1. Introduction
As of 2023, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to circulate with continued emergence of variants (such as Alpha, Beta, Gamma, Delta, and Omicron) [
1,
2]. Vaccines against the virus have been developed to combat coronavirus disease 2019 (COVID-19) [
3]. To establish effectiveness of these vaccines, especially in light of recent immune-evasive variants [
4], correlates of protection (CoPs) are needed.
Most current COVID-19 vaccines target the SARS-CoV-2 spike (S) protein on the viral surface [
3]. Neutralizing antibodies which target the S protein are a known CoP for vaccine efficacy for COVID vaccines [
5,
6,
7,
8,
9]. Microneutralization assays (MN50) are used to quantify functional neutralizing antibodies against SARS-CoV-2 in human serum [
10,
11]. Such neutralizing antibodies may develop as a result of natural infection or after receipt of a SARS-CoV-2 spike protein vaccine [
12].
This article describes the validation of an in vitro MN50 assay (360biolabs, Melbourne, Australia) to detect/quantitate SARS-CoV-2 neutralizing antibodies against ancestral and variant strains (Beta, Delta, Omicron BA.1, Omicron BA.5 and XBB.1.5). The aim was to assess suitability of the assay for detection of SARS-CoV-2 neutralizing antibodies in human serum from SARS-CoV-2 vaccine clinical trials. The microneutralization assay was assessed for precision, specificity, selectivity, linearity, robustness, stability, and other validation parameters. The MN50 assay described here is suitable for detection of SARS-CoV-2 neutralizing antibodies (a CoP for vaccine efficacy) in a robust, precise, and accurate manner, including in clinical trial serum samples.
2. Materials and Methods
2.1. Assay Procedure
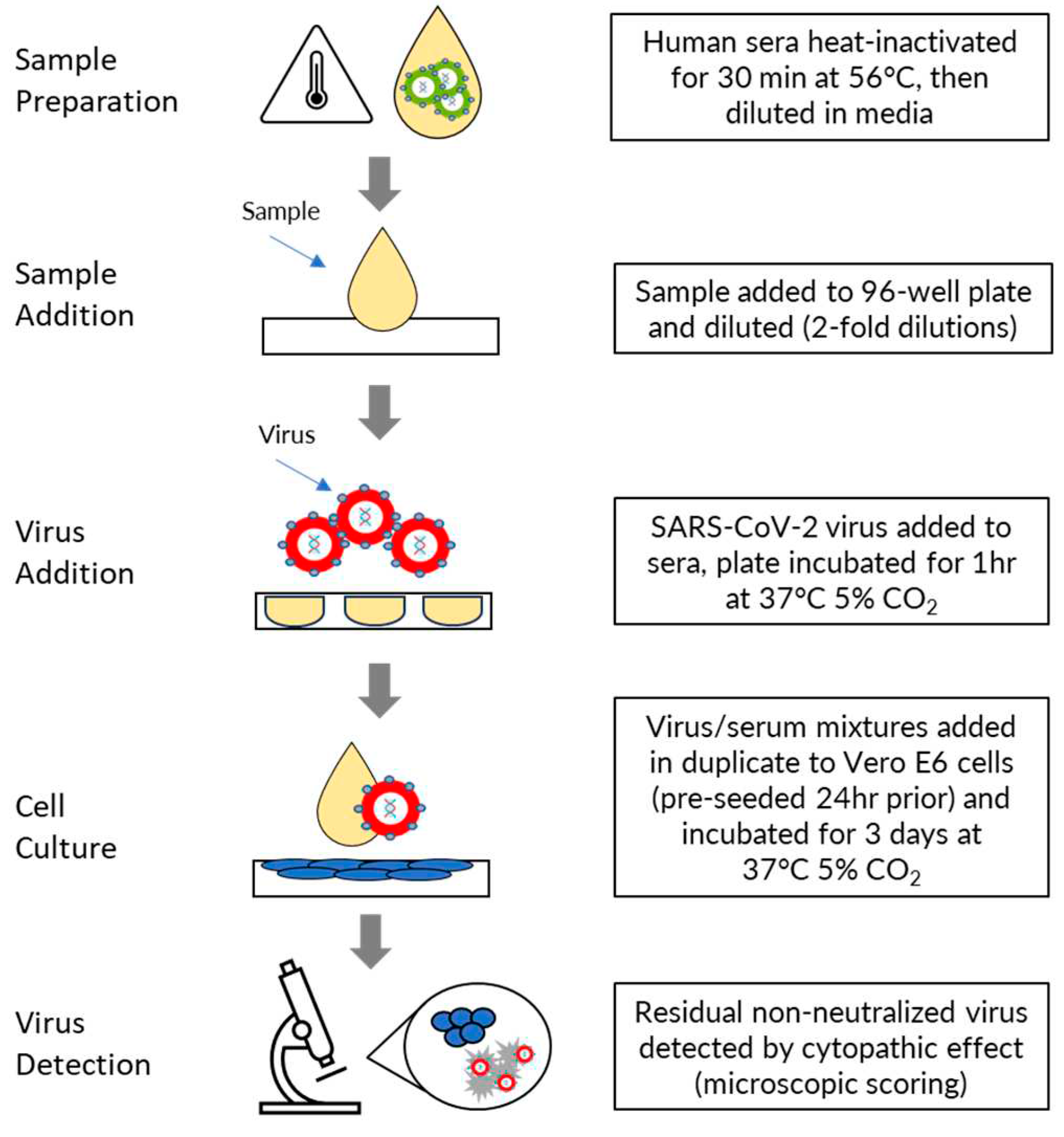
Human sera were heat inactivated for 30 minutes at 56⁰C and then diluted in MN50 assay media comprising Dulbecco Essential Medium without L-glutamine (DMEM; Thermo Fisher Scientific, Cat. No. 10313-021) supplemented with 2% FBS (Bovagen SFBS), 1% Glutamax (Thermo Fisher Scientific, Cat. No. 35050-061), and 1% Pen/Strep (Thermo Fisher Scientific, Cat. No.15140-122). Serum was diluted by addition of 28μL to 252μL of assay media in the first column of a 96 well dilution plate and an eleven-point two-fold serial dilution was then prepared by transferring 140μL from the wells of each column into 140μL of medium in the wells of the next column. Diluted sera were then mixed with an equal volume (140μL) of SARS-CoV-2 virus (4000 TCID50 units/mL for ancestral strain and 2000 TCID50 units/mL for delta, BA.1, BA.5 and XBB.1.5 strains) and incubated for 1 hour at 37oC, 5% CO2. Following this incubation, 100μL of the virus/serum mixtures (200 TCID50 units/well for ancestral strain, 100 TCID50 units/well for delta, BA.1, BA>5 and XBB.1.5 strains) were added in duplicate to Vero E6 cells, pre-seeded 24 hours prior in 96 well plates in 100μL of assay media at 1.5 x 104 cells/well. Plates were incubated for 3 days at 37oC, 5% CO2.
The residual non-neutralized virus was detected via cytopathic effect (CPE) assessed by microscopic scoring by a fully trained experienced subject matter expert, in comparison to the cell controls without virus infection. Two replicate wells per dilution were scored as either positive (SARS-CoV-2 cytopathology is present) or negative (healthy Vero E6 monolayer). The neutralization titer was expressed as the reciprocal of the highest dilution at which ≥50% of the replicate wells were protected from infection (MN50). The final serum dilution range of the assay was an MN50 titer range of 20 - 20,480. If the test samples were >20,480, then the samples were diluted in negative serum in repeat testing to generate precise MN50 values. If at least 50% protection was not observed for an individual serum at any dilution, the MN50 titer was recorded as ≤20. (
Figure 1).
2.2. Samples
Human serum samples included SARS-CoV-2 convalescent reference serum from PRECISION for Medicine, US (2020), sera from a COVID-19 vaccine clinical trial (2019nCoV-311 part 1 Novavax, Inc., Gaithersburg, MD, USA), and pre- and post-vaccinated serum from Novavax non-SARS-CoV-2 vaccine studies prior to circulation of SARS-CoV-2 virus in the human population. Convalescent serum samples (N=18) were from vaccinated subjects (primary vaccination series) but tested positive for Omicron BA.1 strain by PCR.
2.3. Viral stocks
For this manuscript, “ancestral strain” refers to SARS-CoV-2 hCoV-19/Australia/VIC01/2020 (GenBank MT007544.1; Melbourne Health at the Doherty Institute, Australia). Genome sequence analysis of the SARS-CoV-2 hCoV-19/Australia/VIC01/2020 spike protein gene shows high sequence homology with the Wuhan strain, with only one nucleotide difference in the spike protein (S247R) for the virus bank compared to the Wuhan strain. The working stock of SARS-CoV-2 hCoV-19/Australia/VIC01/2020 also had one additional spike protein amino acid change (T95I), but no additional changes in the furin cleavage site were introduced during passage of SARS-CoV-2 hCoV-19/Australia/VIC01/2020 in Vero E6 cells. To avoid genetic drift, one fully characterized working stock was generated for validation and all associated clinical testing. All subsequent virus strain stocks were confirmed by PCR and sequence identity compared against GEN bank.
