Submitted:
02 December 2023
Posted:
04 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
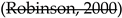 . These birds therefore demand a great deal of costs, such as feed, labour and importantly, treatment of disease.
. These birds therefore demand a great deal of costs, such as feed, labour and importantly, treatment of disease.2. Results
2.1. Questionnaire returns
2.2. Participants’ charactaeristics
2.2.1. Practice type
2.2.2. Associated veterinarians
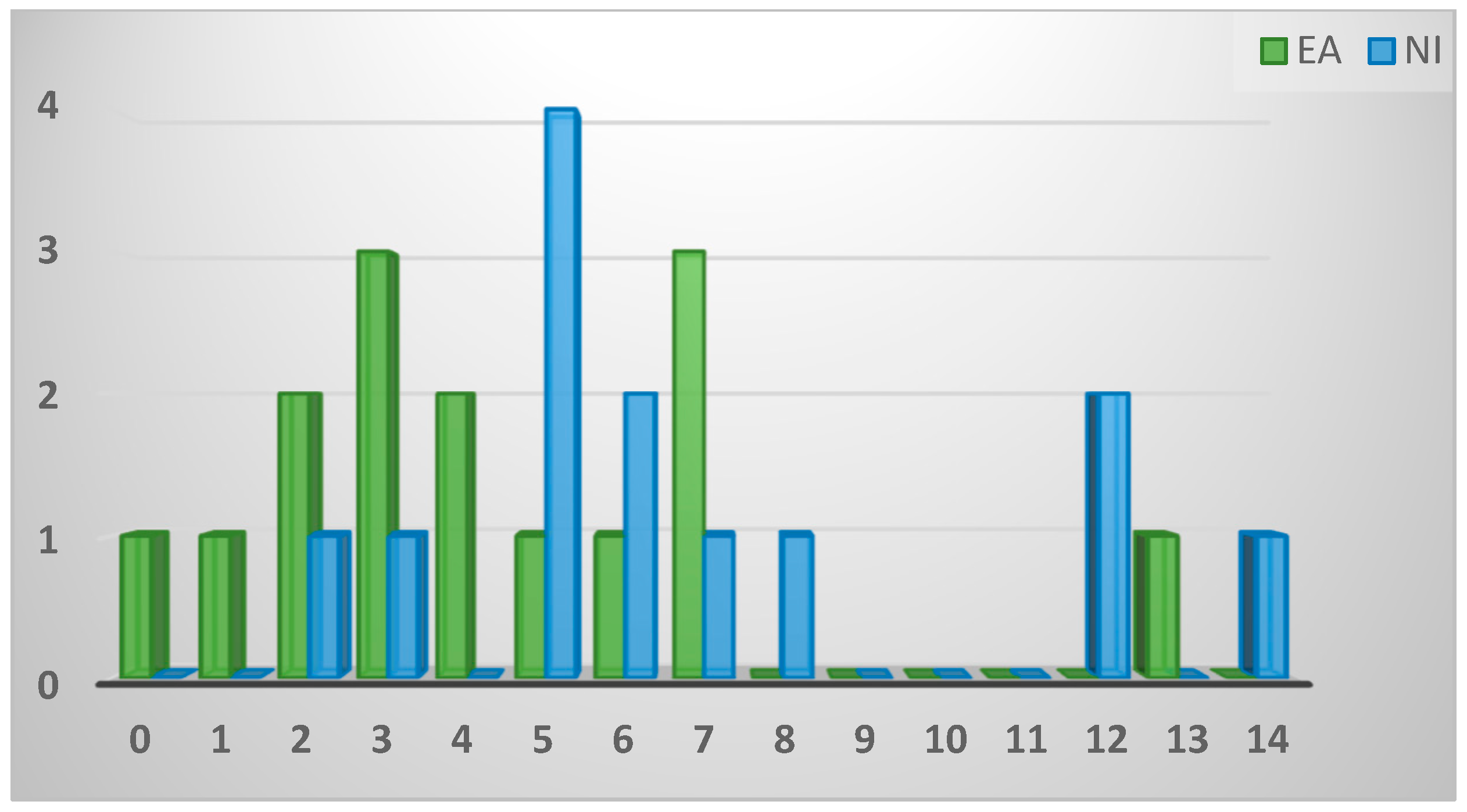
2.2.3. Game bird caseload
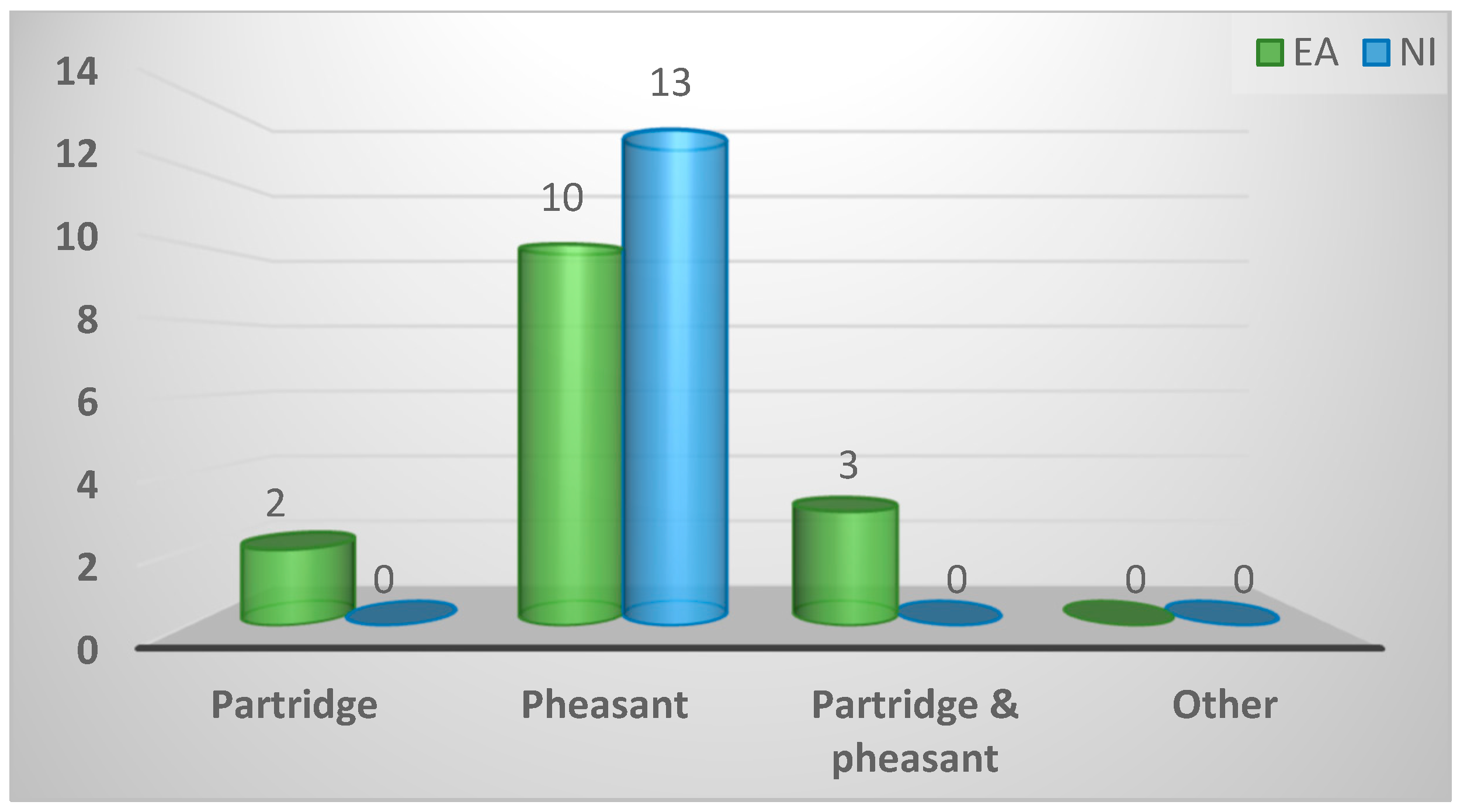
2.2.4. Most common breed of game birds
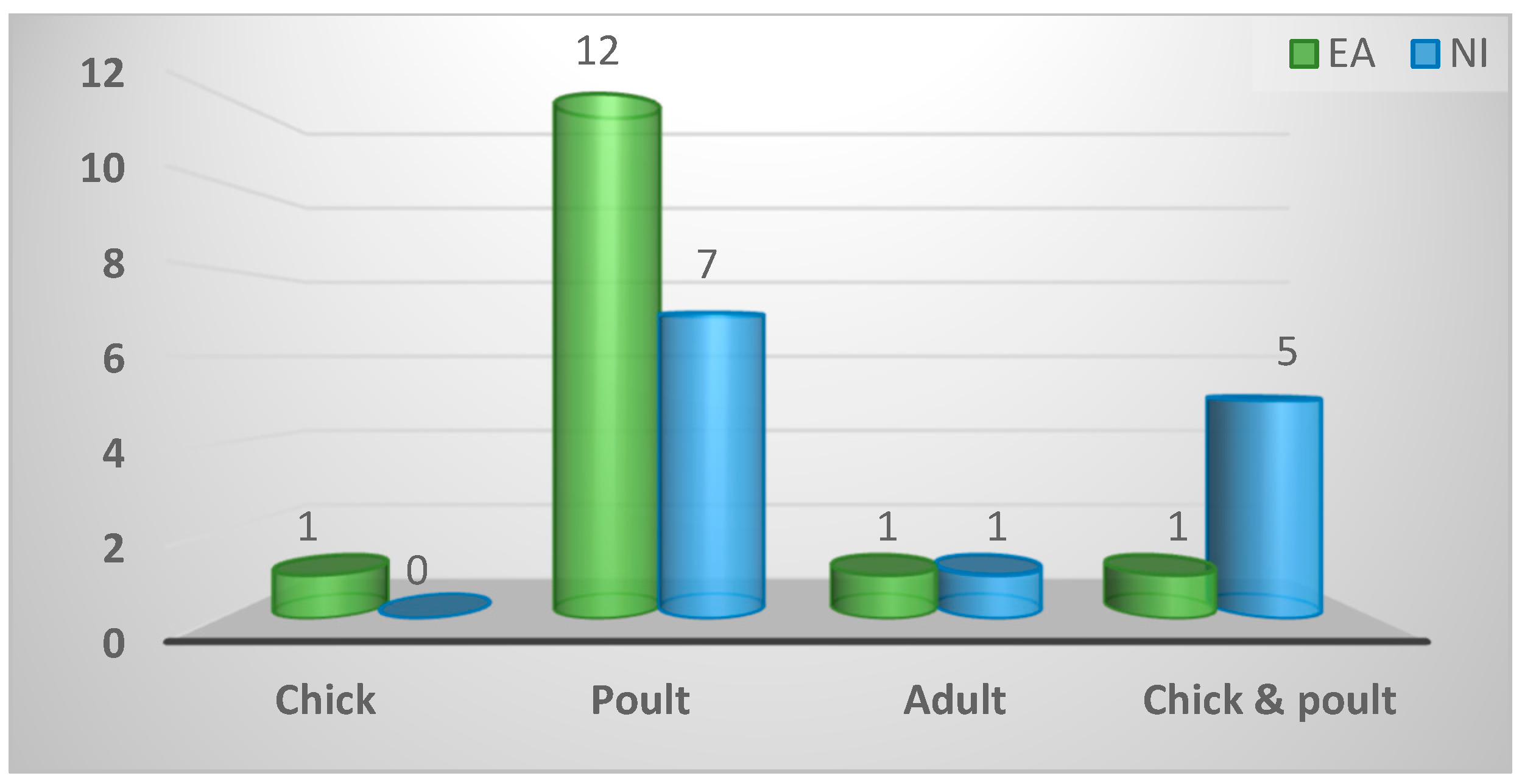
2.2.5. Most common age of game birds
2.3. Preliminary questions
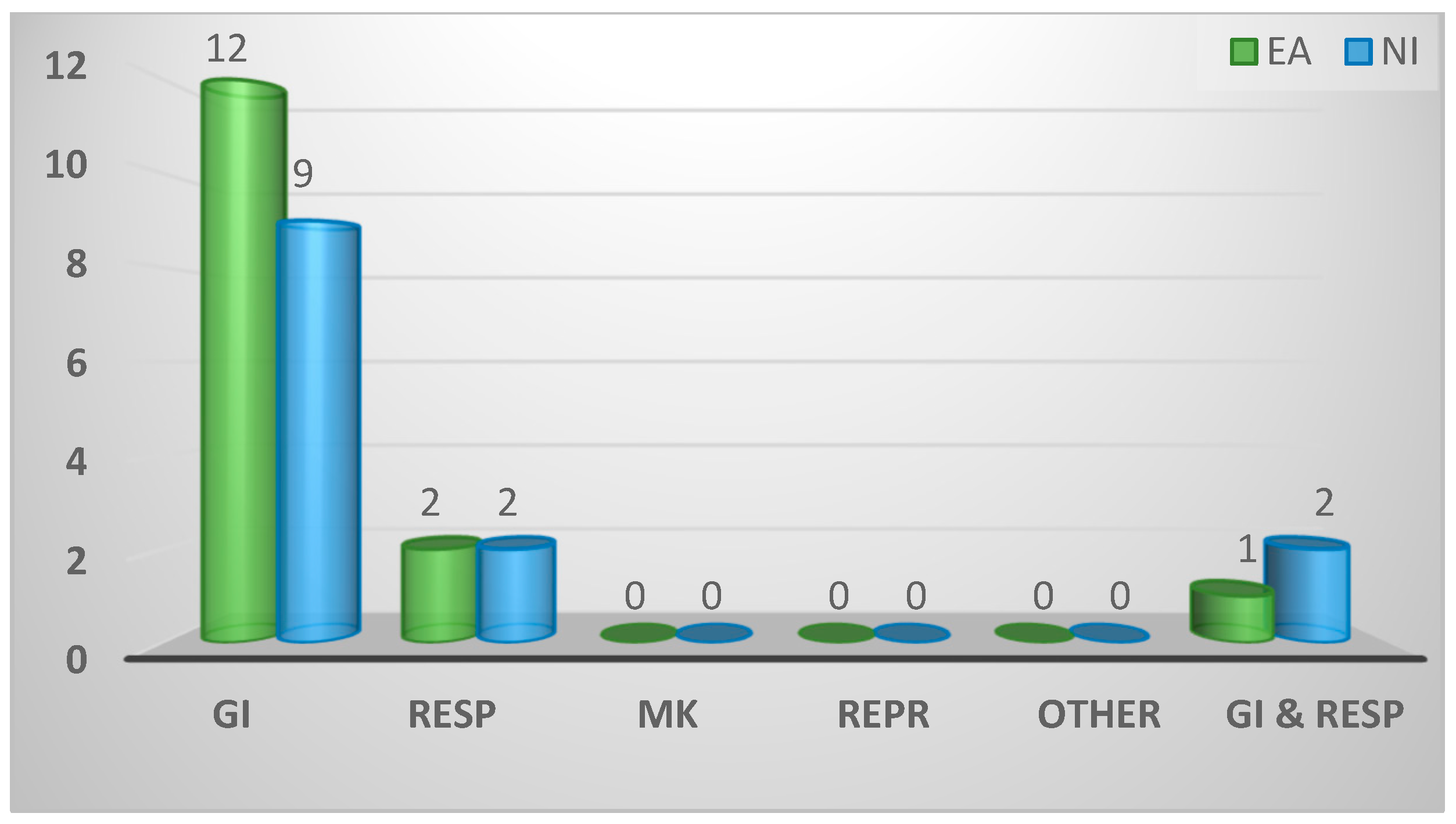
2.3.1. Bodily system most often treated/diagnosed
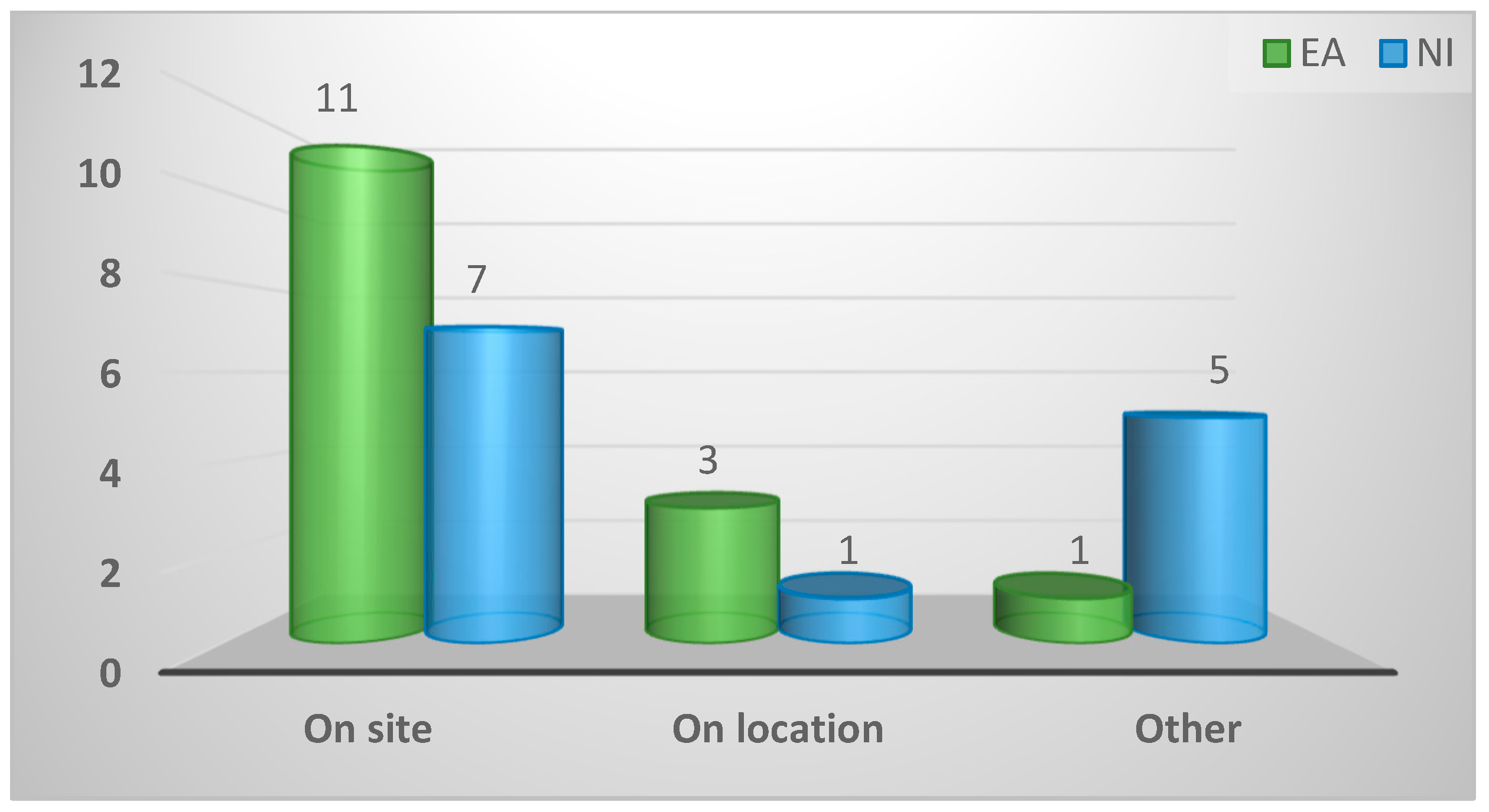
2.3.2. Location of diagnosis
2.3.3. Drug prescription
2.3.4. Treatment protocol
2.4. Importance and perception of enteric disease
2.4.1. Eagerness of game farmers to consult veterinarians
2.4.2. Enteric disease investigation
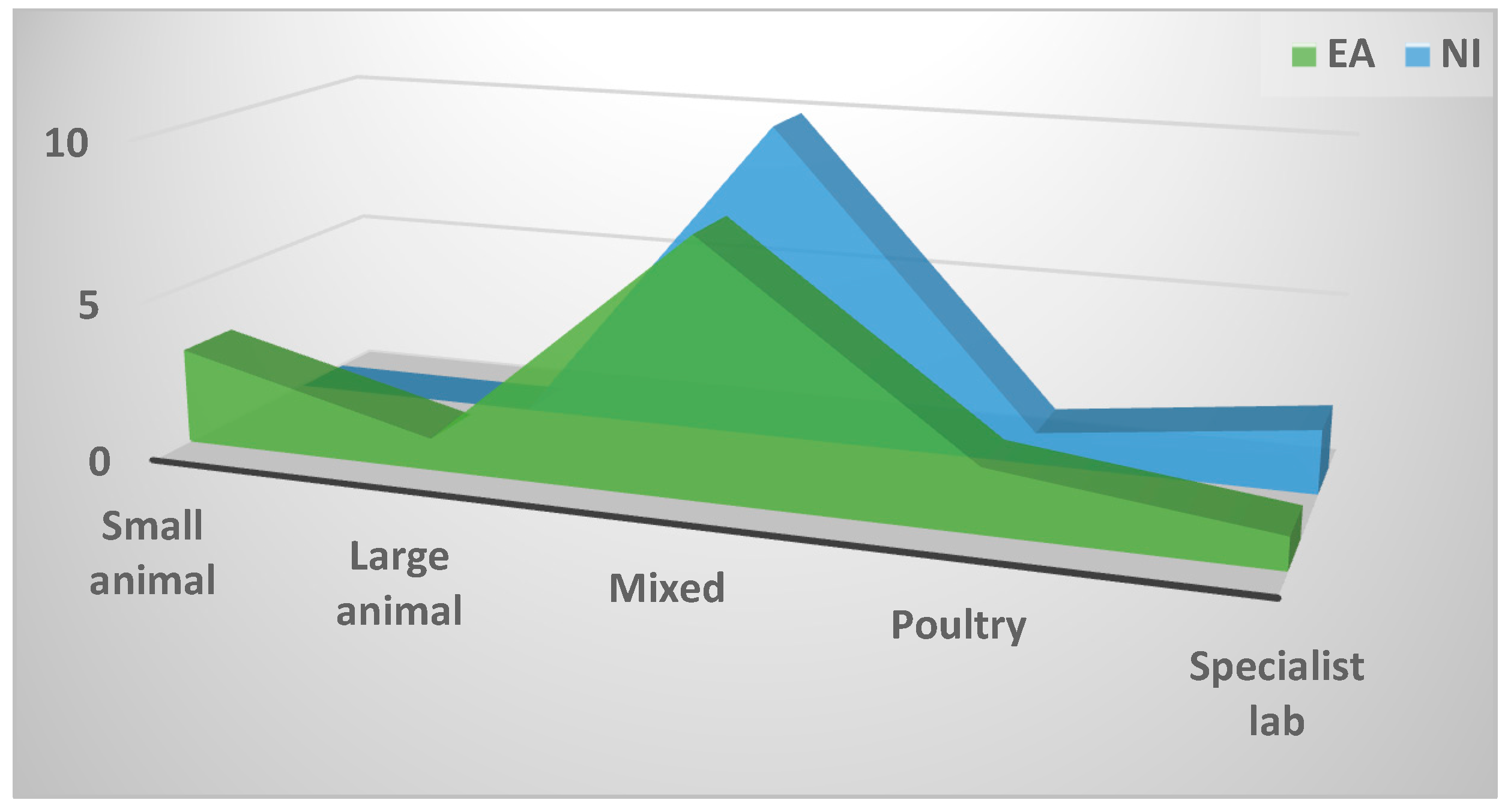
2.4.3. Importance assigned to enteric disease
2.4.4. Most common causative agent of enteric disease
2.4.5. Importance assigned to spironucleosis
2.4.6. Rearing stage(s) most closely associated with Spironucleus infection
2.5. Sample collection and adoption of existing methods
2.5.1. Selection of sample birds
2.5.2. Sample birds removed from flocks
2.5.3. Clinically infected flock
2.5.4. Method of euthanasia
2.5.5. Time between death and examination
2.5.6. Time limit for diagnosis of spironucleosis
2.6. Spironucleosis diagnosis questions
2.6.1. Clinical signs associated with spironucleosis
2.6.2. Post-mortem protocol for cases of enteric disease
2.6.3. Pathological changes visible on external examination
2.6.4. Pathological changes visible on internal examination
2.6.5. Sites used for spironucleosis diagnosis
2.6.6. Spironucleosis diagnosis
2.6.7. Positive result confirmation
2.6.8. Utilisation of faeces for spironucleosis diagnosis
2.6.9. Morphological appearance of Spironucleus
2.6.10. Spironucleus differentiation
2.6.11. Confirmation of infection in a bird
2.6.12. Confirmation of infection in a flock
2.6.13. Quantifying spironucleosis
2.6.14. Reliance on stated method
2.6.15. Knowledge of other methods
2.6.16. Reproducibility in general practice
2.6.17. Spironucleosis diagnosis in general practice
2.6.18. Differences between regions
