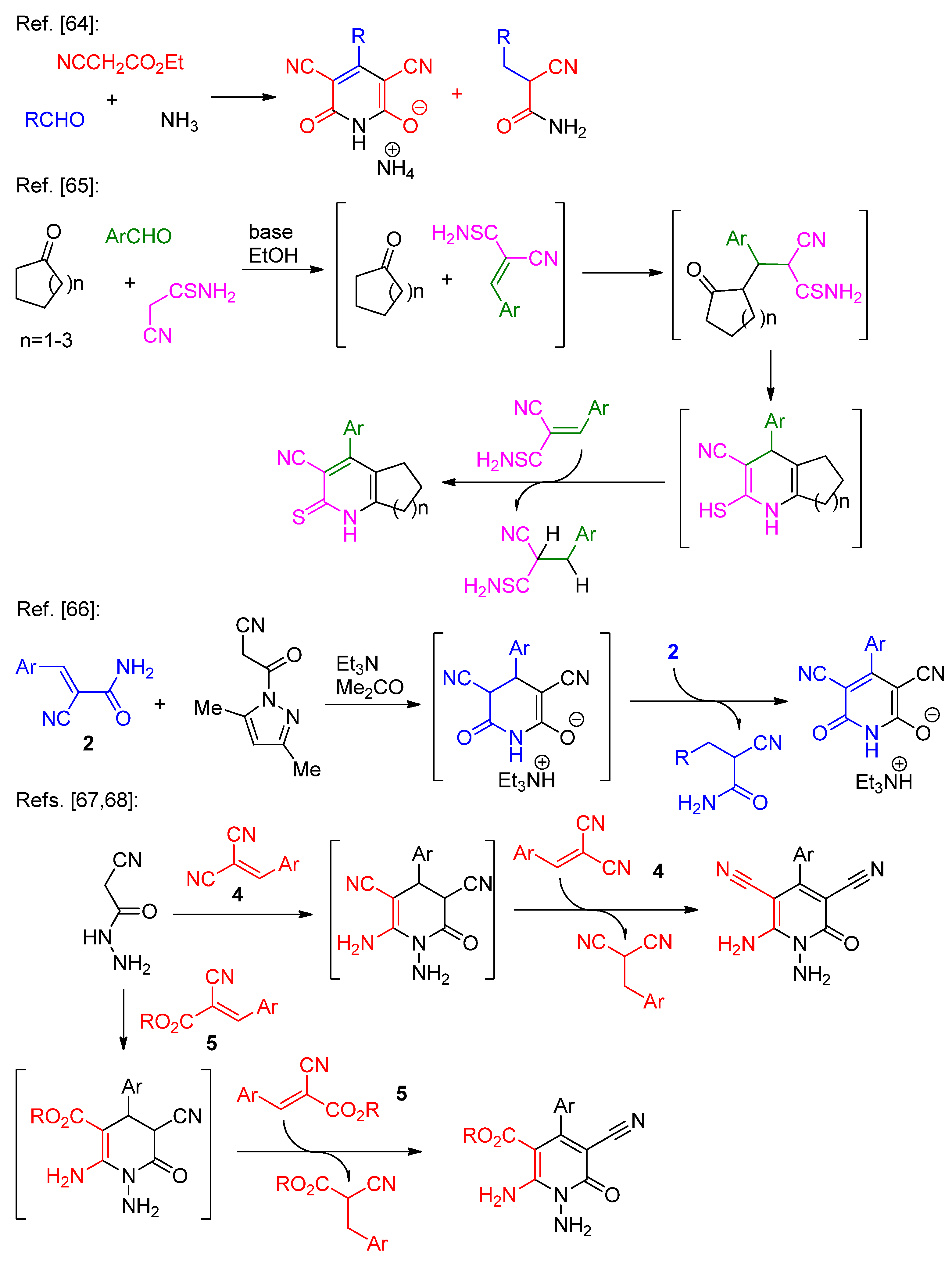
2.1. Synthesis
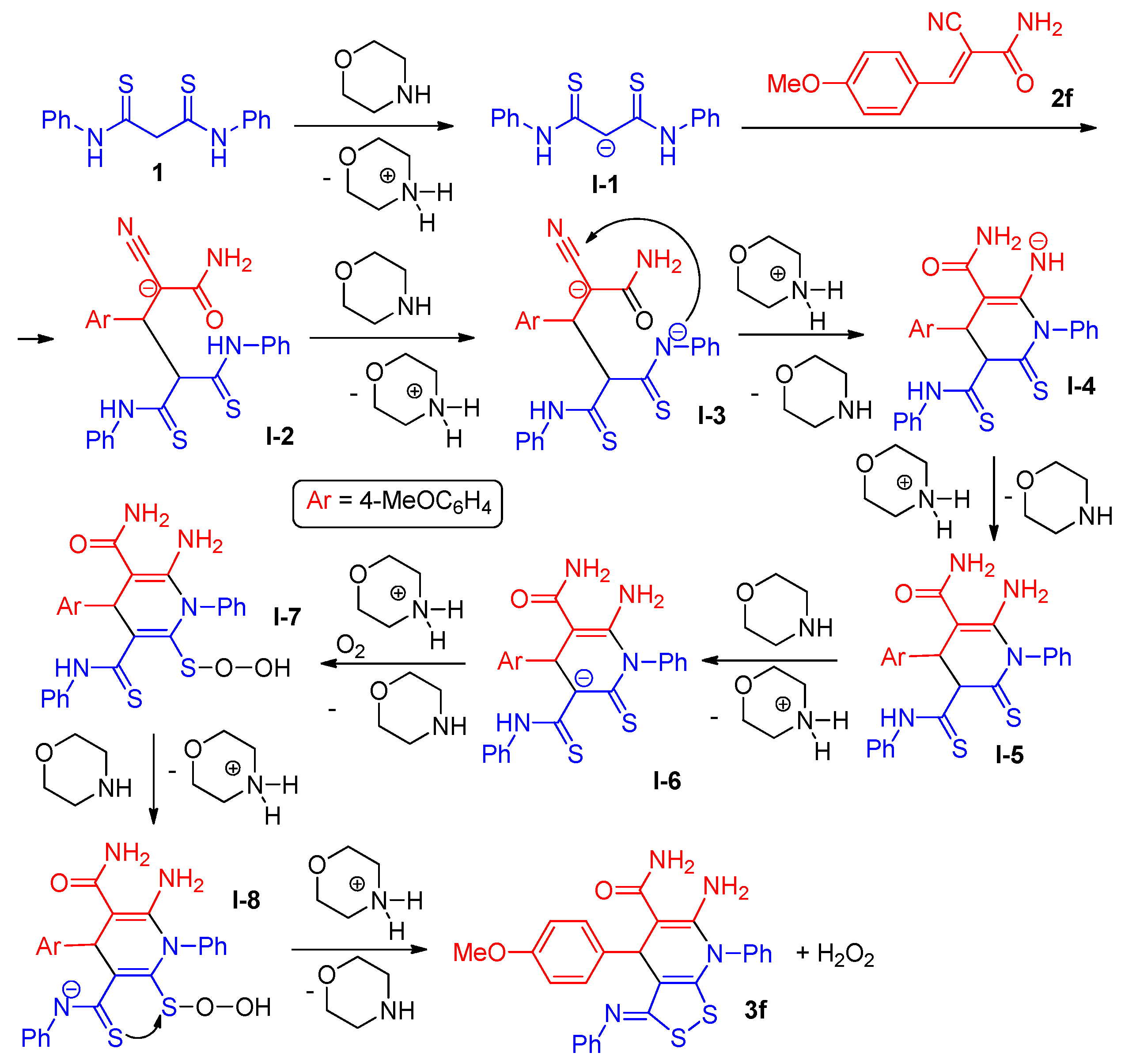
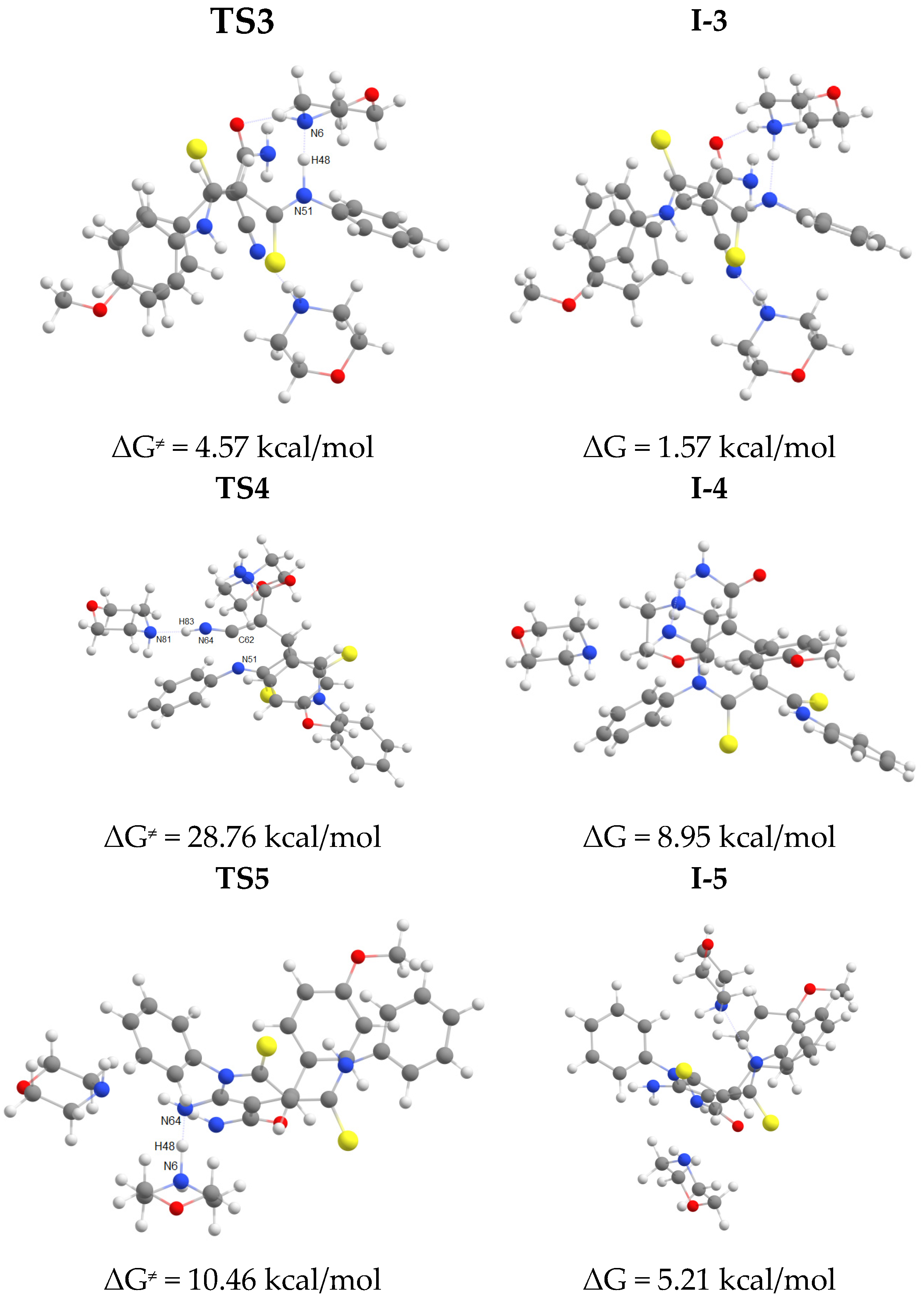
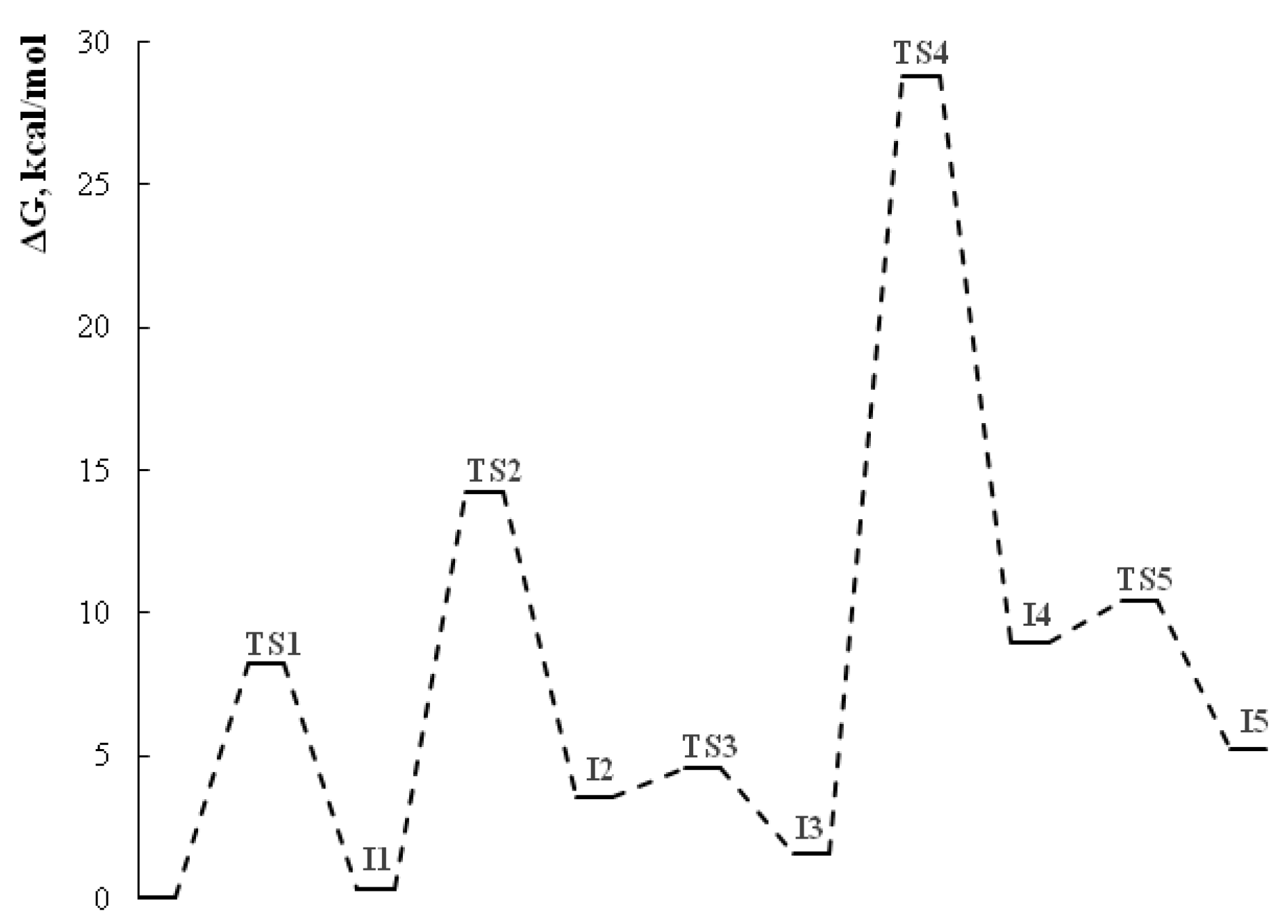
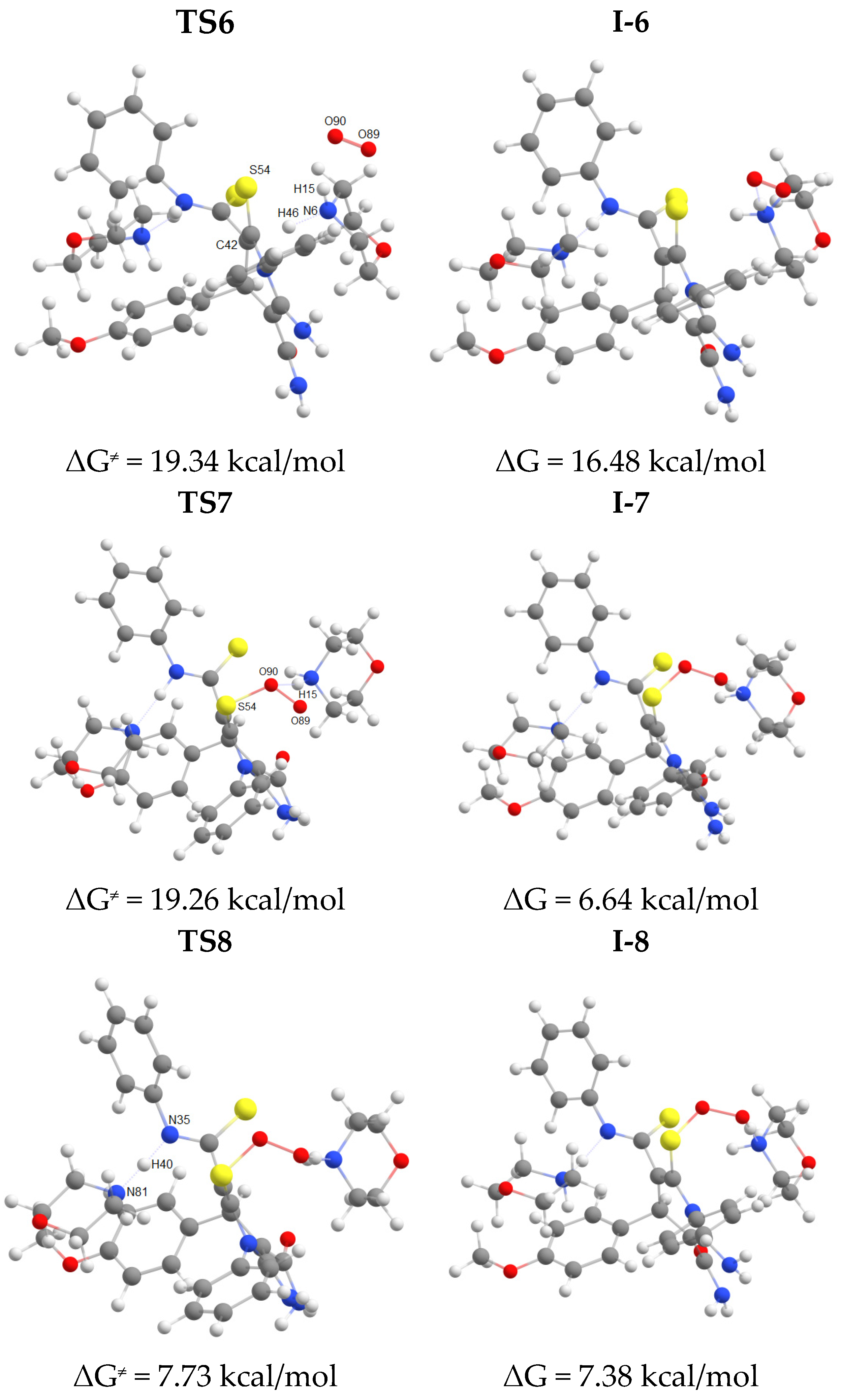
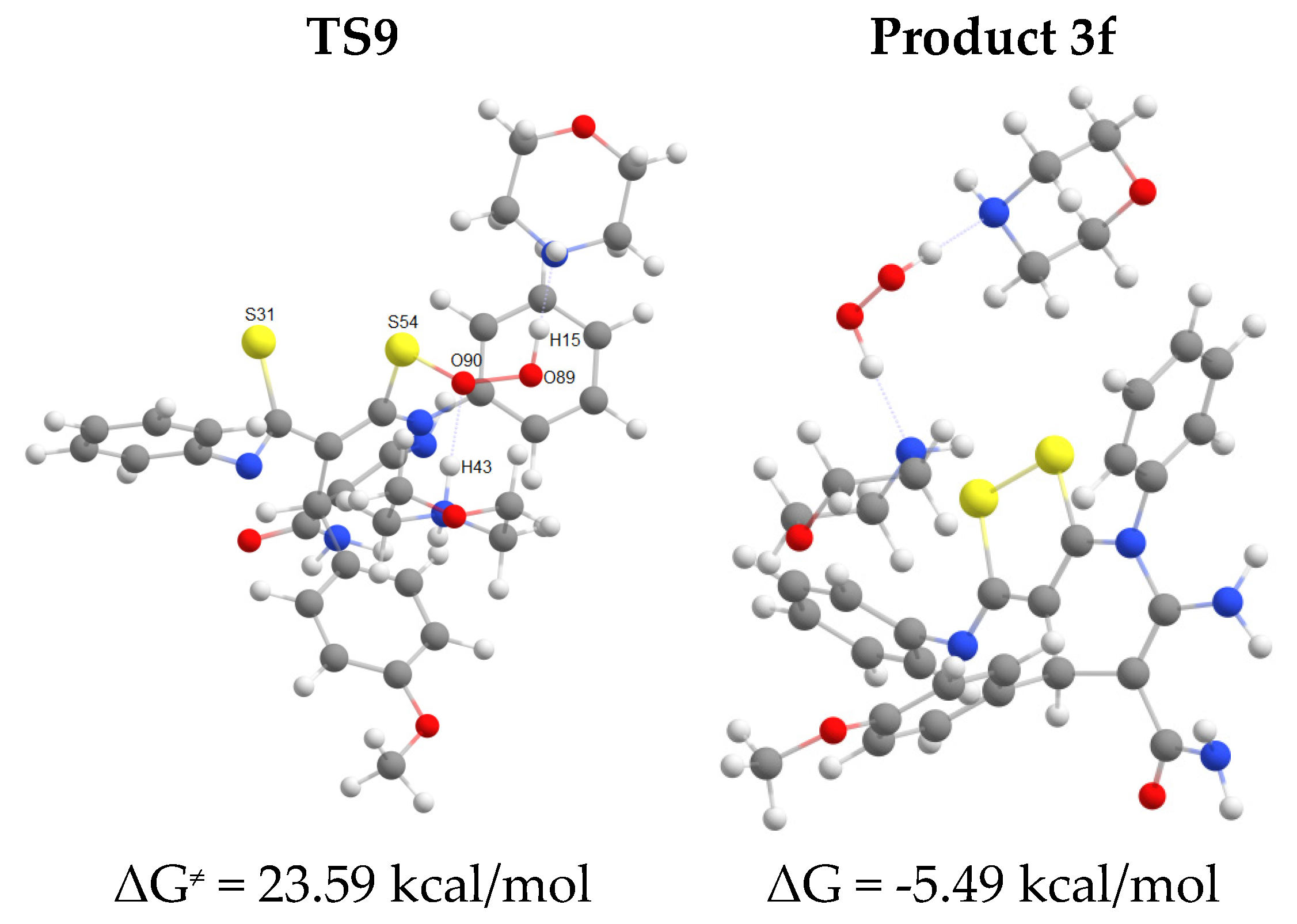
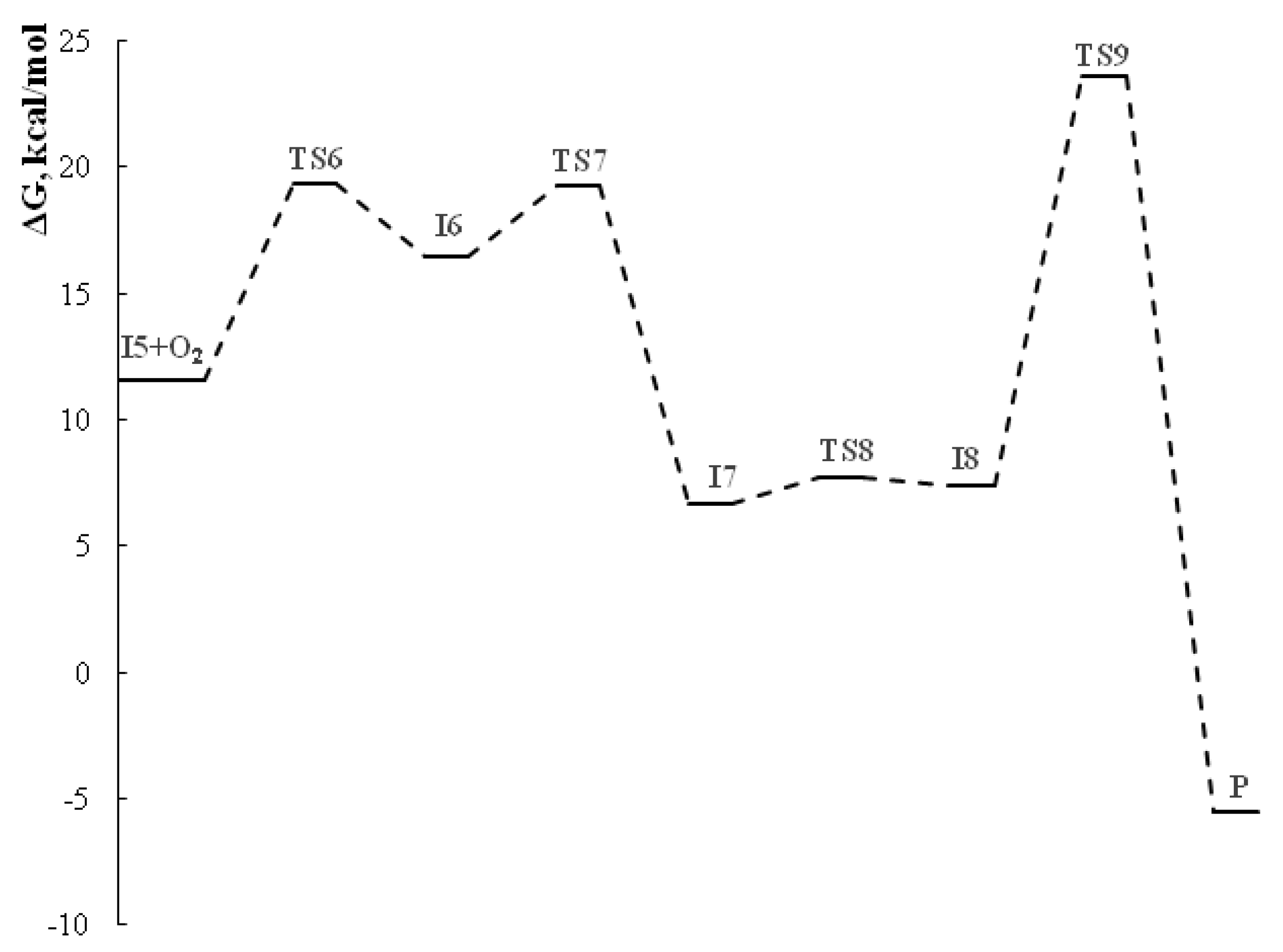
2.2. The studies of the reaction mechanism
- (1)
- under argon flow to exclude the effect of oxygen, at a ratio of 2f:1 = 2:1;
- (2)
- under air stream at the ratio 2f:1 = 1:1 (Table 1, entry 6);
- (3)
- under air atmosphere at a ratio of 2f:1 = 2:1;
- (4)
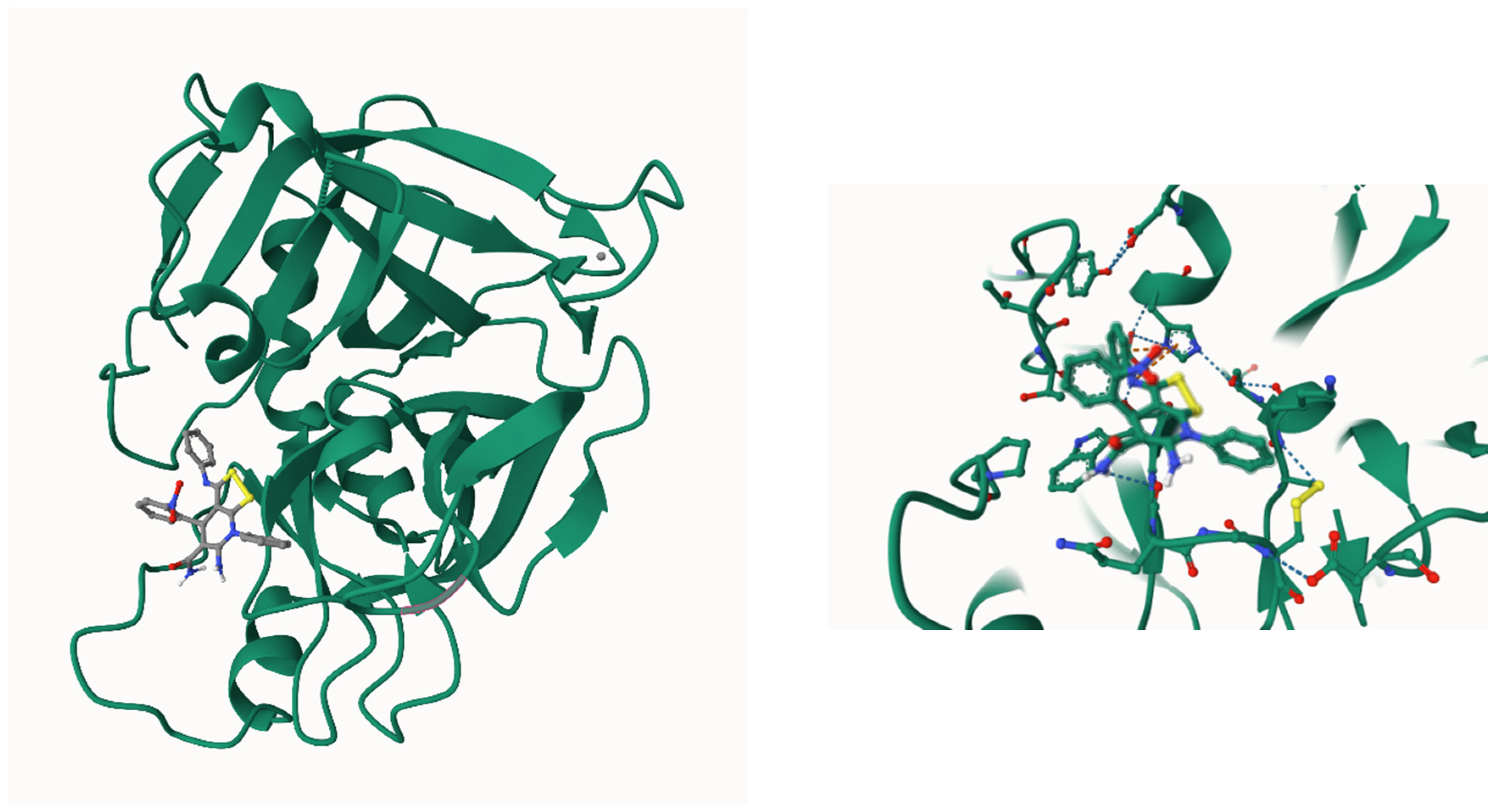
2.3. ADMET calculations and molecular docking studies
2.4. Agrochemical studies
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devendar, P.; Yang, G.F. Sulfur-containing agrochemicals. Top. Curr. Chem. 2017, 375, 82. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Jiang, X. Synthesis and perspective of organosulfur chemicals in agrochemicals. Advanced Agrochem. 2023, 2, 3–14. [Google Scholar] [CrossRef]
- Pathania, S.; Narang, R.K.; Rawal, R.K. Role of sulphur-heterocycles in medicinal chemistry: An update. Eur. J. Med. Chem. 2019, 180, 486–508. [Google Scholar] [CrossRef] [PubMed]
- Sosnovskikh, V.Y. Synthesis and properties of 2,3-heteroannulated thiochromones-hetero analogs of thioxanthone. Chem. Heterocycl. Compd. 2019, 55, 103–125. [Google Scholar] [CrossRef]
- Taubert, K.; Kraus, S.; Schulze, B. Isothiazol-3(2H)-Ones, Part I: Synthesis, reactions and biological activity. J. Sulfur Chem. 2002, 23, 79–121. [Google Scholar] [CrossRef]
- Kletskov, A.V.; Bumagin, N.A.; Zubkov, F.I.; Grudinin, D.G.; Potkin, V.I. Isothiazoles in the design and synthesis of biologically active substances and ligands for metal complexes. Synthesis 2020, 52, 159–188. [Google Scholar] [CrossRef]
- Shafran, Y.; Glukhareva, T.; Dehaen, W.; Bakulev, V. Recent developments in the chemistry of 1,2,3-thiadiazoles. Adv. Heterocycl. Chem. 2018, 126, 109–172. [Google Scholar]
- Podlesný, J.; Bureš, F. Thienothiophene scaffolds as building blocks for (opto)electronics. Organics 2022, 3, 446–469. [Google Scholar] [CrossRef]
- Larionova, N.A.; Shestopalov, A.M.; Rodinovskaya, L.A.; Zubarev, A.A. Synthesis of biologically active heterocycles via a domino sequence involving an SN2/Thorpe–Ziegler Reaction Step. Synthesis 2022, 54, 217–245. [Google Scholar] [CrossRef]
