1. Introduction
Sarcoma is a heterogeneous group of cancers of the connective tissue, which are inclusive of over 100 subtypes presenting as soft tissue, bone tumours and gastrointestinal stromal tumours (GIST). Tumours can therefore be present in any part of the body, with the majority presenting in the upper and lower extremities. The treatment burden for sarcoma is high, often requiring extensive surgery, high dose chemotherapy and radiotherapy. Sarcomas are also associated with a high risk of developing metastases, recurrence, and poorer survival in comparison to the common cancers [
1,
2,
3]. Further heterogeneity exists among patients with sarcoma because they can present from birth to old adulthood and some subtypes are a common cancer in younger people [
4].
The high risk of developing metastases and recurrence has resulted in treatment pathways in many countries, including the United Kingdom (UK), to comprise annual follow-up often accompanied with a scan. Repeated hospital attendances have a significant impact on patients’ emotional well-being [
5], as these require patients to revisit the experiences, they had at diagnosis [
6]. For this and other reasons, fears of sarcoma recurrence are common [
7]. Clinical consultations are driven by these emotions so patients either focus on what their treatment options are if the scans are positive or experience relief if they are clear. This relief overwhelms everything, so patients often forget anything additional they want to discuss with their clinician [
8,
9]. One way to overcome this is using outcome measures within clinical practice to help direct meaningful communication between clinician and patients. However, to be meaningful, the outcome measure needs to reflect issues that are important to the patient and are representative of the disease.
Documenting patient-reported experience of healthcare and outcomes is now considered a central component in providing and evaluating quality cancer care in the UK [
10]. Aligned to the importance of patient-reported outcomes (PRO) has been the increase in instruments to measure them. This has progressed from generic measures that can be used across all disease states, such as the Short-Form-36 (SF36) [
11], to condition-specific versions, for example, the European Organisation for Research and Treatment in Cancer Quality of Life Questionnaire (EORTC QLQ-C30) [
12]. Adoption of patient-reported outcome measures (PROM) into clinical practice can improve health-related processes, outcomes and satisfaction with care, however there are often reported significant barriers to adoption and implementation, including length and perceived burden on clinical teams [
13], fear of bringing up topics of conversation that clinicians are not trained or equipped to follow through on [
14], or that more generalisable measures may not be perceived as being patient-centred enough to be clinically useful [
15].
The importance of using condition- or disease-specific measures also follows the observation that generic measures are often not responsive to small changes in clinical condition and functioning, and they may overlook clinically relevant aspects related to the specific condition [
16]. Interpretation of results from generic measures may also be challenging in discerning assessment of overall health in relation to the patient’s specific condition. Conversely, disease-specific measures may not be comprehensive enough to allow comparison to other conditions, where the normative data from generic measures enables interpretation to be more meaningful [
16,
17,
18]. Administering PROMs alongside condition-specific measures is, therefore, recommended to capture the burden of disease whilst also facilitating comparisons to other populations.
Relating specifically to the age distribution of those diagnosed with sarcoma, very few validated PROMs span all age ranges and accommodate variance relating to developmental stages. Validation in specific target populations is critical; however, it is important to ensure that PROMs can be appropriately used in clinical practice and are applicable for assessing population-specific challenges over longer periods of follow-up and surveillance.
While many generic and generic-cancer PROMs often provide an additional cancer-type module (see EORTC:
https://qol.eortc.org/; FACIT:
https://www.facit.org/; PROMIS:
https://www.promishealth.org/), there are currently no PROMs specific for sarcoma. Measures currently available for sarcoma include the BtDux, which is specifically for teenagers and young adults with bone tumours [
19], the Soft Tissue Sarcoma questionnaire, which specifically focuses on symptoms associated with six soft tissue subtypes [
20], the Toronto Extremity Sarcoma Scale (TESS), which measures the impact of upper and lower limb sarcoma on activities of daily living [
21], and the Gounder/DTRF Desmoid Symptom/Impact Scale (GODDESS), which has been developed for patients with desmoid tumours or aggressive fibromatosis [
22].
To address this existing gap in available PROMs for sarcoma, we aimed to develop and validate an outcome measure that better reflects the patient experience of living with a sarcoma diagnosis across the lifespan. The PROM was based on the following definition of health-related quality of life: “… subjective, multidimensional, and dynamic. It is unique to each individual and includes aspects of physical, psychological and social function. It is dependent upon not only the stage of development but also the illness trajectory. This involves the achievement of goals and aspirations and the constraints imposed through ill health and treatment” [
23].