2.4. Cell lines/passaging
African Green Monkey Kidney cells (Vero-E6 ATCC® CRL-1586) were sub-cultured to generate cell bank stocks in cell growth medium (Minimal Essential Medium without L-glutamine supplemented with 10% [v/v] heat-inactivated Fetal Bovine Serum and 1% [w/v] L-glutamine). Cell stocks were frozen at -80°C overnight and were then transferred to liquid nitrogen for longer term storage. Vero-E6 cells passaged up to a maximum of 10 passages post -thawing were used for this validation (excepting robustness testing, where cells were passaged for an additional six passages up to 16 passages post thawing), at which point a new working cell bank stock was retrieved from liquid nitrogen for further use. The maximum number of cell passages currently permitted was determined empirically (although supported by the present data), and further future studies evaluating the virus back titration and MN50 data for a representative panel of convalescent and healthy donor sera will be used to determine whether the maximum acceptable passage number might be expanded.
2.5. Cell Viability measurement (MTT assay)
Cells seeded in 96 well plates at 1.5 x 104 cells/well density were infected in quadruplicates with serial dilutions of the prototype and variant strains starting from 200 TCID50/well. CPE in the infected wells was scored using the microscope as described in section 2.1 above.
Cell viability was determined at day 2 and 3 post-infection by staining with MTT cell viability assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) using the standard kit protocol (Promega, Australia). A 100µL volume of a 3 mg/mL solution of MTT was added to plates and incubated for 2 hours at 37°C in a 5% CO2 incubator. Liquid in the culture wells were aspirated to dryness using a multichannel manifold attached to a vacuum chamber and formazan crystals were solubilized by the addition of 200µL 100% 2-Propanol at room temperature for 30 minutes. Absorbance was measured at 540 – 650nm on a plate reader. MTT values in the wells were normalized to "no virus control cells only wells”.
2.6. Validation assays
2.6.1. Precision (inter-assay, intra-assay)
Thirty-two samples were tested twice per assay run (in duplicate), in 2 different assay runs performed by 3 different analysts (6 total runs). The geometric mean MN50 titer (GMT) was calculated for each sample from all of the runs. Inter- and intra-assay precision were then estimated by calculating percent geometric coefficient of variation (%GCV), based on variance component analysis using analyst and day as random effects, and the samples as a fixed effect. The acceptance criterion for precision was that at least 80% of samples should have a %GCV≤35% (for Ancestral strain) and ≤40% for variants for both intra and inter assay measurements.
2.6.2. Sensitivity (LLoQ)
Lower limits of quantification (LLoQ) were determined using precision and linearity assessment data. LLoQ was defined by the lowest MN50 dilution that could be quantitatively determined with acceptable precision (%GCV ≤35% for at least 80% of samples);
2.6.3. Assay range (ULoQ)
Four serum samples with high titers were tested in the assay with measurement of inter assay % GCV. ULoQ was defined by the highest MN50 dilution with acceptable precision (%CV should be ≤35% for at least 80% of the validation samples).
2.6.4. Specificity and selectivity
To confirm specific detection of SARS-CoV-2 rS-induced neutralization, 6 representative positive serum samples were spiked with SARS-CoV-2 rS protein at 4ug/mL or 2 ug/mL (homologous inhibitor), or with RSV F protein (non-specific) at 4ug/mL (heterologous inhibitor). Samples were incubated with the spiked protein for 30 minutes prior to addition of 4000 TCID50 units/mL SARS-CoV-2. The target criteria were that homologous protein incubation would result in a ≥50% reduction in detected MN50 titer in at least 80% of samples, and the MN50 titer would remain at the same level (≤20% reduction) for RSV F protein in at least 80% of samples.
Selectivity was confirmed by analyzing twenty paired samples of pre- and post-vaccination sera. These sera included 14 pairs from the Novavax 2019nCoV-101 SARS-CoV-2 phase 1 study, 2 pairs from the EBOV-H-101 Novavax Ebola vaccine study, 2 pairs from the qNIV-E-201 Novavax influenza vaccine study, and 2 pairs from the RSV-M-301 Novavax RSV vaccine study. Subjects in the non-SARS-CoV-2 studies received vaccines manufactured on the same expression platform and purification strategy used to generate the Novavax SARS-CoV-2 antigen, and thus may have contained a similar set of process-related impurities. To assess the impact of any potential immune responses to impurities on SARS-CoV-2 neutralization in the assay, these 20 pairs of sera were analyzed for seroconversion. The target criteria were 100% seroconversion (defined as a titer ≥4 fold above the matched pre-vaccination serum) for SARS-CoV-2 vaccinated serum, and 0% seroconversion for non-SARS-CoV-2-vaccinated pairs.
2.6.5. Linearity
Two phase 1 clinical trial samples with high MN50 titers and 1 convalescent serum sample were tested in the assay undiluted and diluted from 1:2 to 1:256 in 4 assay runs. Samples were diluted in SARS-CoV-2 antibody-negative serum independently for each dilution. Simple linear regression was then performed with log10 expected MN50 GMT as the independent variable and log10 observed MN50 GMT as the dependent variable. The target criteria were as follows: 1) each dilution point should show % relative bias between -50% and 100%, and %GCV ≤ 61.4% based on MN50 assay qualification results; 2) the point estimate and the 95% CI of the slope should fall within the range 0.7 to 1.43; and 3) the coefficient of determination (R
2) should be ≥ 0.95. Percent relative bias was calculated as follows:
2.6.6. Stability
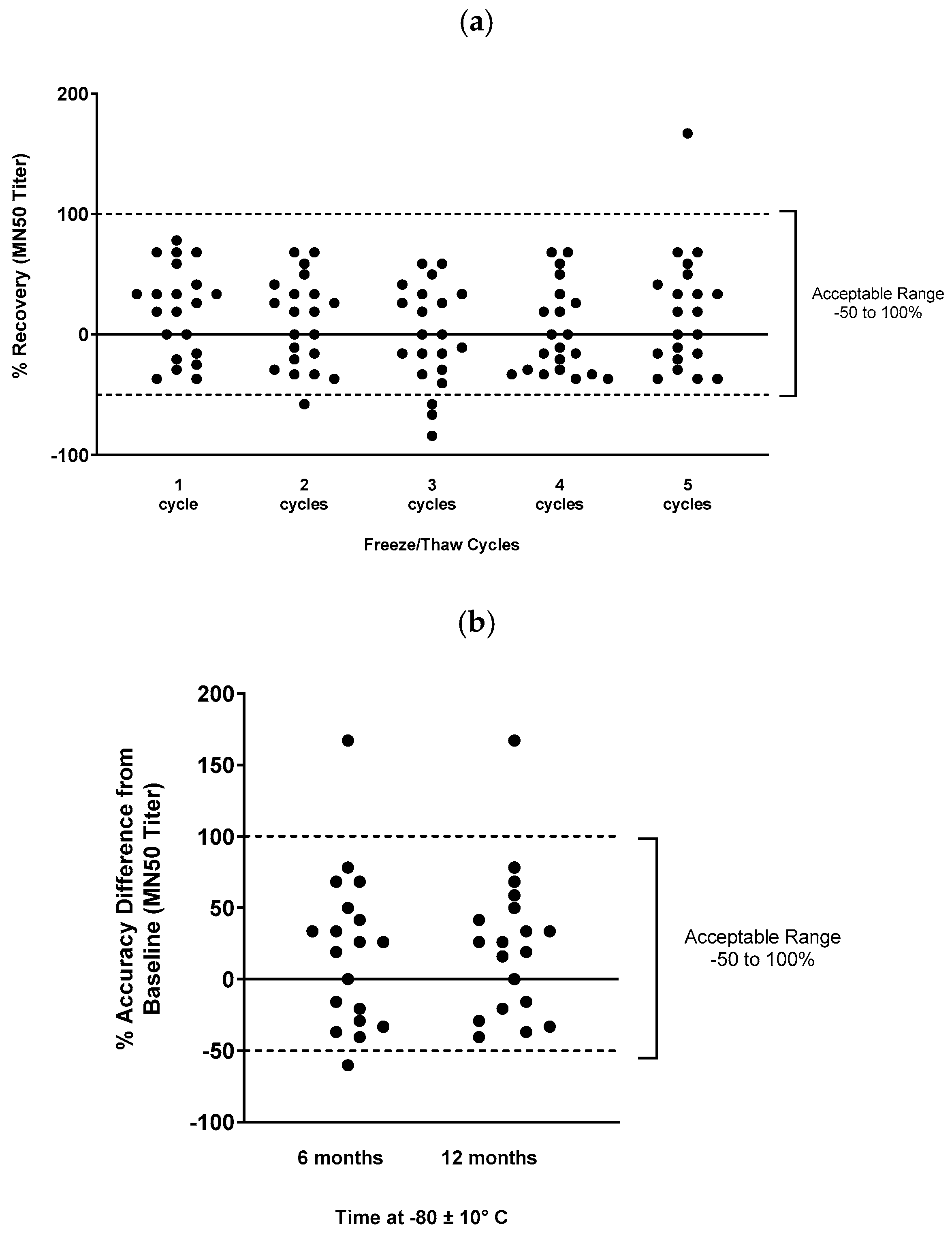
To assess freeze/thaw stability of the serum samples for the assay, 20 representative phase 1 samples and 1 convalescent donor sample were aliquoted and stored at –80 ± 10°C after 1 to 5 additional freeze/thaw events. Samples were then tested in the assay and compared to fresh samples. The target criterion was that the percent recovery (percent difference between baseline MN50 values in fresh samples and the observed MN50 value after freeze/thaw) should be within -50 to 100% for at least 80% of samples.
To assess long-term stability, 19 representative phase 1 or convalescent donor positive control samples were aliquoted and stored at –80 ± 10°C for 6 months or 12 months, then used in the assay. Again, the target criterion was for percent recovery to be within -50 to 100% of baseline for at least 80% of samples.
