1. Introduction
Endophthalmitis is a severe intraocular infection, which can be classified into an exogenous and endogenous form. The vast majority of endophthalmitis cases have an exogenous origin. The infection typically occurs after surgical procedures, trauma, or pathogen infiltration following infections of the ocular adnexa [
1]. On the other hand, endogenous endophthalmitis is caused by the hematogenous dissemination of microorganisms from the primary site of inflammation [
2,
3,
4]. It represents only 2% to 8% of all endophthalmitis cases [
2]. Several risk factors for endogenous endophthalmitis have been identified, including indwelling catheters, prolonged hospitalization, diabetes, immunosuppression, renal pathology, human immunodeficiency virus infection, influenza A virus infection, intravenous drug abuse, or malignancies [
2,
3,
4,
5,
6,
7]. The primary pathological factor may be either bacterial or fungal. In fungal endophthalmitis,
Candida albicans stands out as the most prevalent etiological factor, followed by
Cryptococcus neoformans,
Aspergillus spp., and
Paecilomyce spp. [
8] Recent reports indicated that the incidence of endogenous fungal inflammation in patients with fungemia has decreased significantly and does not exceed 2.2% [
9]. However, its prevalence depends on a geographical region. A meta-analysis by Phongkhun et al. [
10] showed that the prevalence of fungal endophthalmitis reported in studies from Asian countries was 2- and 4-fold higher as compared with studies from European countries and as reported by the American Academy of Ophthalmology (AAO).
Endogenous
Candida endophthalmitis (ECE) is a serious sight-threatening disease. Bilateral involvement is observed in more than 25% of cases [
4,
9]. The diagnosis of ECE is difficult and is based on clinical findings supported by additional diagnostic tests, including blood culture, aqueous or vitreous culture, and microscopic examination of the sample. However, the sensitivity of these tests is limited [
11,
12,
13,
14]. Techniques based on the amplification and quantification of DNA, such as a polymerase chain reaction test, were shown to be more sensitive than culture methods, but low microorganism load in a sample can yield false negative results [
15].
Along with advances in intravitreal medical treatment, there has also been an ongoing improvement in modern imaging techniques such as optical coherence tomography (OCT) and OCT angiography (OCTA). These tools allow a noninvasive imaging of the retina and choroid, enabling an early diagnosis and immediate treatment of numerous diseases, especially those involving the macula. Recently, it was also shown that the characteristic findings on OCT facilitate a correct diagnosis in ECE cases [
16,
17,
18,
19].
This case report highlights the usefulness of OCT and OCTA in the diagnosis and follow-up of ECE in a patient with a history of prolonged hospitalization for severe intermittent porphyria leading to the placement of a permanent central vein catheter 3 weeks before the onset of visual symptoms.
2. Case description
A 41-year-old woman was admitted to the Metabolic Disorders Unit of the University Hospital in Kraków, Poland, due to a life-threatening attack of acute intermittent porphyria with severe visceral manifestations. The patient was treated with an intravenous injection of hemin and morphine. Due to peripheral venous access problems, the internal jugular vein cannulation was performed and the treatment was continued. During systemic therapy, the patient developed fever and started to complain of blurred vision in the left eye. Systemic antibiotic therapy with meropenem at a dose of 2 g every 8 hours was administered. However, the patient complained of a further deterioration of vision, pain, and photophobia in her left eye.