The study used the broad methodology employed for developing quality of life measures in cancer [
24], significantly content selection decisions were driven by patient experience rather than researcher or clinician bias to remain true to the ‘subjective’ aspect of the above definition. Previous reports on the development of our novel Sarcoma Assessment Measure (SAM) are presented in detail elsewhere [
8,
9,
25] and the methods of development are presented in
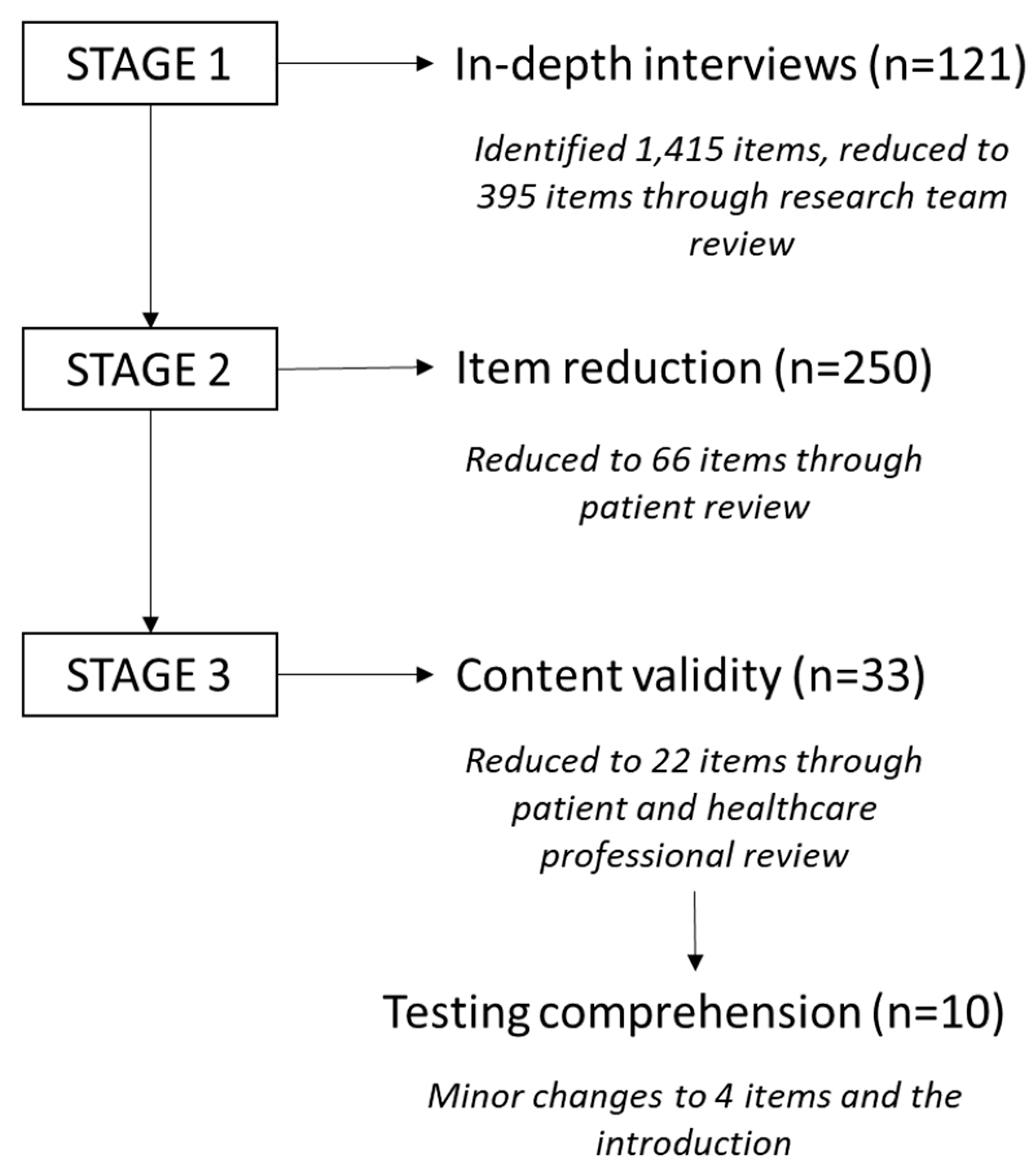
Figure 1.
In summary, text reflecting an outcome or experience following a sarcoma diagnosis was extracted from the transcripts and assigned to one of the domains of quality of life (physical, emotional, and social wellbeing). Additional fields of finance and sexuality were added as these were frequently discussed. Each of the 1,415 items were assigned a subtheme, e.g., fatigue, self-esteem. These were reviewed by the research team and reduced to 395 items after removing duplicates and redundant items. Patients rated the 395-item questionnaire on two scales based on worry and importance, which allowed an impact score to be calculated. Those items which were deemed primarily to reflect the core construct of existing PROMs were removed, i.e., we wanted to capture the additional areas sarcoma impacted rather than developing another measure of quality of life [
26]. Sixty-six items were reviewed by patients and healthcare professionals and rated on their relevance. The final 22 items were tested for comprehension and following minor changes to the language, were included in the SAM questionnaire. Significantly, this was an innovative collaboration between researchers, patients, and healthcare professionals to capture the elements of experience that are most subjectively important to patients, that are not captured in existing measures [
25].
