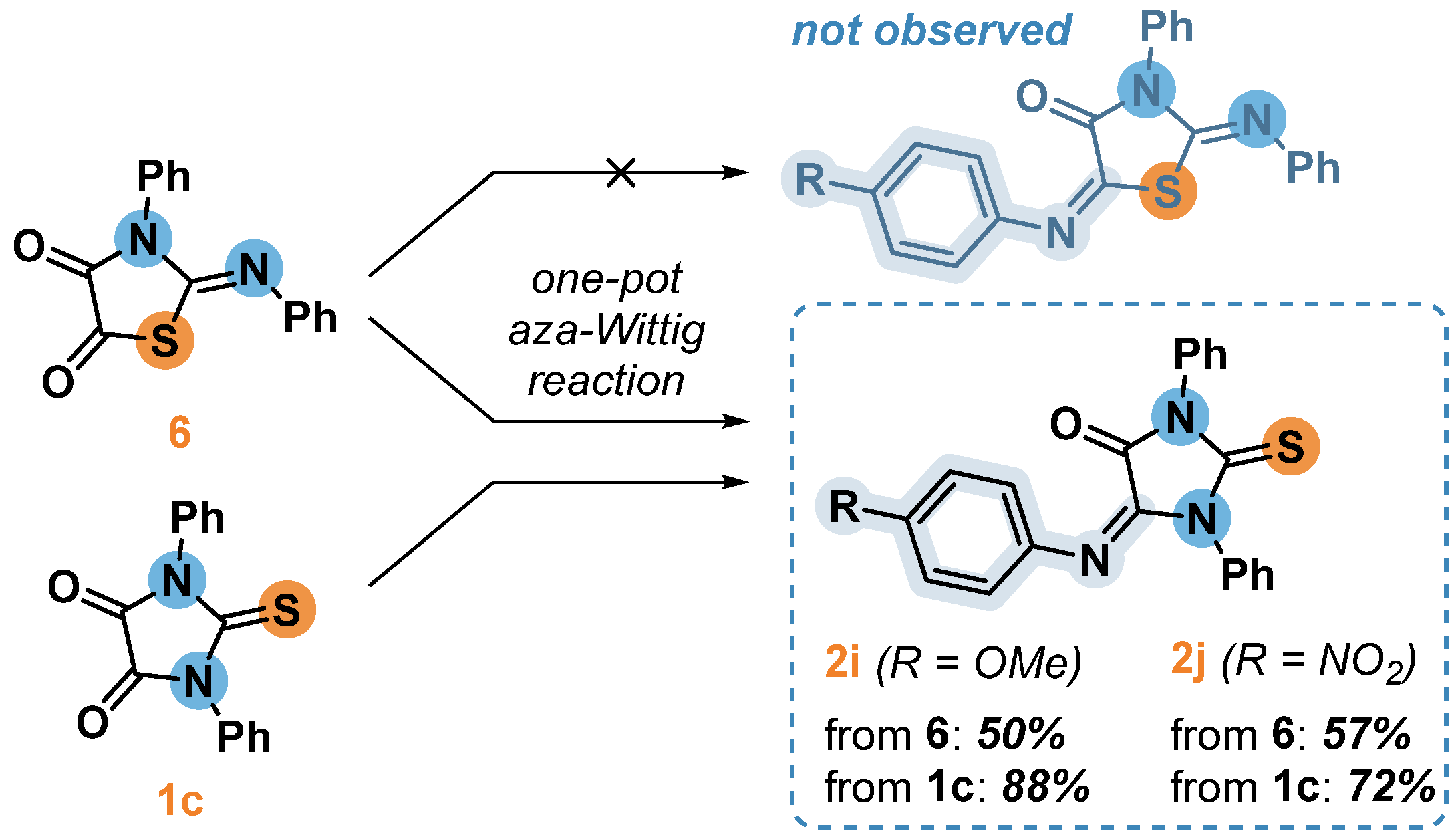
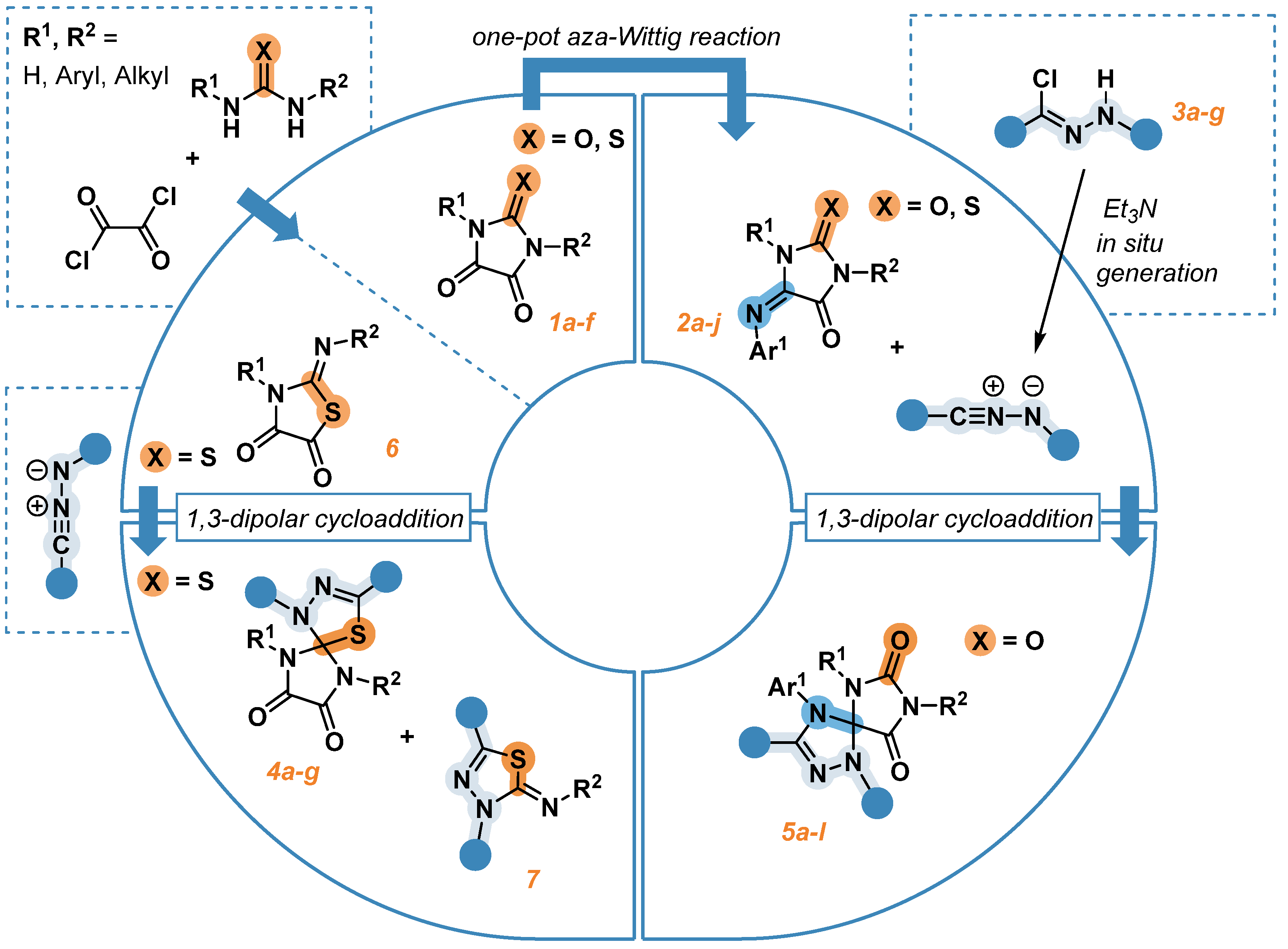
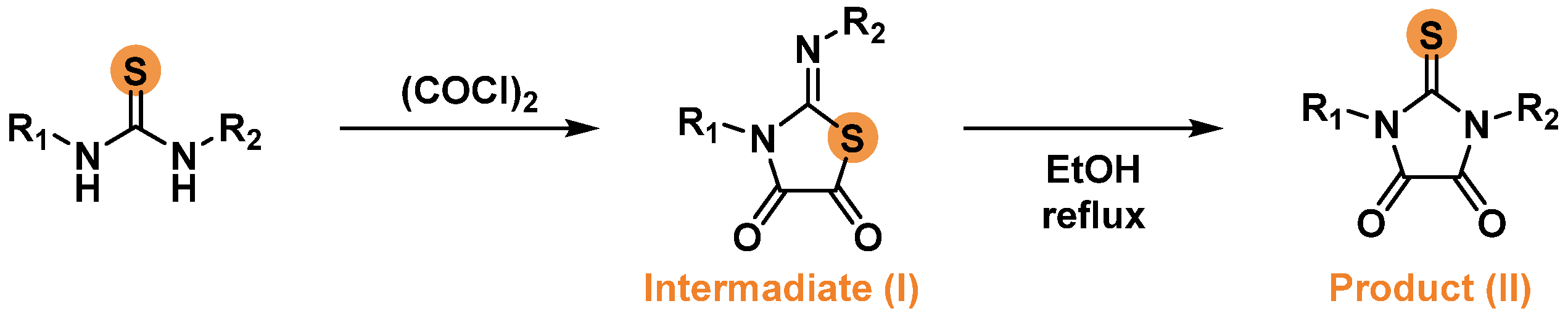
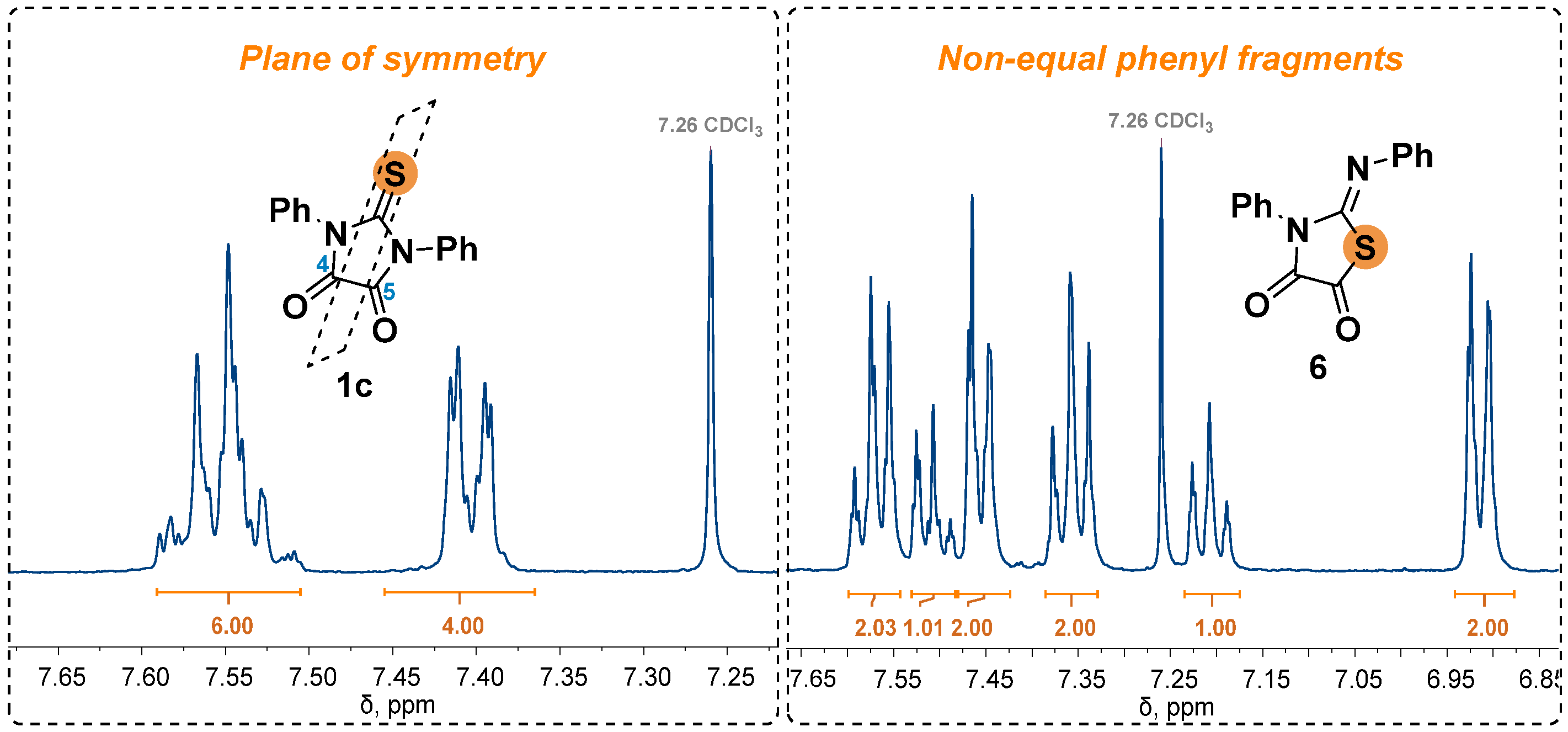
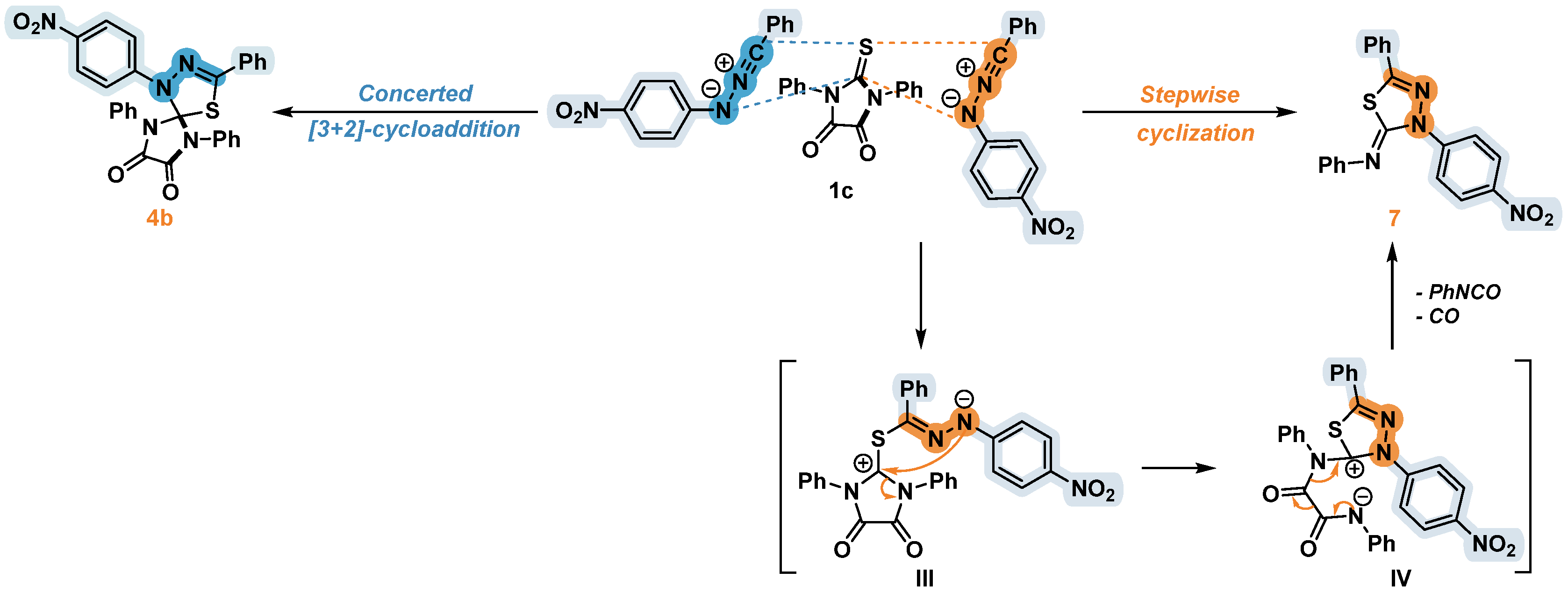
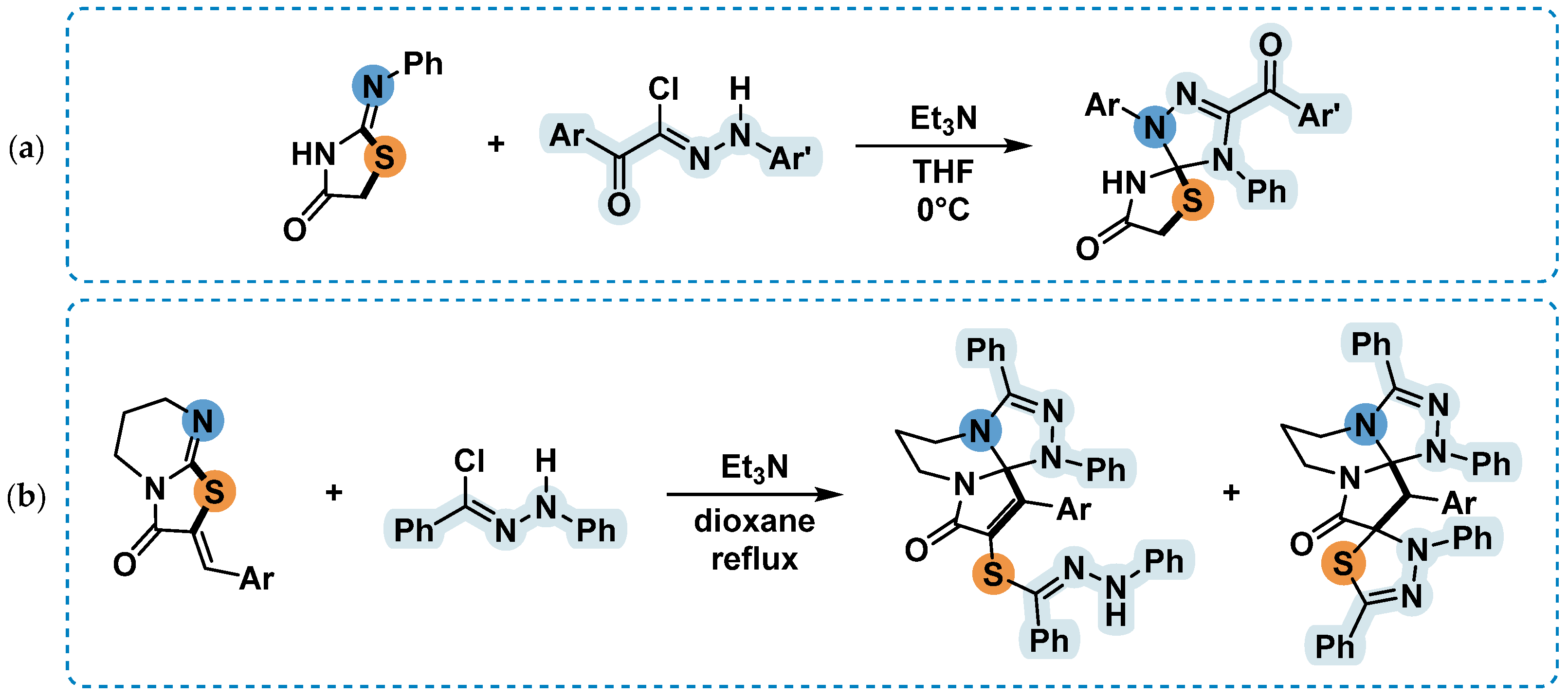
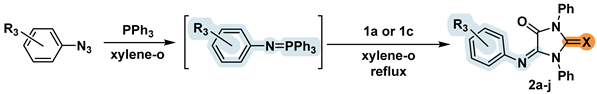
3.4. General Procedure for the Synthesis of the 5-Aryliminoimidazolidine-2,4-diones 2a-l
Triphenylphosphine (1.1 eq) and arylazide (1.1 eq) solution in xylene (0.1 M) was stirred at 50℃ for 0.5-2 hours (until nitrogen release becomes visually undetectable) and charged with imidazolidine-4,5-dione 1a-c (1 eq). The reaction mixture was refluxed for 6 hours and then was left for 16 hours with stirring without additional heating. The solvent was removed in vacuo and the resulting oily mass was purified by flash chromatography with CHCl3 as eluent. Compounds 2a-j were additionally recrystallized from isopropyl alcohol. Compounds 2k and 2l were isolated by column chromatography with hexane-Et2O system as eluent (gradient elution 80/20 to 55/45).
(E)-5-((4-Chlorophenyl)imino)-1,3-diphenylimidazolidine-2,4-dione (2a) was obtained from 1a (0.10 g, 0.4 mmol), 1-azido-4-chlorobenzene (0.07 g, 0.44 mmol) and PPh3 (0.12 g, 0.44 mmol) as orange solid (0.12 g, 79%). 1H NMR (400 MHz, CDCl3) δ 7.57 – 7.51 (m, 4H, Ar), 7.50 – 7.40 (m, 6H, Ar), 7.28 (d, J = 8.5 Hz, 2H, Ar), 6.94 (d, J = 8.5 Hz, 2H, Ar). 