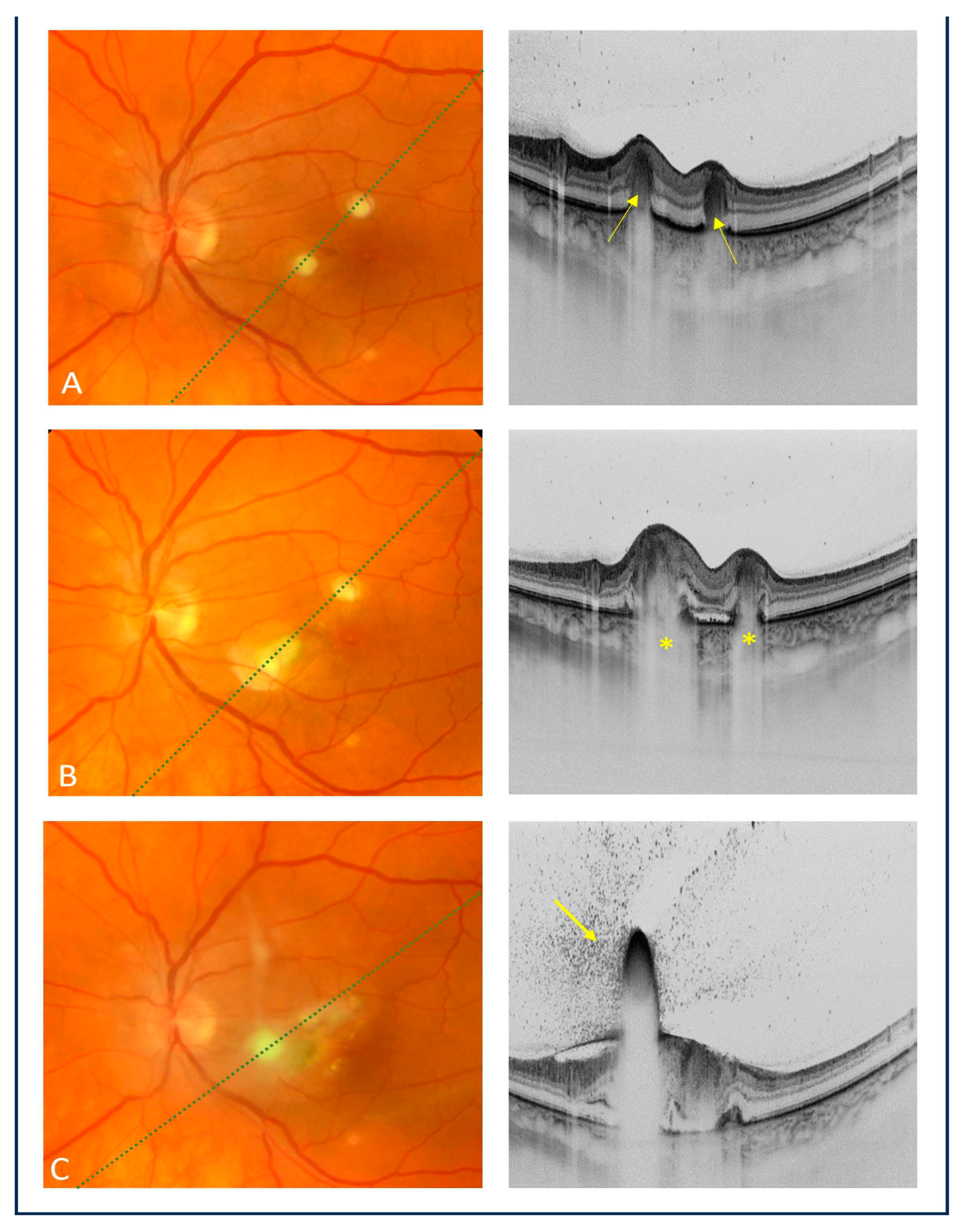
The patient was consulted at the Department of Ophthalmology and Ocular Oncology of the University Hospital in Kraków. On ophthalmic examination, the best corrected visual acuity (BCVA) was 1.0 in the right eye and 0.8 in the left eye. No abnormalities were present on a slit-lamp examination of both eyes. The fundus examination of the right eye showed no pathology. However, in the macula of the left eye, there were two creamy-white, consolidated inflammatory lesions. Swept-source OCT (Topcon, Japan) revealed hyperreflective lesions involving the choroid and outer retinal layers, corresponding to the inflammatory foci found on fundoscopy (
Figure 1a). During the follow-up, the patient experienced a recurrent attack of acute intermittent porphyria, suggesting that meropenem could be a porphyrinogen agent that triggered the attack. The ophthalmic examination showed further deterioration of vision and progression of inflammatory chorioretinal inflammatory foci on both fundoscopic and OCT examinations (
Figure 1b).
