Submitted:
04 December 2023
Posted:
06 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
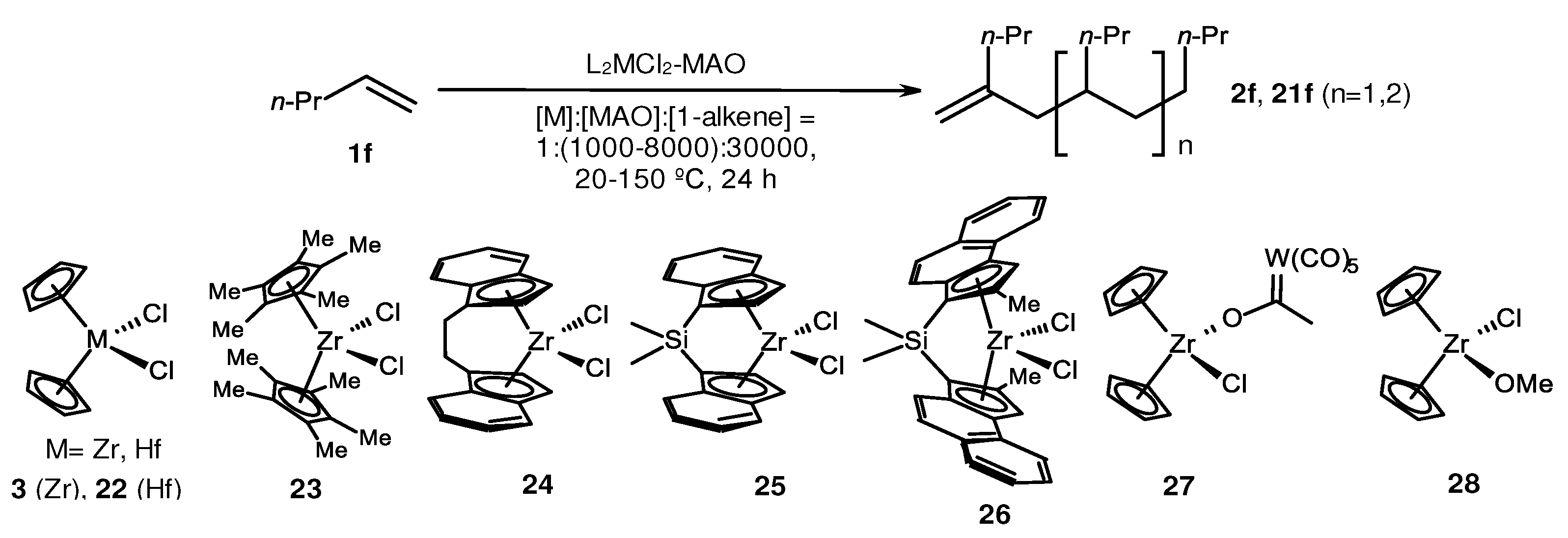
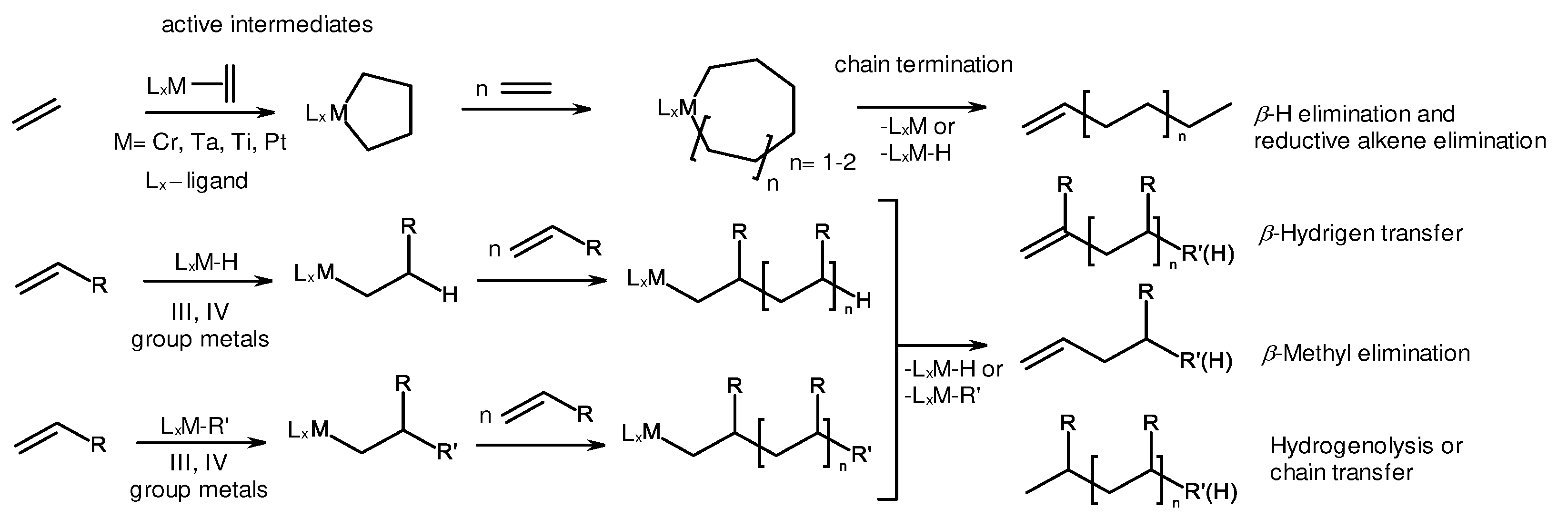
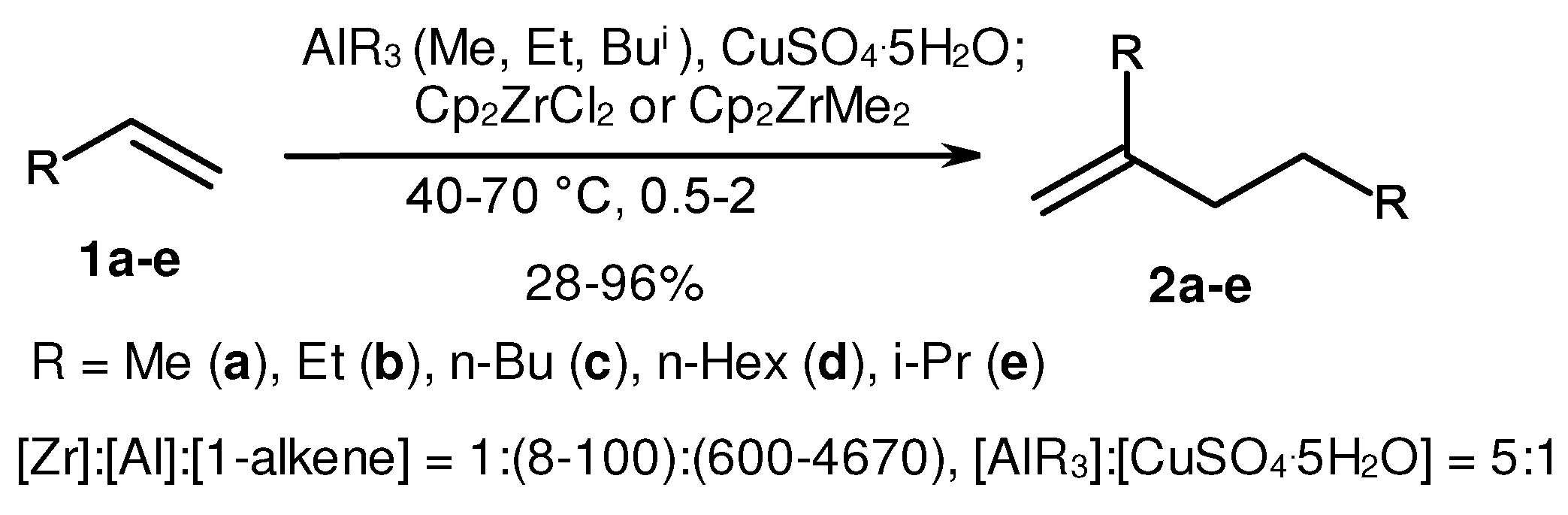
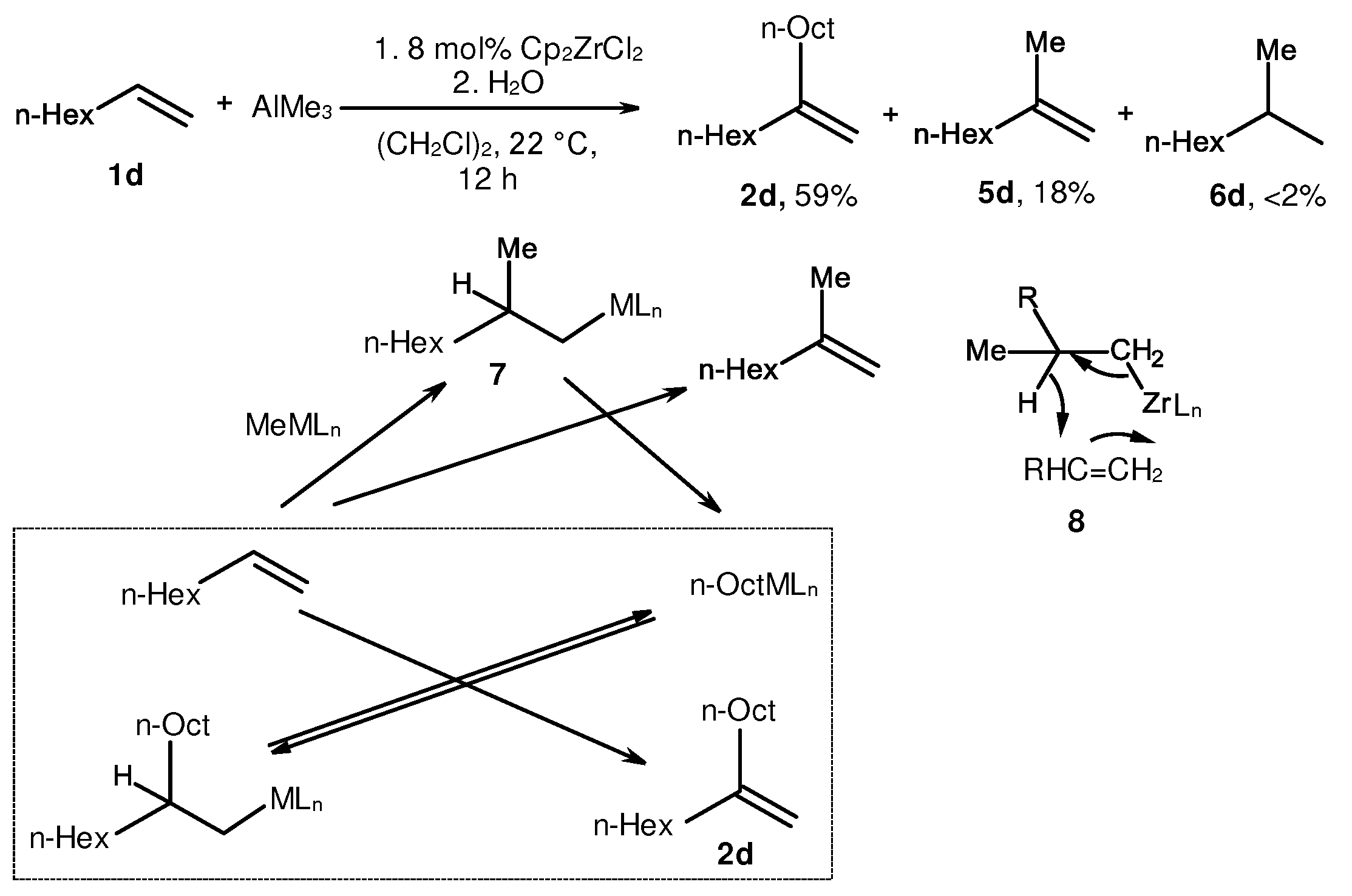
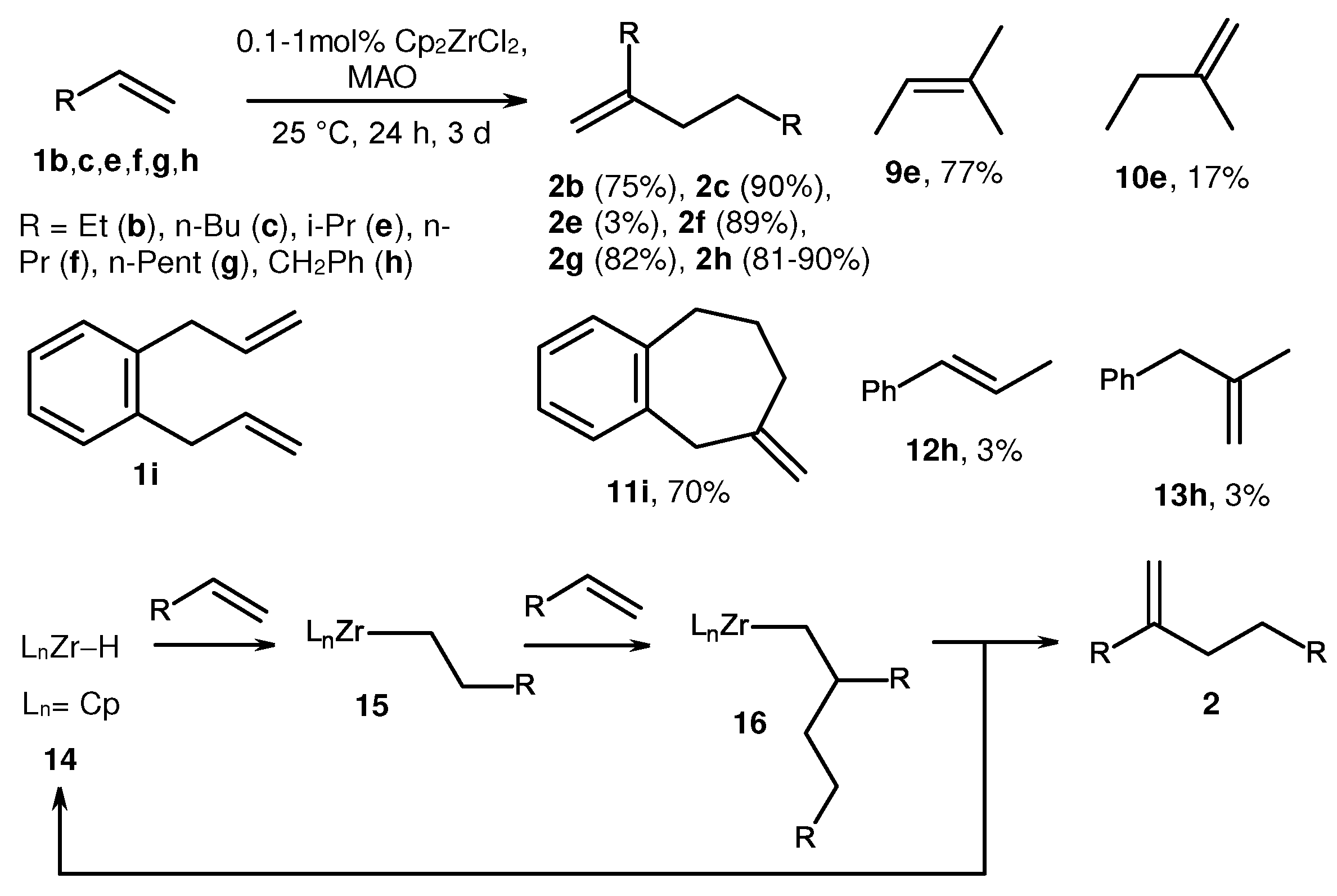
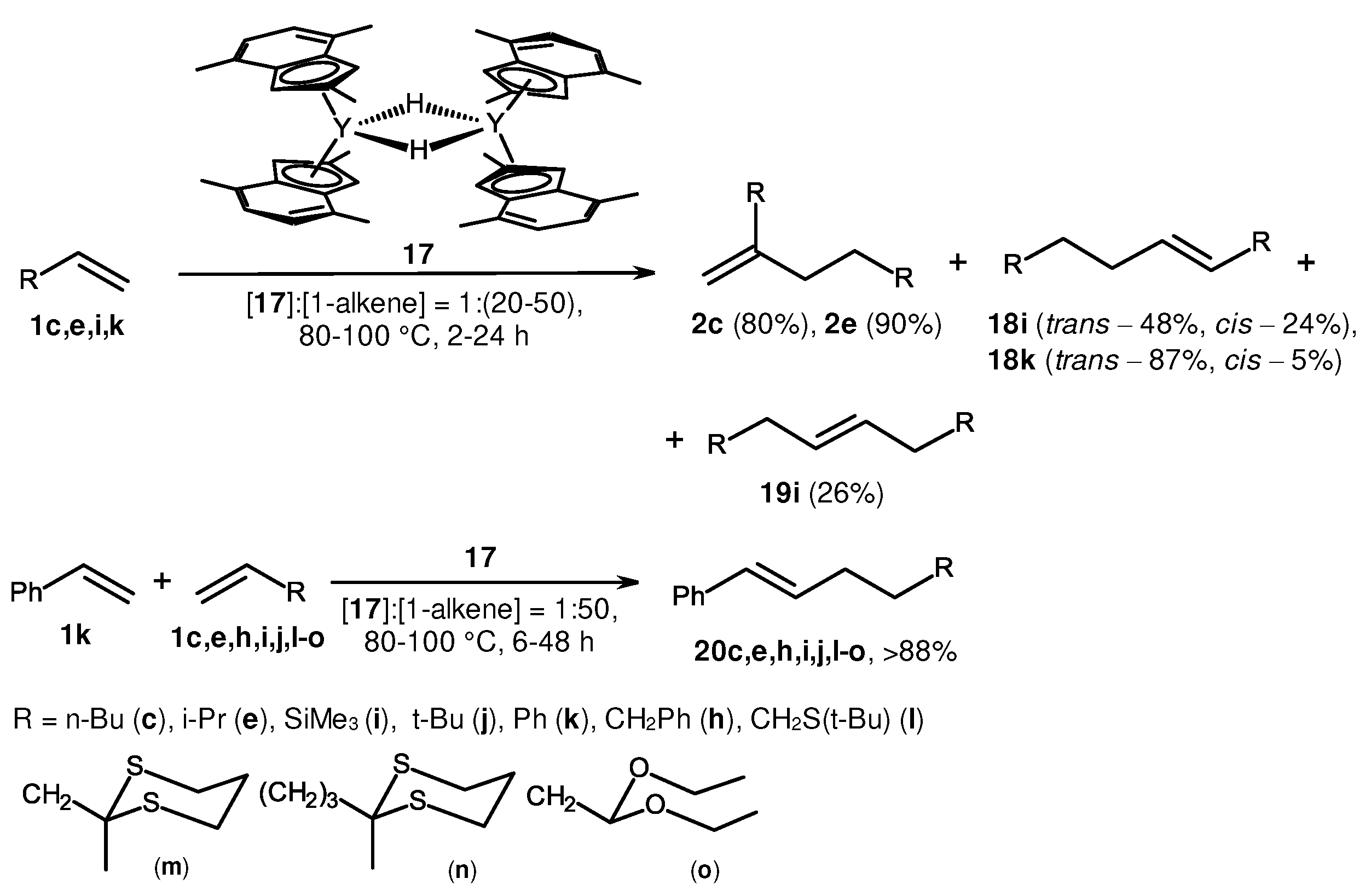
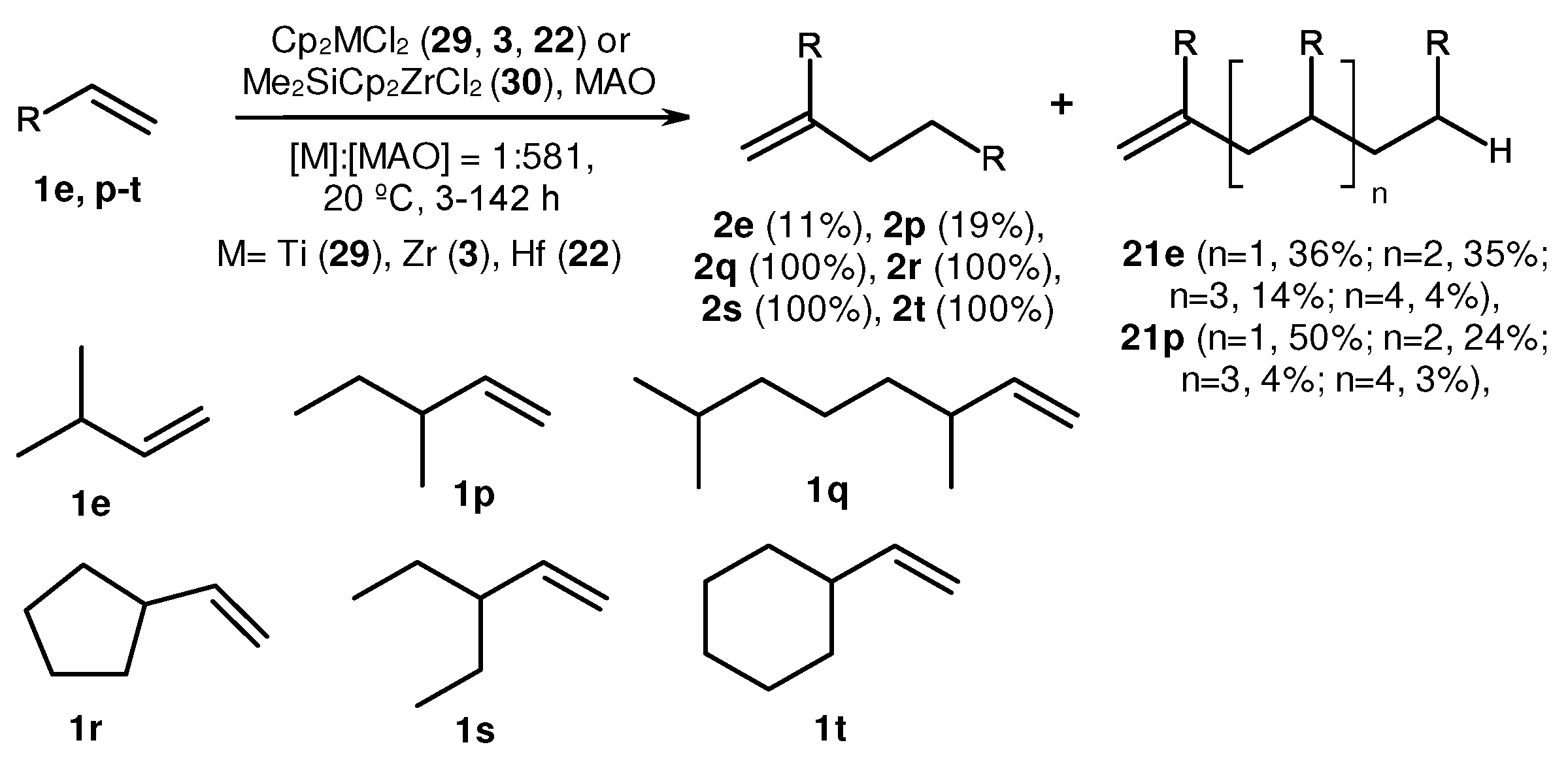
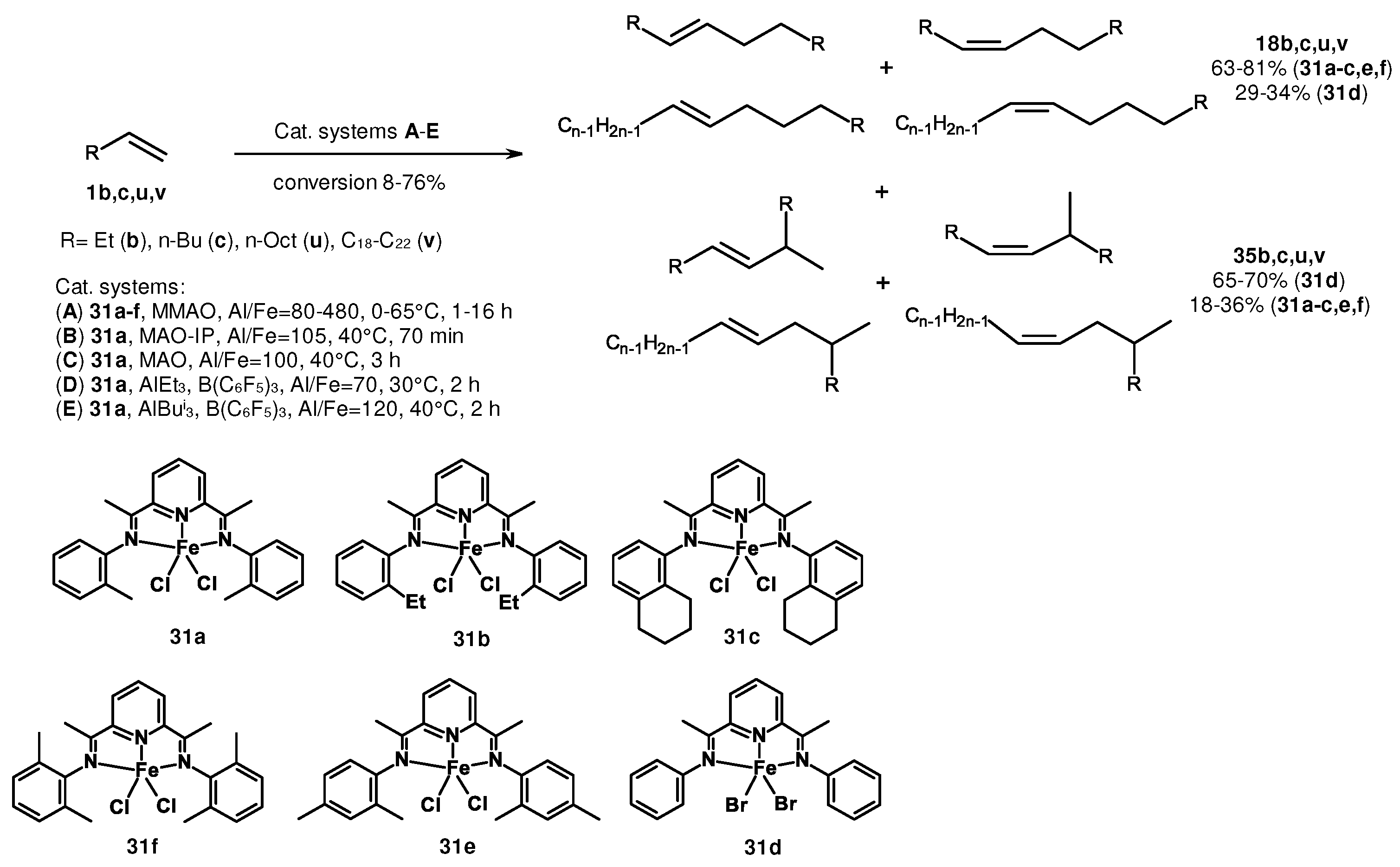
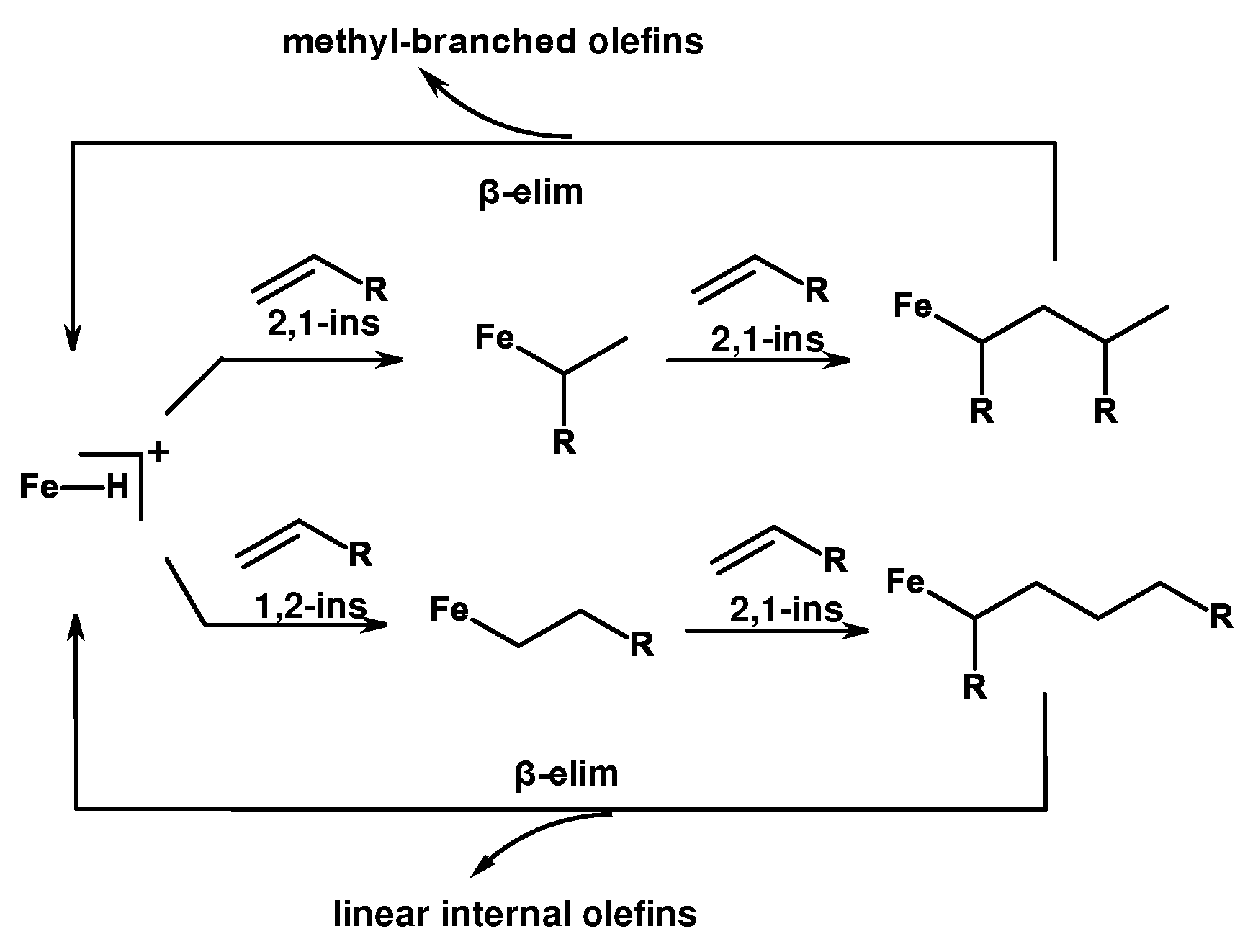
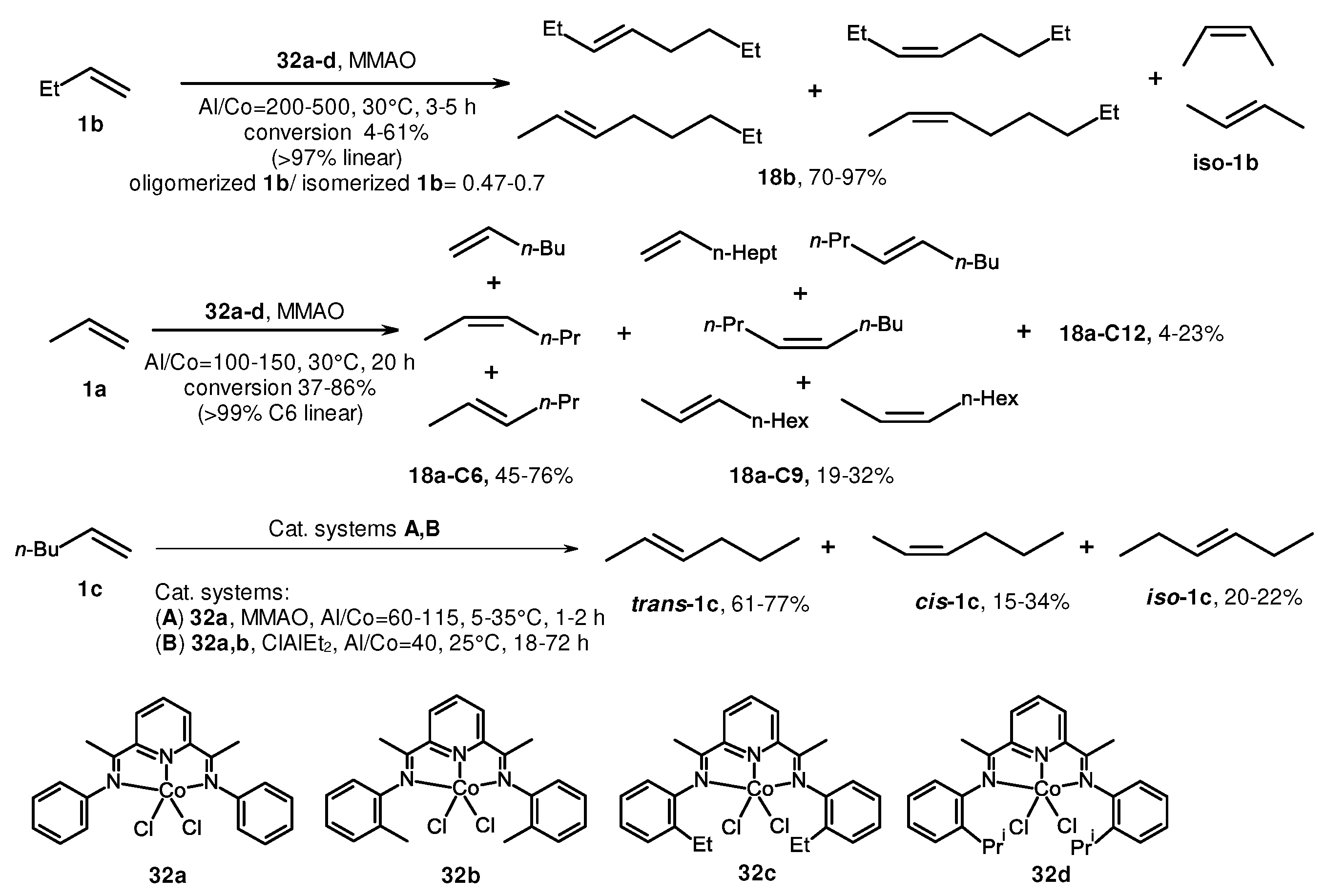
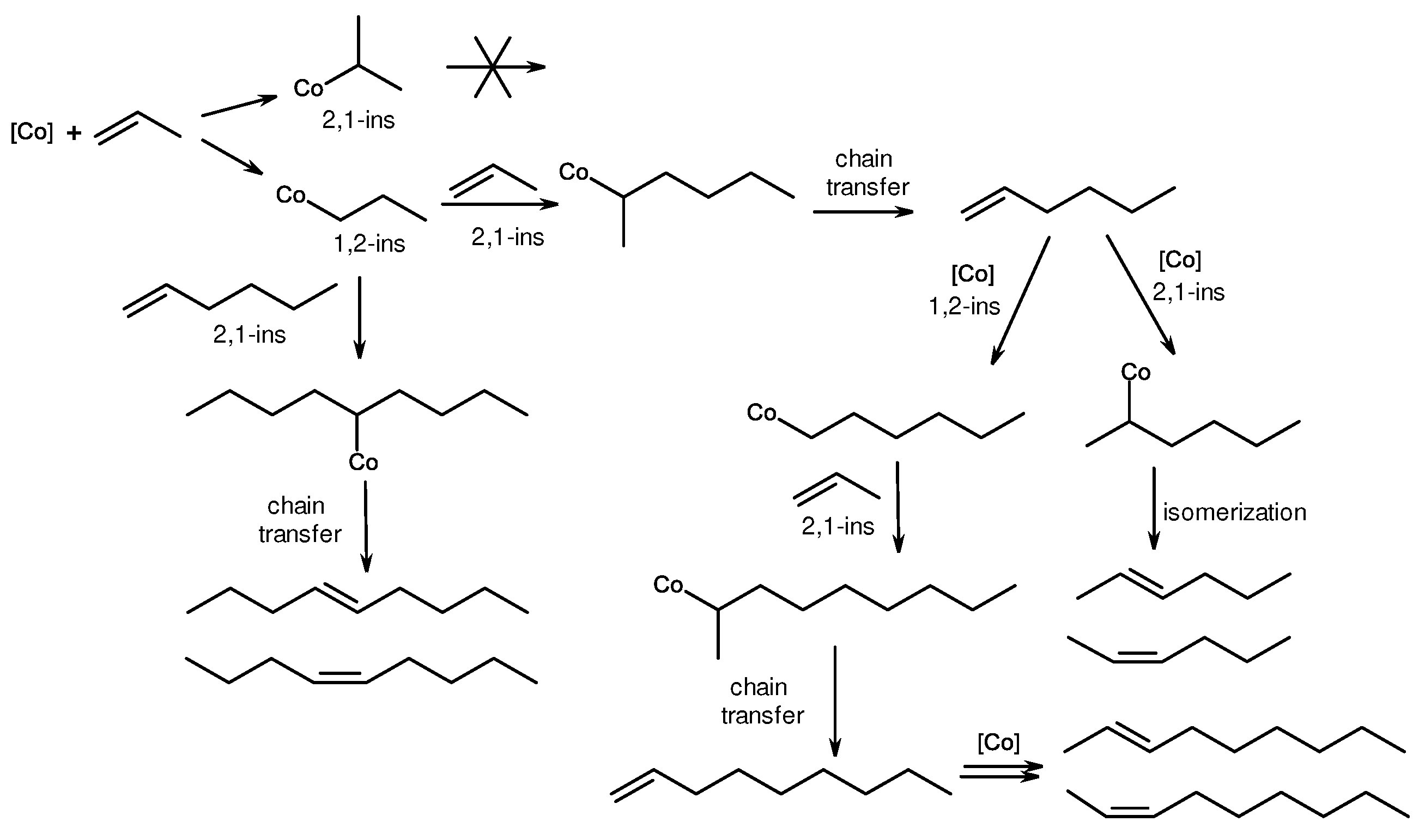
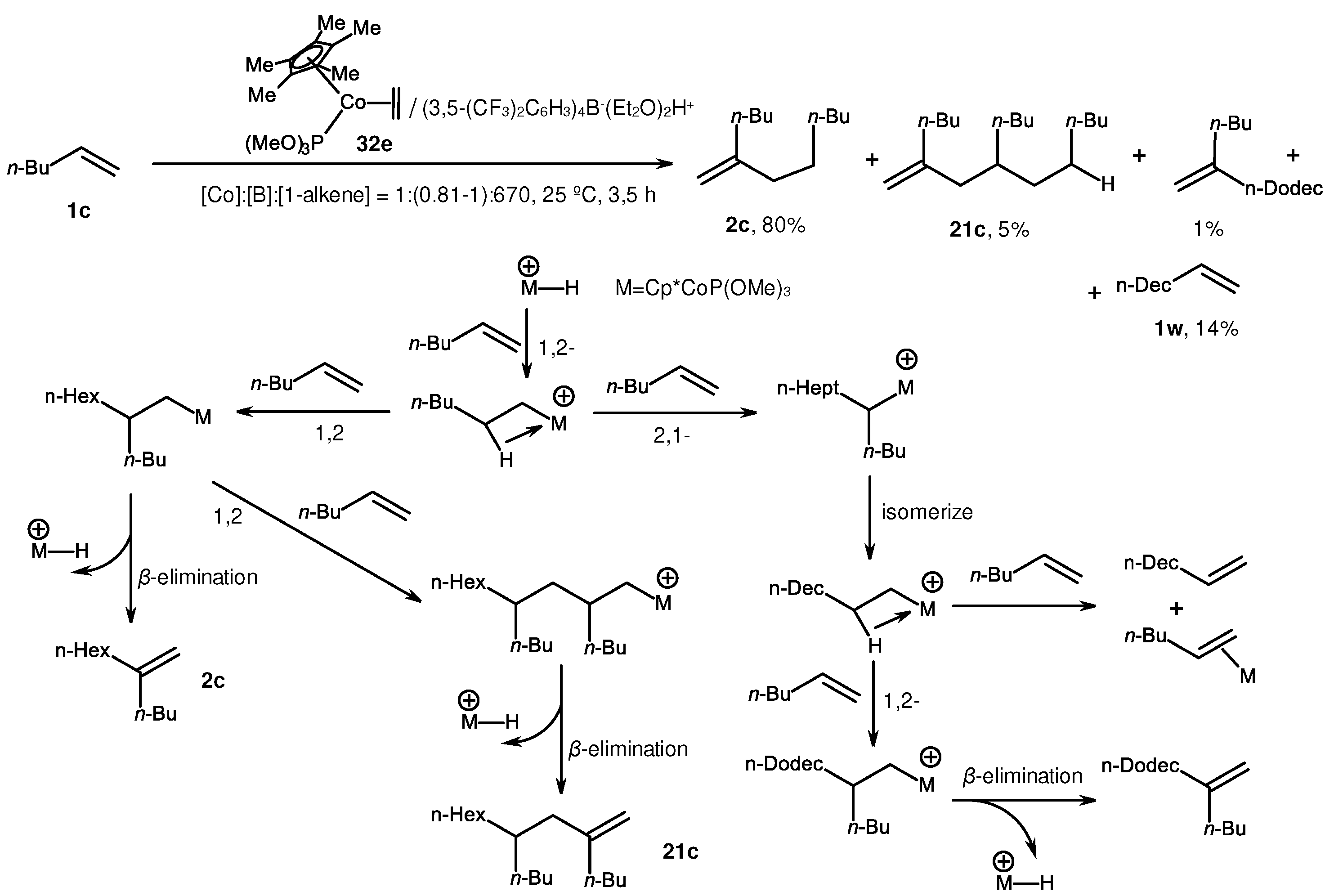
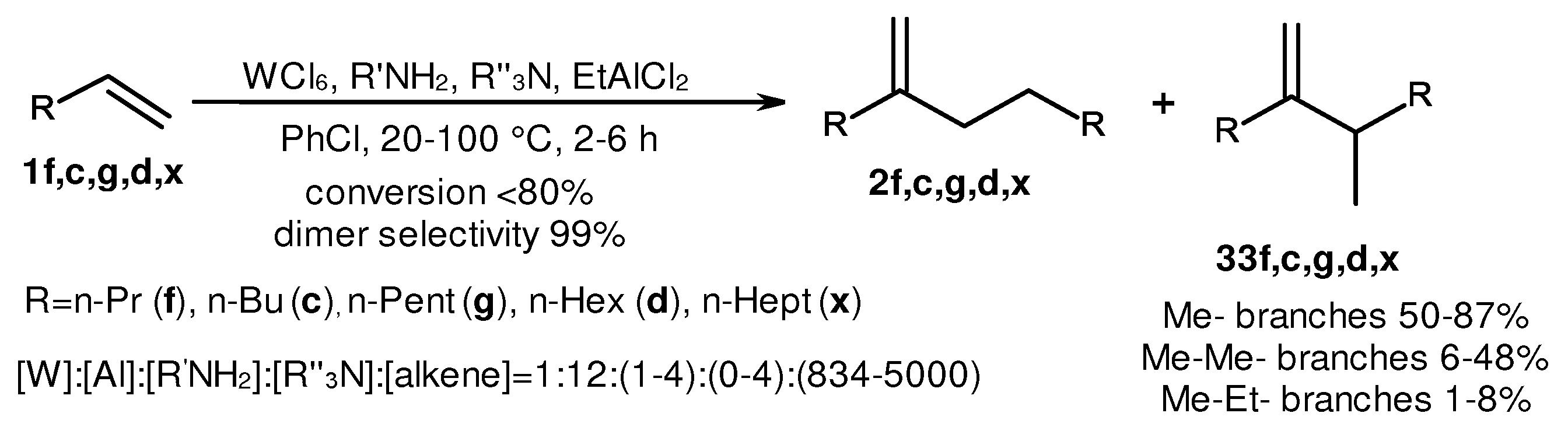
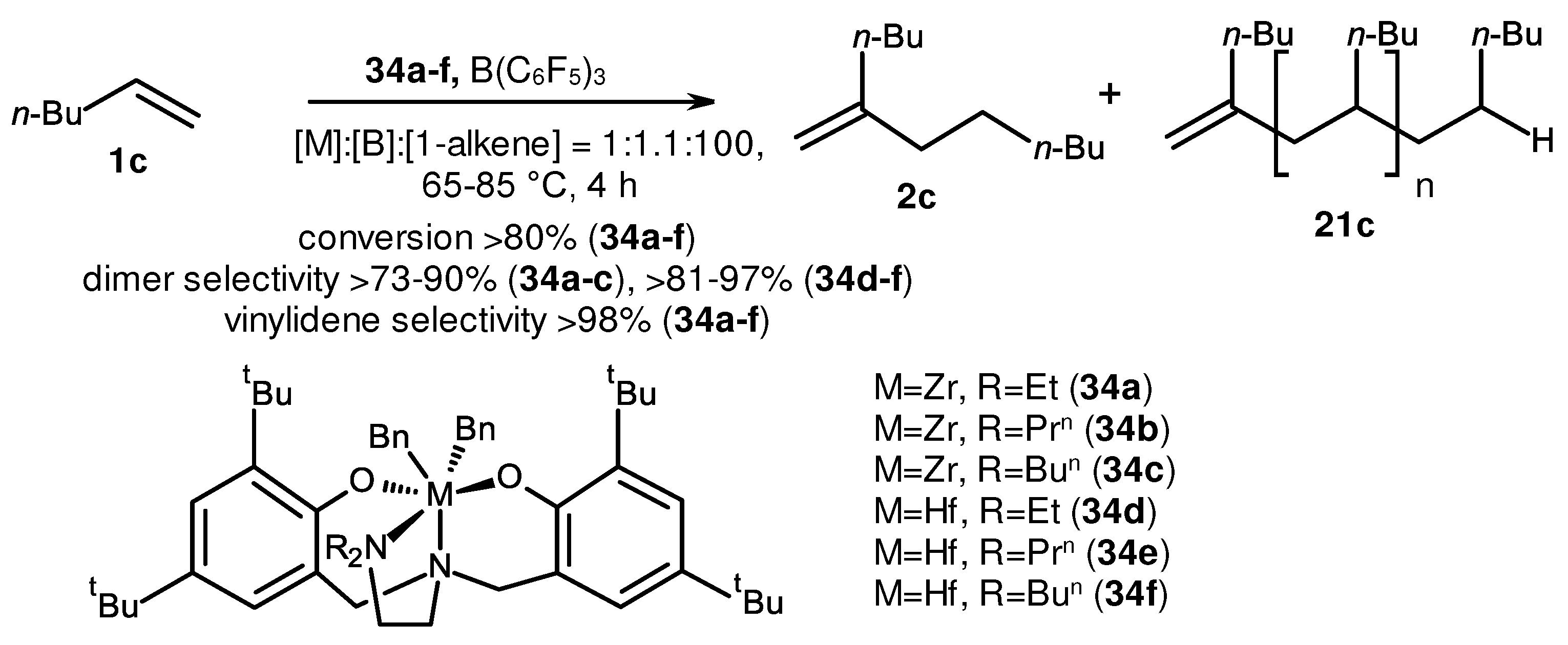
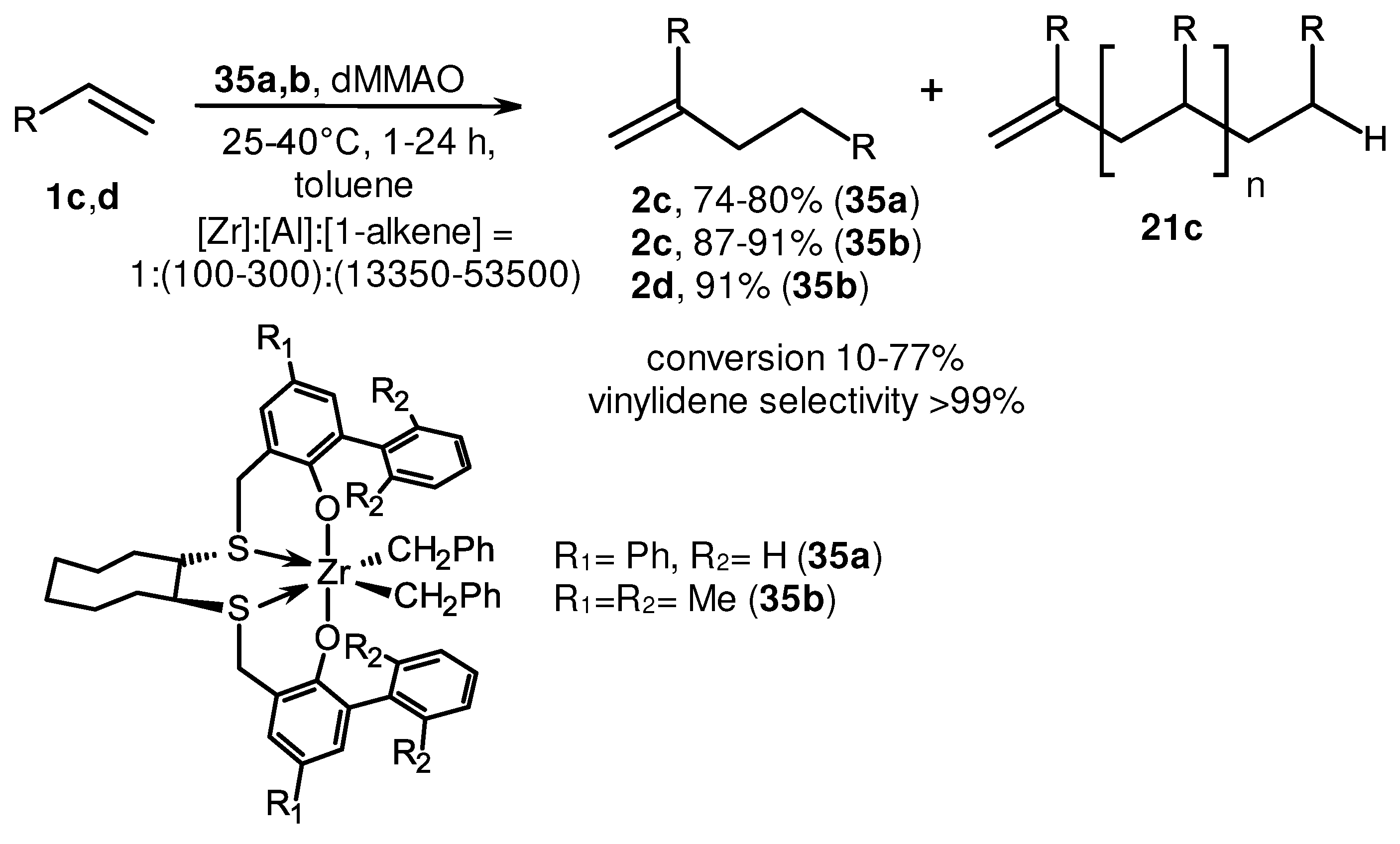
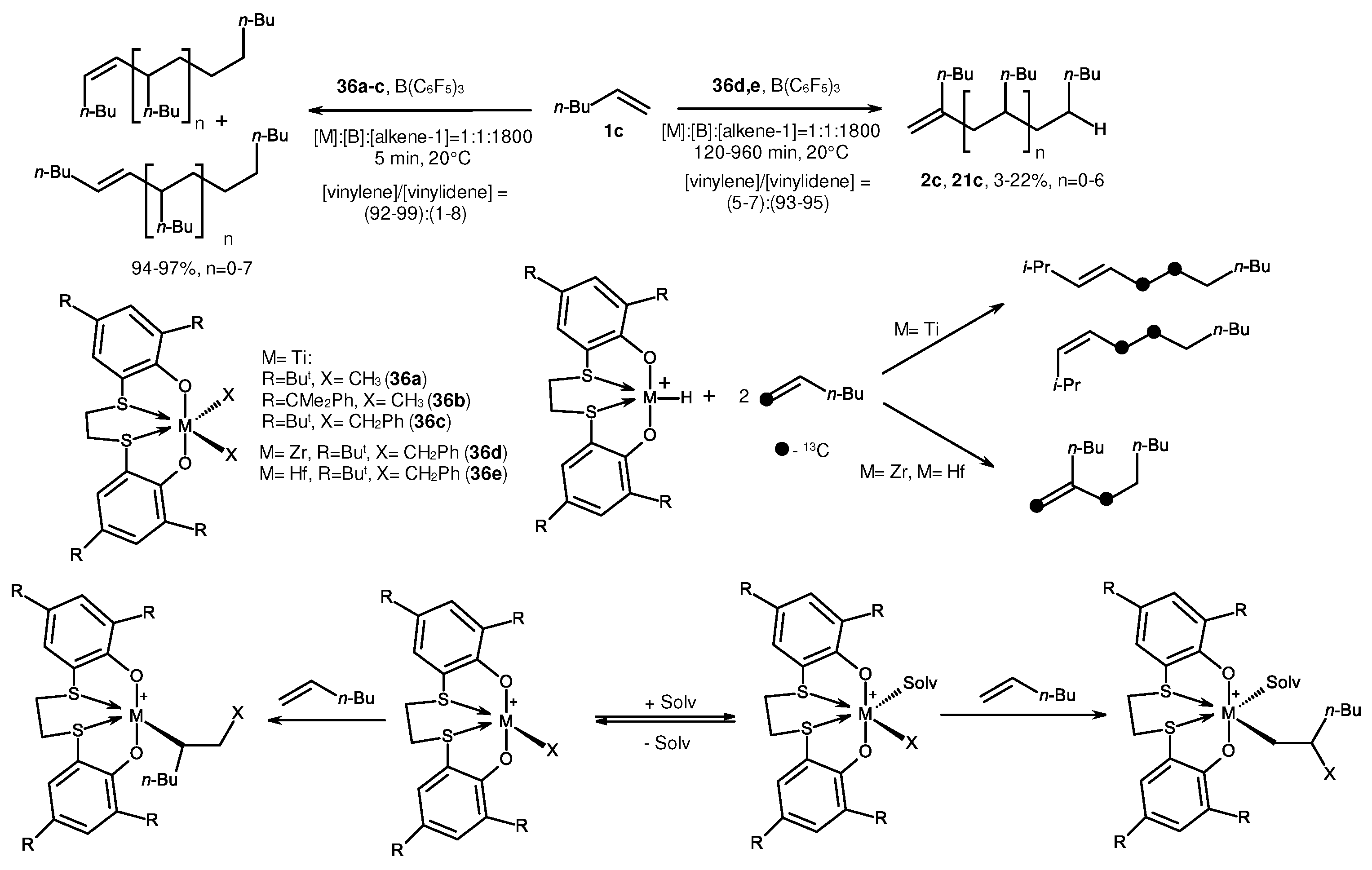
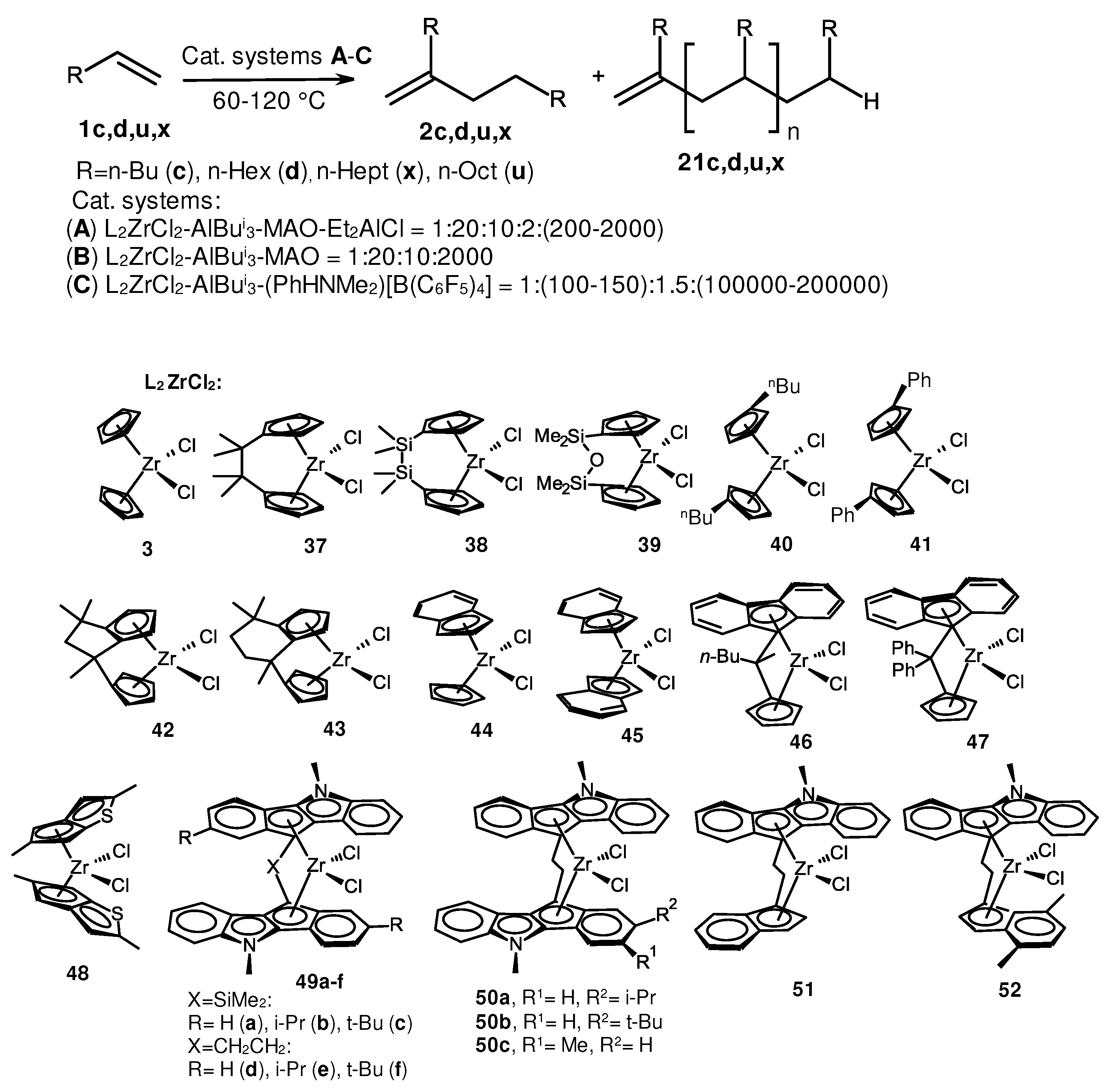
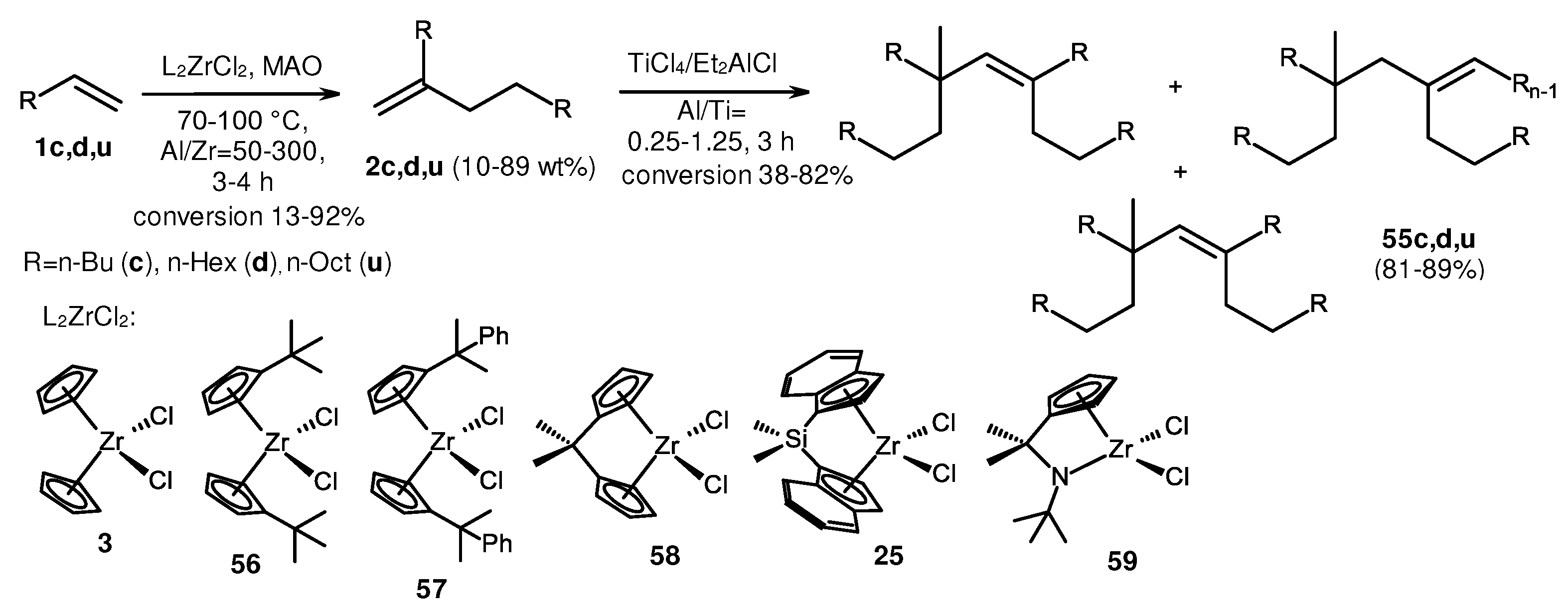
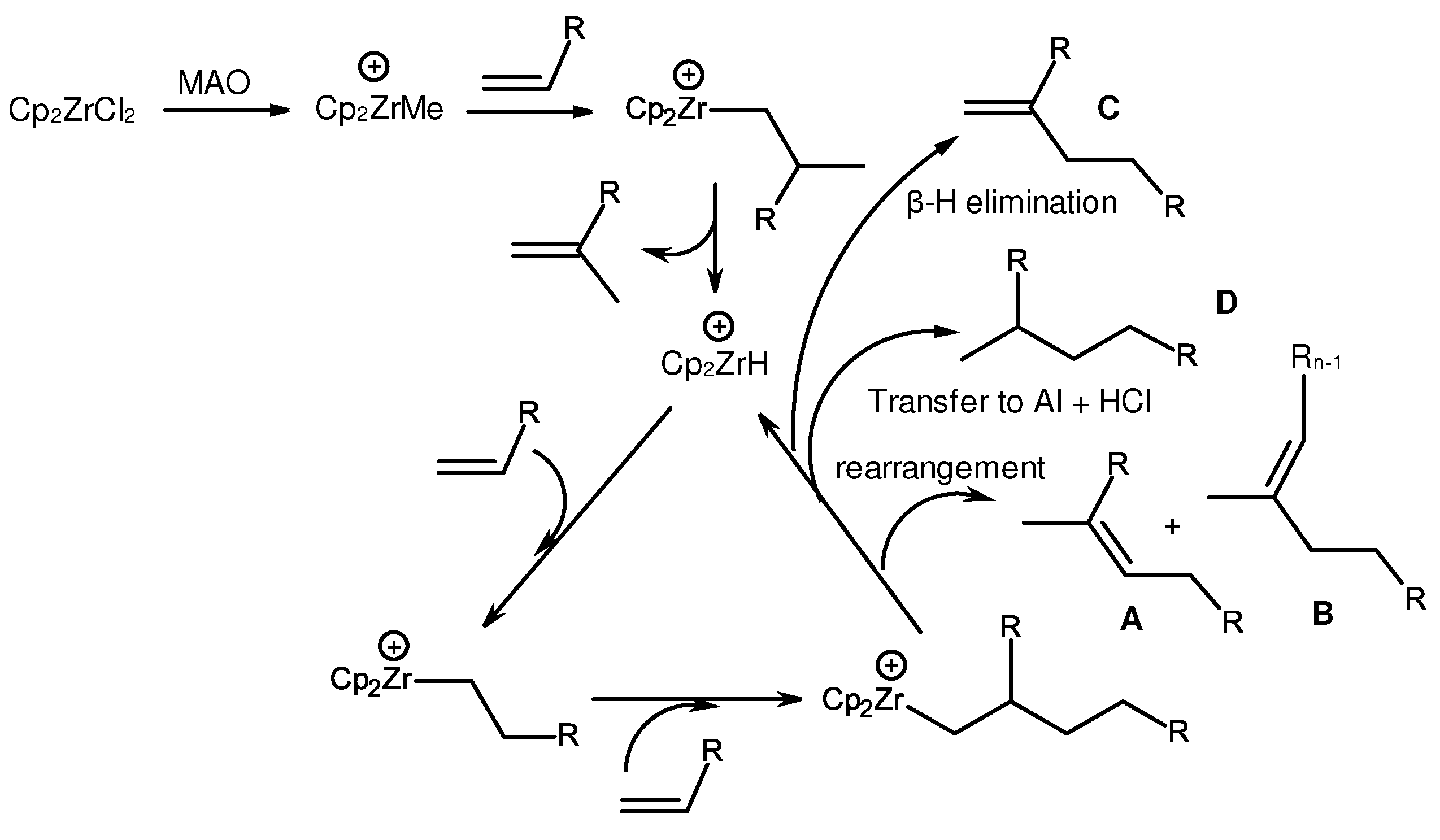
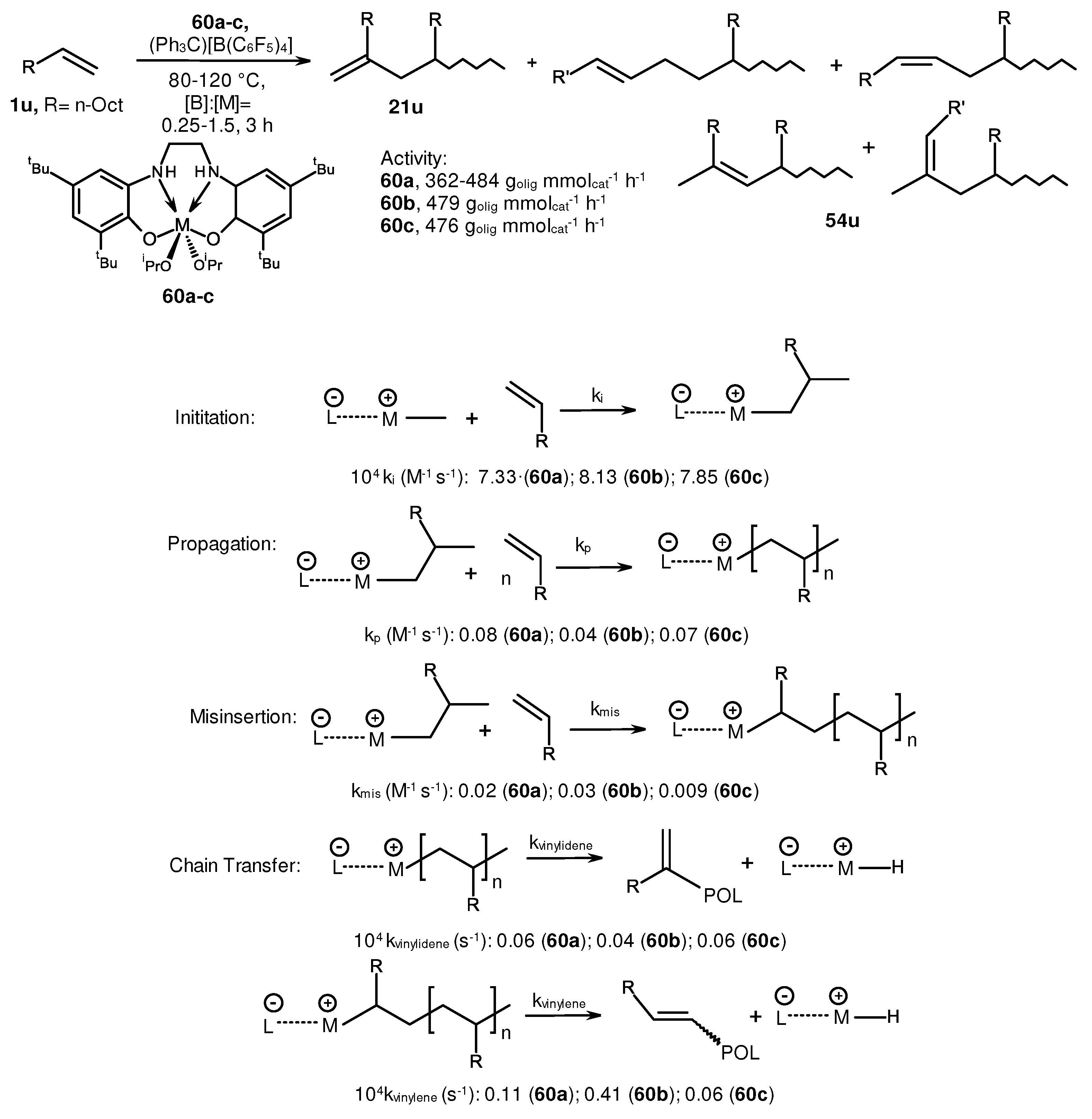
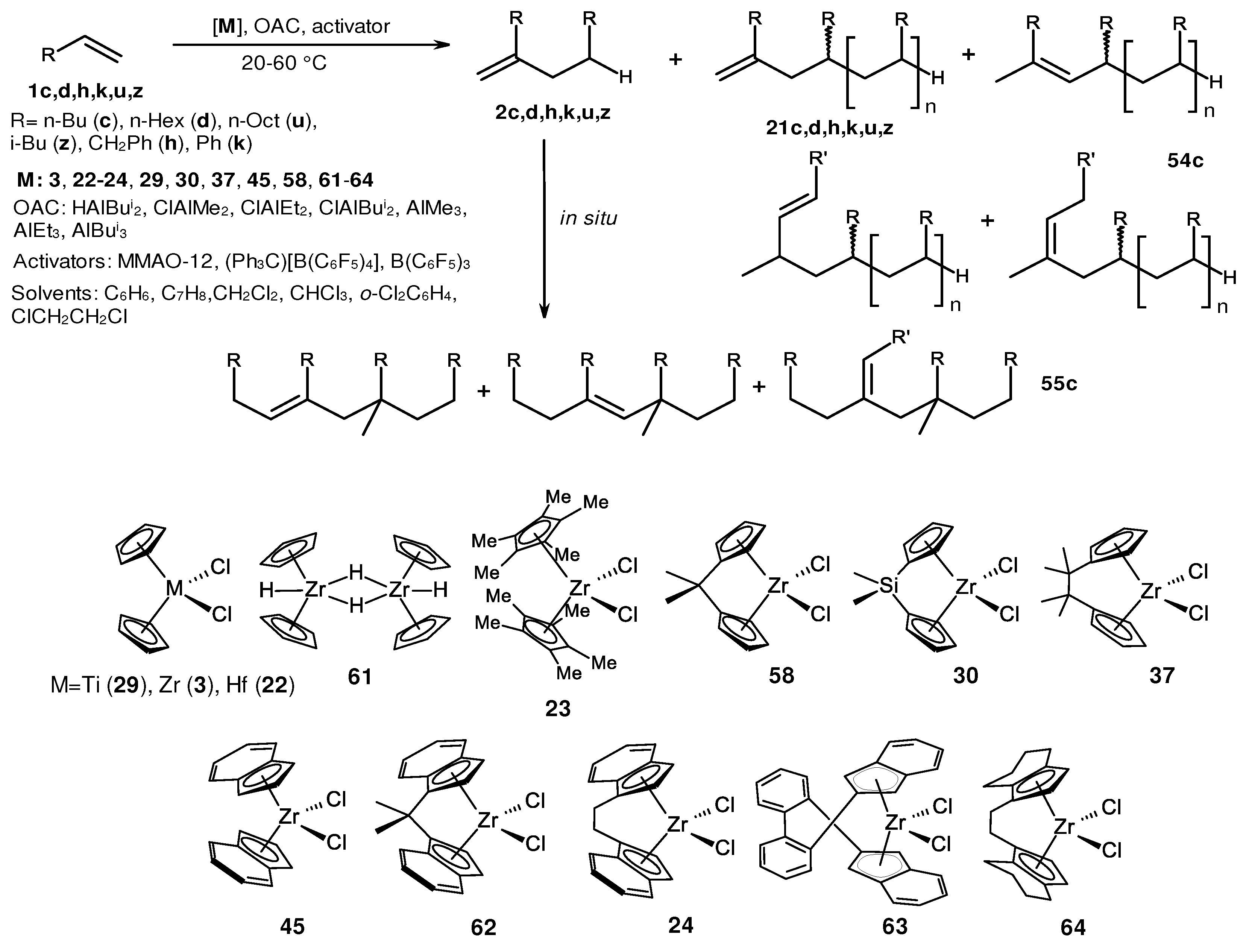
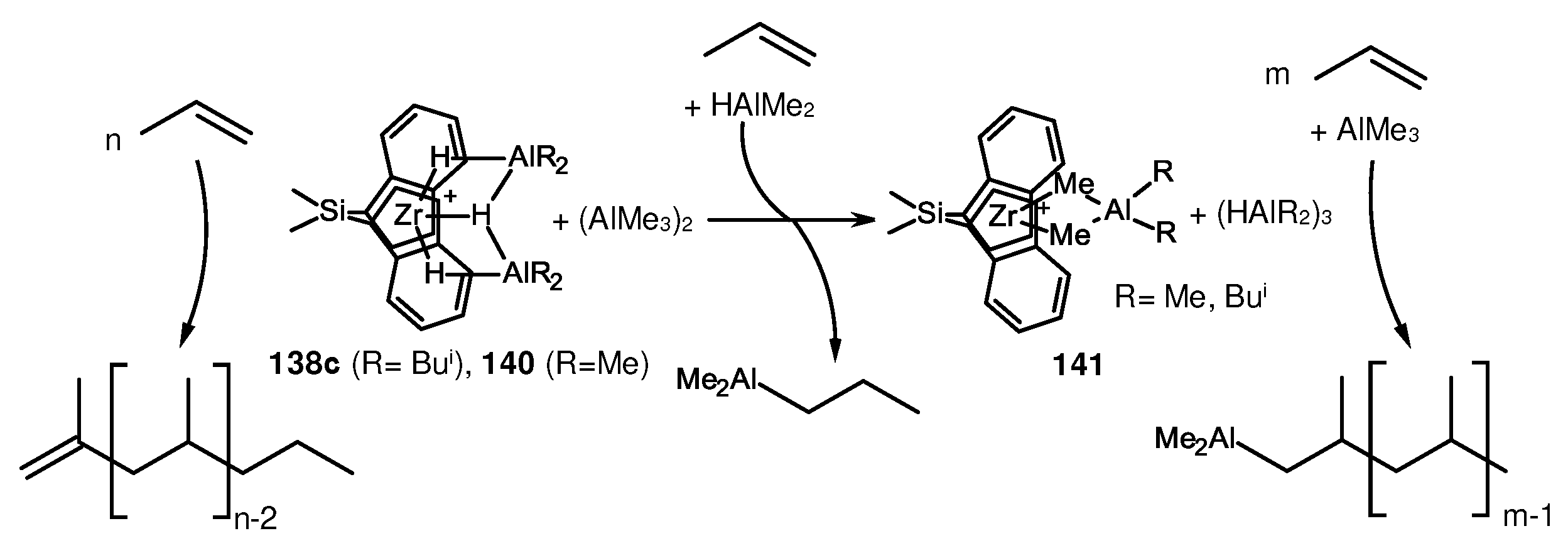
2. Catalytic synthesis of terminal alkene dimers and oligomers
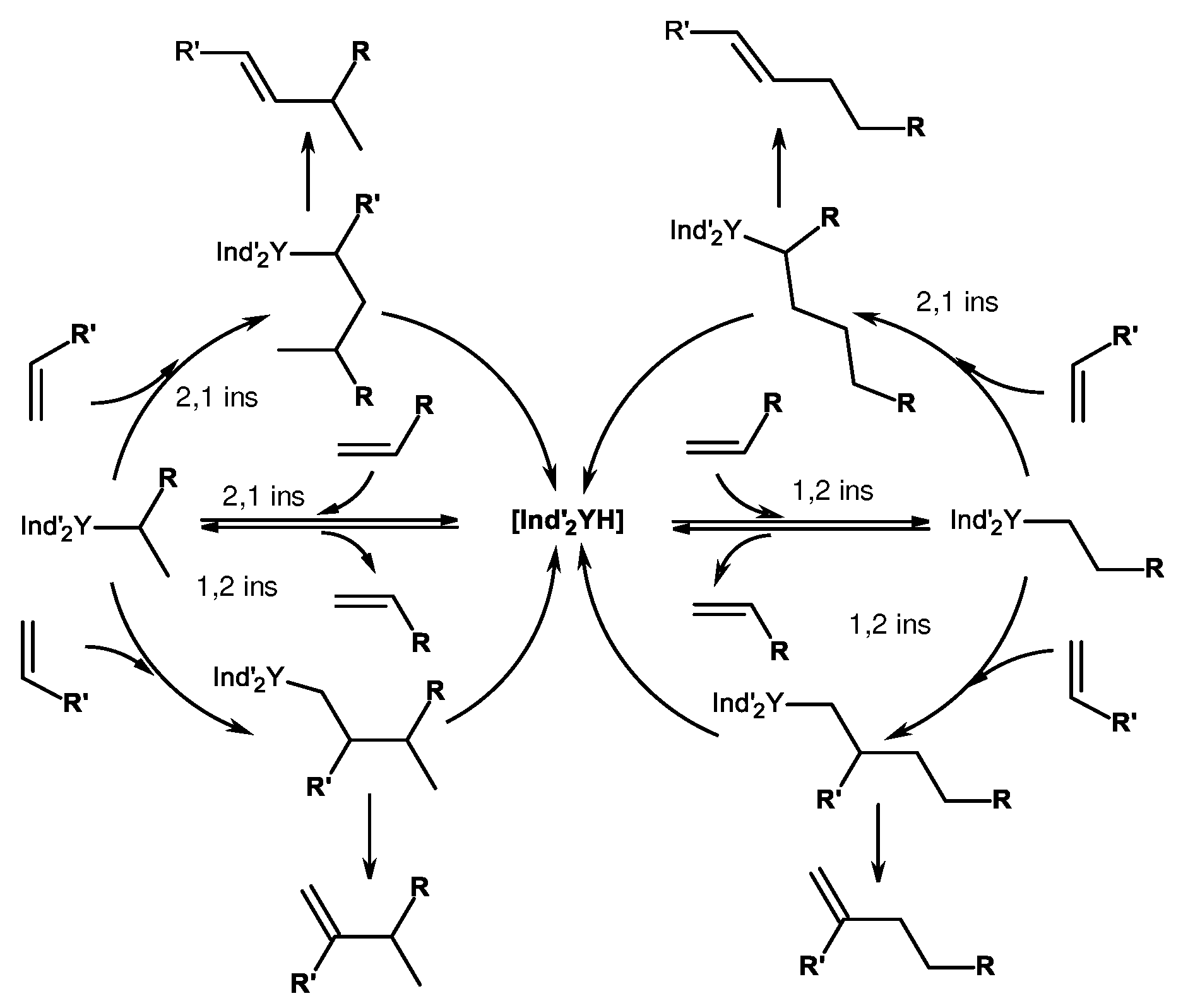
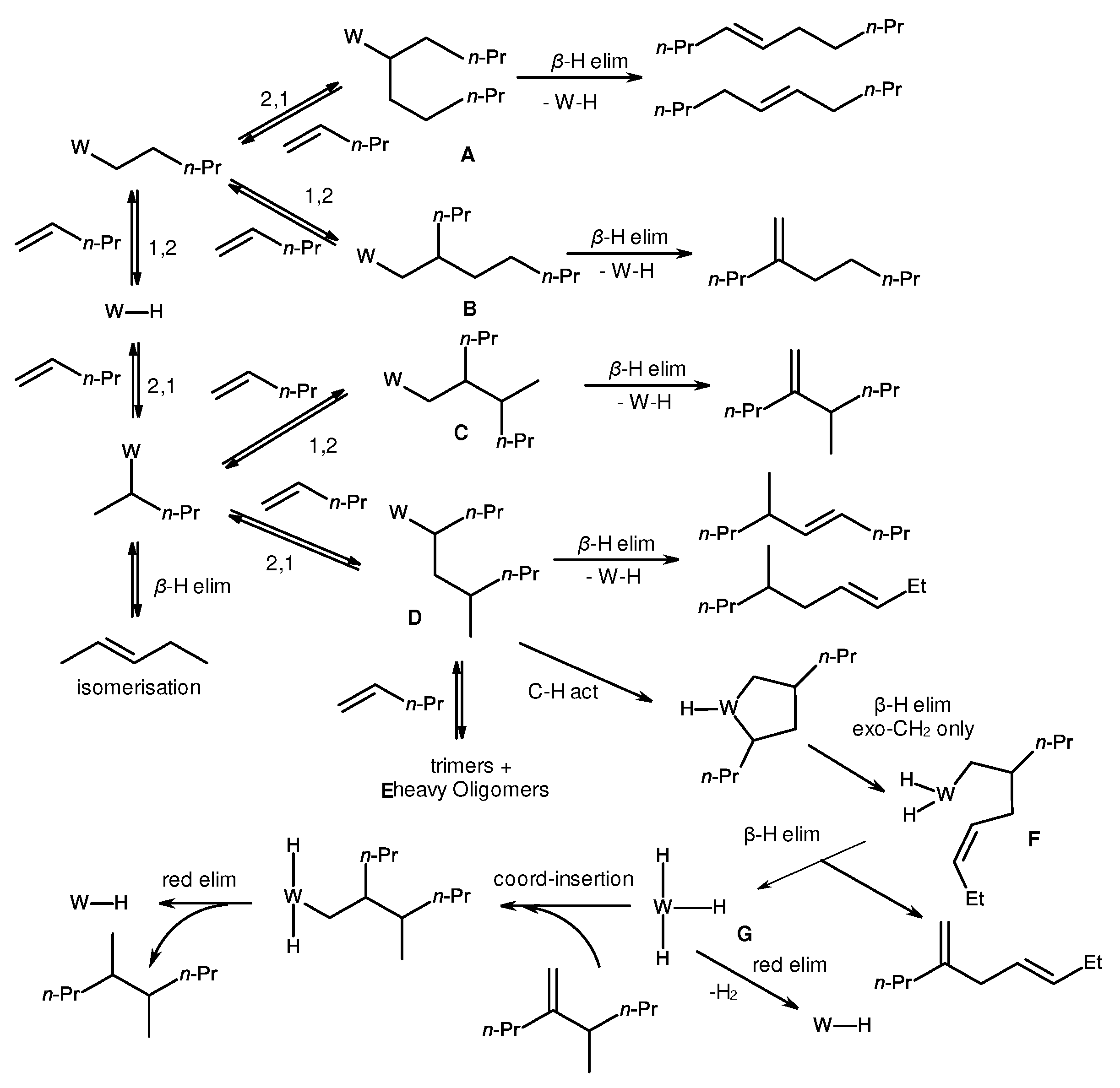
3. Structure of catalytically active centers
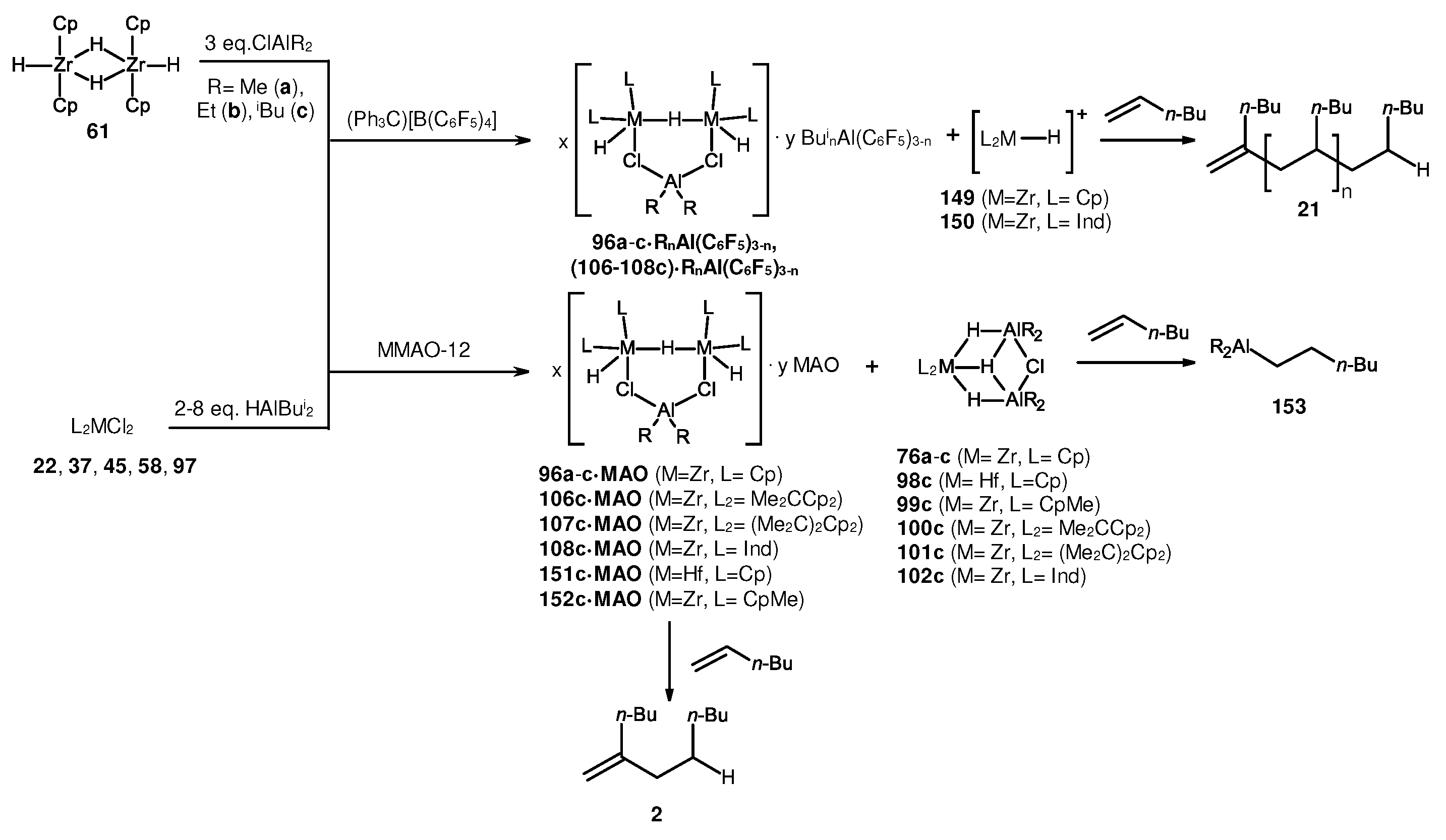
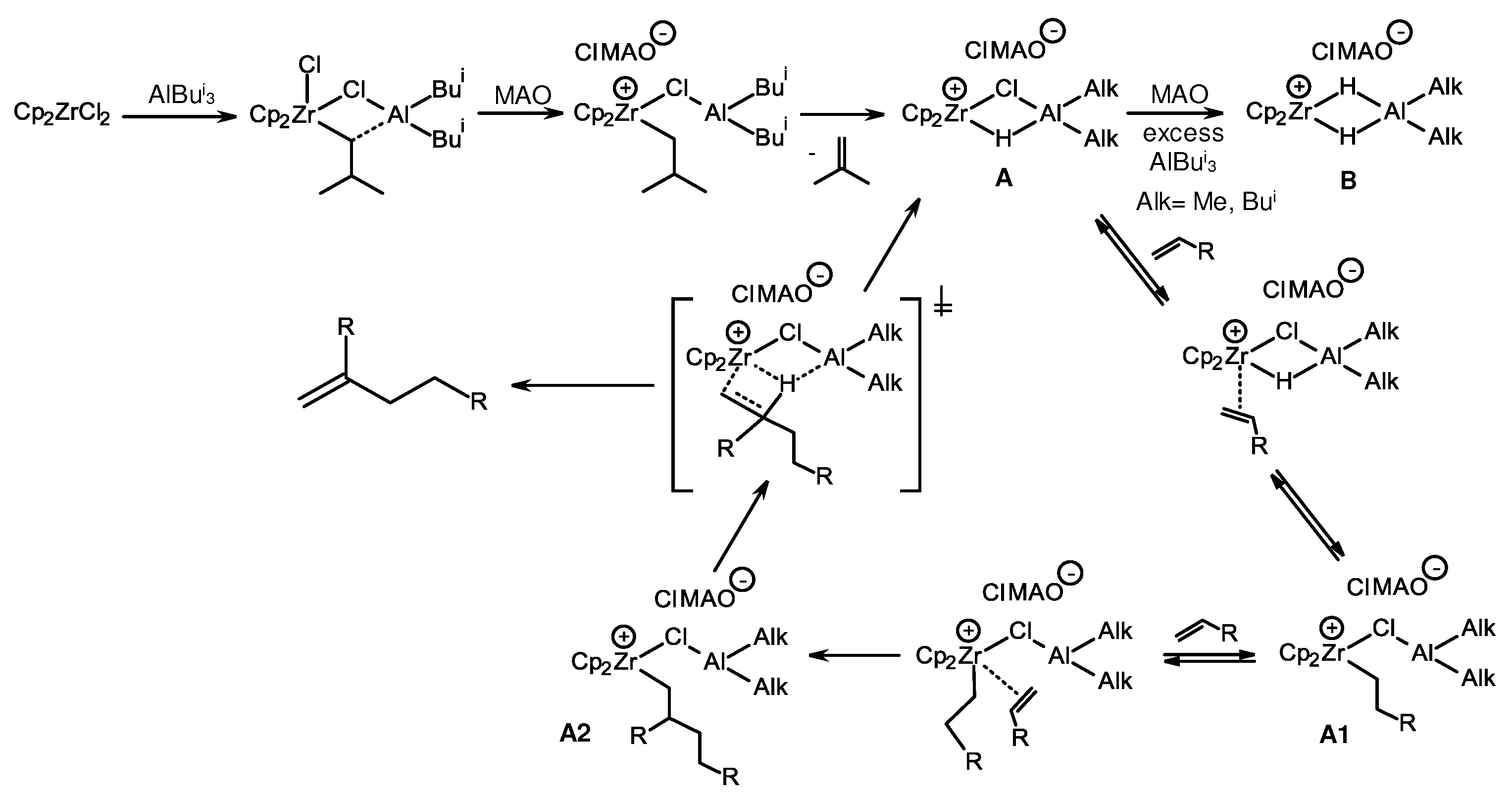
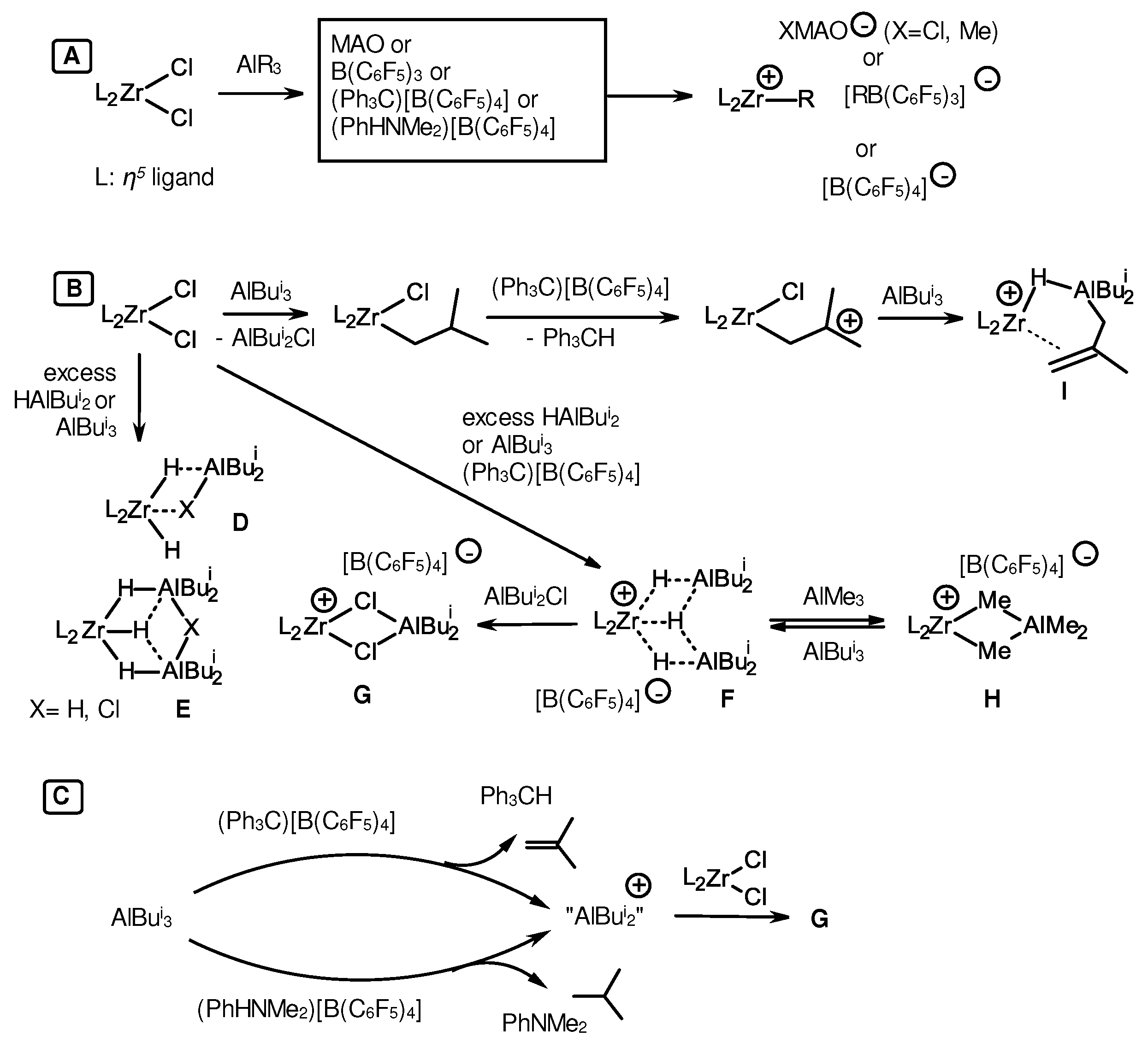
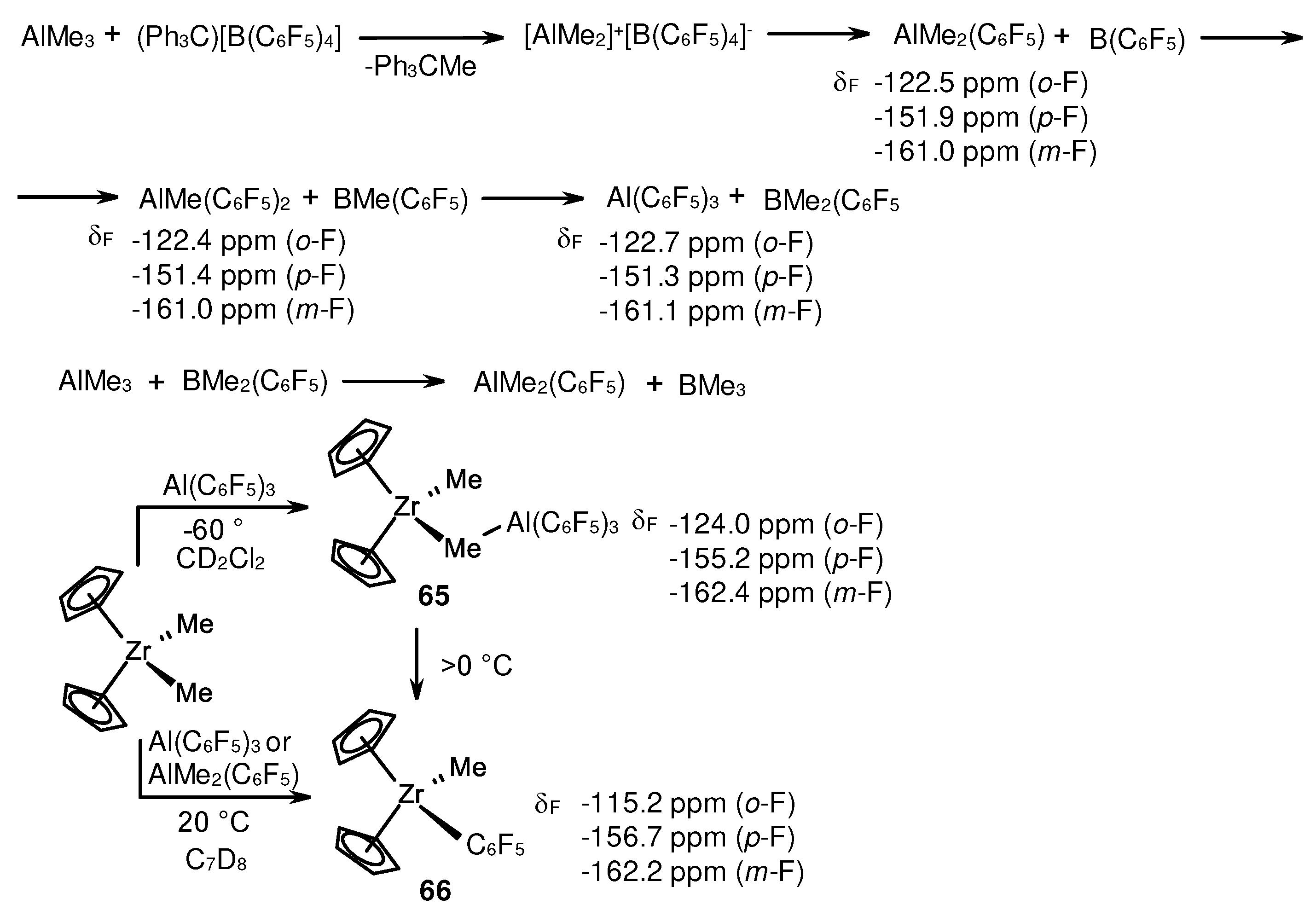
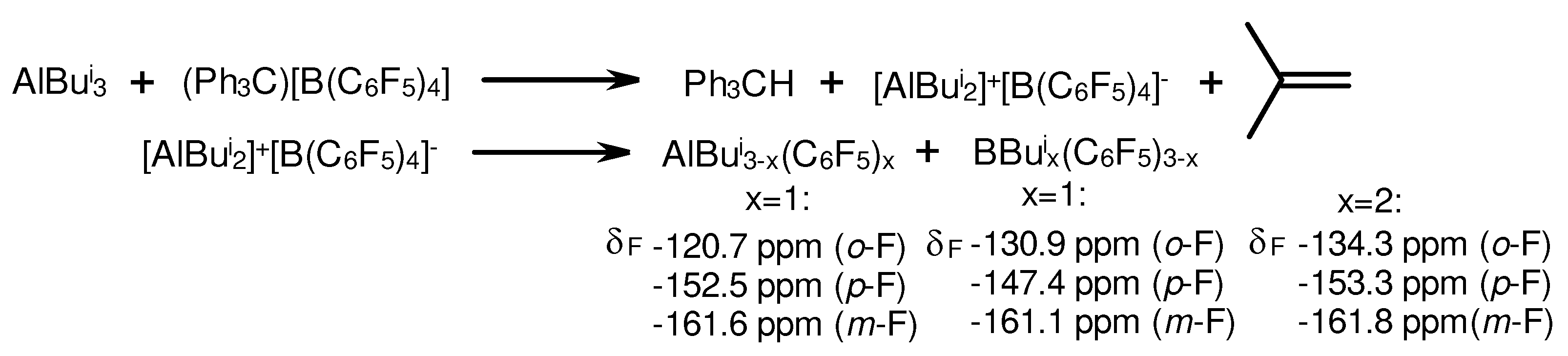
3.1. Reactions of organoaluminum compounds with activators and metal complexes
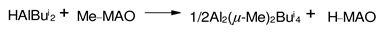
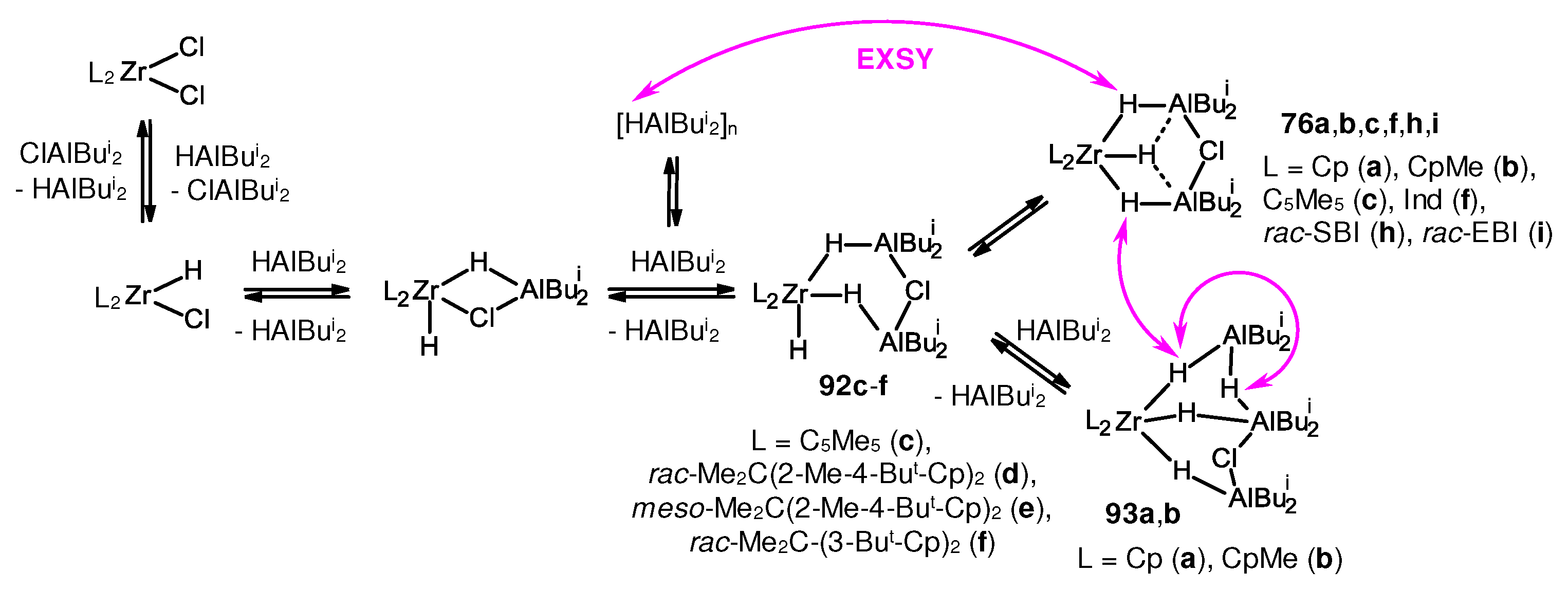
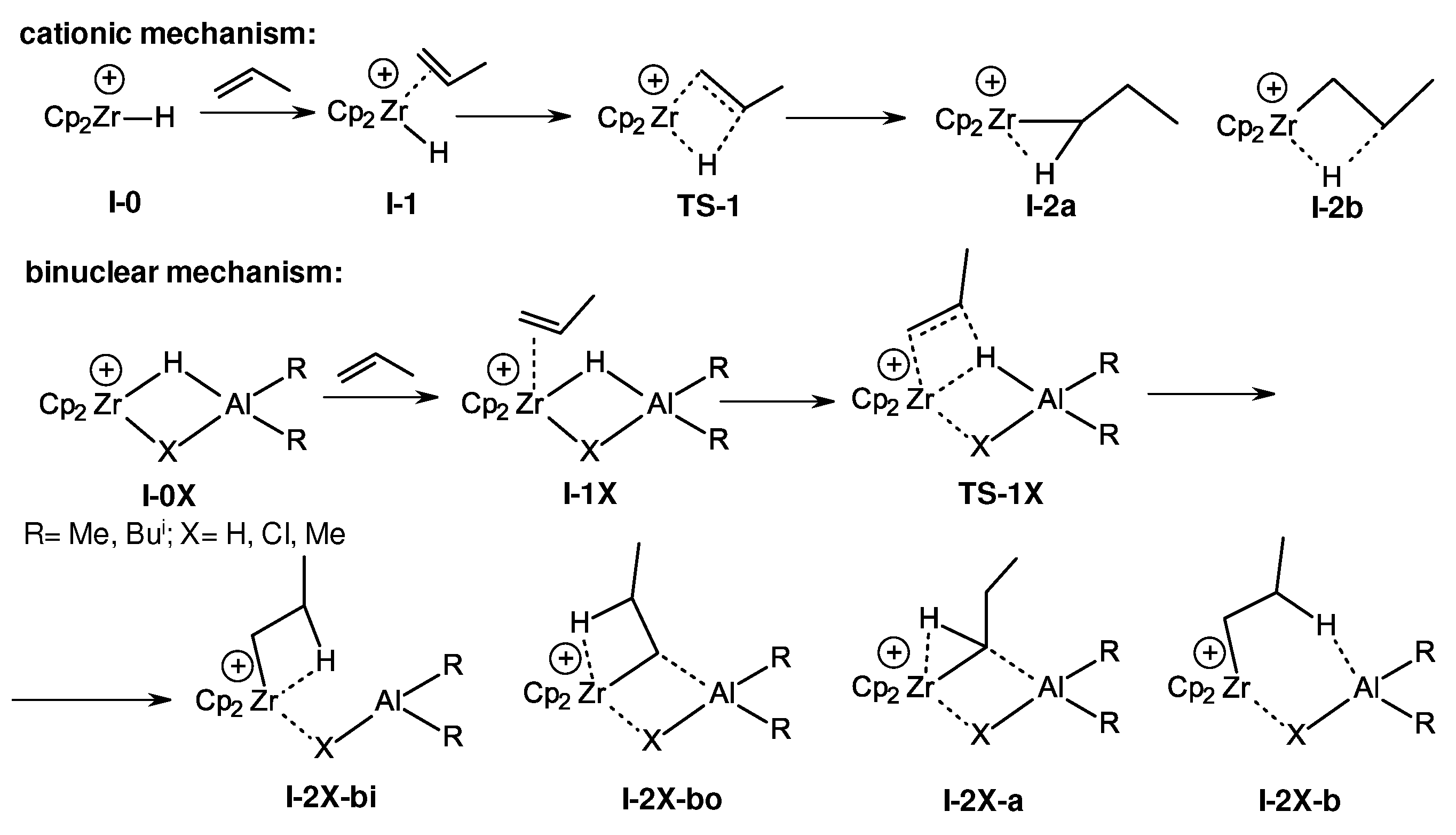
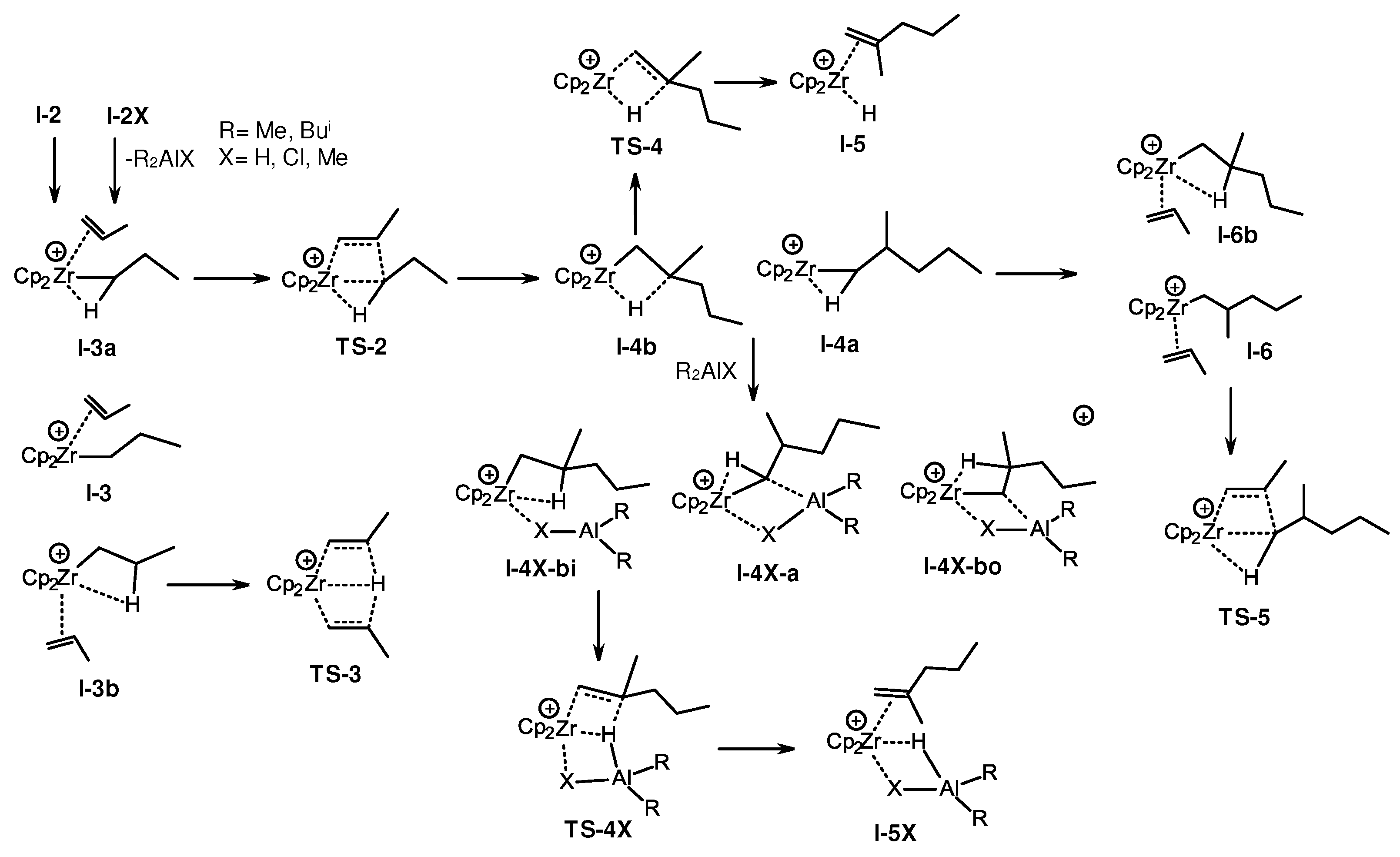
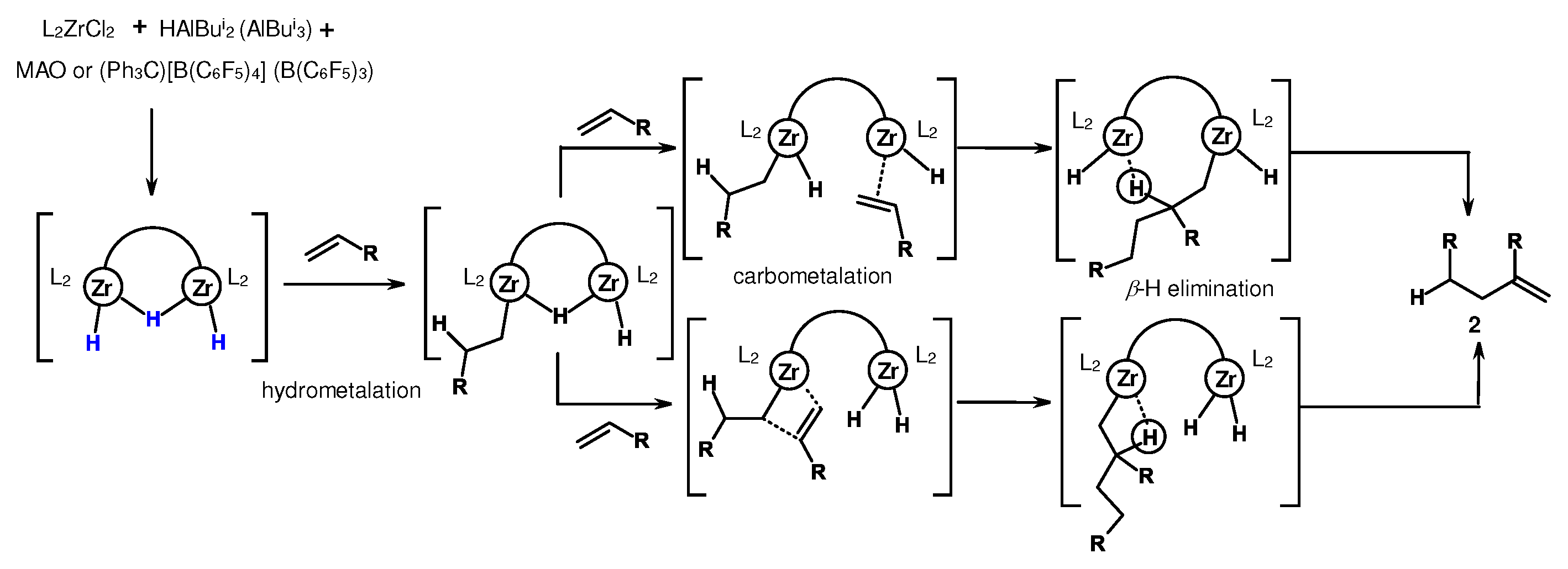
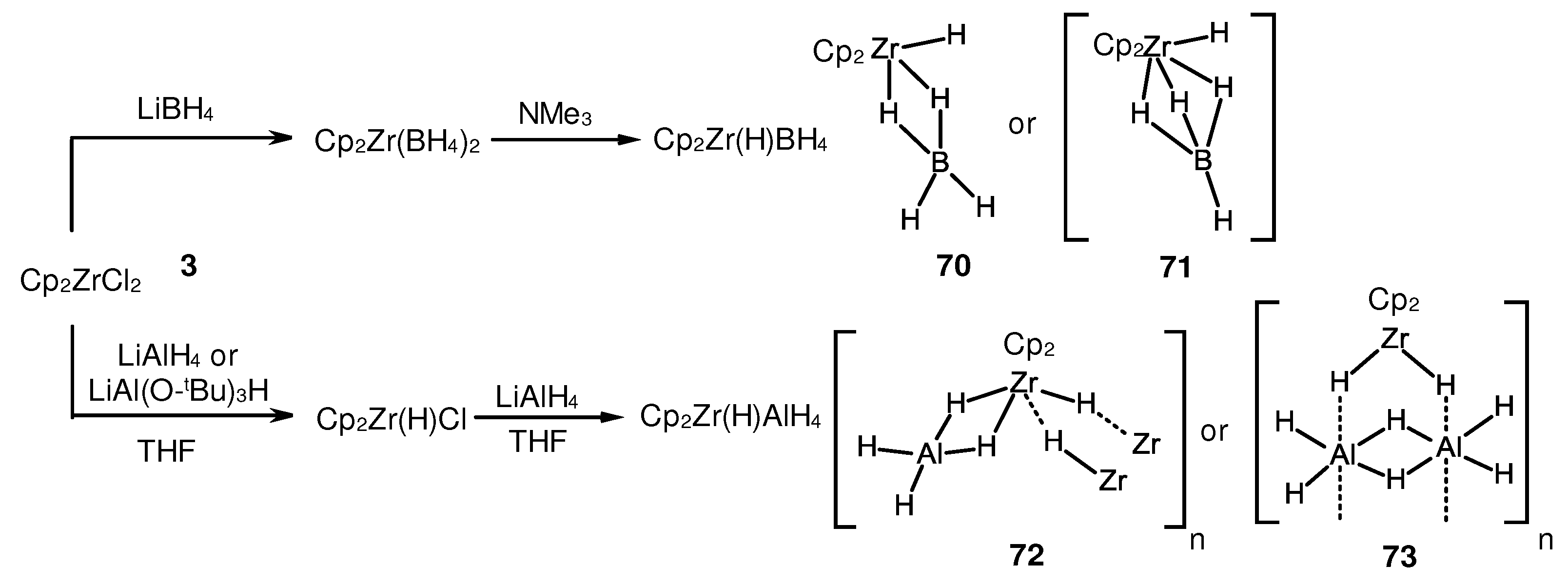
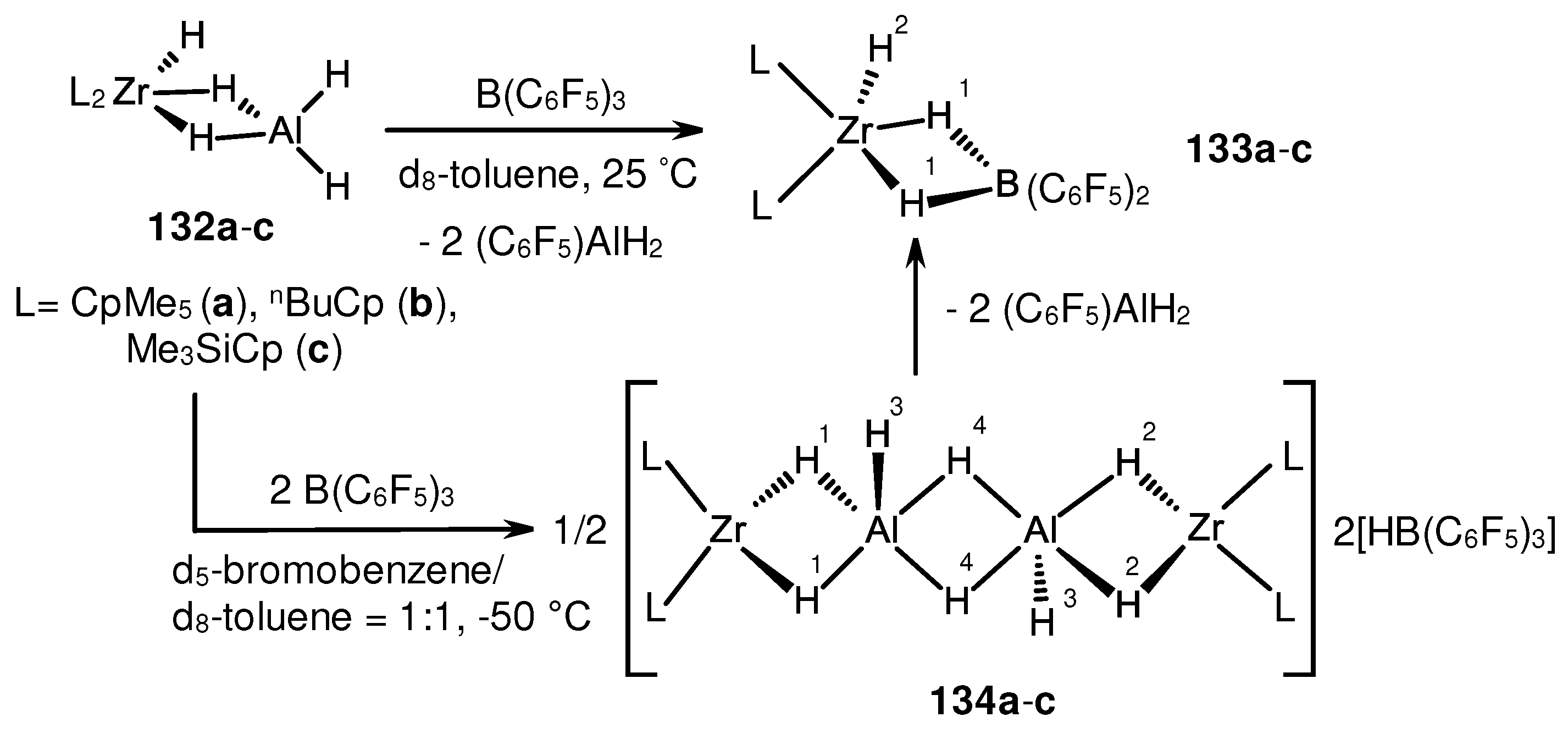
3.2. Structure and reactivity of Zr,Al- and Zr,B-hydride complexes