- Konovalov, A.I.; Antipin, I.S.; Burilov, V.A.; Madzhidov, T.I.; Kurbangalieva, A.R.; Nemtarev, A.V.; Solovieva, S.E.; Stoikov, I.I.; Mamedov, V.A.; Zakharova, L.Ya.; Gavrilova, E.L.; Sinyashin, O.G.; Balova, I.A.; Vasilyev, A.V.; Zenkevich, I.G.; Krasavin, M.Yu.; Kuznetsov, M.A.; Molchanov, A.P.; Novikov, M.S.; Nikolaev, V.A.; Rodina, L.L.; Khlebnikov, A.F.; Beletskaya, I.P.; Vatsadze, S.Z.; Gromov, S.P.; Zyk, N.V.; Lebedev, A.T.; Lemenovskii, D.A.; Petrosyan, V.S.; Nenaidenko, V.G.; Negrebetskii, V.V.; Baukov, Yu.I.; Shmigol’, T.A.; Korlyukov, A.A.; Tikhomirov, A.S.; Shchekotikhin, A.E.; Traven’, V.F.; Voskresenskii, L.G.; Zubkov, F.I.; Golubchikov, O.A.; Semeikin, A.S.; Berezin, D.B.; Stuzhin, P.A.; Filimonov, V.D.; Krasnokutskaya, E.A.; Fedorov, A.Yu.; Nyuchev, A.V.; Orlov, V.Yu.; Begunov, R.S.; Rusakov, A.I.; Kolobov, A.V.; Kofanov, E.R.; Fedotova, O.V.; Egorova, A.Yu.; Charushin, V.N.; Chupakhin, O.N.; Klimochkin, Yu.N.; Osyanin, V.A.; Reznikov, A.N.; Fisyuk, A.S.; Sagitullina, G.P.; Aksenov, A.V.; Aksenov, N.A.; Grachev, M.K.; Maslennikova, V.I.; Koroteev, M.P.; Brel’, A.K.; Lisina, S.V.; Medvedeva, S.M.; Shikhaliev, Kh.S.; Suboch, G.A.; Tovbis, M.S.; Mironovich, L.M.; Ivanov, S.M.; Kurbatov, S.V.; Kletskii, M.E.; Burov, O.N.; Kobrakov, K.I.; Kuznetsov, D.N. Modern Trends of Organic Chemistry in Russian Universities. Russ. J. Org. Chem. 2018, 54, 157–371. [Google Scholar]
- Rakitin, O.A. Synthesis and reactivity of 3H-1, 2-dithiole-3-thiones. Molecules 2021, 26, 3595. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Aggarwal, N.; Marwaha, M.G.; Deep, A.; Chopra, H.; Matin, M.M.; Roy, A.; Emran, T.B.; Mohanta, Y.K.; Ahmed, R.; Mohanta, T.K.; Saravanan, M.; Marwaha, R.K.; Al-Harrasi, A. Thiazolidin-2,4-Dione Scaffold: An Insight into Recent Advances as Antimicrobial, Antioxidant, and Hypoglycemic Agents. Molecules 2022, 27, 6763. [Google Scholar] [CrossRef] [PubMed]
- Shainyan, B.A.; Zhilitskaya, L.V.; Yarosh, N.O. Synthetic Approaches to Biologically Active C-2-Substituted Benzothiazoles. Molecules 2022, 27, 2598. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, H.; Suárez, M.; Albericio, F. Thiadiazines, N, N-heterocycles of biological relevance. Molecules 2012, 17, 7612–7628. [Google Scholar] [CrossRef] [PubMed]
- Dotsenko, V.V.; Frolov, K.A.; Krivokolysko, S.G. Synthesis of partially hydrogenated 1,3,5-thiadiazines by Mannich reaction. Chem. Heterocycl. Comp. 2015, 51, 109–127. [Google Scholar] [CrossRef]
- Litvinov, V.P. The chemistry of 3-cyanopyridine-2(1H)-chalcogenones. Russ. Chem. Rev. 2006, 75, 577–599. [Google Scholar] [CrossRef]