Considering the clinical presentation and imaging findings, a fungal etiology of intraocular inflammation in the left eye was suspected. The blood culture from the internal jugular venous catheter was positive for
Candida albicans, thus confirming the diagnosis of endogenous fungal endophthalmitis. Intravenous therapy with fluconazole at a dose of 400 mg/day was initiated. However, due to an underlying disease (porphyria), fluconazole was poorly tolerated. The patient experienced another acute attack of porphyria. The results of systemic therapy also proved to be unsatisfactory, as the chorioretinal lesions continued to deteriorate clinically (
Figure 1c). The follow-up showed progression of inflammatory lesions. Moreover, vitreous exudation over the posterior pole was noted, and the BCVA of the left eye decreased to 0.16 (
Figure 1c). The patient was referred for intravitreal injections of amphotericin B (5 μg/0.1 ml). A total of four injections every 7 days were administered.
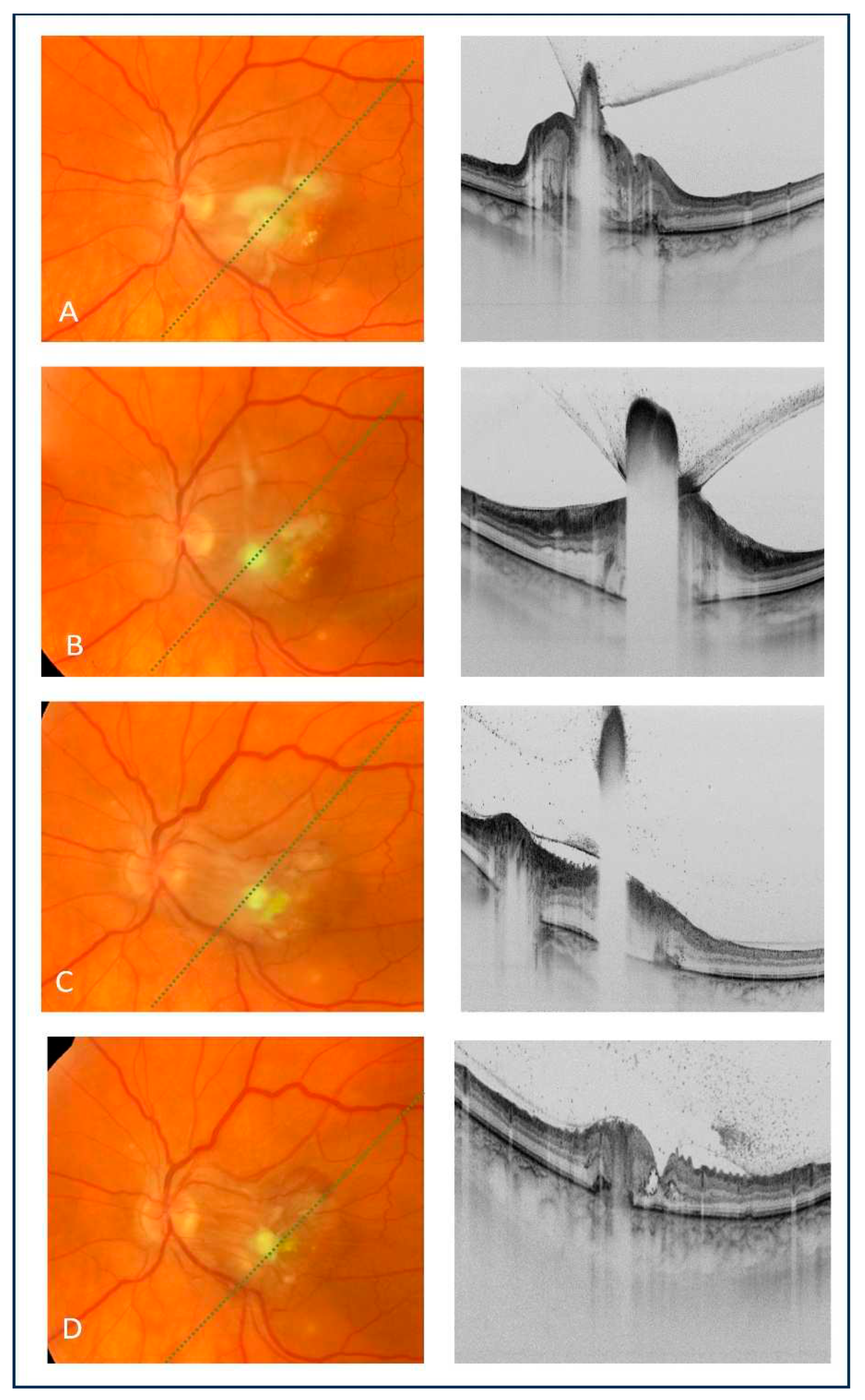
Intravitreal amphotericin B administration resulted in the regression of inflammatory foci and vitreous exudates, as revealed by an fundoscopy and OCT (
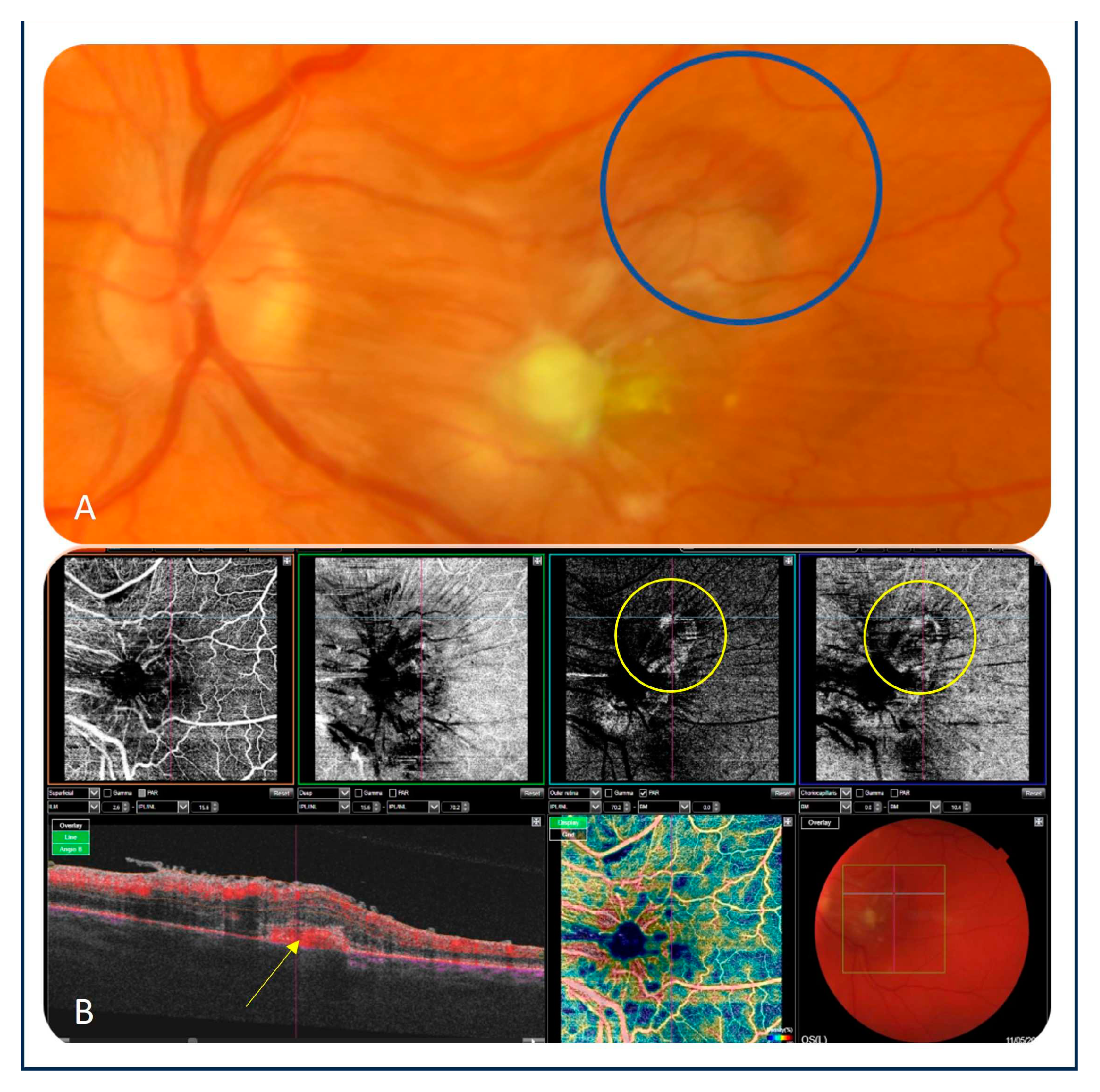