2.6.7. Assay robustness
To assess assay robustness, different assay parameters were intentionally varied from standard protocol and assay results were compared to baseline. The following parameters were varied for this analysis: virus/serum incubation time (60 minutes varied to 30 or 90 minutes), cell passage number (varied from P2 to P16), cell seeding density (1.5x104/well varied to 1x104/well or 2x104/well), SARS-CoV-2 TCID50 units/well (varied from 200 to 100 or 400 TCID50 units/well), and serum interference (addition of hemolysate [10mg/mL, Sun Diagnostics], triglyceride [15mg/mL Sun Diagnostics], or bilirubin [0.4mg/mL Sun Diagnostics] to serum prior to the assay). For all parameters, the target criterion was that percent recovery should be within -50 to 100% of baseline for at least 80% of samples.
2.7. SARS-CoV-2 Variant assays
The MN50 assay was originally developed using ancestral strain SARS-CoV-2 as defined above. However, the assay was later adapted to variant strains including Beta (B.1.351) (African Health Research Institute), Delta, and Omicron (BA.1, BA.5, XBB.1.5) viruses. A similar validation process was followed as described above, but the viral stocks were different based on the variant being tested. For Beta variant, the SARS-CoV-2 hCoV-19/501Y.V2 strain was used (Pango lineage B.1.351 confirmed). For Delta variant, the SARS-CoV-2-hCoV-19/Australia/VIC18440/2021 strain was used (Delta B.1.617.2 lineage. For Omicron BA.1, the SARS-CoV-2-hCoV-19/Australia/VIC29890/2021 strain was used (Omicron B.1.1.529 lineage). For Omicron BA.5, the SARS-CoV-2-hCoV-19/Australia/VIC61194/2022 strain was used. Delta, BA.1 and BA.5 strains were provided by (Melbourne Health at the Doherty Institute, Australia. For XBB.1.5, the SARS-CoV-2-hCoV-19/USA/MD-HP40900/2022 (XBB.1.5 variant) strain was used (BEI Resources, Manassas, VA, USA).
3. Results
3.1. Comparison of cytopathic effect (CPE) between different strains (A/Vic, Delta, Omicron BA.1/BA5 and XBB.1.5)
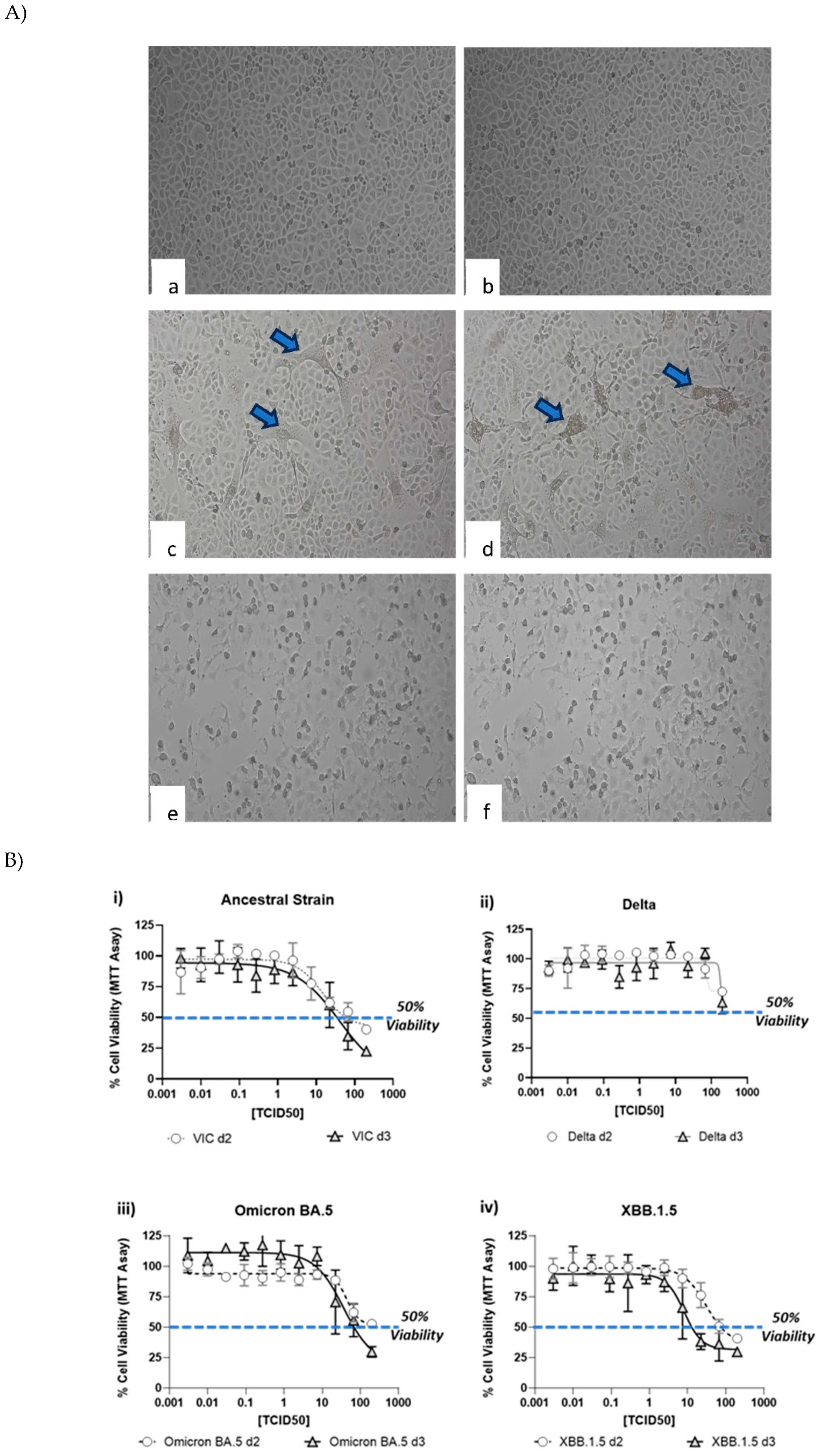
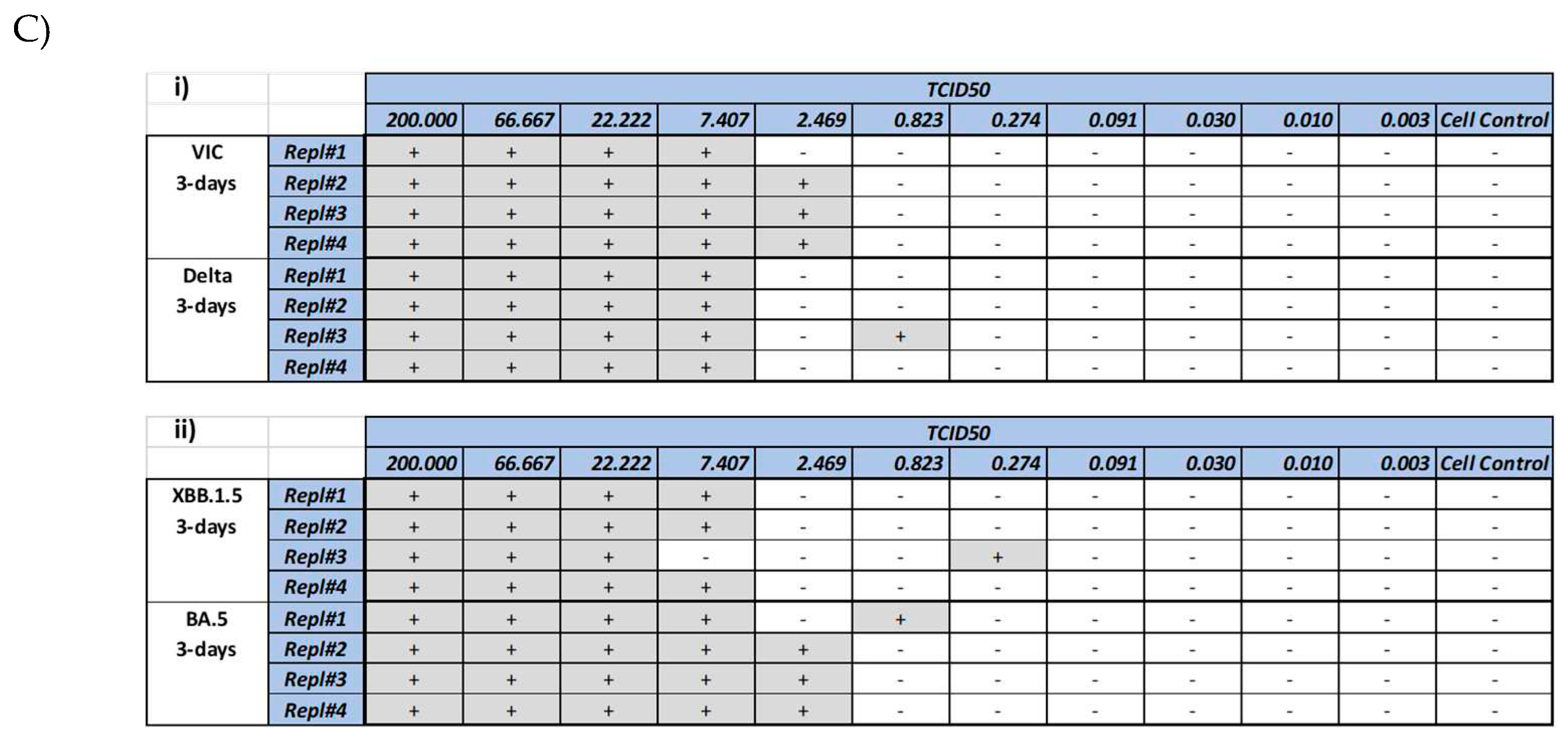
CPE induced by various SARS-CoV-2 strains was documented by visual inspection using microscopic imaging. In comparison to the no virus cell controls, cells infected with SARS-CoV-2 strains, showed significant CPE, which included cell rounding, detachment from the culture surface and cell death (
Figure 2A). Though most of the strains showed similar CPE (rounding, detachment and loss of viability), delta strain showed cell fusion and syncytia (
Figure 2A c, d), followed by cell death. In contrast, cells infected with XBB.1.5 showed rounding and cell death. To further quantitate the CPE, MTT assay was performed at day 2 and day 3 to understand the optimal time point of CPE. As shown in
Figure 2B, a dose dependent CPE was observed with all the 4 strains. A small increase in the CPE (as measured by lower MTT reading) was observed at higher dose of virus. Another method of CPE quantitation was also evalauted by microscopic scoring of the CPE at day 3 (
Figure 2C). Comparison of the microscopic scoring with the MTT based quantitation revealed that microscopic scoring by human eye was more sensitive, as CPE could be observed and documented in wells, with >50% and <75% viability. In light of these observations, microscopic scoring method was used for the assay methodology for validation.