- Bakhite, E.A.-G. Recent trends in the chemistry of thienopyridines. Phosphorus, Sulfur, Silicon Relat. Elem. 2003, 178, 929–992. [Google Scholar] [CrossRef]
- Litvinov, V.P.; Dotsenko, V.V.; Krivokolysko, S.G. Thienopyridines: synthesis, properties, and biological activity. Russ. Hcem. Bull. Int. Ed. 2005, 54, 864–904. [Google Scholar] [CrossRef]
- Litvinov, V.P.; Dotsenko, V.V.; Krivokolysko, S.G. The chemistry of thienopyridines. Adv. Heterocycl. Chem. 2007, 93, 117–178. [Google Scholar]
- Sajadikhah, S.S.; Marandi, G. Recent approaches to the synthesis of thieno[2,3-b]pyridines (microreview). Chem. Heterocycl. Compd. 2019, 55, 1171–1173. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Buryi, D.S.; Lukina, D.Yu.; Krivokolysko, S.G. Recent advances in the chemistry of thieno[2,3-b]pyridines 1. Methods of synthesis of thieno[2,3-b]pyridines. Russ. Chem. Bull. Int. Ed. 2020, 69, 1829–1858. [Google Scholar] [CrossRef]
- Pregnolato, M.; Terreni, M.; Ubiali, D.; Pagani, G.; Borgna, P.; Pastoni, F.; Zampollo, F. 3H-[1,2]Dithiolo[3,4-b]pyridine-3-thione and its derivatives. Synthesis and antimicrobial activity. Il Farmaco 2000, 55, 669–679. [Google Scholar] [CrossRef]
- Salvetti, R.; Martinetti, G.; Ubiali, D.; Pregnolato, M.; Pagani, G. 1,2-Dithiolan-3-ones and derivatives structurally related to leinamycin. Synthesis and biological evaluation. Il Farmaco 2003, 58, 995–998. [Google Scholar] [CrossRef]
- Martinez-Merino, V.; Gil, M.J.; Gonzalez, A.; Zabalza, J.M.; Navarro, J.; Manu, M.A. New 5-substituted derivatives of ethyl 2,3-dihydro-3-oxoisothiazolo[5,4-b]pyridine-2-acetate. Heterocycles 1994, 38, 333–344. [Google Scholar] [CrossRef]
- Borgna, P.; Pregnolato, M.; Invernizzi, A.G.; Mellerio, G. On the reaction between 3H-1,2-dithiolo[3,4-b]pyridine-3-thione and primary alkyl and arylalkylamines. J. Heterocycl. Chem. 1993, 30, 1079–1084. [Google Scholar] [CrossRef]
- Davis, R.C.; Grinter, T.J.; Leaver, D.; O’Neil, R.M.; Thomson, G.A. The dithiole series. Part 8. Synthesis of ring-fused 1,2-dithiolylium and isothiazolium salts from complexes containing cyclopalladated ligands. J. Chem. Soc. Perkin Trans. 1 1990, 2881–2887. [Google Scholar] [CrossRef]
- Pagani, G.; Pregnolato, M.; Ubiali, D.; Terreni, M.; Piersimoni, C.; Scaglione, F.; Fraschini, F.; Rodríguez Gascón, A.; Pedraz Muñoz, J.L. Synthesis and in vitro anti-mycobacterium activity of N-alkyl-1,2-dihydro-2-thioxo-3-pyridinecarbothioamides. Preliminary toxicity and pharmacokinetic evaluation. J. Med. Chem. 2000, 43, 199–204. [Google Scholar] [CrossRef]