Figure 2a–d). However, the BCVA of the left eye remained unchanged. Three weeks after the fourth intravitreal injection of amphotericin B, a small hemorrhage in the upper-temporal part of the macula was noted. An OCT scan showed a hyperreflective subretinal lesion suspected of macular neovascularization (MNV) (
Figure 3a). As there were difficulties with the vascular access, fluorescein angiography could not be performed. Moreover, the patient was in a poor general condition. Therefore, OCTA (Topcon, Japan) was done, which confirmed the diagnosis of MNV (
Figure 3b). The patient was scheduled for an intravitreal injection of bevacizumab (1.25 mg/0.05 ml) in the left eye. While follow-up OCT and OCTA examinations revealed anatomical improvement, visual acuity of the left eye remained poor, as assessed based on counting fingers.
3. Discussion
The diagnosis of ECE may be challenging. Previous studies demonstrated that endogenous endophthalmitis can be missed in 16% to 63% of cases [
3,
20]. Therefore, it is necessary to raise the awareness about this condition among clinicians, especially in the presence of risk factors for ECE.
As the choroid is a rich vascularized tissue, it is the primary site affected by fungal infection. Subsequently, the inflammation spreads and progresses anteriorly from the choroid to the outer and then to the inner retinal layers. Consequently, endogenous fungal endophthalmitis often presents with choroiditis or chorioretinitis. As the infection worsens, it involves the vitreous body [
