1. Introduction
Hepatocellular carcinoma (HCC) is a common and deadly cancer worldwide [
1]. It is associated with underlying chronic liver pathological conditions, including chronic viral hepatitis. Chronic hepatitis B (CHB) contributes to more than 50% of global HCC occurrence [
2]. Surgery is usually the curative treatment modality for early-stage HCC. However, cancer recurrence is frequently observed. Tumor recurrence rate is approximately 50% and 70% within 5 and 7 years after surgery, respectively [
3,
4]. Currently, there are no approved therapies to inhibit recurrence. Effective and well-tolerated adjuvant therapies are urgently needed to reduce recurrence.
Programmed cell death 1 (PD-1) is a negative costimulatory receptor expressed primarily on the surface of activated T cells. The binding of PD-1 to its ligand, programmed cell death-1 ligand-1/2 (PD-L1/2), inhibits cytotoxic T cell-mediated immunological responses and elicits an immune checkpoint [
5]. Tumor cells upregulate PD-L1 and utilize the PD-1 pathway to escape T-cell-mediated immune responses. Anti-PD1 and anti-PD-L1/2 therapeutic antibodies can interfere with the interactions between PD-1 and its ligands, resulting in enhanced anti-tumor immunological response by cytotoxic T cells [
5,
6]. Anti-PD1 therapy causes a decline or seroclearance of hepatitis B surface antigen (HBsAg) in patients with CHB [
7]. HBsAg seroclearance is associated with a lower risk of hepatitis B virus (HBV)-related HCC [
8]. Anti-PD1 therapies have been approved to treat advanced HCC, melanoma, metastatic non-small cell lung cancer, and other advanced malignancies [
9]. However, anti-PD-1 therapies are also associated with known toxicities, which may not be suitable in post-surgical adjuvant settings for patients with HCC. A combination therapy that can potentially minimize toxicity and produce a significant anti-cancer effect may be applicable in this setting.
Ropeginterferon alfa-2b represents a new generation, PEGylated interferon alpha (IFN-α)-based therapy with a favorable pharmacokinetic profile. It can be injected less frequently, e.g., once every two weeks [10-12]. It has been approved for the treatment of polycythemia vera (PV), a myeloproliferative neoplasm (MPN), in the United States and Europe and is currently under development for other indications [13-21]. It appeared to be well-tolerated and showed anti-HBV activities when administered once every two weeks at the dose of 450 μg in a Phase II clinical study [22]. Here, we report our findings in an HBV mouse model sequentially treated with a recombinant mouse-IFN-alpha (rmIFN-α) and an anti-mouse-PD1 antibody and clinical Phase I results of sequential therapy with ropeginterferon alfa-2b and anti-human PD-1 antibody nivolumab in patients with HBV-related HCC after curative surgery.
2. Results
Animal Modeling Data
An HBV mouse model (HBV-HDI) was generated by intravenous injection of an HBV genotype A DNA plasmid into CBA/CaJ mice [23, 24]. In this HBV-HDI mouse model, the sequential combination treatment with rmIFN-α and an anti-mouse PD1 antibody (RMP-17) continuously caused a decline in the mean HBsAg values compared to the phosphate-buffered saline (PBS) control. After the sequential combination treatment, the mean HBsAg level was one log lower than that in the PBS control group (
Figure 1A). In contrast, the treatment alone with either rmIFN-α or anti-mouse PD1 showed no significant decline in the HBsAg level when compared to the PBS control (
Figure 1A). Compared to the rmIFN-α and anti-mouse PD1 treatment alone, the sequential combination of rmIFN-α and anti-mouse PD1 antibody significantly reduced the HBsAg levels and HBV viral titers in the HBV-carrying CBA/CaJ mice (
Figure 1A to B). In addition, the HBsAg clearance rate was 44.4% (4/9) in those mice receiving 6-week treatment of rmIFN-α and anti-mouse PD1, which was higher than that in the treatment alone groups (
Table 1). The animal data showed a synergistic effect between rmIFN-α and anti-PD1 antibody for HBV suppression or even clearance in the HBV mouse model. We did not observe any adverse effect on animal body weight, liver function and blood cell production (data not shown).