3. Discussion
4. Materials and Methods
4.1. Sampling and recruitment
4.2. Approach
4.3. Strategy of data collection
3.4. Data recording and coding
3.5. Data analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Lloyd, S.; Irvine, K.L.; Eves, S.M.; Gibson, J.S. Fluid absorption in the small intestine of healthy game birds and those infected with Spironucleus spp. Avian Pathol 2005, 34, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Lister, S. Disease of Game Birds. In Practice 1989, 11, 170–174. [Google Scholar] [CrossRef]
- Swarbrick, O. Hexamitiasis and an emaciation syndrome in pheasant poults: clinical aspects and differential diagnosis. Vet Rec 1990, 126, 265–267. [Google Scholar]
- Pennycott, T.W. Carriage of trichomonads, Hexamita species and Blastocystis species by adult pheasants. Vet Rec 1998, 143, 142–143. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.L.; Charlton, B.R.; Bickford, A.A.; Nordhausen, R. Hexamita meleagridis (Spironucleus meleagridis) infection in chukar partridges associated with high mortality and intracellular trophozoites. Avian Dis 2004, 48, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, S.; Gibson, J.S. Haematology and biochemistry in healthy young pheasants and red-legged partridges and effects of spironucleosis on these parameters. Avian Pathol 2006, 35, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Welchman, D. Avian Practice: Diseases in young pheasants. In Practice 2008, 30, 86–89. [Google Scholar] [CrossRef]
- Swarbrick, O. Pheasant rearing: associated husbandry and disease problems. Vet Rec 1985, 116, 610–617. [Google Scholar] [CrossRef]
- Wood, A.M.; Smith, H.V. Spironucleosis (Hexamitiasis, Hexamitosis) in the ring-necked pheasant (Phasianus colchicus): detection of cysts and description of Spironucleus meleagridis in stained smears. Avian Dis 2005, 49, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Harper, F.D. Hexamita species present in some avian species in South Wales. Vet Rec 1991, 128, 130. [Google Scholar] [CrossRef]
- Stoikov, D. Detection of Hexamita meleagridis in Bulgaria. Vet Med Nauki. 1985, 22, 44–50. [Google Scholar] [PubMed]
- Trees, A.J. Parasitic conditions in poultry 1: Protozoal diseases. In Practice 1987, 9, 86–89. [Google Scholar] [CrossRef]
- Dezfoulian, O.; Gharagozlou, M.J.; Rahbari, S. Hexamita infection associated with diarrhoea and stunting in native turkey poults. Trop Biomed 2010, 27, 504–508. [Google Scholar] [PubMed]
