Strengths and limitations of this study
- -
Systematic reviews, mainly with meta-analysis, play a crucial role for heath professional’s decision-making;
- -
Systematic reviews of systematic reviews provide reliable and strong information in fields in which a lot of systematic reviews can be found;
- -
Nutritional interventions for sarcopenia have been a big target for primary (clinical trials and observational) and secondary (reviews) research;
- -
This study is only to describe an ongoing study protocol, and it aims to improve the quality and standardize future reviews of this type.
BACKGROUND
Sarcopenia has emerged as a subject of extensive study in recent years, resulting in a proliferation of clinical trials (CTs) being published. This surge in published CTs has, in turn, led to a substantial number of systematic reviews (SRs) aimed at consolidating the data from various sources. SRs play a crucial role in facilitating decision-making for healthcare professionals working with older individuals who either have or are at risk of developing sarcopenia. They excel at summarizing results and, when coupled with meta-analyses, can combine these findings into a single, powerful analysis that enhances the reliability of the conclusions while mitigating the impact of random results (1-3).
However, the abundance of SRs, particularly those attempting to answer the overarching question regarding sarcopenia management – i.e., which strategies are most effective for preventing or treating sarcopenia – has also introduced a degree of complexity. Instead of simplifying matters for healthcare professionals, this proliferation of SRs has, at times, made it even more challenging to discern the most effective approaches. Consequently, systematic reviews of systematic reviews have emerged as a valuable alternative, consolidating the data collected from various SRs into a single comprehensive document. In essence, a systematic review of systematic reviews serves as a meta-analysis of CTs and SRs, offering a comprehensive overview of topics with a high volume of publications, such as sarcopenia (3-7).
In 2023, a study of this nature was published by our laboratory (1), aiming to provide insights into the most effective exercise interventions for the treatment of sarcopenia in older individuals. However, it is crucial to recognize that nutritional interventions are equally vital in the context of sarcopenia management (2, 8). Bearing this in mind, the primary objective of this study is to examine the impact of dietary interventions in isolation on the three diagnostic criteria for sarcopenia. It seeks to determine which dietary interventions, or combination thereof, yield the most favorable outcomes for older individuals.
METHODS
This study is a protocol for a systematic review of systematic reviews, designed following the recommendations of the Cochrane Collaboration To Intervention Systematic Reviews Book (9) and the PRISMA Statement (5). Also, this project is registered in PROSPERO under the code CRD42023468286.
Is important to notice that, however this is not a systematic review of interventions, but a systematic review of systematic reviews of interventions, there are no guidelines to this kind of study. So was choose to use the Cochrane Collaboration as a guideline to conduct this review, and the recommendations by Smith et al, 2011 (4), “Methodology in conducting a systematic review of systematic reviews of healthcare interventions”. In addition, all criteria included in PRISMA Statement (5) were met.
All the items recommended by the PRISMA-P (Preferred reporting items for systematic review and meta-analysis protocols)(10) for this study can be found in appendix 1, signalizing in which page it can be found..
Search Strategy
The selection of eligible papers will occur on the following databases: Pubmed/MedLine, Embase, Scopus, Cinahl, web of Science and Cochrane. The search terms used included the MeSH ‘sarcopenia’ and its subheadings; ‘aged’ and its subheadings; ‘nutrition’ and its subheadings; and the filter for ‘systematic review’. The search strategies used on all databases are available in appendix 2.
Eligibility Criteria
Only systematic reviews (SR) of controlled clinical trials with human patients or volunteers will be included. Non-systematic reviews, overviews, clinical trials and reviews of non-clinical investigations will be excluded. All articles will be evaluated by two blinded authors for its inclusion or not.
In addition, after the SR selection, the clinical trials included in the SRs analysis will be listed to found duplicates, and, if the reference do not meet the inclusion criteria, the trial will be excluded. Only articles that follows the PICO of this study will be included.
Population: Adults over 60 years, male or female, sarcopenic or at risk of, with or without comorbidities.
Intervention: Any kind of diet or nutritional intervention, such as macro or micronutrients, food intake and/ or reduction of those nutrients, among others, since not combined with other interventions, such as physical exercises programs or pharmaceutical approaches, in order to reduce the risk of bias regarding the nutritional interventions.