- Ubiali, D.; Pagani, G.; Pregnolato, M.; Piersimoni, C.; Pedraz Muñoz, J.L.; Rodríguez Gascón, A.; Terreni, M. New N-Alkyl-1,2-dihydro-2-thioxo-3-pyridinecarbothioamides as antituberculous agents with improved pharmacokinetics. Bioorg. Med. Chem. Lett. 2002, 12, 2541–2544. [Google Scholar] [CrossRef]
- Ogurtsov, V.A.; Karpychev, Y.V.; Rakitin, O.A. Synthesis of 1-[(1,2-dithiol-3-ylidene)methyl]pyrrolo[1,2-a]pyrazines and 2-[(1,2-dithiol-3-ylidene)methyl]pyridines from 1,2-dithiole-3-thiones. Russ. Chem. Bull., Int. Ed. 2013, 62, 1076–1079. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Krivokolysko, S.G.; Frolov, K.A.; Chigorina, E.A.; Polovinko, V.V.; Dmitrienko, A.O.; Bushmarinov, I.S. Synthesis of [1,2]dithiolo[3,4-b]pyridines via the reaction of dithiomalondianilide with arylmethylidenemalononitriles. Chem. Heterocycl. Comp. 2015, 51, 389–392. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Aksenov, A.V.; Sinotsko, A.E.; Varzieva, E.A.; Russkikh, A.A.; Levchenko, A.G.; Aksenov, N.A.; Aksenova, I.V. The reactions of N,N′-diphenyldithiomalondiamide with arylmethylidene Meldrum’s acids. Int. J. Mol. Sci. 2022, 23, 15997. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Sinotsko, A.E.; Strelkov, V.D.; Varzieva, E.A.; Russkikh, A.A.; Levchenko, A.G.; Temerdashev, A.Z.; Aksenov, N.A.; Aksenova, I.V. Alkyl 4-aryl-6-amino-7-phenyl-3-(phenylimino)-4,7-dihydro-3H-[1,2]dithiolo[3,4-b]pyridine-5-carboxy- lates: Synthesis and Agrochemical Studies. Molecules 2023, 28, 609. [Google Scholar] [CrossRef]
- Sinotsko, A.E.; Bespalov, A.V.; Pashchevskaya, N.V.; Dotsenko, V.V.; Aksenov, N.A.; Aksenova, I.V. N,N′-Diphenyldithiomalonodiamide: Structural Features, Acidic Properties, and In Silico Estimation of Biological Activity. Russ. J. Gen. Chem. 2021, 91, 2136–2150. [Google Scholar] [CrossRef]
- Barnikow, G.; Kath, V.; Richter, D. Isothiocyanate. II. N,N′-Aryl-substituierte Dithiomalonsäurediamide. J. Prakt. Chem. 1965, 30, 63–66. [Google Scholar] [CrossRef]
- Barnikow, G.; Kunzek, H. Nickel-Chelate von N,N′-Diaryl-dithiomalonsäure-diamiden. Z. Chem. 1966, 6, 343–343. [Google Scholar] [CrossRef]
- Peyronel, G.; Pellacani, G.C.; Benetti, G.; Pollacci, G. Nickel(II) complexes with dithiomalonamide and NN′-diphenyldithiomalonamide. J. Chem. Soc. Dalton Trans. 1973, 879–882. [Google Scholar] [CrossRef]
- Pellacani, G.C. Palladium(II) complexes with dithiomalonamide and N,N′-diphenyldithiomalonamide. Can. J. Chem. 1974, 52, 3454–3458. [Google Scholar] [CrossRef]