This phase of the study aimed to validate the SAM in a larger sample, in order to derive an appropriate scoring method, and assess suitability for use of the SAM in clinical care.
2. Materials and Methods
2.1. Study design
This was a prospective cross-sectional survey, recruiting patients from across the United Kingdom (UK). The study was approved by London-Riverside Research Ethics Committee (ref: 18/LO/0023) and survey administration was coordinated by Quality Health (IQVIA Ltd.).
2.2. Sample and setting
Patients were eligible to participate if they had a diagnosis of sarcoma, were aged 13 or older, and were able to communicate verbally or in written English. In line with general recommendations for PROM development (e.g., Brysbaert [
27]) our aim was to recruit a minimum of 220 participants (10 participants per item in SAM). However, due to the heterogeneous nature of sarcoma, a larger sample was sought in order that variance according to clinical factors could be explored [
9].
Patients were recruited through three mechanisms. First, recruitment directly though participating hospitals. For young people aged 13-15 years, the study was explained to the parent/guardian and if they were happy for their child to participate it was explained to the young person, who then received the questionnaire if they too expressed interest. Second, through the National Cancer Patient Experience Survey (NCPES), which was coordinated by Quality Health, the original contracted hosts of the NCPES. When patients returned this survey, they had the opportunity to leave contact details to be invited to participate in future research. Patients participating in the 2014-2017 NCPES with a diagnosis of sarcoma, who provided future research consent were approached for this study. Finally, we re-invited those who had participated in the development phases of the study [
25]. Details of these participants were transferred securely to Quality Health. Regardless of identification method, all patients were given or sent information and the return of the questionnaire was implicit of consent.
2.3. Data collection
Data to test the psychometric properties of the SAM were collected using established, validated questionnaires. These were administered as a single questionnaire pack in paper format sent postally by Quality Health or given directly to patients in participating hospitals. Patients were instructed to complete the questionnaires without help and leave anything blank that was unclear. These were returned in a pre-paid envelope. No reminders were sent to patients recruited through participating hospitals. Patients sent questionnaires by Quality Health were sent two reminders after 2 and 4 weeks, and the data collection process was entirely managed by Quality Health.
The version of the SAM used in this study (version 1.0) contained 22 items, each scored on a 5-point Likert-type scale ranging from ‘strongly agree’ to ‘strongly disagree’ with the option of responding as ‘not applicable’ (see tables in results section for included items). The purpose of this study was to establish whether any items were redundant, and to establish a method of scoring the SAM.
Alongside our novel SAM, we included two additional questionnaires with established reliability and validity in cancer patients. These were selected due to the frequency with which they have previously been used in other studies involving patients with sarcoma [
26]. First, we included the EORTC QLQ-C30 [
12], a 30-item measure of Quality of Life, incorporating nine multiple-item scales: five functional scales (physical, role, emotional, social and cognitive); three symptom scales (fatigue, pain, and nausea and vomiting) and a global health and quality-of-life scale. Five single items assessed physical symptoms of dyspnoea, insomnia, appetite, diarrhoea, and constipation, and one item evaluated the financial impact of the disease. The first 28 items on the questionnaire are rated on a response scale from 1 (“not at all”) to 4 (“very much”), and compose the functioning, symptom, and financial difficulties scales. Items 29 and 30, which assess global health/QOL, use a response scale ranging from 1 (“very poor”) to 7 (“excellent”). Scores on the EORTC QLQ-C30 are transformed to a 0-100 point scale. Higher mean scores for the functional scales and global health status/QOL scale represent better functioning and overall QOL. While the QLQ-C30 is demonstrably reliable and valid for patients over 18 years, it has also been used in younger adolescents [
28]. Higher mean values for the multi-item symptom scales and higher scores for single items represent more frequent and/or more intense symptoms and a higher financial impact.
Second, we included the TESS. There are two versions of TESS to reflect upper and lower extremity limitations in daily life, such as restrictions in body movement, mobility, self-care, and performance of daily tasks and routine [
21]. These are commonly used in clinical practice in patients with extremity sarcoma. Items reflect activities of daily living that could be impacted by upper/lower limb disability rather than treatment side-effects. With permission from the author (personal correspondence), the upper and lower extremity versions were combined so it could be administered as a single measure. It included 17 unique upper and 16 lower extremity items, and 13 that were common across both (total of 46 items). Patients can answer questions concerning activities they do not perform in daily life with “not applicable.” The degree of physical disability is rated from 0 (not possible) to 5 (without any problem). Higher total scores indicate fewer functional limitations. The two final items which related to overall perceptions of activity and disability were left as stand-alone items.