13C NMR (101 MHz, CDCl3) δ 153.3, 152.1, 144.6, 131.4, 131.0, 130.4, 130.2, 129.6, 129.4, 129.3, 128.9, 128.9, 128.8, 127.2, 126.0, 125.9, 121.5. HRMS (ESI): calcd for C21H14ClN3O2 (M+H)+ 376.0847, found 376.0852.
(E)-5-((2-Chlorophenyl)imino)-1,3-diphenylimidazolidine-2,4-dione (2b) was obtained from 1a (0.40 g, 1.5 mmol), 1-azido-2-chlorobenzene (0.25 g, 1.65 mmol) and PPh3 (0.43 g, 1.65 mmol) as yellow solid (0.34 g, 60%). 1H NMR (400 MHz, CDCl3) δ 7.66 (d, J = 7.9 Hz, 2H, Ar), 7.56 (t, J = 7.7 Hz, 2H, Ar), 7.47 (d, J = 4.2 Hz, 5H, Ar), 7.40 (d, J = 8.2 Hz, 2H, Ar), 7.21 (t, J = 7.6 Hz, 1H, Ar), 7.06 (t, J = 7.6 Hz, 1H, Ar), 6.95 (d, J = 8.0 Hz, 1H, Ar). 13C NMR (101 MHz, CDCl3) δ 153.2, 152.1, 144.0, 141.5, 131.3, 130.2, 129.6, 129.4, 129.3, 128.9, 127.2, 127.2, 125.9, 125.4, 120.6. HRMS (ESI): calcd for C21H14ClN3O2 (2M+Na)+ 773.1441, found 773.1443.
(E)-5-((4-Bromophenyl)imino)-1,3-diphenylimidazolidine-2,4-dione (2c) was obtained from 1a (0.39 g, 1.45 mmol), 1-azido-4-bromobenzene (0.32 g, 1.60 mmol) and PPh3 (0.42 g, 1.60 mmol) as yellow solid (0.37 g, 60%). 1H NMR (400 MHz, CDCl3) δ 7.57 – 7.53 (m, 4H, Ar), 7.51 – 7.45 (m, 5H, Ar), 7.42 (d, J = 8.3 Hz, 3H, Ar), 6.87 (d, J = 8.2 Hz, 2H, Ar). 13C NMR (101 MHz, CDCl3) δ 153.2, 152.0, 145.1, 139.9, 131.7, 131.4, 130.2, 129.4, 129.3, 128.9, 128.9, 127.2, 125.9, 121.8, 118.1. HRMS (ESI): calcd for C21H14BrN3O2 (M+H)+ 420.0342, found 420.0344.