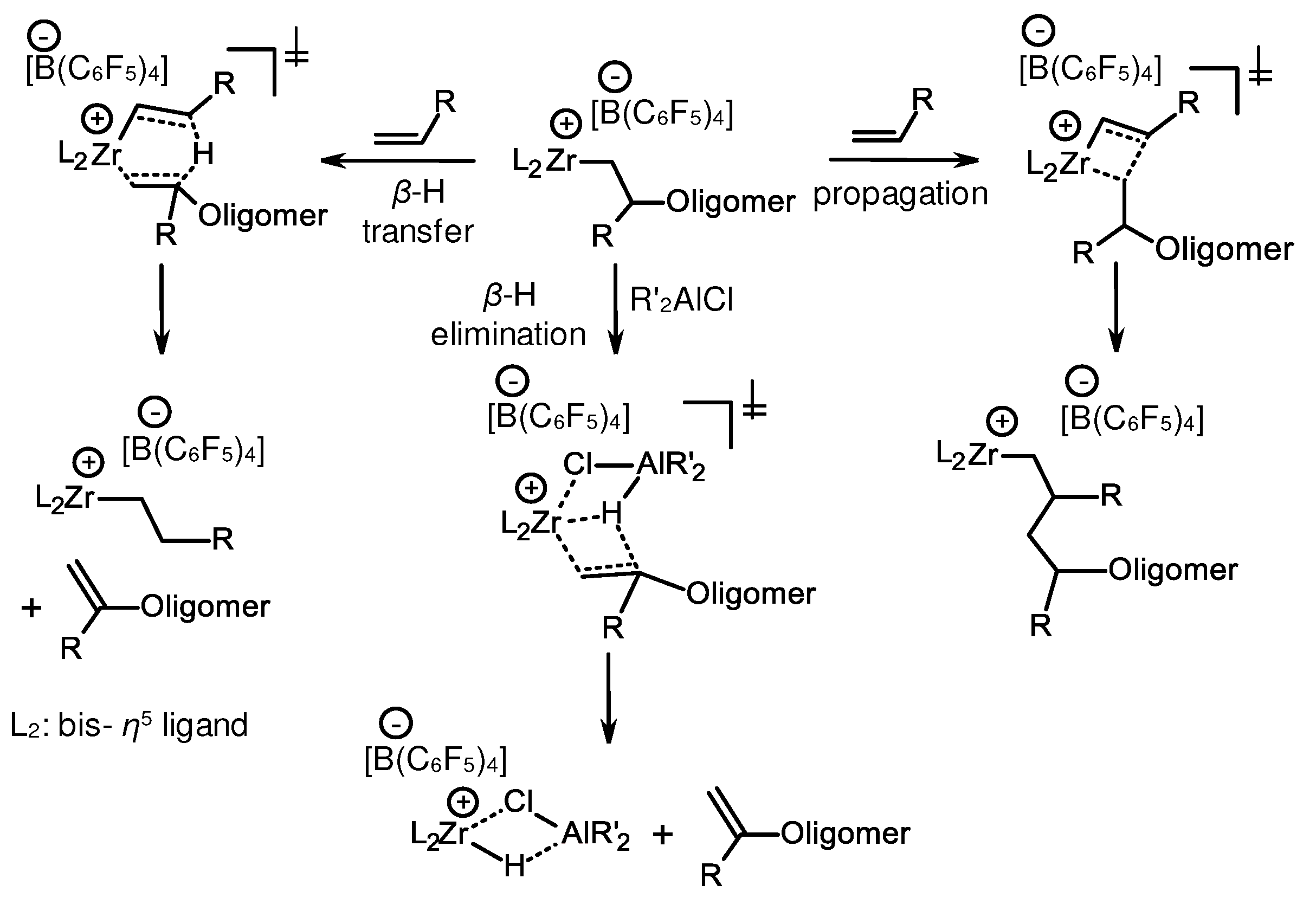
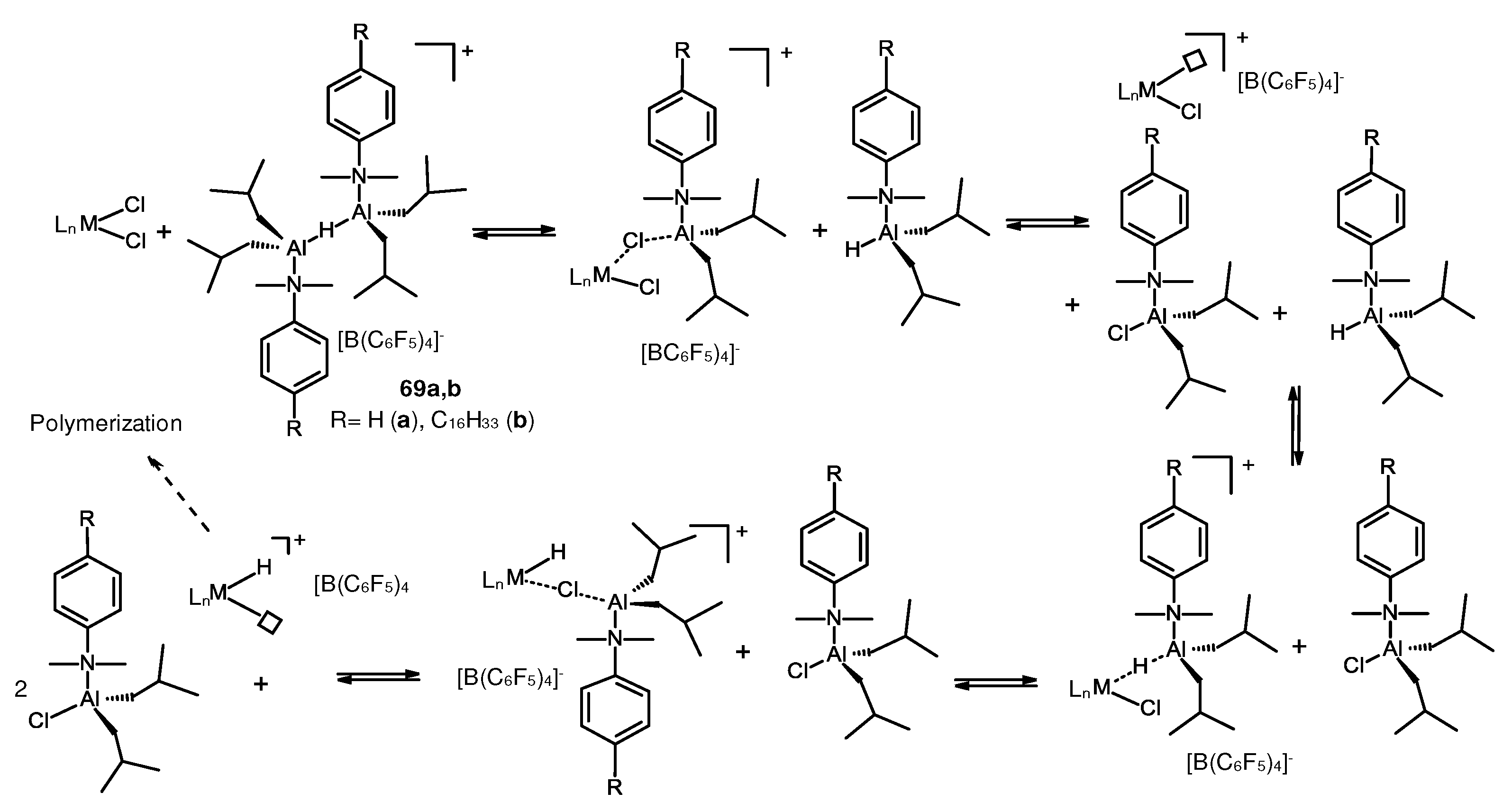
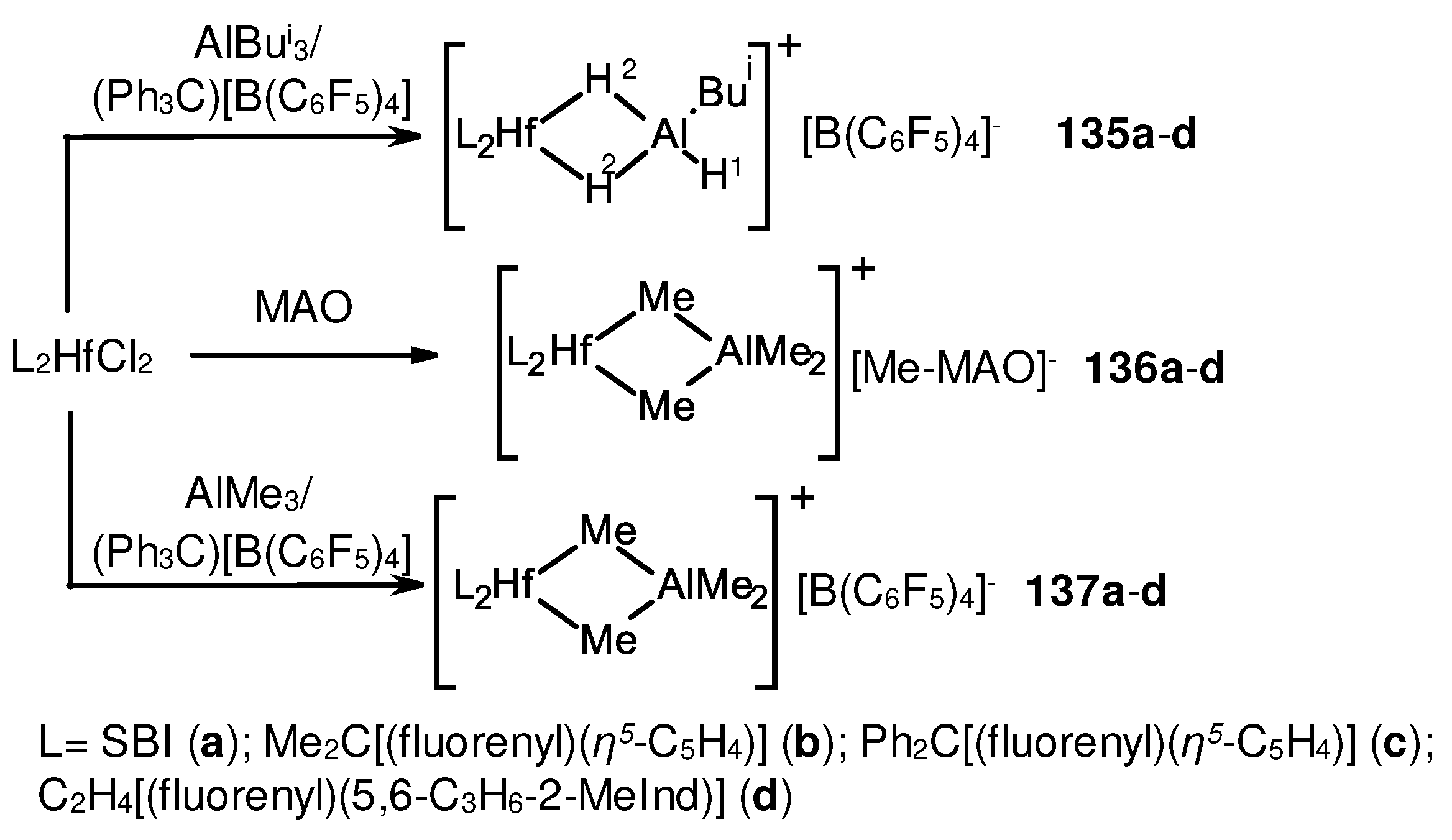
3.3. Influence of Al- and B-containing activators on structure and reactivity of metallocene hydrides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Janiak, C. Metallocene and related catalysts for olefin, alkyne and silane dimerization and oligomerization. Coord. Chem. Rev. 2006, 250, 66–94. [Google Scholar] [CrossRef]
- de Klerk, A. Oligomerization. In Fischer-Tropsch Refining, de Klerk, A., Ed.; Wiley-VCH Verlag: Hoboken, NJ, USA, 2011; pp. 369–391. [Google Scholar]
- McGuinness, D.S. Olefin Oligomerization via Metallacycles: Dimerization, Trimerization, Tetramerization, and Beyond. Chem. Rev. 2011, 111, 2321–2341. [Google Scholar] [CrossRef] [PubMed]
- Organometallic Reactions and Polymerization; Osakada, K., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2014; p. 301. [Google Scholar]
- Nifant’ev, I.; Ivchenko, P.; Tavtorkin, A.; Vinogradov, A.; Vinogradov, A. Non-traditional Ziegler-Natta catalysis in a-olefin transformations: reaction mechanisms and product design. Pure Appl. Chem. 2017, 89, 1017–1032. [Google Scholar] [CrossRef]
- Nicholas, C.P. Applications of light olefin oligomerization to the production of fuels and chemicals. Appl. Cat. A Gen. 2017, 543, 82–97. [Google Scholar] [CrossRef]
- Busca, G. Acid Catalysts in Industrial Hydrocarbon Chemistry. Chem. Rev. 2007, 107, 5366–5410. [Google Scholar] [CrossRef] [PubMed]
- Lavrenov, A.V.; Karpova, T.R.; Buluchevskii, E.A.; Bogdanets, E.N. Heterogeneous oligomerization of light alkenes: 80 years in oil refining. Catal. Ind. 2016, 8, 316–327. [Google Scholar] [CrossRef]
- Arlman, E.J.; Cossee, P. Ziegler-Natta catalysis III. Stereospecific polymerization of propene with the catalyst system TiCl3*AlEt3. J. Catal. 1964, 3, 99–104. [Google Scholar] [CrossRef]