20,
21]. Progression is typically slow.
Candida chorioretinitis typically manifests as creamy-white, well-circumscribed chorioretinal lesions accompanied by white vitreous opacities, representing the characteristic “string of pearls” sign [
8,
21].
The differential diagnosis includes noninfectious uveitis (e.g., in association with sarcoidosis, Behçet syndrome, sympathetic ophthalmia, or Vogt-Koyanagi-Harada disease) as well as infectious entities such as bacterial endophthalmitis, tuberculosis, syphilis, herpes virus infections, toxoplasmosis, and masquerade syndromes (intraocular lymphoma) [
8,
9,
23].
Recently, it has been shown that characteristic OCT findings may facilitate the correct diagnosis of fungal infection in cases with ECE [
16,
17,
19]. Invernizzi et al. [
16] described two distinct patterns of choroidal and retinal involvement (intraretinal and chorioretinal) influencing the final BCVA, which was shown to be worse in eyes with the chorioretinal pattern. Additionally, all eyes with ECE presented with hyperreflective preretinal lesions that obscured the underlying retina and created a shadowing effect called the “rain-cloud” sign. Chorioretinal involvement was also noted in our patient, along with the “rain-cloud” sign on OCT as a characteristic feature of early intraretinal fungal lesions (
Figure 1b).