Phase I Clinical Study
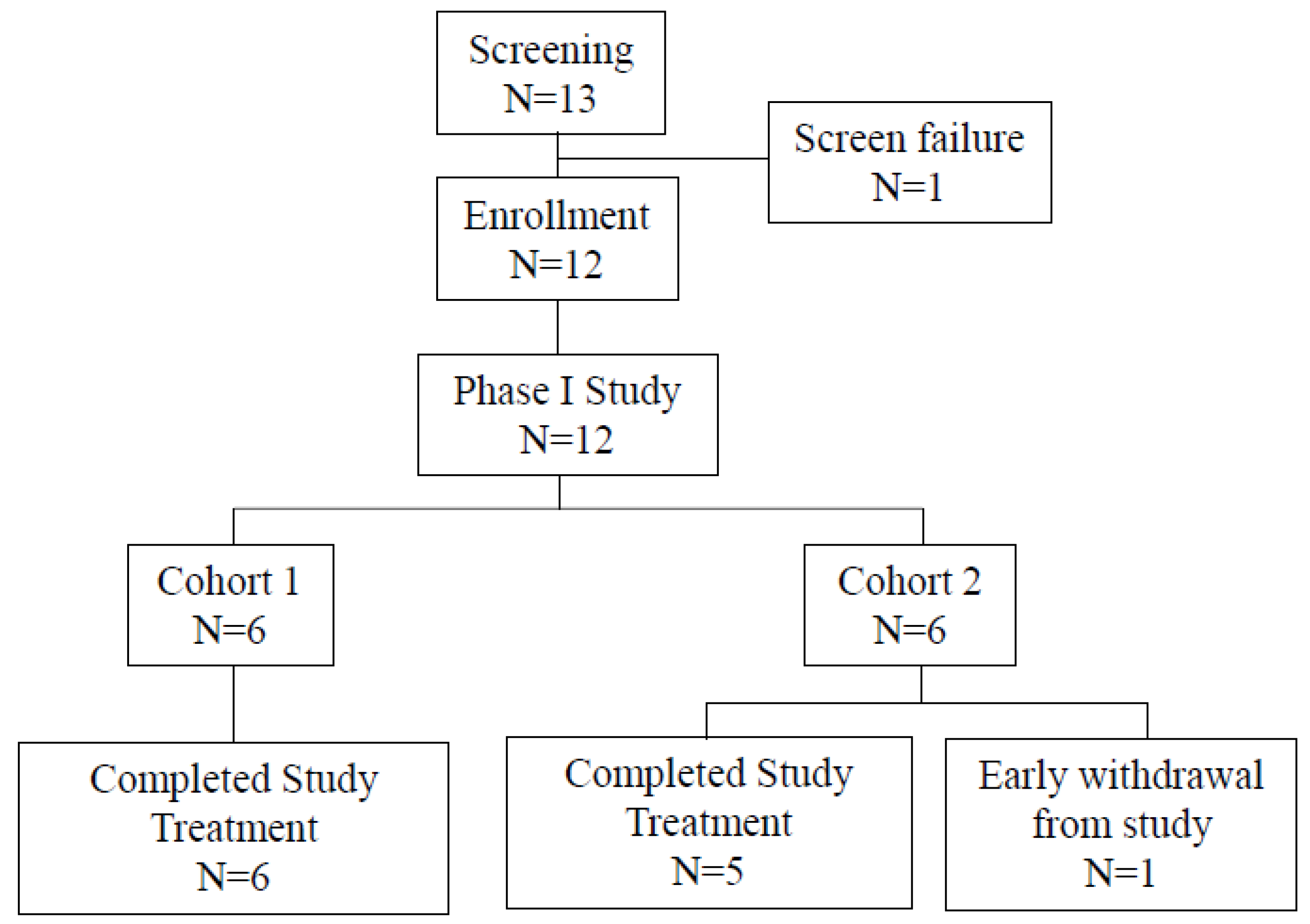
A phase I study was completed to determine the maximum-tolerated dose (MTD). A total of 12 eligible patients were enrolled (
Figure 2), including six patients in Cohort 1 and six in Cohort 2. Patients received six doses of ropeginterferon alfa-2b at the dose of 450 μg once every two weeks followed by three doses of nivolumab at 0.3 mg/kg in Cohort 1 or followed by three doses of nivolumab at 0.75 mg/kg in Cohort 2. All patients completed the study treatment except one who withdrew early because of grade 3 anorexia after receiving one dose of ropeginterferon alfa-2b in Cohort 2.