2.4. Analysis
Neither traditional factor analytic approaches using Classical Test Theory (CTT) nor alternative Item Response Theory (IRT) approaches were suitable for this kind of checklist measure. Both CTT and IRT approaches assume that within a potential new measure, items will group together because they are each assessing a different aspect of a coherent underlying psychological construct; for example, the Hospital Anxiety and Depression Scale (HADS; Zigmond & Snaith [
29]) is suitable for these analyses because a sub-set of items collectively assess anxiety as a construct, and the remainder assess depression. Here, we had developed a list of items that could be best described as a problem checklist: not all participants were expected to identify with the relevance of all items (e.g., not everyone will be using a prosthesis or taking painkillers). Though the items on the checklist might all be caused by sarcoma, there is substantial variation in the presentation of sarcoma such that the measure would not meet the strict assumption of causation by a latent variable which underlies CTT and IRT (see Loehlin & Beaujean [
30] for a discussion).
An alternative approach might have been to ask respondents to weight each item on the perception of importance as has been done with psychometric scales such as the Social Readjustment Rating Scale [
31]. However, Cox et al. [
32] warn against such approaches as subjective weighting techniques can be erratic and lead to implausible conclusions. As Cox et al. (p.34) conclude: “Given the general lack of a pre-eminent weighting scheme, a prudent course includes checks on the robustness of conclusions to alternative, but still arbitrary, choices of weighting schemes.”
Consequently, to test whether a scoring scheme might be desirable in principle, we adopted a pragmatic approach to calculating a total score. First, we undertook a separate regression analysis for each item of the SAM to calculate how much each one predicted Global QoL as measured by the EORTC QLQ-C30 (our measure of convergent validity). To minimise respondent burden, it is generally desirable to remove items which contribute poorly to convergent validity and so we removed the three items which predicted 0% of the variance in Global QoL (rounded to 2 decimal places). We then compared two approaches of calculating an overall score: first, a simple mean score from all items; and second, a weighted mean with weights determined by their regression coefficient of prediction of global quality of life. We used an odd-even data split, creating Subsamples A and B, to avoid over-fitting the data: the first sample (odd numbered respondents) were used to create scoring weights using the regression analysis, and the second sample (even numbered respondents) were used to calculate total scores on which to test comparative efficacy of the two approaches (see Table 4). Given the level of ‘not applicable’ responses we adopted a generous cut-off for pro-rating in calculating total scores: where 10 or more items had been completed, a mean score was generated from the questions they did answer. Higher scores represent better functioning.
3. Results
Valid questionnaires were received from a total of 762 participants (
Table 1). The majority of patients had soft tissue sarcoma (72%) and were off treatment (77%). Despite our best efforts to recruit a younger sample, the mean age was 63 years (SD=17).
The mean score on the TESS was 180 (SD=52; N=614). Of the total sample, 68 (9.1%) rated their participation in activities of daily living as being ‘extremely difficult’ or ‘impossible’ to do, and 34 (4.5%) stated that they were either ‘severely disabled’ or ‘completely disabled’. For this paper we use only the QLQ-C30 Global Quality of Life Score: within this sample we recorded a mean score of 70.3 (SD=20.6).
3.1. Acceptability of the SAM
Table 2 summarises the number of respondents, the median and inter-quartile range (IQR) of the scores for each item. Sixteen questions were responded to by more than 75% participants. The six questions with a lower response related to items targeted at specific groups: amputations (questions 5 and 6), extremity surgery (question 4), younger patients (questions 8 and 19) and those experiencing pain (question 7).
3.2. Scoring the SAM
Table 3 summarises results from the regression coefficient models in which we used each item of the SAM to predict Global QoL. Based on these data, three items predicted <2% of the variance (number 2, 11, and 15), so these were removed from further analysis.
Pearson’s r correlation tests were then used to compare correlations between the weighted and unweighted mean scores with both Global Quality of Life and TESS total scores (see
Table 4). The SAM weighted mean score explained 1.7% more variance in Total TESS scores, and approximately 1.2% extra variance in Global QoL than the simple unweighted mean. SAM therefore explained 34.8% of the variance in Global QoL and 18.5% of TESS.
4. Discussion
The purpose of this study was to develop an outcome measure that could be used to guide clinical consultations. We also wanted to be able to derive a scoring formula to enable patients and/or clinicians to monitor changes over time. Health models suggest clinical care only contributes to 20% of clinical outcome [
33] and therefore we wanted to base the content of the measure on the aspects of living with a sarcoma diagnosis that were most important to patients. While some authors equate ‘experience’ to ‘experience of healthcare’ [
34] - the biomedical model of assessing outcome - we aimed to take a more holistic person-centred approach. Patients are more than their illness and after a sarcoma diagnosis we strive to support patients to reintegrate to their pre-diagnosis lives, or to find their ‘new normal’ [
8,
9]. Having an outcome measure that goes beyond symptoms is therefore important. Whilst symptom-based items were included in SAM, (e.g., pain), the measure assessed the personal impact of that symptom, for example whether it was manageable using pain-relieving medications.