3.2. Ancestral strain validation parameters
The overall inter-assay and intra-assay %GCV were 0% and 28.6%, respectively (
Table 1). A total of 100% (32 of 32) and 78% (25 of 32) of samples showed inter- and intra-assay %GCV ≤35%, respectively. 100% of samples had intra-assay %GCV <37%.
For sensitivity, LLoQ was assigned at an MN50 titer of 20, based on the lowest titer values that were accurately and precisely detected (as well as the precision and accuracy estimates of the assay [
Table 1]). Further based on the tested samples, the ULoQ was found to be an MN
50 of at least 10,240 for ancestral strain and the assay range was from 20 to 10,240 titer (MN
50).
Specificity of the microneutralization assay was successfully demonstrated (
Table 2). 100% of samples incubated with homologous rS protein 4 ug/mL showed a reduction in MN50 ≥50%. All samples incubated with RSV F protein 4ug/mL (nonspecific protein) showed a reduction in MN50 ≤20%. Selectivity was also shown, with none of the sample pairs showing seroconversion after vaccination for non-SARS-CoV-2 pathogens, and all sample pairs showing seroconversion after SARS-CoV-2 vaccination (
Table 2).
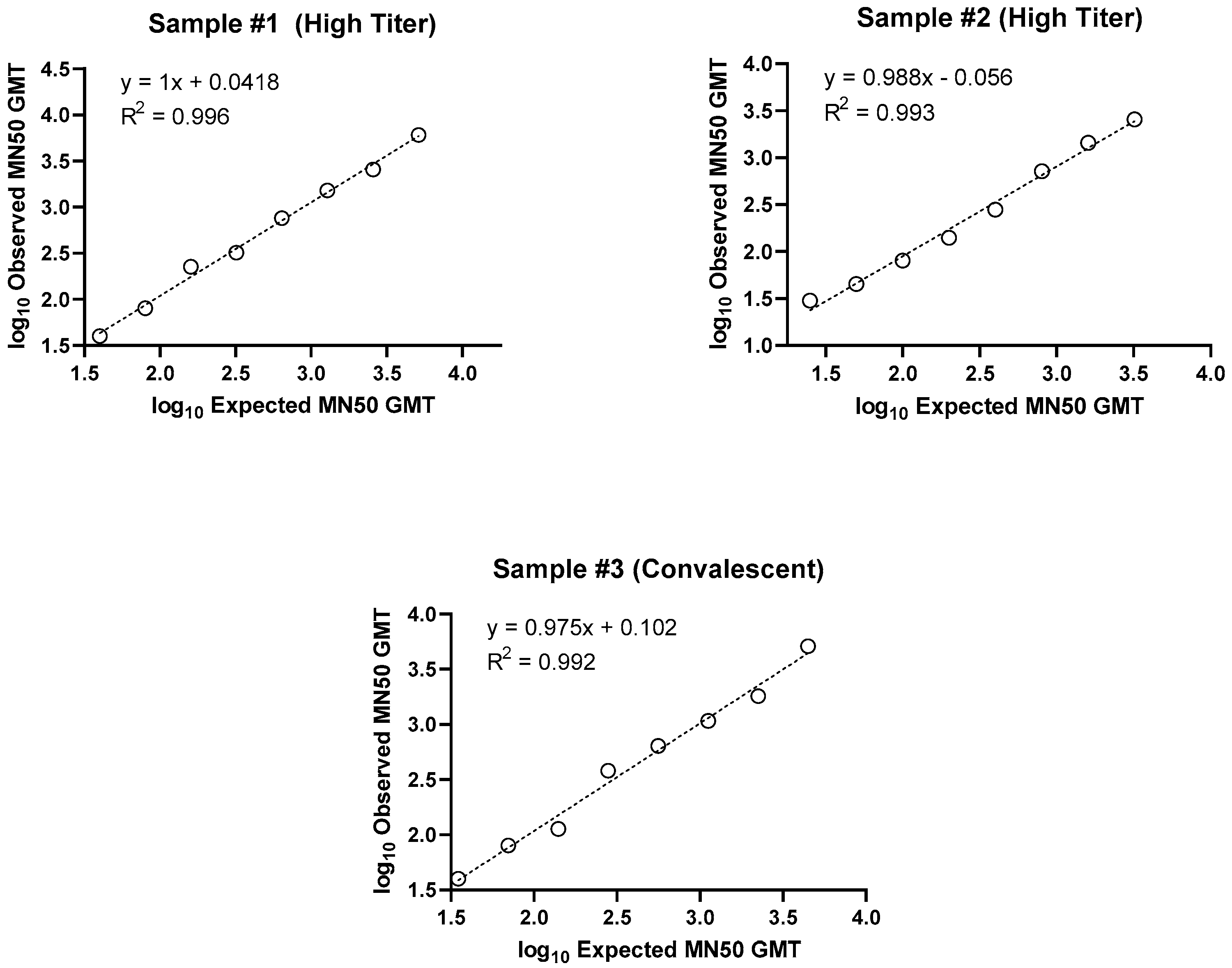
Linearity of the assay was demonstrated, with R
2 values of 0.996, 0.993, and 0.992 for the 3 individual samples examined (
Figure 3 and
Table 3). Relative bias criteria were met for 100% of dilutions for both high titer samples and 90% of dilutions for the convalescent serum.
Freeze/thaw and temperature stability of serum samples in MN assay were assessed, with 85-100% of samples meeting acceptance criteria at each condition (
Figure 4).
Robustness was assessed for various assay parameters (
Table 4). For virus incubation, 80% of samples showed acceptable levels of difference from baseline with 30 or 90 minutes of incubation instead of 60 minutes (
Table S1). For cell passage number, robustness was demonstrated up to P10 (tested at P2, P5, P6, and P10); 60% of samples met acceptance criteria even at P12 (
Table S2). For cell seeding density, robustness was shown for 1x10
4 cells/well (100%) and 2x10
4 cells/well (80%) instead of 1.5x10
4 cells/well (
Table S3). For SARS-CoV-2 TCID50 units/well, robustness was demonstrated for 100 TCID50 units/well (100%) and 400 TCID50 units/well (80%) instead of 200 TCID50 units/well (
Table S4). For serum interference, selectivity was shown for addition of triglyceride (83%), hemolysate (83%), and bilirubin (100%) in the assay (
Table S5).
3.3. Variant assay validation parameters
The original assay was developed using ancestral strain, and later validated for variant strains (including Beta, Delta, Omicron BA.1, Omicron BA.5 and XBB.1.5). Results for the assay validation parameters for the Beta, Delta, and Omicron variants were similar to those for ancestral strain (
Table 5). For each variant tested, more than 90% of samples achieved the ≤40% criterion for intra-assay precision and more than 75% of samples achieved the ≤40% criterion for inter-assay precision. All variant assays demonstrated selectivity, with 6 of 6 non-SARS-CoV-2 vaccinated pairs (from Ebola, Influenza, and RSV vaccine clinical trials) failing to show seroconversion in the assay. Each variant assay showed linearity, with LLoQ of 20 and ULoQ of 10,240. More than 80% of samples passed the ≤40% criterion for sensitivity for each variant assay.