The mean (standard deviation) age of the eligible patients was 61.8 (10.3) years old (
Table 2). Six (50%) patients had liver cirrhosis before participating in this study. All patients experienced at least one adverse event (AE) after treatment. Most AEs were mild or moderate (
Table 3). No Grade 4 or 5 AEs were observed. No serious AEs (SAEs) were observed. Four patients (33.3 %) experienced Grade 3 AEs. The most frequent AE was pyrexia (50%), followed by alanine transaminase (ALT) increase (41.7%), aspartate aminotransferase (AST) increase (41.7%), fatigue (33.3%), and neutrophil count decrease (25%).
Dose-limiting toxicities (DLTs) were observed in only one patient. Drug-related Grade 3 ALT and AST increases were observed in one patient in Cohort 1. The patient completed ropeginterferon alfa-2b treatment and received one dose of nivolumab. No DLTs were observed in Cohort 2. However, given that the DLT of Grade 3 ALT and AST increases was observed in Cohort 1 and that there were greater levels of Grade 2 ALT and AST increases in Cohort 2, we determined that Cohort 2 reached the MTD of the study based on the overall safety assessment. Therefore, ropeginterferon alfa-2b at 450 ug for six doses followed by nivolumab at 0.75 mg/kg for three doses is the MTD for the adjuvant, sequential combination therapy.
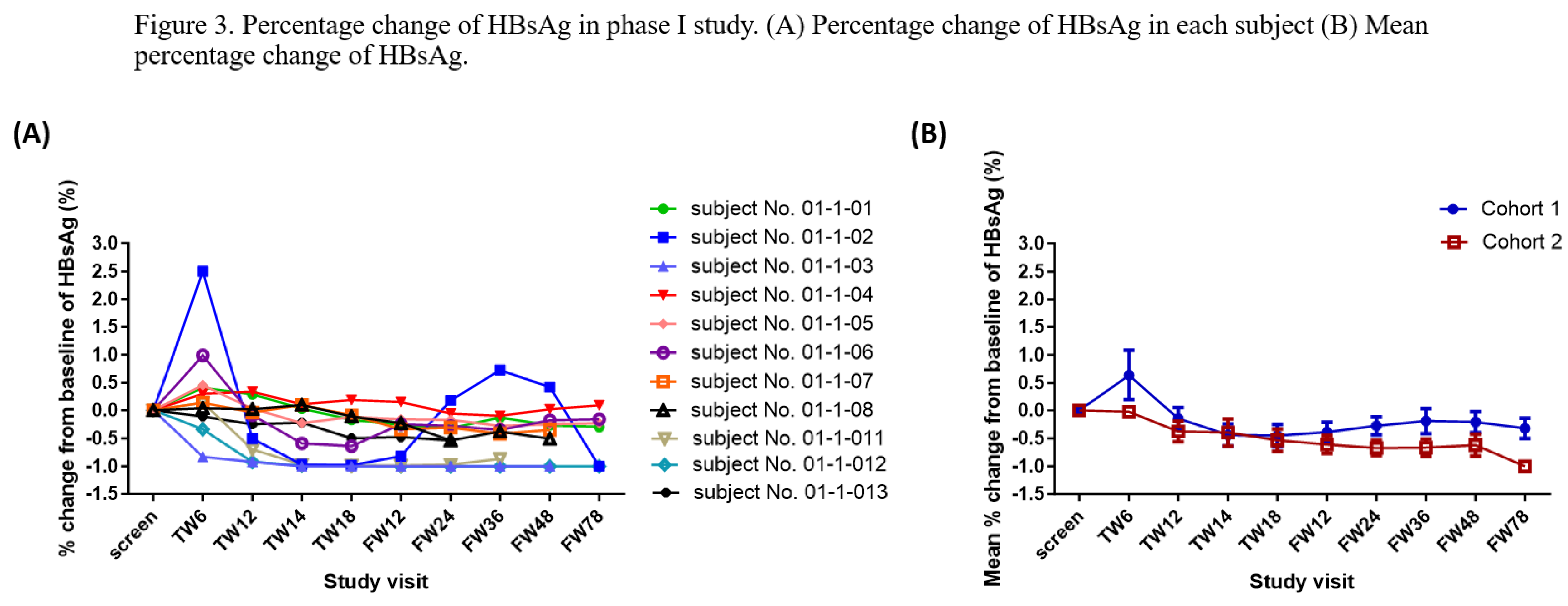
All patients are alive without cancer recurrence as of the date. The mean follow-up period was 716.75 days (minimum: 114 days; maximum: 1416). HBsAg was undetectable in one patient in Cohort 1 at follow-up week 12 and after (
Figure 3A). The mean HBsAg levels decreased over time in Cohort 2 during the treatment period (
Figure 3B).
3. Discussion
HCC is a common cause of cancer-related deaths, and most HCC cases are associated with HBV infection. Patients with HCC have a high risk of tumor recurrence after surgical resection. Currently, no approved therapies for recurrence inhibition exist. In this report, we showed our animal modeling data that sequential combination treatment with rmIFN-α and anti-PD1 antibody led to a synergistic effect in HBV suppression and clearance. Our Phase I clinical study further suggested that the sequential combination therapy with ropeginterferon alfa-2b and nivolumab was well-tolerated and might help clear residual HBV infection and inhibit cancer recurrence in patients with HBV-related HCC after curative surgery.
