Submitted:
24 November 2023
Posted:
27 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Study area and period
2.2. Water sample collection
2.3. Isolation of bacterial pathogens in the screened water samples
2.4. Identification of bacterial pathogens in screened water samples
2.4.1. Biochemical and serological identification of bacterial pathogens
2.4.2. Molecular identification of the bacterial pathogens
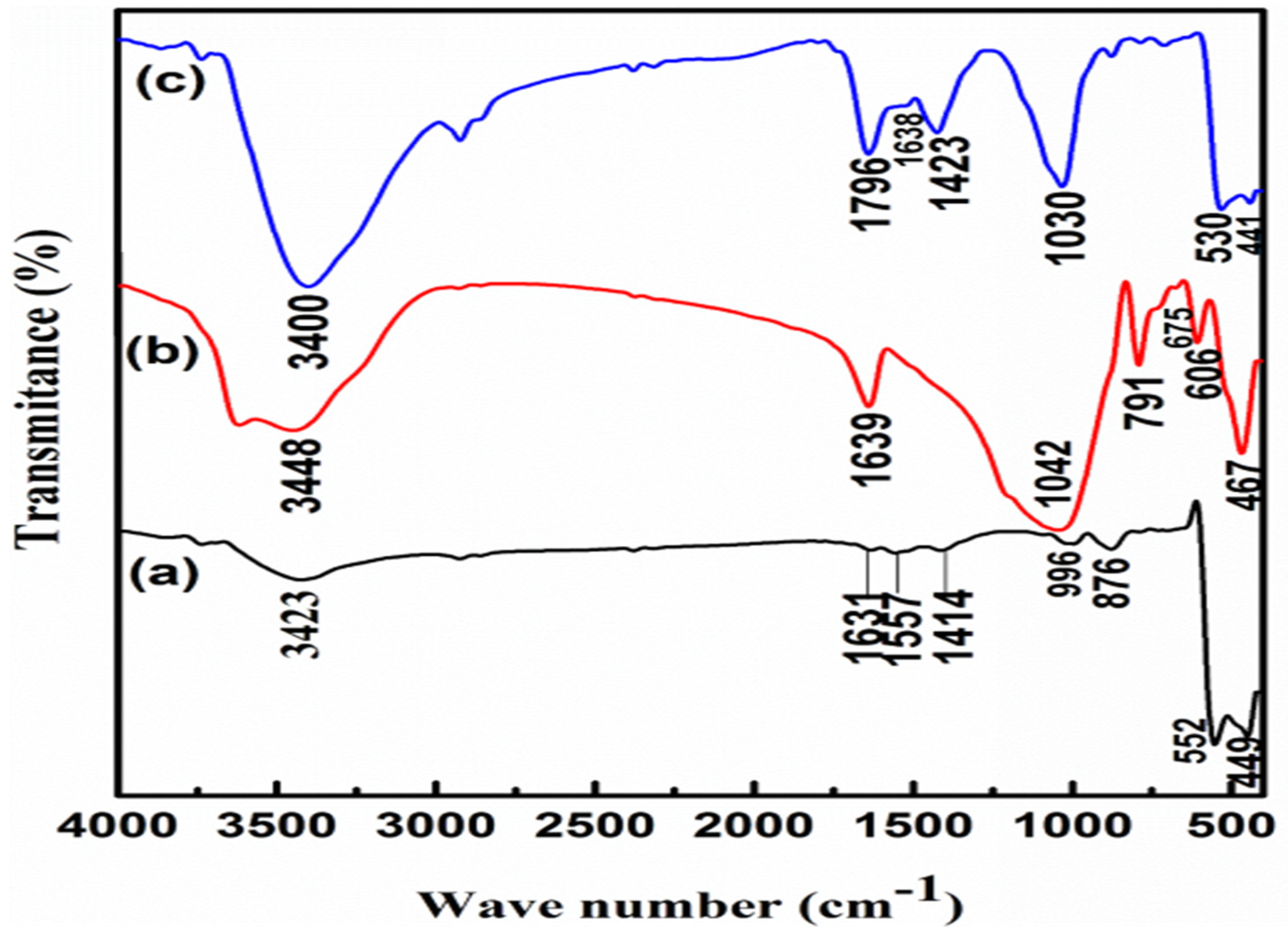
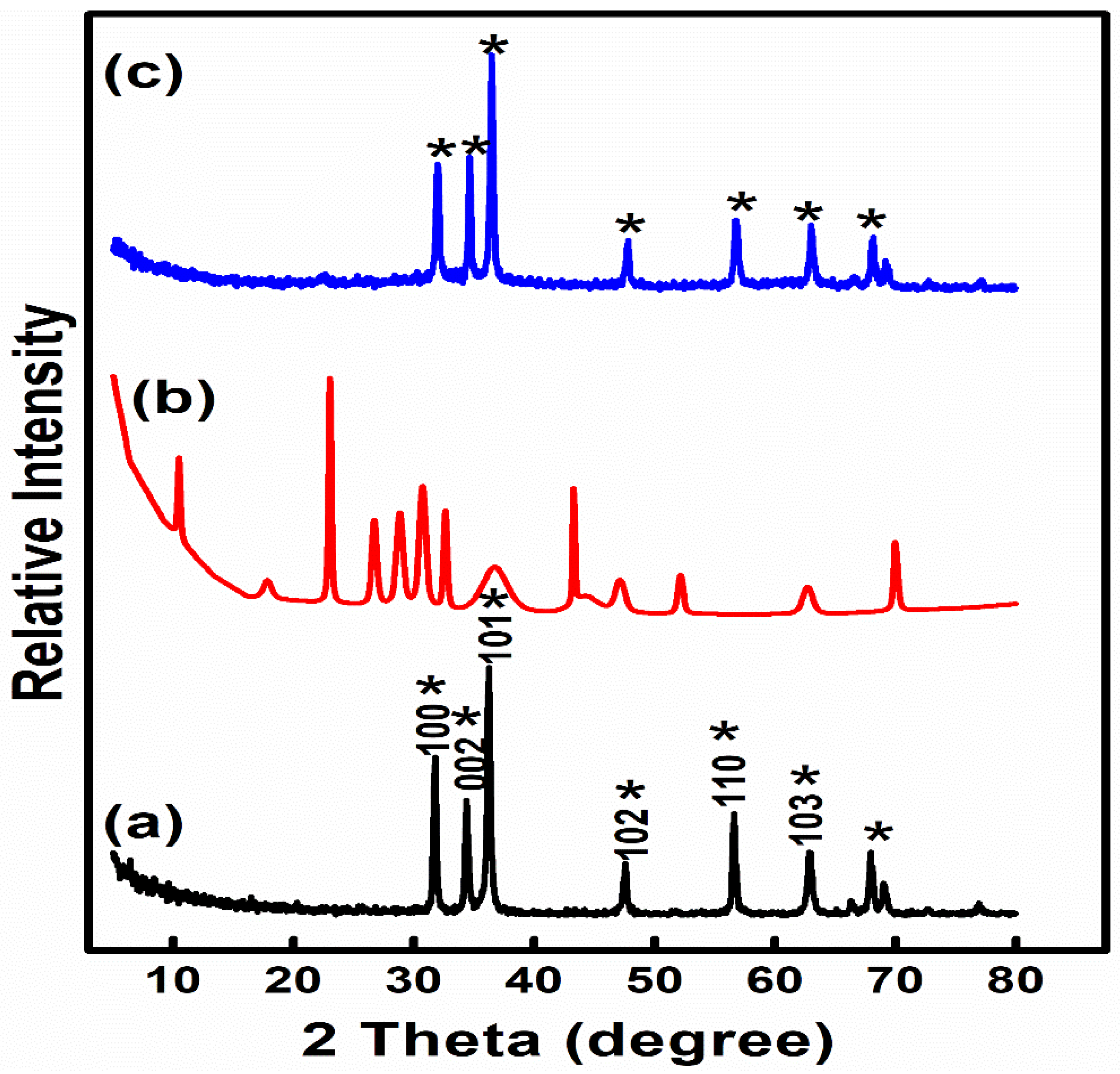
2.5. Synthesis and characterization of ZnO NPs, Z NPs and ZnOnanocomposite
2.6. Evaluation of biocidal activity of tested disinfectants
2.7. Experimental animals
2.7.1. Experimental groups and medications for 10 days’ acute study
2.7.2. The assessment of LD50, LD90 as a measure of toxicity (Probit analysis)
2.7.3. Estimation of the maximum LD100 and median lethal dose LD50 for the tested nanomaterials
2.7.4. Mortality and Toxic Signs
2.8. Histopathological observation
3. Statistical analysis
4. Results and discussion
5. Conclusion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviation:
| E. | Escherichia |
| P. | Pseudomonas |
| S. | Salmonella |
| A. | Aeromonas |
| H2O2 | hydrogen peroxide |
| NPs | Nanoparticles |
| ZnO | Zinc oxide |
| Z | Zeolite |
| LD | Lethal Dose |
| QAC | quaternary ammonium compounds |
| TSB | tryptic soya broth |
| XRD | X-ray diffraction |
| FT-IR | Fourier-transform infrared spectrum |
| SEM | Scanning Electron microscope |
References
- Umar, S. , Munir, M.T., Azeem, T., Ali, M., Shah, A. Effects of Water Quality on Productivity and Performance of Livestock: A Mini Review. Veterinaria 2014, 2, 11–15. [Google Scholar]
- Guppy, L. , Shantz, H. Groundwater Quality in Rural Cambodia: Measures and Perceptions. Geograph. Res. 2011, 49, 384–394. [Google Scholar] [CrossRef]
- Amna, M.M. , Abdelga, A. Bacteriological evaluation of the drinking water quality in dairy farms in Khartoum state, Sudan. J of Vet Med. and Anim. Hlth. 2004, 6, 95–100. [Google Scholar] [CrossRef]
- Abdel-Shafey, H.I. , Aly, F.O. Water issue in Egypt: resources, pollution and protection endeavours. Cent.Eurp. J. of Environ. Med. 2002, 8, 3–21. [Google Scholar]
- El-Sadek, A. Upscaling field scale hydrology and water quality modeling to catchment scale. Water Res. Managem. 2007, 21, 149–169. [Google Scholar] [CrossRef]