- Hussain, A.Z. Morphometric evaluation of the small intestines and caeca of pheasants infected with Hexamita and Trichomonas species. Vet Rec 2001, 148, 484–485. [Google Scholar] [CrossRef]
- Pennycott, T.W. Effect of four therapeutic agents on Trichomonas phasiani carriage in pheasants. Vet Rec 1996, 139, 214–215. [Google Scholar] [CrossRef] [PubMed]
- McNeil, E.H.; Hinshaw, W.R.; Kofoid, C.A. Hexamita meleagridis sp nov from the turkey. American Journal of Hygiene 1941, 34, 71–82. [Google Scholar]
- Zwart, P.; Hooimeijer, J. Hexamitiasis in carrier pigeons in the Netherlands. Tijdschr Diergeneeskd 1985, 110, 1074–1075. [Google Scholar] [PubMed]
| Variable | Total (n = 28) No. (%) |
EA (n = 15) No. (%) |
NI (n = 13) No. (%) |
P value |
|---|---|---|---|---|
| Type of practice | ||||
| Mixed practice | 18 (64%) | 8 (53%) | 10 (77%) | 0.43 |
| Small animal | 3 (11%) | 3 (20%) | 0 (0%) | |
| Large animal | 1 (4%) | 1 (7%) | 0 (0%) | |
| Poultry | 3 (11%) | 2 (13%) | 1 (8%) | |
| Laboratory | 3 (11%) | 1 (7%) | 2 (15%) | |
| No. of veterinarians per practice | ||||
| <7 | 19 (68%) | 11 (73%) | 8 (62%) | 0.7 |
| ≥7 | 9 (32%) | 4 (27%) | 5 (38%) | |
| Gamebird caseload | ||||
| <5 | 26 (93%) | 14 (93%) | 12 (92%) | 1.0 |
| 5-25 | 2 (7%) | 1 (7%) | 1 (8%) | |
| >25 | 0 (0%) | 0 (0%) | 0 (0%) | |
| Most common breed of game birds seen | ||||
| Pheasant | 23 (82%) | 10 (67%) | 13 (100%) | 0.07 |
| Partridge | 3 (11%) | 3 (20%) | 0 (0%) | |
| Partridge and pheasant | 2 (7%) | 2 (13%) | 0 (0%) | |
| Most common age of game birds seen | ||||
| Poult | 19 (68%) | 12 (80%) | 7 (54%) | 0.13 |
| Chick and poult | 6 (21%) | 1 (7%) | 5 (38%) | |
| Chick | 1 (4%) | 1 (7%) | 0 (0%) | |
| Adult | 2 (7%) | 1 (7%) | 1 (8%) | |
| Variables | Total (n = 28) N (%) |
EA (n = 15) N (%) |
NI (n = 13) N (%) |
P value |
|---|---|---|---|---|
| Bodily system of game birds most frequently diagnosed | ||||
| Gastrointestinal | 21 (75%) | 12 (80%) | 9 (69%) | 0.8 |
| Respiratory | 4 (14%) | 2 (13%) | 2 (15%) | |
| Gastrointestinal and respiratory | 3 (11%) | 1 (7%) | 2 (15%) | |
| Musculoskeletal | 0 (0%) | 0 (0%) | 0 (0%) | |
| Reproductive | 0 (0%) | 0 (0%) | 0 (0%) | |
| Location of diagnosis of disorders in game birds | ||||
| On site (Practice) | 18 (64%) | 11 (73%) | 7 (54%) | 0.16 |
| On location (Game farm) | 4 (14%) | 3 (20%) | 1 (8%) | |
| Other | 6 (21%) | 1 (7%) | 5 (38%) | |
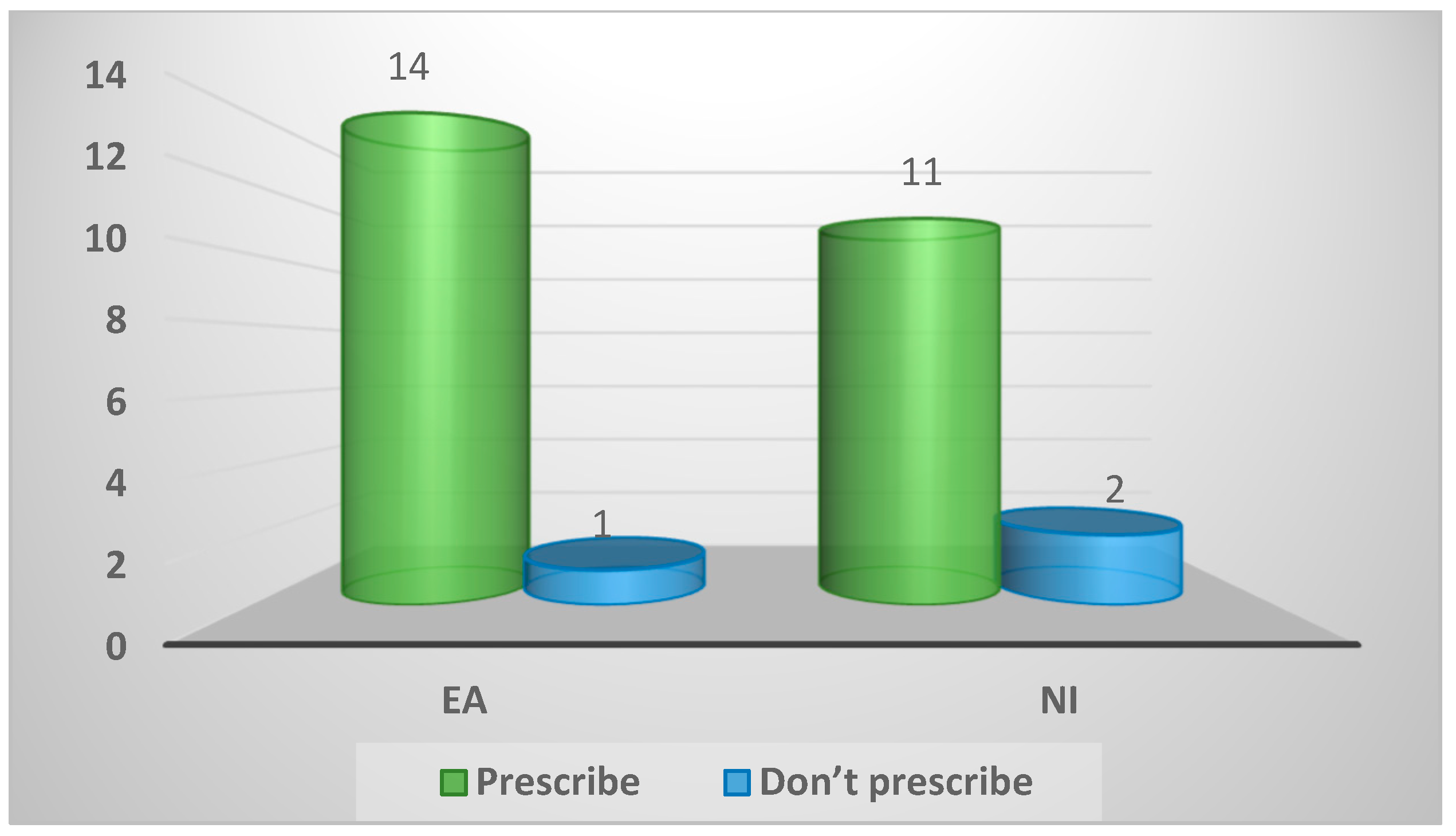
| Practice position on prescribing drugs to game birds | ||||
