1. Introduction
Chronicization of pain is frequently occurring after surgery, thus leading often to functional limitations and psychological disorders, with a negative impact on quality of life [
1].
Chronic post-surgical pain (CPSP) was first defined as “pain that develops after surgical intervention and lasts at least 2 months; other causes of pain have to be excluded, in particular, pain from a condition existing before the surgery” [
2]. An updated definition of CPSP, was later proposed as “pain persisting at least three months after surgery, that was not present before surgery, or that had different characteristics or increased intensity from preoperative pain, localized to the surgical site or a referred area, and other possible causes of the pain were excluded (e.g., cancer recurrence, infection)” [
3].
Chronic pain persisting after mastectomy is a major individual and public health problem. The etiology of persistent pain after mastectomy is still unclear, because of its possible multifactoriality [
4,
5,
6] with a partial neuropathic origin [
7]. While surgical factors, including axillary lymph node dissection, and reconstruction, have been postulated to serve as important risk factors for chronic pain, many studies do not fully support this association. Adjuvant treatment, such as radiation, chemotherapy, and hormone therapy, has also been occasionally associated with persistent pain consequent to mastectomy [
8,
9,
10].
Psychosocial factors such as anxiety and catastrophizing are being revealed as crucial contributors to individual differences in pain processing and outcomes. Some researchers have reported the associations between the development of persistent pain catastrophizing and depression or with psychological distress and reduced physical activity [
11,
12,
13,
14,
15]. This condition may lead to disability worsening individual quality of life [
16]. Due to the potential benefits induced by physical activity, it was recommended by the American Cancer Society recommends to begin it as soon as possible in people for which cancer is diagnosed [
17].
A recent meta-analysis indicates anxiety as the main psychological risk factor for raising of CPSP (and to a lesser degree, depression, catastrophising, kinesiophobia and impaired self-efficacy) [
18]. Furthermore, many other studies have proven that neuroinflammation is involved in multiple steps of chronic pain, promoting central [
19] and peripheral sensitization [
20]. Moreover, the relationship between depression, anxiety and inflammation has been proposed by several works, indicating that levels of inflammatory markers such as C-reactive protein, Interleukin-(IL)6, IL-1β, IL-17 and brain derived neurotrophic factor (BDNF), are modified in people with depression and anxiety [
21,
22,
23].
It is well documented that exercise and physical activity undertaken before or after a cancer diagnosis reduces risk of tumor recurrence, improves overall health thus increasing survival [
24,
25,
26]. In oncologic patients, exercise and physical activity accelerate the recovery of the functional capacities, reduce lymphedema, increase overall energy levels thus increasing strength and flexibility, reduce fatigue, improve pain symptom and physical capability to carry out daily activities and reduce the risk of chronic diseases [
27,
28].
It has been shown that physical activity could inhibit the release of inflammatory cytokines and has beneficial effects on the immune system and influences the intensity of pain as it is perceived by patients [
29,
30,
31].
Moreover, several studies suggest that physical activity improves anxiety, sleep and mood disorders such as depression by a modulation of cortisol level, increases Brain Derived Neutrophic Factor (BDNF) levels [
32,
33].
On the light of the above exposed concepts, objective of this study was to assess the effects of physical activity on the intensity and interference of chronic pain in daily activities and the effect on depression and anxiety in patient underwent mastectomy.
2. Materials and Methods
2.1. Study Design
A prospective observational unicentric cohort study was conducted. Population of the study was represented by female patients underwent unilateral or bilateral mastectomy due to removal of stage II and III breast cancer not yet subjected to breast reconstruction, chemotherapy and radiation aging 18 years or over. One hundred and eighty (180) female patients were selected for the study. Pain evaluation of each participant in the study was assessed at 3 and 6 months after the surgery through verbal administration of the Numerical Rating Scale (NRS). For depression and anxiety assessment Beck’s Depression Inventory (BDI) and General Anxiety Disorders-7 (GAD-7) were used, respectively. Physical activity was measured with the International Physical Activity Questionnaire (IPAQ). At the same timepoints the following blood biomarkers associated with inflammation, depression and anxiety were evaluated: Interleukin (IL)-17, IL-1β, cortisol, adrenocorticotropic hormone (ACTH) and brain-derived neurotrophic factor (BDNF).