(E)-5-((2-Bromophenyl)imino)-1,3-diphenylimidazolidine-2,4-dione (2d) was obtained from 1a (0.19 g, 0.71 mmol), 1-azido-2-bromobenzene (0.15 g, 0.78 mmol) and PPh3 (0.20 g, 0.78 mmol) as yellow solid (0.23 g, 76%). 1H NMR (400 MHz, CDCl3) δ 7.73 (d, J = 7.9 Hz, 2H, Ar), 7.63 (d, J = 8.0 Hz, 1H, Ar), 7.58 (t, J = 7.8 Hz, 2H, Ar), 7.53 – 7.45 (m, 5H, Ar), 7.43 – 7.39 (m, 1H, Ar), 7.29 (t, J = 7.6 Hz, 1H, Ar), 7.06 – 6.97 (m, 2H, Ar). 13C NMR (101 MHz, CDCl3) δ 153.0, 145.3, 132.7, 131.3, 130.2, 129.4, 129.3, 128.9, 127.8, 127.2, 125.9, 125.7, 120.4, 114.1. HRMS (ESI): calcd for C21H14BrN3O2 (M+Na)+ 442.0162, found 442.0167.
(E)-1,3-Diphenyl-5-(p-tolylimino)imidazolidine-2,4-dione (2e) was obtained from 1a (0.27 g, 1.00 mmol), 1-azido-4-methylbenzene (0.15 g, 1.10 mmol) and PPh3 (0.29 g, 1.10 mmol) as light orange solid (0.35 g, 84%). 1H NMR (400 MHz, CDCl3) δ 7.61 – 7.52 (m, 4H, Ar), 7.52 – 7.44 (m, 5H, Ar), 7.43 – 7.38 (m, 1H, Ar), 7.13 (d, J = 7.9 Hz, 2H, Ar), 6.92 (d, J = 7.9 Hz, 2H, Ar), 2.33 (s, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 153.2, 143.4, 139.4, 134.8, 130.4, 129.3, 129.2, 128.8, 128.8, 128.7, 127.3, 126.1, 120.2, 120.1, 120.1, 21.1. HRMS (ESI): calcd for C22H17N3O2 (M+H)+ 356,1394, found 356.1396.