- Wu, M.M.-s.; Coker, C.L.; Walzer Jr, J.F.; Jiang, P. Process to produce low viscosity poly-alpha-olefins. U.S. Patent 8,207,390 B2, 26 June 2012. [Google Scholar]
- Wu, M.M.; Hagemeister, M.P.; Yang, N. Process to produce polyalphaolefins. U.S. Patent 8,513,478 B2, 20 August 2013. [Google Scholar]
- Comyns, A.E. Encyclopedic Dictionary of Named Processes in Chemical Technology, 4th ed.; CRC Press: Boca Raton, FL, USA, 2014; p. 416. [Google Scholar]
- Martin, R.W.; Deckman, D.E.; Kelly, K.J.; Emett, C.J.; Hagemeister, M.P.; Harrington, B.A.; Lin, C.-y.; Matsunaga, P.T.; Ruff, C.J.; Stavens, K.B. Low viscosity engine oil compositions. U.S. Patent 9,234,150 B2, 12 January 2016. [Google Scholar]
- Patil, A.O.; Bodige, S. Synthetic lubricant basestocks and prepared from vinyl-terminated olefin macromonomers. U.S. Patent 9,422,497 B2, 23 August 2016. [Google Scholar]
- Harvey, B.G.; Meylemans, H.A. 1-Hexene: a renewable C6 platform for full-performance jet and diesel fuels. Green Chem. 2014, 16, 770–776. [Google Scholar] [CrossRef]
- Natta, G.; Danusso, F. Stereoregular Polymers and Stereospecific Polymerizations: The Contributions of Giulio Natta and His School to Polymer Chemistry; Symposium Publications Division, Pergamon Press: Michigan, USA, 1967; p. 888. [Google Scholar]
- Fink, G. Polymerization on Molecular Catalysts. In Handbook of Heterogeneous Catalysis, Ertl, G., Helmut Knözinger, H., Schüth, F., Weitkamp, J., Eds.; Wiley-VCH Verlag: Fairford, UK, 2008; pp. 3792–3830. [Google Scholar]
- Nowlin, T.; Mink, R.; Kissin, Y. Supported Magnesium/Titanium-Based Ziegler Catalysts for Production of Polyethylene. In Handbook of Transition Metal Polymerization Catalysts, Hoff, R., Mathers, R.T., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 131–155. [Google Scholar]