- Pellacani, G.C.; Peyronel, G.; Pollacci, G.; Coronati, R. Zinc(II) complexes of dithiomalonamide, N,N′-dimethyl- and N,N′-diphenyl-dithiomalonamide: ZnLX2 (X = Cl, Br, I) and ZnL2(ClO4)2. J. Inorg. Nucl. Chem. 1976, 38, 1619–1621. [Google Scholar] [CrossRef]
- Pellacani, G.C.; Peyronel, G.; Malavasi, W.; Menabue, L. Antimony and bismuth trihalide complexes of dithiomalonamide, N,N′-dimethyl- and N,N′-diphenyl-dithiomalonamide. J. Inorg. Nucl. Chem. 1977, 39, 1855–1857. [Google Scholar] [CrossRef]
- Pal, T.; Ganguly, A.; Maity, D.S.; Livingstone, S.E. N,N′-diphenyldithiomalonamide as a gravimetric reagent for nickel and cobalt. Talanta 1986, 33, 973–977. [Google Scholar] [CrossRef]
- Pal, T.; Ganguly, A.; Pal, A. Spectrophotometric determination of cobalt and nickel with N,N′-diphenyldithiomalonamide and elucidation of structures of the compounds. J. Ind. Chem. Soc. 1988, 65, 655–657. [Google Scholar]
- Battaglia, L.P.; Bonamartini Corradi, A.; Marzotto, A.; Menabue, L.; Pellacani, G.C. Nickel(II) and palladium(II) complexes of dithiomalonamides: Ligands which favor the formation of π-conjugation systems in the coordination. J. Crystallogr. Spectrosc. Res. 1988, 18, 101–112. [Google Scholar] [CrossRef]
- Battaglia, L.P.; Bonamartini Corradi, A.; Marzotto, A.; Menabue, L.; Pellacani, G.C. Co-ordinative abilities of ligands which favour S,S chelation: copper(I) halide complexes of N,N′-diphenyldithiomalonamide. The crystal and molecular structure of bis(N,N′-diphenyldithiomalonamide)copper(I) iodide–methanol (2/1). J. Chem. Soc. Dalton Trans 1988, 1713–1718. [Google Scholar] [CrossRef]
- Singh, T.; Singh, R.; Verma, V.K. Evaluation of 2,4-dithiomalonamides as extreme pressure additives in the four-ball test. Proc. Inst. Mech. Eng., Part D: J. Automob. Eng. 1990, 204, 103–107. [Google Scholar] [CrossRef]
- Singh, T. Tribochemistry and EP activity assessment of Mo-S complexes in lithium-base greases. Adv. Tribol. 2008, 2008, 947543. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, M.M. Substituted dithiomalonamides as inhibitor for the corrosion of AISI 304SS in phosphoric acid – hydrochloric acid mixture. Anti-Corros. Methods Mater. 1993, 40, 4–7. [Google Scholar] [CrossRef]
- Schmidt, U. Synthesen mit den Thioamiden der Malonsäure, I. 3,5-Diamino-dithiyliumsalze. Ein neuer pseudoaromatischer Fünfring mit 1,2-Stellung der S-Atome. Chem. Ber. 1959, 92, 1171–1176. [Google Scholar] [CrossRef]