(E)-5-((4-Methoxyphenyl)imino)-1,3-diphenylimidazolidine-2,4-dione (2f) was obtained from 1a (0.53 g, 2.00 mmol), 1-azido-4-methoxybenzene (0.33 g, 2.20 mmol) and PPh3 (0.58 g, 2.20 mmol) as orange solid (0.48 g, 65%). 1H NMR (400 MHz, DMSO-d6) δ 7.58 – 7.54 (m, 4H, Ar), 7.52 (d, J = 7.6 Hz, 2H, Ar), 7.48 – 7.44 (m, 4H, Ar), 7.02 (d, J = 8.7 Hz, 2H, Ar), 6.84 (d, J = 8.8 Hz, 2H, Ar), 3.72 (s, 3H, OCH3). 13C NMR (101 MHz, DMSO-d6) δ 156.4, 153.9, 152.4, 140.7, 139.0, 132.5, 131.0, 129.0, 128.9, 128.5, 128.2, 127.9, 127.0, 122.3, 113.5, 55.2. HRMS (ESI): calcd for C22H17N3O3 (M+H)+ 372.1343, found 372.1347.
(E)-5-((4-Nitrophenyl)imino)-1,3-diphenylimidazolidine-2,4-dione (2g) was obtained from 1a (0.53 g, 2.00 mmol), 1-azido-4-nitrobenzene (0.36 g, 2.20 mmol) and PPh3 (0.58 g, 2.20 mmol) as light yellow solid (0.58 g, 75%). 1H NMR (400 MHz, CDCl3) δ 8.19 (s, 2H, Ar), 7.57 (m, 3H, Ar), 7.52 – 7.38 (m, 7H, Ar), 7.03 (s, 2H, Ar). 13C NMR (101 MHz, CDCl3) δ 153.4, 152.8, 152.0, 144.5, 140.3, 131.1, 130.0, 129.5, 129.4, 129.2, 129.1, 127.1, 125.8, 124.8, 120.2. HRMS (ESI): calcd for C21H14N4O4 (M+H)+ 409.0907, found 409.0911.
(E)-5-((4-bromophenyl)imino)-1,3-diphenyl-2-thioxoimidazolidin-4-one (2h) was obtained from 1c (0.50 g, 1.77 mmol), 1-azido-4-bromobenzene (0.39 g, 1.94 mmol) and PPh3 (0.51 g, 1.94 mmol) as yellow solid (0.64 g, 82%). 1H NMR (400 MHz, CDCl3) δ 7.60 – 7.55 (m, 2H, Ar), 7.50 (q, J = 9.2, 8.3 Hz, 6H, Ar), 7.43 – 7.36 (m, 4H, Ar), 6.91 (d, J = 8.3 Hz, 2H, Ar). 13C NMR (101 MHz, CDCl3) δ 179.2, 153.1, 144.5, 133.8, 132.0, 131.7, 129.8, 129.7, 129.5, 129.4, 128.9, 128.3, 125.4, 122.4, 118.7. HRMS (ESI): calcd for C22H17N3O2S (2M+Na)+ 797.1975, found 797.1986.
(E)-5-((4-Methoxyphenyl)imino)-1,3-diphenyl-2-thioxoimidazolidin-4-one (2i) was obtained from 1c (0.71 g, 2.50 mmol), 1-azido-4-methoxybenzene (0.41 g, 2.75 mmol) and PPh3 (0.72 g, 2.75 mmol) as red solid (0.86 g, 88%). 1H NMR (400 MHz, CDCl3) δ 7.58 – 7.53 (m, 2H, Ar), 7.53 – 7.46 (m, 6H, Ar), 7.40 (d, J = 7.4 Hz, 2H, Ar), 7.17 (d, J = 6.5 Hz, 2H, Ar), 6.83 (d, J = 6.6 Hz, 2H, Ar), 3.78 (s, 3H, OCH3). 13C NMR (101 MHz, CDCl3) δ 178.9, 158.3, 153.1, 139.3, 137.6, 134.2, 132.3, 129.6, 129.3, 129.0, 128.4, 123.9, 113.7, 55.5. HRMS (ESI): calcd for (M+H)+ 388.1114, found 388.1122.
(E)-5-((4-Nitrophenyl)imino)-1,3-diphenyl-2-thioxoimidazolidin-4-one (2j) was obtained from 1c (0.47 g, 1.66 mmol), 1-azido-4-nitrobenzene (0.30 g, 1.83 mmol) and PPh3 (0.48 g, 1.83 mmol) as light yellow solid (0.48 g, 72%). 1H NMR (400 MHz, CDCl3) δ 8.19 (s, 2H, Ar), 7.64 – 7.46 (m, 8H, Ar), 7.41 (d, J = 7.4 Hz, 2H, Ar), 7.08 (s, 2H, Ar). 13C NMR (101 MHz, CDCl3) δ 179.2, 152.3, 144.6, 141.1, 133.3, 131.8, 129.8, 129.5, 129.4, 128.7, 128.1, 124.6, 120.4. HRMS (ESI): calcd for C21H14N4O3S (M+H)+ 403.0859, found 403.0864.
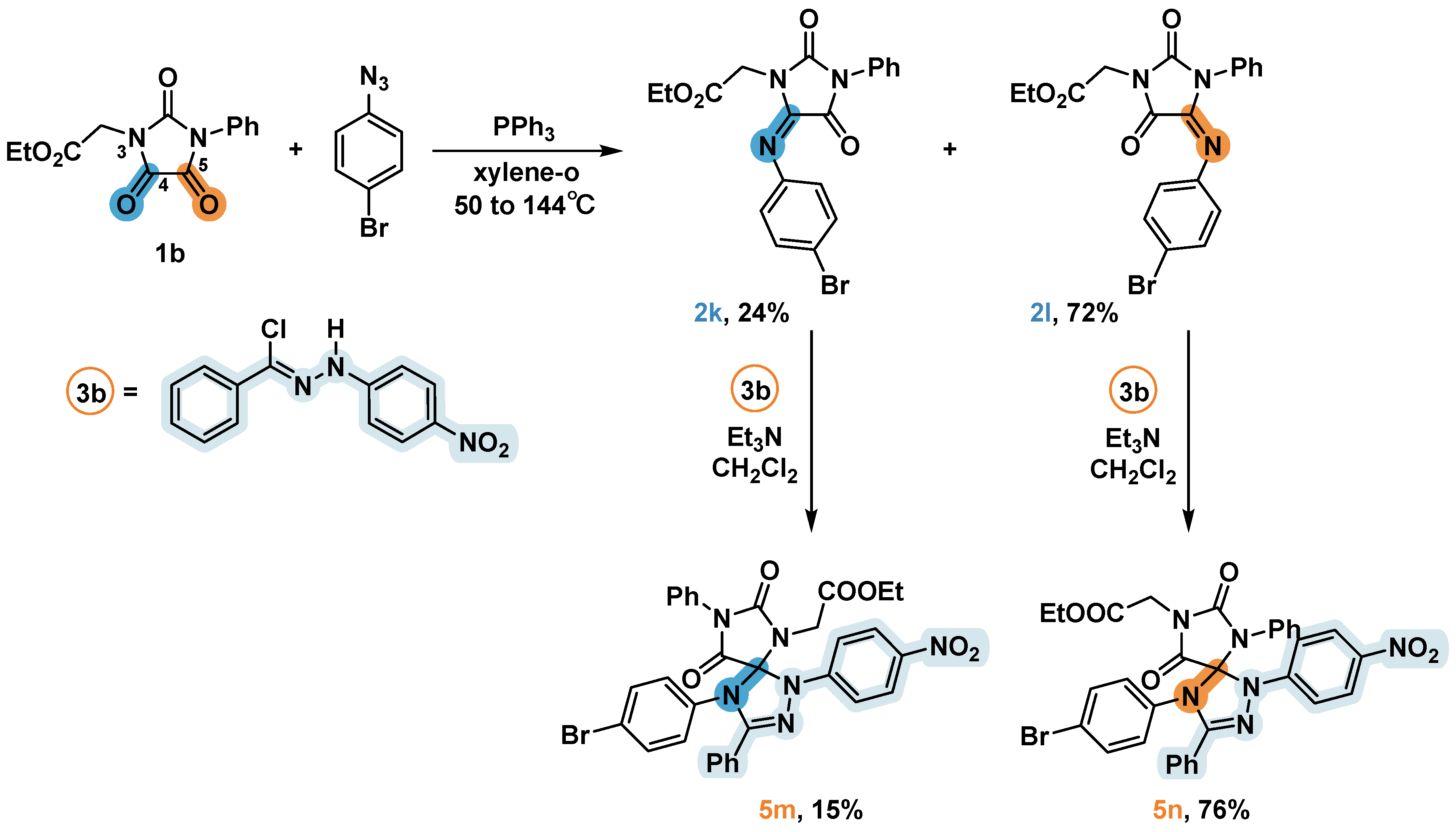
Ethyl (E)-2-(5-((4-bromophenyl)imino)-2,4-dioxo-3-phenylimidazolidin-1-yl)acetate (2k) was obtained from 1b (0.46 g, 1.66 mmol), 1-azido-4-bromobenzene (0.36 g, 1.83 mmol) and PPh3 (0.48 g, 1.83 mmol) as orange solid (0.17 g, 24%). 1H NMR (400 MHz, CDCl3) δ 7.48 – 7.43 (m, 3H, Ar), 7.43 – 7.37 (m, 4H, Ar), 6.92 – 6.87 (m, 2H, Ar), 4.59 (s, 2H, NCH2), 4.29 (q, J = 7.2 Hz, 2H, COOCH2), 1.33 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 166.8, 153.5, 152.6, 144.6, 139.3, 131.8, 130.2, 129.3, 128.9, 125.9, 122.3, 118.5, 62.3, 40.7, 14.3. HRMS (ESI): calcd for C19H16BrN3O4 (M+Na)+ 452.0216, found 452.0222.