3.4. Comparison of MN assay with other immunological assays
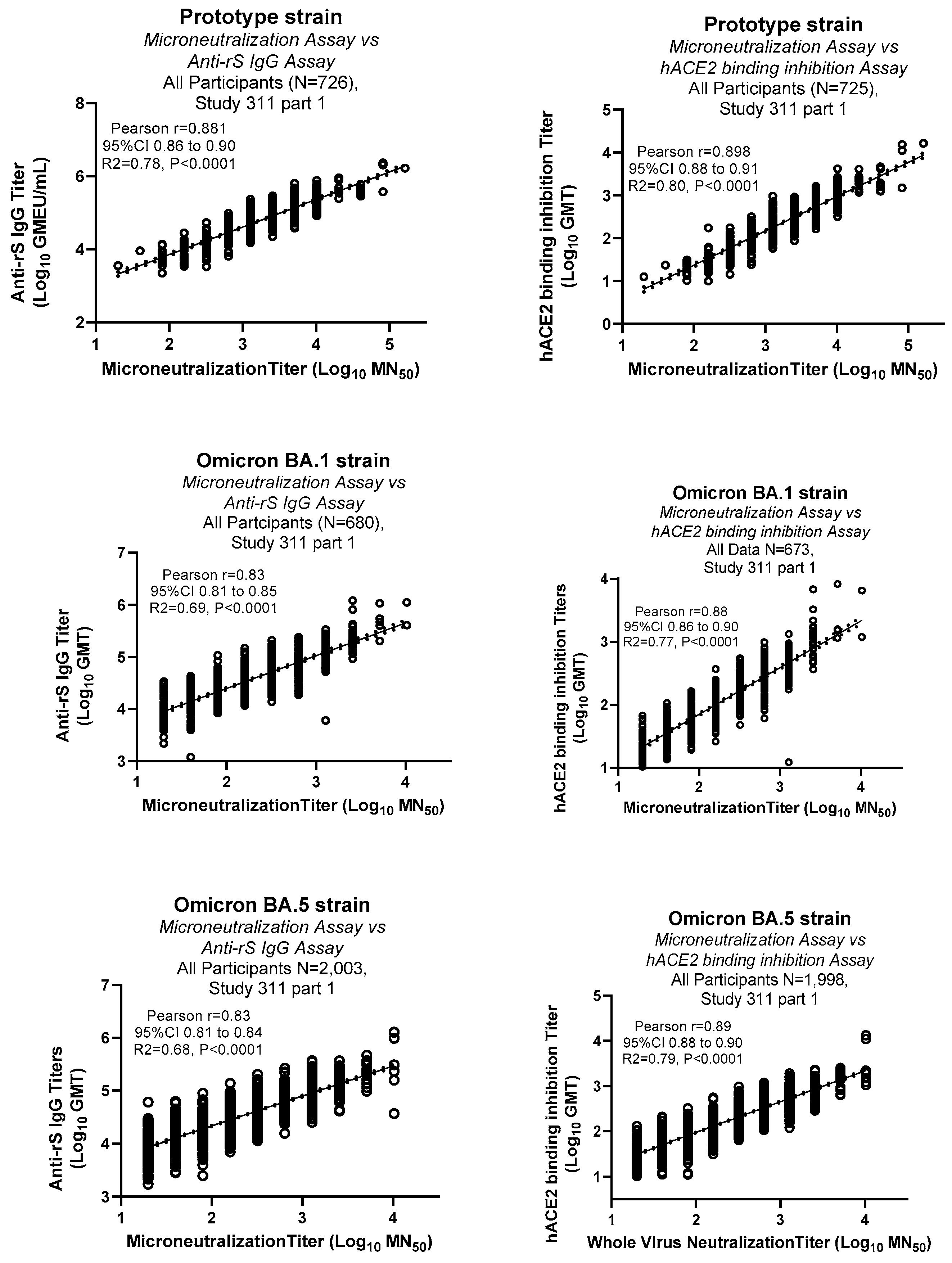
Clinical samples were tested in the MN assay for various strains including ancestral prototype, Omicron BA.1 and BA.5 strains. Results from the MN assays were compared with the binding antibody titers (Anti-rSpike IgG ELISA assay) [
13] and hACE2 binding inhibition assay [
14] (
Figure 5). These results showed strong positive correlations of MN assays data with both anti-rSpike IgG and hACE2 binding inhibition assays (Pearson r 0.83 to 0.9).
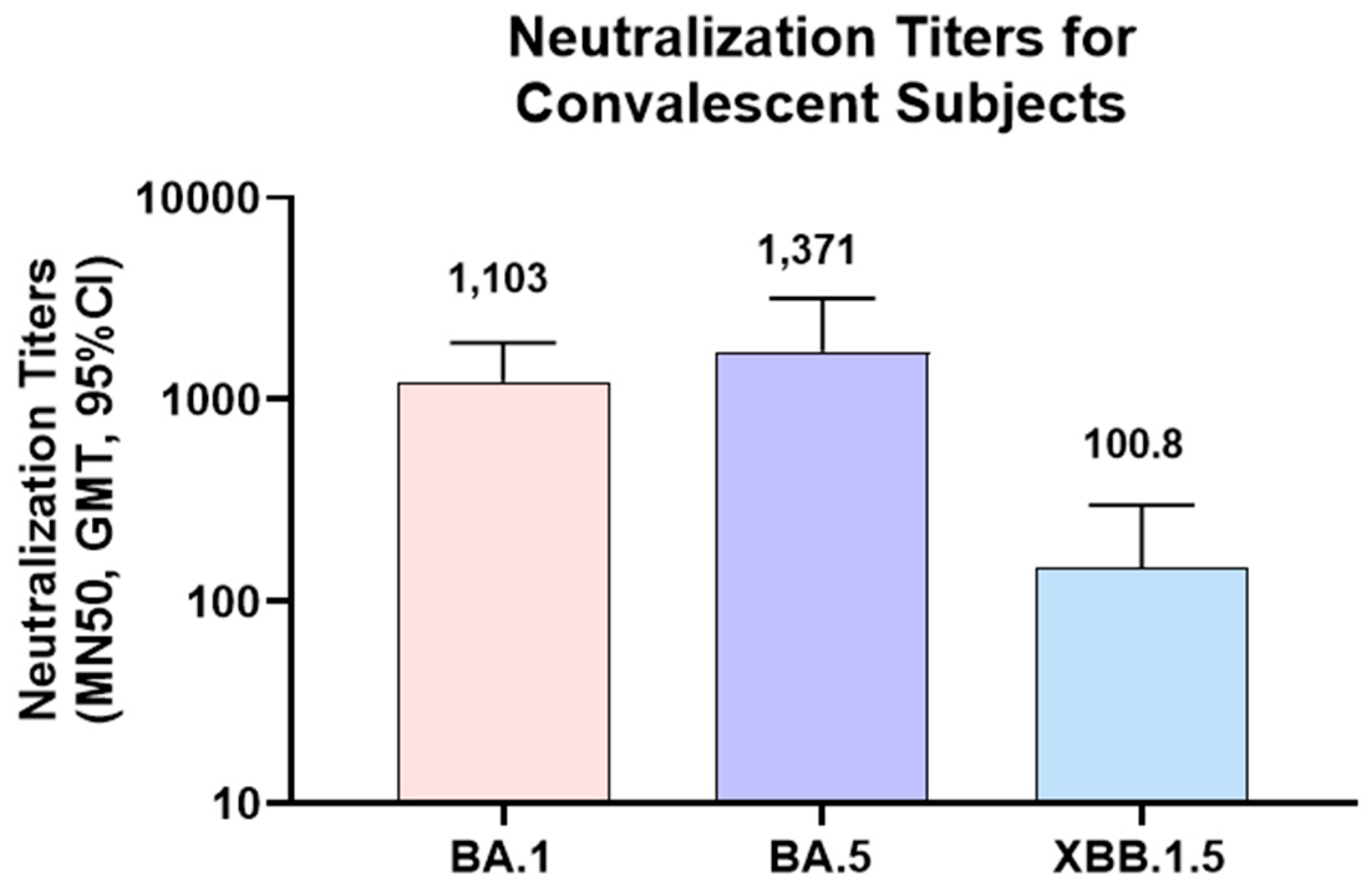
3.5. Convalescent serum sample testing against Omicron strains
To further confirm the utility of the MN assay in clinical sample testing, serum samples from convalescent subjects were tested in MN assays for Omicron strains. As the infections of these subjects were PCR confirmed for Omicron BA.1 strain, MN assay was focused on Omicron strains (BA.1, BA.5 and XBB.1.5). As expected, higher titers were observed for Omicron BA.1 and BA.5 (GMT of 1,103 and 1,371 respectively) than XBB.1.5 (GMT of 100.8). Lower titers in XBB.1.5 might be due to the significant differences in the XBB.1.5 RBD sequence compared to Omicron BA.1 and BA.5 strains.
3.6. Conversion to WHO International Units
To convert and compare between different assay methods the units using WHO international standard units (IU/mL) were determined. The NIBSC international standard 20/136 was run in duplicate by three operators in two independent assays alongside the MN50 assay quality control samples. The GMT expressed in 1/dilution units of the NIBSC International Standard across all runs was 1612.70. This titer corresponds with an arbitrary assigned value of 1,000 IU/mL, resulting in a conversion factor of 0.62 (i.e., multiply assay unit values by 0.62 to find IU/mL values) (
Table 6).
3.5. Correlation of microneutralization assay data with other immunological assays
4. Discussion
The MN50 assay as implemented here provides a robust and precise method for quantitation of neutralizing antibodies in clinical trial sera. This assay will be useful for assessment of vaccine immunogenicity, as neutralizing antibodies are a CoP for vaccine efficacy [
5,
6,
7,
8,
9]. The MN50 assay was precise, linear, and selective for ancestral strain as well as the Beta, Delta, Omicron BA.1, Omicron BA.5 and XBB.1.5 variants. Current recommendations from regulatory authorities suggest use of Omicron-based vaccines [WHO 2023, FDA 2023], so the ability of the assay to perform well for Omicron subvariants is critical (including for XBB.1.16, XBB.1.5, and others).