- Barnikow, G. Isothiocyanate, XIV. 3.5-Bis-arylamino-1.2-dithioliumsalze aus N.N′-Diaryl-dithiomalonsäure-diamiden. Chem. Ber. 1967, 100, 1389–1393. [Google Scholar] [CrossRef]
- Nizovtseva, T.V.; Komarova, T.N.; Nakhmanovich, A.S.; Larina, L.I.; Lopyrev, V.A.; Kalistratova, E.F. Reaction of Dithiomalonamide and Dianilide with α-Acetylene Ketones. Russ. J. Org. Chem. 2002, 38, 1205–1207. [Google Scholar] [CrossRef]
- Nizovtseva, T.V.; Komarova, T.N.; Nakhmanovich, A.S.; Larina, L.I.; Lopyrev, V.A. Synthesis of 1,3-dithiinium salts. Arkivoc 2003, xiii, 191–195. [Google Scholar] [CrossRef]
- Elokhina, V.N.; Yaroshenko, T.I.; Nakhmanovich, A.S.; Larina, L.I.; Amosova, S.V. Reaction of dithiomalonic acid dianilide with substituted acetylenic ketones. Russ. J. Gen. Chem. 2006, 76, 1916–1918. [Google Scholar] [CrossRef]
- Volkova, K.A.; Nakhmanovich, A.S.; Elokhina, V.N.; Yaroshenko, T.I.; Larina, L.I.; Shulunova, A.M.; Amosova, S.V. Reaction of dithiomalonic acid dianilide with methyl propiolate. Russ. J. Org. Chem. 2007, 43, 768–770. [Google Scholar] [CrossRef]
- Obydennov, K.L.; Golovko, N.A.; Kosterina, M.F.; Pospelova, T.A.; Slepukhin, P.A.; Morzherin, Yu.Yu. Russ. Chem. Bull. Int Ed. 2014, 63, 1330–1336. [CrossRef]
- Beckert, R.; Gruner, M. Regioselektive Cyclisierung von Thiocarbonsäureamiden mit Bisimidoylchloriden – Synthese von Thiazolidinen mit einer Ketenacetal-Substruktur. Z. Naturforsch. B: Chem. Sci. 1997, 52, 1245–1250. [Google Scholar] [CrossRef]
- Barnikow, G. Isothiocyanate, X. Pyrazole und Thiophene aus N.N′-Diaryl-dithiomalonsäure-diamiden. Liebigs Ann. Chem. 1966, 700, 46–49. [Google Scholar] [CrossRef]
- Degorce, S.; Jung, F.H.; Harris, C.S.; Koza, P.; Lecoq, J.; Stevenin, A. Diversity-orientated synthesis of 3,5-bis(arylamino)pyrazoles. Tetrahedron Lett. 2011, 52, 6719–6722. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Sinotsko, A.E.; Varzieva, E.A.; Buryi, D.S.; Vasilin, V.K.; Aksenov, N.A.; Aksenova, I.V. Reaction of N,N′-diphenyldithiomalondiamide with aromatic aldehydes and cyanoacetamide. Russ. J. Gen. Chem. 2023, 93, 2518–2522. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Sinotsko, A.E.; Varzieva, E.A.; Chigorina, E.A.; Aksenov, N.A.; Aksenova, I.V. N,N′-Diphenyldithiomalon- amide as methylene active thioamide: A first synthesis of stable Michael adducts. Russ. J. Gen. Chem. 2022, 92, 2530–2535. [Google Scholar] [CrossRef]
- Koval, I.V. The chemistry of disulfides. Russ. Chem. Rev. 1994, 63, 735–750. [Google Scholar] [CrossRef]
- Witt, D. Recent developments in disulfide bond formation. Synthesis 2008, 2008, 2491–2509. [Google Scholar] [CrossRef]