Ethyl (E)-2-(4-((4-bromophenyl)imino)-2,5-dioxo-3-phenylimidazolidin-1-yl)acetate (2l) was obtained from 1b (0.46 g, 1.66 mmol), 1-azido-4-bromobenzene (0.36 g, 1.83 mmol) and PPh3 (0.48 g, 1.83 mmol) as yellow solid (0.52 g, 72%). 1H NMR (400 MHz, CDCl3) δ 7.54 – 7.48 (m, 4H, Ar), 7.46 – 7.39 (m, 3H, Ar), 6.84 (d, J = 8.5 Hz, 2H, Ar), 4.38 (s, 2H, NCH2), 4.25 (q, J = 7.1 Hz, 2H, COOCH2), 1.30 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 166.4, 153.9, 152.4, 144.9, 140.3, 131.8, 131.3, 129.4, 128.9, 127.2, 122.0, 118.3, 62.5, 39.7, 14.2. HRMS (ESI): calcd for C19H16BrN3O4 (M+Na)+ 452.0216, found 452.0220.
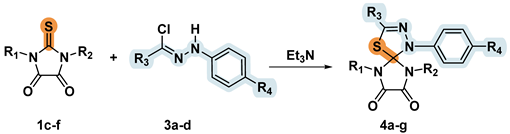
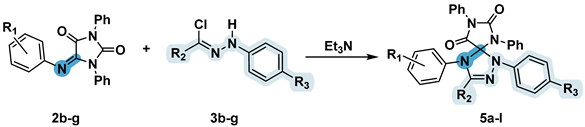
3.5. General Procedure for the Synthesis of the Spirocyclic Products 4a-g and 5a-n
Diffusion mixing technique (a): Small vial (15 ml volume, diameter 1.3 cm) equipped with a magnetic bar was charged with a mixture of dipolarophile 1 or 2 (1 eq, 0.150 mmol) and hydrazonoyl chloride 3 (1.1 eq, 0.165 mol) in 4 ml of chloroform and then placed in bigger vial (50 ml volume, diameter 3.5 cm) containing triethylamine (35.85 mmol, 5 ml). The outer vial was tightly closed with a lid and the reaction mixture was stirred at room temperature for 24–48 h.
Triethylamine dropwise addition (b): A solution of TEA (1.2 eq) in DCM (3 mL) was slowly added for 30 min to a stirring solution dipolarophile 1 or 2 (1 eq, 0.150 mmol) and hydrazonoyl chloride 3 (1.1 eq, 0.165 mol) in 4 ml of DCM. After the addition, the reaction mixture was stirred at room temperature for 24–48 h.
In both cases after the reactions were completed (TCL-control) the solvent was removed in vacuo and the residue was purified by column chromatography on silica gel with EtOAc/hexane or DCM as eluent.
3-(4-Chloro-3-nitrophenyl)-1,6,9-triphenyl-4-thia-1,2,6,9-tetraazaspiro[4.4]non-2-ene-7,8-dione (4a) was obtained from 1c and 3a as pale yellow solid (96%a/88%b). 1H NMR (400 MHz, DMSO-d6) δ 8.12 (dd, J = 1.9, 0.7 Hz, 1H, Ar), 7.72 – 7.66 (m, 2H, Ar), 7.52 (dd, J = 8.8, 7.3 Hz, 2H, Ar), 7.42 (m, 8H, Ar), 7.27 – 7.20 (m, 5H, Ar). 13C NMR (101 MHz, DMSO-d6) δ 156.3, 148.1, 140.5, 139.4, 132.8, 132.3, 130.1, 130.1, 129.8, 129.5, 129.4, 127.7, 125.8, 124.3, 121.9, 117.4, 112.1. HRMS (ESI): calcd for C28H18ClN5O4S (M+H)+ 578.0660, found 578.0665.
1-(4-Nitrophenyl)-3,6,9-triphenyl-4-thia-1,2,6,9-tetraazaspiro[4.4]non-2-ene-7,8-dione (4b) was obtained from 1c and 3b as beige solid (44%a/91%b). 1H NMR (400 MHz, CDCl3) δ 8.33 – 8.27 (m, 2H, Ar), 7.55 – 7.51 (m, 2H, Ar), 7.46 – 7.42 (m, 2H, Ar), 7.42 – 7.33 (m, 9H, Ar), 7.25 – 7.20 (m, 4H, Ar). 13C NMR (101 MHz, CDCl3) δ 155.0, 151.7, 148.8, 145.2, 144.4, 131.4, 130.0, 130.0, 129.2, 126.6, 124.9, 124.7, 120.8, 120.8. HRMS (ESI): calcd for C28H19N5O4S (M+H)+ 522.1231, found 522.1226.
3-(4-Bromophenyl)-1,6,9-triphenyl-4-thia-1,2,6,9-tetraazaspiro[4.4]non-2-ene-7,8-dione (4c) was obtained from 1c and 3c as white solid (99%a,b). 1H NMR (400 MHz, CDCl3) δ 7.46 – 7.42 (m, 2H, Ar), 7.42 – 7.37 (m, 2H, Ar), 7.36 – 7.31 (m, 8H, Ar), 7.30 – 7.27 (m, 2H, Ar), 7.26 – 7.19 (m, 5H, Ar). 13C NMR (101 MHz, CDCl3) δ 155.78, 140.64, 140.00, 132.55, 132.00, 129.99, 129.65, 129.51, 128.98, 127.55, 127.52, 124.79, 124.43, 118.21, 112.00. HRMS (ESI): calcd for C28H19BrN4O2S (M+H)+ 555.0485, found 555.0487.
3-Methyl-1,6,9-triphenyl-4-thia-1,2,6,9-tetraazaspiro[4.4]non-2-ene-7,8-dione (4d) was obtained from 1c and 3d as white solid (88%a/74%b). 1H NMR (400 MHz, CDCl3) δ 7.39 – 7.32 (m, 8H, Ar), 7.26 – 7.20 (m, 6H, Ar), 7.14 (t, J = 7.3 Hz, 1H, Ar), 2.00 (s, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 155.8, 140.9, 139.2, 132.8, 129.9, 129.6, 129.4, 127.6, 124.1, 117.7, 112.0, 16.5. HRMS (ESI): calcd for C23H18N4O2S (2M+Na)+ 851.2193, found 851.2186.
Ethyl 2-(3-(4-bromophenyl)-9-(4-ethoxyphenyl)-7,8-dioxo-1-phenyl-4-thia-1,2,6,9-tetraazaspiro[4.4]non-2-en-6-yl)acetate (4e) was obtained from 1d and 3c as beige solid (77%a/49%b). 1H NMR (400 MHz, CDCl3) δ 7.48 (d, J = 8.5 Hz, 2H, Ar), 7.39 – 7.32 (m, 4H, Ar), 7.24 (d, J = 8.4 Hz, 2H, Ar), 7.18 (t, J = 7.3 Hz, 1H, Ar), 7.08 (d, J = 9.0 Hz, 2H, Ar), 6.79 (d, J = 9.0 Hz, 2H, Ar), 4.31 (d, J = 17.6 Hz, 1H, NCH2), 4.28 – 4.13 (m, 2H, COOCH2CH3), 4.01 – 3.90 (m, 3H, NCH2+OCH2CH3), 1.34 (t, J = 7.0 Hz, 3H, OCH2CH3), 1.24 (t, J = 7.1 Hz, 3H, COOCH2CH3). 13C NMR (101 MHz, CDCl3) δ 166.1, 159.6, 156.4, 155.4, 140.4, 140.0, 132.1, 129.9, 129.0, 128.9, 127.6, 125.2, 124.5, 124.2, 118.8, 115.2, 111.0, 63.7, 62.4, 41.9, 14.7, 14.1. HRMS (ESI): calcd for C28H25BrN4O5S (M+H)+ 609.0802, found 609.0803.
