Submitted:
27 November 2023
Posted:
28 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Test Material
2.2. Animals and Ethical Aspects
2.3. Experimental design and ovariectomy
- Negative control group (OVW): ovariectomized rats treated with distilled water (1 mL/kg, p.o);
- Sodium alendronate group (ADS): ovariectomized rats treated with Sodium Alendronate (4 mg/kg, p.o).
- Estrogen group (EST): ovariectomized rats treated with estrogen (2 µg/kg, p.o);
- Ormona® group (ORM): ovariectomized rats treated with Ormona® (20 mg/kg, p.o);
- Experimental group (ORM + EST): ovariectomized rats treated with Ormona® (20 mg/kg + estrogen 2 µg/kg, p.o);
2.4. Hormonal and Biochemical Analysis
2.5. Scanning electron microscopy (SEM) of the femur
2.6. Quantification of Calcium in Bone Matrix by atomic absorption spectrophotometry
2.7. Histopathological analysis of bone tissue
2.8. Statistical Analysis
3. Results
3.1. Ponderal development
3.2. Hormonal and biochemical analysis
3.3. Scanning electron microscopy (SEM) of the femur and calcium quantification
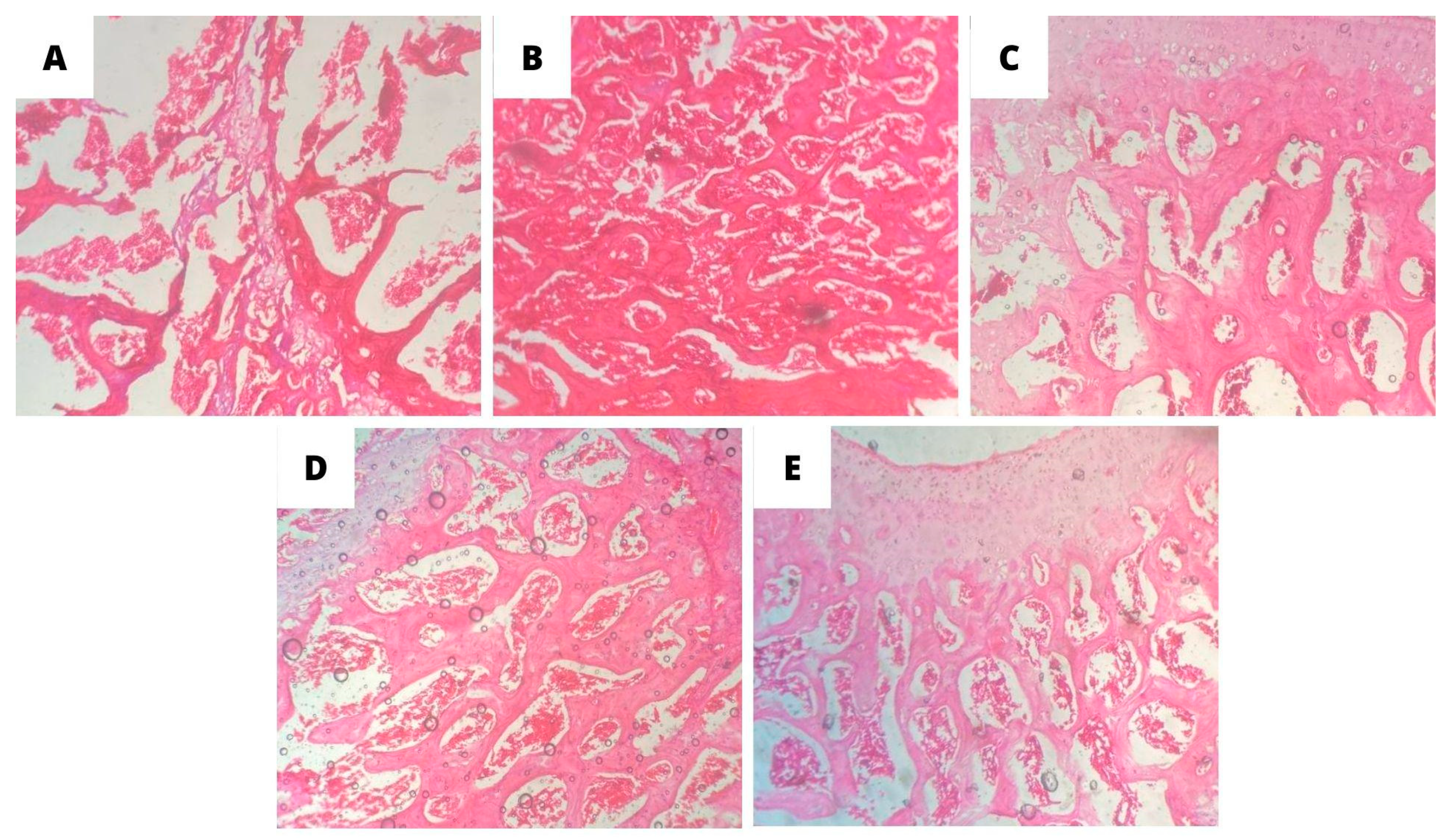
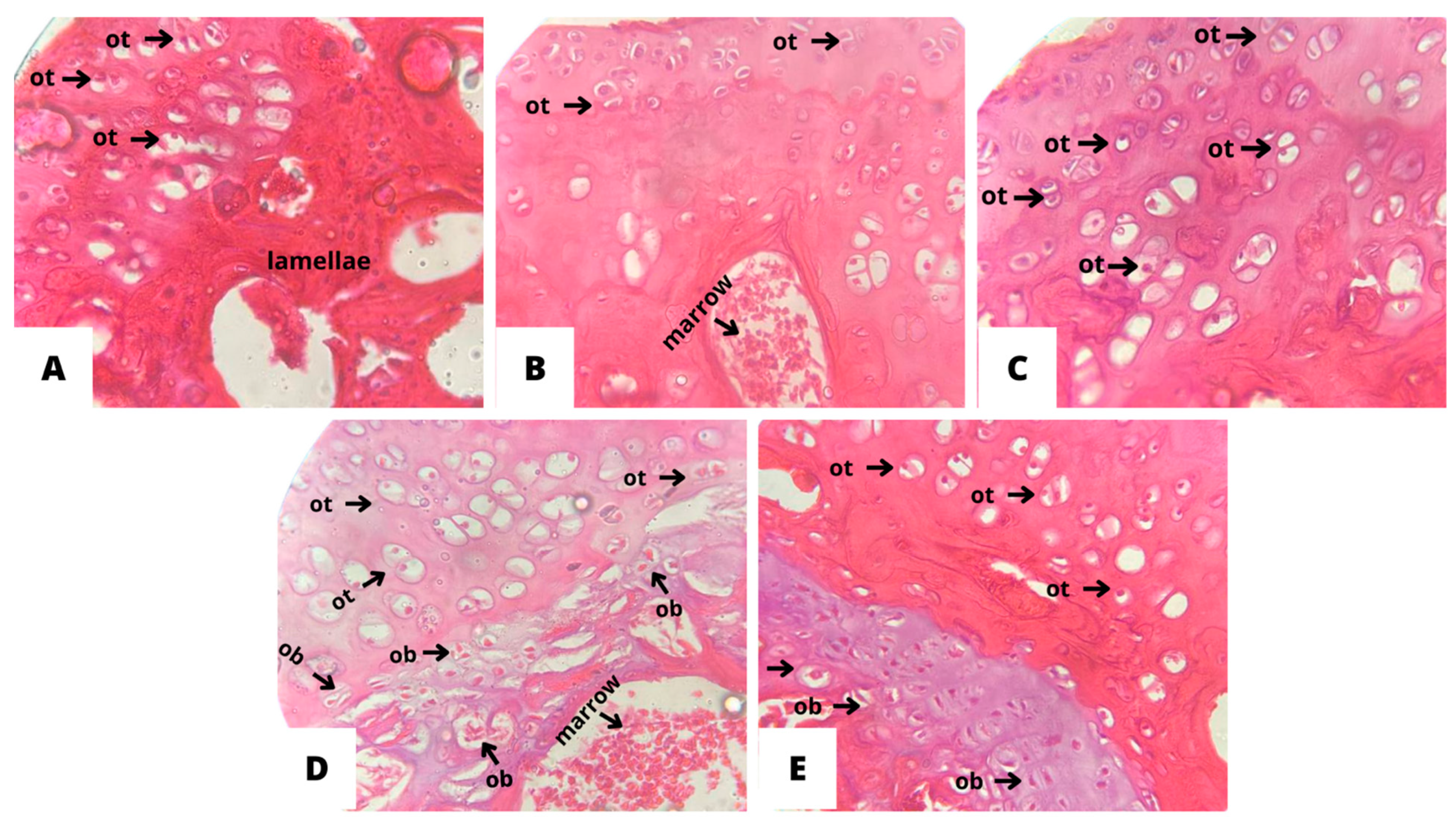
3.4. Histopathological analysis of bone tissue
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, A.C.V.; Rosa, M.I.; Fernandes, B.; Lumertz, S.; Diniz, R.M.; Damiani ME dos, R. Fatores associados à osteopenia e osteoporose em mulheres submetidas à densitometria óssea. Rev Bras Reumatol, 2015, 55:223–8. [CrossRef]
- Radominski, S.C.; Bernardo, W.; Patrícia De Paula, A.; Albergaria, B.H.; Moreira, C.; Fernandes, C.E.; et al. Brazilian guidelines for the diagnosis and treatment of post-menopausal osteoporosis. Revista brasileira de reumatologia, 2017. [CrossRef]
- Costa, J.R.G da, Tavares A.L de F, Bertolini G.R.F, Costa R.M, Ribeiro L. de F.C. Efeito da reposição fitoterápica com isoflavonas na histomorfometria do tecido ósseo de ratas Wistar ooforectomizadas. Biosaúde. (2021).
- Deli, T.; Orosz, M.; Jakab, A. Hormone Replacement Therapy in Cancer Survivors – Review of the Literature. Pathology & Oncology Research 2019, 26, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y., Ma L., Yang X., Bie J., Li D., Sun C., et al. Menopausal Hormone Replacement Therapy and the Risk of Ovarian Cancer: A Meta-Analysis. Front Endocrinol (Lausanne). 3 de dezembro de 2019, 10:486180. [CrossRef]
- Frigo, M., Barros E. de, Santos P.C. de B. dos, Koehnlein E.A. Isoflavonas como tratamento alternativo na sintomatologia climatérica: uma revisão sistemática. Rev Inst Adolfo Lutz, 2021. [CrossRef]
- Viggiani, M.T.; Polimeno, L.; Di Leo, A.; Barone, M. Phytoestrogens: Dietary Intake, Bioavailability, and Protective Mechanisms against Colorectal Neoproliferative Lesions. Nutrients. 24 de julho de 2019, 11, 1709. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K. Defining ’nutraceuticals’: neither nutritious nor pharmaceutical. British journal of clinical pharmacology. 2017, 83, 8–19. [Google Scholar] [CrossRef]
- Rocha, C.F, Flexa C de N.N, de Souza G.C, Pereira A.C.M, Carvalho H. de O., Nascimento A.L, et al. Acute and Reproductive Toxicity Evaluation of Ormona® SI and Ormona® RC-Two New Nutraceuticals with Geranylgeraniol, Tocotrienols, Anthocyanins, and Isoflavones-In Adult Zebrafish. Pharmaceuticals (Basel), 2022 15(11). [CrossRef]
- Rodrigues A.P.S, da Silva B.R, Pereira A.C.M, Batista M.A, Sales P.F, Ferreira A.M, et al. Ormona® SI and Ormona® RC—New Nutraceuticals with Geranylgeraniol, Tocotrienols, Anthocyanins, and Isoflavones—Decrease High-Fat Diet-Induced Dyslipidemia in Wistar Rats. Nutraceuticals, 2022, 2, 311–22. [CrossRef]
- Tsuang Y.H, Chen L.T, Chiang C.J, Wu L.C, Chiang Y.F, Chen P.Y, et al. Isoflavones prevent bone loss following ovariectomy in young adult rats. J Orthop Surg Res, 2008, 3, 1–9. [CrossRef]
- Yousefzadeh, N.; Kashfi, K.; Jeddi, S.; Ghasemi, A. Ovariectomized rat model of osteoporosis: a practical guide. EXCLI J. 10 de janeiro de 2020, 19:89. [CrossRef]