PD-L1 is often overexpressed during CHB infection [25]. In HCC patients with CHB, treatment with nivolumab decreased HBsAg levels [7, 26]. Nivolumab is approved for HCC treatment in combination with ipilimumab [27]. It is reasonable to assume that anti-PD-1 antibodies may suppress cancer recurrence in HBV-infected patients with HCC who have undergone surgical resection. However, the toxicities associated with the anti-PD-1 treatment at approved dose levels and HBV reactivation due to the residual viral genome may pose hurdles for its use alone as a prophylactic measure against HCC occurrence [28-30].
Type 1 IFNs, including IFN-α and beta (IFN-β), share the same receptor components. They can slow S phase progression by the activation of an intra-S phase checkpoint and induce senescence entry accompanied by a loss of tumorigenicity in solid tumor cells [31]. In addition, they stimulate the immune system to elicit anti-tumor activities, including inducing natural killer cell-dependent and CD8+ T cell-mediated anti-tumor responses [32, 33]. These combined anti-tumor activities can inhibit tumor formation. Pegylated IFN-α treatment has been shown to inhibit HBV and is approved for patients with HBV infection. Therefore, a PEGylated IFN-α therapy that can exert both anti-cancer and anti-HBV activities may reduce the need of the anti-PD1 treatment at a high dose level. Their sequential combination treatment may potentially eradicate residual tumor cells or newly formed tumor cells due to HBV infection in patients with HBV-related HCC after curative surgery. Our results suggest that the sequential combination therapy with ropeginterferon alfa-2b and nivolumab may be a promising regimen for inhibiting cancer recurrence in patients with HBV-related HCC after curative surgery.