Ethyl 2-(9-(4-methoxyphenyl)-1-(4-nitrophenyl)-7,8-dioxo-3-phenyl-4-thia-1,2,6,9-tetraazaspiro[4.4]non-2-en-6-yl)acetate (4f) was obtained from 1e and 3b as beige solid (93%a/94%b). 1H NMR (400 MHz, CDCl3) δ 8.25 (d, J = 9.3 Hz, 2H, Ar), 7.58 – 7.52 (m, 2H, Ar), 7.47 – 7.39 (m, 5H, Ar), 7.03 (d, J = 9.0 Hz, 2H, Ar), 6.82 (d, J = 9.0 Hz, 2H, Ar), 4.39 (d, J = 17.5 Hz, 1H, NCH2), 4.20 (qq, J = 10.8, 7.1 Hz, 2H, COOCH2CH3), 4.06 (d, J = 17.5 Hz, 1H, NCH2), 3.74 (s, 3H, OCH3), 1.24 (t, J = 7.1 Hz, 3H, COOCH2CH3). 13C NMR (101 MHz, CDCl3) δ 165.8, 160.5, 145.7, 131.4, 129.4, 129.2, 129.0, 126.7, 125.7, 123.8, 117.0, 115.1, 110.6, 62.6, 55.6, 42.1, 14.2. HRMS (ESI): calcd for C27H23N5O7S (M+Na)+ 584.1210, found 584.1217.
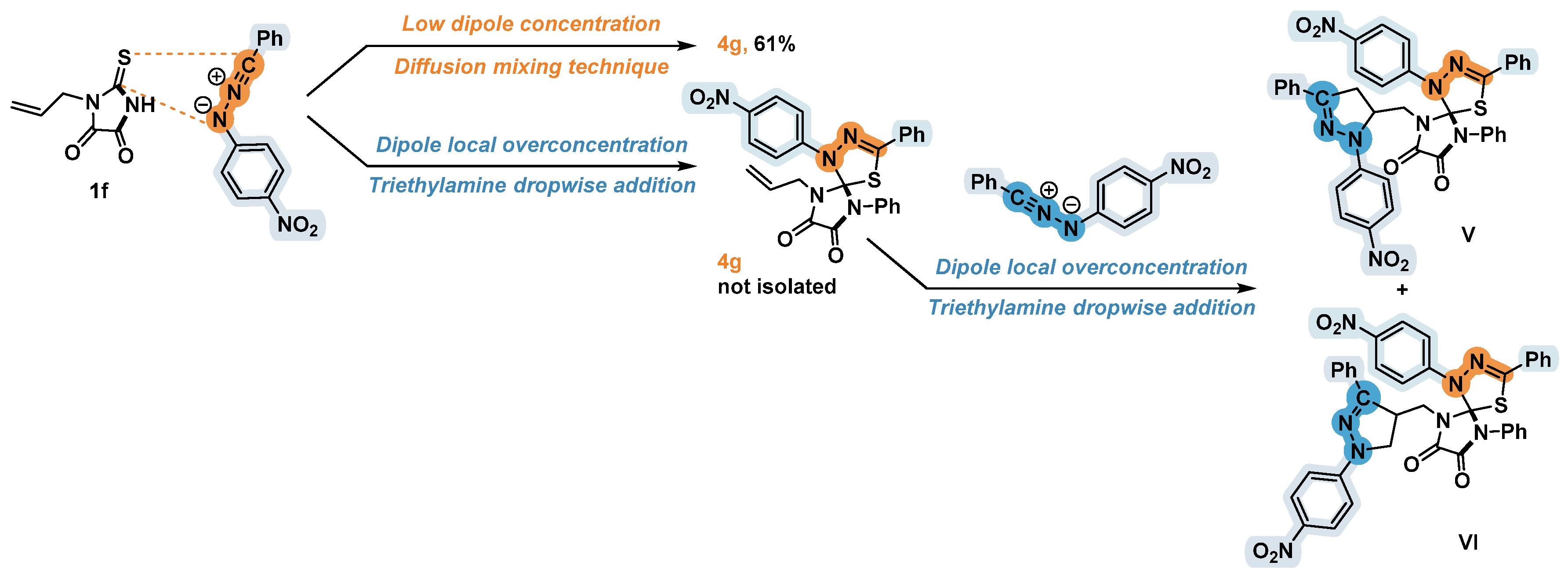
6-Allyl-1-(4-nitrophenyl)-3-phenyl-4-thia-1,2,6,9-tetraazaspiro[4.4]non-2-ene-7,8-dione (4g) was obtained from 1f and 3b as light yellow solid (61%a). 1H NMR (400 MHz, CDCl3) δ 8.53 – 8.39 (m, 4H, Ar), 7.96 – 7.91 (m, 2H, Ar), 7.62 – 7.53 (m, 3H, Ar), 7.51 – 7.44 (m, 1H, NH), 5.90 (ddt, J = 17.2, 10.2, 5.6 Hz, 1H, CH), 5.29 – 5.18 (m, 2H, =CH2), 4.06 – 4.00 (m, 2H, NCH2). 13C NMR (101 MHz, CDCl3) δ 168.9, 167.4, 160.2, 158.2, 146.7, 143.6, 133.4, 133.4, 132.4, 129.6, 128.8, 127.1, 124.9, 124.7, 117.1, 42.2. HRMS (ESI): calcd for C19H15N5O4S (2M+Na)+ 841.1582, found 841.1582.
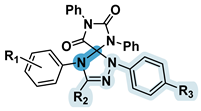
4-(4-Bromophenyl)-1,3,6,8-tetraphenyl-1,2,4,6,8-pentaazaspiro[4.4]non-2-ene-7,9-dione (5a) was obtained from 2d and 3e as beige solid (71%a/62%b). 1H NMR (400 MHz, CDCl3) δ 7.53 – 7.50 (m, 2H, Ar), 7.47 – 7.42 (m, 3H, Ar), 7.42 – 7.38 (m, 3H, Ar), 7.38 – 7.35 (m, 4H, Ar), 7.35 – 7.26 (m, 7H, Ar), 7.15 – 7.11 (m, 2H, Ar), 7.07 – 7.02 (m, 1H, Ar), 6.98 – 6.93 (m, 2H, Ar). 13C NMR (101 MHz, CDCl3) δ 165.0, 151.8, 146.6, 141.4, 135.7, 133.9, 132.9, 130.6, 130.0, 129.7, 129.5, 129.5, 129.0, 128.7, 128.6, 127.9, 127.6, 126.3, 126.1, 124.9, 122.7, 121.9, 115.6, 97.5. HRMS (ESI): calcd for C34H24BrN5O2 (M+H)+ 614.1186, found 614.1193.
1,4-Bis(4-nitrophenyl)-6,8-diphenyl-3-(p-tolyl)-1,2,4,6,8-pentaazaspiro[4.4]non-2-ene-7,9-dione (5b) was obtained from 2g and 3f as orange solid (40%a/13%b). 1H NMR (400 MHz, CDCl3) δ 8.28 – 8.23 (m, 2H, Ar), 8.20 – 8.16 (m, 2H, Ar), 7.52 – 7.44 (m, 3H, Ar), 7.39 – 7.34 (m, 4H, Ar), 7.33 – 7.27 (m, 3H, Ar), 7.25 – 7.18 (m, 6H, Ar), 7.13 (d, J = 8.1 Hz, 2H, Ar), 2.35 (s, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 164.0, 151.3, 147.8, 146.5, 145.5, 142.1, 141.9, 141.8, 132.8, 130.1, 130.0, 129.9, 129.8, 129.6, 128.6, 128.0, 126.6, 126.1, 125.7, 125.2, 125.1, 122.2, 113.6, 95.5, 21.6. HRMS (ESI): calcd for C35H25N7O6 (M+Na)+ 662.1759, found 662.1765.
4-(4-Bromophenyl)-1-(4-nitrophenyl)-6,8-diphenyl-3-(p-tolyl)-1,2,4,6,8-pentaazaspiro[4.4]non-2-ene-7,9-dione (5c) was obtained from 2d and 3f as beige solid (90%a/85%b). 1H NMR (400 MHz, DMSO-d6) δ 8.34 (d, J = 9.4 Hz, 2H, Ar), 7.69 (d, J = 8.7 Hz, 2H, Ar), 7.55 – 7.50 (m, 3H, Ar), 7.49 – 7.43 (m, 4H, Ar), 7.39 – 7.35 (m, 3H, Ar), 7.33 (d, J = 8.2 Hz, 2H, Ar), 7.23 – 7.17 (m, 4H, Ar), 7.15 (d, J = 8.6 Hz, 2H, Ar), 2.29 (s, 3H,CH3). 13C NMR (101 MHz, DMSO-d6) δ 163.5, 148.2, 145.2, 140.7, 135.0, 133.1, 132.5, 129.9, 129.9, 129.8, 129.6, 129.5, 128.9, 128.8, 128.6, 127.9, 126.8, 126.5, 126.1, 122.1, 121.5, 113.1, 95.3, 21.0. HRMS (ESI): calcd for C35H25BrN6O4 (M+Na)+ 695.1013, found 695.1015.