- Oliveira Carvalho H., Farias e Souza B.S, dos Santos I.V.F, Resque R.L, Keita H, Fernandes C.P, et al. Hypoglycemic effect of formulation containing hydroethanolic extract of Calophyllum brasiliense in diabetic rats induced by streptozotocin. Revista Brasileira de Farmacognosia. setembro de 2016, 26, 634–9. [CrossRef]
- Pereira A.C.M, de Oliveira Carvalho H., Gonçalves D.E.S., Picanço K.R.T, de Lima Teixeira dos Santos A.V.T, da Silva H.R, et al. Co-Treatment of Purified Annatto Oil (Bixa orellana L.) and Its Granules (Chronic®) Improves the Blood Lipid Profile and Bone Protective Effects of Testosterone in the Orchiectomy-Induced Osteoporosis in Wistar Rats. Molecules. 4 de agosto de 2021, 26, 4720. [CrossRef]
- Palma, M.N.N.; Rocha, G.C.; Filho, S.C.V.; Detmann, E. Evaluation of Acid Digestion Procedures to Estimate Mineral Contents in Materials from Animal Trials. Asian-Australas J Anim Sci. 4 de agosto de 2015, 28, 1624–8. [Google Scholar] [PubMed]
- Hou, T.; Zhang, L.; Yang, X. Ferulic acid, a natural polyphenol, protects against osteoporosis by activating SIRT1 and NF-κB in neonatal rats with glucocorticoid-induced osteoporosis. Biomedicine & Pharmacotherapy. 1o de dezembro de 2019, 120:109205. [CrossRef]
- Turner, A.S. Animal models of osteoporosis - necessity and limitations. European Cells & Materials. 22 de junho de 2001, 1:66–81. [CrossRef]
- Yamagishi T., Otsuka E., Hagiwara H. Reciprocal Control of Expression of mRNAs for Osteoclast Differentiation Factor and OPG in Osteogenic Stromal Cells by Genistein: Evidence for the Involvement of Topoisomerase II in Osteoclastogenesis. Endocrinology. 1o de agosto de 2001, 142, 3632–7. [CrossRef]
- Thompson D.D., Simmons H.A., Pirie C.M., Ke H.Z. FDA guidelines and animal models for osteoporosis. Bone. Outubro de 1995, 17, S125–33. [CrossRef]
- Silveira, V.A.S. Efeitos iniciais da ovariectomia e do tratamento com estrógeno e isoflavonas da soja, isolados e associados, na reparação óssea alveolar e no útero de ratas [tese]. Faculdade de Odontologia de São José dos Campos, UNESP. 10 de julho de 2007.
- Sharaf, H.A.; Shaffie, N.M.; Morsy, F.A.; Badawi, M.A.; Abbas, N.F. Role of some phytoestrogens in recovering bone loss: histological results from experimental ovariectomized rat models. Journal of The Arab Society for Medical Research. 2015, 10, 65. [Google Scholar] [CrossRef]
- Kalleny, N.K. Histological and morphometric studies on the effect of alpha-lipoic acid on post ovariectomy osteoporosis induced in adult female albino rats. The Egyptian Journal of Histology. Março de 2011, 34, 139–55. [Google Scholar] [CrossRef]
- Park, J.A., Ha S.K., Kang T.H., Oh M.S., Cho M.H., Lee S.Y., et al. Protective effect of apigenin on ovariectomy-induced bone loss in rats. Life Sci. 20 de junho de 2008, 82(25–26):1217–23. [CrossRef]
- Weber K., Kaschig C., Erben R.G. 1α-Hydroxyvitamin D2 and 1α-hydroxyvitamin D3 have anabolic effects on cortical bone, but induce intracortical remodeling at toxic doses in ovariectomized rats. Bone. Setembro de 2004, 35, 704–10. [CrossRef]
- Barlet J.P., Picherit C., Coxam V., Bennetau-Pelissero C., Lebecque P., Kati-Coulibaly S., et al. Daidzein Is More Efficient than Genistein in Preventing Ovariectomy-Induced Bone Loss in Rats. J Nutr. Julho de 2000, 130, 1675–81. [CrossRef]
- Fanti P., Monier-Faugere M.C., Geng Z., Schmidt J., Morris P.E., Cohen D., et al. The phytoestrogen genistein reduces bone loss in short-term ovariectomized rats. Osteoporosis International. 1998, 8, 274-81. [CrossRef]
- López, M.; Tena-Sempere, M. Estrogens and the control of energy homeostasis: a brain perspective. Trends in Endocrinology & Metabolism. 1o de agosto de 2015, 26, 411–21. [Google Scholar] [CrossRef]
- Martínez de Morentin, P.B.; González-García, I.; Martins, L.; Lage, R.; Fernández-Mallo, D.; Martínez-Sánchez, N.; et al. Estradiol Regulates Brown Adipose Tissue Thermogenesis via Hypothalamic, A. M.P.K. Cell Metab. 1º de julho de 2014, 20, 41–53. [Google Scholar] [CrossRef]