Comparison: To have at least a second group, subjected to a different diet or nutritional intervention, another intervention of any kind, a combined intervention, or a control group.
Outcome: Studies that analyzed or evaluated the results of nutritional interventions in their outcomes, as long as these results were related to some of the sarcopenia indicators, according to the EWGSOP2 (2): muscle strength, physical performance and/ or skeletal muscle mass.
Studies Selection
The studies selection will occur in two phases, by two blinded and independent reviewers. On first phase will be analyzed title and abstracts. When selected for at least one reviewer, the articles will be maintained on the list. On the second phase will be read the selected full texts papers. Having disagreement between reviewers, a third reviewer will be necessary.
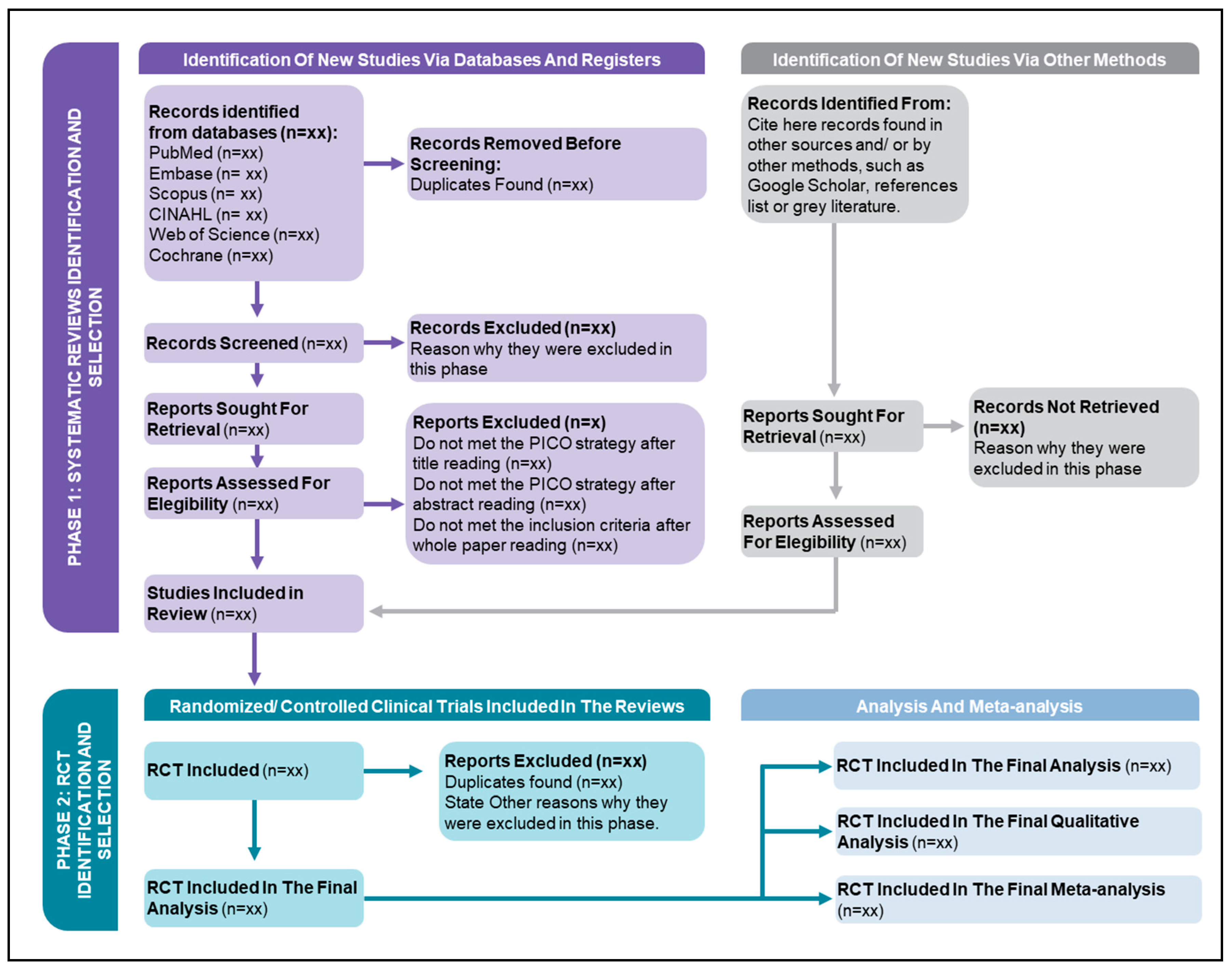
The studies found will be organized and selected using the reference manager software EndNote, Version X9. This selection will be presented in a PRISMA 2020 flowchart adapted for this purpose (
Figure 1).