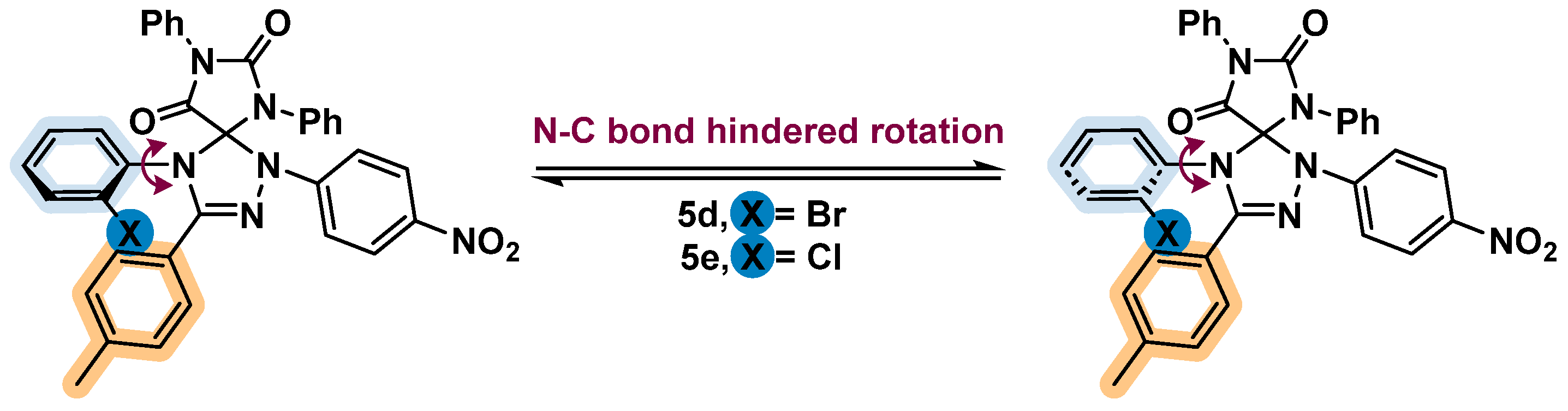
4-(2-Bromophenyl)-1-(4-nitrophenyl)-6,8-diphenyl-3-(p-tolyl)-1,2,4,6,8-pentaazaspiro[4.4]non-2-ene-7,9-dione (5d) was obtained from 2c and 3f as yellow solid (84%a/83%b). Mixture of isomers I and II (1:2.3 in CDCl3). Isomer I: 1H NMR (400 MHz, CDCl3) δ 8.20 – 8.15 (m, 2H, Ar), 7.68 – 7.65 (m, 1H, Ar), 7.53 – 7.49 (m, 2H, Ar), 7.47 – 7.41 (m, 5H, Ar), 7.29 – 7.24 (m, 2H, Ar), 7.23 – 7.17 (m, 4H, Ar), 7.17 – 7.11 (m, 6H, Ar), 2.34 (s, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 163.6, 151.6, 150.0, 145.8, 141.4, 141.3, 137.1, 134.2, 133.5, 133.2, 130.6, 130.5, 129.7, 129.6, 129.4, 129.2, 128.7, 127.8, 127.7, 126.0, 125.9, 125.1, 123.3, 122.9, 113.2, 96.2, 21.6. HRMS (ESI): calcd for C35H25BrN6O4 (M+Na)+ 695.1013, found 695.1016. Isomer II: 1H NMR (400 MHz, CDCl3) δ 8.24 – 8.19 (m, 2H, Ar), 7.60 – 7.55 (m, 2H, Ar), 7.45 – 7.39 (m, 6H, Ar), 7.34 – 7.27 (m, 5H, Ar), 7.26 – 7.21 (m, 3H, Ar), 7.10 – 7.04 (m, 4H, Ar), 2.33 (s, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 163.5, 151.5, 149.0, 145.6, 141.3, 141.1, 135.6, 134.9, 133.4, 130.8, 130.4, 129.8, 129.6, 129.5, 129.3, 128.9, 128.8, 128.0, 127.3, 126.1, 126.0, 125.1, 124.6, 124.1, 113.3, 95.5, 21.7. HRMS (ESI): calcd for C35H25BrN6O4 (M+Na)+ 695.1013, found 695.1022.
4-(2-Chlorophenyl)-1-(4-nitrophenyl)-6,8-diphenyl-3-(p-tolyl)-1,2,4,6,8-pentaazaspiro[4.4]non-2-ene-7,9-dione (5e) was obtained from 2a and 3f as orange solid (87%a/81%b). Mixture of isomers I and II (1:1.75 in DMSO-d6). 1H NMR (400 MHz, DMSO-d6) δ 8.33 (d, J = 9.3 Hz, 2HII, Ar), 8.28 (d, J = 9.2 Hz, 2HI, Ar), 7.88 (d, J = 7.8 Hz, 1HII, Ar), 7.71 – 7.64 (m, 1HI+1HII, Ar), 7.56 – 7.45 (m, 5HI+7HII, Ar), 7.44-7.35 (m, 3HI+3HII, Ar), 7.35-7.30 (m, 5HI+4HII, Ar), 7.29-7.26 (m, 1HI, Ar), 7.23 (d, J = 8.0 Hz, 2HI, Ar), 7.19-7.12 (m, 3HI+2HII, Ar), 7.06 – 7.02 (m, 2HII, Ar), 2.29 (s, 3HI+3HII, CH3). 13C NMR (101 MHz, DMSO-d6) δ 163.1, 163.0, 150.9, 150.7, 149.4, 148.7, 145.2, 145.0, 141.2, 141.0, 140.6, 140.5, 134.4, 133.1, 132.9, 132.6, 131.9, 131.7, 131.7, 131.3, 131.1, 130.9, 130.0, 129.8, 129.5, 129.5, 129.4, 129.3, 129.3, 128.8, 128.7, 128.6, 127.9, 127.3, 126.9, 126.8, 126.7, 126.5, 126.3, 126.0, 125.1, 123.4, 122.7, 113.1, 113.1, 113.0, 95.8, 95.1, 40.2, 40.0, 39.7, 39.5, 39.3, 39.1, 38.9, 21.0, 20.8. HRMS (ESI): calcd for C35H25ClN6O4 (M+Na)+ 651.1518, found 651.1527.
1-(4-Nitrophenyl)-6,8-diphenyl-3,4-di-p-tolyl-1,2,4,6,8-pentaazaspiro[4.4]non-2-ene-7,9-dione (5f) was obtained from 2e and 3f as beige solid (71%a/88%b). 1H NMR (400 MHz, DMSO-d6) δ 8.32 (d, J = 9.0 Hz, 2H, Ar), 7.52 (d, J = 8.0 Hz, 3H, Ar), 7.46 (dd, J = 14.1, 6.4 Hz, 4H, Ar), 7.39 – 7.31 (m, 5H, Ar), 7.28 (d, J = 7.9 Hz, 2H, Ar), 7.18 (d, J = 7.9 Hz, 2H, Ar), 7.07 (d, J = 7.6 Hz, 4H, Ar), 2.32 (s, 3H, CH3), 2.28 (s, 3H, CH3). 13C NMR (101 MHz, DMSO-d6) δ 163.7, 150.8, 148.7, 145.4, 140.8, 140.5, 138.6, 132.8, 132.8, 130.5, 130.0, 129.7, 129.4, 129.4, 128.3, 127.8, 127.1, 126.8, 126.4, 125.6, 122.4, 112.9, 95.6, 20.9, 20.6. HRMS (ESI): calcd for C36H28N6O4 (M+Na)+ 631.2064, found 631.2072.
4-(4-Methoxyphenyl)-1-(4-nitrophenyl)-6,8-diphenyl-3-(p-tolyl)-1,2,4,6,8-pentaazaspiro[4.4]non-2-ene-7,9-dione (5g) was obtained from 2f and 3f as orange solid (52%a/38%b). 1H NMR (400 MHz, DMSO-d6) δ 8.31 (d, J = 9.5 Hz, 2H, Ar), 7.55 – 7.50 (m, 3H, Ar), 7.50 – 7.43 (m, 4H, Ar), 7.39 – 7.31 (m, 5H, Ar), 7.19 (d, J = 7.5 Hz, 2H, Ar), 7.13 (d, J = 8.4 Hz, 2H, Ar), 7.10 – 7.06 (m, 2H, Ar), 7.04 (d, J = 9.3 Hz, 2H, Ar), 3.76 (s, 3H, OCH3), 2.28 (s, 3H, CH3). 13C NMR (101 MHz, DMSO-d6) δ 163.8, 159.3, 150.9, 148.9, 145.4, 140.9, 140.4, 133.0, 130.0, 129.7, 129.5, 129.4, 129.4, 129.1, 128.2, 127.8, 127.6, 126.8, 126.4, 125.3, 122.3, 115.2, 112.9, 95.8, 55.6, 21.0. HRMS (ESI): calcd for C36H28N6O5 (M+H)+ 625.2194, found 625.2199.