- Annual Drinking Report, UNEP GEMS/Water Programme, 2005. Workshop report: Development and use of global water quality indicators and indices. Vienna, Austria 4-6th May 2005. (http://www.gemswater.org/publications/pdfs/indicators_workshop_report.pdf).
- LeJeune, J.T. , Thomas, E., Hancock, D.D. Cattle water troughs as reservoirs of E. coli 0157. Appl. Envir. Microbiol. 2001, 67, 3053–3057. [Google Scholar] [CrossRef]
- Murinda, L. , Miliwebsky, E., Gioffre, A., Chinen, I., Basckier, A., Chillemi, G., Guth, B.E.C., Masana, M.O., Cataldi, A., Rodriguez, H.R. Novel single-tube agar-based test system for motility enhancement and immunocapture of E.coli O157:H7 by H7 flagellar antigen-specific antibodies. J ClinMicrobiol. 2002, 40, 4685–4690. [Google Scholar] [CrossRef]
- Ezzat, S.M. , Mohammed, T., Moustafa, Fouda, A., S. El-Gamal, M., and Ibrahim, A. M. Assessment of some drinking water purification plants efficiency at Great Cairo in Egypt. Curr.Scie. Inter. 2017, 06, 761–776. [Google Scholar]
- Aksoy A, El Kahlout KEM, Yardimci H. Comparative Evaluation of the Effects of Binzalkonium Chloride, Iodine, Gluteraldehyde and Hydrogen Peroxide Disinfectants against Avian Salmonellae Focusing on Genotypic Resistance Pattern of the Salmonellae Serotypes toward Benzalkonium Chloride. Brazilian Journal of Poultry Science. [CrossRef]
- Davies, R. , Wales, A.. Antimicrobial resistance on farms: A review including biosecurity and the potential role of disinfectants in resistance selection. Comp. Rev. Food Sci. Food Saf 2019, 18, 753–774. [Google Scholar] [CrossRef] [PubMed]
- Jegadeesan, G. , Mondal,K., Lalvani S.B. Arsenate remediation using nanosized modified zerovalent iron particles. Environ. Progress 2005, 24, 289–296. [Google Scholar] [CrossRef]
- Hardiljeet, K.B. , Meera, J., Dennis, M.O. Kinetics and thermodynamics of Cadmium ion removal by adsorption onto nanozervalent iron particles. J. Harzard. Mater 2010, 186, 458–65. [Google Scholar] [CrossRef]
- Li, W.R. , Xie, X.B., Shi, Q.S., Zeng, H.Y., Ou-Yang, Y.S., Chen, Y.B. Antibacterial activity and mechanism of silver nanoparticles on E. coli. ApplMicrobiol Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef]
- Petrik, L. , Missengue, R., Fatoba, M., Tuffin, M., Sachs, J. 2014. Silver/zeolite nano composite-based clay filters for water disinfection. Report to the Water Research Commission. No KV 297/12. Available online: http://www.ircwash.org/resources/silver-zeolite-nano-composite-based-clay-filters-water-disinfection.