2.2. Participants
Enrollment was performed during the April 2023 and October 2023 at the Breast Unit of San Vincenzo Hospital of Taormina in collaboration with the Azienda Ospedaliera Universitaria (AOU) Policlinico “G. Martino” of Messina, Italy. Inclusion criteria were, women aging over 18 years with a diagnosis of prior Phase II or III breast cancer, who had undergone mastectomy due to cancer removal three months earlier.
Exclusion criteria considered were: chemotherapy and radiation throughout the six months after surgery, anamnesis including other cancer types, immune system disorders (multiple sclerosis, HIV, lupus), and fresh symptoms of flu (cough, fever). Women taking anxiolytic and/or antidepressant and anti-inflammatory drugs, in the 15 days before the recruitment, were also excluded. Patients with breast cancer at Stages 0 and I were not included because of possible and frequent lack of pain. Women with cancer at Stage IV were not included as pain can derive from metastases. Women reporting pain prior to surgery and those affected by other types of tumors or by other diseases characterized by chronic pain were also excluded. All the patients were asked to sign the informed consent form to be included in the study. The study was approved by the Ethics Committee of AOU Policlinico “G. Martino”: Approval Number: Prot. 70-23, 18/04/2023, Board Name: Comitato Etico Interaziendale Messina. The trial was conducted according to the ethical principles of the Declaration of Helsinki and Good Clinical Practice principles were adopted. To enroll subjects in the study, sample size was calculated by Clinical software (ClinicalTrials.gov Identifier: NCT06123559).
2.3. Methodology
2.3.1. Demographic and Surgical Variables
Demographic variables influencing pain-related conditions including age, marriage, and school level, were considered. Italian education system comprises primary (five years), secondary (three years), post-secondary (five years), and graduation phases (three-six years). Data about lymph nodes dissection were asked for each participant. All the participants were Caucasian.
2.3.2. Numerical Rating Scale (NRS)
The numeric rating scale (NRS), commonly used to assess pain severity [
34], has been administered 3 and 6 months after surgery, as previously described [
29].
NRS, it is important to establish the degree of change representing a clinical improvement. NRS is a 0-11 point-scale with end points representing the extremes meaning no pain (point 0) and the worst possible pain (point 10) [
34,
35]. Three months after surgery, based on the collection of medical history (in particular, pain lasting three months after surgery) and the evaluation of NRS score, women participating in the study was divided in two groups: “PMP group” with women with pain totalizing
≥ 5 at NRS and, “Non-PMP group” composed by women totalizing a NRS score < 5. NRS was newly self-administered six months after surgery.
2.3.3. Beck’s Depression Inventory (BDI)
The BDI questionnaire, is one of the most widely used psychometric tests for the evaluation of depression. BDI consists of twenty-one questions about how the subject has been feeling in the last week. Each question has at least four possible answer choices; a score of 0 to 3 is assigned for every answer and then the total score establishes the severity of depression as follows: 0–9: for normal or minimal depression; 10–18: for mild depression; 19–29: for moderate depression; 30-63: for severe depression [
36]. BDI was administered 3 and 6 months after surgery.
2.3.4. Generalized Anxiety Disorders-7
The Generalized Anxiety Disorder Assessment (GAD-7) is a seven-item instrument that is used to measure or assess the severity of generalised anxiety disorder (GAD). Each item asks the individual to rate the severity of his or her symptoms over the past two weeks.
A score of 0, 1, 2, and 3 is assigned to the response categories, respectively, of “not at all,” “several days,” “more than half the days,” and “nearly every day.” GAD-7 total score for the seven items ranges from 0 to 21 describred as follow: 0–4: minimal anxiety; 5–9: mild anxiety; 10–14: moderate anxiety; 15–21: severe anxiety [
37]. GAD-7 was administered 3 and 6 months after surgery.