- Gardner, B.M.; Seechurn, C.C.C.J.; Colacot, T.J. Industrial Milestones in Organometallic Chemistry. In Organometallic Chemistry in Industry, Colacot, T.J., Seechurn, C.C.C.J., Eds.; Wiley-VCH Verlag: Weinheim, Germany, 2020; pp. 1–22. [Google Scholar]
- Kumawat, J.; Gupta, V.K. Fundamental aspects of heterogeneous Ziegler–Natta olefin polymerization catalysis: an experimental and computational overview. Polym. Chem. 2020, 11, 6107–6128. [Google Scholar] [CrossRef]
- Pawlak, M.; Drzeżdżon, J.; Jacewicz, D. The greener side of polymers in the light of d-block metal complexes as precatalysts. Coord. Chem. Rev. 2023, 484, 215122. [Google Scholar] [CrossRef]
- Wilkinson, G.; Birmingham, J.M. Bis-cyclopentadienyl Compounds of Ti, Zr, V, Nb and Ta. J. Am. Chem. Soc. 1954, 76, 4281–4284. [Google Scholar] [CrossRef]
- Long, W.P.; Breslow, D.S. Der Einfluß von Wasser auf die katalytische Aktivität von Bis(π-cyclopentadienyl)titandichlorid-Dimethylaluminiumchlorid zur Polymerisation von Äthylen. Justus Liebigs Ann. Chem. 1975, 1975, 463–469. [Google Scholar] [CrossRef]
- Andresen, A.; Cordes, H.-G.; Herwig, J.; Kaminsky, W.; Merck, A.; Mottweiler, R.; Pein, J.; Sinn, H.; Vollmer, H.-J. Halogen-Free Soluble Ziegler Catalysts for the Polymerization of Ethylene. Control of Molecular Weight by Choice of Temperature. Angew. Chem. Int. Ed. Engl. 1976, 15, 630–632. [Google Scholar] [CrossRef]
- Bolesłlawski, M.; Pasynkiewicz, S.; Kunicki, A.; Serwatowski, J. Proton magnetic resonance studies on the structure of tetraethylalumoxane. J. Organomet. Chem. 1976, 116, 285–289. [Google Scholar] [CrossRef]
- Yang, X.; Stern, C.L.; Marks, T.J. Cation-like homogeneous olefin polymerization catalysts based upon zirconocene alkyls and tris(pentafluorophenyl)borane. J. Am. Chem. Soc. 1991, 113, 3623–3625. [Google Scholar] [CrossRef]