- Azam, A. , Ahmed, A.S., Oves, M., Khan, M.S., Habib, S.S., Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. Int. J. Nanomedicine 2012, 7, 6003–6009. [Google Scholar] [CrossRef] [PubMed]
- APHA, A.W.W. A, W.E.F. 2012.Standard methods for examination of water and wastewater, 22nd. American Public Health Association, Washington. ISBN: 978-087553-013-0. https://www.scirp.org/(S(vtj3fa45qm1ean45vvffcz55))/ reference/ References Papers.aspx?.
- Palleroni, N.J. Genus I.Pseudomonas. In Bergey’s Manual of Systematic Bacteriology; Krieg, N. R. and Holt, J. C., Ed.; Williams and Wilkins: Baltimore; Volume 1, pp. 141–199.
- Holtz, J.G. , Krieg, N.R., Sneath, P.H.A., Staley, J.T., Williams, S.T. Bergey’s manual of determinative bacteriology, 9th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000. [Google Scholar]
- Ashiru, A. , Uaboi-Egbeni, P., Oguntowo, J., Idika, C. 2011. Isolation and antibiotic profile of Aeromonas species from tilapia fish (Tilapia nilotica) and catfish (Clariasbetrachus). Pakistan J. Nutr. 2011, 10, 982–986. [Google Scholar] [CrossRef]
- Ewing, W.H. The genus Salmonella. In Edwards and Ewing's identification of Enterobacteriaceae, 4th Ed.; Ewing WH, Ed.; Elsevier: New York, 1986; pp. 181–245. [Google Scholar]
- Akiba, M. , Rice, D.H., Davis, M.A. Masuda, T., Sameshima, T., Nakazawa, M., D. Hancock, D. A comparison of E. coli O157 isolates from cattle in Japan and the USA by molecular biological methods. Epidemiol. Infect. 2000, 125, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Konemann, E.; Allen, S.; Janda, W.; Schreckenberger, C.; Winn, W. Color Atlas and Textbook of Diagnostic Microbiology, 5th ed.; Lippincott, Philadelphia, New York, 1997. [Google Scholar]
- Morifnigo, A. , Borrego, J.J., Romero, P. Comparative study of different method for detection and enumeration of Salmonella spp. in natural waters.Appl. Bacteriol. 1986, 61, 169–176. [Google Scholar] [CrossRef] [PubMed]
- ChirinoM. J.,Trejo, A. A more sensitive procedure for the detection of Salmonella carriers in swine. A more sensitive procedure for the detection of Salmonella carriers in swine. Canad. Assoc. Swine. Pract. 1999, 50–53. [Google Scholar]
- Gordon, L. , Giraud, E., Ganiẽre, G.P., Armand, F., Bouju-Albert, A., de la Cotte, N., Mangion, C. Le Bri, H. Antimicrobial resistance survey in a river receiving effluents from freshwater fish farms. J. of Applied Microb. 2007, 102, 11670–1176. [Google Scholar] [CrossRef]
- Nawaz, M. , Khan, S. A., Khan, A.A., Sung, K., Tran, Q., Kerdahi, K., Steele, R. Detection and characterization of virulence genes and integronsinA. veronii isolated from catfish. Food Microbiology 2010, 327–331. [Google Scholar] [CrossRef]
- Spilker, T. , Coenye, T., Vandamme, P., LiPuma, J.J. 2004. PCR-Based Assay for Differentiation of P. aeruginosa from other Pseudomonas Species Recovered from Cystic Fibrosis Patients. J. OF Clin. Micr, 42(5):2074-2079. [CrossRef]
- Matar, G.M.; Ramlawi, F.; Hijazi, N.; Khneisser, I. Abdelnoor, A.M. 2002. Transcription Levels of P. aeruginosa Exotoxin A Gene and Severity of Symptoms in Patients with Otitis Externa. Curr.Micro. 45,350–354. [CrossRef]
- Ghadaksaz, A. , Fooladi, A.A.A., Hosseini,, H.H., Amin, M. 2015. The prevalence of some Pseudomonas virulence genes related to biofilm formation and alginate production among clinical isolates. J. of Applied Biomed, 13(1), 61-68. [CrossRef]
- Hu, Q. , Tu, J., Han, X., Zhu, Y., Ding, C., Yu, S. 2011.Development of multiplex PCR assay for rapid detection of Riemerellaanatipestifer, E. coli, and S. enterica simultaneously from ducks.J. of Microbiol. Methods 87, (2011) 64-69. [CrossRef]
- Yaguchi, K. , Ogitani, T., Osawa, R., Kawano, M., Kokumai, N., Kaneshige, T., Noro,T., Masubuchi, K., Shimizu, Y. 2007. Virulence Factors of Avian Pathogenic E. coli Strains Isolated from Chickens with Colisepticemia in Japan. Avian Dis., 51(3):656-62. [CrossRef]
- Ghanbarpour, R. , Salehi, M. Determination of Adhesion Encoding Genes in Escherichia coli isolates from omphalitis of chicks. American Journal of Animal and Veterinary Sciences 2010, 5, 91–96. [Google Scholar] [CrossRef]
- Liu, B. , Zhou, X., Zhang, L., Liu, W., Dan, X., Shi, C., Shi, X. 2012.Development of a novel multiplex PCR assay for the identification of S. enterica Typhimurium and Enteritidis. Food Control, 27, 8-93. [CrossRef]
- Murugkar, H.V. , Rahman, H., Dutta, P.K.. Distribution of virulence genes in Salmonella serovars isolated from man & animals. Indian J Med Res. 2003, 117, 66–70. [Google Scholar] [PubMed]
- Huehn, S. , La Ragione, R.M., Anjum, M., Saunders, M., Woodward, M.J., Bunge, C., Helmuth, R., Hauser, E., Guerra, B., Beutlich, J., Brisabois, A., Peters, T., Svensson, L., Madajczak, G., Litrup, E., Imre, A., Herrera-Leon, S., Mevius, D., Newell, D.G., Malorny, B. 2010. Virulo-typing and antimicrobial resistance typing of S. entericaserovars relevant to human health in Europe. Food-borne Pathogens Dis., 7,523-35. [CrossRef]
- Finney, D.J. Probit Analysis, 3th ed. , University Press, Cambridge. Ghanbarpour, R., Salehi, S., 2010.Determination of Adhesin Encoding Genes in E. coli Isolates from Omphalitis of Chicks. Amer. J. of Anim. and Vet.Sci. 1971, 5, 91–96. [Google Scholar]
- Arambaši, M.B. , Kondi, S., Piti, L.J. Stojanovi, M. Review of some mathematical statistical methods for processing toxicological-pharmacological experimental results. Acta Pharm. Jugosl. 1991, 41, 177–190. [Google Scholar]
- Randhawa, M.A. Calculation of LD50 values from the method of Miller and Tainter, 1944. J. Ayub. Med. Coll. Abbottabad 2009, 21, 184–185. [Google Scholar]