Anvari et al. [
19] used the term “inverted snowing-cloud sign” to describe a preretinal lesion with vitreous aggregates resembling a white cloud with snowflakes (
Figure 1c and
Figure 2a). This finding was also noted in our patient, corresponding to the more advanced stage of fungal inflammation extending to the vitreous body. However, according to the authors, the “inverted snowing-cloud” sign may not be specific for
Candida endophthalmitis and can be observed in other retinal and choroidal diseases associated with vitritis.
Zhuang et al. [
17] described the following four types of OCT manifestations of ECE: type 1 representing subretinal lesions in the macula, type 2 lesions located in the inner retinal layers, type 3 lesions involving the full-thickness macula, and type 4 representing the subinner limiting membrane lesions. After antifungal treatment, the final visual acuity of eyes with type 2 lesions was improved [
17]. Based on this classification, in our patients, we observed type 3 lesions, which translated into a poor prognosis for the affected eye.
The treatment strategy for patients with ECE depends on the clinical presentation and includes systemic therapy with fluconazole or voriconazole, intravitreal injections of amphotericin B or voriconazole, and—in more complicated cases—vitrectomy [
23]. There is evidence that systemic therapy with fluconazole and voriconazole is effective in patients with ECE [
24,
25]. While it is thought to have a generally good safety profile with only a few side effects, it may have a significant impact on the clinical course of acute intermittent porphyria [
26]. Antifungal agents also may have a porphyrogenic effect and trigger disease relapse [
26]. Drug delivery directly at the site of the pathology is more effective than the administration of intravenous or oral medication. Moreover, local therapy is safer because it reduces the risk of systemic complications caused by the medication. This is especially important in patients who cannot tolerate systemic therapy, and exposure to the therapy may cause exacerbation of systemic disease. In our patient, systemic therapy with fluconazole triggered acute attacks of porphyria [
27]. Because of poor tolerance of systemic fluconazole and macula involvement, the patient received intravitreal injections of amphotericin B. She responded well to antifungal intravitreal therapy, and regression of intraocular inflammation was observed after 4 injections. However, follow-up OCT scans showed a new lesion suspected of MNV, and OCTA confirmed the diagnosis. Therefore, a decision was made to administer the intraretinal injection of bevacizumab.
4. Conclusions
Patients with ECE require early and aggressive treatment strategies, including systemic and intravitreal drug administration with or without vitrectomy. This highlights the importance of diagnostic tools that can accelerate the diagnostic process and ensure close disease monitoring.
The characteristic OCT features of ECE found in our patient confirm that OCT is a useful tool to diagnose this type of intraocular inflammation. As a noninvasive repeatable imaging technique, OCT can be used to assess the response to treatment and monitor long-term outcomes. On the other hand, OCTA aids a quick diagnosis and therapy decision-making if the patient presents with secondary MNV, which is a rare macular complication of intraocular inflammation.
Author Contributions
Conceptualization, A.K.-T.; investigation, A.K.-T., I.K.-B. and W.P.-M.; data curation, D.B., K.Ż-Ł and A.M; writing—original draft preparation, A.K.-T., D.B. and I.K.-B.; writing—review and editing, A.K.-T., I.K.-B. and D.B.; visualization, W.P.-M. and K.Ż.-Ł; supervision, B.R.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All procedures were carried out in accordance with guidelines set forth by the Declaration of Helsinki.