- Yang, X.; Stern, C.L.; Marks, T.J. Cationic Zirconocene Olefin Polymerization Catalysts Based on the Organo-Lewis Acid Tris(pentafluorophenyl)borane. A Synthetic,Structural, Solution Dynamic, and Polymerization Catalytic Study. J. Am. Chem. Soc. 1994, 116, 10015–10031. [Google Scholar] [CrossRef]
- Brintzinger, H.H.; Fischer, D.; Mülhaupt, R.; Rieger, B.; Waymouth, R.M. Stereospecific Olefin Polymerization with Chiral Metallocene Catalysts. Angew. Chem. Int. Ed. 1995, 34, 1143–1170. [Google Scholar] [CrossRef]
- Chen, E.Y.-X.; Marks, T.J. Cocatalysts for Metal-Catalyzed Olefin Polymerization: Activators, Activation Processes, and Structure−Activity Relationships. Chem. Rev. 2000, 100, 1391–1434. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.A.; Russell, A.F.; Mountford, P. Group 4 metal complexes for homogeneous olefin polymerisation: a short tutorial review. Appl. Petrochem. Res. 2015, 5, 153–171. [Google Scholar] [CrossRef]
- M. Dzhemilev, U.; G. Ibragimov, A. Metal complex catalysis in the synthesis of organoaluminium compounds. Russ. Chem. Rev. 2000, 69, 121–135. [Google Scholar] [CrossRef]
- Guiry, P.J.; Coyne, A.G.; Carroll, A.M. 10.19 – C–E bond formation through hydroboration and hydroalumination. In: Crabtree RH, Mingos DMP, editors. Comprehensive Organometallic Chemistry III. Elsevier Ltd.; Oxford. 2007. pp. 839-869.
- Dzhemilev, U.M.; Ibragimov, A.G. Hydrometallation of Unsaturated Compounds. In Modern Reduction Methods, Andersson, P.G., Munslow, I.J., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 447–489. [Google Scholar]
- Tolstikov, G.A.; Dzhemilev, U.M.; Tolstikov, A.G. Aluminiyorganicheskie soedineniya v organicheskom sinteze (Organoaluminum compounds in organic synthesis). Akad. izd. GEO: Novosibirsk, 2009; pp. 645. ISBN 978-645-9747-0147-9744.