- Arambaši, M.B. , Piti, L.J., Jeremi, D., Adnaðevi, D. Applicational possibilities of the application rd of regression analysis and analysis of variance.II. Assessment and comparison of acute toxicity: Presentation and practical application of interactive computer programm "LD50 -mortality". Boll.Chim.Farmaceutico 2002, 141, 290–298. [Google Scholar]
- Bancroft, J. D, Layton, C. 2013. The hematoxylin and eosin. In: Suvarna, S.K, Layton, C, Bancroft, J.D, editors. Theory Practice of histological techniques. 7thed. (Ch.10 and 11), Philadelphia: Churchill Livingstone of El Sevier, 179-220. [CrossRef]
- Selim, S.A. , Ahmed, S.F., Aziz, M.H.A., Zakaria, A.M., Klena, J.D., Pangallo, D. 2013. Prevalence and characterization of Shiga-toxin O157:H7 and Non-O157:H7 enterohemorrhagic E. coli isolated from different sources. Biotech.Biotech.Equ.,27:3834-3842. [CrossRef]
- Barbosa, M.M. , Pinto, F.D., Ribeiro, L.F., Guriz, C.S., Maluta, P.R., Rigobelo, E.C., Avila, F.A. and Amaral, L.A. Serology and patterns of antimicrobial susceptibility in E. coli isolates from pay- to fish ponds. Arq. Int. Biol. Sao Paulo 2014, 81, 43–48. [Google Scholar]
- Momtaz, H. , Dehkord, F. S., Rahimi, E., Aagarifar, A. 2013. Detection of E. coli,Salmonella species, and Vibrio cholerae in tap water and bottled drinking water in Isfahan, Iran. BMC Public Health, 13(1): 556. [CrossRef]
- Yam, W.C. , Chan, C.Y., Ho Bella, S.W., Tam, T.Y., Kueh, C. Lee, T. Abundance of clinical enteric bacterial pathogens in coastal waters and shellfish. Water Res. 2000, 34, 51–56. [Google Scholar] [CrossRef]
- Haley, B.J. , Cole, D.J., Lipp, E.K. 2009.Distribution, diversity, and seasonality of water-borne Salmonellae in a rural watershed.Appl. and Env.Micr., 75(5), 1248-55. [CrossRef]
- Adingra, A.A. , Kouadio, A.N., Blé, M.C., Kouassi, A.M. 2012. Bacteriological analysis of surface water collected from the Grand-Lahou lagoon, Côte d’ivoire. Afri. J. of Micr. Res. 6(13), 3097-3105. [CrossRef]
- Tracogna, M.F. , Lösch, L.S., Alonso, J.M., Merino, L.A., 2013. Detection and characterization of Salmonella spp. in recreational aquatic environments in the Northeast of Argentina.RevistaAmbiente&Água-An Interdisciplinary Journal of Applied Science, 8 (2), 18-26. [CrossRef]
- Yhils, N. , Bassey, E. Primary Sources of Salmonella Species in Poultry Production Settings in Calabar, Cross River State. Nigeria.Donnish J. of Medi.and Med. Sc. 2015, 2, 047–051. [Google Scholar]
- Abd El-Tawab,A. A., Abdelbaset, E., AbdElhalim, M., Abd-Elmonem, R. Bacteriological and molecular studies on Salmonella species isolated from poultry farms. Benha Vet. Med. J. 2019, 36, 280–393. [Google Scholar] [CrossRef]
- Mohamed, G.M. Comparative Study between raw and cooked fish sold in Assiut city on the incidence of some food-borne pathogens. Assiut Vet Med J 2012, 58, 99–108. [Google Scholar]
- Carr, L.E. , D.W., Wabeck, C.J. Livestock Environment. In III Proceedings of the International Livestock Environment Symposium.; pp. 279–285.dp/0916150925.
- Carpenter, S. R. , Fisher, S. G., Grimm, N. B., Kitchell, J. F., 1992.Global change and fresh water ecosystems. Annual Rev of Ecol. and Syst, 23, 119-139. [CrossRef]
- Renwick, S.A. , Irwin, R.J., Clarke, R.C., McNab, W.B., Poppe, C., McEwen, S.A. Epidemiological associations between characteristics of registered broiler chicken flocks in Canada and the Salmonella culture status of floor litter and drinking water. Can. Vet. J. https://europepmcorg/article/med/17424037. 1992, 33, 449–458. [Google Scholar] [PubMed]
- Morgan-Jones, S.C. 1980. The occurrence of Salmonellae during the rearing of broiler birds. British Poultry Sci. 21(6):463-470. [CrossRef]
- Poppe, C. , Irwin, R.J., Messier, S., Finley, G.G., Oggel, J. 1991. The prevalence of S. enteritidis and other Salmonella spp. among Canadian registered commercial chicken broiler flocks. Epidem.and Infect. 107, 201–211. [CrossRef]
- Donald, D.J. , Yi-Chen, T., Miao-Chi, T., Mei-Man, H., Yu-Lan, L., Lien-Ching, C.C, Jar-Fun, L., Kuo-Sh,i Y. Investigation of a collective diarrhea outbreak among cadets of a certain training unit located in Neipu Township, Pingtung County. Epidem. Bulletin 2006, 25, 269–279. [Google Scholar]
- Hinton, A. , Holser, J.R. 2009. Role of water hardness in rinsing bacteria from the skin of processed broiler chickens. J. of Intern. Poultry Sci., 2, 112–115. [CrossRef]
- Momba,M.N.B., Abong, B.O., Mwambakana, J.N. 2008. Prevalence of enterohaemorrhagicE. coli O157: H7 in drinking water and its predicted impact on diarrhoeic HIV/AIDS patients in the Amathole District, Eastern Cape Province, South Africa. Water SA 34(3), 365-372. [CrossRef]
- Mersha, D. , Asrat, B., Zewde, M., Kyule M. 2010. Occurrence of E. coli O157:H7 in faeces, skin and carcasses from sheep and goats in Ethiopia Lett. Appl. Microbiol., 50 (1), 71-76. [CrossRef]
- El-Liethy,M. A., El-Shatoury, E., Morsy, W., El-Senousy, W.M., El-Taweel, G.,. Detection of six E. coli O157 virulence genes in water samples using multiplex PCR. egypt J. microbiol. 2012, 47, 171–188. [Google Scholar] [CrossRef]
- Goma, M.K.E. , Indraswar,iA., Haryanto, A., Widiasih, D.A. 2019. Detection of E. coli O157:H7 and Shiga toxin 2a gene in pork, pig feces, and clean water at Jagalan slaughterhouse in Surakarta, Central Java Province, Indonesia.Vet. World, 12(10),1584-1590. [CrossRef]
- Hassan, A.H.A. , Salam, H.S.H., AbdelLatef, G.K. 2016. Serological identification and antimicrobial resistance of Salmonella isolates from broiler carcasses and human stools in Beni-Suef, Egypt. Beni-suef University J. of basic and applied sciences 5, 202-207. [CrossRef]
- Djeffal, S. , Mamache, B., Elgroud, R., Hireche, S., Bouaziz, O. 2018. Prevalence and risk factors for Salmonella spp. contamination in broiler chicken farms and slaughterhouses in the northeast of Algeria Vet. World, 11, 1102-1108. [CrossRef]