Different strains of SARS-CoV-2 exhibited differences in the presentation of CPE. Though cell rounding, death and detachment was observed for most strains, delta strain showed fusogenic CPE, as reported in the literature [
15]. Differences in the CPE and kinetics were observed between strains. Two methods of SARS-CoV-2 induced CPE were compared, as part of the assay development effort to develop a sensitive assay. Though the MTT method provides objective and quantitative reading, there might be a threshold for recording the 50% cell death/CPE by the MTT endpoint. In contrast, the microscopic-based readout was more sensitive as the visual CPE scoring can identify any evidence of CPE. Due to the higher sensitivity associated with microscopic reading, it was chosen further for our assay development and validation. Using microscopic scoring method as the assay endpoint also helped to overcome the intrinsic differences between the strains in CPE induction.
The intra assay precision results for ancestral strain showed 78% of the 32 samples tested met the stringent acceptance criterion of a %GCV of ≤35%. Deviation from the target performance, when noted, was marginal. The average margin of failure in the failing specimens was 1.39%, and all 32 samples tested (100%) demonstrated a %GCV of <37%. Further, every individual result fell within a ≤2-fold range about the overall GMT for that specimen, which is the expected performance of a classical 2-fold serial dilution virological assay. Similarly, for the Omicron BA.1 assay, the variability associated with assay precision assessment is captured exclusively in the inter-assay component, wherein 76% of samples met the acceptance criteria despite an overall inter-assay precision of 13.7%. In fact, 23 of 41 samples (56%) showed a % GCV of 0, and no individual result fell >2-fold away from the geometric means. Further, the supplementary set of sera from Omicron-BA.1-infected subjects did satisfy the target criteria. It is important to note that the %GCV target of ≤=40 was set based on the original qualification and validation of the assay with the ancestral strain, wherein a larger number of total replicate assays was used, which may have buffered the probability of %GCV results >40 arising from small numbers of data with 2-fold variations. For the Omicron BA.1 assay, the finding of all results within 2-fold of the GMT is consistent with the long-standing performance of two-fold dilution assays. In contrast, for XBB.1.5 strain, 91% and 97% of the tested samples showed % GCV ≤40%. In summary, although some variation in precision was observed in %GCV between variant strains, all strains ≤40% GCV.
MN assay showed strong correlation with other immunological assays (anti-rS IgG and hACE2 binding inhibition) for prototype, Omicron BA.1 and Omicron BA.5, which demonstrates a high degree of concordance between MN and other assays. As the XBB.1.5 MN assay was recently validated, samples tested in the MN assays are being evaluated in other assays. Correlation of MN data with other assay data gives us confidence in the MN assay developed and validated for multiple strains.
One limitation of the MN50 assay is that, due to its dependence on the virus induced CPE, differences between the capacities of virus variants to induce CPE, might pose challenges in harmonizing the assay method across different variants and confound the comparison of neutralization titers between different variants. In our assay method, to some extent we circumvented the strain differences in CPE induction by using microscopic scoring method. Additionally, performing the MN50 assay requires a BSL-3 laboratory due to the use of live SARS-CoV-2 virus and CPE scoring could be subjective. However, as this is a phenotypic assay, it is a robust and reliable method to measure virus neutralization. Other assays such as plaque reduction neutralization assays, pseudovirus assays or foci reduction neutralization assays may have automated readouts [
16], which could be less laborious/less hands on time. However, pseudovirus assays require additional effort and time in constructing and characterizing pseudoviruses, followed by demonstration of their concordance with the live virus assay data. Importantly, our assay uses live whole virus with strains representative of actual wild-type viruses and containing all viral proteins (including S and N proteins), in contrast to the pseudovirus assay which uses only a part of the virus (e.g., spike) needed to infect cells. Therefore, MN assay provides a readout that is directly applicable to circulating viruses in the population, including the impact of antibodies evoked in the clinical setting. This is critical, given that the intended use of this assay is to assess neutralizing antibodies for vaccine immunogenicity assessments. To our knowledge, this is the first report describing the extensive assay validation of SARS-CoV-2 microneutralization assay for various virus strains including the recent XBB.1.5 variant, which could be useful for the evaluation of the clinical samples from studies using the updated vaccine strain for 2023-24.
5. Conclusions
The MN50 assay described here was robust, specific, selective, linear, and precise for measurement of SARS-CoV-2 neutralizing antibodies in human serum, including from clinical trials. The assay maintained acceptable validation parameters even for immune-evasive Omicron variants. This assay will be useful for vaccine immunogenicity analyses in clinical trial samples and is applicable to variants in addition to ancestral strain SARS-CoV-2. Future work will focus on adapting the assay for currently circulating viral variants and emerging strains.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org, Table S1. Microneutralization assay ancestral strain robustness – virus incubation time; Table S2. Microneutralization assay ancestral strain robustness – cell passage; Table S3. Microneutralization assay ancestral strain robustness – cell seeding density; Table S4. Microneutralization assay ancestral strain robustness – SARS-CoV-2 TCID50 units/well; Table S5. Microneutralization assay ancestral strain robustness – serum interference; Table S6: Microneutralization assay results for SARS-CoV-2 variant strains.
Author Contributions
Conceptualization, JP, SH, MZ. LFF, MP, SCC; methodology, JP, MZ, SCC, MP, SH.; software, SH, PM.; validation, SH, PM, JP, SCC, MP, LFF., RK and MZ.; formal analysis, MZ, RC.; investigation, JP.; resources, MP, JP; data curation, SH; writing—original draft preparation, RK.; writing—review and editing, JP, LFF, RK, MP, SH, SCC, MZ, PM, JF, LC, RC; visualization, RK.; supervision, JP, MP.; project administration, PM.; funding acquisition, JP. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Novavax, Inc., and the APC was funded by Novavax, Inc.
Institutional Review Board Statement
All the clinical samples used in this study were either from commercial vendors or from vaccine clinical trials conducted by Novavax. The study protocols for the human clinical trials were conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Novavax (protocols 2019nCoV-311 part 1, dated 1 June 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Requests for the data presented in this study will be considered by the corresponding author. The data are not publicly available because of proprietary subject and sample information.
Acknowledgments
Writing and editorial support for the preparation of this manuscript was funded by Novavax, Inc. and provided by Kelly Cameron, PhD, and Rebecca Harris, PhD, of Ashfield MedComms (New York, NY, USA), an Inizio company. The authors acknowledge help from Doherty Institute Australia and African Health Research Institute, Durban, South Africa for providing SARS_CoV-2 strains. The following reagent was obtained through BEI Resources, NIAID, NIH: SARS-Related Coronavirus 2, Isolate hCoV-19/USA/MD-HP40900/2022 (Lineage XBB.1.5; Omicron Variant), NR-59104, contributed by Dr. Andrew S. Pekosz. The authors acknowledge help from the Novavax Clinical Operations and 2019nCoV-311 study team. The authors had full control of the manuscript and provided their final approval of all content.
Conflicts of Interest
All the authors that are employees of Novavax are stockholders of Novavax, Inc.
References
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect 2022, 28, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rocklov, J. The effective reproductive number of the Omicron variant of SARS-CoV-2 is several times relative to Delta. J Travel Med 2022, 29. [Google Scholar] [CrossRef] [PubMed]
- Firouzabadi, N.; Ghasemiyeh, P.; Moradishooli, F.; Mohammadi-Samani, S. Update on the effectiveness of COVID-19 vaccines on different variants of SARS-CoV-2. Int Immunopharmacol 2023, 117, 109968. [Google Scholar] [CrossRef] [PubMed]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; Consortium, C.-G.U.; Peacock, S.J.; Barclay, W.S.; de Silva, T.I.; Towers, G.J.; et al. SARS-CoV-2 variant biology: immune escape, transmission and fitness. Nat Rev Microbiol 2023, 21, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med 2021, 27, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.; Huang, Y.; Benkeser, D.; Carpp, L.N.; Anez, G.; Woo, W.; McGarry, A.; Dunkle, L.M.; Cho, I.; Houchens, C.R.; et al. Immune correlates analysis of the PREVENT-19 COVID-19 vaccine efficacy clinical trial. Nat Commun 2023, 14, 331. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.B.; Montefiori, D.C.; McDermott, A.B.; Fong, Y.; Benkeser, D.; Deng, W.; Zhou, H.; Houchens, C.R.; Martins, K.; Jayashankar, L.; et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022, 375, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Theel, E.S. Immunity to SARS-CoV-2: What Do We Know and Should We Be Testing for It? J Clin Microbiol 2022, 60, e0048221. [Google Scholar] [CrossRef] [PubMed]
- Van Tilbeurgh, M.; Lemdani, K.; Beignon, A.S.; Chapon, C.; Tchitchek, N.; Cheraitia, L.; Marcos Lopez, E.; Pascal, Q.; Le Grand, R.; Maisonnasse, P.; et al. Predictive Markers of Immunogenicity and Efficacy for Human Vaccines. Vaccines (Basel) 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Bewley, K.R.; Coombes, N.S.; Gagnon, L.; McInroy, L.; Baker, N.; Shaik, I.; St-Jean, J.R.; St-Amant, N.; Buttigieg, K.R.; Humphries, H.E.; et al. Quantification of SARS-CoV-2 neutralizing antibody by wild-type plaque reduction neutralization, microneutralization and pseudotyped virus neutralization assays. Nat Protoc 2021, 16, 3114–3140. [Google Scholar] [CrossRef] [PubMed]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H.; et al. Phase 1-2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N Engl J Med 2020, 383, 2320–2332. [Google Scholar] [CrossRef] [PubMed]
- Morales-Nunez, J.J.; Munoz-Valle, J.F.; Torres-Hernandez, P.C.; Hernandez-Bello, J. Overview of Neutralizing Antibodies and Their Potential in COVID-19. Vaccines (Basel) 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Cloney-Clark, S.; Feng, S.L.; Parekh, A.; Gorinson, D.; Silva, D.; Skonieczny, P.; Wilson, A.; Kalkeri, R.; Woo, W.; et al. A Severe Acute Respiratory Syndrome Coronavirus 2 Anti-Spike Immunoglobulin G Assay: A Robust Method for Evaluation of Vaccine Immunogenicity Using an Established Correlate of Protection. Microorganisms 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Massuda, E.; Patel, U.; Klindworth, A.; Massare, M.J.; Cai, R.; Fries, L.; Glenn, G.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 Receptor (Human Angiotensin-Converting Enzyme 2) Binding Inhibition Assay: A Rapid, High-Throughput Assay Useful for Vaccine Immunogenicity Evaluation. Microorganisms 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Irie, T.; Suzuki, R.; Maemura, T.; Nasser, H.; Uriu, K.; Kosugi, Y.; Shirakawa, K.; Sadamasu, K.; Kimura, I.; et al. Enhanced fusogenicity and pathogenicity of SARS-CoV-2 Delta P681R mutation. Nature 2022, 602, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.P.C.; Quadros, H.C.; Fernandes, A.M.S.; Goncalves, L.P.; Badaro, R.; Soares, M.B.P.; Machado, B.A.S. An Overview of the Conventional and Novel Methods Employed for SARS-CoV-2 Neutralizing Antibody Measurement. Viruses 2023, 15. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Procedure for performing the microneutralization assay.