- Mandal, B.; Basu, B. Recent advances in S–S bond formation. RSC Adv. 2014, 4, 13854–13881. [Google Scholar] [CrossRef]
- Chiu, J.; Hogg, P.J. Allosteric disulfides: Sophisticated molecular structures enabling flexible protein regulation. J. Biol. Chem. 2019, 294, 2949–5908. [Google Scholar] [CrossRef]
- Tyler, T.J.; Durek, T.; Craik, D.J. Native and Engineered Cyclic Disulfide-Rich Peptides as Drug Leads. Molecules 2023, 28, 3189. [Google Scholar] [CrossRef] [PubMed]
- Brunskill, J.S.A. Some cyano-amides and dicyano-glutaconimides derived from pyridine aldehydes. J. Chem. Soc. Perkin Trans. 1 1972, 2946–2950. [Google Scholar] [CrossRef]
- Shestopalov, A.M.; Rodinovskaya, L.A.; Zubarev, A.A.; Nesterov, V.N.; Ugrak, B.I.; Dutova, T.Y. Synthesis and domino reactions of polymethylene-3-cyanopyridine-2 (1Н)-thiones. J. Heterocycl. Chem. 2020, 57, 913–922. [Google Scholar] [CrossRef]
- Chigorina, E.A. 1-(Cyanoacetyl)-3,5-dimethylpyrazole as an effective alternative to cyanoacetic ester in the synthesis of 2, 6-dioxopiperidine-3, 5-dicarbonitrile derivatives. Chem. Heterocycl. Comp. 2013, 49, 574–585. [Google Scholar] [CrossRef]
- Soto, J.L.; Seoane, C.; Zamorano, P.; Cuadrado, F.J. A convenient synthesis of N-amino-2-pyridones. Synthesis 1981, 1981, 529–530. [Google Scholar] [CrossRef]
- Seoane, C.; Soto, J.L.; Zamorano, P. Preparation of substituted 1,6-diamino-2-oxopyridines. Org. Prep. Proced. Int. 1984, 16, 393–400. [Google Scholar] [CrossRef]
- Grabenko, A.D.; Kulaeva, L.N.; Pel’kis, P.S. Research on a number of substituted arylamides of dithiocarboxylic acids: XV. Oxidation of dithiomalonic acid arylamides to substituted 1,2-dithiols. Chem. Heterocycl. Compd. 1974, 10, 806–809. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: the ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Brandenburg, J.G.; Bannwarth, C.; Hansen, A.; Grimme, S. B97-3c: A revised low-cost variant of the B97-D density functional method. J. Chem. Phys. 2018, 148, 064104. [Google Scholar] [CrossRef]
- Caldeweyher, E.; Brandenburg, J.G. Simplified DFT methods for consistent structures and energies of large systems. J. Phys.: Condens. Matter 2018, 30, 213001. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug. Delivery Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- OECD series on testing and assessment. Number 24. Guidance document on acute oral toxicity testing. Avail. URL is: https://one.oecd.org/document/env/jm/mono(2001)4/en/pdf.