- Calenge, E. , Kaiser, F., Vignal, P.,A., Beaumont, C. Genetic control of resistance to salmonellosis and to Salmonella carrier state in fowl: a review. Gen. Sel. Evol. 2010, 42, 11. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.N. , Mohamed, D.A., Mohamed, Mohamed M. B. E., El Bably, A. Assessment of Drinking Water Quality and New Disinfectants for Water Treatment in a Small Commercial Poultry Farm. J.ofAdv.Vet. Res. 2020, 10, 206–212. [Google Scholar]
- Tapouk,F.A., Nabizadeh,R., Mirzaei,N., Amin, M., Hasanloei, V. 2020. Comparative efficacy of hospital disinfectants against nosocomial infection pathogens.Antimicrobial Resistance & Infection Control 9(1):115-122. [CrossRef]
- de Souza, R.C. , Haberbeck, L.U., Humberto, G., Riella, Deise, H. B., Ribeiro, Bruno Carciof, A.M. Antibacterial activity of zinc oxide nanoparticles synthesized by solochemical process. Braz. J.of Chem. Eng. 2019, 36, 885–893. [Google Scholar] [CrossRef]
- Stanković, A. , Dimitrijević, S., Uskoković, D., 2013. Influence of size scale and morphology on antibacterial properties of ZnO powders hydrothermally synthesized using different surface stabilizing agents. Colloids and Surfaces B: Biointerfaces, 102, 21-28. [CrossRef]
- Talebian, N. , Amininezhad, S. M., Doudi, M., 2013.Controllable synthesis of ZnO nanoparticles and their morphology-dependent antibacterial and optical properties. Journal of Photochemistry and Photobiology B: Biology, 120, 66-73. [CrossRef]
- Wang, M. , Wang, J., Liu, Y. 2019. Subcellular targets of zinc oxide nanoparticles during the aging process: role of cross-talk between mitochondrial dysfunction and endoplasmic reticulum stress in the genotoxic response. Toxicol Sci.,171(1): 159-171. [CrossRef]
- Wakweya,B., Jifar, W.W. 2023. In vitro Evaluation of Antibacterial Activity of Synthetic Zeolite Supported AgZno Nanoparticle Against a Selected Group of Bacteria.J. of Exper. Pharm. 15, 39-147. [CrossRef]
- Azizi-Lalabadi, M. , Ehsani, A., Ghanbarzadeh, B., Divband, B. 2020.Polyvinyl alcohol/gelatin nanocomposite containing ZnO, TiO2 or ZnO/TiO2 nanoparticles doped on 4A zeolite: Microbial and sensory qualities of packaged white shrimp during refrigeration. Int. J. of Food Microb., 312,108375. [CrossRef]
- Fal, H.N. and Farzaneh, F. Synthesis of ZnOnanocrystals with hexagonal (Wurtzite) structure in water using microwave irradiation. J. of Sci., Islamic Republic of Iran. 2006, 17, 231–234. [Google Scholar]
- Xiong, G. Pal, U. , Serrano, J.G., Ucer, K.B. Williams, R.T. Photoluminesence and FTIR study of ZnO nanoparticles: the impurity and defect perspective. phys. stat. sol. (c), 2006, 3, 3577–3581. [Google Scholar] [CrossRef]
- Azam, A. ,Ahmed, F., Arshi, N., Naqvi, A. H. Low temperature synthesis of nO nanoparticles using mechano-chemical route: A green chemistry approach. Intern.J.of Theoretical & Applied Sci. https://wwwresearchgatenet/publication/265938211. 2009, 1, 12. [Google Scholar]
- Bhuyan, T. , Mishra, K., Khanuja, M., Varma. A., 2015.Biosynthesis of zinc oxide nanoparticles from Azadirachtaindica for antibacterial and photocatalytic applications.Materials Science in Semiconductor Processing, 32, 55-61. [CrossRef]
- Parthasarathi, V. , Thilagavathi, G. Synthesis and characterization of zinc oxide nanopartilce and its application on fabrics for microbe resistant defence clothing. International Journal of Pharmacy 2011, 3, 392–398. [Google Scholar]
- Fereshteh, Z. , Reza, M. , Estarki, L., Razavi, R.S.,Taheran, M. Template synthesis of zinc oxide nanoparticles entrapped in the zeolite Y matrix and applying them for thermal control paint, Materials Science in Semiconductor Processing. 2013, 16, 547–553. [Google Scholar] [CrossRef]
- Hara, K. , Horiguchi, T., Kinoshita, T., Sayama, K., Sugihara H., Arakawa, H., 2000. Highly efficient photon-to-electron conversion with mercurochrome-sensitized nanoporous oxide semiconductor solar cells.Solar Energy Materials and Solar Cells. 64(2), 115-134. [CrossRef]
- Hadri, A. , Nassiri, C.,Chafi,F. Z., Loghmarti, M., Mzerd, A.2013. Effect of acetic acid adding on structural, optical and electrical properties of sprayed ZnO thin films.Energy and Environment Focus, 4(1), 12-17. [CrossRef]
- Srivastava, V. , Gusain, D., Sharma, Y.C., 2013.Synthesis, characterization and application of zinc oxide nanoparticles (n-ZnO). Ceramics International, 39(8), 9803-9808. [CrossRef]
- Yadav, A. , Prasad, V., Kathe, A. A., Raj, S., Yadav, D., Sundaramoorthy, C Vigneshwaran, N., 2006. Functional finishing in cotton fabrics using zinc oxide nanoparticles.Bulletin of Mat. Sci., 29, 641-645. [CrossRef]
- Alswat, A.A. , Bin Ahmad, M.N., Hussein, M.Z., Ibrahim, N., Saleh, T., 2017. Copper oxide nanoparticles-loaded zeolite and its characteristics and antibacterial activities. J. of Mat. Sci. and Techn., 33(8), 889-896. [CrossRef]
- Lu, C.H. , Yeh, H. C. 2000. Influence of hydrothermal conditions on the morphology and particle size of zinc oxide powder.CeramicsInternl., 2000, 26, 351–357. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M. , Ehsani, A., Divband, B., Alizadeh-Sani M., 2019. Antimicrobial activity of Titanium dioxide and Zinc oxide nanoparticles supported in 4A zeolite and evaluation the morphological characteristic.Sci., Rep., 9(1)., 17439. [CrossRef]
- Hong, T. , Tripathy, N., Jin Son, H., Ki-Tae, H., Sol Jeong, H. Bong Hahn, Y. 2013. A comprehensive in vitro and in vivo study of ZnO nanoparticles toxicity. J. Mater. Chem. B, 1(23), 2985-2992. [CrossRef]
- Kura, A.U. , Saifullah, B., Cheah, P., Hussein, M., Azmi, N., Fakurazi, S., 2015. Acute oral toxicity and biodistribution study of zinc-aluminium-levodopa nanocomposite. Nanoscale Res. Lett., 10, 105. [CrossRef]
- Clarke, E.G.C. , Clarke, M.L., 1977. In Veterinary toxicology. (Cassel and Collier Macmilan, London), pp. 268-277.