- Xu, Y.; López, M. Central regulation of energy metabolism by estrogens. Mol Metab. 23 de maio de 2018, 15:104–15. [CrossRef]
- Hirschberg, A.L. Sex hormones, appetite and eating behaviour in women. Maturitas. Março de 2012, 71, 248–56. [Google Scholar] [CrossRef]
- Liu Z.P., Li W.X., Yu B., Huang .J, Sun J., Huo J.S., et al. Effects of trans-Resveratrol from Polygonum cuspidatum on Bone Loss Using the Ovariectomized Rat Model. J Med Food. 25 de março de 2005, 8, 14–9. [CrossRef]
- Yu, K., He Y., Hyseni I., Pei Z., Yang Y., Xu P., et al. 17β-estradiol promotes acute refeeding in hungry mice via membrane-initiated ERα signaling. Mol Metab. Dezembro de 2020, 42:101053. [CrossRef]
- Li L., Yang Y., Ma C., Li X., Bian X., Fu Y., et al. Effects of soybean isoflavone aglycone on osteoporosis in ovariectomized rats. Front Nutr. 5 de junho de 2023, 10. [CrossRef]
- Xu, H., Liu T., Hu L., Li J., Gan C., Xu J., et al. Effect of caffeine on ovariectomy-induced osteoporosis in rats. Biomedicine & Pharmacotherapy. Abril de 2019, 112:108650. [CrossRef]
- Zhang, Q., Song X., Chen X., Jiang R., Peng K., Tang X., et al. Antiosteoporotic effect of hesperidin against ovariectomy-induced osteoporosis in rats via reduction of oxidative stress and inflammation. J Biochem Mol Toxicol. 24 de agosto de 2021, 35(8). [CrossRef]
- Khattab, H.A.H.; Ardawi, M.S.; Ateeq, R.A.M. Effect of Phytoestrogens Derived from Red Clover (Trifolium Pratense L.) in Ovariectomized Rats. Life Science Journal. Janeiro de 2013, 10, 1485–1497. [Google Scholar]
- Chen, Y.M.; Wang, I.L.; Zhu, X.Y.; Chiu, W.C.; Chiu, Y.S. Red Clover Isoflavones Influence Estradiol Concentration, Exercise Performance, and Gut Microbiota in Female Mice. Front Nutr. 14 de abril de 2021, 8: 623698. [CrossRef]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: estrogenic activity, biological effect and bioavailability. Eur J Drug Metab Pharmacokinet. 17 de março de 2013, 38, 15–25. [Google Scholar] [CrossRef]
- Liu J., Burdette J.E., Xu H., Gu C., van Breemen R.B., Bhat K.P.L., et al. Evaluation of Estrogenic Activity of Plant Extracts for the Potential Treatment of Menopausal Symptoms. J Agric Food Chem. 1o de maio de 2001, 49, 2472–9. [CrossRef]
- Burdette J.E., Liu J., Lantvit D., Lim E., Booth N., Bhat K.P.L., et al. Trifolium pratense (Red Clover) Exhibits Estrogenic Effects In Vivo in Ovariectomized Sprague-Dawley Rats. J Nutr. Janeiro de 2002, 132, 27–30. [CrossRef]
- Greenwood S., Barnes S., Clarkson T.B., Eden J., Helferich W.G., Hughes C., et al. The role of isoflavones in menopausal health: Consensus opinion of the North American Menopause Society. Menopause: The Journal of the North American Menopause Society. 27 de março de 2000, 7, 215–29. [CrossRef]
- Tripathi A., Singh S., Raju K.S.R., Wahajuddin, Gayen J. Effect of Red Clover on CYP Expression: An Investigation of Herb-Drug Interaction at Molecular Level. Indian Journal of Pharmaceutical Sciences. 2014, 76, 261. [CrossRef]
- Hwang, C.S., Kwak H.S., Lim H.J., Lee S.H., Kang Y.S., Choe T.B., et al. Isoflavone metabolites and their in vitro dual functions: They can act as an estrogenic agonist or antagonist depending on the estrogen concentration. J Steroid Biochem Mol Biol. Novembro de 2006, 101(4–5): 246–53. [CrossRef]
- Stubert, J.; Gerber, B. Isoflavones – Mechanism of Action and Impact on Breast Cancer Risk. Breast Care. 20 de fevereiro de 2009, 4, 22–9. [Google Scholar] [CrossRef]
- Medina-Contreras, J.; Villalobos-Molina, R.; Zarain-Herzberg, A.; Balderas-Villalobos, J. Ovariectomized rodents as a menopausal metabolic syndrome model. A minireview. Mol Cell Biochem. 27 de dezembro de 2020, 475(1–2): 261–76. [CrossRef]
- Nigro M., Santos A.T., Barthem C.S., Louzada R.A.N., Fortunato R.S., Ketzer L.A., et al. A Change in Liver Metabolism but Not in Brown Adipose Tissue Thermogenesis Is an Early Event in Ovariectomy-Induced Obesity in Rats. Endocrinology. 1o de agosto de 2014, 155, 2881–91. [CrossRef]