| Prescribe | 25 (89%) | 14 (93%) | 11 (85%) | 0.58 |
| Do not prescribe | 3 (11%) | 1 (7%) | 2 (15%) | |
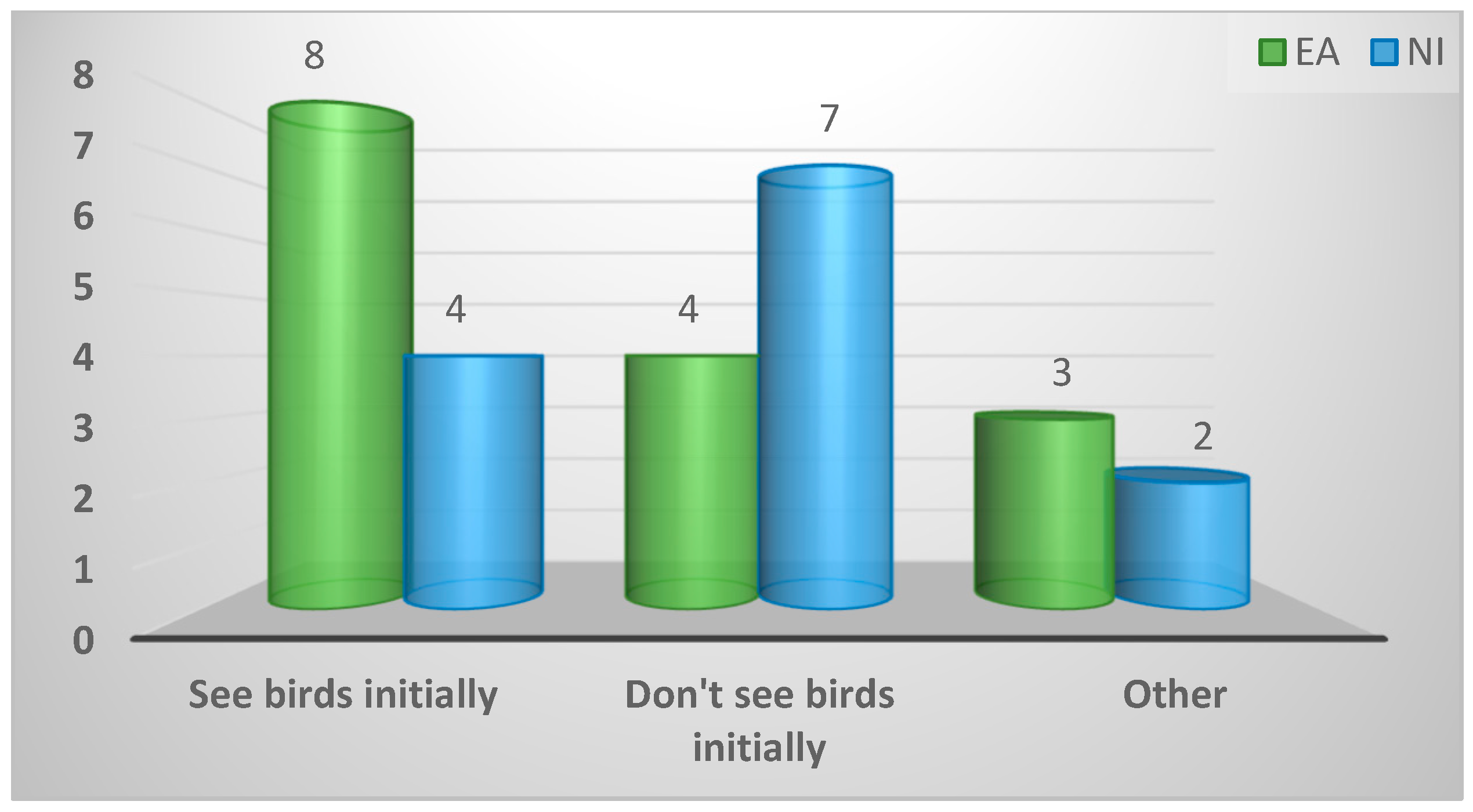
| Prescription protocol on seeing affected birds prior to treatment | ||||
| See birds initially | 12 (43%) | 8 (53%) | 4 (31%) | 0.37 |
| Do not see birds initially | 11 (39%) | 4 (27%) | 7 (54%) | |
| Other | 5 (18%) | 3 (20%) | 2 (16%) | |
| Variables | Total (n = 28) N (%) |
EA (n = 15) N (%) |
NI (n = 13) N (%) |
P value |
|---|---|---|---|---|
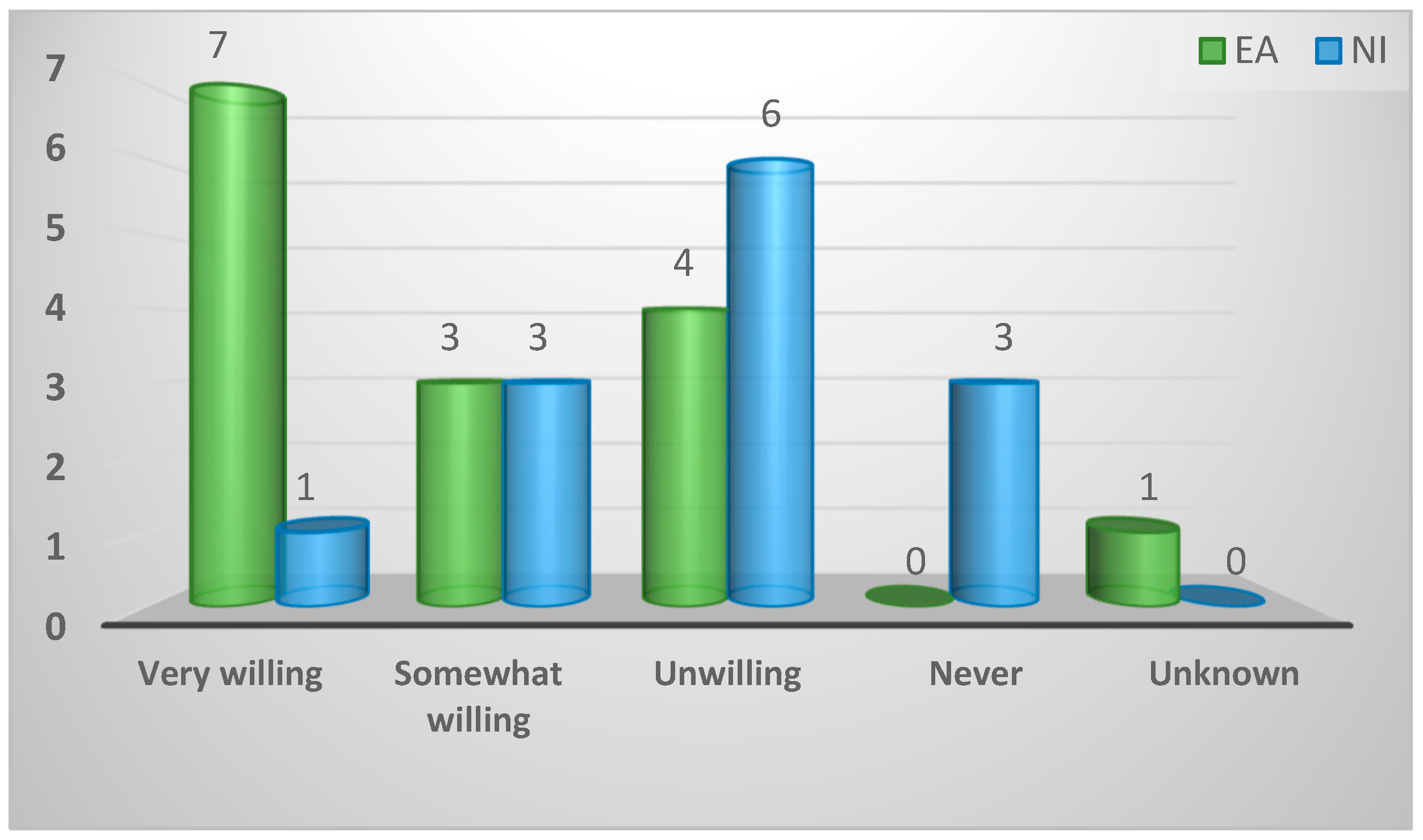
| Eagerness of gamekeepers to consult vet | ||||
| Very Willing | 8 (29%) | 7 (47%) | 1 (8%) | 0.04 |
| Somewhat Willing | 6 (21%) | 3 (20%) | 3 (23%) | |
| Unwilling | 10 (36%) | 4 (27%) | 6 (46%) | |
| Never | 3 (11%) | 0 (0%) | 3 (23%) | |
| Unknown | 1 (4%) | 1 (7%) | 0 (0%) | |
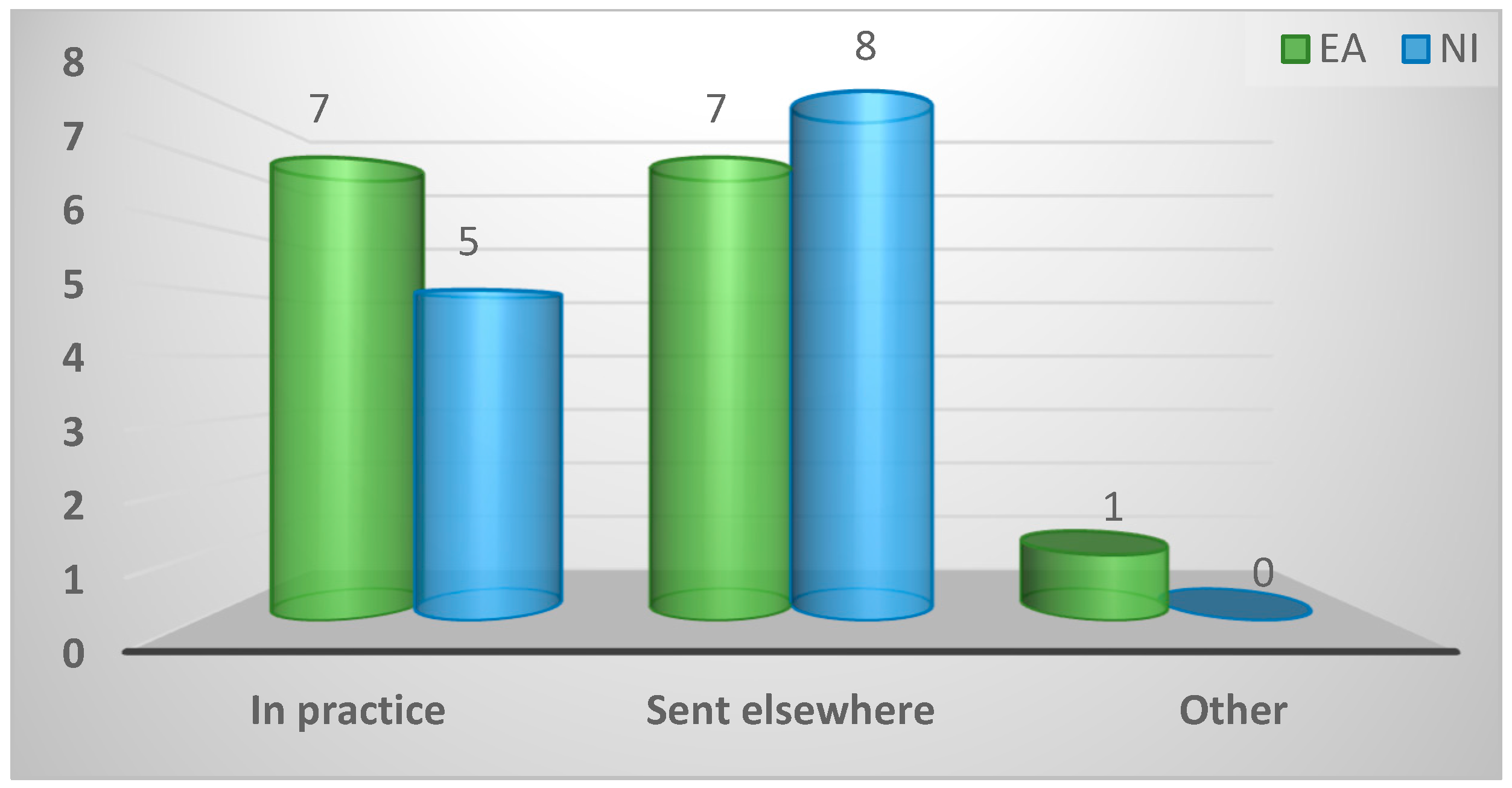
| Location of diagnosis of enteric disease | ||||
| In practice | 12 (43%) | 7 (47%) | 5 (38%) | 0.84 |
| Sent elsewhere | 15 (54%) | 7 (47%) | 8 (62%) | |
| Other | 1 (4%) | 1 (7%) | 0 (0%) | |
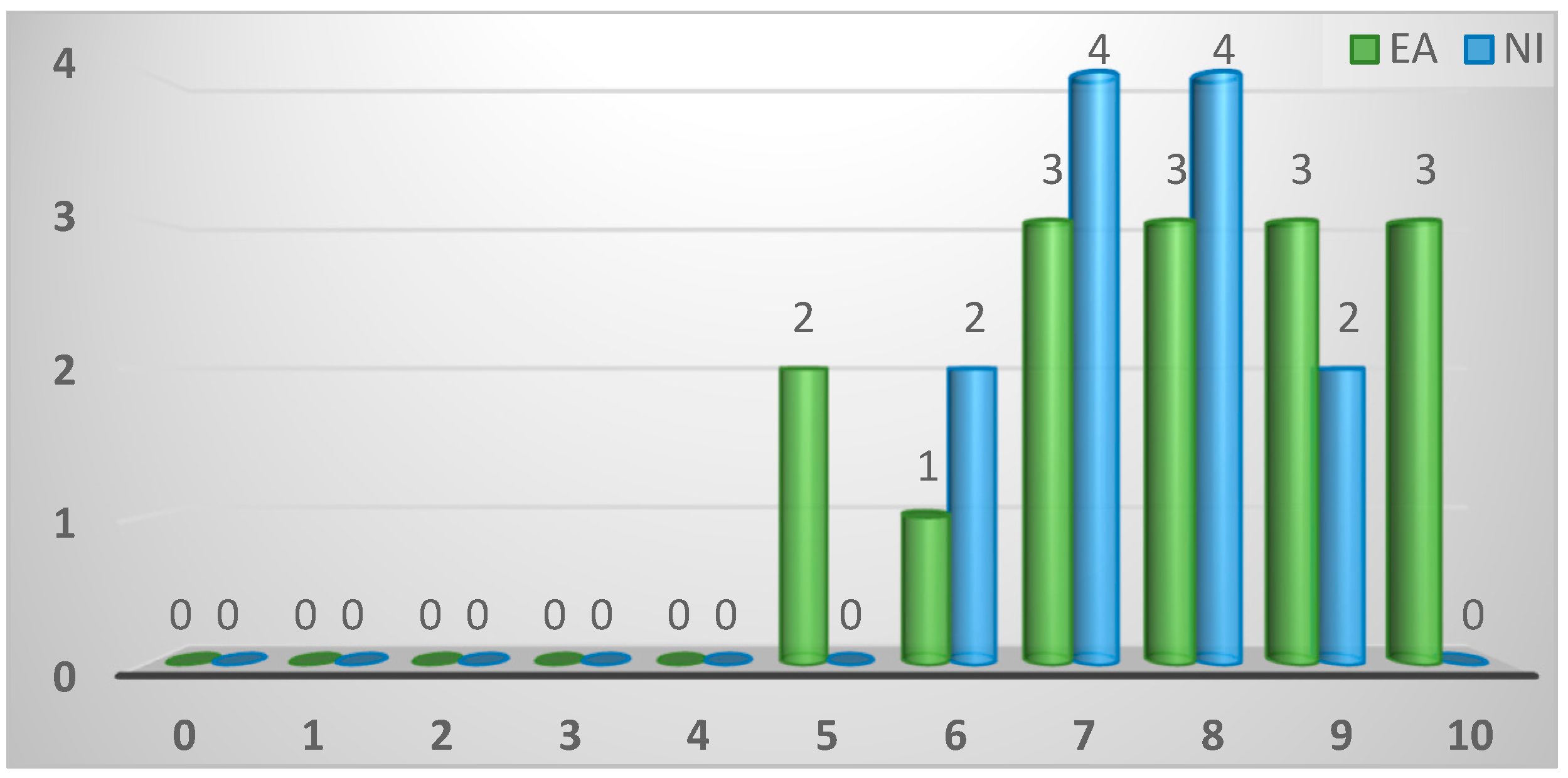
| Importance assigned to enteric disease of gamebirds (1-10) | ||||
| ≤4 | 0 (0%) | 0 (0%) | 0 (0%) | 0.45 |
| 5 | 2 (7%) | 2 (13%) | 0 (0%) | |
| 6 | 3 (11%) | 1 (7%) | 2 (15%) | |
| 7 | 7 (25%) | 3 (20%) | 4 (31%) | |
| 8 | 7 (25%) | 3 (20%) | 4 (31%) | |
| 9 | 5 (18%) | 3 (20%) | 2 (15%) | |
| 10 | 3 (11%) | 3 (20%) | 0 (0%) | |
| Missing | 1 (4%) | 0 (0%) | 1 (4%) | |
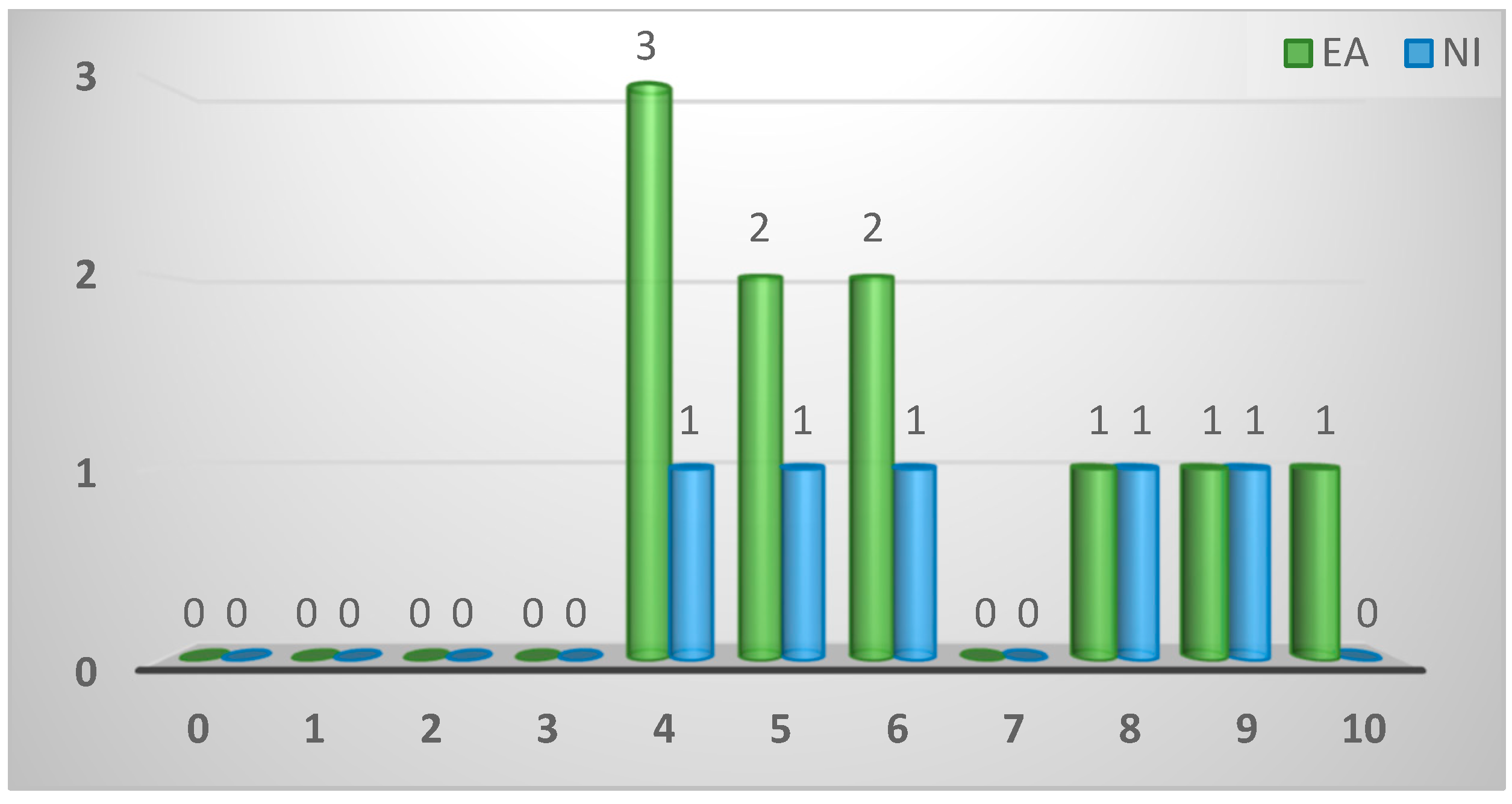
| Importance assigned to spironucleosis (1-10) | ||||
| ≤3 | 0 (0%) | 0 (0%) | 0 (0%) | 1.0 |
| 4 | 4 (27%) | 3 (30%) | 1 (20%) | |
| 5 | 3 (20%) | 2 (20%) | 1 (20%) | |
| 6 | 3 (20%) | 2 (20%) | 1 (20%) | |
| 7 | 0 (0%) | 0 (0%) | 0 (0%) | |
| 8 | 2 (13%) | 1 (10%) | 1 (20%) | |
| 9 | 2 (13%) | 1 (10%) | 1 (20%) | |
| 10 | 1 (7%) | 1 (10%) | 0 (0%) | |
| Variables |
Total (n = 28) n (%) |
EA (n = 15) n (%) |
NI (n = 13) n (%) |
P Value |
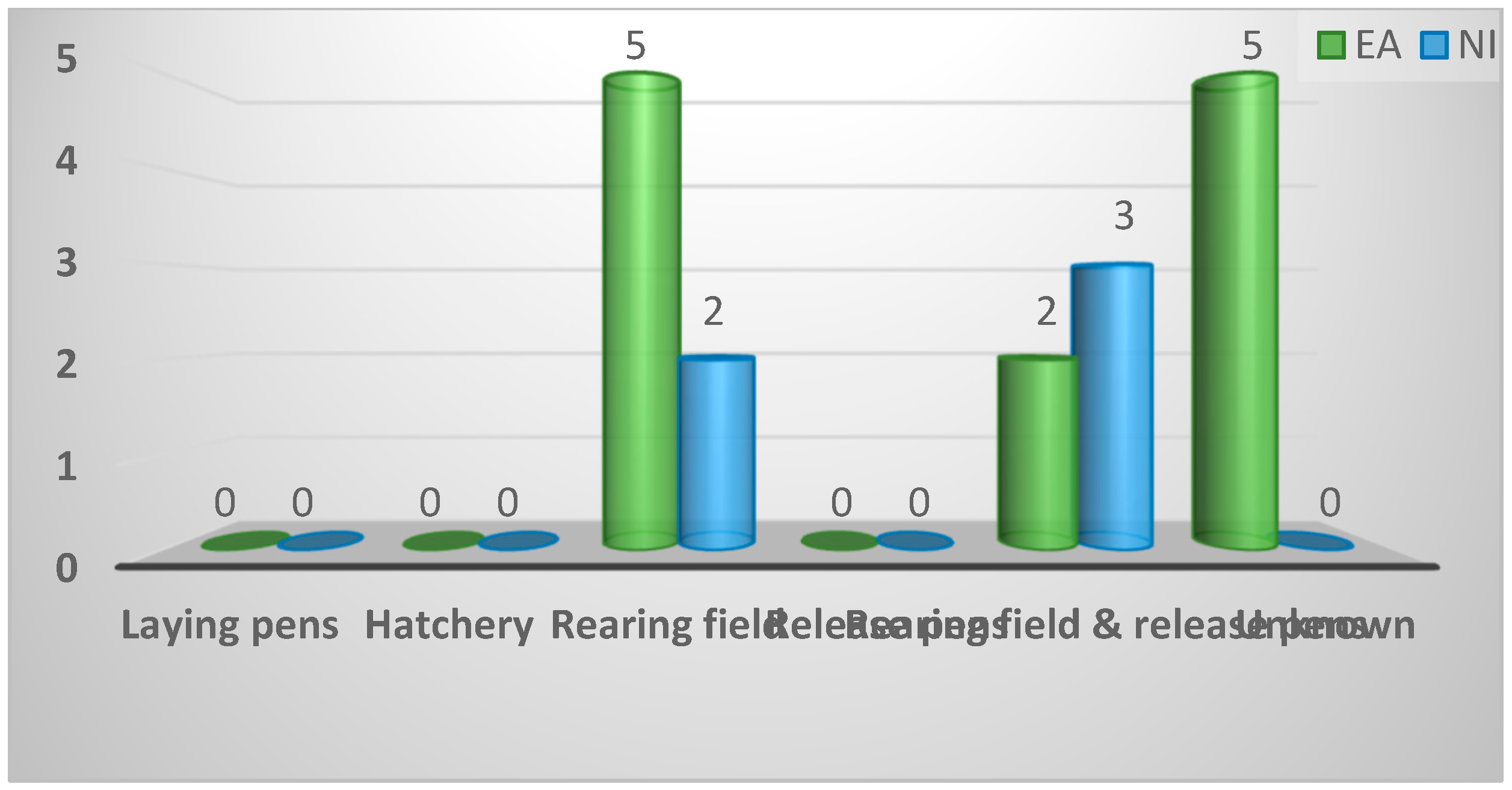
| Rearing stage(s) most closely associated with spironucleosis | ||||
| Rearing field | 7 (41%) | 5 (42%) | 2 (40%) | 0.18 |
| Rearing field and release pens | 5 (29%) | 2 (17%) | 3 (60%) | |
| Release pens | 0 (0%) | 0 (0%) | 0 (0%) | |
| Laying pens | 0 (0%) | 0 (0%) | 0 (0%) | |
| Hatchery | 0 (0%) | 0 (0%) | 0 (0%) | |
| Unknown | 5 (29%) | 5 (42%) | 0 (0%) | |
| Variables |
Total (n = 33) N (%) |
EA (n = 19) N (%) |
NI (n = 14) N (%) |
P value |
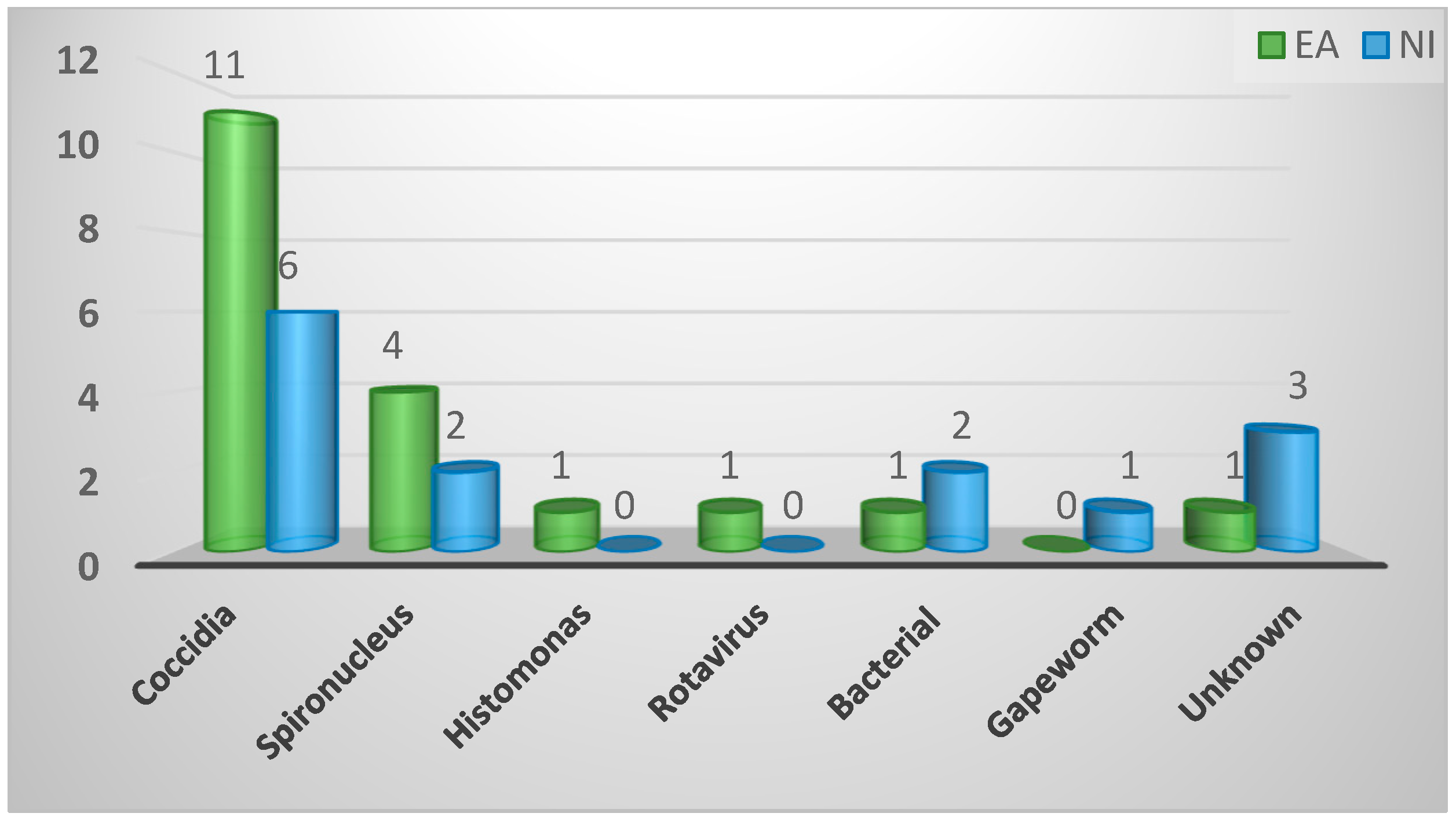