2.3.5. International Physical Activity Questionnaire (IPAQ)
The International Physical Activity Questionnaire (IPAQ), was used to collect information about self-reported physical activity. It was administered 3 and 6 months after surgery, as previously described [
29]. This questionnaire measures the type and amount of physical activity. It assesses the number of days and quantity of time spent for physical activity (PA) as moderate or vigorous intensity and walking of at least 10-min during the last 7 days, and also comprises the time spent sitting during the last week. The IPAQ includes four PA levels (work-related activity, leisure-time activity, transport-related activity, and domestic activities) each of 3 degrees of intensities: walking, moderate, and vigorous. Whole weekly physical activity was evaluated by weighing time consumed in each activity intensity together with its calculated metabolic equivalent energy expenditure (Metabolic equivalent of task; MET). According to the answers, patients were classified into three categories: inactive, if presenting a METs less than 700, adequate active women presenting a METs value raging between 700 and 2519 and highly active if presenting METs > 3000 [
38].
2.3.6. Haematological Biomarkers Associated with Depressive Disorders, Anxiety and Inflammation
Serum levels of biomarkers considered were measured 3 and 6 months after surgical intervention, according with the protocol of ELISA kits. We evaluated the following biomarkers: IL-17, IL-1β, cortisol, ACTH and BDNF. Blood sample were collected between 8-9 a.m. The following kits were used: IL-17 (R&D System Catalog #: D1700); IL1-β (R&D System Catalog #: DLB50); BDNF (R&D System Catalog #: DBD00), ACTH (Novus biologicals NBP2-66401) and cortisol (Novus biologicals NBP3-18003.
3. Results
One hundred and sixty (160) women undergone for mastectomy were enrolled in the study. The mean age was 50.34 ± 11.9 years (range 28-72 years; median age 53.5 years). The mean BMI was 21.59 ± 1.49.
3.1. Numerical Rating Scale Score (NRSs)
Clinical examination and estimation of NRS results collected 3 months after surgery, showed that the 54.4% (n = 87) did not report any significant pain (Non-PMP group), while the 45.6% (n =73) of women recruited for the study, manifested PMP Syndrome (PMP group). Participants were divided into two groups, PMP and non-PMP group.
In
Table 1, age and percentage of surgical variables of patients of the two groups are reported. PMP pain was not associated with any demographic or surgery-related variables taken in account.
In the group of PMP patients, assessment of NRS performed 3 and 6 months after surgery, revealed a statistically significant increase of pain intensity in PMP patients compared with those of non-PMP group (
Table 2).
3.2. BDI, Anxiety and Physical Activity
BDI and GAD-7 results, showed that 60.2% (n = 44) of the PMP group totalized scores associated with depression and anxiety; specifically, the 45.45% showed scores linked to severe depression and anxiety and 34% showed scores associated with moderate depression and anxiety, while 20.55% showed scores associated with scores associated with mild depression and anxiety. According to the BDI and GAD-7 score, PMP group was divided into two subgroups: depression anxiety (DA)-PMP subgroup (women reporting a BDI score ≥ 10 and GAD-7 score ≥ 5); and non DA-PMP subgroup (women reporting a BDI ≤ 9; and GAD-7 ≤ 4) (
Table 3).
3.3. Biomarkers Related to Depression and Anxiety
IL-17, IL1-β, cortisol and ACTH levels were significantly increased in PMP group compared to non-PMP group. These biomarkers were significantly more elevated in DA-PMP subgroup in comparison with non-DA-PMP subgroup, either 3 or 6 months after surgery (
Table 2 and
Table 3). BDNF level was statistically significantly reduced in DA-PMP subgroup compared to non DA-PMP subgroup (
Table 3).
3.4. IPAQ Score
Physical activity at IPAQ was evaluated among the 44 PMP women showing anxiety and depression (DA-PMP). According to IPAQ questionnaire, 20 patients have been categorized as inactive (< 700 METs) and 24 as adequately active (> 700 METs).