The conceptual basis for the SAM was a model of quality of life [
23], so it included items related to the three domains of health (physical, emotional, and social functioning) [
35]. However, it was interesting to note in the development phase of SAM, there was a preponderance of items rated highly important and impactful by patients within the emotional wellbeing domain [
25]. This concurs with previous literature demonstrating a prevalence of anxiety and depression in patients with sarcoma in a fifth to a third of patients [
36,
37]. We have also identified fear of recurrence as having a significant impact on patients’ lives [
5], which was higher than reports in other types of cancer [
7]. Given the seemingly higher weighting patients place on the emotional impact of sarcoma, and the relatively superficial exploration of this in the literature, this is an area that warrants more detailed investigation.
Based on our regression analyses we excluded three of the twenty-two items from further analysis (2. I am more conscious of what I eat since I was diagnosed with sarcoma; 11. Since my diagnosis I appreciate everyday things more; 15. I try and cope emotionally on my own). Given our sample size, we are confident that these items do not contribute to our knowledge of the impact of sarcoma for most participants and so can be excluded from future iterations of the SAM.
Our results show that a weighted mean score on the SAM explained slightly more variance in TESS Total scores than a simple mean, suggesting that not all questions are equally important in predicting outcomes. It is likely that for maximised predictive validity, future users of the SAM may want to apply a weighting in calculating total scores. Whilst we provide the regression weights from our sample, researchers and clinicians will need to decide whether our sample is sufficiently similar to their own participants and clients in deciding whether to use these weights or to re-weight the items based on fresh data. It is also worth noting that the increased validity lent to SAM by using a more complex scoring approach is apparently minimal and so users may prefer to calculate a more parsimonious unweighted total score.
During the development of SAM, there appeared to be increased awareness across the sarcoma community of patient-reported outcomes and an interest in using SAM, not just for clinical practice but also as an endpoint in clinical trials and biomarker studies, e.g., ICONiC (Improving Outcome Through Collaboration in Osteosarcoma). The lack of sensitivity and/or specificity of existing generic cancer PROMs [
26] has highlighted the challenge of including these in research in sarcoma if there is the potential no difference will be detected over time. Consequently, there have been several subtype-specific PROMs developed for leiomyosarcoma, synovial sarcoma, liposarcoma or undifferentiated [
20] and desmoid tumours or aggressive fibromatosis [
22]. These are undoubtably valuable during the treatment phase of the patient journey, but we found a significant flooring effect (data not shown) with the EORTC QLQ-C30 symptom scales; given most of our participants were off treatment, this suggests that these were not symptoms experienced by this broader population of cancer survivors. As patients with sarcoma were not included in the development of the original EORTC measure and given that it was developed over 30 years ago when treatment for sarcoma was quite different, this is not surprising. It also raises the question whether a PROM developed from a biomedical perspective or focused more on the physical aspects of health will be suitable as an outcome in complex intervention trials, for example those testing psychological and behavioural interventions.
We are confident that SAM has clinical relevance, especially as clinicians and patients were involved in every stage of development and testing. However, we are cautious about the use of the current version as a sole outcome measure in biomedical studies, as this is beyond the reason for its development. We are currently making changes to the wording and structure of SAM, for example, adding filter questions for those items targeted at specific populations, e.g., amputees, childbearing age. We are now revalidating SAM-2 with these additional changes to be a PROM that can be used in research.
4.1. Limitations
The current study had several limitations. First, although we aimed to capture a broad range of participants through multiple recruitment methods, we had under representation of patients with GIST. The number of adolescents and young adults participating was also small (11%), so whilst the SAM as presented here might reflect the sarcoma-related experience overall, it may miss the nuances of developing sarcoma at this earlier life stage, which is recognised as being a challenging time to be diagnosed with cancer [
38,
39,
40]. Further validation in these groups may therefore be required. Second, we struggled with the amount of missing data in this dataset because of the response options provided to participants. It is obvious from the list of items that not all items in SAM will be applicable to all sarcoma patients. For example, not all will be taking painkillers (item 7) or living with a prosthesis (items 5 and 6). Closer analysis of these items shows that although a substantial number of participants responded, ‘not applicable’, a large proportion also missed these items out entirely. We can assume here that some participants were leaving the question blank because it was irrelevant to them. A consequence of this is that we cannot be certain that data in SAM were missing not at random, which complicates how we can interpret and deal with missing data [
41]. It will be necessary in developing the next version of SAM to alter item response options. One way to do this might be to replace statements of agreement with statements of scale of the problem (i.e., instead of
strongly disagree to
strongly agree, we could use
definitely not a problem for me to
definitely a problem for me). Alternatively, it might be possible to present the ‘not applicable’ option in a way that makes the explicit meaning clearer; for example, for item 5 (‘my prosthesis is heavy and uncomfortable’) the ‘not applicable’ option could be clarified as ‘I don’t wear a prosthesis’, or for Item 7 it could be clarified as ‘I don’t take painkillers’. Despite these limitations, this study recruited a substantial number of people who had been diagnosed with a range of sarcoma types, from across the UK, and represents the first PROM for patients with all types of sarcomas. Moreover, we were able to demonstrate the broad applicability and acceptability of a PROM designed to capture the holistic experience of those living both with and beyond treatment for sarcoma.