| Most commonly diagnosed causative agent of enteric disease | ||||
| Coccidia | 17 (52%) | 11 (58%) | 6 (43%) | 0.53 |
| Spironucleus | 6 (18%) | 4 (21%) | 2 (14%) | |
| Bacterial | 3 (9%) | 1 (5%) | 2 (14%) | |
| Gapeworm | 1 (3%) | 0 (0%) | 1 (7%) | |
| Histomonas | 1 (3%) | 1 (5%) | 0 (0%) | |
| Rotavirus | 1 (3%) | 1 (5%) | 0 (0%) | |
| Unknown | 4 (12%) | 1 (5%) | 3 (21%) | |
| Variables | Total (n = 13) No. (%) |
EA (n = 9) No. (%) |
NI (n = 4) No. (%) |
P value |
|---|---|---|---|---|
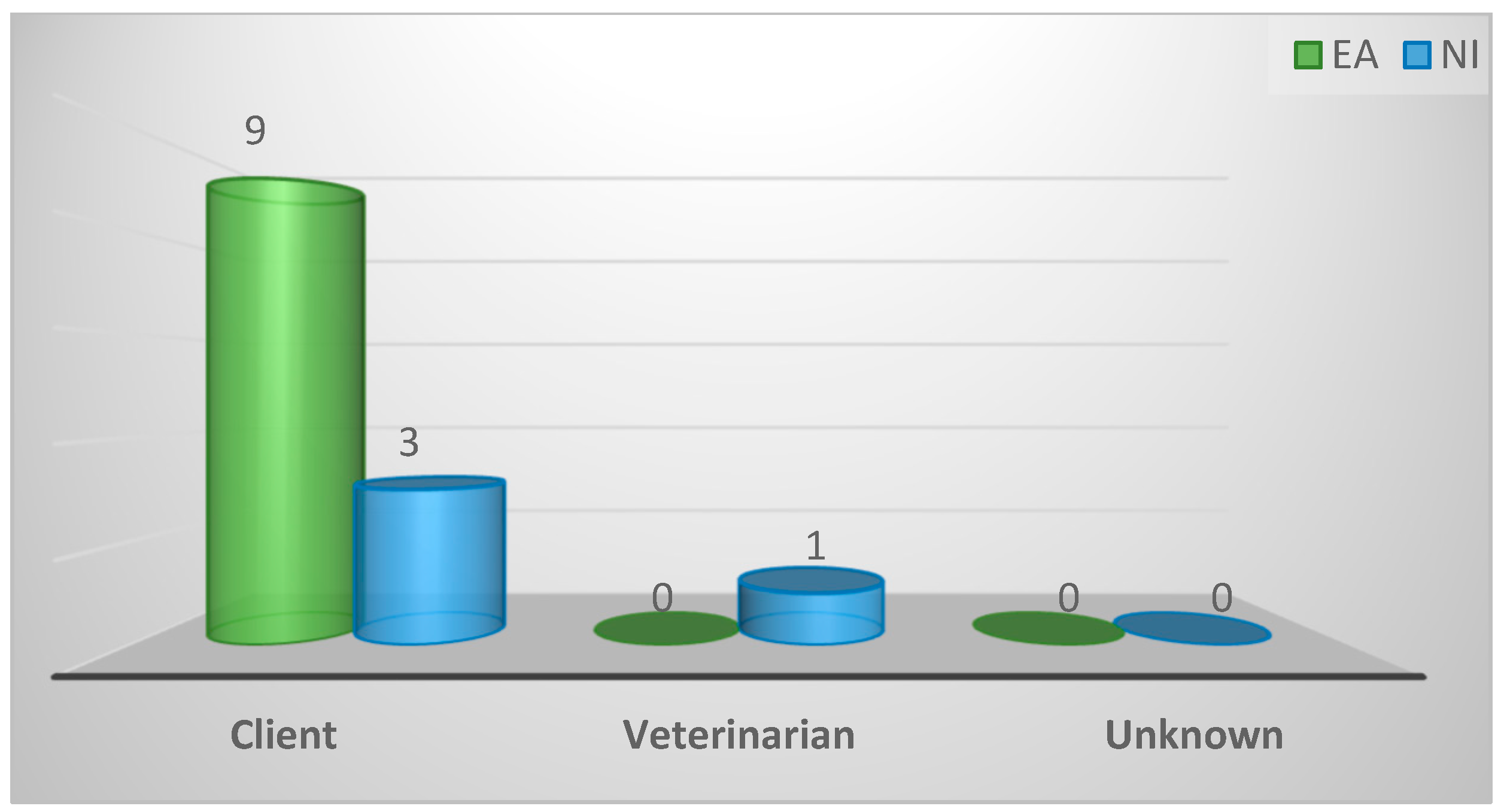
| Stakeholder responsible for selection of birds for diagnosis | ||||
| Client | 12 (92%) | 9 (100%) | 3 (75%) | 0.31 |
| Vet | 1 (8%) | 0 (0%) | 1 (25%) | |
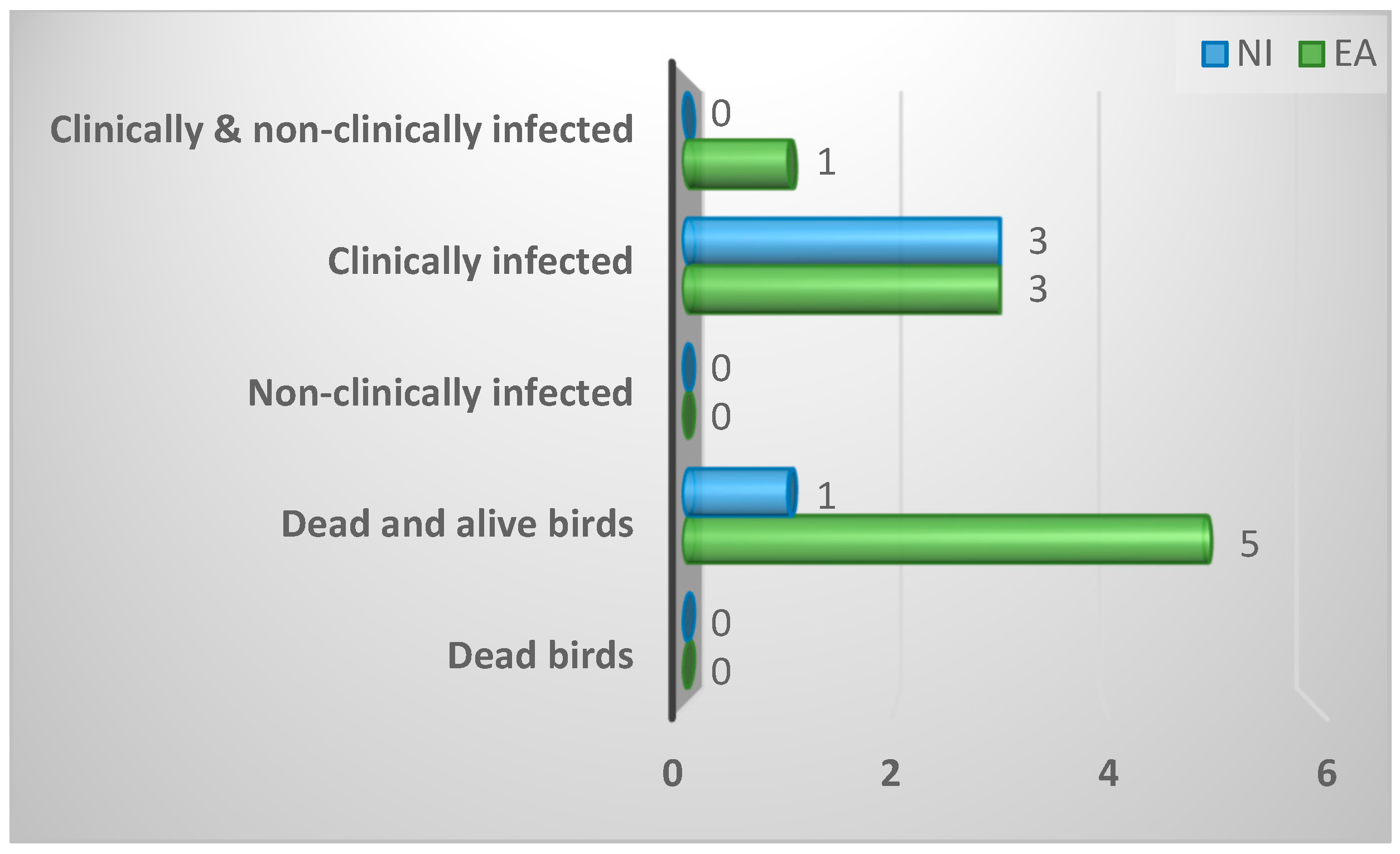
| Nature of birds removed from flock for diagnosis | ||||
| Dead and alive birds | 6 (46%) | 5 (56%) | 1 (25%) | 0.69 |
| Dead Birds | 0 (0%) | 0 (0%) | 0 (0%) | |
| Clinically affected birds | 6 (46%) | 3 (33%) | 3 (75%) | |
| Clinically affected and non- clinically affected Birds | 1 (8%) | 1 (11%) | 0 (0%) | |
| Variables |
Total (n = 12) No. (%) |
EA (n = 8) No. (%) |
NI (n = 4) No. (%) |
P value |
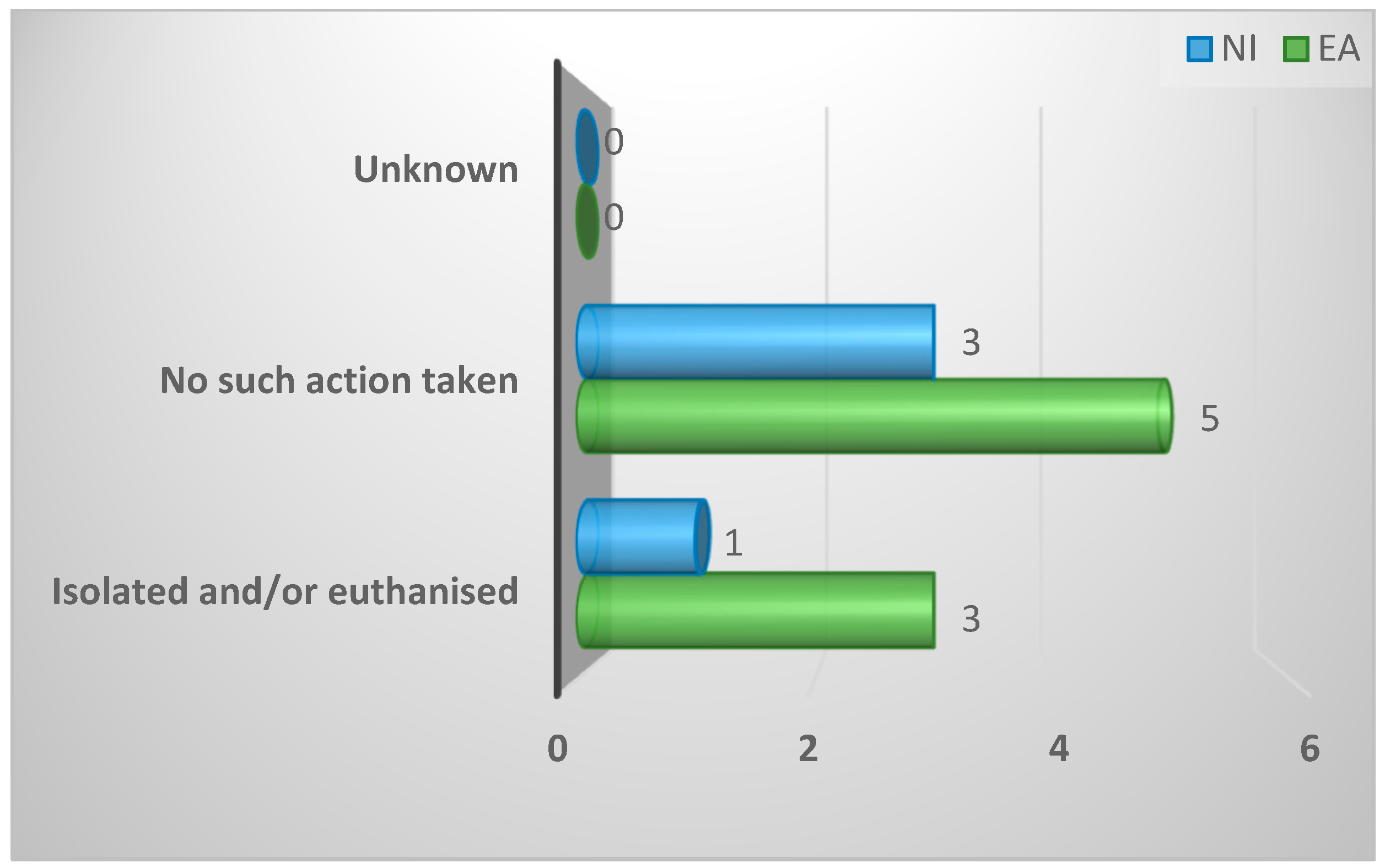
| Fate of remaining clinically infected birds in flock | ||||
| Isolate and euthanise | 4 (33%) | 3 (38%) | 1 (25%) | 1.0 |
| No action taken | 8 (67%) | 5 (63%) | 3 (75%) | |
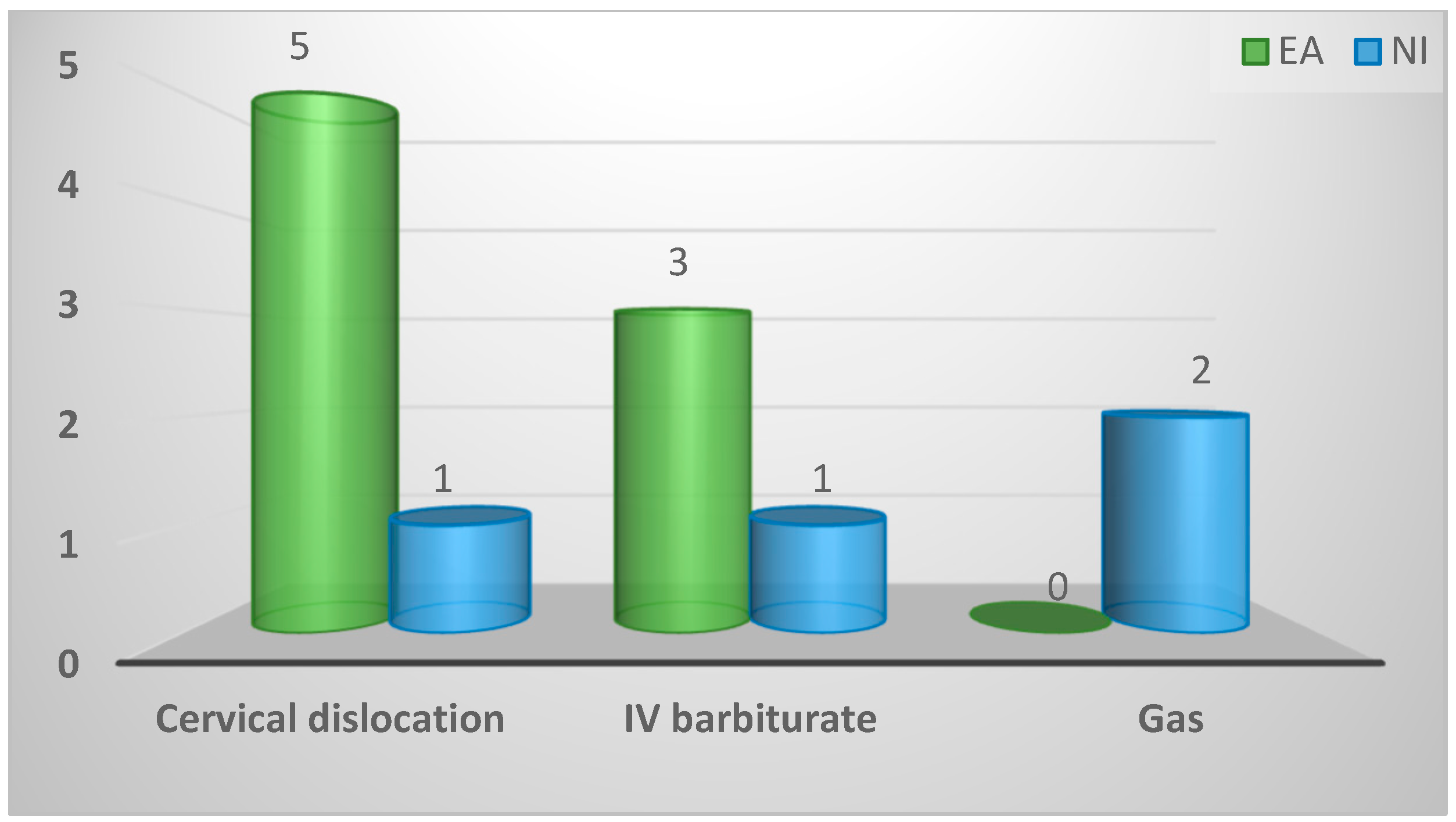
| Euthanasia method of collected birds | ||||
| Cervical dislocation | 6 (50%) | 5 (63%) | 1 (25%) | 0.19 |
| IV barbiturate | 4 (33%) | 3 (38%) | 1 (25%) | |
| Gas | 2 (17%) | 0 (0%) | 2 (50%) | |
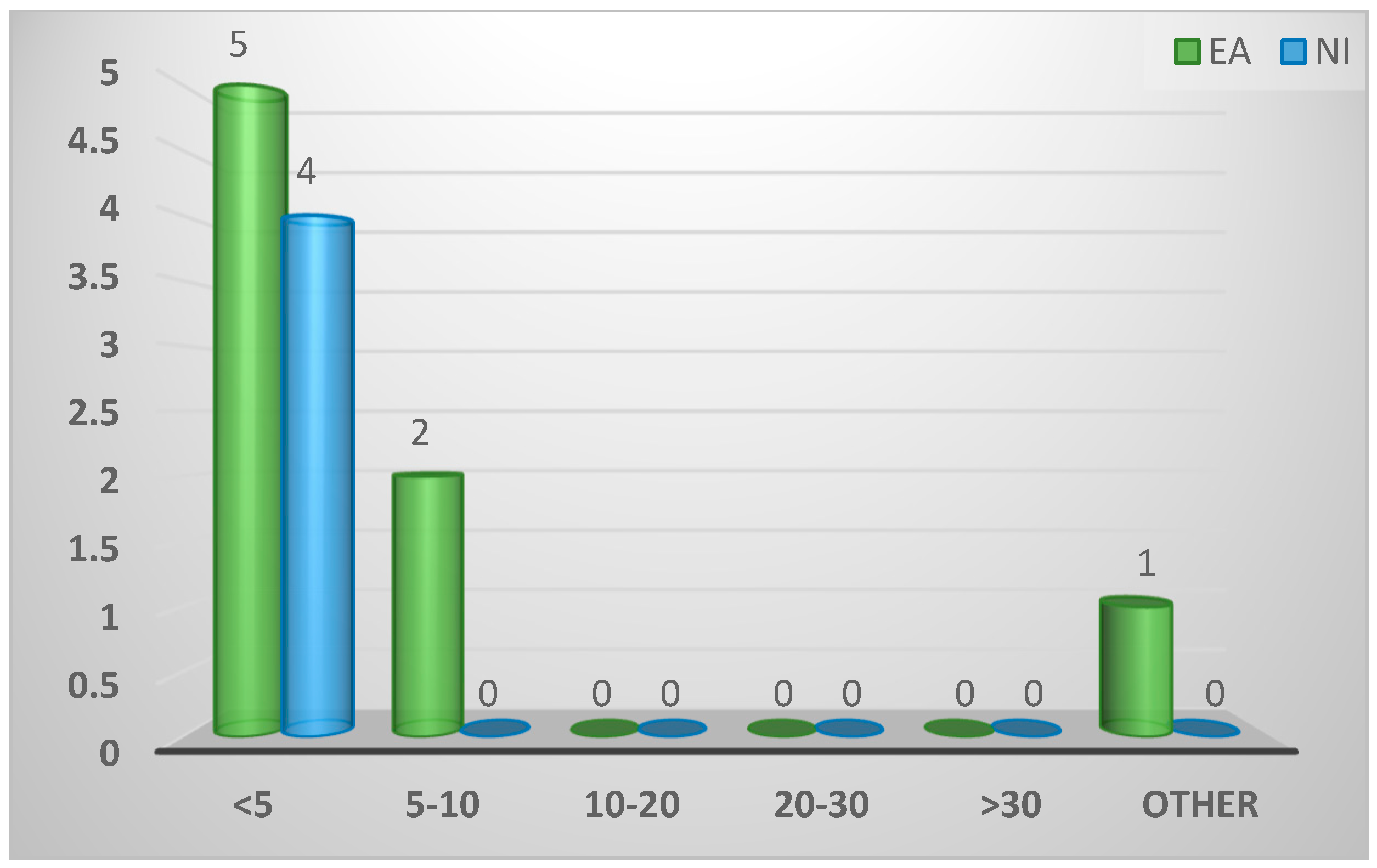
| Time (minutes) between death of bird and examination for Spironucleus | ||||
| <5 | 9 (75%) | 5 (63%) | 4 (100%) | 0.66 |
| 5-10 | 2 (17%) | 2 (25%) | 0 (0%) | |
| >10 | 0 (0%) | 0 (0%) | 0 (0%) | |
| Other | 1 (8%) | 1 (13%) | 0 (0%) | |
| Variables |
Total (n = 11) No. (%) |
EA (n = 7) No. (%) |
NI (n = 4) No. (%) |
P value |
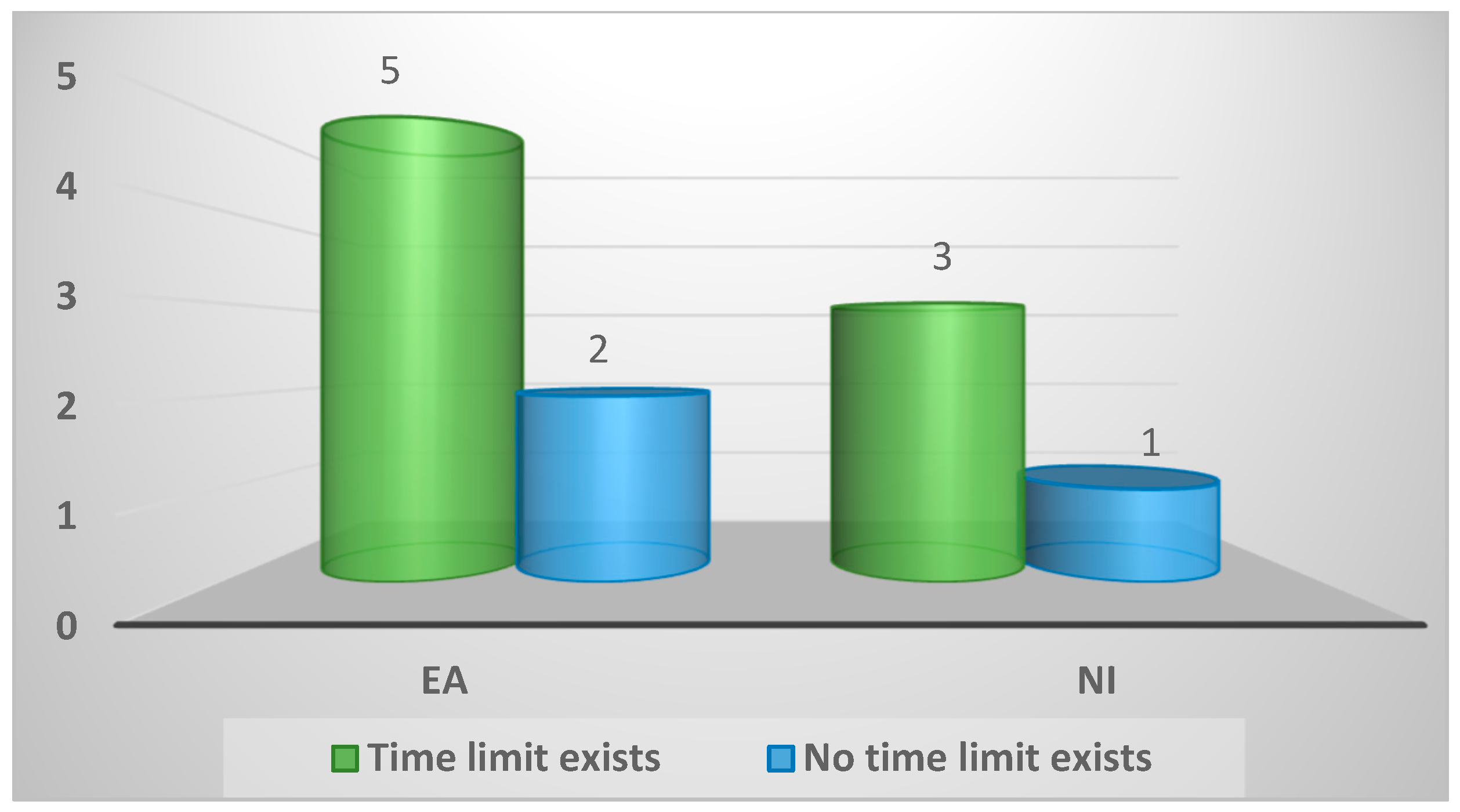
| Existence of a time limit between death and examination for Spironucleus | ||||
| Time limit exists | 8 (73%) | 5 (71%) | 3 (75%) | 1.0 |
| No time limit exists | 3 (27%) | 2 (29%) | 1 (25%) | |
| Variables | Total (n = 34) No. (%) |
EA (n = 21) No. (%) |
NI (n = 13) No. (%) |
P value |
|---|---|---|---|---|
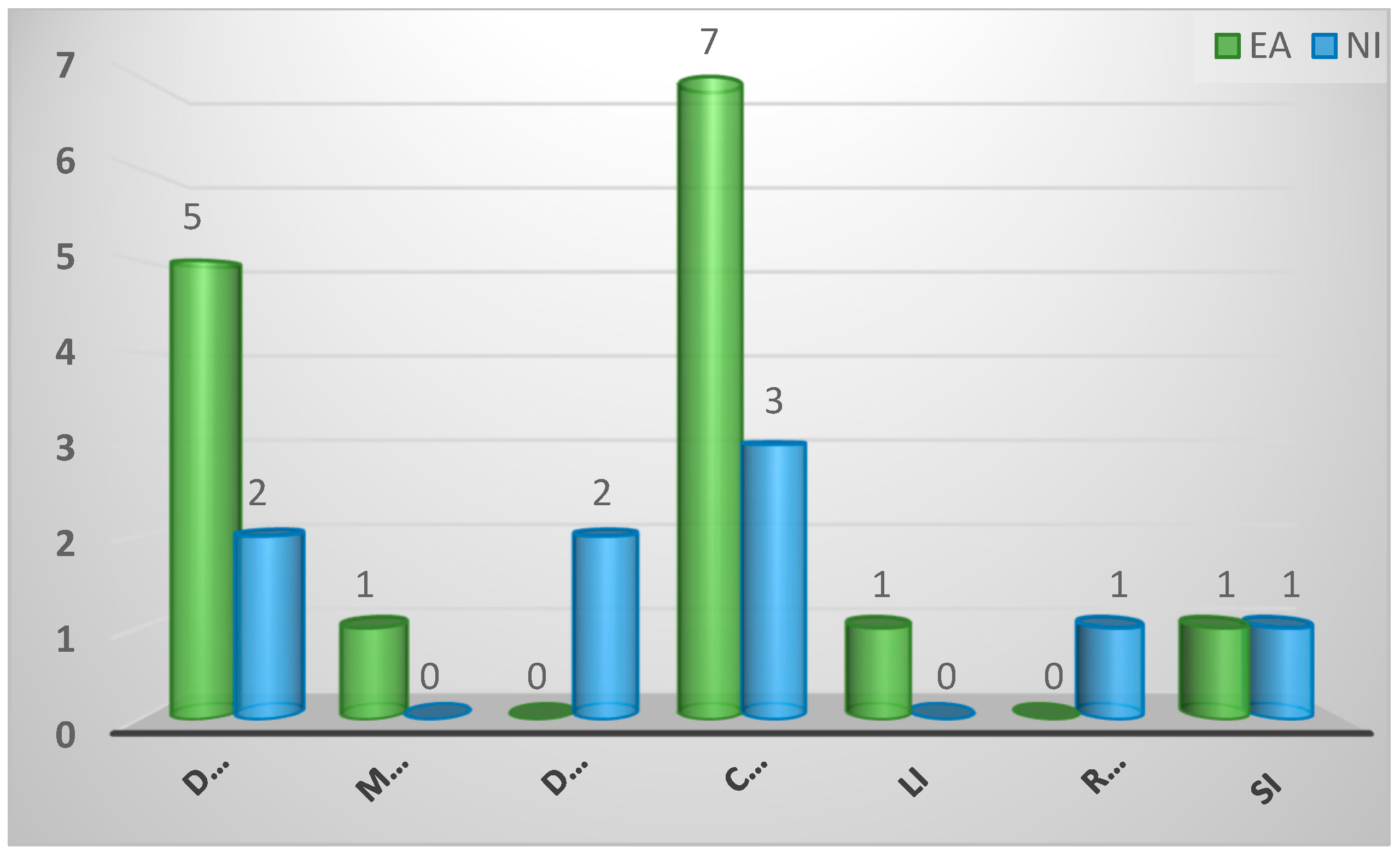
| Clinical signs associated with spironucleosis | ||||
| Diarrhoea | 9 (27%) | 6 (29%) | 3 (23%) | 0.56 |