4. Methods
HBV-HDI Mouse Model:
Six- to eight-week-old male CBA/Caj mice were bred at the National Taiwan University Laboratory Animal Center. The mice were intravenously injected with 10 μg of HBV genotype A DNA plasmid dissolved in PBS equivalent to approximately 8% of the mouse’s body weight as previously described [23, 24]. Serum HBsAg and HBV DNA were measured to monitor the HBV persistence. All experiments were performed according to the guidelines established by the Institutional Animal Care and Use Committee at the National Taiwan University College of Medicine.
Preclinical Materials:
Anti-mouse PD1 (RMP-17) is a monoclonal antibody targeting mouse PD-1 generated by hybridoma screening at the PharmaEssentia Corporation Research Laboratory. rmIFN-α was produced using the Escherichia coli expression system at PharmaEssentia Corporation.
Treatment of HBV-Carrying CBA/CaJ Mice:
Mice were divided into eight groups to investigate the effect of rmIFN-α in sequential combination with the anti-mouse PD1. The drug dosages and administration routes are described below:
Group 1 (control): Six HBV-carried CBA/CaJ mice were subcutaneously (s.c.) injected with 200 μl PBS at every other day [Q2D] x 8 (Days 0~14).
Group 2: Six HBV-carried CBA/CaJ mice were s.c. injected with 800 IU/g of rmIFN-α (Q2D x 8, Day 0~14).
Group 3: Six HBV-carried CBA/CaJ mice were s.c. injected with 800 IU/g of rmIFN-α (Q2D x 22, day 0~42)
Group 4: Ten HBV-carried CBA/CaJ mice were intraperitoneally (i.p.) injected with 32 μg/g of the anti-mouse PD1 antibody (Q2D x 6, days 16~26).
Group 5: Ten HBV-carried CBA/CaJ mice were i.p. injected with 32 μg/g of anti-mouse PD1 antibody (Q2D x 10, days 16~34).
Group 6: Ten HBV-carried CBA/CaJ mice were s.c. injected with 800 IU/g of rmIFN-α (Q2D x 8, days 0~14), and then, i.p. injected with 32 μg/g of the anti-PD1 antibody (Q2D x 6, days 16~26).
Group 7: Ten HBV-carried CBA/CaJ mice were s.c. injected with 800 IU/g of rmIFN-α (Q2D x 22, days 0~42), and then, i.p. injected with 32 μg/g of the anti-PD1 antibody (Q2D x 6, days 44~54).
Group 8: Ten HBV-carried CBA/CaJ mice were s.c. injected with 800 IU/g of rmIFN-α (Q2D x 43, days 0~84), and then, i.p. injected with 32 μg/g of the anti-PD1 antibody (Q2D x 6, days 86~96).
Clinical Materials:
Ropeginterferon alfa-2b was produced by PharmaEssentia Corporation. It was provided as a prefilled syringe of 500 µg/1.0 mL. Nivolumab (OPDIVO®, Bristol-Myers Squibb Company) was obtained via investigator prescription in the dosage form of 20 mg/2 mL or 100 mg/10 mL per vial.
Study Design:
This clinical study was designed as a Phase I/II trial. The Phase I study aimed to evaluate the safety and tolerability and define the MTD of the sequential administration of ropeginterferon alfa-2b and nivolumab in patients who had received curative surgery of hepatitis B-related HCC. The Phase II trial was designed to further evaluate the safety and prophylactic effect of sequential administration of ropeginterferon alfa-2b and nivolumab at the MTD. Phase I was conducted at the National Taiwan University Hospital (NTUH) Taiwan (approval number: 201710061MIPB). The Phase I study was completed, and Phase II has not yet started.