- Debbage, P. 2009. Targeted drugs and nanomedicine: present and future. Curr. Pharm. Design 15, 153-172. [CrossRef]
- Scheurich, D. , Woeltje, W. Skin and soft tissue infections due to CA-MRSA. Mo. Med. 2009, 106, 274–276. [Google Scholar] [PubMed]
- Islam, T. , Harisinghani, M. G. 2009.Overview of nanoparticle use in cancer imaging.Cancer Biomark. 5, 61-67. [CrossRef]
- Lai, S. K. , WanLai, S. K., Wang, Y. Y., Hanes, Y., Hanes, J. 2009. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv.DrugDel.Rev. J., 61, 158-171. [CrossRef]
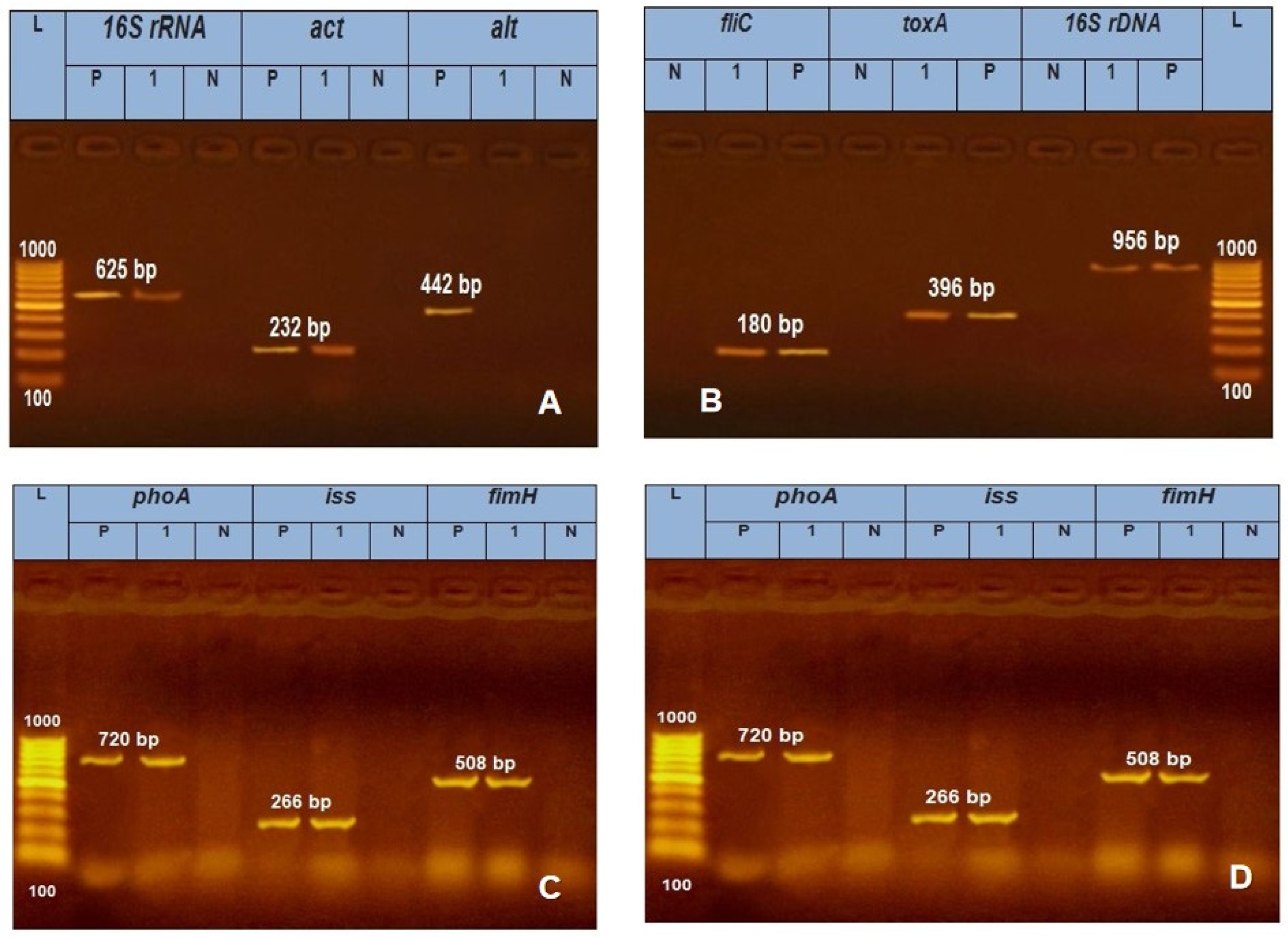


| Microbial agent | Target gene | Primers sequences | Amplified segment (bp) | Primary denaturation |
Amplification (35 cycles) | Final extension | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| Secondary denaturation | Annealing | Extension | |||||||
|
A.. hydrophila |
16S rRNA | GAAAGGTTGATGCCTAATACGTA | 625 | 94˚C 5 min. |
94˚C 30 sec. |
50˚C 40 sec. |
72˚C 45 sec. |
72˚C 10 min. |
Gordon et al. [26] |
| CGTGCTGGCAACAAAGGACAG | |||||||||
| Act | AGAAGGTGACCACCACCAAGAACA | 232 | 94˚C 5 min. |
94˚C 30 sec. |
55˚C 30 sec. |
72˚C 30 sec. |
72˚C 7 min. |
Nawaz et al. [27] | |
| AACTGACATCGGCCTTGAACTC | |||||||||
| alt | TGACCCAGTCCTGGCACGGC | 442 | 94˚C 5 min. |
94˚C 30 sec. |
55˚C 40 sec. |
72˚C 40 sec. |
72˚C 10 min. |
||
| GGTGATCGATCACCACCAGC | |||||||||
| P. aeruginosa | 16S rDNA | GGGGGATCTTCGGACCTCA | 956 | 94˚C 5 min. |
94˚C 30 sec. |
52˚C 40 sec. |
72˚C 45 sec. |
72˚C 10 min. |
Spilkeret al. [28] |
| TCCTTAGAGTGCCCACCCG | |||||||||
| toxA | GACAACGCCCTCAGCATCACCAGC | 396 | 94˚C 5 min. |
94˚C 30 sec. |
55˚C 40 sec. |
72˚C 40 sec. |
72˚C 10 min. |
Mataret al. [29] | |
| CGCTGGCCCATTCGCTCCAGCGCT | |||||||||
| fliC | TGAACGTGGCTACCAAGAACG | 180 | 94˚C 5 min. |
94˚C 30 sec. |
56.2˚C 30 sec. |
72˚C 30 sec. |
72˚C 7 min. |
Ghadaksazet al. [30] 2015 | |
| TCTGCAGTTGCTTCACTTCGC | |||||||||
| E. coli | phoA | CGATTCTGGAAATGGCAAAAG | 720 | 94˚C 5 min. |
94˚C 30 sec. |
55˚C 40 sec. |
72˚C 45 sec. |
72˚C 10 min. |
Hu et al. [31] |
| CGTGATCAGCGGTGACTATGAC | |||||||||
| Iss | ATGTTATTTTCTGCCGCTCTG | 266 | 94˚C 5 min. |
94˚C 30 sec. |
54˚C 30 sec. |
72˚C 30 sec. |
72˚C 7 min. |
Yaguchiet al. [32] | |
| CTATTGTGAGCAATATACCC | |||||||||
| fimH | TGCAGAACGGATAAGCCGTGG | 508 | 94˚C 5 min. |
94˚C 30 sec. |
50˚C 40 sec. |
72˚C 45 sec. |
72˚C 10 min. |
Ghanbarpour and Salehi [33] | |
| GCAGTCACCTGCCCTCCGGTA | |||||||||
| S. tTphimurium | STM4495 | GGT GGC AAG GGA ATG AA | 915 | 94˚C 5 min. |
94˚C 30 sec. |
50˚C 40 sec. |
72˚C 50 sec. |
72˚C 10 min. |
Liu et al. [34] |
| CGC AGC GTA AAG CAA CT | |||||||||
| Stn | TTG TGT CGC TAT CAC TGG CAA CC | 617 | 94˚C 5 min. |
94˚C 30 sec. |
59˚C 40 sec. |
72˚C 45 sec. |
72˚C 10 min. |
Murugkaret al. [35] | |
| ATT CGT AAC CCG CTC TCG TCC | |||||||||