| Ill thrift | 7 (21%) | 5 (24%) | 2 (15%) | |
| Depression | 6 (18%) | 4 (19%) | 2 (15%) | |
| Mortality | 5 (15%) | 1 (5%) | 4 (31%) | |
| Malaise | 3 (9%) | 1 (5%) | 2 (15%) | |
| Altered gait | 1 (3%) | 1 (5%) | 0 (0%) | |
| Dehydration | 1 (3%) | 1 (5%) | 0 (0%) | |
| Lethargy | 1 (3%) | 1 (5%) | 0 (0%) | |
| Ruffled feathers | 1 (3%) | 1 (5%) | 0 (0%) | |
| Variables |
Total (n = 11) No. (%) |
EA (n = 7) No. (%) |
NI (n = 4) No. (%) |
P value |
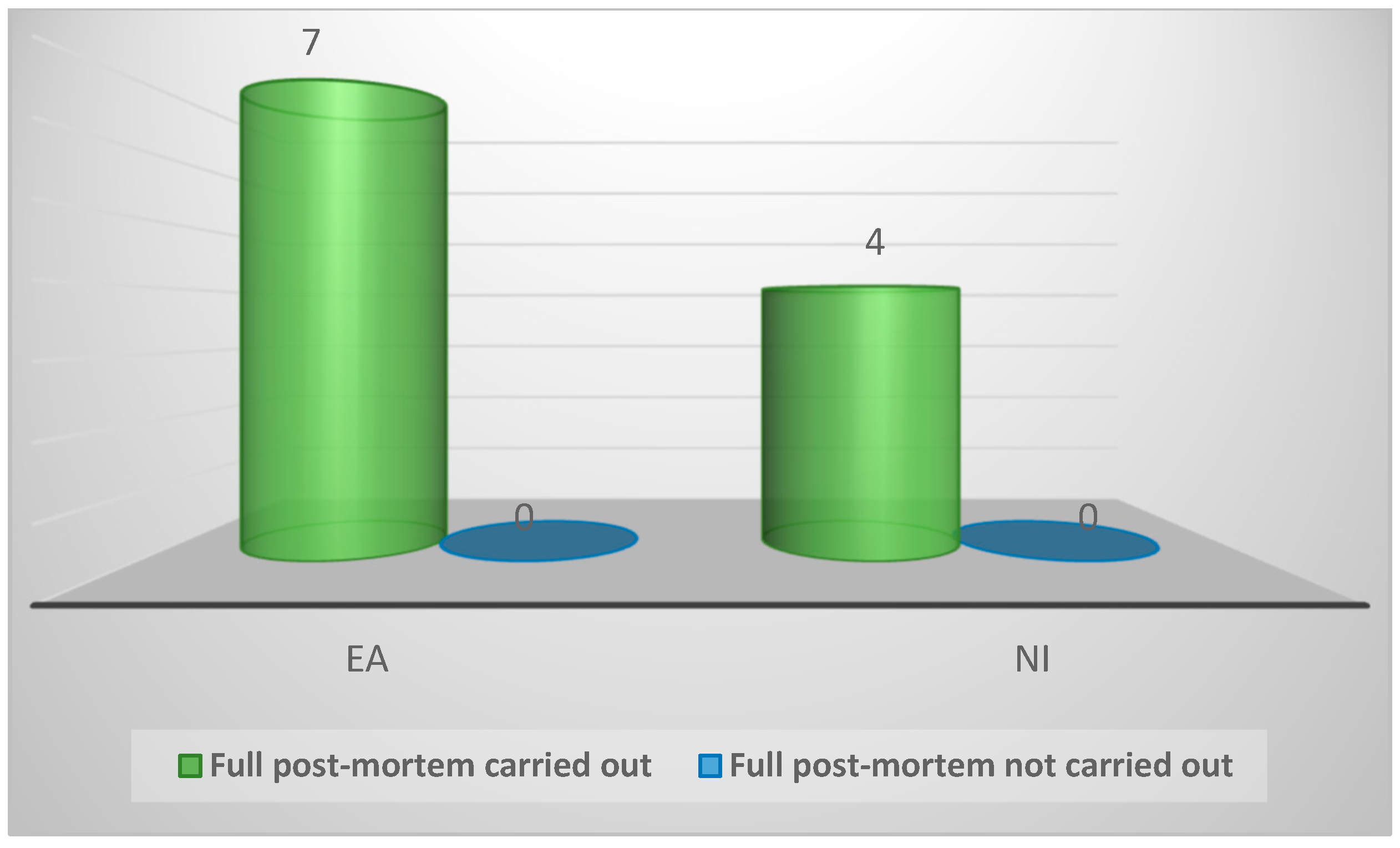
| Practice protocol for post-mortem of suspected enteric disease | ||||
| Full post-mortem carried out | 11 (100%) | 7 (100%) | 4 (100%) | 1.0 |
| Full post-mortem not carried out | 0 (0%) | 0 (0%) | 0 (0%) | |
| Variables |
Total (n = 21) No. (%) |
EA (n = 9) No. (%) |
NI (n = 12) No. (%) |
P value |
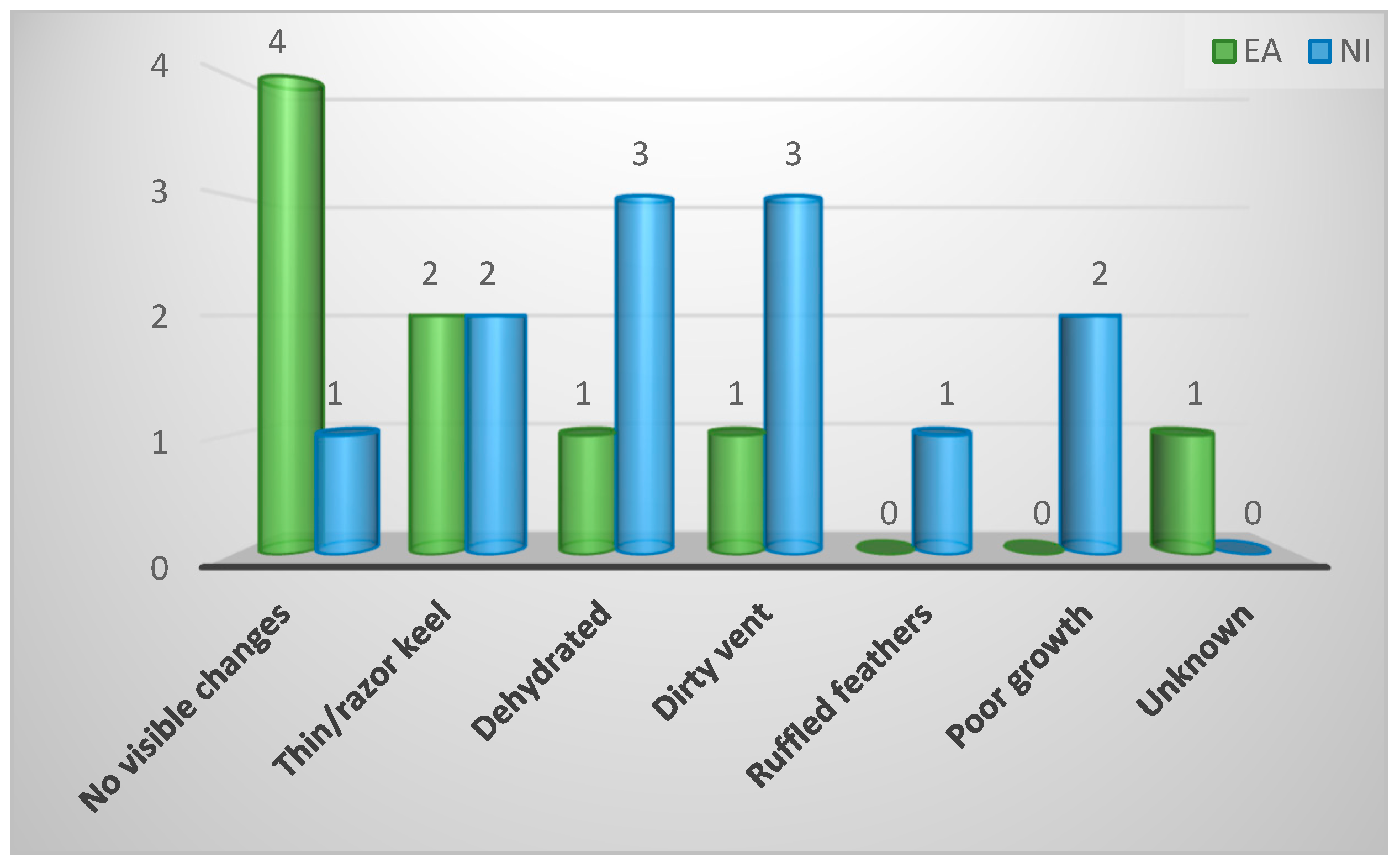
| Pathological changes visible on gross external examination | ||||
| No visible changes | 5 (24%) | 4 (44%) | 1 (8%) | 0.35 |
| Dehydrated | 4 (19%) | 1 (11%) | 3 (25%) | |
| Dirty vent | 4 (19%) | 1 (11%) | 3 (25%) | |
| Thin keel | 4 (19%) | 2 (22%) | 2 (17%) | |
| Poor growth | 2 (10%) | 0 (0%) | 2 (17%) | |
| Ruffled feathers | 1 (5%) | 0 (0%) | 1 (8%) | |
| Unknown | 1 (5%) | 1 (11%) | 0 (0%) | |
| Variables |
Total (n = 27) No. (%) |
EA (n = 18) No. (%) |
NI (n = 9) No. (%) |
P value |
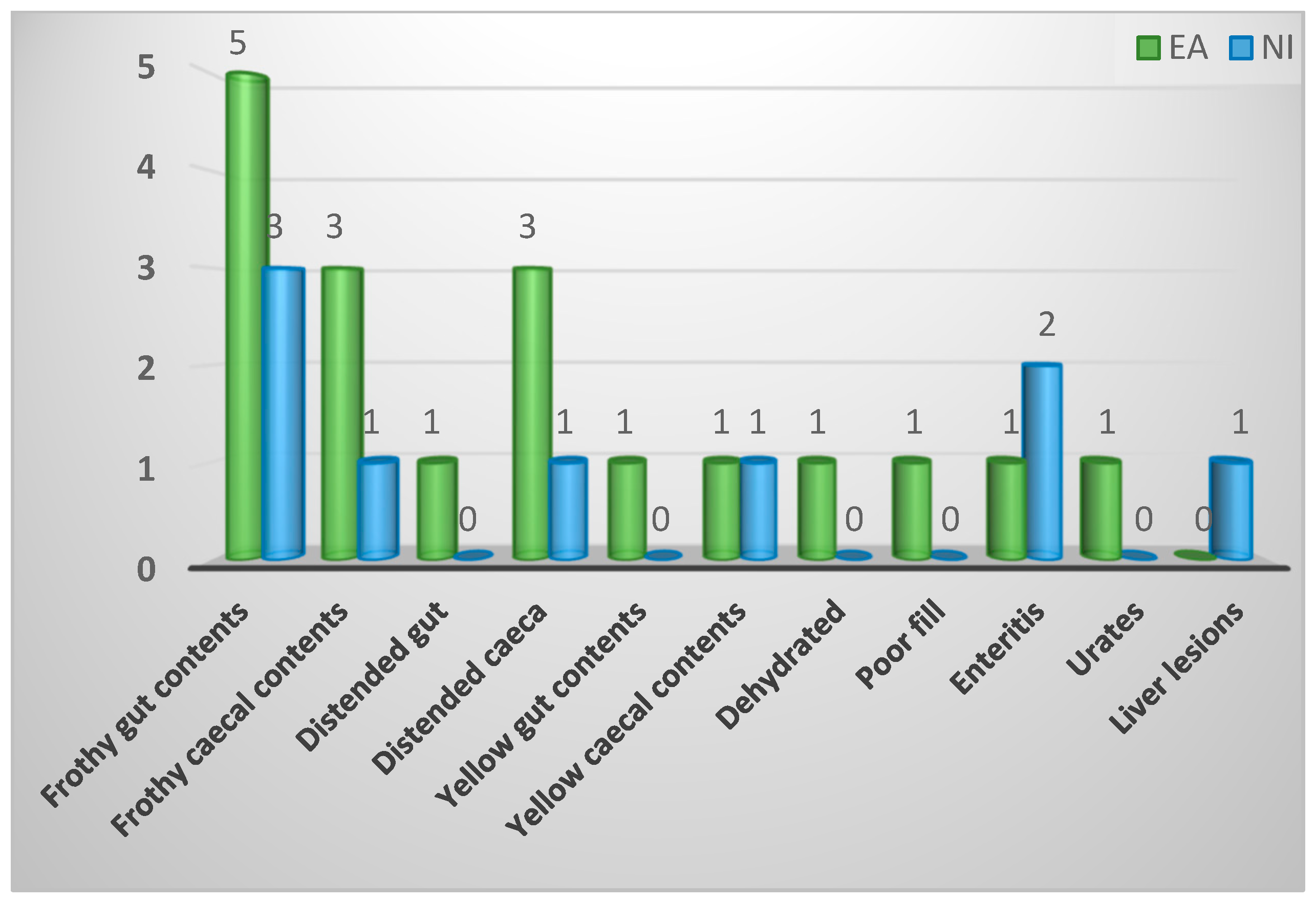
| Pathological changes visible on gross internal examination | ||||
| Frothy gut contents | 8 (30%) | 5 (28%) | 3 (33%) | 0.94 |
| Frothy caecal contents | 4 (15%) | 3 (17%) | 1 (11%) | |
| Distended gut | 1 (4%) | 1 (6%) | 0 (0%) | |
| Distended caeca | 4 (15%) | 3 (17%) | 1 (11%) | |
| Yellow gut contents | 1 (4%) | 1 (6%) | 0 (0%) | |
| Yellow caecal contents | 2 (7%) | 1 (6%) | 1 (11%) | |
| Enteritis | 3 (11%) | 1 (6%) | 2 (22%) | |
| Dehydrated | 1 (4%) | 1 (6%) | 0 (0%) | |
| Poor fill | 1 (4%) | 1 (6%) | 0 (0%) | |
| Urates | 1 (4%) | 1 (6%) | 0 (0%) | |
| Liver lesions | 1 (4%) | 0 (0%) | 1 (11%) | |
| Variables |
Total (n = 24) No. (%) |
EA (n = 15) No. (%) |
NI (n = 9) No. (%) |
P value |
| Sites of sample bird utilised for spironucleosis diagnosis | ||||
| Caecum | 10 (42%) | 7 (47%) | 3 (33%) | 0.36 |
| Duodenum | 7 (29%) | 5 (33%) | 2 (22%) | |
| Distal small intestine | 2 (8%) | 0 (0%) | 2 (22%) | |
| Small intestine | 2 (8%) | 1 (7%) | 1 (11%) | |
| Mid small intestine | 1 (4%) | 1 (7%) | 0 (0%) | |
| Large intestine | 1 (4%) | 1 (7%) | 0 (0%) | |
| Rectum | 1 (4%) | 0 (0%) | 1 (11%) | |
| Variables |