The sequential administration of ropeginterferon alfa-2b and nivolumab was assessed using a 3+3 dose escalation scheme. Eligible patients were planned to be enrolled into four dose cohorts to receive the six doses of ropeginterferon alfa-2b at the dose of 450 μg once every two weeks, followed by three doses of nivolumab every two weeks at a pre-determined dose level by cohort, i.e., 0.3 mg/kg in Cohort 1; 0.75 mg/kg in Cohort 2; 1.5 mg/kg in Cohort 3, and 3 mg/kg in Cohort 4. Patients were followed up by a site visit for an additional 48 weeks after completion of the study treatment. Disease progression and survival status were planned to be continuously monitored.
Patients:
Patients with HBV-related HCC who received the surgical resection within eight weeks were enrolled. Other major inclusion criteria included positive results for HBsAg, undetectable HBV DNA, compensated liver disease, normal fundoscopic examination, and an Eastern Cooperative Oncology Group Performance Status score of 0 to 1. The major exclusion criteria included HCC that was not related to HBV, vascular invasion of HCC on imaging diagnosis, patients who had undergone transcatheter arterial embolization or chemoembolization, transcatheter arterial infusion, or chemolipiodolization in combination with surgery, and a concurrent active malignancy other than HCC.
5. Conclusions
Our animal data demonstrated a synergistic effect between rmIFN-α and anti-PD1 treatment for HBV suppression or even clearance. This effect was observed in patients with HCC who received the sequential combination therapy of ropeginterferon alfa-2b and nivolumab in our Phase 1 clinical study. Most AEs were mild or moderate. Increased liver transaminase increases were common but not associated with increased bilirubin levels or clinical symptoms. No unexpected AEs were observed. The MTD of sequential combination therapy with ropeginterferon alfa-2b and nivolumab was determined. Further exploration and clinical development of the combination therapy are warranted.
Author Contributions
All authors contributed to the work. P-J. C. and M-C. H. enrolled and treated patients. All authors participated in the writing and review of the manuscript and approved it for publication.
Funding
This precclinical work and phase I/II study were partially sponsored by the PharmaEssentia Corporation.
Ethics approval and Institutional Review Board Statement
Animal experiments were performed by following the guidelines established by the Institutional Animal Care and Use Committee of the National Taiwan University College of Medicine. The phase I/II clinical study was approved by the Institutional Review Board of NTUH (201710061MIPB) and conducted according to the principles of the Declaration of Helsinki for all human experimental investigations. Phase I/II clinical studies were registered at ClinicalTrials.gov (NCT04233840).
Informed Consent Statement
Informed consent was obtained from all participating patients.
Data Availability Statement
Data will be available to external researchers upon reasonable request from the investigator and PharmaEssentia.
Acknowledgments
The authors thank all the other participants, including the study nurse, coordinator, other investigators, and PharmaEssentia team members involved in this study. We are grateful to the patients and their families.
Conflicts of Interest
Albert Qin and Chanyen Tsai work for PharmaEssentia Corporation. Pei-Jer Chen served as a consultant for PharmaEssentia Corporation. Other authors declare no conflicts of interest.