Informed Consent Statement
The patient signed an institutional informed consent for the use of medical records and laboratory data and the publication of this information for research purposes.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schwartz, S.G.; Flynn, H.W., Jr.; Das, T.; Mieler, W.F. Ocular infection: endophthalmitis. Dev. Ophthalmol. 2016, 55, 176–188. [Google Scholar] [CrossRef]
- Sadiq, M.A.; Hassan, M.; Agarwal, A.; Sarwar, S.; Toufeeq, S.; Soliman, M.K.; Hanout, M.; Sepah, Y.J.; Do, D.V.; Nguyen, Q.D. Endogenous endophthalmitis: diagnosis, management, and prognosis. J. Ophthalmic Inflamm. Infect. 2015, 5, 32. [Google Scholar] [CrossRef]
- Jackson, T.L.; Paraskevopoulos, T.; Georgalas, I. Systematic review of 342 cases of endogenous bacterial endophthalmitis. Surv. Ophthalmol. 2014, 59, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Danielescu, C.; Anton, N.; Stanca, H.T.; Munteanu, M. Endogenous endophthalmitis: a review of case series published between 2011 and 2020. J. Ophthalmol. 2020, 2020, 8869590. [Google Scholar] [CrossRef]
- Bhagali, R.; Prabhudesai, N.P.; Prabhudesai, M.N. Post COVID-19 opportunistic candida retinitis: a case report. Indian J. Ophthalmol. 2021, 69, 987–989. [Google Scholar] [CrossRef]
- Menia, N.K.; Sharma, S.P.; Bansal, R. Fungal retinitis following influenza virus type A (H1N1) infection. Indian J. Ophthalmol. 2019, 67, 1483–1484. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Shin, Y.U.; Siegel, N.H.; Yu, H.G.; Sobrin, L.; Patel, A.; Durand, M.L.; Miller, J.W.; Husain, D. Endogenous endophthalmitis in the American and Korean population: an 8-year retrospective study. Ocul. Immunol. Inflamm. 2018, 26, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.U.; Yu, Y.H.; Kim, S.S.; Oh, TH.; Kim, S.E.; Kim, U.J.; Kang, S.J.; Jang, H.C.; Park, K.H.; Jung, S.I. Clinical characteristics and risk factors for complications of candidaemia in adults: Focus on endophthalmitis, endocarditis, and osteoarticular infections. Int. J. Infect. Dis. 2020, 93, 126–132. [Google Scholar] [CrossRef]
- Haseeb, A.A.; Elhusseiny, A.M.; Siddiqui, M.Z.; Ahmad, K.T.; Sallam, A.B. Fungal endophthalmitis: a comprehensive review. J. Fungi (Basel). 2021, 7, 996. [Google Scholar] [CrossRef]
- Phongkhun, K.; Pothikamjorn, T.; Srisurapanont, K.; Manothummetha, K.; Sanguankeo, A.; Thongkam, A.; Chuleerarux, N.; Leksuwankun, S.; Meejun, T.; Thanakitcharu, J.; et al. Prevalence of ocular candidiasis and candida endophthalmitis in patients with candidemia: a systematic review and meta-analysis. Clin. Infect. Dis. 2023, 76, 1738–1749. [Google Scholar] [CrossRef]
- Reginatto, P.; Agostinetto, G.J.; Fuentefria, R.D.N.; Marinho, D.R.; Pizzol, M.D.; Fuentefria, A.M. Eye fungal infections: a mini review. Arch. Microbiol. 2023, 205, 236. [Google Scholar] [CrossRef] [PubMed]
- Lingappan, A.; Wykoff, C.C.; Albini, T.A.; Miller, D.; Pathengay, A.; Davis, J.L.; Flynn, H.W., Jr. Endogenous fungal endophthalmitis: causative organisms, management strategies, and visual acuity outcomes. Am. J. Ophthalmol. 2012, 15, 162–166.e1. [Google Scholar] [CrossRef]