No active women (> 2510 METs) were present in the DA-PMP group (
Table 4).
DA-PMP inactive women showed a statistically significant increase of intensity of pain (p < 0.01) and an increase of anxiety and depression scores (p < 0.01) compared to adequate active DA-PMP women, either 3 or 6 months after surgery (
Table 5).
IL-17, IL-1-β, cortisol and ACTH levels were statistically significantly increased in inactive DA-PMP women compared to active DA-PMP women (
Table 5), while BDNF was statistically significantly reduced in inactive DA-PMP women compared to active women of the same group (
Table 5).
3.5. Spearman’s Correlation
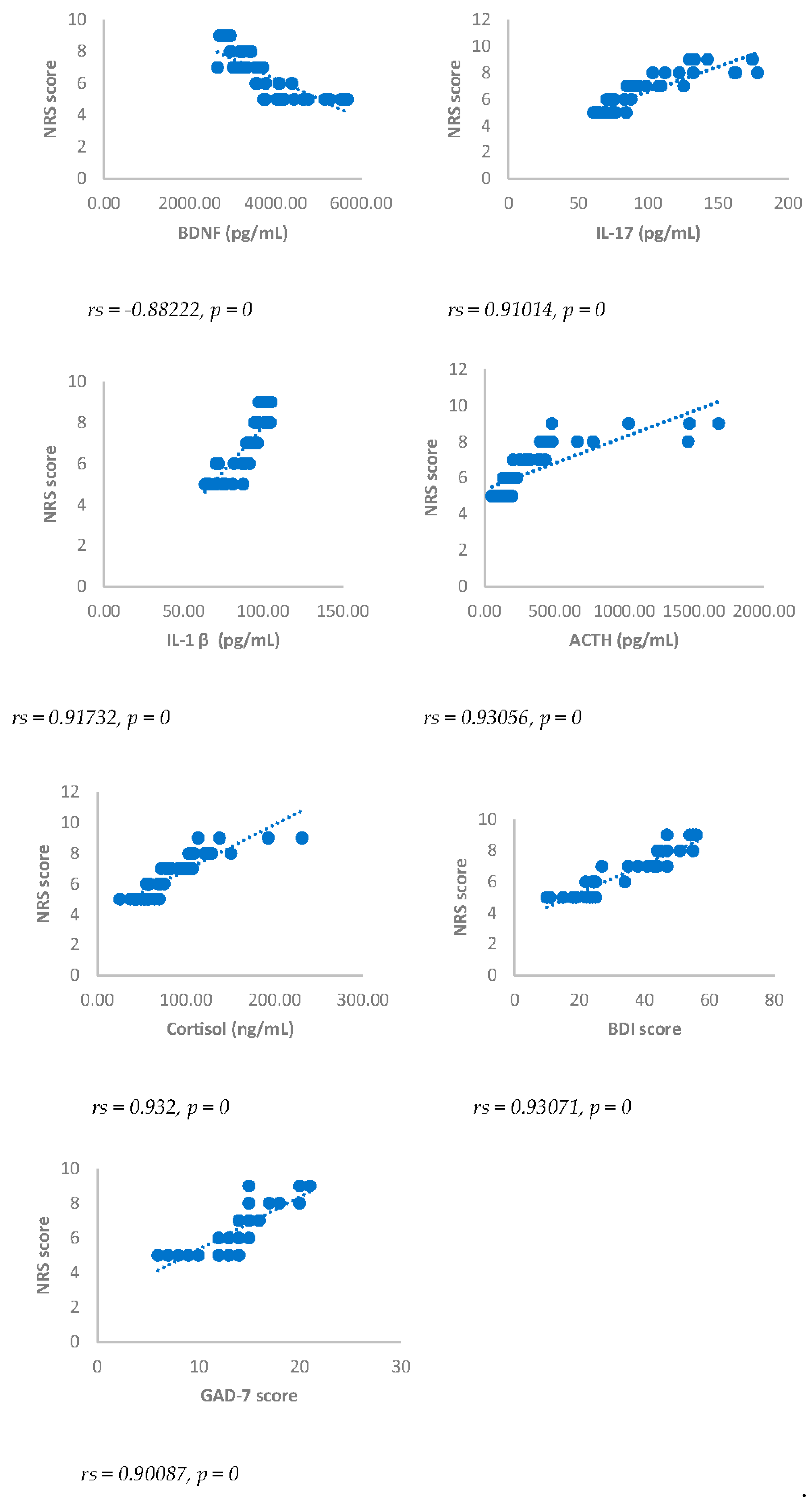