Total (n = 46) No. (%) |
EA (n = 26) No. (%) |
NI (n = 20) No. (%) |
P value |
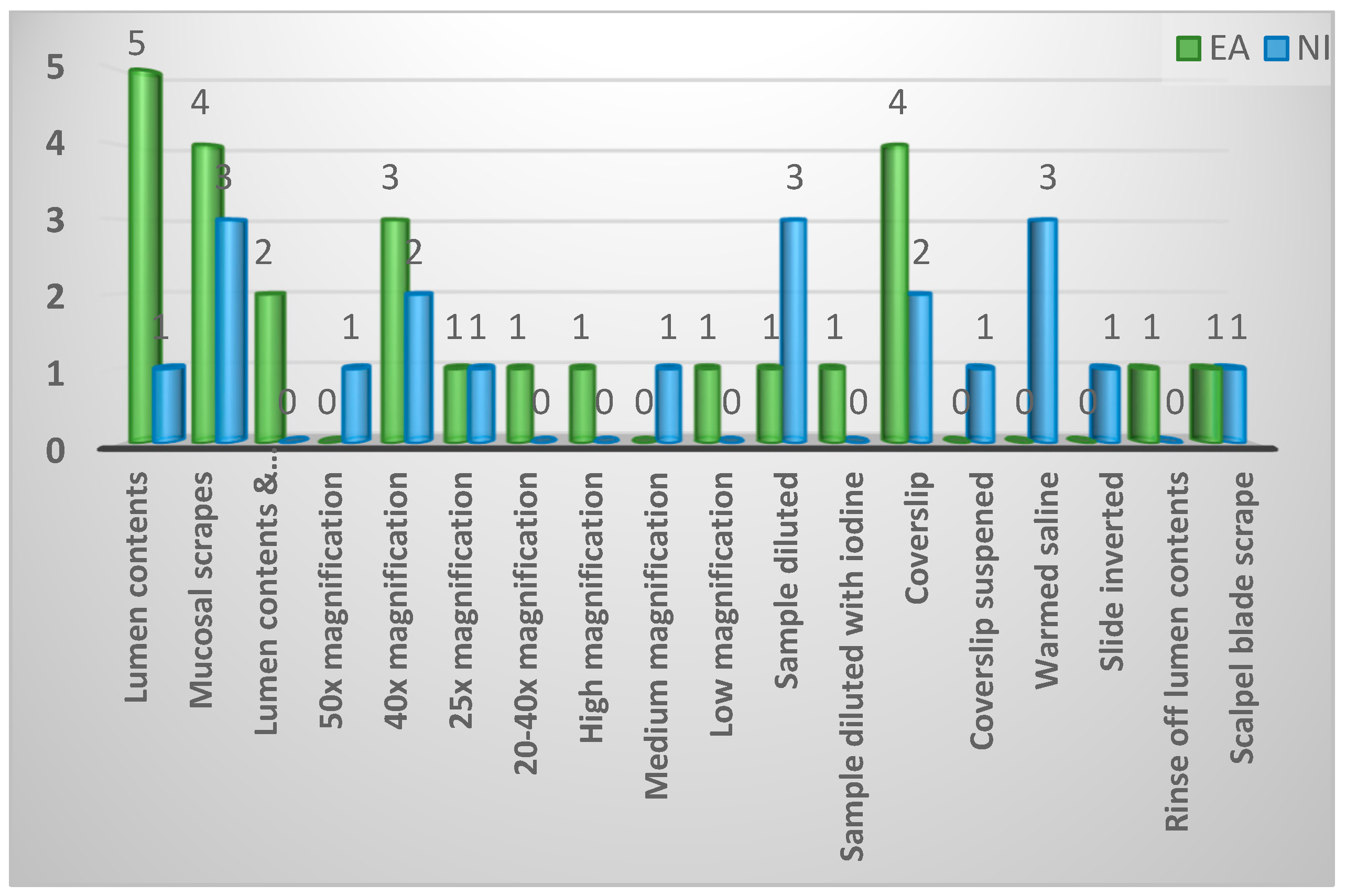
| Diagnostic techniques described for spironucleosis diagnosis | ||||
| Mucosal scrapes | 7 (15%) | 4 (15%) | 3 (15%) | 0.38 |
| Lumen contents | 6 (13%) | 5 (19%) | 1 (5%) | |
| Lumen contents and mucosal scrapes | 2 (4%) | 2 (8%) | 0 (0%) | |
| 50x Magnification | 1 (2%) | 0 (0%) | 1 (5%) | |
| 40x Magnification | 5 (11%) | 3 (12%) | 2 (10%) | |
| 25x Magnification | 2 (4%) | 1 (4%) | 1 (5%) | |
| 20-40x Magnification | 1 (2%) | 1 (4%) | 0 (0%) | |
| High magnification | 1 (2%) | 1 (4%) | 0 (0%) | |
| Medium magnification | 1 (2%) | 0 (0%) | 1 (5%) | |
| Low magnification | 1 (2%) | 1 (4%) | 0 (0%) | |
| Sample diluted | 4 (9%) | 1 (4%) | 3 (15%) | |
| Sample diluted with iodine | 1 (2%) | 1 (4%) | 0 (0%) | |
| Coverslip | 6 (13%) | 4 (15%) | 2 (10%) | |
| Coverslip suspended | 1 (2%) | 0 (0%) | 1 (5%) | |
| Warmed saline | 3 (7%) | 0 (0%) | 3 (15%) | |
| Slide inverted | 1 (2%) | 0 (0%) | 1 (5%) | |
| Rinse off lumen contents | 1 (2%) | 1 (4%) | 0 (0%) | |
| Scalpel blade scrape | 2 (4%) | 1 (4%) | 1 (5%) | |
| Variables |
Total (n = 16) No. (%) |
EA (n = 12) No. (%) |
NI (n = 4) No. (%) |
P value |
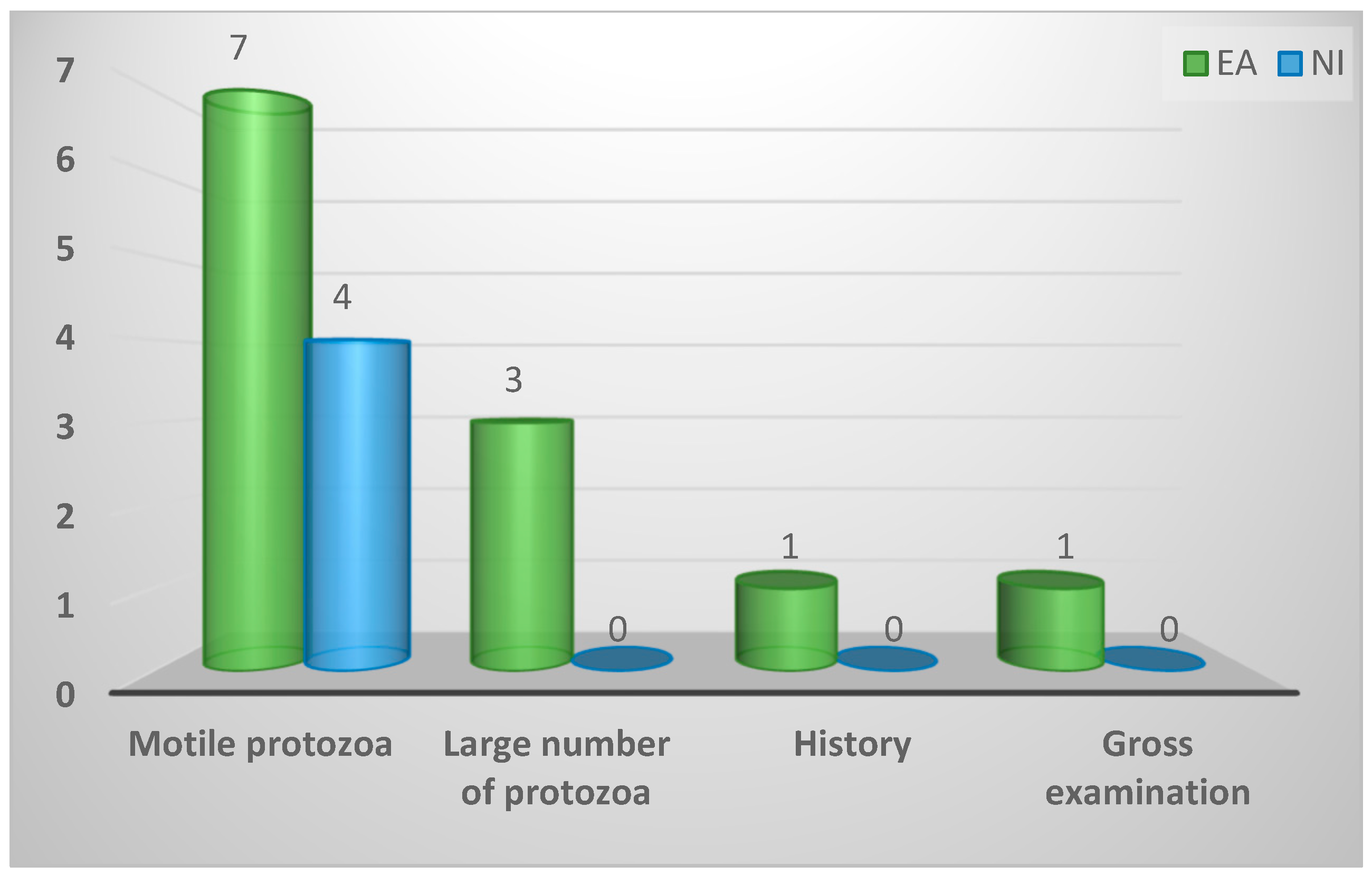
| Most crucial factor of a positive result | ||||
| Motile protozoa | 11 (69%) | 7 (58%) | 4 (100%) | 0.73 |
| Large number of protozoa | 3 (19%) | 3 (25%) | 0 (0%) | |
| History | 1 (6%) | 1 (8%) | 0 (0%) | |
| Gross examination | 1 (6%) | 1 (8%) | 0 (0%) | |
| Variables |
Total (n = 31) No. (%) |
EA (n = 19) No. (%) |
NI (n = 12) No. (%) |
P value |
| Morphological appearance of Spironucleus | ||||
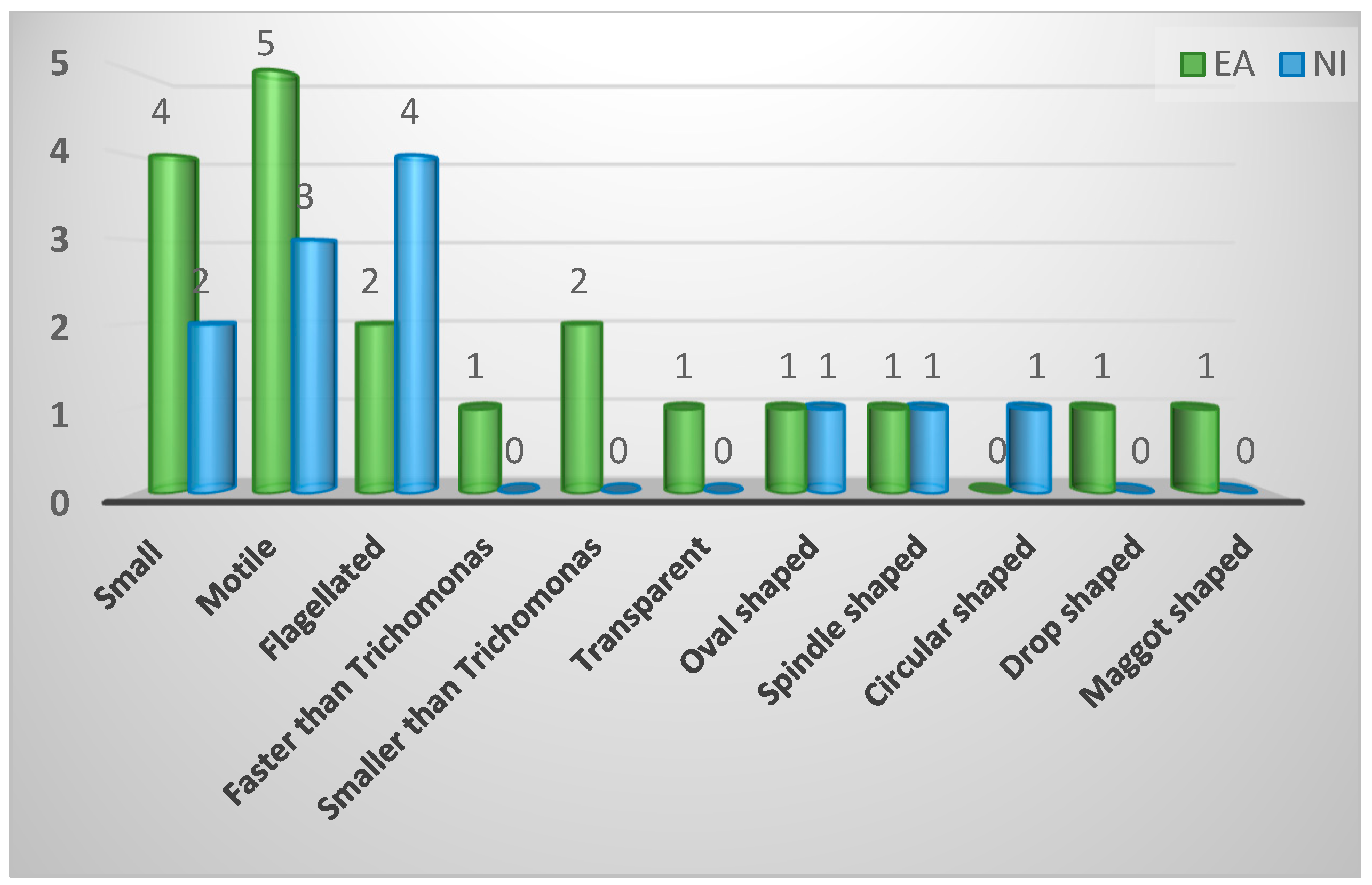
| Small | 6 (19%) | 4 (21%) | 2 (17%) | 0.86 |
| Motile | 8 (26%) | 5 (26%) | 3 (25%) | |
| Flagellated | 6 (19%) | 2 (11%) | 4 (33%) | |
| Faster than Trichomonas | 1 (3%) | 1 (5%) | 0 (0%) | |
| Smaller than Trichomonas | 2 (7%) | 2 (11%) | 0 (0%) | |
| Transparent | 1 (3%) | 1 (5%) | 0 (0%) | |
| Oval shaped | 2 (7%) | 1 (5%) | 1 (8%) | |
| Spindle shaped | 2 (7%) | 1 (5%) | 1 (8%) | |
| Circular shaped | 1 (3%) | 0 (0%) | 1 (8%) | |
| Drop shaped | 1 (3%) | 1 (5%) | 0 (0%) | |
| Maggot shaped | 1 (3%) | 1 (5%) | 0 (0%) | |
| Variables |
Total (n = 11) No. (%) |
EA (n = 7) No. (%) |
NI (n = 4) No. (%) |
P value |
| Utilisation of Faeces for Spironucleosis Diagnosis | ||||
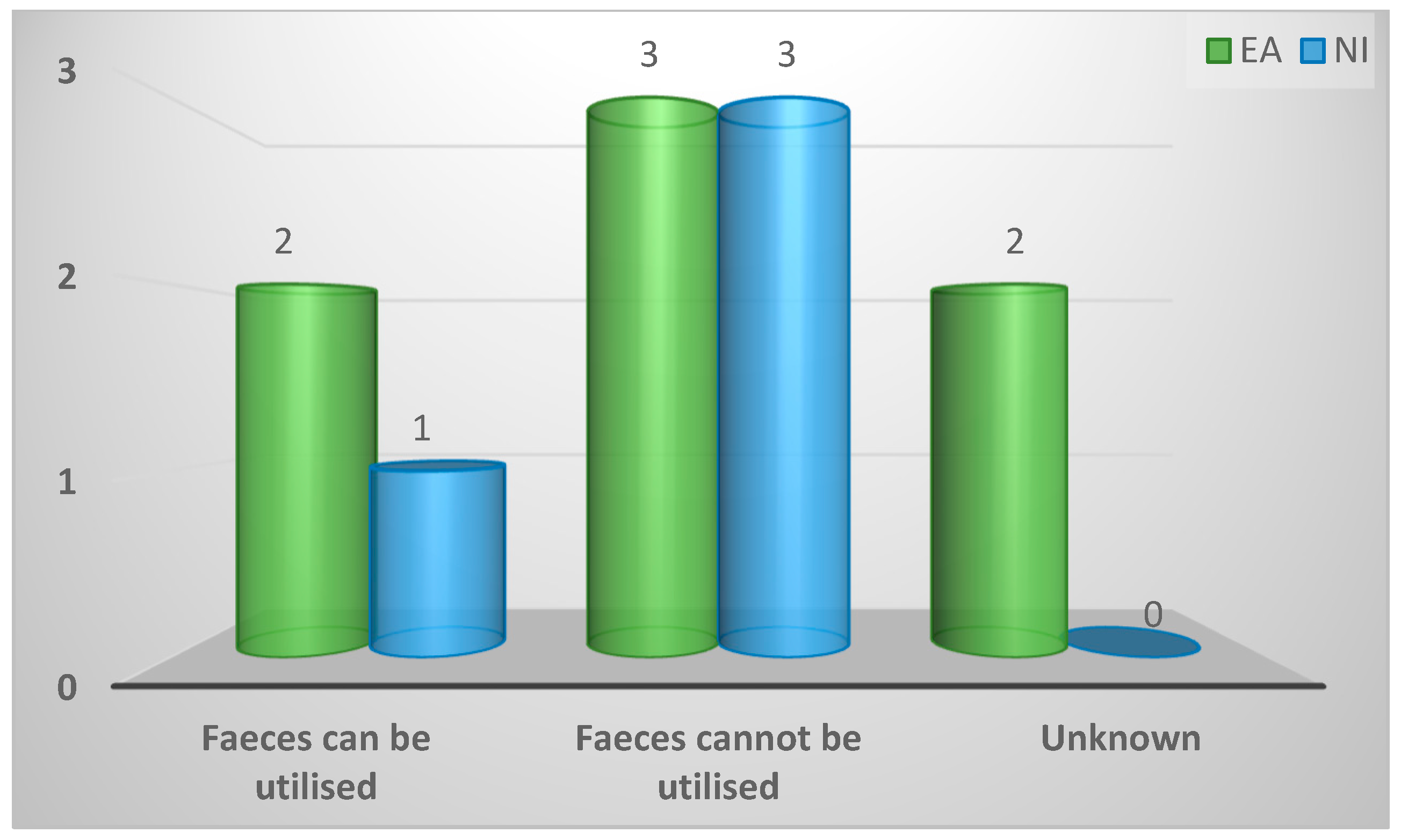
| Faeces can be utilised | 3 (27%) | 2 (29%) | 1 (25%) | 0.73 |