References
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Vilarinho, S.; Calvisi, D.F. New advances in precision medicine for hepatocellular carcinoma recurrence prediction and treatment. Hepatology 2014, 60, 1812–1814. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Kudo, M. Adjuvant therapy after radical surgery for hepatocellular carcinoma: still an unmet need. Hepatobiliary Surg. Nutr. 2019, 8, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, K.; Anagnostou, T.; Boussiotis, V.A. The PD1:PD-L1/2 Pathway from Discovery to Clinical Implementation. Front. Immunol. 2016, 7, 550. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Targeting the PD-1/B7-H1 (PD-L1) pathway to activate anti-tumor immunity. Curr. Opin. Immunol. 2012, 24, 207–212. [Google Scholar] [CrossRef]
- Gane, E.; Verdon, D.J.; Brooks, A.E.; Gaggar, A.; Nguyen, A.H.; Subramanian, G.M.; Schwabe, C.; Dunbar, P.R. Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: A pilot study. J. Hepatol. 2019, 71, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Du, S.; Yang, H. HBsAg seroclearance reduces the risk of late recurrence in HBV-related HCC. J Hepatol. 2022, 77, 1469–1470. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Chen, J.; Yan, H.; He, Q.; Luo, P.; Xu, Z.; Yang, X. Research Status and Outlook of PD-1/PD-L1 Inhibitors for Cancer Therapy. Drug Des. Devel. Ther. 2020, 14, 3625–3649. [Google Scholar] [CrossRef]
- Miyachi, N.; Zagrijtschuk, O.; Kang, L.; Yonezu, K.; Qin, A. Pharmacokinetics and pharmacodynamics of ropeginterferon alfa-2b in healthy Japanese and Caucasian Subjects After Single Subcutaneous Administration. Clin. Drug Investig. 2021, 41, 391–404. [Google Scholar] [CrossRef]
- Huang, Y.W.; Qin, A.; Fang, J.; Wang, T.F.; Tsai, C.W.; Lin, K.C.; Teng, C.L.; Larouche, R. Novel long-acting ropeginterferon alfa-2b: Pharmacokinetics, pharmacodynamics, and safety in a phase 1 clinical trial. Br. J. Clin. Pharmacol. 2022, 88, 2396–2407. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.W.; Tsai, C.Y.; Tsai, C.W.; Wang, W.; Zhang, J.; Qin, A.; Teng, C.; Song, B.; Wang, MX. Pharmacokinetics and pharmacodynamics of novel long acting ropeginterferon alfa-2b in healthy Chinese subjects. Adv. Ther. 2021, 38, 4756–4770. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. FDA Approves Treatment for Rare Blood Disease: Treatment is First FDA-Approved Option Patients Can Take Regardless of Previous Therapies [Press release; date: Nov 12, 2021]. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-treatment-rare-blood-disease#:~:text=Rare%20Blood%20Disease-,FDA%20NEWS%20RELEASE,-FDA%20Approves%20Treatment (accessed on 7 September 2023).
- European Medicines Agency. Besremi: EPAR-Medicine Overview. 2019. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/besremi (accessed on 7 September 2023).
- Verstovsek, S.; Komatsu, N.; Gill, H.; Jin., J.; Lee, S.E.; Hou, H.A.; Sato, T.; Qin, A.; Urbanski, R.; Shih, W. SURPASS-ET: phase III study of ropeginterferon alfa-2b versus anagrelide as second-line therapy in essential thrombocythemia. Future Oncol. 2022, 18, 2999–3009. [Google Scholar] [CrossRef]
- Huang, Y.W.; Qin, A.; Tsai, C.Y.; Chen, P.J. Novel Pegylated Interferon for the Treatment of Chronic Viral Hepatitis. Viruses 2022, 14, 1128. [Google Scholar] [CrossRef]
- Chen, C.T.; Chuang, W.L.; Qin, A.; Zhang, W.H.; Zhu, L.Y.; Zhang, G.Q.; Chen, J.J.; Lo, C.C.; Zhou, X.; Mao, X. A phase 3 clinical trial validating the potency and safety of an innovative, extra-long-acting interferon in chronic hepatitis C. JGH Open. 2022, 6, 782–791. [Google Scholar] [CrossRef]