- Heine, P.A.; Taylor, J.A.; Iwamoto, G.A.; Lubahn, D.B.; Cooke, P.S. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proceedings of the National Academy of Sciences. 7 de novembro de 2000, 97, 12729–34. [Google Scholar] [CrossRef] [PubMed]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ. 24 de dezembro de 2017, 8, 33. [Google Scholar] [CrossRef]
- Palmisano, B.T.; Zhu, L.; Stafford, J.M. Role of Estrogens in the Regulation of Liver Lipid Metabolism. Adv Exp Med Biol. 10 de dezembro de 2017, 1043: 227–56. [CrossRef]
- Berman, D.M.; Nicklas, B.J.; Ryan, A.S.; Rogus, E.M.; Dennis, K.E.; Goldberg, A.P. Regulation of Lipolysis and Lipoprotein Lipase after Weight Loss in Obese, Post-menopausal Women. Obes Res. Janeiro de 2004, 12, 32–9. [Google Scholar] [CrossRef]
- Faulds M.H., Zhao C., Dahlman-Wright K/, Gustafsson J.Å. The diversity of sex steroid action: regulation of metabolism by estrogen signaling. Journal of Endocrinology. Janeiro de 2012, 212, 3–12. [CrossRef]
- Pearce, B.C.; Parker, R.A.; Deason, M.E.; Qureshi, A.A.; Wright, J.J.K. Hypocholesterolemic activity of synthetic and natural tocotrienols. J Med Chem. 1o de outubro de 1992, 35, 3595–606. [Google Scholar] [CrossRef]
- Sever, N.; Song, B.L.; Yabe, D.; Goldstein, J.L.; Brown, M.S.; DeBose-Boyd, R.A. Insig-dependent Ubiquitination and Degradation of Mammalian 3-Hydroxy-3-methylglutaryl-CoA Reductase Stimulated by Sterols and Geranylgeraniol. Journal of Biological Chemistry. Dezembro de 2003, 278, 52479–90. [Google Scholar] [CrossRef]
- Qureshi, A.; Khan, D.; Mahjabeen, W.; Qureshi, N. Dose-dependent Modulation of Lipid Parameters, Cytokines and RNA by δ-tocotrienol in Hypercholesterolemic Subjects Restricted to AHA Step-1 Diet. Br J Med Med Res. 10 de janeiro de 2015, 6, 351–66. [Google Scholar] [CrossRef]
- Luís, Â.; Domingues, F.; Pereira, L. Effects of red clover on perimenopausal and post-menopausal women’s blood lipid profile: A meta-analysis. Climacteric. 3 de setembro de 2018, 21, 446–53. [Google Scholar] [CrossRef]
- Chen, M.J.; Chiu, H.M.; Chen, C.L.; Yang, W.S.; Yang, Y.S.; Ho, H.N. Hyperandrogenemia Is Independently Associated with Elevated Alanine Aminotransferase Activity in Young Women with Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 1o de julho de 2010, 95, 3332–41. [Google Scholar] [CrossRef]
- Grigoryan A.V., Dimitrova A.A., Kostov K.G., Russeva A.L., Atanasova M.A., Blagev A.B., et al. Changes of Serum Concentrations of Alkaline Phosphatase and Metalloproteinase-9 in an Ovariectomized Wistar Rat Model of Osteoporosis. Journal of Biomedical and Clinical Research. 1o de novembro de 2017, 10, 32–6. [CrossRef]
- Kim, S.K.; Lee, M.H.; Rhee, M.H. Studies on the Effects of Biomedicinal Agents on Serum Concentration of Ca2+, P and ALP Activity in Osteoporosis-Induced Rats. J Vet Sci. Agosto de 2003, 4, 151–4. [Google Scholar] [CrossRef]
- Devareddy, L.; Hooshmand, S.; Collins, J.K.; Lucas, E.A.; Chai, S.C.; Arjmandi, B.H. Blueberry prevents bone loss in ovariectomized rat model of post-menopausal osteoporosis. J Nutr Biochem. Outubro de 2008, 19, 694–9. [Google Scholar] [CrossRef]
- Zhang, R.; Hu, S.J.; Li, C.; Zhang, F.; Gan, H.Q.; Mei, Q.B. Achyranthes bidentata root extract prevent OVX-induced osteoporosis in rats. J Ethnopharmacol. Janeiro de 2012, 139, 12–8. [Google Scholar] [CrossRef]
- Chang K.L., Hu Y.C., Hsieh B.S., Cheng H.L., Hsu H.W., Huang L.W., et al. Combined effect of soy isoflavones and vitamin D3 on bone loss in ovariectomized rats. Nutrition. Janeiro de 2013, 29, 250–7. [CrossRef]
- Hooshmand, S.; Juma, S.; Arjmandi, B.H. Combination of Genistin and Fructooligosaccharides Prevents Bone Loss in Ovarian Hormone Deficiency. J Med Food. Abril de 2010, 13, 320–5. [Google Scholar] [CrossRef]
- Gao, Y.H.; Yamaguchi, M. Suppressive Effect of Genistein on Rat Bone Osteoclasts: Apoptosis Is Induced through Ca2+ Signaling. Biol Pharm Bull. 15 de agosto de 1999, 22, 805–9. [Google Scholar] [CrossRef]