5. Conclusions
Our study aimed to validate a PROM for patients aged 13 onward with sarcoma. We are confident that SAM provides a comprehensive patient-reported outcome of multiple domains of the impact of sarcoma and its treatment, and that it is possible to generate a meaningful score of the total impact for individual patients. Given correlations with both global quality of life and total TESS scores, the SAM has high clinical relevance and could be a useful adjunct to clinical consultation discussions. Importantly, we have demonstrated that SAM picks up issues that are important to patients with sarcoma that are not reflected in other measures. With some minor alterations to response options, our data indicate that the SAM is likely a useful outcome measure for treatment trials and patient experience research in this population.
Author Contributions
Conceptualization, R.M.T., J.S.W., L.A.F., M.L., R.W., C.G., L.B., M.W., A.M. and L.S.; methodology, R.M.T., J.S.W., L.A.F., M.L., R.W., C.G., L.B., M.W., A.M. and L.S.; formal analysis, N.J.H. and L.H.; data curation, J.B. and H.O’S.; writing—original draft preparation, N.J.H., L.H., and R.M.T.; writing—review and editing, R.M.T., N.J.H. and L.H.; supervision, R.M.T.; project administration, A.M., R.M.T., J.B. and H.O’S.; funding acquisition, R.M.T., J.S.W., L.A.F, M.L., R.W., C.G., L.B., M.W., A.M. and L.S.; Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Sarcoma UK, grant number SUK102.2016. Rachel Taylor is partially funded by UCLH Charity. Lorna Fern is funded by Teenage Cancer Trust. Mary Wells acknowledges support from the Imperial Biomedical Research Centre. The views are of the authors and do not necessarily reflect those of Sarcoma UK, UCLH Charity, Department of Health and Social Care or Teenage Cancer Trust.
Institutional Review Board Statement
This study was approved by London-Riverside NHS Research Ethics Committee reference 18/LO/0023.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available on request from the authors.
Acknowledgments
The authors would like to thank the following sarcoma teams for recruiting to the study: Dr Fiona Cowie (Beatson Oncology Centre, Glasgow), Dr Helen Hatcher (Cambridge University Hospitals), Dr Dan Stark (Leeds Teaching Hospitals NHS Foundation Trust), Dr Sherron Furtado (Newcastle Upon Tyne Hospitals), Suriya Kirkpatrick (North Bristol NHS Trust ), Mr Robert Ashford (Nottingham University Hospitals NHS Foundation Trust and University Hospitals Leicester NHS Trust), Clare McKenzie (Oxford University Hospitals NHS Foundation Trust), Jayne Edwards and Mr Paul Cool (Robert Jones and Agnes Hunt Orthopaedic Hospital), Julie Woodford (Royal National Orthopaedic Hospital), Mr Jonathan Gregory (Royal Orthopaedic Hospital), Dr Robin Young (Sheffield Teaching Hospitals NHS Trust), Dr Mariam Jafri (University Hospitals Birmingham NHS Foundation Trust), Dr Paula Wilson (University Hospitals Bristol NHS Trust), Rebecca Burt (University Hospitals Coventry and Warwickshire NHS Trust), Dr Nicola Keay (University Hospitals Southampton NHS Foundation Trust), Sarah Massey (Royal Liverpool and Broadgreen University Hospitals), Mr Amit Kumar (Central Manchester University Hospital NHS Trust), Prof Jeremy Whelan (University College London Hospitals NHS Foundation Trust)
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thomas, D.M.; Whelan, J. Bone sarcomas in the adolescent and young adult population. In Cancer in Adolescents and Young Adults, 2nd ed. 2016. ed.; Bleyer Archie, Barr Ronald, Ries Lynn, Whelan Jeremy and Ferrari Andrea, Eds., 2017, pp. 417-427.
- Ferrari, A.; Whelan, J.; Ries, L.; Barr, R.; Bleyer, A. Soft tissue sarcoma. In Cancer in Adolescents and Young Adults, 2nd ed. ed.; Bleyer, A., Barr, R., Ries, L., Whelan, J. and Ferrari, A., Eds., 2017, pp. 383-416.