- Zaidlewicz, M.; Wolan, A.; Budny, M. M. 8.24 Hydrometallation of C=C and C=C bonds. Group 3. In Comprehensive Organic Synthesis II, Knöchel P., M.G.A., Ed.; Elsevier Science & Technology Books: Amsterdam, 2014; Volume 877. [Google Scholar] [CrossRef]
- Skupinska, J. Oligomerization of alpha-olefins to higher oligomers. Chem. Rev. 1991, 91, 613–648. [Google Scholar] [CrossRef]
- Blank, F. Metallocene Catalysts for Olefin Oligomerization. Macromol. Symp. 2006, 236, 14–22. [Google Scholar]
- Belov, G.P. Selective dimerization, oligomerization, homopolymerization and copolymerization of olefins with complex organometallic catalysts. Russ. J. Appl. Chem. 2008, 81, 1655–1666. [Google Scholar] [CrossRef]
- Breuil, P.-A.R.; Magna, L.; Olivier-Bourbigou, H. Role of Homogeneous Catalysis in Oligomerization of Olefins : Focus on Selected Examples Based on Group 4 to Group 10 Transition Metal Complexes. Catal. Letters 2015, 145, 173–192. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Ivchenko, P. Fair Look at Coordination Oligomerization of Higher α-Olefins. Polymers 2020, 12, 1082. [Google Scholar] [CrossRef]
- Olivier-Bourbigou, H.; Breuil, P.A.R.; Magna, L.; Michel, T.; Espada Pastor, M.F.; Delcroix, D. Nickel Catalyzed Olefin Oligomerization and Dimerization. Chem. Rev. 2020, 120, 7919–7983. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Valodkar, V.; Tembe, G. Recent developments in catalyst systems for selective oligomerization and polymerization of higher α-olefins. Polym. Chem. 2023, 14, 2542–2571. [Google Scholar] [CrossRef]
- Slaugh, L.H.; Schoenthal, G.W. Vinylidene Olefin Process. U.S. Patent 4,658,078, 14 April 1987. [Google Scholar]
- Kondakov, D.Y.; Negishi, E.-i. Zirconium-catalyzed enantioselective methylalumination of monosubstituted alkenes. Journal of the American Chemical Society 1995, 117, 10771–10772. [Google Scholar] [CrossRef]
- Christoffers, J.; Bergman, R.G. Catalytic Dimerization Reactions of α-Olefins and α,ω-Dienes with Cp2ZrCl2/Poly(methylalumoxane): Formation of Dimers, Carbocycles, and Oligomers. J. Am. Chem. Soc. 1996, 118, 4715–4716. [Google Scholar] [CrossRef]
- Christoffers, J.; Bergman, R.G. Zirconocene-alumoxane (1:1) - a catalyst for the selective dimerization of α-olefins. Inorg. Chim. Acta 1998, 270, 20–27. [Google Scholar] [CrossRef]
- Kretschmer, W.P.; Troyanov, S.I.; Meetsma, A.; Hessen, B.; Teuben, J.H. Regioselective Homo- and Codimerization of α-Olefins Catalyzed by Bis(2,4,7-trimethylindenyl)yttrium Hydride. Organometallics 1998, 17, 284–286. [Google Scholar] [CrossRef]
- Wahner, U.M.; Brüll, R.; Pasch, H.; Raubenheimer, H.G.; Sanderson, R.D. Oligomerisation of 1-pentene with metallocene catalysts. Angew. Makromol. Chem. 1999, 270, 49–55. [Google Scholar] [CrossRef]
- Brüll, R.; Kgosane, D.; Neveling, A.; Pasch, H.; Raubenheimer, H.; Sanderson, R.; Wahner, U. Synthesis and properties of poly-1-olefins. Macromol. Symp. 2001, 165, 11–18. [Google Scholar] [CrossRef]
- Boccia, A.C.; Costabile, C.; Pragliola, S.; Longo, P. Selective Dimerization of γ-Branched α-Olefins in the Presence of C2v Group-4 Metallocene-Based Catalysts. Macromol. Chem. Phys. 2004, 205, 1320–1326. [Google Scholar] [CrossRef]
- Small, B.L.; Marcucci, A.J. Iron Catalysts for the Head-to-Head Dimerization of α-Olefins and Mechanistic Implications for the Production of Linear α-Olefins. Organometallics 2001, 20, 5738–5744. [Google Scholar] [CrossRef]
- Small, B.L. Tridentate Cobalt Catalysts for Linear Dimerization and Isomerization of α-Olefins. Organometallics 2003, 22, 3178–3183. [Google Scholar] [CrossRef]
- Broene, R.D.; Brookhart, M.; Lamanna, W.M.; Volpe, A.F. Cobalt-Catalyzed Dimerization of α-Olefins to Give Linear α-Olefin Products. J. Am. Chem. Soc. 2005, 127, 17194–17195. [Google Scholar] [CrossRef] [PubMed]
- Hanton, M.J.; Daubney, L.; Lebl, T.; Polas, S.; Smith, D.M.; Willemse, A. Selective dimerisation of α-olefins using tungsten-based initiators. Dalton Trans. 2010, 39, 7025–7037. [Google Scholar] [CrossRef]
- Gunasekara, T.; Preston, A.Z.; Zeng, M.; Abu-Omar, M.M. Highly Regioselective α-Olefin Dimerization Using Zirconium and Hafnium Amine Bis(phenolate) Complexes. Organometallics 2017, 36, 2934–2939. [Google Scholar] [CrossRef]
- Flory, P.J. Molecular Size Distribution in Linear Condensation Polymers1. J. Am. Chem. Soc. 1936, 58, 1877–1885. [Google Scholar] [CrossRef]
- Nakata, N.; Nakamura, K.; Ishii, A. Highly Efficient and 1,2-Regioselective Method for the Oligomerization of 1-Hexene Promoted by Zirconium Precatalysts with [OSSO]-Type Bis(phenolate) Ligands. Organometallics 2018, 37, 2640–2644. [Google Scholar] [CrossRef]
- Lian, B.; Beckerle, K.; Spaniol, T.P.; Okuda, J. Regioselective 1-Hexene Oligomerization Using Cationic Bis(phenolato) Group 4 Metal Catalysts: Switch from 1,2- to 2,1-Insertion. Angew. Chem. Int. Ed. 2007, 46, 8507–8510. [Google Scholar] [CrossRef] [PubMed]
- Nifant'ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Ivchenko, P.V. Zirconocene-catalyzed dimerization of 1-hexene: Two-stage activation and structure–catalytic performance relationship. Catal. Commun. 2016, 79, 6–10. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Sedov, I.V.; Dorokhov, V.G.; Lyadov, A.S.; Ivchenko, P.V. Structurally uniform 1-hexene, 1-octene, and 1-decene oligomers: Zirconocene/MAO-catalyzed preparation, characterization, and prospects of their use as low-viscosity low-temperature oil base stocks. Appl. Catal. A-Gen. 2018, 549, 40–50. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Churakov, A.V.; Ivchenko, P.V. Synthesis of zirconium(III) complex by reduction of O[SiMe2(η5-C5H4)]2ZrCl2and its selectivity in catalytic dimerization of hex-1-ene. Mendeleev Commun. 2018, 28, 467–469. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Vinogradov, A.; Vinogradov, A.; Karchevsky, S.; Ivchenko, P. Zirconocene-Catalyzed Dimerization of α-Olefins: DFT Modeling of the Zr-Al Binuclear Reaction Mechanism. Molecules 2019, 24, 3565. [Google Scholar] [CrossRef]
- Kuklin, M.S.; Hirvi, J.T.; Bochmann, M.; Linnolahti, M. Toward Controlling the Metallocene/Methylaluminoxane-Catalyzed Olefin Polymerization Process by a Computational Approach. Organometallics 2015, 34, 3586–3597. [Google Scholar] [CrossRef]
- Nifant'ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Bagrov, V.V.; Churakov, A.V.; Minyaev, M.E.; Kiselev, A.V.; Salakhov, I.I.; Ivchenko, P.V. A competetive way to low-viscosity PAO base stocks via heterocene-catalyzed oligomerization of dec-1-ene. Mol. Catal. 2022, 529, 112542. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Bagrov, V.V.; Kiselev, A.V.; Minyaev, M.E.; Samurganova, T.I.; Ivchenko, P.V. Heterocene Catalysts and Reaction Temperature Gradient in Dec-1-ene Oligomerization for the Production of Low Viscosity PAO Base Stocks. Ind. Eng. Chem. Res. 2023, 62, 6347–6353. [Google Scholar] [CrossRef]
- Bryliakov, K.P.; Talsi, E.P.; Semikolenova, N.V.; Zakharov, V.A.; Brand, J.; Alonso-Moreno, C.; Bochmann, M. Formation and structures of cationic zirconium complexes in ternary systems rac-(SBI)ZrX2/AlBu3i/[CPh3][B(C6F5)4] (X=Cl, Me). J. Organomet. Chem. 2007, 692, 859–868. [Google Scholar] [CrossRef]
- Götz, C.; Rau, A.; Luft, G. Ternary metallocene catalyst systems based on metallocene dichlorides and AlBu3i/[PhNMe2H][B(C6F5)4]: NMR investigations of the influence of Al/Zr ratios on alkylation and on formation of the precursor of the active metallocene species. J. Mol. Catal. A Chem. 2002, 184, 95–110. [Google Scholar] [CrossRef]
- Shao, H.; Wang, R.; Li, H.; Guo, X.; Jiang, T. Synthesis of low-molecular-weight poly-α-olefins using silicon-bridged zirconocene catalyst for lubricant basestock. Arab. J. Chem. 2018, 13, 2715–2721. [Google Scholar] [CrossRef]