Methodological quality assessment
After the final selection, two independent and blinded examiners will assess the selected studies regarding the quality of the review report using the AMSTAR instruments (Assessment of Multiple Systematic Reviews) (11) and PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement (12). During the quality assessment of the report of the selected reviews, eventual disagreements between the examiners will be resolved through discussion until consensus, or by a third reviewer decision.
Synthesis and data analyses
To quantitative analyses will be employed the statistic method of Inverse Variance, with analysis model in Random Effects, and the effect measures Mean Difference. The heterogeneity assessment of studies will be made with the Cochran’s Q Test, and the inconsistency with I2 Test, which values of <50% were considered as low heterogeneity, <75% moderate heterogeneity, and >75% high heterogeneity (13, 14). A p value lower than 0,05, and confidence interval of 95% will be considered statistically significant. All analyses will be conducted in Software Review Manager, version 5.3.
RESULTS
The results must be presented in, at least, 3 tables: one table (
Table 1) summarizing the results from the SRs, such as authors, year, aims, search strategy, conclusions and, if possible, number of studies and patients included.
The second table should include the primary information from the RCTs included, having, at least, sample (total, intervention and control), age (median and standard deviation), gender (proportion between female and male, in absolute and relative data). In addition,
Table 2 must include the information of patient’s inclusion. In this case, how sarcopenia was diagnosed.
The third table must include the intervention’s data and results, such as the intervention model, duration, which macro or micro nutrient was controlled, and other relevant information found in the SRs. This table may vary, due to the paper’s heterogeneity, and it may include different information, depending on the included papers.
Methodological quality assessment
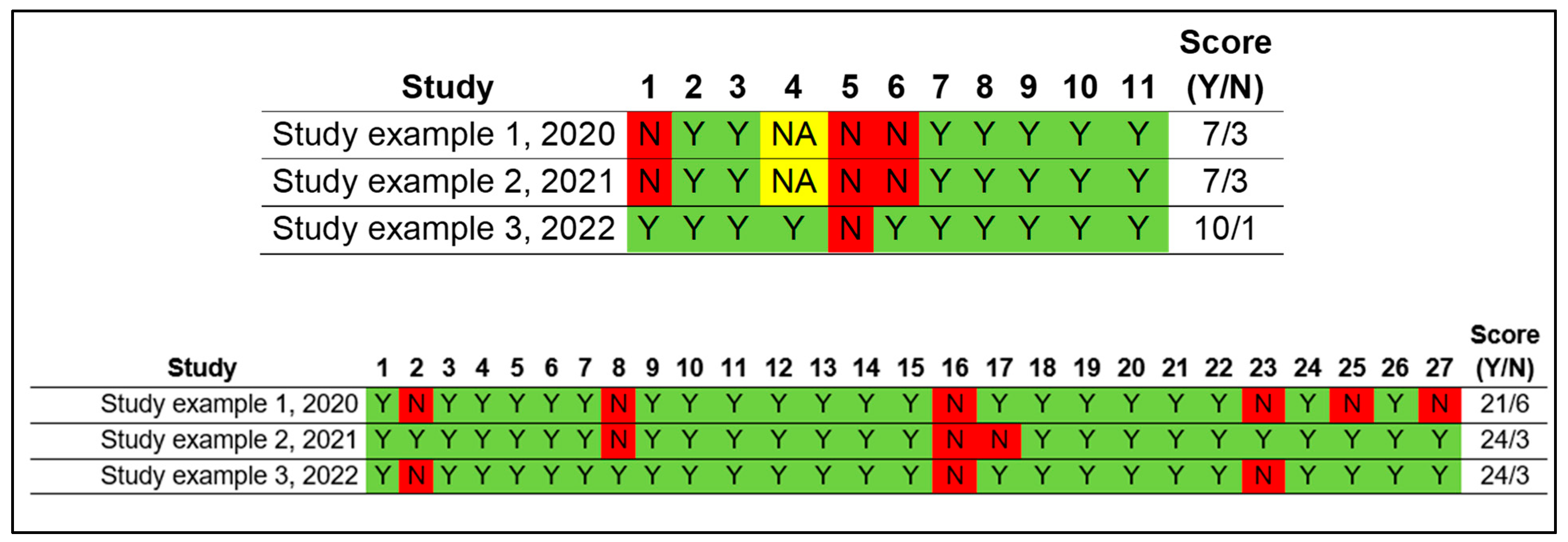
The methodological quality assessment must be presented in a clearly and direct way, as the example from Ferreira et al, 2020 (
1): it may presented dividing by each field/ question in AMSTAR and PRISMA (
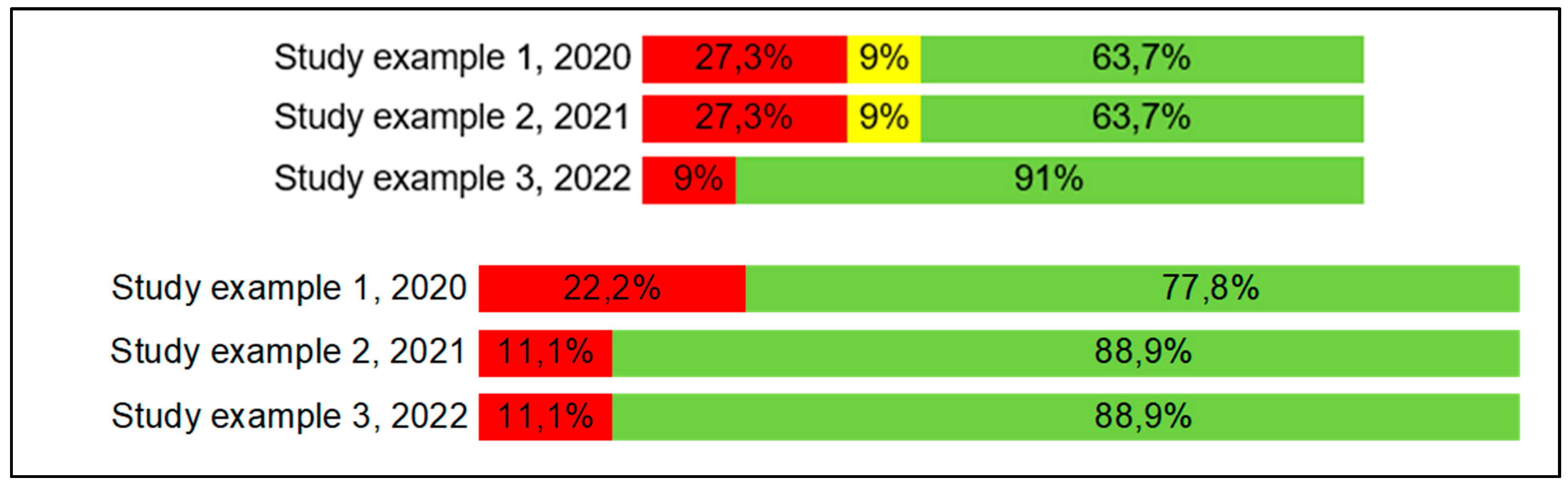
Figure 2), in percentage of each answer (
Figure 3), or both ways.
Table 1.
Summary table of scope of reviews in a systematic review of reviews.
Table 1.
Summary table of scope of reviews in a systematic review of reviews.
| Review, Year |
Aim (participants) |
Search strategy |
Conclusions |
Total studies included |
Total partici-pants |
| Author, year |
Preferably the aim ipsis literis from the original paper. If the aim is not presented in a clearly and direct way, summarize it, encompassing the main key-terms |
If the search strategy is presented in the original paper, or; the paragraph that summarize the MeSH, subheadings and keywords. |
The original conclusions reached by the original study, in a short form, encompassing the main keywords. |
Nº |
Nº |
Table 2.