- Chen, K.Y.; Lee, K.Y.; Qin, A.; Luo, C.S.; Yeh, Y.K.; Zheng, J.Q.; Chen, C.M.; Tsai, C.Y.; Lin, S.; Liao, J. Clinical experience with ropeginterferon alfa-2b in the off-label use for the treatment of COVID-19 patients in Taiwan. Adv. Ther. 2022, 39, 910–922. [Google Scholar] [CrossRef]
- Jin, J.; Qin, A.; Zhang, L.; Shen, W.; Wang, W.; Zhang, J.; Li, Y.; Wu, D.; Xiao, Z. A Phase 2 Trial to Assess the Efficacy and Safety of Ropeginterferon alfa-2b in Chinese Patients with Polycythemia Vera. Future Oncol. 2023, 19, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Urbansky, R.W.; Yu, L.; Ahmed, T.; Mascrenhas, J. An Alternative Dosing Strategy for Ropeginterferon alfa-2b may Help Improve Outcomes in Myeloproliferative Neoplasms: An Overview of Previous and Ongoing studies with Perspectives on the Future. Front Oncol. 2023, 13, 1109866. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Zhang, L.; Qin, A.; Wu, D.; Shao, Z.; Bai, J.; Chen, S.; Duan, M.; Zhou, H.; Xu, N.; et al. A new dosing regimen of ropeginterferon alfa-2b is highly effective and tolerable: findings from a phase 2 study in Chinese patients with polycythemia vera. Exp. Hematol. Oncol. 2023, 12, 55. [Google Scholar] [CrossRef]
- Huang, Y.W.; Hsu, C.W.; Lu, S.N.; Yu, M.L.; Su, C.W.; Su, W.W.; Chien, R.N.; Hsu, C.S.; Hsu, S.J.; Lai, H.C. Ropeginterferon alfa-2b every 2 weeks as a novel pegylated interferon for patients with chronic hepatitis B. Hepatol. Int. 2020, 14, 997–1008. [Google Scholar] [CrossRef]
- Huang, LR.; Wu, HL.; Chen, PJ.; Chen, DS. An immunocompetent mouse model for the tolerance of human chronic hepatitis B virus infection. Proc. Natl. Acad. Sci. USA 2006, 103, 17862–17867. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Song, Y.; Liu, D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999, 6, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Cao, L.J.; Song, H.F.; Xu, P.; Chen, H.; Xu, J.C.; Zhu, X.Y.; Zhang, X.G.; Wang, X.F. Expression of PD-L1 on CD4+CD25+Foxp3+ Regulatory T Cells of Patients with Chronic HBV Infection and Its Correlation with Clinical Parameters. Viral. Immunol. 2015, 28, 418–24. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H. R.d.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- Saung, M.T.; Pelosof, L.; Casak, S.; Donoghue, M.; Lemery, S.; Yuan, M.; Rodriguez, L.; Schotland, P.; Chuk, M.; Davis, G.; et al. FDA Approval Summary: Nivolumab Plus Ipilimumab for the Treatment of Patients with Hepatocellular Carcinoma Previously Treated with Sorafenib. Oncologist 2021, 26, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Pu, D.; Yin, L.; Zhou, Y.; Li, W.; Huang, L.; Cai, L.; Zhou, Q. Safety and efficacy of immune checkpoint inhibitors in patients with HBV/HCV infection and advanced-stage cancer: A systematic review. Medicine 2020, 99, e19013. [Google Scholar] [CrossRef] [PubMed]
- Burns, E.A.; Muhsen, I.N.; Anand, K.; Xu, J.; Umoru, G.; Arain, A.N.; Abdelrahim, M. Hepatitis B Virus Reactivation in Cancer Patients Treated with Immune Checkpoint Inhibitors. J. Immunother. 2021, 44, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Féray, C.; López-Labrador, F.X. Is PD-1 blockade a potential therapy for HBV? JHEP Rep. 2019, 1, 142–144. [Google Scholar] [CrossRef]
- Qin, A. An anti-cancer surveillance by the interplay between interferon-beta and retinoblastoma protein RB1. Front. Oncol. 2023, 13, 1173467. [Google Scholar] [CrossRef]
- Qin, X.Q.; Beckham, C.; Brown, J.L.; Lukashev, M.; Barsoum, J. Human and mouse IFN-β gene therapy exhibits different anti-tumor mechanisms in mouse models. Mol. Therapy 2001, 4, 356–64. [Google Scholar] [CrossRef]
- Brown, J.L.; Barsoum, J.; Qin, X.Q. CD4+ T helper cell-independent anti-tumor response mediated by murine IFN-beta gene delivery in immunocompetent mice. J. Interferon Cytokine Res. 2002, 22, 719–28. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).