| Faeces cannot be utilised | 6 (55%) | 3 (43%) | 3 (75%) | |
| Unknown | 2 (18%) | 2 (29%) | 0 (0%) | |
| Differentiation of Spironucleus from other protozoa | ||||
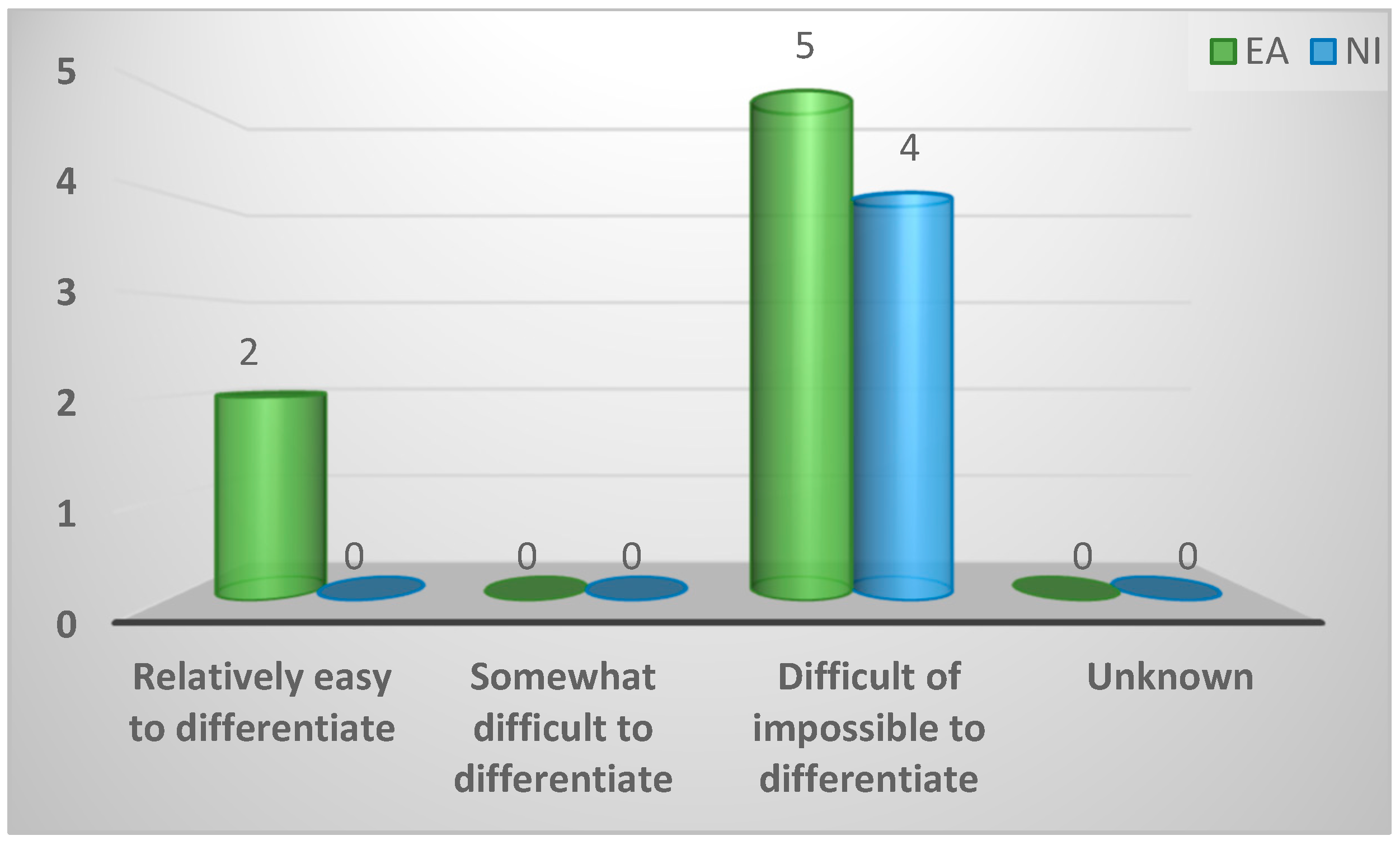
| Relatively easy to differentiate | 2 (18%) | 2 (29%) | 0 (0%) | 0.49 |
| Somewhat difficult to differentiate | 0 (0%) | 0 (0%) | 0 (0%) | |
| Difficult to differentiate | 9 (82%) | 5 (71%) | 4 (100%) | |
| No. of positive samples needed to confirm infection in an individual bird | ||||
| One | 10 (91%) | 6 (86%) | 4 (100%) | 1.0 |
| Two | 1 (9%) | 1 (14%) | 0 (0%) | |
| No. of infected birds needed to confirm Spironucleus in a flock | ||||
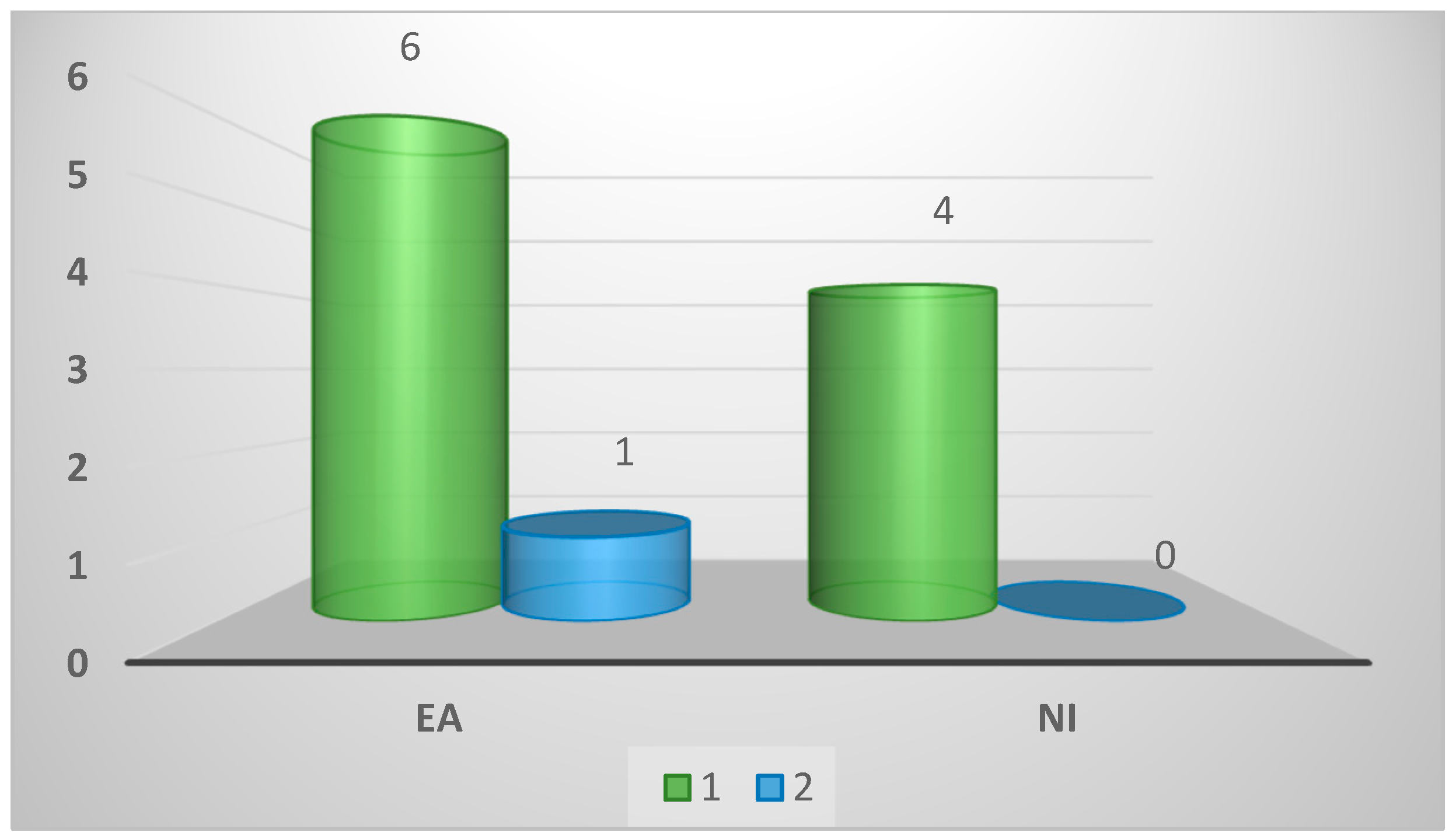
| One | 7 (64%) | 3 (43%) | 4 (100%) | 0.53 |
| Two | 1 (9%) | 1 (14%) | 0 (0%) | |
| Three | 1 (9%) | 1 (14%) | 0 (0%) | |
| Other | 2 (18%) | 2 (29%) | 0 (0%) | |
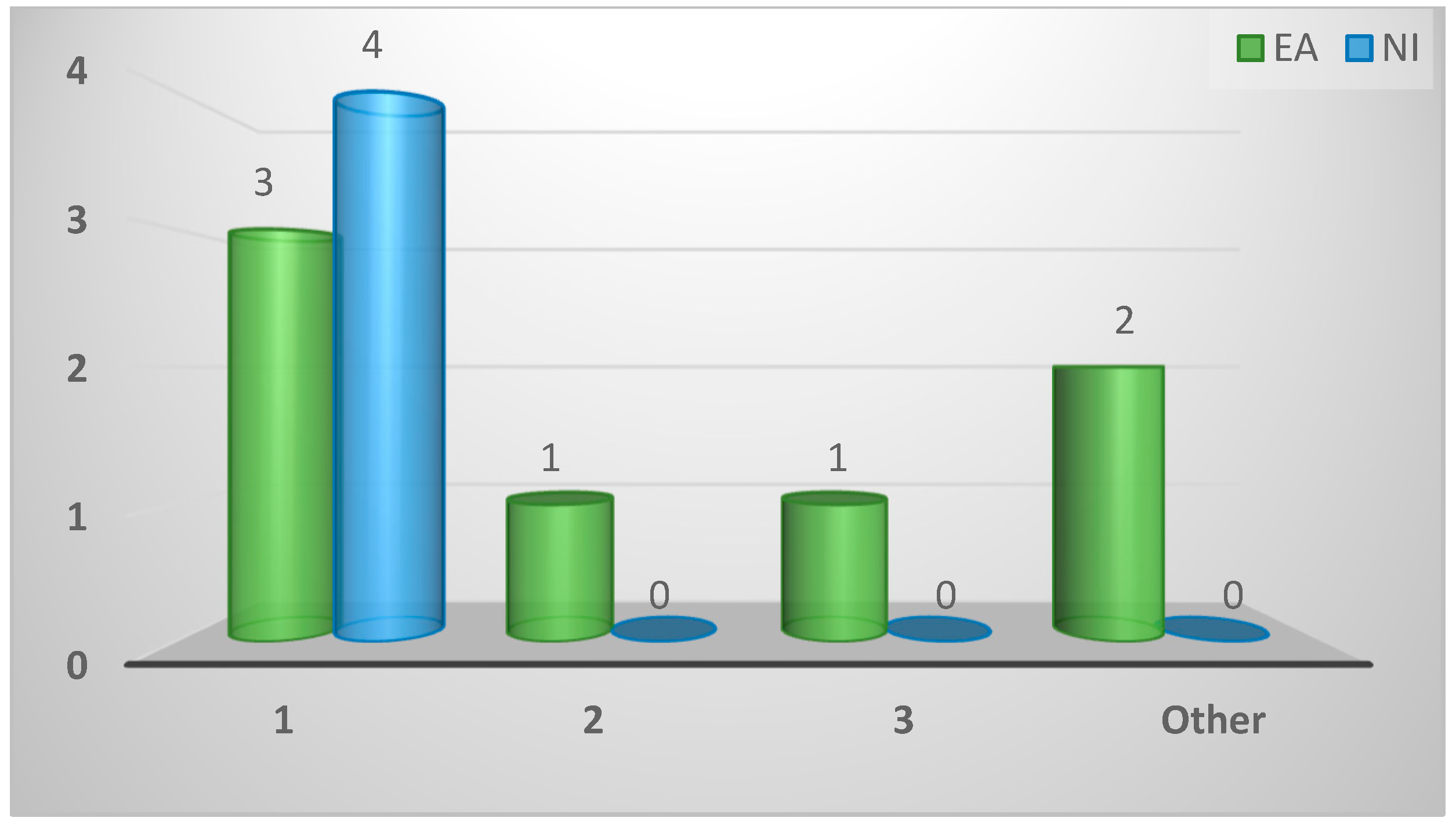
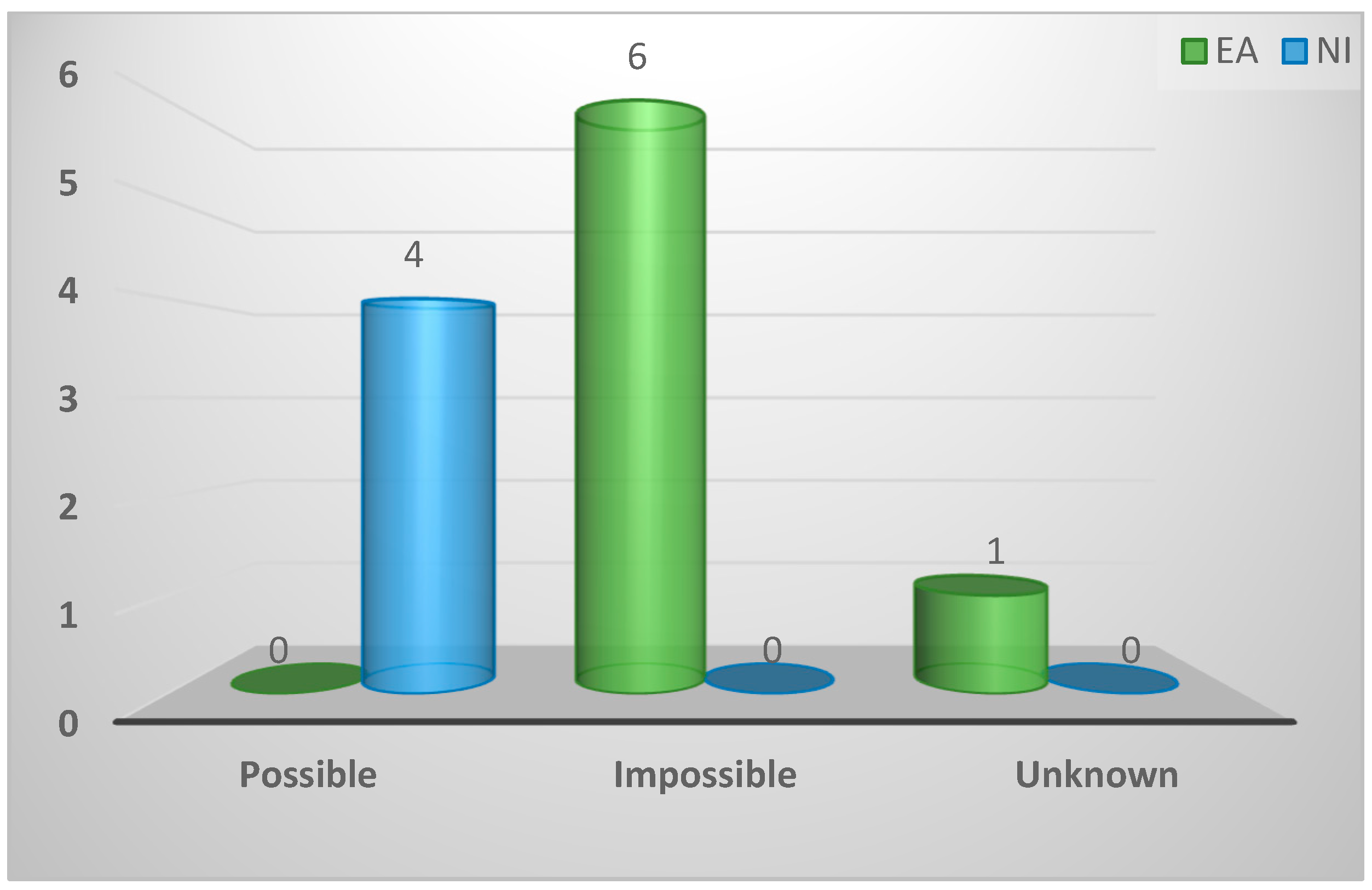
| Ability to quantify Spironucleus infection levels | ||||
| Possible | 4 (36%) | 0 (0%) | 4 (100%) | 0.003 |
| Impossible | 6 (55%) | 6 (86%) | 0 (0%) | |
| Unknown | 1 (9%) | 1 (14%) | 0 (0%) | |
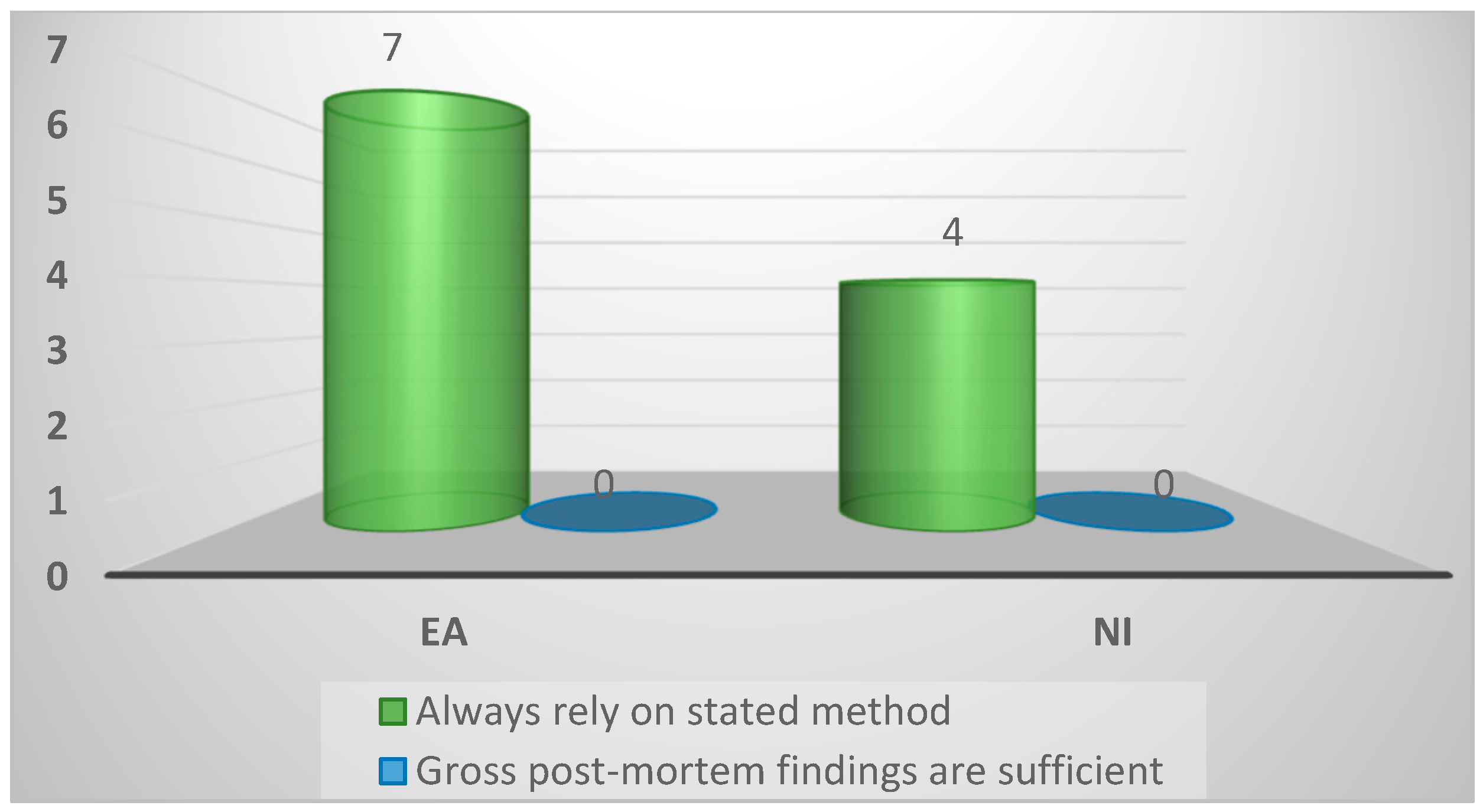
| Reliance on stated method of Spironucleus diagnosis | ||||
| Always rely on stated method | 11 (100%) | 7 (100%) | 4 (100%) | 1.0 |
| Gross post-mortem findings sufficient | 0 (0%) | 0 (0%) | 0 (0%) | |
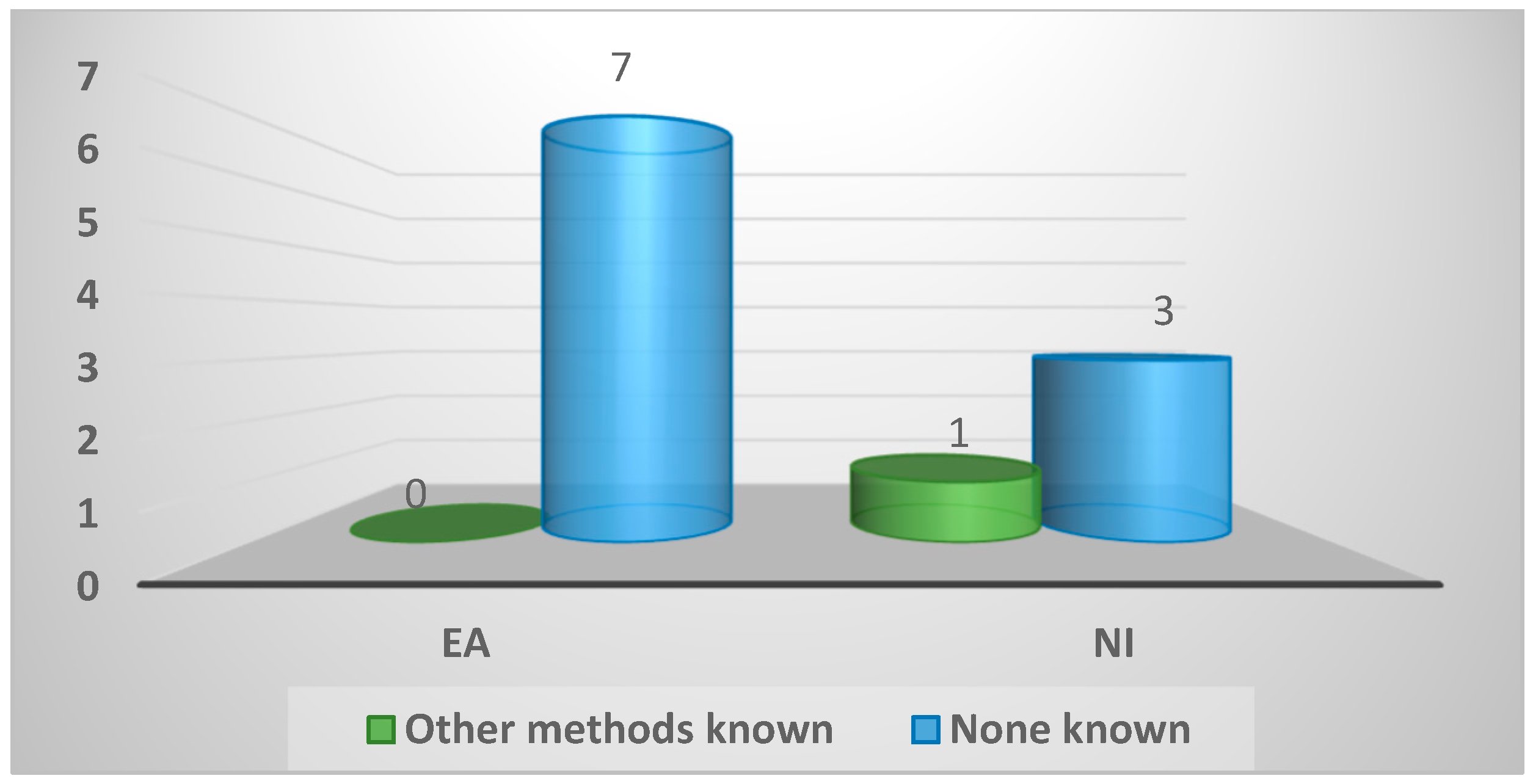
| Knowledge of other methods used for spironucleosis diagnosis | ||||
| Other methods known | 1 (9%) | 0 (0%) | 1 (25%) | 0.36 |
| None known | 10 (91%) | 7 (100%) | 3 (75%) | |
| Reproducibility of diagnostic method in general practice | ||||
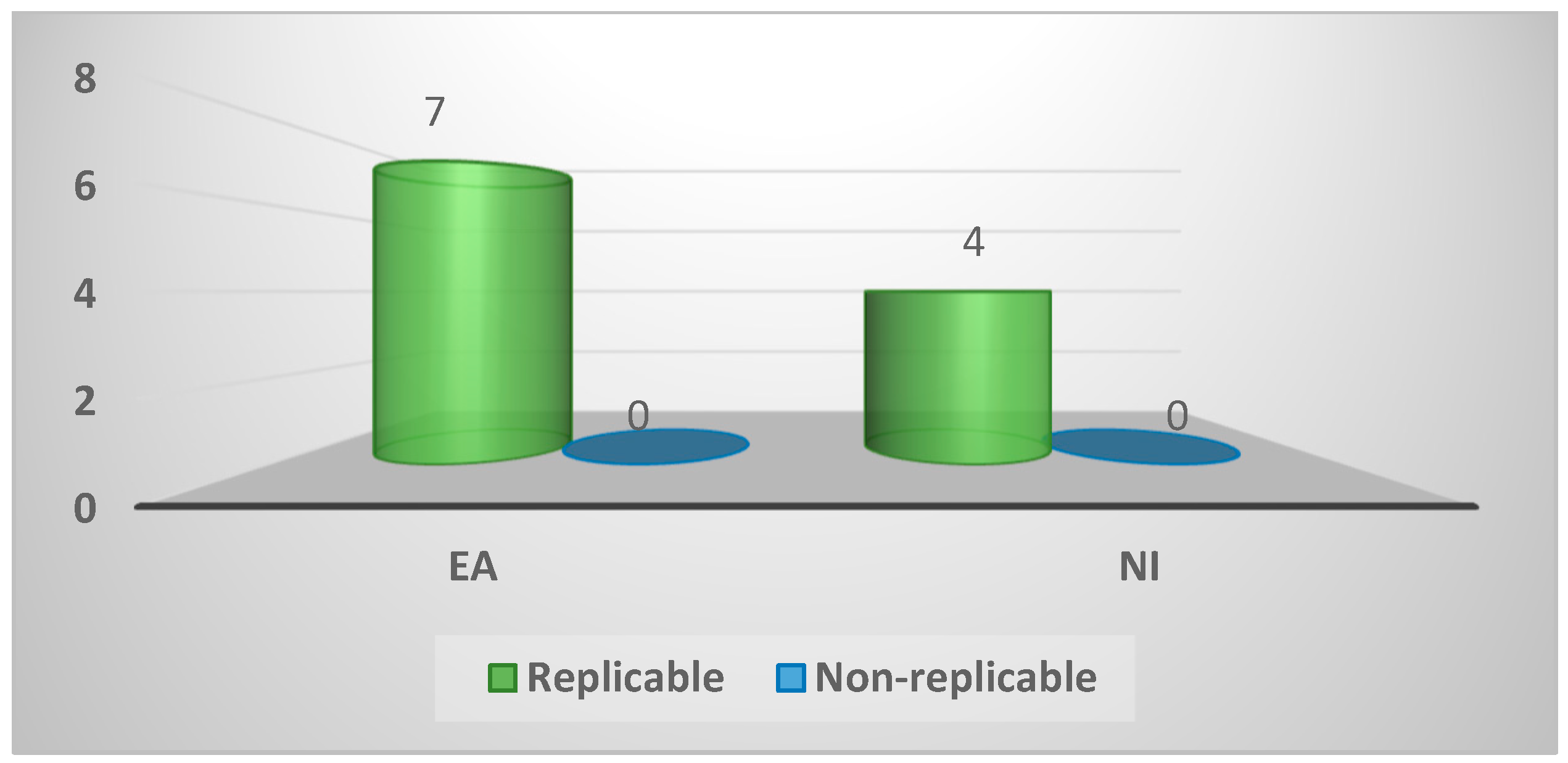
| Replicable | 11 (100%) | 7 (100%) | 4 (100%) | 1.0 |
| Non-Replicable | 0 (0%) | 0 (0%) | 0 (0%) | |
| Variables |
Total (n = 12) No. (%) |
EA (n = 7) No. (%) |
NI (n = 5) No. (%) |
P value |
| Opinion on spironucleosis diagnosis in general practice | ||||
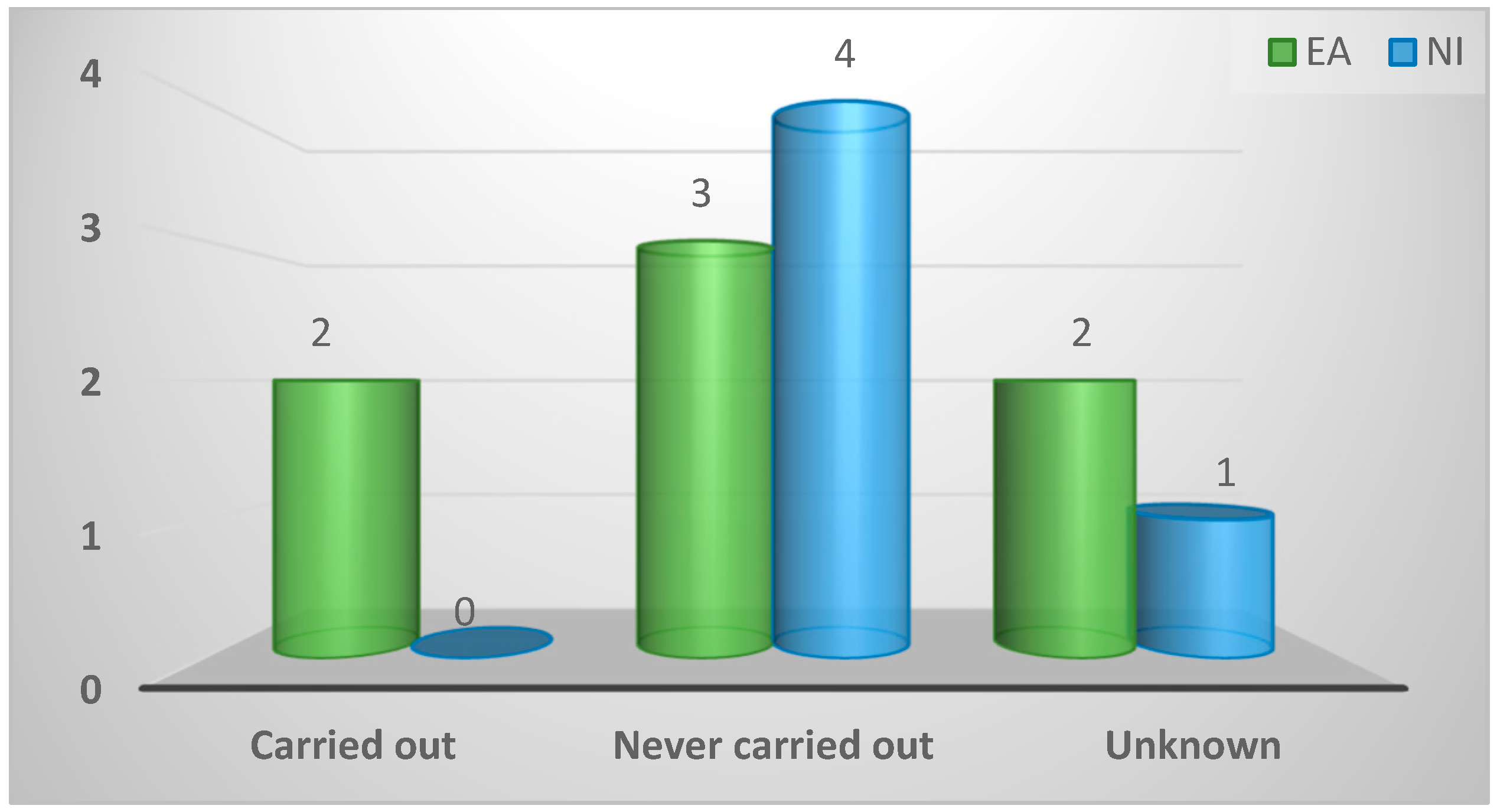
| Carried out | 2 (17%) | 2 (29%) | 0 (0%) | 0.72 |
| Never carried out | 8 (67%) | 4 (57%) | 4 (80%) | |
| Unknown | 2 (17%) | 1 (14%) | 1 (20%) | |
| Difference in spironucleosis diagnosis between regions | ||||
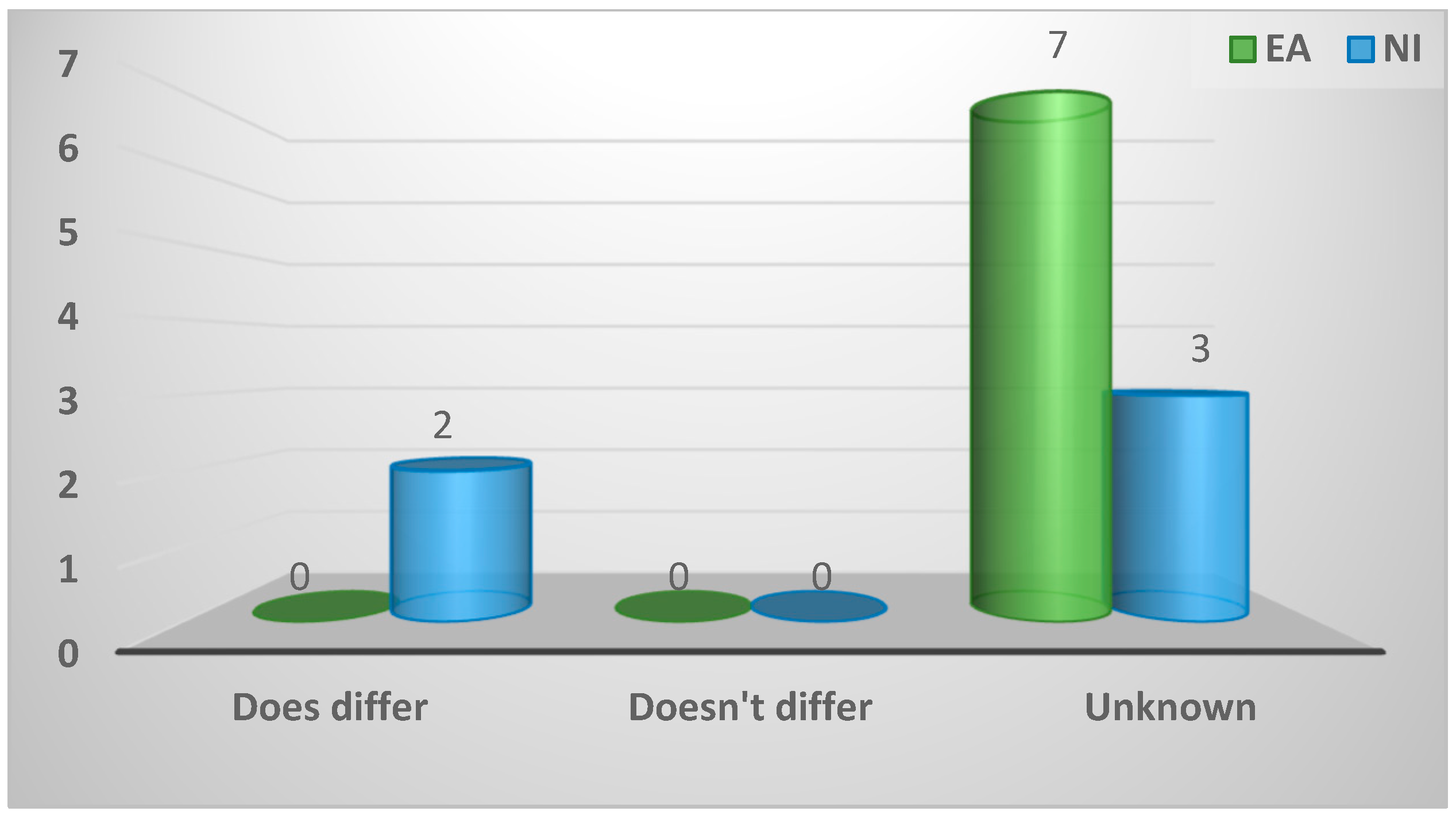
| Does differ | 2 (17%) | 0 (0%) | 2 (40%) | 0.15 |
| Does not differ | 0 (0%) | 0 (0%) | 0 (0%) | |
| Unknown | 10 (83%) | 7 (100%) | 3 (60%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