- Furtado, S.; Briggs, T.; Fulton, J.; Russell, L.; Grimer, R.; Wren, V.; Cool, P.; Grant, K.; Gerrand, C. Patient Experience After Lower Extremity Amputation for Sarcoma in England: A National Survey. Disability & Rehabilitation 2016, 1-20. [CrossRef]
- Birch, J.M.; Alston, R.D.; Kelsey, A.M.; Quinn, M.J.; Babb, P.; McNally, R.J.Q. Classification and Incidence of Cancers in Adolescents and Young Adults in England 1979-1997. Br. J. Cancer 2002, 87, 1267-1274. [CrossRef]
- Vindrola-Padros, C.; Fern, L.A.; Gerrand, C.; Hulbert-Williams, N.J.; Lawal, M.; Storey, L.; Wells, M.; Windsor, R.; Woodford, J.; Taylor, R.M. Experiences of Fear of Recurrence in Patients with Sarcoma. Journal of psychosocial oncology research and practice 2023, 5. [CrossRef]
- Paterson, B.L. The Shifting Perspectives Model of Chronic Illness. Journal of Nursing Scholarship 2001, 33, 21-26. [CrossRef]
- Petrella, A.; Storey, L.; Hulbert-Williams, N.J.; Fern, L.A.; Lawal, M.; Gerrand, C.; Windsor, R.; Woodford, J.; Bradley, J.; O'Sullivan, H. et al. Fear of Cancer Recurrence in Patients with Sarcoma in the United Kingdom. Cancers 2023, 15, 956. [CrossRef]
- Martins, A.; Whelan, J.S.; Bennister, L.; Fern, L.A.; Gerrand, C.; Onasanya, M.; Storey, L.; Wells, M.; Windsor, R.; Woodford, J. et al. Qualitative Study Exploring Patients Experiences of being Diagnosed and Living with Primary Bone Cancer in the UK. BMJ Open 2019, 9, e028693. [CrossRef]
- Martins, A.; Bennister, L.; Fern, L.A.; Gerrand, C.; Onasanya, M.; Storey, L.; Wells, M.; Whelan, J.S.; Windsor, R.; Woodford, J. et al. A Qualitative Study of the Factors Influencing Patient’s Experience of Soft Tissue Sarcoma in the United Kingdom. Cancer Nursing 2022, In press. [CrossRef]
- Department of Health. Equity and Excellence: Liberating the NHS.; The Stationery Office Ltd: UK, 2010.
- Ware, J.R.; Sherbourne, C. The MOS 36-Item Short-Form Health Survey: 1. Conceptual Framework and Item Selection. Med. Care 1992, 30, 473-483.
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for use in International Clinical Trials in Oncology. J. Natl. Cancer Inst. 1993, 85, 365-376. [CrossRef]
- Glenwright, B.G.; Simmich, J.; Cottrell, M.; O’Leary, S.P.; Sullivan, C.; Pole, J.D.; Russell, T. Facilitators and Barriers to Implementing Electronic Patient-Reported Outcome and Experience Measures in a Health Care Setting: A Systematic Review. J Patient Rep Outcomes 2023, 7, 13. [CrossRef]
- Ozakinci, G.; Swash, B.; Humphris, G.; Rogers, S.N.; Hulbert-Williams, N.J. Fear of Cancer Recurrence in Oral and Oropharyngeal Cancer Patients: An Investigation of the Clinical Encounter. European journal of cancer care 2018, 27, e12785-n/a. [CrossRef]
- Mercieca-Bebber, R.; King, M.T.; Calvert, M.J.; Stockler, M.R.; Friedlander, M. The Importance of Patient-Reported Outcomes in Clinical Trials and Strategies for Future Optimization. Patient Related Outcome Measures 2018, 9, 353-367. [CrossRef]
- Drotar, D. Validating Measures of Pediatric Health Status, Functional Status and Health-Related Quality of Life: Key Methodological Challenges and Strategies. Ambulatory Pediatrics 2004, 4, 358-364. [CrossRef]
- Joyce, C.R. Use, Misuse and Abuse of Questionnaires on Quality of Life. Patient Education & Counseling 1995, 26, 319-323. [CrossRef]
- Joyce, C.R.B.; Hickey, A.; McGee, H.M.; O'Boyle, C.A. A Theory-Based Method for the Evaluation of Individual Quality of Life: The SEIQoL. Quality of Life Research 2003, 12, 275-280. [CrossRef]
- Bekkering, W.P.; Vlieland, T.P.M.V.; Koopman, H.M.; Schaap, G.R.; Schreuder, H.W.B.; Beishuizen, A.; Tissing, W.J.E.; Hoogerbrugge, P.M.; Anninga, J.K.; Taminiau, A.H.M. The Bt-DUX: Development of a Subjective Measure of Health-Related Quality of Life in Patients Who Underwent Surgery for Lower Extremity Malignant Bone Tumor. Pediatric Blood & Cancer 2009, 53, 348-355. [CrossRef]
- Skalicky, A.M.; Ghate, S.R.; Perez, J.R.; Rentz, A.M. Results of a Qualitative Study to Develop a Patient Reported Outcome Measure for Patients with 4 Subtypes of Soft Tissue Sarcoma. Sarcoma 2017, 2017, 6868030. [CrossRef]
- Davis, A.M.; Bell, R.S.; Badley, E.M.; Yoshida, K.; Williams, J.I. Evaluating Functional Outcome in Patients with Lower Extremity Sarcoma. Clinical Orthopaedics & Related Research 1999. [CrossRef]
- Gounder, M.M.; Maddux, L.; Paty, J.; Atkinson, T.M. Prospective Development of a Patient-reported Outcomes Instrument for Desmoid Tumors Or Aggressive Fibromatosis. Cancer 2020, 126, 531-539. [CrossRef]
- Taylor, R.M.; Gibson, F.; Franck, L.S. A Concept Analysis of Health-Related Quality of Life in Young People with Chronic Illness. J. Clin. Nurs. 2008, 17, 1823-1833. [CrossRef]
- EORTC. Https://Qol.Eortc.Org/. , 2019.