| sopB | TCA GAA GRC GTC TAA CCA CTC | 517 | 94˚C 5 min. |
94˚C 30 sec. |
58˚C 40 sec. |
72˚C 45 sec. |
72˚C 10 min. |
Huehnet al. [36] | |
| TAC CGT CCT CAT GCA CAC TC | |||||||||
| Water source | Examined samples | No. (%) of positive samples | No. (%) of bacterial isolates | ||||
|---|---|---|---|---|---|---|---|
| E. coli | Salmonella Spp. | P. aeruginosa | A. hydrophila | ||||
| Tap water | |||||||
| Main source | 10 | 1(10.0) | 0 (0.0) | 0 (0.0) | 1(100.0) | 0 (0.0) | |
| Drinkers | 30 | 24 (80.0) | 10 (41.7) | 6 (25.0) | 3 (12.5) | 5 (20.8) | |
| Hand pump | |||||||
| Main source | 10 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Drinkers | 30 | 12 (40.0) | 5 (41.6) | 2 (16.7) | 3 (25.0) | 2 (16.7) | |
| Surface water | |||||||
|
Main source |
10 | 10 (100.0) | 4 (40.0) | 4 (40.0) | 1 (10.0) | 1(10.0) | |
| Drinkers | 30 | 28 (93.3) | 15 (53.0) | 9 (32.1) | 2 (7.1) | 2 (7.1) | |
| Total | 120 | 75 (62.5) | 34 (45.3) | 21 (28.0) | 10 (13.3) | 10 (13.3) | |
| X2= 56.250 P>0.000 | X2=20,840 P>0.000 | ||||||
| Bacteria isolated | Serogroups | No. (%) |
|---|---|---|
|
E. coli |
O157 O26 O144 Ountyped |
16 (47.1) 4 (11.8) 8 (23.5) 6 (17.6) |
| Total 34 (45.3) | ||
|
Salmonella spp. |
S. Kentucky S. typhimurium S. infantis S. ferruch S. kottobus |
10 (47.6) 5 (23.8) 4 (19.0) 1 (4.8) 1 (4.8) |
| Total 21 (28.0) | ||
| X2= 29.273, P >0.000 | ||
| Disinfectant tested | Conc.mg/l | Biocidal activity of tested disinfectant against bacterial isolates at different contact times | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli (20) | Salmonella spp. (20) | P.Aerogenosa (10) | A. hydrophila(10) | ||||||||||||||||||||||
| 30min | 2h | 24h | 30min | 2h | 24h | 30min | 2h | 24h | 30min | 2h | 24h | ||||||||||||||
| S | R | S | R | S | R | S | R | S | R | S | R | S | R | S | R | S | R | S | R | S | R | S | R | ||
| Iodine | 0.5 | 0 | 100.0 | 0 | 100.0 | 15.0 | 85.0 | 5.0 | 95.0 | 15.0 | 85.0 | 30.0 | 70.0 | 5.0 | 95.0 | 20.0 | 80.0 | 35.0 | 65.0 | 0 | 100.0 | 10.0 | 90.0 | 25.0 | 75.0 |
| 1.0 | 5.0 | 95.0 | 10.0 | 90.0 | 25.0 | 75.0 | 5.0 | 95.0 | 20.0 | 80.0 | 25.0 | 75.0 | 5.0 | 95.0 | 25.0 | 75.0 | 50.0 | 50.0 | 0 | 100.0 | 15.0 | 85. 0 | 30.0 | 70.0 | |
| H2O2 | 3.0 | 5.0 | 95.0 | 25.0 | 75.0 | 30.0 | 70.0 | 10.0 | 90.0 | 20.0 | 80.0 | 25.0 | 75.0 | 5.0 | 95.0 | 20.0 | 80.0 | 40.0 | 60.0 | 0 | 100.0 | 0 | 10.0 | 20.0 | 80.0 |
| 5.0 | 15.0 | 85.0 | 45.0 | 55.0 | 55.0 | 45.0 | 20.0 | 80.0 | 30.0 | 70.0 | 30.0 | 70.0 | 10.0 | 90.0 | 35.0 | 65.0 | 40.0 | 60.0 | 5.0 | 95.0 | 15.0 | 85.0 | 30.0 | 70.0 | |
| Terminator | 0.3 | 10.0 | 90.0 | 25.0 | 75.0 | 45.0 | 55.0 | 0 | 100.0 | 5.0 | 95.0 | 20.0 | 80.0 | 15.0 | 85.0 | 25.0 | 75.0 | 45.0 | 55.5 | 5.0 | 95.0 | 20.0 | 80.0 | 50.0 | 50.0 |