- Ko, J.; Park, H.; Heo, L.; Seok, C. GalaxyWEB server for protein structure prediction and refinement. Nucl. Acids Res. 2012, 40, W294–W297. [Google Scholar] [CrossRef]
- Seok, C.; Baek, M.; Steinegger, M.; Park, H.; Lee, G.R.; Won, J. Accurate protein structure prediction: what comes next? Biodesign 2021, 9, 47–50. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Buryi, D.S.; Lukina, D.Y.; Stolyarova, A.N.; Aksenov, N.A.; Aksenova, I.V.; Strelkov, V.D.; Dyadyuchenko, L.V. Substituted N-(thieno[2,3-b]pyridine-3-yl)acetamides: synthesis, reactions, and biological activity. Monatsh. Chem. 2019, 150, 1973–1985. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Muraviev, V.S.; Lukina, D.Y.; Strelkov, V.D.; Aksenov, N.A.; Aksenova, I.V.; Krapivin, G.D.; Dyadyuchenko, L.V. Reaction of 3-Amino-4,6-diarylthieno[2,3-b]pyridine-2-carboxamides with ninhydrin. Russ. J. Gen. Chem. 2020, 90, 948–960. [Google Scholar] [CrossRef]
- Buryi, D.S.; Dotsenko, V.V.; Aksenov, N.A.; Aksenova, I.V.; Krivokolysko, S.G.; Dyadyuchenko, L.V. Synthesis and properties of 4,6-dimethyl-5-pentyl-2-thioxo-1,2-dihydropyridine-3-carbonitrile and 3-amino-4,6-dimethyl-5-pentylthieno[2,3-b]pyridi- nes. Russ. J. Gen. Chem. 2019, 89, 1575–1585. [Google Scholar] [CrossRef]
- Peterson, M.A.; McMaster, S.A.; Riechers, D.E.; Skelton, J.; Stahlman, P.W. 2,4-D past, present, and future: a review. Weed Technol. 2016, 30, 303–345. [Google Scholar] [CrossRef]
- Chkanikov, N.D.; Spiridonov, Y.Y.; Khalikov, S.S.; Muzafarova, A.M. Antidotes for reduction of phytotoxicity of the residues of sulfonylurea herbicides. INEOS OPEN 2019, 2, 145–152. [Google Scholar] [CrossRef]
- Deng, X. Current advances in the action mechanisms of safeners. Agronomy 2022, 12, 2824. [Google Scholar] [CrossRef]
- Jia, L.; Jin, X.Y.; Zhao, L.X.; Fu, Y.; Ye, F. Research progress in the design and synthesis of herbicide safeners: A review. J. Agric. Food Chem. 2022, 70, 5499–5515. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Krivokolysko, S.G.; Rusanov, E.B.; Gutov, A.V.; Litvinov, V.P. Synthesis of 3-(4-chlorophenyl)oxirane- 2,2-dicarboxamide. Russ. Chem. Bull. 2007, 56, 1470–1473. [Google Scholar] [CrossRef]
- Bezgin, D.A.; Ershov, O.V.; Ievlev, M.Y.; Belikov, M.Y.; Bardasov, I.N. Aqueous-Phase Synthesis and Solid-Phase Fluorescence of 3-(Methoxyphenyl)-2-cyanoacrylamides. Russ. J. Org. Chem. 2018, 54, 1100–1102. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinementand analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
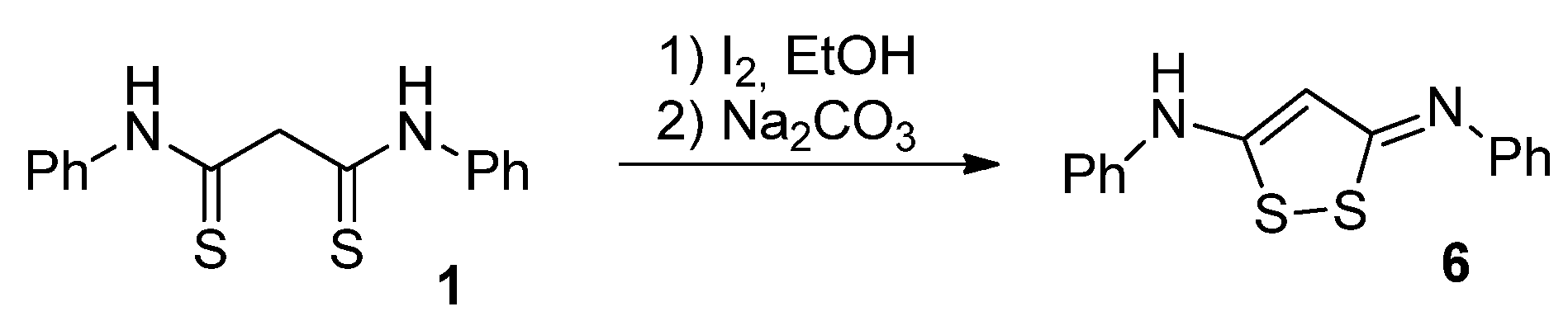

| Entries | Product | Methods | Yields, % |
|---|---|---|---|
| entry 1 | 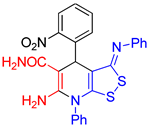 3a 3a |
A B |
54 43 |
| entry 2 | 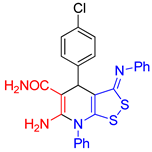 3b 3b |
A B |
40 33 |
| entry 3 |  3c 3c |
A B |
37 27 |
| entry 4 | 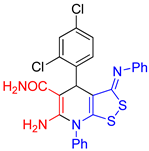 3d 3d |
A B |
39 31 |
| entry 5 | 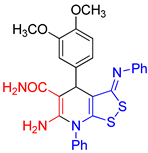 3e 3e |
A B |
40 22 |
| entry 6 | 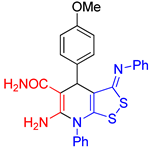 3f 3f |
A B |
54 35 |
| N | compound | Organ | Antidote effect A at different concentrations, % 1 | |||
|---|---|---|---|---|---|---|
| 10-2 | 10-3 | 10-4 | 10-5 | |||
| 1 | 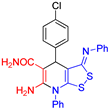 3b 3b |
roots | 123 | 121 | 121 | 116 |
| hypocotyls | 127 | 130 | 124 | 120 | ||
| 2 | 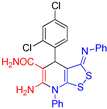 3d 3d |
roots | 130 | 131 | 123 | 128 |
| hypocotyls | 132 | 127 | 120 | 128 | ||
| 3 | 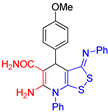 3f 3f |
roots | 133 | 127 | 132 | 130 |
| hypocotyls | 132 | 132 | 116 | 119 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