Figure 1.
Procedure for performing the microneutralization assay.
Figure 2.
Cytopathic effect (CPE) induced by SARS-CoV-2 virus strains. Vero cells were infected with various SARS-CoV-2 virus strains as mentioned in the methods section, followed by microscopic imaging (A), cell viability measurement (B) and CPE scoring. Imaging was performed at day 2 post-infection. Panel 2A shows uninfected cells (a, b), cells infected with delta strain (c, d), cells infected with XBB.1.5 (e, f). Arrows show syncytia observed after infection with Delta strain. Circles and triangles in panel (B) show the viability at day 2 and 3 respectively. Gray shades in panel (C) show wells marked positive (+) for CPE by manual microscopic observation. Wells marked (-) did not show any CPE observable by microscopy.
Figure 2.
Cytopathic effect (CPE) induced by SARS-CoV-2 virus strains. Vero cells were infected with various SARS-CoV-2 virus strains as mentioned in the methods section, followed by microscopic imaging (A), cell viability measurement (B) and CPE scoring. Imaging was performed at day 2 post-infection. Panel 2A shows uninfected cells (a, b), cells infected with delta strain (c, d), cells infected with XBB.1.5 (e, f). Arrows show syncytia observed after infection with Delta strain. Circles and triangles in panel (B) show the viability at day 2 and 3 respectively. Gray shades in panel (C) show wells marked positive (+) for CPE by manual microscopic observation. Wells marked (-) did not show any CPE observable by microscopy.
Figure 3.
Microneutralization assay ancestral strain (SARS-CoV-2 hCoV-19/Australia/VIC01/2020) demonstrating analysis of linearity for three serum samples from individuals (either vaccinated or convalescent following infection). Two samples with high SARS-CoV-2 MN50 titers and one convalescent serum sample were diluted in series and tested in 4 assay runs. Linearity was then evaluated by calculating precision and accuracy of the titer at each dilution, and the slope of linear regression lines for each sample. (a) Sample #1 (high titer), (b) Sample #2 (high titer), and (c) Sample #3 (convalescent serum) are presented.
Figure 3.
Microneutralization assay ancestral strain (SARS-CoV-2 hCoV-19/Australia/VIC01/2020) demonstrating analysis of linearity for three serum samples from individuals (either vaccinated or convalescent following infection). Two samples with high SARS-CoV-2 MN50 titers and one convalescent serum sample were diluted in series and tested in 4 assay runs. Linearity was then evaluated by calculating precision and accuracy of the titer at each dilution, and the slope of linear regression lines for each sample. (a) Sample #1 (high titer), (b) Sample #2 (high titer), and (c) Sample #3 (convalescent serum) are presented.
Figure 4.
Temperature and freeze/thaw stability of clinical serum samples for the microneutralization assay. Samples were stored frozen for various lengths of time, or were subjected to multiple freeze/thaw cycles, then were used in the microneutralization assay to determine sample stability. The acceptable range of deviation was -50% to 100% of the reference condition values (shown by dashed lines). (a) Freeze/thaw cycle stability, and (b) temperature stability is shown.
Figure 4.
Temperature and freeze/thaw stability of clinical serum samples for the microneutralization assay. Samples were stored frozen for various lengths of time, or were subjected to multiple freeze/thaw cycles, then were used in the microneutralization assay to determine sample stability. The acceptable range of deviation was -50% to 100% of the reference condition values (shown by dashed lines). (a) Freeze/thaw cycle stability, and (b) temperature stability is shown.
Figure 5.
Correlation of MN assay data with other immunological assays: Serum samples from a clinical study (2019nCoV-311 study part 1) were tested in MN assay for various strains and other immunological assays (Anti-rSpike IgG and hACE2 binding inhibition assays. Pearson correlation analysis with 95% Confidence interval (dotted lines) and R2 values are shown in the figure. Correlation analysis was conducted using GraphPad Prism (Version 9.3.1).
Figure 5.
Correlation of MN assay data with other immunological assays: Serum samples from a clinical study (2019nCoV-311 study part 1) were tested in MN assay for various strains and other immunological assays (Anti-rSpike IgG and hACE2 binding inhibition assays. Pearson correlation analysis with 95% Confidence interval (dotted lines) and R2 values are shown in the figure. Correlation analysis was conducted using GraphPad Prism (Version 9.3.1).
Figure 6.
Neutralization titers of convalescent subject sera against Omicron variants in MN assay: Serum samples from convalescent subjects were tested in MN assay for Omicron strains (BA.1, BA.5 and XBB.1.5). MN50 titers (GMT, 95% CI) are shown in the figures. N=18 for Omicron BA.1 and XBB.1.5, N=11 for Omicron BA.5. GMT titers are shown on top of the bars.
Figure 6.
Neutralization titers of convalescent subject sera against Omicron variants in MN assay: Serum samples from convalescent subjects were tested in MN assay for Omicron strains (BA.1, BA.5 and XBB.1.5). MN50 titers (GMT, 95% CI) are shown in the figures. N=18 for Omicron BA.1 and XBB.1.5, N=11 for Omicron BA.5. GMT titers are shown on top of the bars.
Table 1.
Microneutralization assay with ancestral strain (SARS-CoV-2 hCoV-19/Australia/VIC01/2020) showing analysis of parameters analyzed for precision (MN50 titer).
Table 1.
Microneutralization assay with ancestral strain (SARS-CoV-2 hCoV-19/Australia/VIC01/2020) showing analysis of parameters analyzed for precision (MN50 titer).
Serum
Sample |
Geometric Mean MN50 |
Inter-assay %GCV |
Intra-assay %GCV |
| Overall |
N/A |
0 |
28.6 |
| 1 |
1810.2 |
24.1 |
30.7 |
| 2 |
56.6 |
10.4 |
37.0 |
| 3 |
508.0 |
12.7 |
34.4 |
| 4 |
38.1 |
31.8 |
27.3 |
| 5 |
403.2 |
13.1 |
33.1 |
| 6 |
6088.7 |
14.0 |
29.6 |
| 7 |
31.8 |
0 |
35.2 |
| 8 |
67.3 |
31.8 |
27.3 |
| 9 |
380.6 |
0 |
32.1 |
| 10 |
806.4 |
13.1 |
33.1 |
| 11 |
239.7 |
29.9 |
33.1 |
| 12 |
59.9 |
0 |
36.9 |
| 13 |
119.9 |
0 |
36.9 |
| 14 |
8127.5 |
0 |
35.2 |
| 15 |
1436.8 |
0 |
27.5 |
| 16 |
8610.0 |
0 |
32.1 |
| 17 |
15.9 |
13.1 |
33.1 |
| 18 |
53.4 |
0 |
36.9 |
| 19 |
30.0 |
0 |
36.9 |
| 20 |
53.4 |
34 |
30.5 |
| 21 |
30.0 |
29.9 |
33.1 |
| 22 |
23.8 |
0 |
32.1 |
| 23 |
<20 |
0 |
0 |
| 24 |
<20 |
0 |
0 |
| 25 |
<20 |
0 |
0 |
| 26 |
<20 |
0 |
0 |
| 27 |
<20 |
0 |
0 |
| 28 |
<20 |
0 |
0 |
| 29 |
<20 |
0 |
0 |
| 30 |
<20 |
0 |
0 |
| 31 |
<20 |
0 |
0 |
| 32 |
<20 |
0 |
0 |
Table 2.
Microneutralization assay with ancestral strain (SARS-CoV-2 hCoV-19/Australia/VIC01/2020) showing analysis of parameters to determine assay specificity.
Table 2.