- William, A.; Spitzer, M.S.; Deuter, C.; Blumenstock, G.; Partsch, M.; Voykov, B.; Ziemssen, F.; Bartz-Schmidt, K.U.; Doycheva, D. Outcomes of primary transconjunctival 23-gauge vitrectomy in the diagnosis and treatment of presumed endogenous fungal endophthalmitis. Ocul. Immunol. Inflamm. 2017, 25, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Wang, W.J. Treatment outcomes after pars plana vitrectomy for endogenous endophthalmitis. Retina. 2005, 25, 746–750. [Google Scholar] [CrossRef]
- Sandhu, H.S.; Hajrasouliha, A.; Kaplan, H.J.; Wang, W. Diagnostic utility of quantitative polymerase chain reaction versus culture in endophthalmitis and uveitis. Ocul. Immunol. Inflamm. 2019, 27, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, A.; Symes, R.; Miserocchi, E.; Cozzi, M.; Cereda, M.; Fogliato, G.; Staurenghi, G.; Cimino, L.; McCluskey, P. Spectral domain optical coherence tomography findings in endogenous candida endophthalmitis and their clinical relevance. Retina. 2018, 38, 1011–1018. [Google Scholar] [CrossRef]
- Zhuang, H.; Ding, X.; Gao, F.; Zhang, T.; Ni, Y.; Chang, Q.; Xu, G. Optical coherence tomography features of retinal lesions in Chinese patients with endogenous Candida endophthalmitis. BMC Ophthalmol. 2020, 20, 52. [Google Scholar] [CrossRef]
- Veronese, C.; Maiolo, C.; Gurreri, A.; Morara, M.; Ciardella, A.P.; Yannuzzi, L.A. Multimodal imaging of multifocal chorioretinitis secondary to endogenous candida infection. Int. Ophthalmol. 2019, 39, 2137–2142. [Google Scholar] [CrossRef]
- Anvari, P.; Mirshahi, R.; Sedaghat, A.; Ghasemi Falavarjani, K. “Inverted snowing-cloud” sign in endogenous candida endophthalmitis. J. Ophthalmic Vis. Res. 2022, 17, 303–305. [Google Scholar] [CrossRef]
- Binder, M.I.; Chua, J.; Kaiser, P.K.; Procop, G.W.; Isada, C.M. Endogenous endophthalmitis: an 18-year review of culture-positive cases at a tertiary care center. Medicine. 2003, 82, 97–105. [Google Scholar] [CrossRef]
- Au, L.; Guduru, K.; Lipscomb, G.; Kelly, S.P. Candida endophthalmitis: a critical diagnosis in the critically ill. Clin. Ophthalmol. 2007, 1, 551–554. [Google Scholar]
- Chhablani, J. Fungal endophthalmitis. Expert Rev. Anti. Infect. Ther. 2011, 9, 1191–1201. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Hamada, Y.; Okuma, R.; Katori, Y.; Takahashi, S.; Hirayama, T.; Ichibe, Y.; Kuroyama, M. Bibliographical investigation (domestic and overseas) on the treatment of endogenous Candida endophthalmitis over an 11-year period. Med. Mycol. J. 2013, 54, 53–67. [Google Scholar] [CrossRef]
- Hariprasad, S.M.; Mieler, W.F.; Holz, E.R.; Gao, H.; Kim, J.E.; Chi, J.; Prince, R.A. Determination of vitreous, aqueous, and plasmaconcentration of orally administered voriconazole in humans. Arch. Ophthalmol. 2004, 122, 42–47. [Google Scholar] [CrossRef]
- Thunell, S.; Pomp, E.; Brun, A. Guide to drug porphyrogenicity prediction and drug prescription in the acute porphyrias. Br. J. Clin. Pharmacol. 2007, 64, 668–679. [Google Scholar] [CrossRef]
- Roveri, G.; Nascimbeni, F.; Rocchi, E.; Ventura, P. Drugs and acute porphyrias: reasons for a hazardous relationship. Postgrad. Med. 2014, 126, 108–120. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).