- Martins, A.; Bennister, L.; Fern, L.A.; Gerrand, C.; Onasanya, M.; Storey, L.; Wells, M.; Whelan, J.S.; Windsor, R.; Woodford, J. et al. Development of a Patient-Reported Experience Questionnaire for Patients with Sarcoma: The Sarcoma Assessment Measure (SAM) . Quality of Life Research 2020. [CrossRef]
- Storey, L.; Fern, L.A.; Martins, A.; Wells, M.; Bennister, L.; Gerrand, C.; Onasanya, M.; Whelan, J.S.; Windsor, R.; Woodford, J. et al. A Critical Review of the Impact of Sarcoma on Psychosocial Wellbeing. Sarcoma 2019, 2019, 9730867. [CrossRef]
- Brysbaert, M. How Many Participants do we have to Include in Properly Powered Experiments? A Tutorial of Power Analysis with Reference Tables. Journal of Cognition 2019, 2, 16. [CrossRef]
- Whelan, J.S.; Bielack, S.S.; Marina, N.; Smeland, S.; Jovic, G.; Hook, J.M.; Krailo, M.; Anninga, J.; Butterfass-Bahloul, T.; Böhling, T. et al. EURAMOS-1, an International Randomised Study for Osteosarcoma: Results from Pre-Randomisation Treatment. Annals of Oncology 2015, 26, 407-414. [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361-370. [CrossRef]
- Loehlin, J.C.; Beaujean, A.A. Latent Variable Models, 5th ed, 2017.
- Holmes, T.H.; Rahe, R.H. The Social Readjustment Rating Scale. J. Psychosom. Res. 1967, 11, 213-218. [CrossRef]
- Cox, D.R.; Fitzpatrick, R.; Fletcher, A.E.; Gore, S.M.; Spiegelhalter, D.J.; Jones, D.R. Quality-of-Life Assessment: Can we Keep it Simple? Journal of the Royal Statistical Society. Series A, Statistics in society 1992, 155, 353-393. [CrossRef]
- Mosku, N.; Heesen, P.; Christen, S.; Scaglioni, M.F.; Bode, B.; Studer, G.; Fuchs, B. The Sarcoma-Specific Instrument to Longitudinally Assess Health-Related Outcomes of the Routine Care Cycle. Diagnostics 2023, 13. [CrossRef]
- Husson, O.; den Hollander, D.; van der Graaf, W.T.A. The Complexity of Assessing Health-Related Quality of Life among Sarcoma Patients. Quality of Life Research 2020, 29, 2613-2614. [CrossRef]
- Schramme, T. Health as Complete Well-being: The WHO Definition and Beyond. Public Health Ethics 2023, 43640, xxxii. [CrossRef]
- Polfer, E.M.; Alici, Y.; Baser, R.E.; Healey, J.H.; Bartelstein, M.K. What Proportion of Patients with Musculoskeletal Sarcomas Demostrate Symptoms of Depression Or Anxiety?. Clinical Orthopaedics & Related Research 2022, 480, 2148-2160. [CrossRef]
- Eichler, M.; Hentschel, L.; Richter, S.; Hohenberger, P.; Kasper, B.; Andreou, D.; Pink, D.; Jakob, J.; Singer, S.; Grützmann, R. et al. The Health-Related Quality of Life of Sarcoma Patients and Survivors in Germany—Cross-Sectional Results of a Nationwide Observational Study (PROSa). Cancers 2020, 12, 3590. [CrossRef]
- Okamura, M.; Fujimori, M.; Sato, A.; Uchitomi, Y. Unmet Supportive Care Needs and Associated Factors among Young Adult Cancer Patients in Japan. BMC Cancer 2021, 21, 17. [CrossRef]
- Martins, A.; Taylor, R.M.; Morgan, S.; Fern, L.A. Young People with Cancer Peer-to-Peer Support Intervention: Process Evaluation of a Two Day Residential Conference. Pediatric Blood & Cancer 2016, Abstract SIOP6-1308.
- Avutu, V.; Lynch, K.A.; Barnett, M.E.; Vera, J.A.; Glade Bender, J.L.; Tap, W.D.; Atkinson, T.M. Psychosocial Needs and Preferences for Care among Adolescent and Young Adult Cancer Patients (Ages 15-39): A Qualitative Study. Cancers 2022, 14. [CrossRef]
- Little, R.J.A.; Rubin, D.B. Statistical Analysis with Missing Data, 2. ed. ed, 2002.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).