- Dong, S.Q.; Mi, P.K.; Xu, S.; Zhang, J.; Zhao, R.D. Preparation and Characterization of Single-Component Poly-α-olefin Oil Base Stocks. Energy Fuels 2019, 33, 9796–9804. [Google Scholar] [CrossRef]
- Hanifpour, A.; Bahri-Laleh, N.; Nekoomanesh-Haghighi, M.; Poater, A. Group IV diamine bis(phenolate) catalysts for 1-decene oligomerization. Mol. Catal. 2020, 493, 111047. [Google Scholar] [CrossRef]
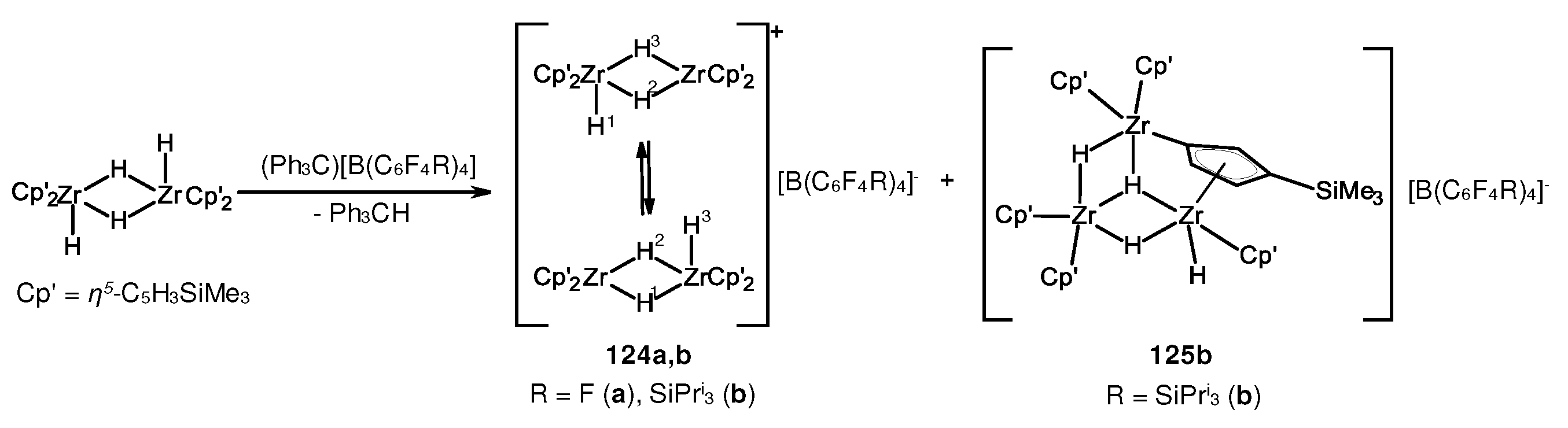
- Parfenova, L.V.; Kovyazin, P.V.; Bikmeeva, A.K. Bimetallic Zr,Zr-Hydride Complexes in Zirconocene Catalyzed Alkene Dimerization. Molecules 2020, 25, 2216. [Google Scholar] [CrossRef] [PubMed]
- Parfenova, L.V.; Kovyazin, P.V.; Bikmeeva, A.K.; Palatov, E.R. Catalytic Systems Based on Cp2ZrX2 (X = Cl, H), Organoaluminum Compounds and Perfluorophenylboranes: Role of Zr,Zr- and Zr,Al-Hydride Intermediates in Alkene Dimerization and Oligomerization. Catalysts 2021, 11, 39. [Google Scholar] [CrossRef]
- Kovyazin, P.V.; Bikmeeva, A.K.; Islamov, D.N.; Yanybin, V.M.; Tyumkina, T.V.; Parfenova, L.V. Ti Group Metallocene-Catalyzed Synthesis of 1-Hexene Dimers and Tetramers. Molecules 2021, 26, 2775. [Google Scholar] [CrossRef] [PubMed]
- Parfenova, L.V.; Kovyazin, P.V.; Bikmeeva, A.K.; Palatov, E.R.; Ivchenko, P.V.; Nifant’ev, I.E.; Khalilov, L.M. Catalytic Properties of Zirconocene-Based Systems in 1-Hexene Oligomerization and Structure of Metal Hydride Reaction Centers. Molecules 2023, 28, 2420. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Kovyazin, P.V.; Bikmeeva, A.K.; Palatov, E.R.; Ivchenko, P.V.; Nifant’ev, I.E. Activation of metallocene hydride intermediates by methylaluminoxane in alkene dimerization and oligomerization. React. Kinet. Mech. Catal. 2023. [Google Scholar] [CrossRef]
- McInnis, J.P.; Delferro, M.; Marks, T.J. Multinuclear Group 4 Catalysis: Olefin Polymerization Pathways Modified by Strong Metal-Metal Cooperative Effects. Acc. Chem. Res. 2014, 47, 2545–2557. [Google Scholar] [CrossRef]
- Rebenstorf, B.; Larsson, R. Why do homogeneous analogs of phillips (CrO3/SiO2) and union carbide (Chromocene/SiO2) polyethylene catalysts fail? Some answers from ir investigations. J. Mol. Catal. 1981, 11, 247–256. [Google Scholar] [CrossRef]
- Brückner, A.; Jabor, J.K.; McConnell, A.E.C.; Webb, P.B. Monitoring Structure and Valence State of Chromium Sites during Catalyst Formation and Ethylene Oligomerization by in Situ EPR Spectroscopy. Organometallics 2008, 27, 3849–3856. [Google Scholar] [CrossRef]
- Rosenthal, U.; Müller, B.H.; Peulecke, N.; Peitz, S.; Wöhl, A.; Müller, W.; Olivier-Bourbigou, H.; Magna, L.; van Leeuwen, P.W.N.M.; Tschan, M.J.L.; et al. Oligomerization, Cyclooligomerization, Dimerization. In Applied Homogeneous Catalysis with Organometallic Compounds, Cornils, B., Herrmann, W.A., Beller, M., Paciello, R., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA.: Weinheim, Germany, 2017; pp. 307–410. [Google Scholar]
- Bochmann; Sarsfield, M. J. Reaction of AlR3 with [CPh3][B(C6F5)4]: Facile Degradation of [B(C6F5)4]- by Transient “[AlR2]+”. Organometallics 1998, 17, 5908–5912. [Google Scholar] [CrossRef]
- Janiak, C.; Lassahn, P.-G. 19F NMR Investigations of the Reaction of B(C6F5)3 with Different Tri(alkyl)aluminum Compounds. Macromol. Symp. 2006, 236, 54–62. [Google Scholar] [CrossRef]
- Bryliakov, K.P.; Semikolenova, N.V.; Panchenko, V.N.; Zakharov, V.A.; Brintzinger, H.H.; Talsi, E.P. Activation of rac-Me2Si(ind)2ZrCl2 by Methylalumoxane Modified by Aluminum Alkyls: An EPR Spin-Probe, 1H NMR, and Polymerization Study. Macromol. Chem. Phys. 2006, 207, 327–335. [Google Scholar] [CrossRef]
- Babushkin, D.E.; Brintzinger, H.H. Modification of Methylaluminoxane-Activated ansa-Zirconocene Catalysts with Triisobutylaluminum-Transformations of Reactive Cations Studied by NMR Spectroscopy. Chem. Eur. J. 2007, 13, 5294–5299. [Google Scholar] [CrossRef] [PubMed]
- Babushkin, D.E.; Panchenko, V.N.; Timofeeva, M.N.; Zakharov, V.A.; Brintzinger, H.H. Novel Zirconocene Hydride Complexes in Homogeneous and in SiO2-Supported Olefin-Polymerization Catalysts Modified with Diisobutylaluminum Hydride or Triisobutylaluminum. Macromol. Chem. Phys. 2008, 209, 1210–1219. [Google Scholar] [CrossRef]
- Zaccaria, F.; Zuccaccia, C.; Cipullo, R.; Budzelaar, P.H.M.; Vittoria, A.; Macchioni, A.; Busico, V.; Ehm, C. Methylaluminoxane’s Molecular Cousin: A Well-defined and “Complete” Al-Activator for Molecular Olefin Polymerization Catalysts. ACS Catal. 2021, 11, 4464–4475. [Google Scholar] [CrossRef]
- Urciuoli, G.; Zaccaria, F.; Zuccaccia, C.; Cipullo, R.; Budzelaar, P.H.M.; Vittoria, A.; Ehm, C.; Macchioni, A.; Busico, V. A Hydrocarbon Soluble, Molecular and "Complete" Al-Cocatalyst for High Temperature Olefin Polymerization. Polymers 2023, 15, 1378. [Google Scholar] [CrossRef]
- Nanda, R.K.; Wallbridge, M.G.H. Dicyclopentadienylzirconium Diborohydride. Inorg. Chem. 1964, 3, 1798–1798. [Google Scholar] [CrossRef]
- James, B.D.; Nanda, R.K.; Walbridge, M.G.H. Reactions of Lewis bases with tetrahydroborate derivatives of the Group IVa elements. Preparation of new zirconium hydride species. Inorg. Chem. 1967, 6, 1979–1983. [Google Scholar] [CrossRef]
- Wailes, P.C.; Weigold, H. Hydrido complexes of zirconium I. Preparation. J. Organomet. Chem. 1970, 24, 405–411. [Google Scholar] [CrossRef]
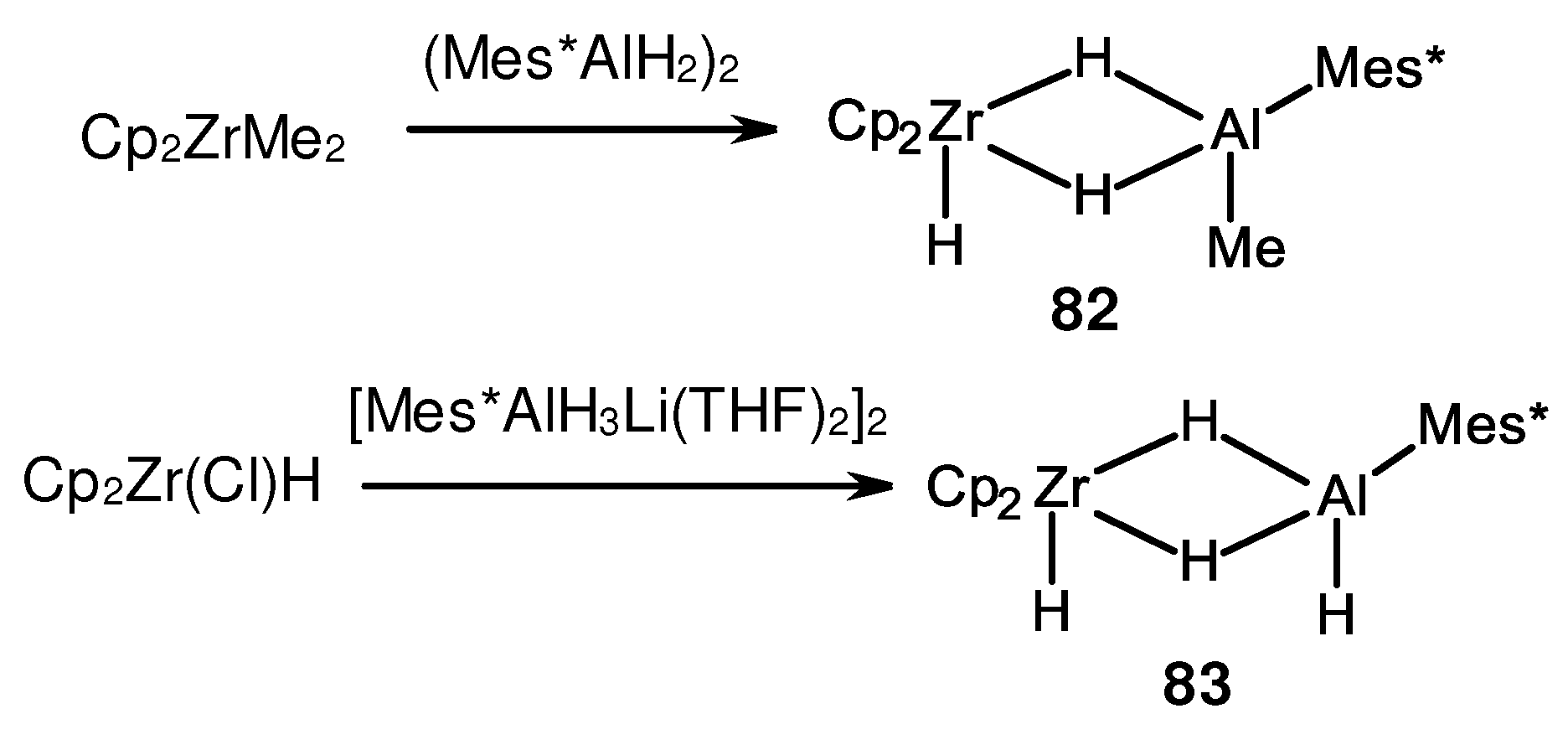
- Wailes, P.C.; Weigold, H.; Bell, A.P. Reaction of dicyclopentadienylzirconium dihydride with trimethylaluminium. Formation of a novel hydride containing both Zr-H-Zr and Zr-H-Al. J. Organomet. Chem. 1972, 43, C29–C31. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Pechatkina, S.V.; Khalilov, L.M.; Dzhemilev, U.M. Mechanism of Cp2ZrCl2-catalyzed olefin hydroalumination by alkylalanes. Russ. Chem. Bull. 2005, 54, 316–327. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Vil’danova, R.F.; Pechatkina, S.V.; Khalilov, L.M.; Dzhemilev, U.M. New effective reagent [Cp2ZrH2·ClAlEt2]2 for alkene hydrometallation. J. Organomet. Chem. 2007, 692, 3424–3429. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Kovyazin, P.V.; Tyumkina, T.V.; Islamov, D.N.; Lyapina, A.R.; Karchevsky, S.G.; Ivchenko, P.V. Reactions of bimetallic Zr,Al- hydride complexes with methylaluminoxane: NMR and DFT study. J. Organomet. Chem. 2017, 851, 30–39. [Google Scholar] [CrossRef]
- Shoer, L.I.; Gell, K.I.; Schwartz, J. Mixed-metal hydride complexes containing Zr-H-Al bridges. synthesis and relation to transition-metal-catalyzed reactions of aluminum hydrides. J. Organomet. Chem. 1977, 136, c19–c22. [Google Scholar] [CrossRef]
- Siedle, A.R.; Newmark, R.A.; Schroepfer, J.N.; Lyon, P.A. Solvolysis of dimethylzirconocene by trialkylaluminum compounds. Organometallics 1991, 10, 400–404. [Google Scholar] [CrossRef]
- Khan, K.; Raston, C.L.; McGrady, J.E.; Skelton, B.W.; White, A.H. Hydride-Bridged Heterobimetallic Complexes of Zirconium and Aluminum. Organometallics 1997, 16, 3252–3254. [Google Scholar] [CrossRef]
- Etkin, N.; Stephan, D.W. The Zirconocene Dihydride−Alane Adducts [(Cp‘)2ZrH(μ-H)2]3Al and [(Cp‘)2ZrH(μ-H)2]2AlH (Cp‘ = Me3SiC5H4). Organometallics 1998, 17, 763–765. [Google Scholar] [CrossRef]
- Lobkovskii, E.B.; Soloveichik, G.L.; Sizov, A.I.; Bulychev, B.M. Structural chemistry of titanium and aluminium bimetallic hydride complexes: III. Synthesis, molecular structure and catalytic properties of [(η5-C5H5)2Ti(μ2-H)2Al(μ2-H)(η1:η5-C5H4)Ti(η5-C5H5)(μ2-H)]2·C6H5CH3. J. Organomet. Chem. 1985, 280, 53–66. [Google Scholar] [CrossRef]
- Bel'sky, V.K.; Sizov, A.I.; Bulychev, B.M.; Soloveichik, G.L. Structural chemistry of titanium and aluminium bimetallic hydride complexes: IV. Molecular structures and catalytic properties of {[η5-C5(CH3)5]2Ti(μ2-H)2Al(H)(μ2-H)}2 and [η5-C5(CH3)5]2Ti(μ2-H)2Al(H)(μ2-H)2Ti[η5-C5(CH3)5]2. J. Organomet. Chem. 1985, 280, 67–80. [Google Scholar] [CrossRef]