4-(4-Bromophenyl)-1-(4-nitrophenyl)-3,6,8-triphenyl-1,2,4,6,8-pentaazaspiro[4.4]non-2-ene-7,9-dione (5h) was obtained from 2d and 3b as beige solid (63%a/50%b). 1H NMR (400 MHz, CDCl3) δ 8.28 – 8.22 (m, 2H, Ar), 7.50 – 7.46 (m, 3H, Ar), 7.46 – 7.41 (m, 4H, Ar), 7.40 – 7.33 (m, 5H, Ar), 7.30 (m, 5H, Ar), 7.16 – 7.11 (m, 2H, Ar), 6.99 – 6.93 (m, 2H, Ar). 13C NMR (101 MHz, CDCl3) δ 164.3, 151.5, 148.7, 145.7, 141.6, 134.8, 133.3, 133.2, 130.9, 130.2, 129.9, 129.7, 129.5, 129.0, 128.9, 128.2, 128.1, 126.1, 126.0, 125.4, 124.7, 122.8, 113.3, 96.0. HRMS (ESI): calcd for C34H23BrN6O4 (M+Na)+ 681.0856, found 681.0853.
4-(4-Methoxyphenyl)-1-(4-nitrophenyl)-3,6,8-triphenyl-1,2,4,6,8-pentaazaspiro[4.4]non-2-ene-7,9-dione (5i) was obtained from 2b and 3b as beige solid (60%a/50%b). 1H NMR (400 MHz, DMSO-d6) δ 8.34 – 8.30 (m, 3H, Ar), 7.54 – 7.51 (m, 3H, Ar), 7.50 – 7.48 (m, 2H, Ar), 7.47 – 7.43 (m, 5H, Ar), 7.40 – 7.36 (m, 4H, Ar), 7.14 (d, J = 8.6 Hz, 2H, Ar), 7.10 – 7.07 (m, 2H, Ar), 7.04 (d, J = 9.1 Hz, 2H, Ar), 3.76 (s, 3H, OCH3). 13C NMR (101 MHz, DMSO-d6) δ 163.9, 159.4, 150.9, 148.9, 145.4, 140.6, 133.0, 130.9, 130.0, 129.8, 129.5, 129.5, 129.1, 128.9, 128.2, 127.9, 127.5, 126.9, 126.4, 125.4, 125.3, 115.3, 113.0, 95.9, 55.6. HRMS (ESI): calcd for C35H26N6O5 (M+H)+ 611.2037, found 611.2041.
3-(4-Chlorophenyl)-1,6,8-triphenyl-4-(p-tolyl)-1,2,4,6,8-pentaazaspiro[4.4]non-2-ene-7,9-dione (5j) was obtained from 2e and 3g as beige solid (57%a/93%b). 1H NMR (400 MHz, CDCl3) δ 7.53 (d, J = 7.7 Hz, 2H, Ar), 7.44 – 7.40 (m, 2H, Ar), 7.40 – 7.34 (m, 4H, Ar), 7.34 – 7.27 (m, 6H, Ar), 7.24 – 7.21 (m, 2H, Ar), 7.13 – 7.09 (m, 4H, Ar), 7.05 – 7.01 (m, 1H, Ar), 6.97 (d, J = 8.3 Hz, 2H, Ar), 2.34 (s, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 165.1, 151.9, 146.2, 141.4, 138.6, 135.8, 134.1, 133.5, 130.7, 130.4, 129.7, 129.4, 129.4, 129.1, 128.9, 128.8, 127.4, 127.3, 126.3, 125.2, 124.9, 122.4, 115.3, 97.8, 21.2. HRMS (ESI): calcd for C35H26ClN5O2 (M+H)+ 584.1848, found 584.1849.
3-Methyl-4-(4-nitrophenyl)-1,6,8-triphenyl-1,2,4,6,8-pentaazaspiro[4.4]non-2-ene-7,9-dione (5k) was obtained from 2g and 3d as orange solid (88%a/59%b). 1H NMR (400 MHz, CDCl3) δ 8.27 – 8.22 (m, 2H, Ar), 7.46 – 7.37 (m, 7H, Ar), 7.36 – 7.31 (m, 3H, Ar), 7.23 – 7.19 (m, 4H, Ar), 7.15 – 7.11 (m, 2H, Ar), 7.05 (m, 1H, Ar), 2.03 (s, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 165.5, 151.5, 147.1, 144.1, 141.7, 140.6, 133.9, 130.4, 129.8, 129.6, 129.5, 129.2, 128.1, 127.7, 126.0, 125.3, 124.8, 122.9, 115.9, 96.9, 12.1. HRMS (ESI): calcd for C29H22N6O4 (M+H)+ 519.1775, found 519.1772.
4-(4-Bromophenyl)-3-methyl-1,6,8-triphenyl-1,2,4,6,8-pentaazaspiro[4.4]non-2-ene-7,9-dione (5l) was obtained from 2d and 3d as orange solid (73%a/57%b). 1H NMR (400 MHz, CDCl3) δ 7.54 – 7.50 (m, 2H, Ar), 7.49 – 7.45 (m, 2H, Ar), 7.45 – 7.36 (m, 5H, Ar), 7.35 – 7.28 (m, 3H, Ar), 7.23 – 7.19 (m, 2H, Ar), 7.15 – 7.10 (m, 2H, Ar), 7.03 – 6.98 (m, 1H, Ar), 6.96 – 6.90 (m, 2H, Ar), 1.93 (s, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ 165.9, 151.6, 145.4, 142.0, 134.3, 133.3, 133.2, 130.6, 130.6, 129.7, 129.4, 129.0, 127.2, 126.2, 124.6, 123.4, 122.3, 115.3, 97.1, 11.6. HRMS (ESI): calcd for C29H22BrN5O2 (M+H)+ 552.1030, found 552.1023.
Ethyl 2-(4-(4-bromophenyl)-1-(4-nitrophenyl)-7,9-dioxo-3,8-diphenyl-1,2,4,6,8-pentaazaspiro[4.4]non-2-en-6-yl)acetate (5m) was obtained from 2k and 3b as orange solid (15%b). 1H NMR (400 MHz, CDCl3) δ 8.23 (d, J = 9.2 Hz, 2H, Ar), 7.51 – 7.47 (m, 3H, Ar), 7.46 – 7.43 (m, 2H, Ar), 7.42 – 7.40 (m, 2H, Ar), 7.37 – 7.33 (m, 3H, Ar), 7.29 (d, J = 9.2 Hz, 2H, Ar), 7.22 (d, J = 8.1 Hz, 2H, Ar), 7.09 – 7.02 (m, 2H, Ar), 4.32 (d, J = 17.6 Hz, 1H, NCH2), 4.21 – 4.07 (m, 3H, NCH2+COOCH2CH3), 1.19 (t, J = 7.1 Hz, 3H, COOCH2CH3). 13C NMR (101 MHz, CDCl3) δ 167.4, 164.0, 152.4, 148.8, 146.0, 141.8, 135.6, 133.1, 131.0, 130.1, 129.6, 129.3, 128.9, 128.7, 128.2, 125.9, 125.7, 125.5, 122.5, 113.7, 95.4, 62.3, 41.0, 14.1. HRMS (ESI): calcd for C32H25BrN6O6 (M+Na)+ 691.0911, found 691,0913.
Ethyl 2-(4-(4-bromophenyl)-1-(4-nitrophenyl)-7,9-dioxo-3,6-diphenyl-1,2,4,6,8-pentaazaspiro[4.4]non-2-en-8-yl)acetate (5n) was obtained from 2l and 3b as orange solid (76%b). 1H NMR (400 MHz, DMSO-d6) δ 8.24 – 8.19 (m, 2H, Ar), 7.60 – 7.56 (m, 2H, Ar), 7.46 – 7.41 (m, 2H, Ar), 7.41 – 7.37 (m, 5H, Ar), 7.37 – 7.31 (m, 3H, Ar), 7.30 – 7.26 (m, 2H, Ar), 7.03 – 6.97 (m, 2H, Ar), 4.56 – 4.45 (m, 2H, NCH2), 4.20 (qd, J = 7.0, 1.4 Hz, 2H, COOCH2CH3), 1.21 (t, J = 7.1 Hz, 3H, COOCH2CH3). 13C NMR (101 MHz, DMSO-d6) δ 167.0, 164.1, 151.1, 148.3, 144.9, 140.7, 134.3, 132.9, 132.3, 130.9, 129.8, 129.0, 128.8, 128.5, 127.9, 126.0, 125.7, 125.0, 121.4, 113.5, 95.3, 61.9, 14.0. HRMS (ESI): calcd for C32H25BrN6O6 (M+Na)+ 691.0911, found 691.0913.