- Orsatti, F.L.; Nahas, E.P.; Nahas-Neto, J.; Orsatti, C.L.; Teixeira, A.S. Efeito do treinamento contrarresistência e isoflavona na densidade mineral óssea em mulheres na pós-menopausa. Revista Brasileira de Cineantropometria e Desempenho Humano. 27 de agosto de 2013, 15(6). [CrossRef]
- Cegieła, U.; Folwarczna, J.; Pytlik, M.; Zgórka, G. Effects of Extracts from Trifolium medium L. and Trifolium pratense L. on Development of Estrogen Deficiency-Induced Osteoporosis in Rats. Evidence-Based Complementary and Alternative Medicine. 29 de novembro de 2012, 2012: 1–11. [CrossRef]
- AL-Ghaban, N.; Jasem, G. Histomorphometric evaluation of the effects of local application of red cloveroil (trifolium pratense) on bone healing in rats. Journal of Baghdad College of Dentistry. 15 de junho de 2020, 32, 26–31. [Google Scholar] [CrossRef]
- Occhiuto F., De Pasquale R., Guglielmo G., Palumbo D.R., Zangla G., Samperi S., et al. Effects of phytoestrogenic isoflavones from red clover (Trifolium pratense L.) on experimental osteoporosis. Phytotherapy Research. Fevereiro de 2007, 21, 130–4. [CrossRef]
- Gao, Y.H.; Yamaguchi, M. Suppressive effect of genistein on rat bone osteoclasts: involvement of protein kinase inhibition and protein tyrosine phosphatase activation. Int J Mol Med. 1o de março de 2000, 5, 261–7. [Google Scholar] [CrossRef]
- Hiruma, Y.; Nakahama, K.; Fujita, H.; Morita, I. Vitamin K2 and geranylgeraniol, its side chain component, inhibited osteoclast formation in a different manner. Biochem Biophys Res Commun. Janeiro de 2004, 314, 24–30. [Google Scholar] [CrossRef]
- Andres, S.; Hansen, U.; Niemann, B.; Palavinskas, R.; Lampen, A. Determination of the isoflavone composition and estrogenic activity of commercial dietary supplements based on soy or red clover. Food Funct. Junho de 2015, 6, 2017–25. [Google Scholar] [CrossRef]
- Pfitscher, A.; Reiter, E.; Jungbauer, A. Receptor binding and transactivation activities of red clover isoflavones and their metabolites. J Steroid Biochem Mol Biol. Novembro de 2008, 112(1–3): 87–94. [CrossRef]
- Mathey J., Mardon J., Fokialakis N., Puel C., Kati-Coulibaly S., Mitakou S., et al. Modulation of soy isoflavones bioavailability and subsequent effects on bone health in ovariectomized rats: the case for equol. Osteoporosis International. 29 de março de 2007, 18, 671–9. [CrossRef]
- Somjen, D.; Katzburg, S.; Kohen, F.; Gayer, B.; Livne, E. Daidzein but not other phytoestrogens preserves bone architecture in ovariectomized female rats in vivo. J Cell Biochem. 15 de abril de 2008, 103, 1826–32. [Google Scholar] [CrossRef]
- Sehmisch S., Uffenorde J., Maehlmeyer S., Tezval M., Jarry H., Stuermer K.M., et al. Evaluation of bone quality and quantity in osteoporotic mice – The effects of genistein and equol. Phytomedicine. Maio de 2010, 17, 424–30. [CrossRef]
- Anderson, J.J.B.; Ambrose, W.W.; Garner, S.C. Biphasic Effects of Genistein on Bone Tissue in the Ovariectomized, Lactating Rat Model. Proc Soc Exp Biol Med. 1o de março de 1998, 217, 345–50. [Google Scholar] [CrossRef]
- Gautam A.K., Bhargavan B., Tyagi A.M., Srivastava K., Yadav D.K., Kumar M., et al. Differential effects of formononetin and cladrin on osteoblast function, peak bone mass achievement and bioavailability in rats. J Nutr Biochem. Abril de 2011, 22, 318–27. [CrossRef]
- Ha H., Lee H.Y., Lee J.H., Jung D., Choi J., Song K.Y., et al. Formononetin prevents ovariectomy-induced bone loss in rats. Arch Pharm Res. 27 de abril de 2010, 33, 625–32. [CrossRef]
- Harahap, I.A.; Suliburska, J. An overview of dietary isoflavones on bone health: The association between calcium bioavailability and gut microbiota modulation. Materials Today Proceedings. Abril de 2022, 63: S368–72. [CrossRef]
- De Souza, A.P., Gonsalves I.F., Schneider M.J.f., Giannini N.M., Kuroiwa V.Y., Quinones E.M., et al. Uso de Isoflavonas em Casos de Osteoporose nas Mulheres Uma Revisão Bibliográfica. Revista Higei@ - Revista Científica de Saúde. 19 de julho de 2022, 4(7).