RCTs Characteristics And Diagnostic Criteria For Sarcopenia.
Table 2.
RCTs Characteristics And Diagnostic Criteria For Sarcopenia.
| Review Reference |
Original Reference |
n total (int.) |
Age (+SD) |
Sex - n (%) |
Diagnostic Criteria For Sarcopenia |
| Cite in which SR (one or more) the RCT was included |
Cite the RCT reference (Author, year) |
Show the total sample. If possible, divide it in intervention and control |
Show the average age from participants. If possible, divide it in intervention and control |
Show in absolute and relative data the number of man and women included. |
If it is a stablished criteria, such as EWGSOP, AWGS or similar, state it. If not, explain how the syndrome was characterized. |
Either way chosen for results presentation, a table showing if each field is satisfied, along with in which page it can be found, should be published with the paper, either in the text body or as an appendix.
Figure 2.
AMSTAR (first image) and PRISMA (second image) quality assessment divided by field/ question.
Figure 2.
AMSTAR (first image) and PRISMA (second image) quality assessment divided by field/ question.
Figure 3.
AMSTAR (first image) and PRISMA (second image) quality assessment summarized by answer.
Figure 3.
AMSTAR (first image) and PRISMA (second image) quality assessment summarized by answer.
CONCLUSION
This protocol is easily reproducible, requires low cost and personnel, and may allow a higher understanding on sarcopenia treatment and management on older people, since all steps been followed.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.
CRediT Statement
Luis Fernando Ferreira: Conceptualization; Data Curation; Formal analysis; Funding Acquisition; Investigations; Methodology; Project administration; Resources; Software; Validation; Visualization; Writing - Original draft preparation; Writing - Review & Editing. Jéssica Roda Cardoso: Data Curation; Investigations; Resources; Software; Visualization; Writing - Original draft preparation. Luis Henrique Telles da Rosa: Conceptualization; Investigations; Methodology; Project administration; Supervision; Writing - Review & Editing.
Registration
This study was approved by UFCSPA Ethics Committee under decision letter 1.553.242, and the study protocol was registered at PROSPERO, under the code CRD42023468286. All ethical procedures follows the Declaration of Helsinki for research with human subjects.
Authors contribution and agreement
all authors have contributed significantly, as stated at CRediT. and all authors are in agreement with the content of the manuscript.
The content has not been published or submitted for publication elsewhere.
Conflict of Interests
The authors of this article declare that there is no conflict of interest or any kind of founding for their realization.
References
- Ferreira LF, Scariot EL, da Rosa LHT. The effect of different exercise programs on sarcopenia criteria in older people: A systematic review of systematic reviews with meta-analysis. Archives of Gerontology and Geriatrics. 2023 2023/02/01/;105:104868. [CrossRef]
- Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jul 1;48(4):601. [CrossRef]
- Linares-Espinós E, Hernández V, Domínguez-Escrig JL, Fernández-Pello S, Hevia V, Mayor J, et al. Methodology of a systematic review. Actas Urol Esp (Engl Ed). 2018 Oct;42(8):499-506. [CrossRef]
- Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol. 2011 Feb 3;11(1):15. [CrossRef]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 Mar 29;372:n71. [CrossRef]
- Ferguson T, Olds T, Curtis R, Blake H, Crozier AJ, Dankiw K, et al. Effectiveness of wearable activity trackers to increase physical activity and improve health: a systematic review of systematic reviews and meta-analyses. Lancet Digit Health. 2022 Aug;4(8):e615-e26. [CrossRef]
- Karlsson M, Bergenheim A, Larsson MEH, Nordeman L, van Tulder M, Bernhardsson S. Effects of exercise therapy in patients with acute low back pain: a systematic review of systematic reviews. Syst Rev. 2020 Aug 14;9(1):182. [CrossRef]
- Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020 Mar;21(3):300-7 e2. [CrossRef]
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Page MJ, Welch V. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester (UK): : John Wiley & Sons; 2019.
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ : British Medical Journal. 2015;349:g7647. [CrossRef]
- Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007 Feb 15;7:10. [CrossRef]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 Jul 21;6(7):e1000097. [CrossRef]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002 Jun 15;21(11):1539-58. [CrossRef]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003 Sep 6;327(7414):557-60. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).