| 0.5 | 15.0 | 85.0 | 40.0 | 60.0 | 50.0 | 50.0 | 15.0 | 85.0 | 25.0 | 75.0 | 25.0 | 75.0 | 20.0 | 80.0 | 30.0 | 70.0 | 60.0 | 40.0 | 10.0 | 90.0 | 25.0 | 75.0 | 60.0 | 40.0 | |
| P.value | X2=522.7, P=0.000 | X2=734.7, P=0.000 | X2=382.7, P=0.000 | X2=669.5, P=0.000 | |||||||||||||||||||||
| Bacteria spp. | Zeolite nanoparticles | Zinc oxide nanoparticles | Zinc oxide-zeolite nanocomposites | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30min | 2hr | 24hr | 30 | 2hr | 24hr | 30min | 2hr | 24hr | |||||||||||||||||||||||
| S % |
I % |
R % |
S % |
I % |
R % |
S % |
I % |
R % |
S % |
I % |
R % |
S % |
I % |
R % |
S % |
I % |
R % |
S % |
I % |
R % |
S % |
I % |
R % |
S % |
I % |
R % |
|||||
| E. coli | 0.0 | 2.0 | 98.0 | 3.0 | 5.0 | 92.0 | 8.0 | 9.0 | 83.0 | 19.0 | 10.0 | 71.0 | 36.0 | 11.0 | 53.0 | 40.0 | 12.0 | 48.0 | 42.0 | 11.0 | 47.0 | 50.0 | 15.0 | 35.0 | 73.0 | 15.0 | 12.0 | ||||
| Salmonellaspp. | 1.0 | 2.0 | 97.0 | 2.0 | 4.0 | 94.0 | 2.0 | 7.0 | 91.0 | 36.0 | 19.0 | 45.0 | 41.0 | 12.0 | 39.0 | 43.0 | 13.0 | 40.0 | 41.0 | 15.0 | 36.0 | 52.0 | 20.0 | 28.0 | 76.0 | 15.0 | 9.0 | ||||
| p.aeroginosa | 2.0 | 3.0 | 95.0 | 5.0 | 2.0 | 93.0 | 5.0 | 5.0 | 90.0 | 39.0 | 12.0 | 49.0 | 29.0 | 15.0 | 56.0 | 42.0 | 19.0 | 39.0 | 57.0 | 12.0 | 31.0 | 43.0 | 27.0 | 30.0 | 69.0 | 18.0 | 13.0 | ||||
| A.hydrophila | 2.0 | 5.0 | 93.0 | 5.0 | 4.0 | 91.0 | 8.0 | 3.0 | 88.0 | 31.0 | 19.0 | 50.0 | 31.0 | 14.0 | 55.0 | 48.0 | 17.0 | 35.0 | 52.0 | 10.0 | 38.0 | 45.0 | 29.0 | 26.0 | 63.0 | 22.0 | 15.0 | ||||
| P-value | X2=864.004, P=0.000 | X2=214.914, P=0.000 | X2=281.951, P=0.000 | ||||||||||||||||||||||||||||
| Dose | ZnO-Z nanocomposite | Z NP | ZnO NP | No. of animals |
|---|---|---|---|---|
| (mg/kgb.wt.) | No. of dead animals/ group | No. of animals / group |
No. of dead animals/ group | / group |
| 200 | 0 | 0 | 0 | 9 |
| 400 | 0 | 0 | 0 | 9 |
| 600 | 0 | 0 | 0 | 9 |
| 800 | 0 | 0 | 0 | 9 |
| 1000 | 0 | 0 | 0 | 9 |
| 1500 | 1 | 0 | 0 | 9 |
| 2000 | 2 | 2 | 0 | 9 |
| 3000 | 5 | 3 | 2 | 9 |
| 4000 | 8 | 6 | 5 | 9 |
| 5000 | 10 | 8 | 8 | 9 |
| 10000 | 10 | 10 | 10 | 9 |
| Treatment | LD50 (%) (LC50) |
95% CL | LD90 or LC50 |
95% CL |
X2 (df = 8) |
P* | ||
|---|---|---|---|---|---|---|---|---|
| LCL | UCL | LCL | UCL | |||||
| Zeolite | 3251 | 2675 | 4054 | 8476 | 5929 | 21737 | 1.63 | 0.99 |
| ZnO NPs | 3709 | 3089 | 4514 | 7620 | 5667 | 21379 | 0.53 | 0.05 |
| Nanocomposite | 2658 | 2187 | 3386 | 6636 | 4627 | 1791 | 0.39 | 0.99 |
| Parameters | Results |
|---|---|
| Zeolite (mg/kg) | |
| LD0 | 1247 |
| LD20 | 1396 |
| LD50 | 3251 |
| LD90 | 5508 |
| LD100 | 8467 |
| ZnO NPs (mg/kg) | |
| LD0 | 1805 |
| LD20 | 1964 |
| LD50 | 3709 |
| LD90 | 5515 |
| LD100 | 7620 |
| Nanocomposite (mg/kg) | |
| LD0 | 1046 |
| LD20 | 1185 |
| LD50 | 2658 |
| LD90 | 4400 |
| LD100 | 6636 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