- De Franciscis P., Colacurci N., Riemma G., Conte A., Pittana E., Guida M., et al. A Nutraceutical Approach to Menopausal Complaints. Medicina (Kaunas). 28 de agosto de 2019, 55, 544. [CrossRef]
- Lambert, M.N.T.; Jeppesen, P.B. Isoflavones and bone health in perimenopausal and post-menopausal women. Curr Opin Clin Nutr Metab Care. Novembro de 2018, 21, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Zhu L.L., Cao J., Sun M., Yuen T., Zhou R., Li J., et al. Vitamin C Prevents Hypogonadal Bone Loss. PLoS One. 8 de outubro de 2012, 7, e47058. [CrossRef]
- Shi, X.; Jiang, J.; Hong, R.; Xu, F.; Dai, S. Circulating IGFBP-3 and Interleukin 6 as Predictors of Osteoporosis in Post-menopausal Women: A Cross-Sectional Study. Mediators Inflamm. 31 de março de 2023, 2023: 1–6. [CrossRef]
- Kong Y.Y., Yoshida H., Sarosi I., Tan H.L., Timms E., Capparelli C., et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. Janeiro de 1999, 397, 315–23. [CrossRef]
- Chen, X.W.; Garner, S.C.; Anderson, J.J.B. Isoflavones regulate interleukin-6 and osteoprotegerin synthesis during osteoblast cell differentiation via an estrogen-receptor-dependent pathway. Biochem Biophys Res Commun. 12 de julho de 2002, 295, 417–22. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Chen, X.W.; Anderson, J.J.B. Physiological concentrations of genistein stimulate the proliferation and protect against free radical-induced oxidative damage of MC3T3-E1 osteoblast-like cells. Nutrition Research. Setembro de 2001, 21, 1287–98. [Google Scholar] [CrossRef]
- Choi, E.M.; Suh, K.S.; Kim, Y.S.; Choue, R.W.; Koo, S.J. Soybean ethanol extract increases the function of osteoblastic MC3T3-E1 cells. Phytochemistry. Abril de 2001, 56, 733–9. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Gao, Y.H. Anabolic effect of genistein on bone metabolism in the femoral-metaphyseal tissues of elderly rats is inhibited by the antiestrogen tamoxifen. Research in Experimental Medicine. 