- Sizov, A.I.; Zvukova, T.M.; Bulychev, B.M.; Belsky, V.K. Synthesis and properties of unsolvated bis(cyclopentadienyl)titanium alumohydride. Structure of {[(η5-C5H5)2Ti(μ-H)]2[(η5-C5H5)Ti(μ-H2]Al3(μ-H4)(H)}2·C6H6 a 12-nuclear titanium aluminum hydride complex with a short Al·Al bond length, and refined structure of LiAlEt4. J. Organomet. Chem. 2000, 603, 167–173. [Google Scholar]
- Wehmschulte, R.J.; Power, P.P. Reaction of cyclopentadienyl zirconium derivatives with sterically encumbered arylaluminum hydrides: X-ray crystal structure of (η5-C5H5)2(H)Zr(μ2-H)2Al(H)C6H2-2,4,6-But3. Polyhedron 1999, 18, 1885–1888. [Google Scholar] [CrossRef]
- Sizov, A.I.; Zvukova, T.; Belsky, V.; Bulychev, B.M. Aluminium zirconium (+3 and +4) heterometallic hydrido complexes of compositions [(η5-C5H5)2Zr(μ-H)]2(μ-H)AlCl2 and [(η5-C5H5)2ZrH(μ-H)2]3Al. J. Organomet. Chem. 2001, 619, 36–42. [Google Scholar] [CrossRef]
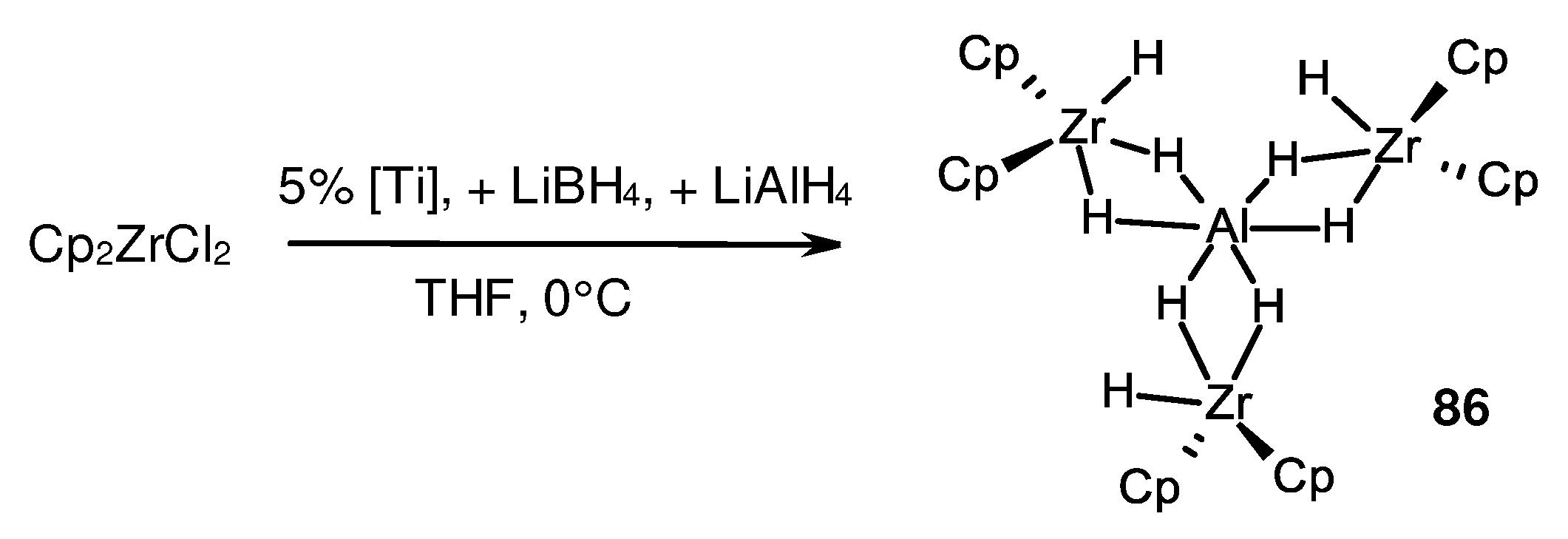
- Sizov, A.I.; Zvukova, T.; Khvostov, A.V.; Belsky, V.; Stash, A.; Bulychev, B.M. Transition metal-catalyzed reduction of ZrIV in Cp2ZrX2-LiAlH4 and Cp2ZrX2-AlH3 (X=Cl, Br, I) systems: Structural study of resulting zirconocene(III) aluminum hydride complexes. J. Organomet. Chem. 2003, 681, 167–173. [Google Scholar] [CrossRef]
- Sizov, A.I.; Zvukova, T.; Khvostov, A.V.; Gorkovskii, A.A.; Starikova, Z.A.; Bulychev, B. Heterometallic (Zr-III)2-Al hydrides [(Cp2Zr)2(µ-H)](µ-H)2AlX2 (X = Cl or Br): preparative synthesis and reactivity. Molecular structure of [(Cp2Zr)2(µ-Cl)](µ-H)2AlCl2. Russ. Chem. Bull. 2005, 54, 2496–2501. [Google Scholar] [CrossRef]
- Baldwin, S.M.; Bercaw, J.E.; Brintzinger, H.H. Alkylaluminum-Complexed Zirconocene Hydrides: Identification of Hydride-Bridged Species by NMR Spectroscopy. J. Am. Chem. Soc. 2008, 130, 17423–17433. [Google Scholar] [CrossRef] [PubMed]
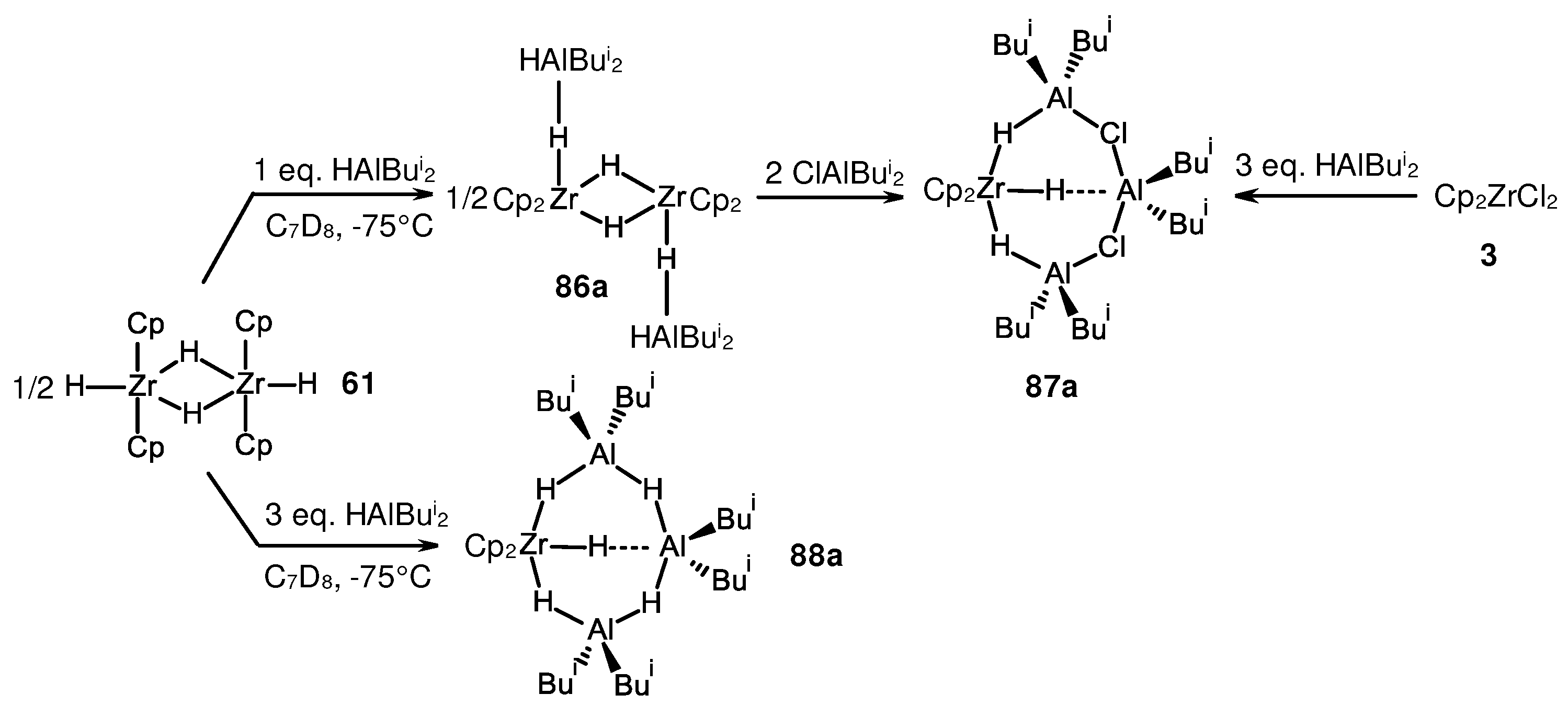
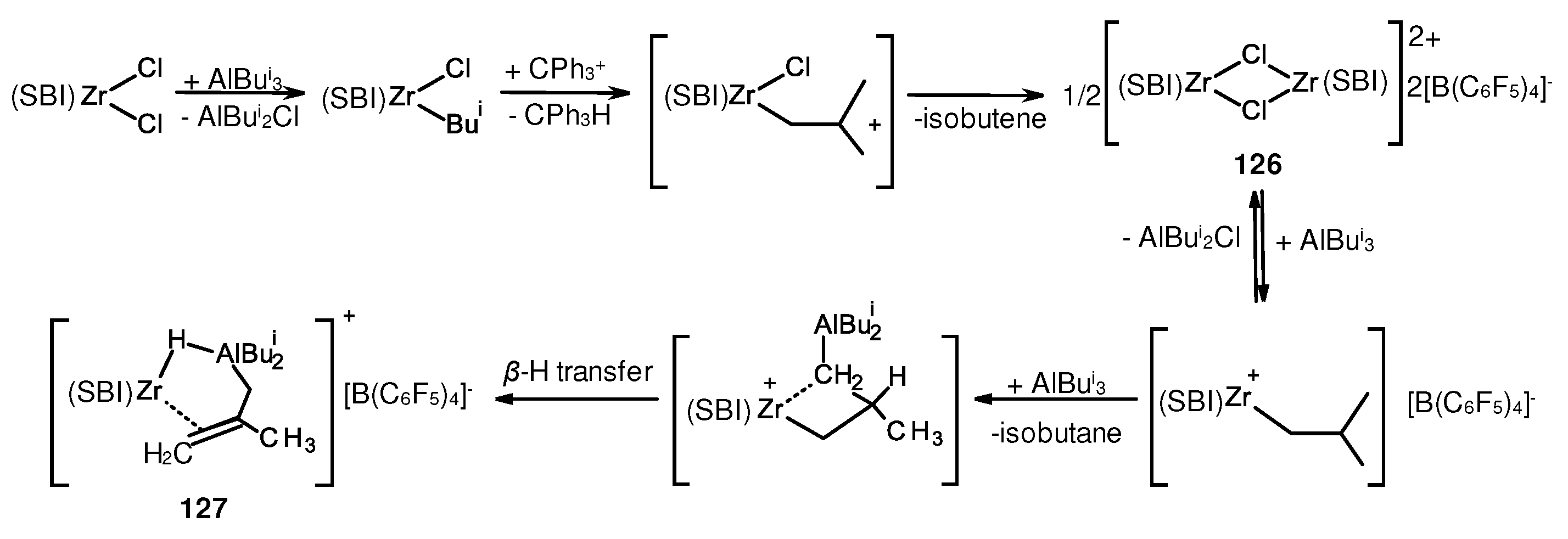
- Parfenova, L.V.; Kovyazin, P.V.; Nifant’ev, I.E.; Khalilov, L.M.; Dzhemilev, U.M. Role of Zr,Al Hydride Intermediate Structure and Dynamics in Alkene Hydroalumination with XAlBui2 (X = H, Cl, Bui), Catalyzed by Zr η5-Complexes. Organometallics 2015, 34, 3559–3570. [Google Scholar] [CrossRef]
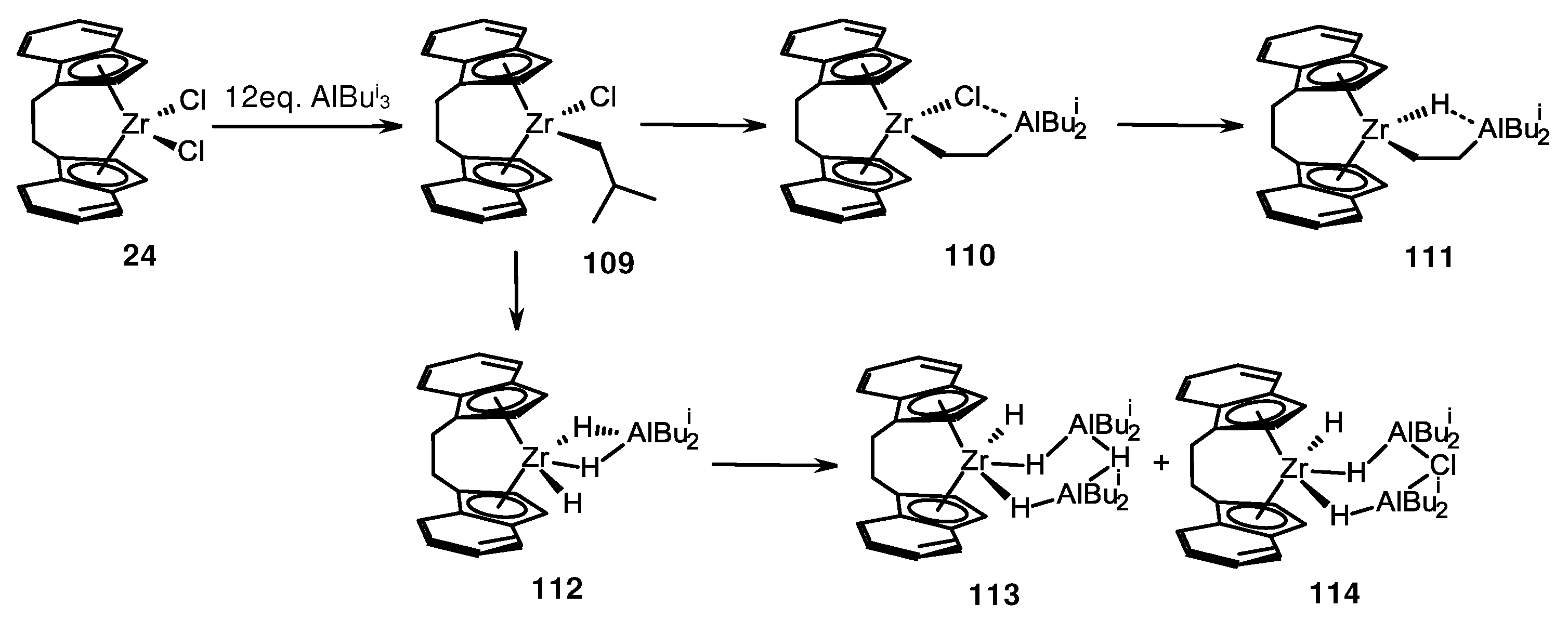
- Culver, D.B.; Corieri, J.; Lief, G.; Conley, M.P. Reactions of Triisobutylaluminum with Unbridged or Bridged Group IV Metallocene Dichlorides. Organometallics 2022, 41, 892–899. [Google Scholar] [CrossRef]
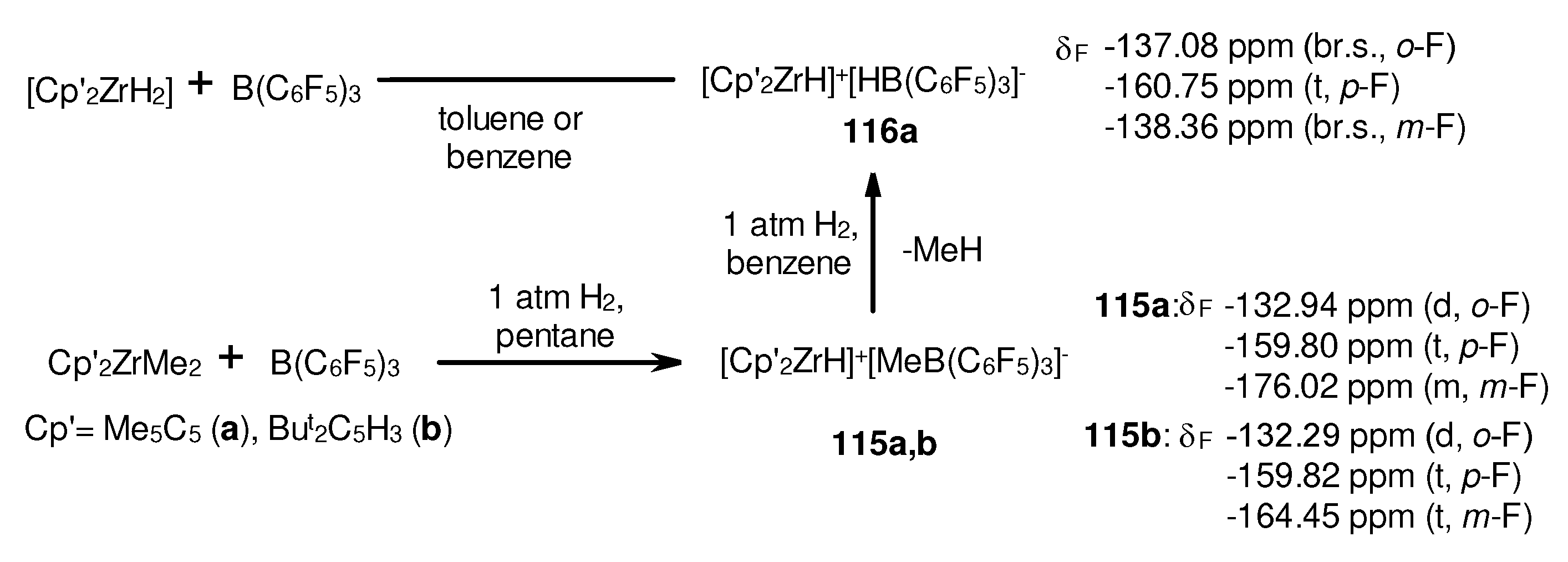
- Yang, X.; Stern, C.L.; Marks, T.J. Cationic Metallocene Polymerization Catalysts. Synthesis and Properities of the First Base-Free Zirconocene Hydride. Angew. Chem. Int. Ed. 1992, 31, 1375–1377. [Google Scholar] [CrossRef]
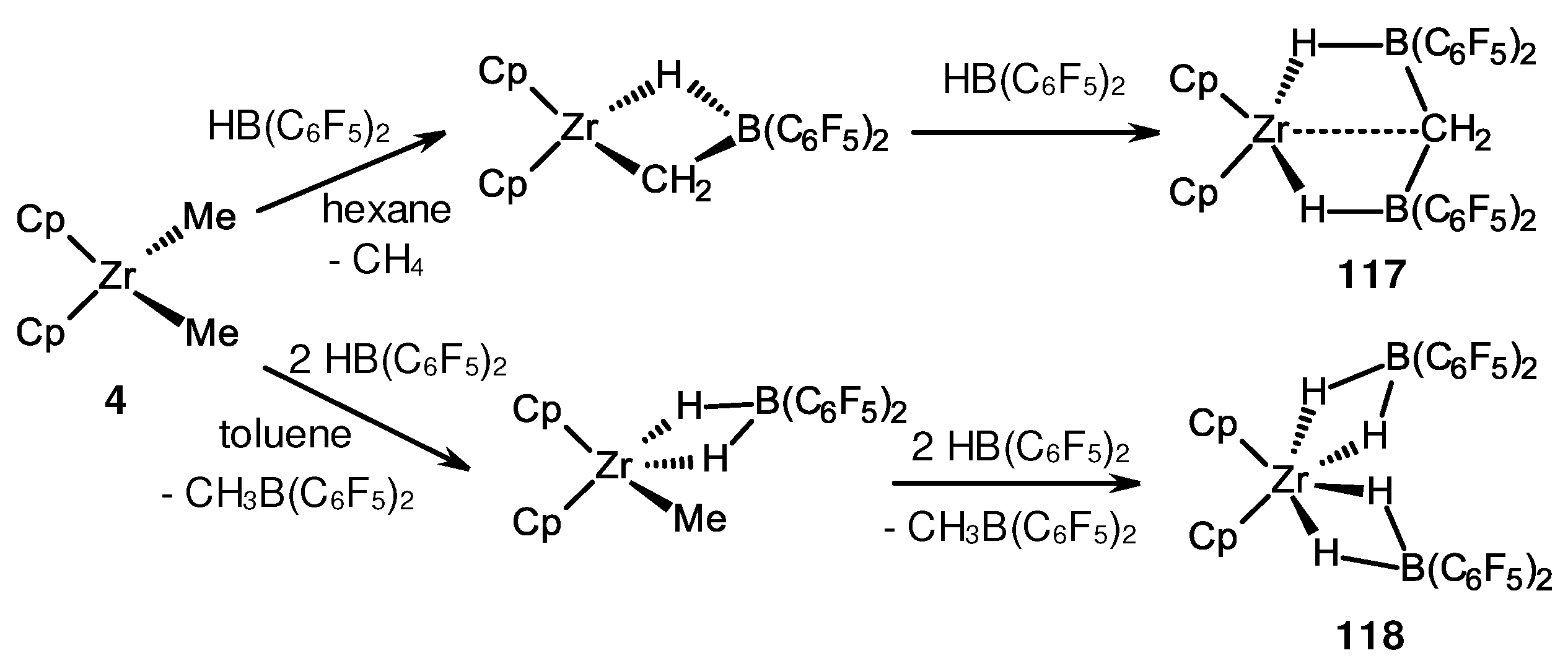
- von, H. Spence, R.E.; Parks, D.J.; Piers, W.E.; MacDonald, M.-A.; Zaworotko, M.J.; Rettig, S.J. Competing Pathways in the Reaction of Bis(pentafluorophenyl)borane with Bis(η5-cyclopentadienyl)dimethylzirconium: Methane Elimination versus Methyl-Hydride Exchange and an Example of Pentacoordinate Carbon. Angew. Chem. Int. Ed. 1995, 34, 1230–1233. [Google Scholar]
- Spence, R.E.v.H.; Piers, W.E.; Sun, Y.; Parvez, M.; MacGillivray, L.R.; Zaworotko, M.J. Mechanistic Aspects of the Reactions of Bis(pentafluorophenyl)borane with the Dialkyl Zirconocenes Cp2ZrR2 (R = CH3, CH2SiMe3, and CH2C6H5). Organometallics 1998, 17, 2459–2469. [Google Scholar] [CrossRef]
- Sun, Y.; Spence, R.E.v.H.; Piers, W.E.; Parvez, M.; Yap, G.P.A. Intramolecular Ion−Ion Interactions in Zwitterionic Metallocene Olefin Polymerization Catalysts Derived from “Tucked-In” Catalyst Precursors and the Highly Electrophilic Boranes XB(C6F5)2 (X = H, C6F5). J. Am. Chem. Soc. 1997, 119, 5132–5143. [Google Scholar] [CrossRef]
- Carr, A.G.; Dawson, D.M.; Thornton-Pett, M.; Bochmann, M. Cationic Zirconocene Hydrides: A New Type of Highly Effective Initiators for Carbocationic Polymerizations. Organometallics 1999, 18, 2933–2935. [Google Scholar] [CrossRef]
- Arndt, P.; Baumann, W.; Spannenberg, A.; Rosenthal, U.; Burlakov, V.V.; Shur, V.B. Reactions of Titanium and Zirconium Derivatives of Bis(trimethylsilyl)acetylene with Tris(pentafluorophenyl)borane: A Titanium(III) Complex of an Alkynylboranate. Angew. Chem. Int. Ed. 2003, 42, 1414–1418. [Google Scholar] [CrossRef]
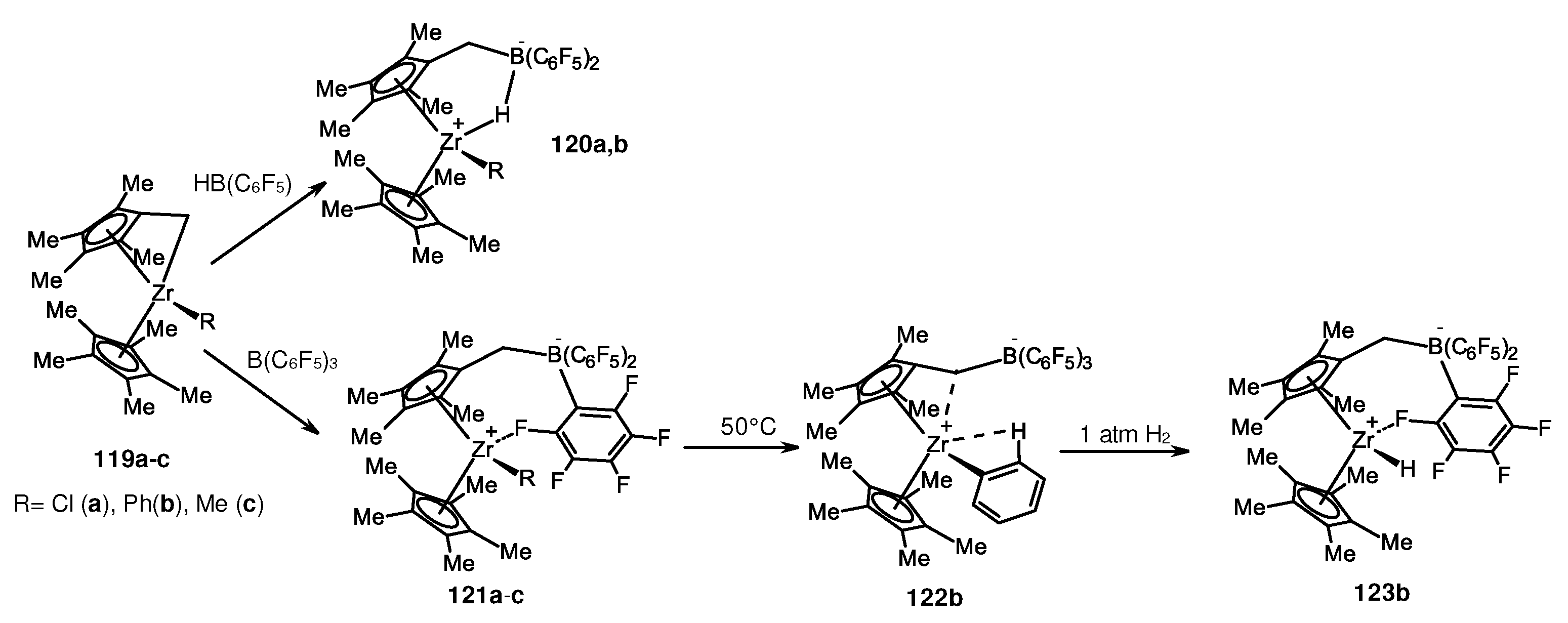
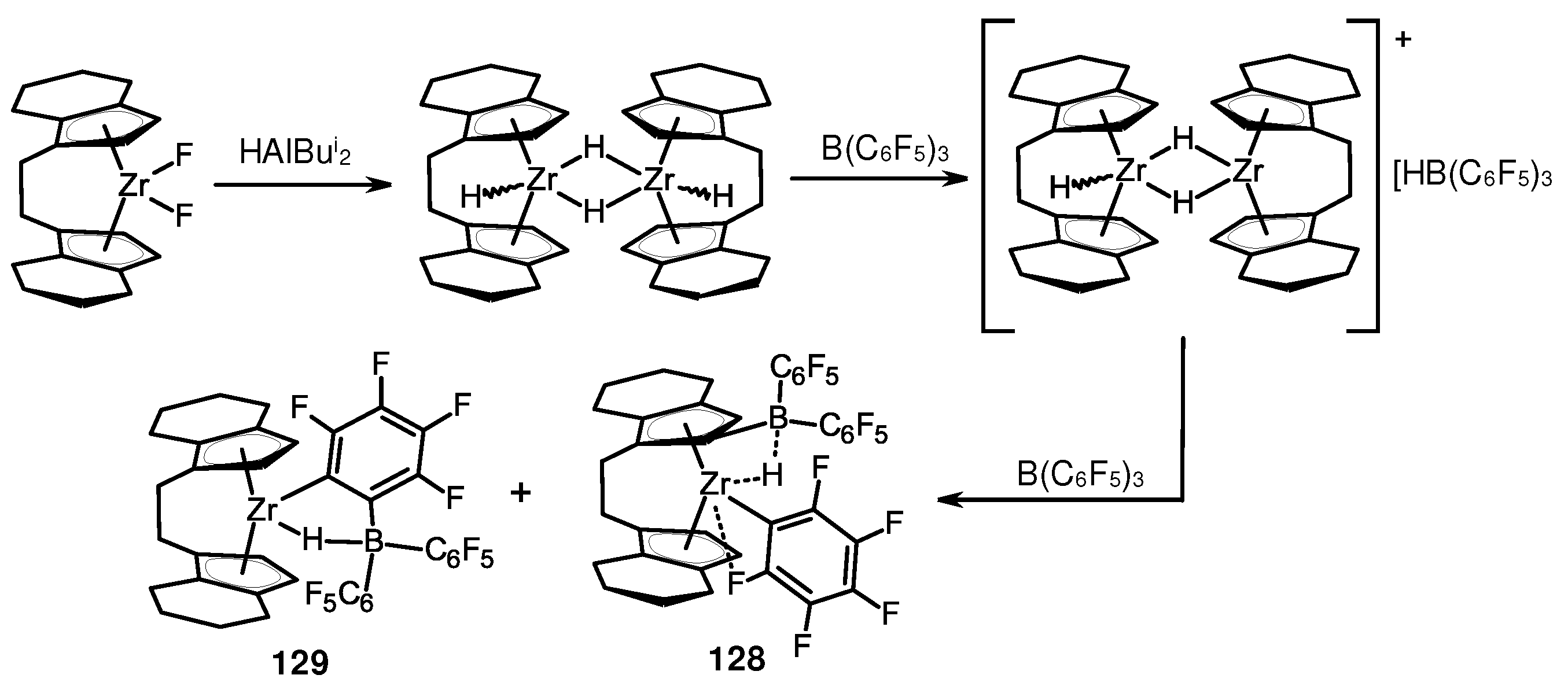
- Arndt, P.; Jäger-Fiedler, U.; Klahn, M.; Baumann, W.; Spannenberg, A.; Burlakov, V.V.; Rosenthal, U. Formation of Zirconocene Fluoro Complexes: No Deactivation in the Polymerization of Olefins by the Contact-Ion-Pair Catalysts [Cp'2ZrR]+[RB(C6F5)3]−. Angew. Chem. Int. Ed. 2006, 45, 4195–4198. [Google Scholar] [CrossRef]
- Al-Humydi, A.; Garrison, J.C.; Mohammed, M.; Youngs, W.J.; Collins, S. Propene polymerization using ansa-metallocenium ions: Catalyst deactivation processes during monomer consumption and molecular structures of the products formed. Polyhedron 2005, 24, 1234–1249. [Google Scholar] [CrossRef]
- González-Hernández, R.; Chai, J.; Charles, R.; Pérez-Camacho, O.; Kniajanski, S.; Collins, S. Catalytic System for Homogeneous Ethylene Polymerization Based on Aluminohydride−Zirconocene Complexes. Organometallics 2006, 25, 5366–5373. [Google Scholar] [CrossRef]
- Bryliakov, K.P.; Talsi, E.P.; Voskoboynikov, A.Z.; Lancaster, S.J.; Bochmann, M. Formation and Structures of Hafnocene Complexes in MAO- and AlBui3/CPh3[B(C6F5)4]-Activated Systems. Organometallics 2008, 27, 6333–6342. [Google Scholar] [CrossRef]
- Baldwin, S.M.; Bercaw, J.E.; Henling, L.M.; Day, M.W.; Brintzinger, H.H. Cationic Alkylaluminum-Complexed Zirconocene Hydrides: NMR-Spectroscopic Identification, Crystallographic Structure Determination, and Interconversion with Other Zirconocene Cations. J. Am. Chem. Soc. 2011, 133, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, S.M.; Bercaw, J.E.; Brintzinger, H.H. Cationic Alkylaluminum-Complexed Zirconocene Hydrides as Participants in Olefin Polymerization Catalysis. J. Am. Chem. Soc. 2010, 132, 13969–13971. [Google Scholar] [CrossRef] [PubMed]
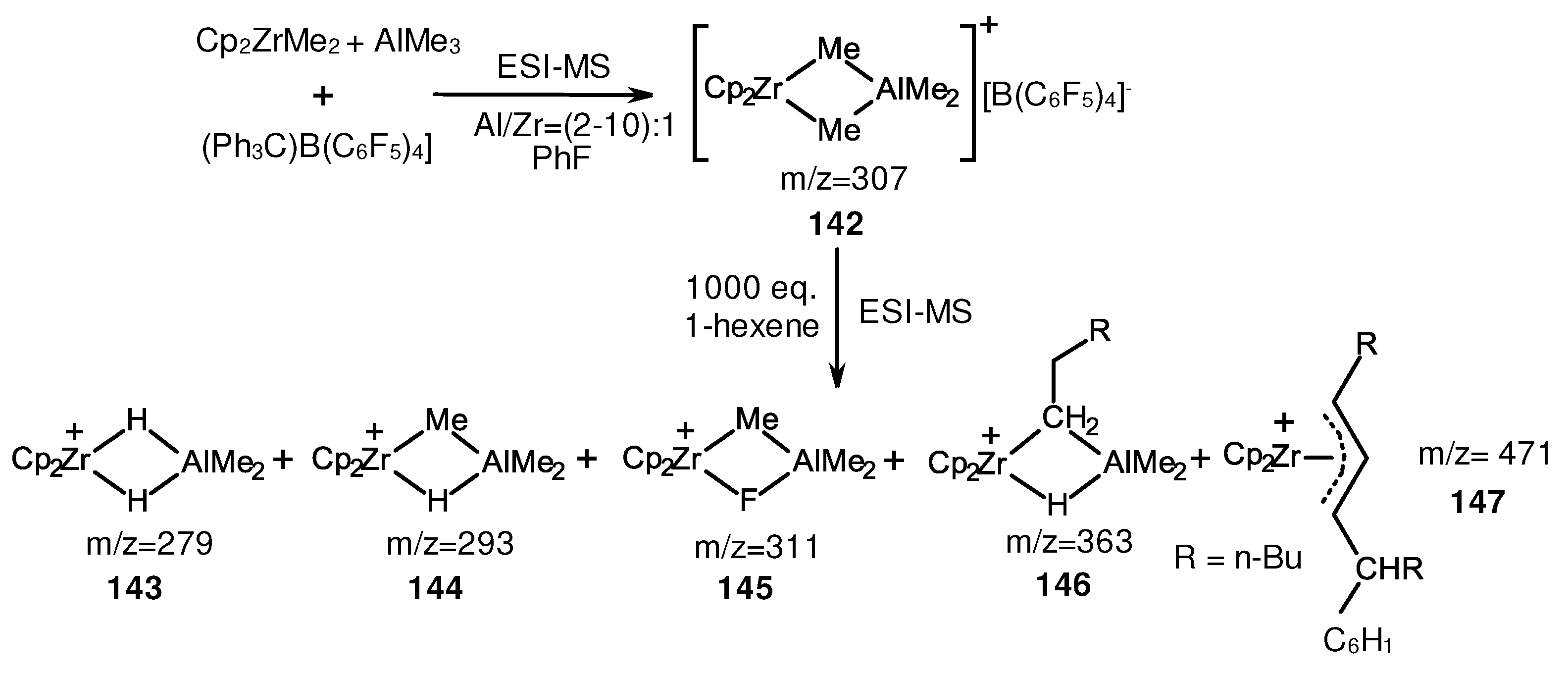
- Joshi, A.; Zijlstra, H.S.; Collins, S.; McIndoe, J.S. Catalyst Deactivation Processes during 1-Hexene Polymerization. ACS Catal. 2020, 10, 7195–7206. [Google Scholar] [CrossRef]
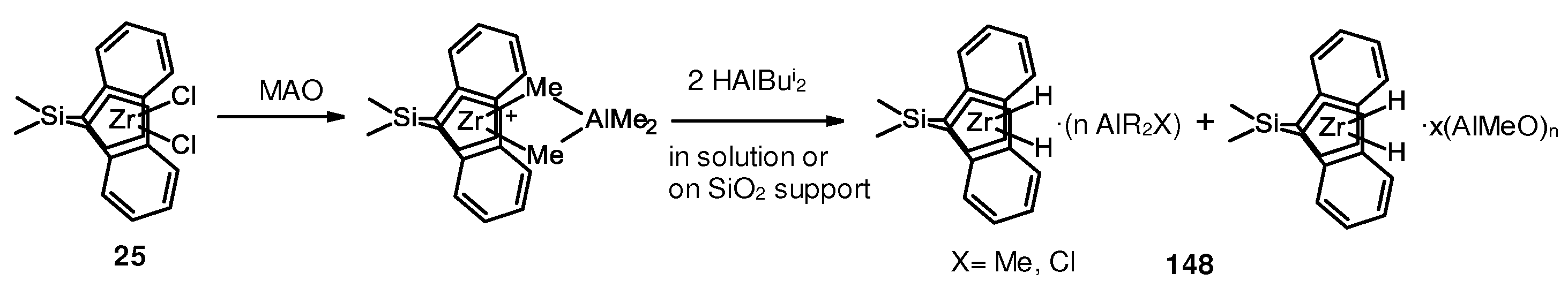
- González, R.; Morales, E.; García, M.; Revilla, J.; Charles, R.; Collins, S.; Cadenas, G.; Lugo, L.; Pérez, O. Heterogeneous Polymerization of Ethylene and 1-Hexene with Me3SiCp2ZrH3AlH2/SiO2 Activated with MAO. Macromol. Symp. 2009, 283–284, 96–102. [Google Scholar] [CrossRef]
- Comparán-Padilla, V.E.; Pérez-Berúmen, C.M.; Cadenas-Pliego, G.; Rodríguez-Hernández, M.T.; Collins, S.; Pérez-Camacho, O. Evaluation of catalyst leaching in silica supported zirconocene alumino hydride catalysts. Can. J. Chem. Eng. 2017, 95, 1124–1132. [Google Scholar] [CrossRef]
- Padilla-Gutiérrez, B.; Ventura-Hunter, C.; García-Zamora, M.; Collins, S.; Estrada-Ramírez, A.N.; Pérez-Camacho, O. Zirconocene Aluminohydride-Methylaluminoxane Clathrates for Ethylene Polymerization in Slurry. Macromol. Symp. 2017, 374, 1600139. [Google Scholar] [CrossRef]

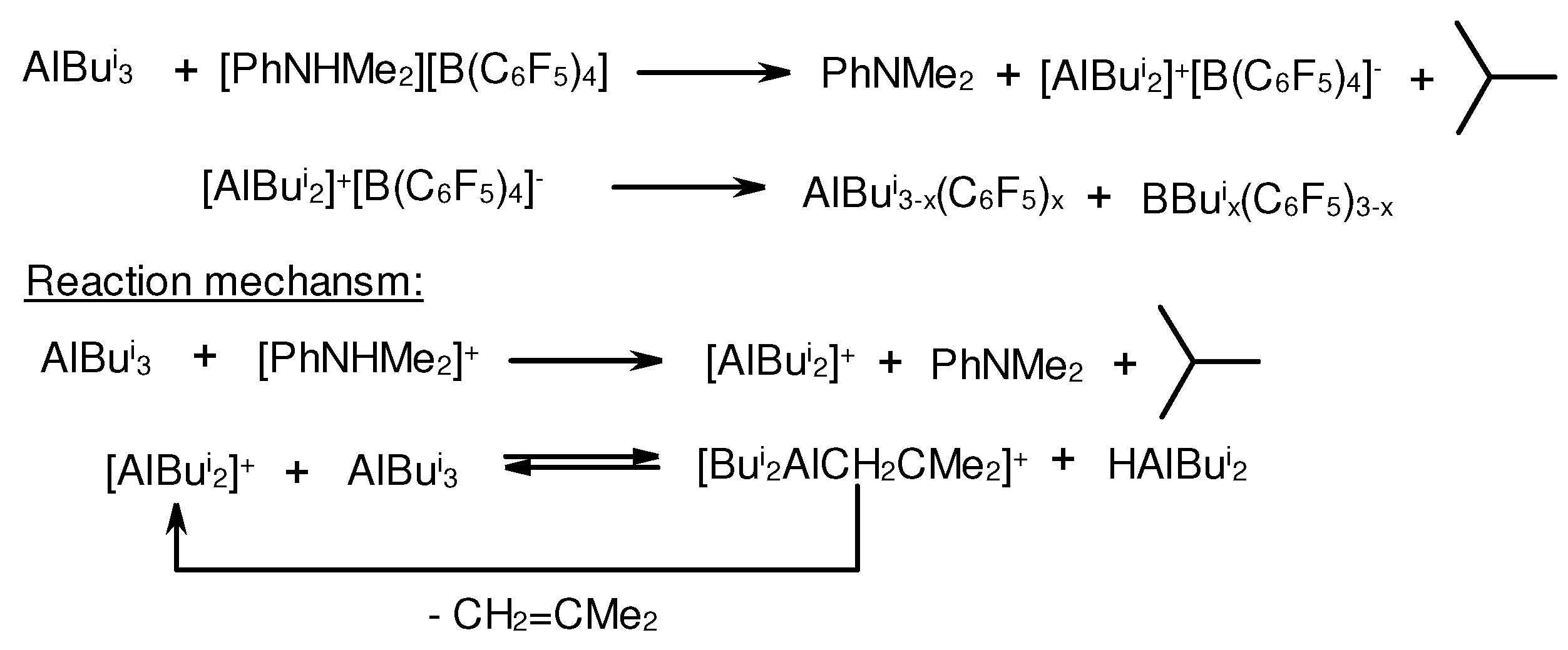

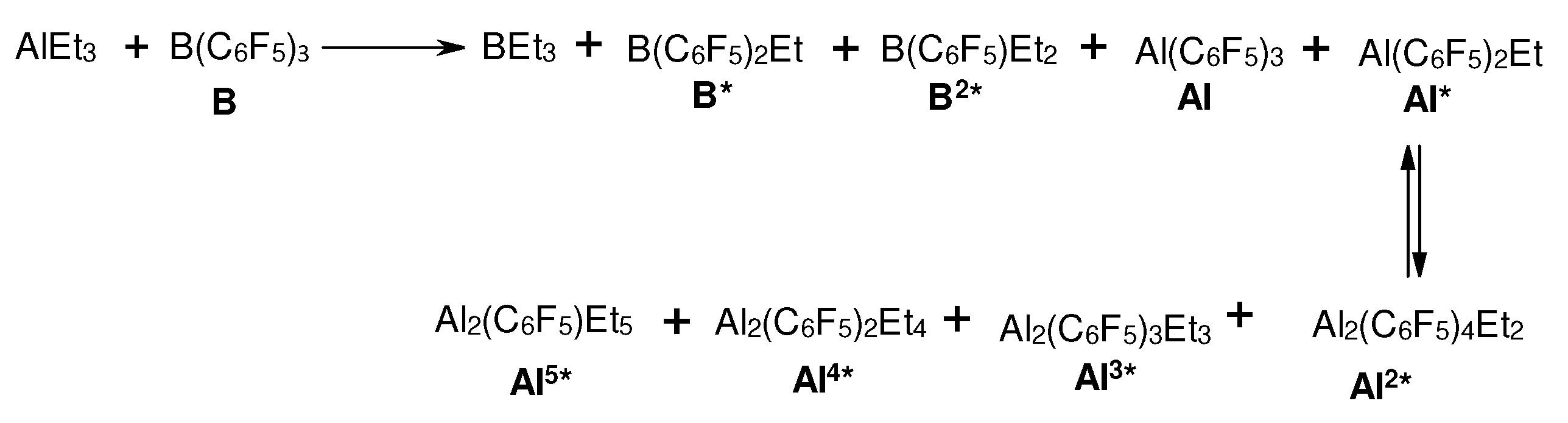
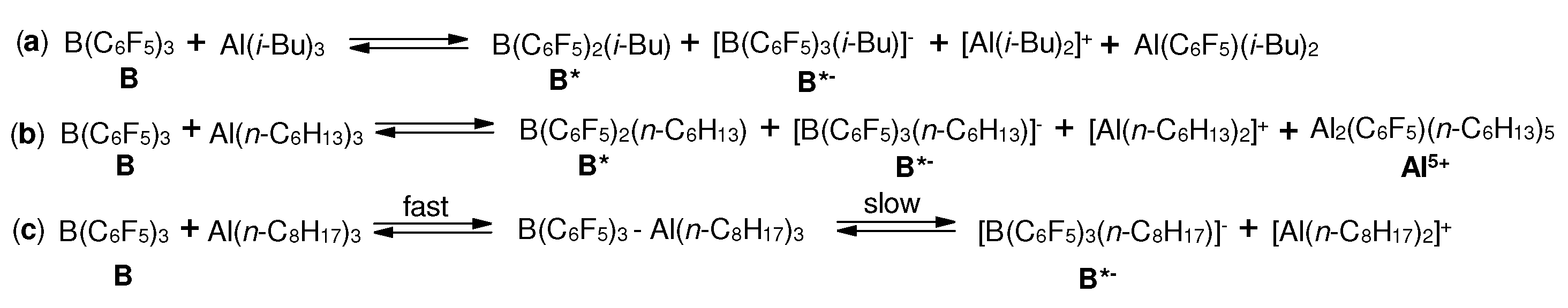
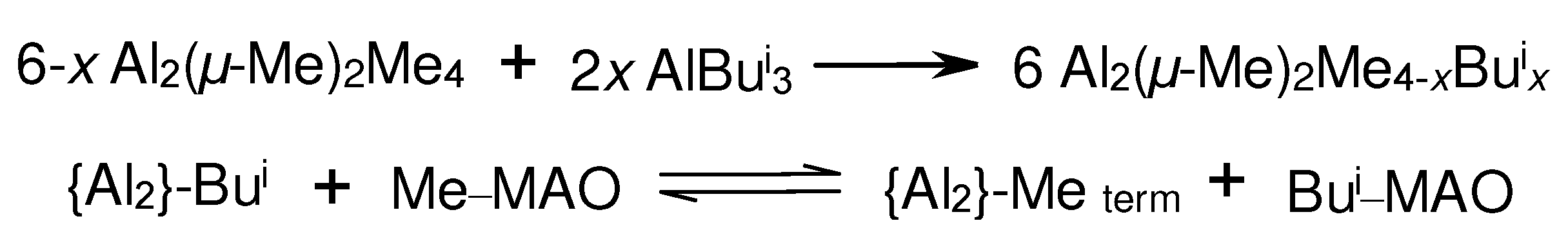
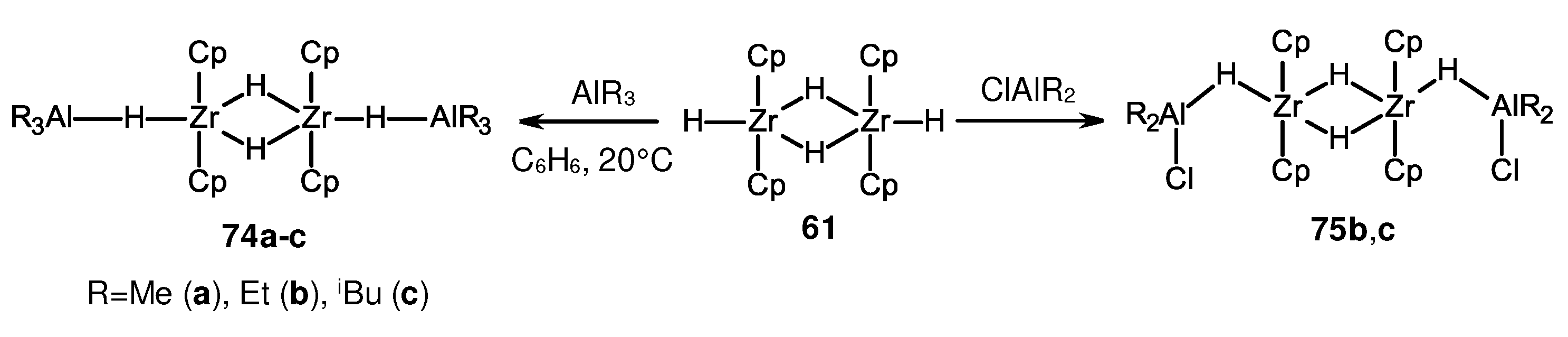
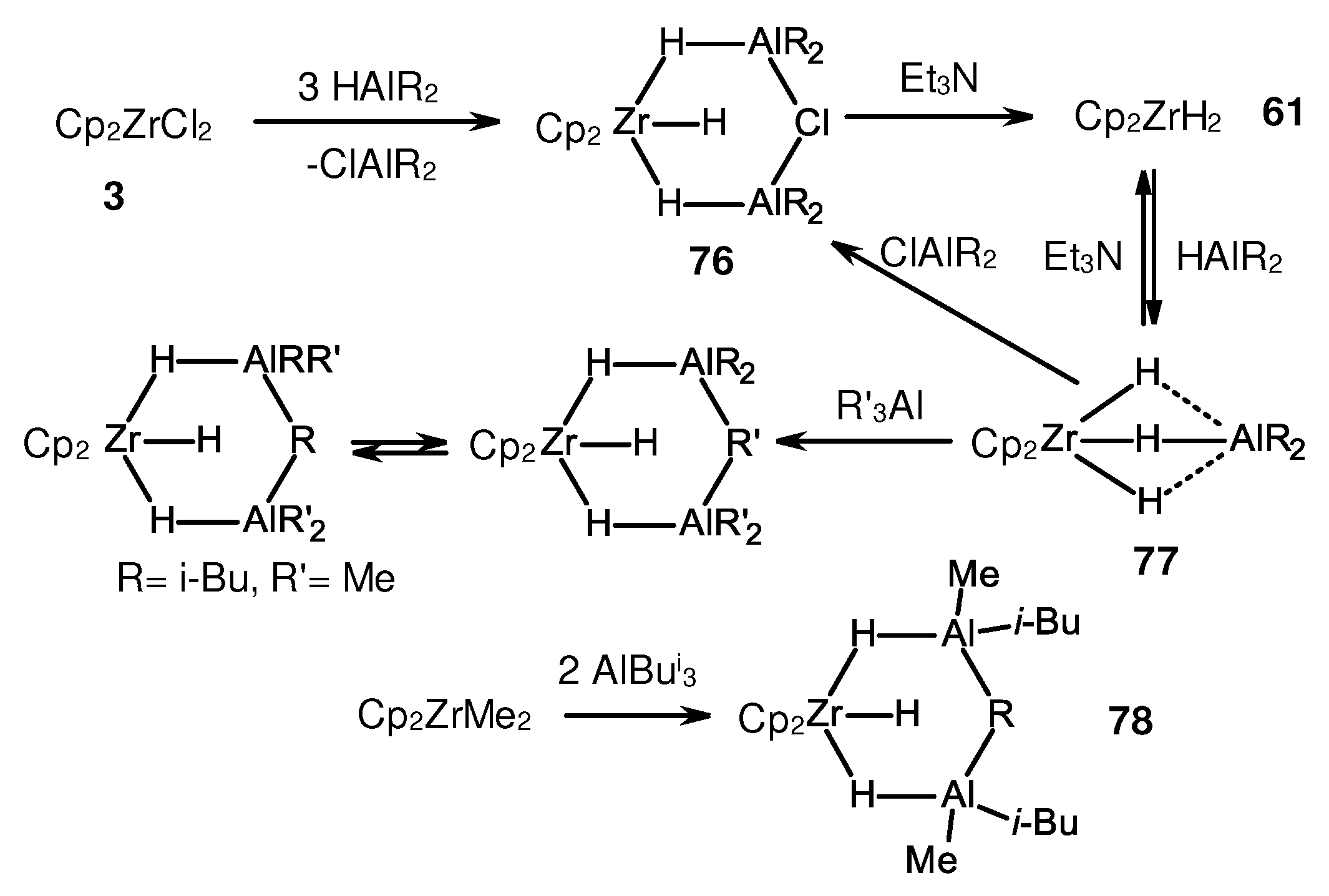
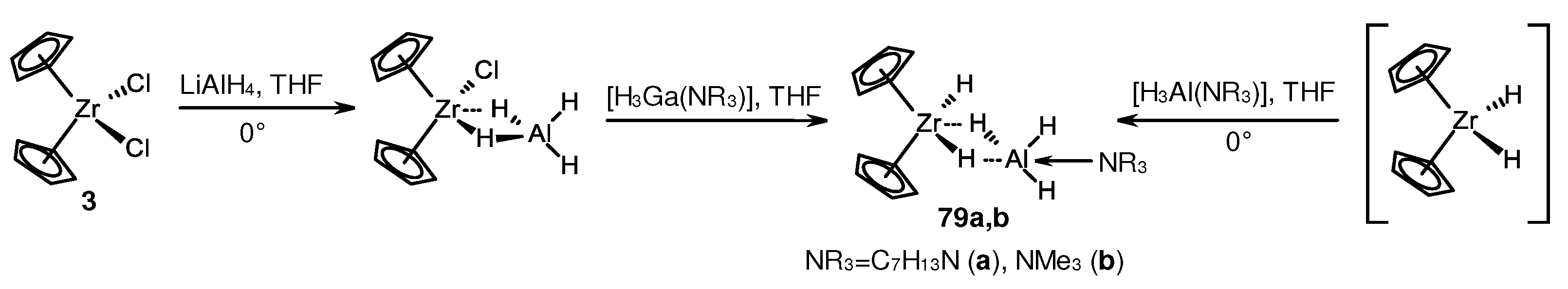
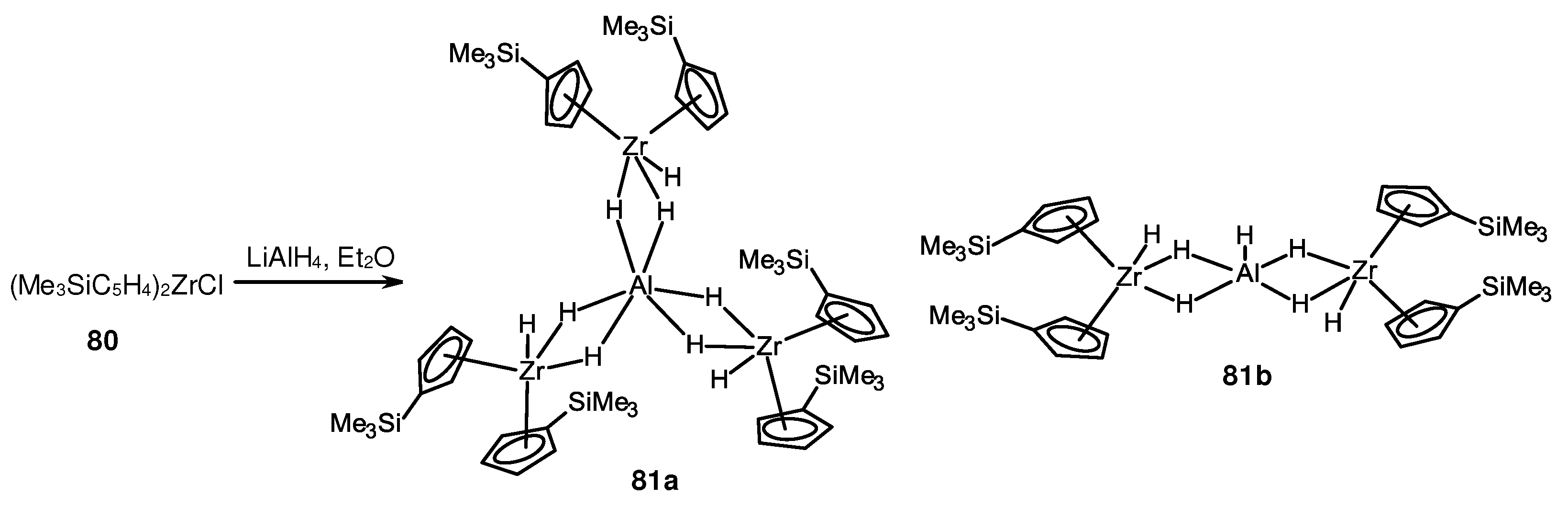
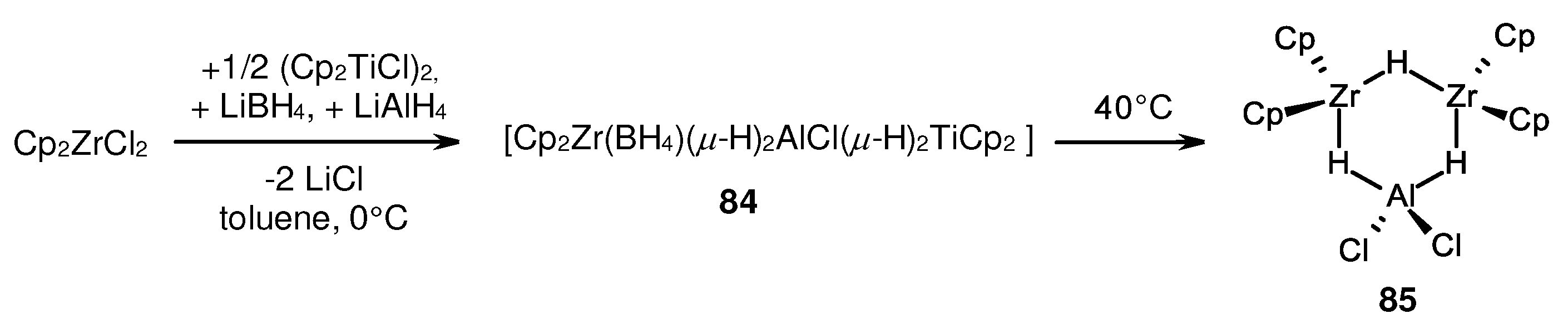
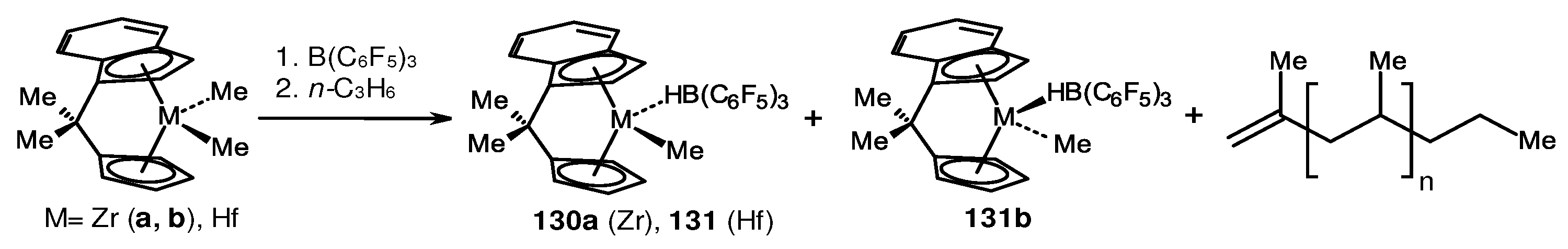
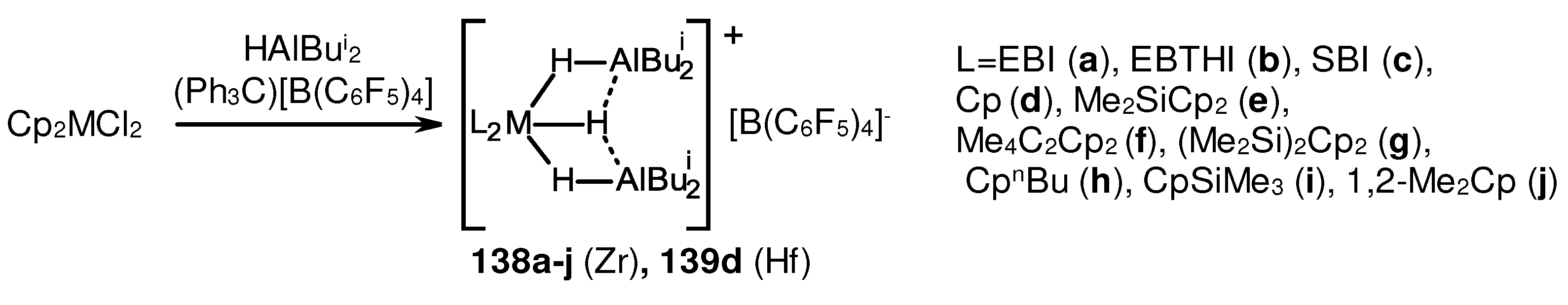
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).