Microneutralization assay with ancestral strain (SARS-CoV-2 hCoV-19/Australia/VIC01/2020) showing analysis of parameters to determine assay specificity.
| Assay Specificity |
|---|
| Sample |
No
competitor protein |
Incubated with
Homologous rS Protein,
2 µg/mL |
Incubated with
Homologous rS Protein,
4 µg/mL |
Incubated with
RSV F Protein (non-specific),
4 µg/mL |
| MN50 |
MN50 |
% Reduction* |
MN50 |
% Reduction* |
MN50 |
% Reduction* |
| 1 |
1280 |
640 |
50 |
320 |
75 |
1280 |
0 |
| 2 |
640 |
640 |
0 |
40 |
93.8 |
640 |
0 |
| 3 |
320 |
160 |
50 |
20 |
93.8 |
320 |
0 |
| 4 |
5120 |
5120 |
0 |
320 |
93.8 |
5120 |
0 |
| 5 |
1280 |
640 |
50 |
40 |
96.7 |
1280 |
0 |
| 6 |
1280 |
1280 |
0 |
40 |
96.9 |
1280 |
0 |
| |
|
| Assay Selectivity |
|
| Sample |
Vaccination Type** |
Pre-Dose |
Post-Dose |
| MN50 |
MN50 |
Fold Shift |
| 1 |
Ebola |
≤20 |
≤20 |
1 |
| 2 |
Ebola |
≤20 |
≤20 |
1 |
| 3 |
Influenza |
≤20 |
≤20 |
1 |
| 4 |
Influenza |
≤20 |
≤20 |
1 |
| 5 |
RSV |
≤20 |
≤20 |
1 |
| 6 |
RSV |
≤20 |
≤20 |
1 |
| 7 |
SARS-CoV-2 |
≤20 |
2560 |
>128 |
| 8 |
SARS-CoV-2 |
≤20 |
5120 |
>256 |
| 9 |
SARS-CoV-2 |
≤20 |
2560 |
>128 |
| 10 |
SARS-CoV-2 |
≤20 |
1810.2 |
>91 |
| 11 |
SARS-CoV-2 |
≤20 |
508.0 |
>25 |
| 12 |
SARS-CoV-2 |
≤20 |
380.5 |
>19 |
| 13 |
SARS-CoV-2 |
≤20 |
6088.7 |
>304 |
| 14 |
SARS-CoV-2 |
≤20 |
380.5 |
>19 |
| 15 |
SARS-CoV-2 |
≤20 |
806.3 |
>40 |
| 16 |
SARS-CoV-2 |
≤20 |
239.7 |
>12 |
| 17 |
SARS-CoV-2 |
≤20 |
119.9 |
>6 |
| 18 |
SARS-CoV-2 |
≤20 |
8127.5 |
>406 |
| 19 |
SARS-CoV-2 |
≤20 |
1436.8 |
>72 |
| 20 |
SARS-CoV-2 |
≤20 |
403.2 |
>20 |
Table 3.
Microneutralization assay validation using ancestral strain to determine linearity.
Table 3.
Microneutralization assay validation using ancestral strain to determine linearity.
| Sample |
Parameter |
Estimate |
95% LCL |
95% UCL |
1
(high titer) |
Slope |
0.998 |
0.913 |
1.062 |
| Intercept |
-0.056 |
-0.259 |
0.147 |
| Residual Variability (% GSD) |
-0.058 (14.4%) |
N/A |
| R2
|
0.9956 |
N/A |
2
(high titer) |
Slope |
0.998 |
0.913 |
1.062 |
| Intercept |
-0.056 |
-0.124 |
0.328 |
| Residual Variability (% GSD) |
-0.074 (18.5%) |
N/A |
| R2
|
0.9929 |
N/A |
3
(convalescent) |
Slope |
0.975 |
0.895 |
1.055 |
| Intercept |
0.102 |
-0.124 |
0.328 |
| Residual Variability (% GSD) |
0.079 (19.8%) |
N/A |
| R2
|
0.9917 |
N/A |
| GSD—geometric standard deviation; LCL—lower confidence limit; N/A—not applicable; UCL—upper confidence limit. |
Table 4.
Microneutralization assay validation using ancestral strain to determine robustness.
Table 4.
Microneutralization assay validation using ancestral strain to determine robustness.
| Condition |
Target Criteria |
Results |
| Virus incubation time |
|
|
| 30 minutes sera/virus incubation |
-50 to 100 % difference between baseline MN50 and testing MN50 for ≥80% of samples |
Pass (80%) |
| 90 minutes sera/virus incubation |
Pass (80%) |
| Cell passage number (Vero E6 passage post-thawing) |
| P2 |
-50 to 100 % difference between baseline MN50 and testing MN50 for ≥80% of samples |
Pass (100%) |
| P5 |
Pass (100%) |
| P8 |
Pass (100%) |
| P10 |
Pass (80%) |
| P12 |
Fail (60%) |
| P16 |
Fail (40%) |
| Cell seeding density |
|
|
| 1x104 cells/well |
-50 to 100 % difference between baseline MN50 and testing MN50 for ≥80% of samples |
Pass |
| 2x104 cells/well |
Pass |
| SARS-CoV-2 Viral dose TCID50 units/well |
| 100 TCID50 units/well |
-50 to 100 % difference between baseline MN50 and testing MN50 for ≥80% of samples |
Pass (100%) |
| 400 TCID50 units/well |
Pass (80%) |
| Serum interference |
|
|
| Sera + 15mg/mL Triglyceride |
-50 to 100 % difference between baseline MN50 and testing MN50 for ≥80% of samples |
Pass (83%) |
| Sera + 10mg/mL Hemolysate |
Pass (83%) |
| Sera + 0.4mg/mL Bilirubin |
Pass (100%) |
Table 5.
Microneutralization assay results for SARS-CoV-2 variant strains.
Table 5.
Microneutralization assay results for SARS-CoV-2 variant strains.
| SARS-CoV-2 strain |
Precision |
Linearity |
LLoQ |
ULoQ |
Selectivity |
Sensitivity
(% of LLoQ samples with GCV ≤40%) |
| Intra-assay |
Inter-assay |
|
|
|
|
|
| Beta |
30% GCVa in 100% of samples
(44 of 44 samples) |
9% GCVa in 80% (35 of 44 samples) |
R2 = 0.9840 and 0.9937 |
20 |
≥ 10,240 |
0% seroconversion in 6 of 6 non-SARS-CoV-2 vaccinated pairs (100%) |
23 of 28 (82%) |
| Delta |
29.6% GCVa in 100% of samples (46 of 46
samples) |
13.2% GCVa in 87% (40 of 46 samples) |
R2 = 0.9877 and 0.9952 |
20 |
≥ 10,240 |
0% seroconversion in 6 of 6 non-SARS-CoV-2 vaccinated pairs (100%) |
17 of 20 (85%) |
| Omicron BA.1 |
28% GCVa in 100% of samples (41 of 41 tested
samplesb or 18 of 18
Omicron-positive
samples) |
15% GCVa in 76% (31 of 41 tested samples1) or 22.5% GCVa in 83% (15 of 18 Omicron-positive samples) |
R2 = 0.9959, 0.9941, and 0.992 |
20 |
≥ 10,240 |
0% seroconversion in 6 of 6 non-SARS-CoV-2 vaccinated pairs (100%) |
9 of 11 (82%) |
| Omicron BA.5 |
31.2% GCVa in 95% of samples (35 of 37
samples) |
5% GCVa in 100% (37 of 37 samples) |
R2 = 0.987 and 0.987 |
20 |
≥ 10,240 |
0% seroconversion in 6 of 6 non-SARS-CoV-2 vaccinated pairs (100%) |
7 of 7 (100%) |
| XBB.1.5 |
≤40% GCVa in 91% of samples (32 of 35 samples) |
≤40% GCVa in 97% (34 of 35 samples) |
R2 = 0.992 and 0.989 |
20 |
≥ 10,240 |
0% seroconversion in 6 of 6 non-SARS-CoV-2 vaccinated pairs (100%) |
17 of 19 (89%) |
Table 6.
Microneutralization assay conversion factor to WHO international standard (20/136) for Ancestral strain.
Table 6.
Microneutralization assay conversion factor to WHO international standard (20/136) for Ancestral strain.
| |
NIBSC International Standard 20/136 GMT1
|
Sample 1 GMT2
|
Sample 2 GMT |
Sample 3 GMT |
| Run 1/Operator 1 |
2560
1280 |
1280
2560 |
640
640 |
2560
1280 |
| Run 1/Operator 2 |
2560
1280 |
1280
1280 |
320
320 |
2560
1280 |
| Run 1/Operator 3 |
1280
1280 |
2560
1280 |
640
320 |
1280
1280 |
| Run 2/Operator 1 |
1280
1280 |
1280
1280 |
640
320 |
1280
1280 |
| Run 2/Operator 2 |
2560
2560 |
1280
1280 |
640
640 |
2560
2560 |
| Run 2/Operator 3 |
1280
1280 |
1280
2560 |
320
320 |
NA |
| Total Mean |
1612.70
1000 IU/mL |
1522.19
943.87 IU/mL |
452.55
280.62 IU/mL |
1688.97
1047.29 IU/mL |
| Run 1 Mean |
1612.70
1000 IU/mL |
1612.70
1000 IU/mL |
452.55
280.62 IU/mL |
1612.70
1000.00 IU/mL |
| Run 2 Mean |
1612.70
1000 IU/mL |
1436.75
890.90 IU/mL |
403.17
250.00 IU/mL |
1436.75
890.90 IU/mL |
| Conversion factor |
0.62 |
| WHO combined GMT3
|
1197 |
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).