24 de março de 1997, 197, 101–7. [Google Scholar] [CrossRef]
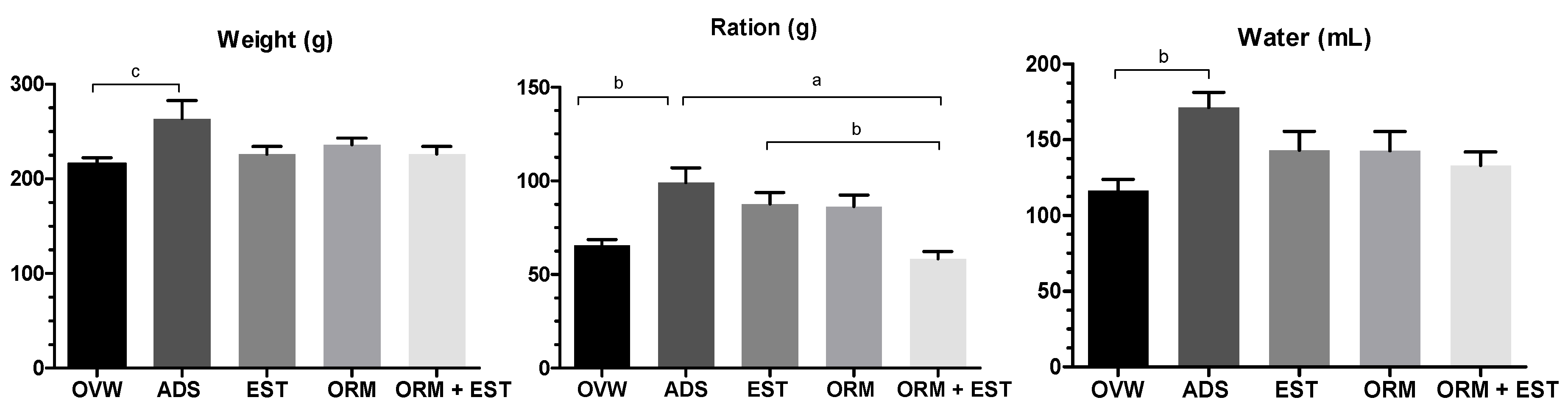

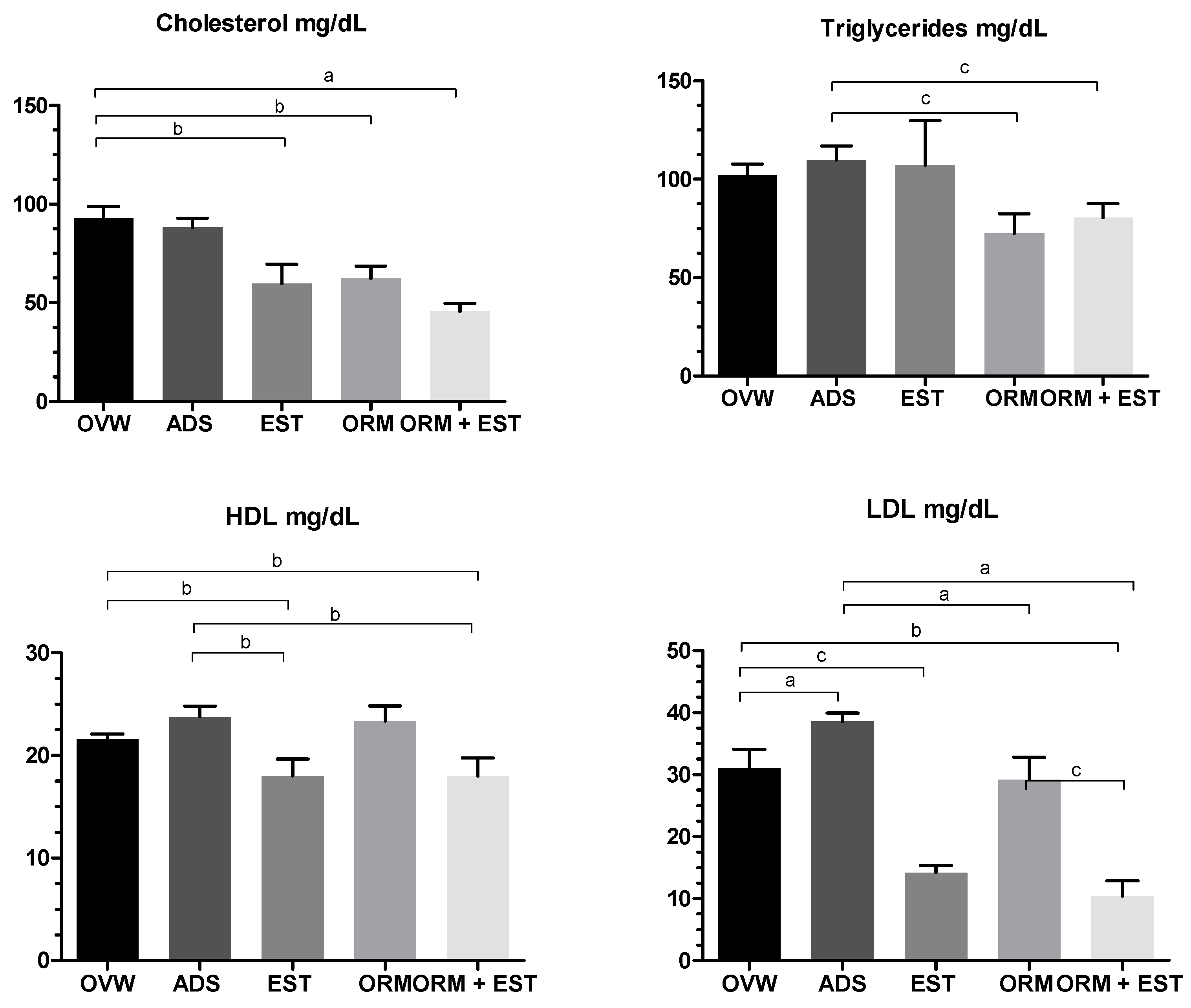
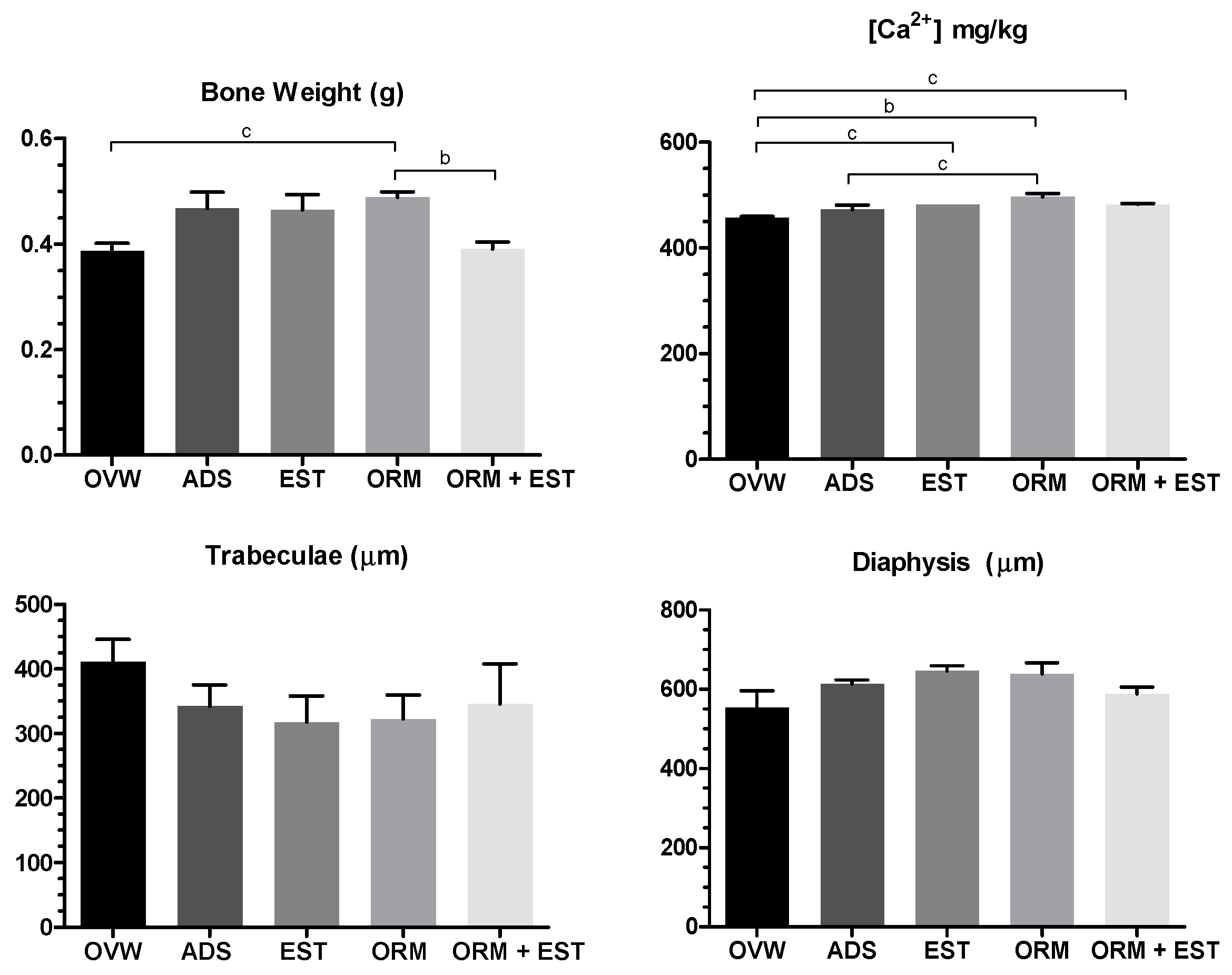
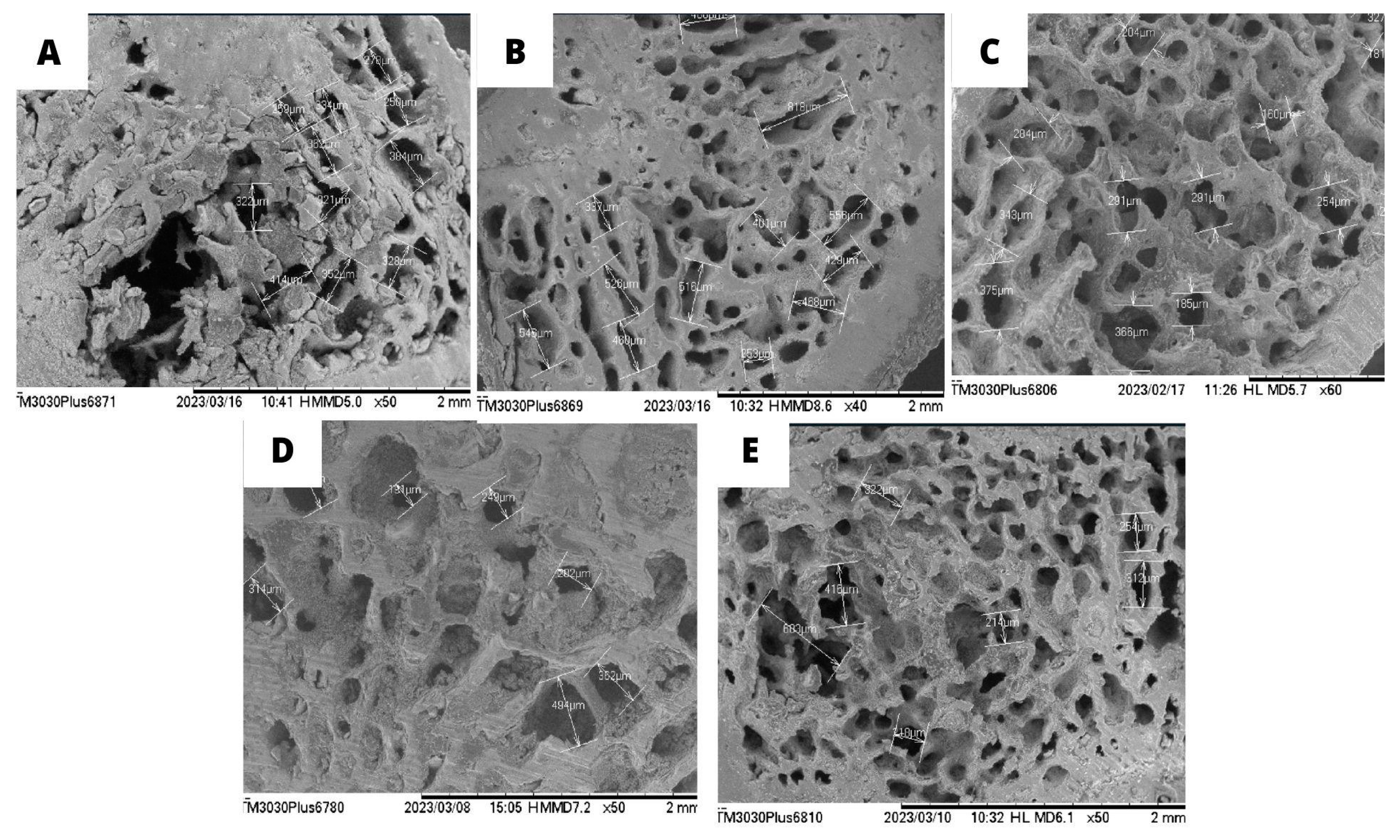
| Parameters | Urea (mg/dL) | Creatinine | AST (U/dL) | ALT (U/dL) | Alkaline Phosphatase (U/L) |
|---|---|---|---|---|---|
| OVW | 29,40 ± 4,10 | 0,42 ± 0,16 | 144,40 ± 24,48 | 20,40 ± 2,07 | 55,00 ± 12,12 |
| ADS | 22,20 ± 6,42 | 0,38 ± 0,08 | 156,60 ± 33,95 | 37,60 ± 2,61*** | 50,80 ± 15,47 |
| EST | 31,40 ± 2,88 | 0,46 ± 0,15 | 276,60 ± 102,10*# | 33,80 ± 7,22** | 44,60 ± 8,53 |
| ORM | 25,40 ± 5,40 | 0,38 ± 0,04 | 201,30 ± 49,27 | 33,00 ± 3,74** | 51,40 ± 19,26 |
| ORM + EST | 26,40 ± 7,27 | 0,48± 0,05 | 156,80 ± 48,02 | 28,80 ± 7,56 | 51,60 ± 4,77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