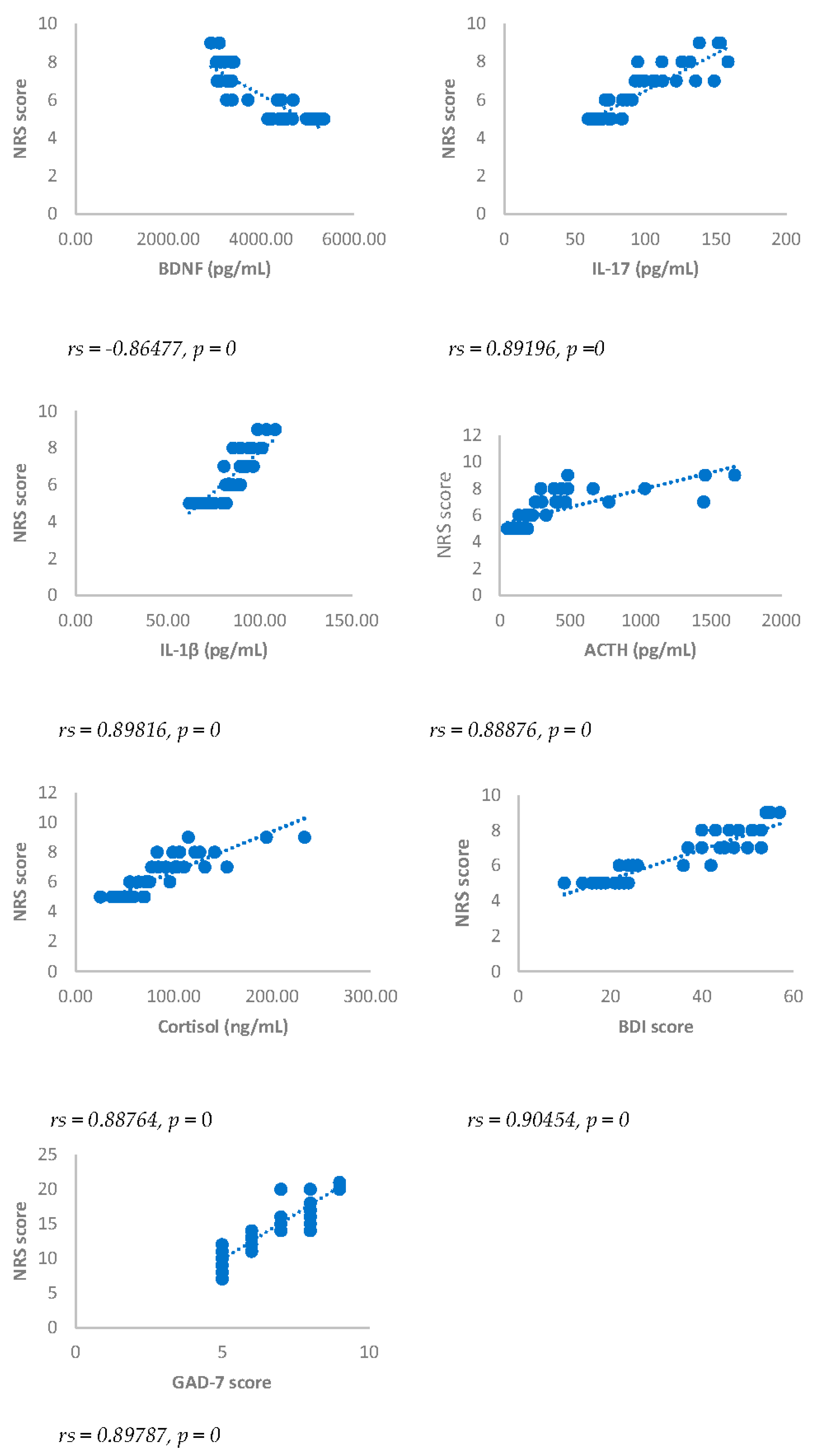
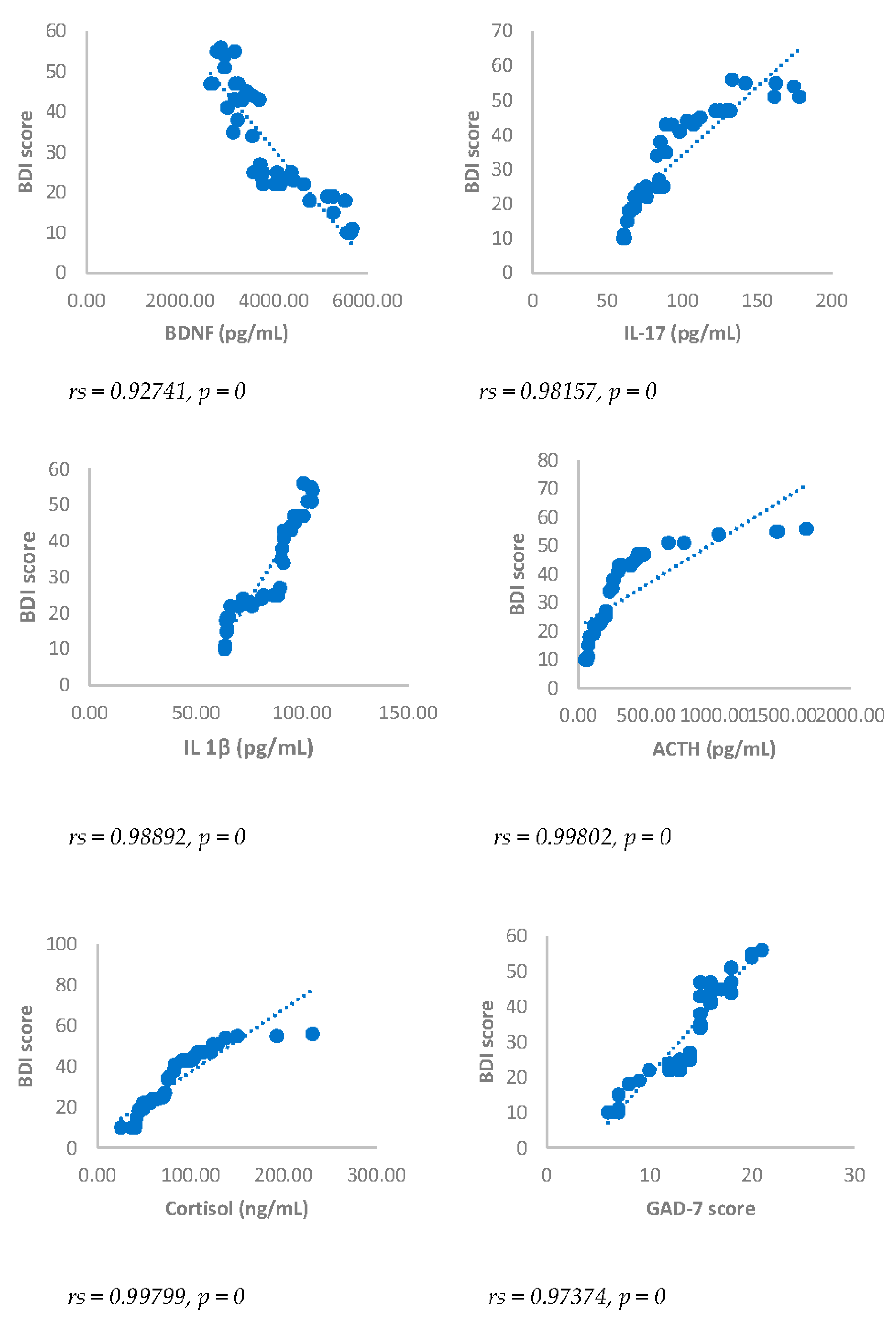
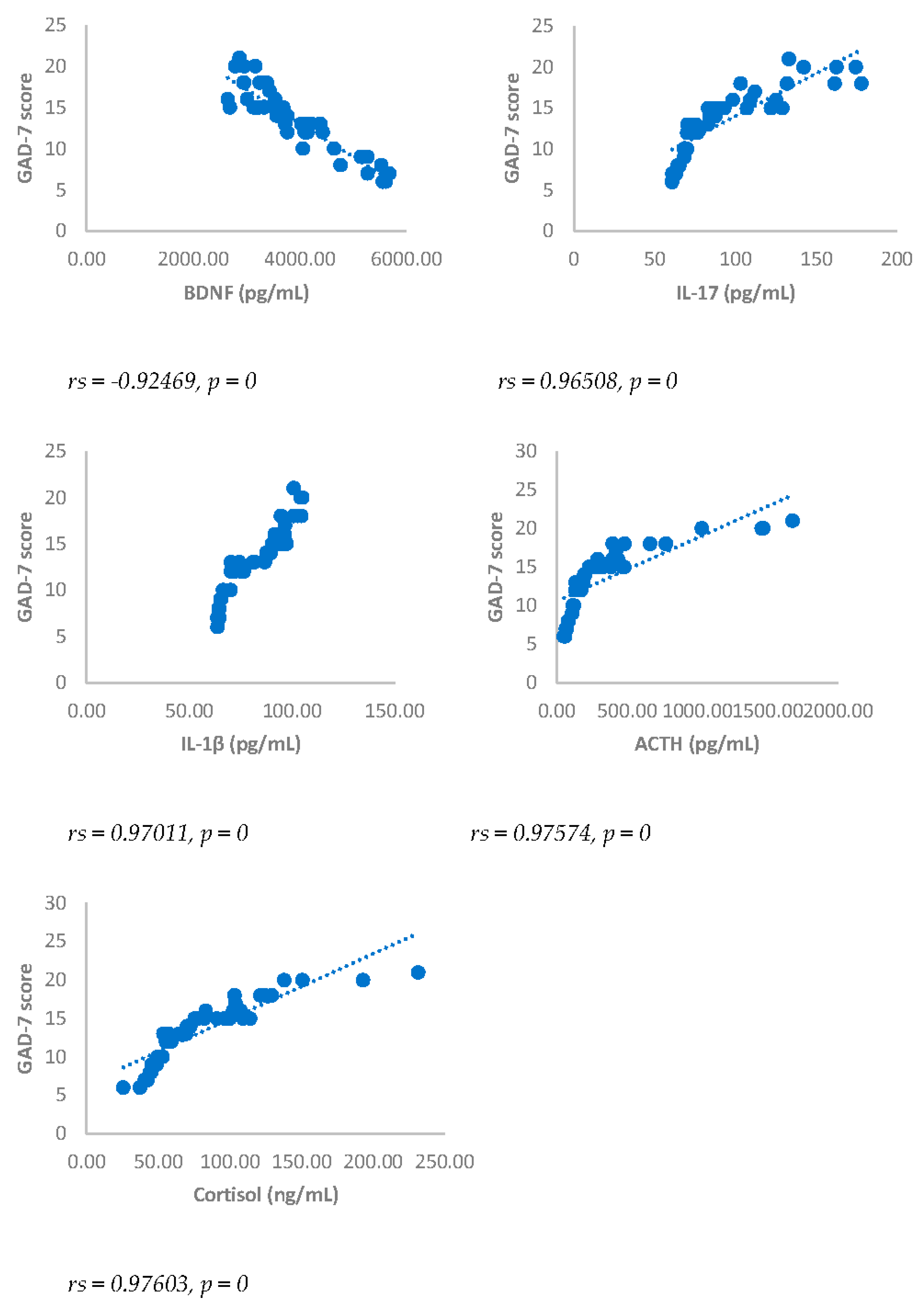
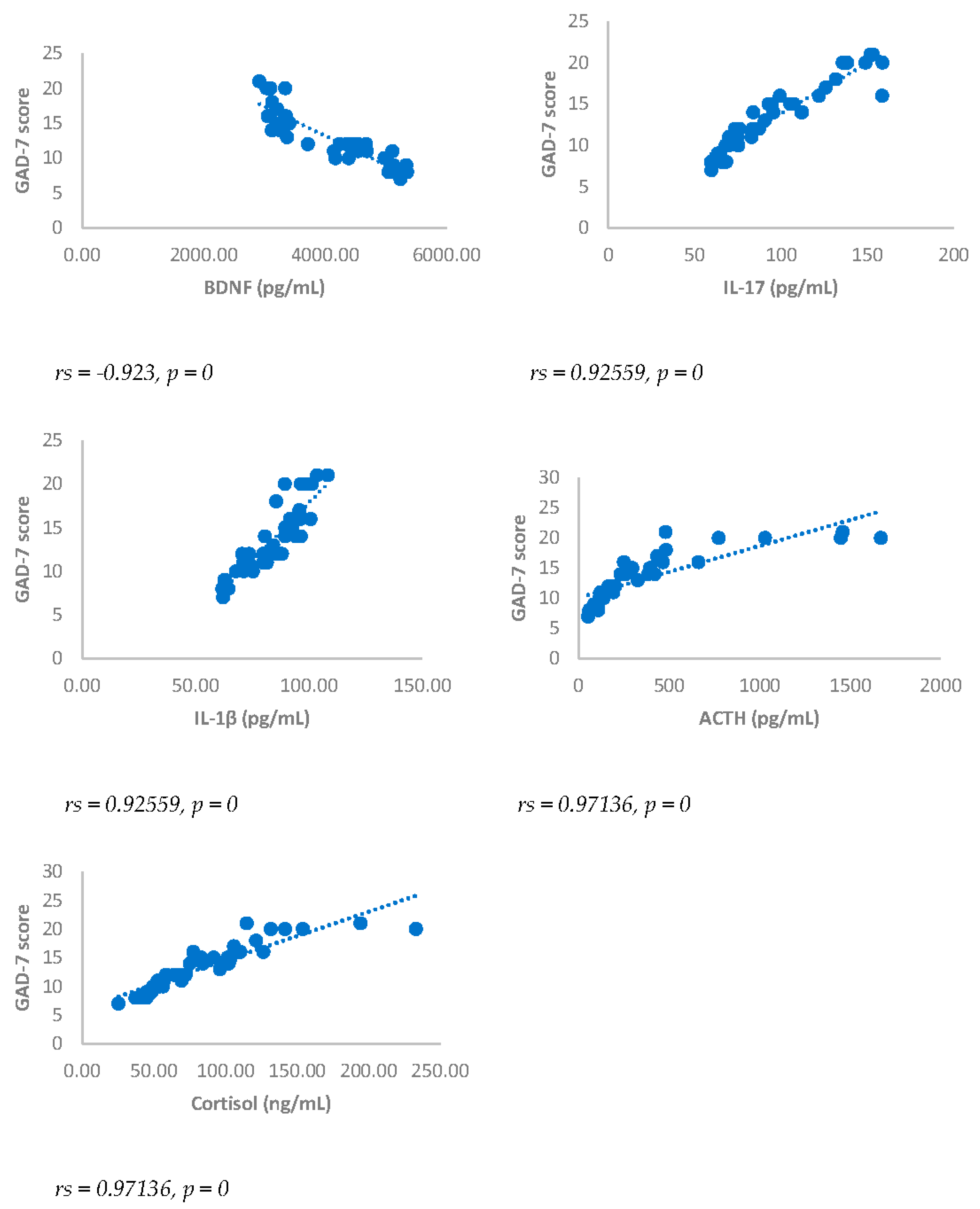
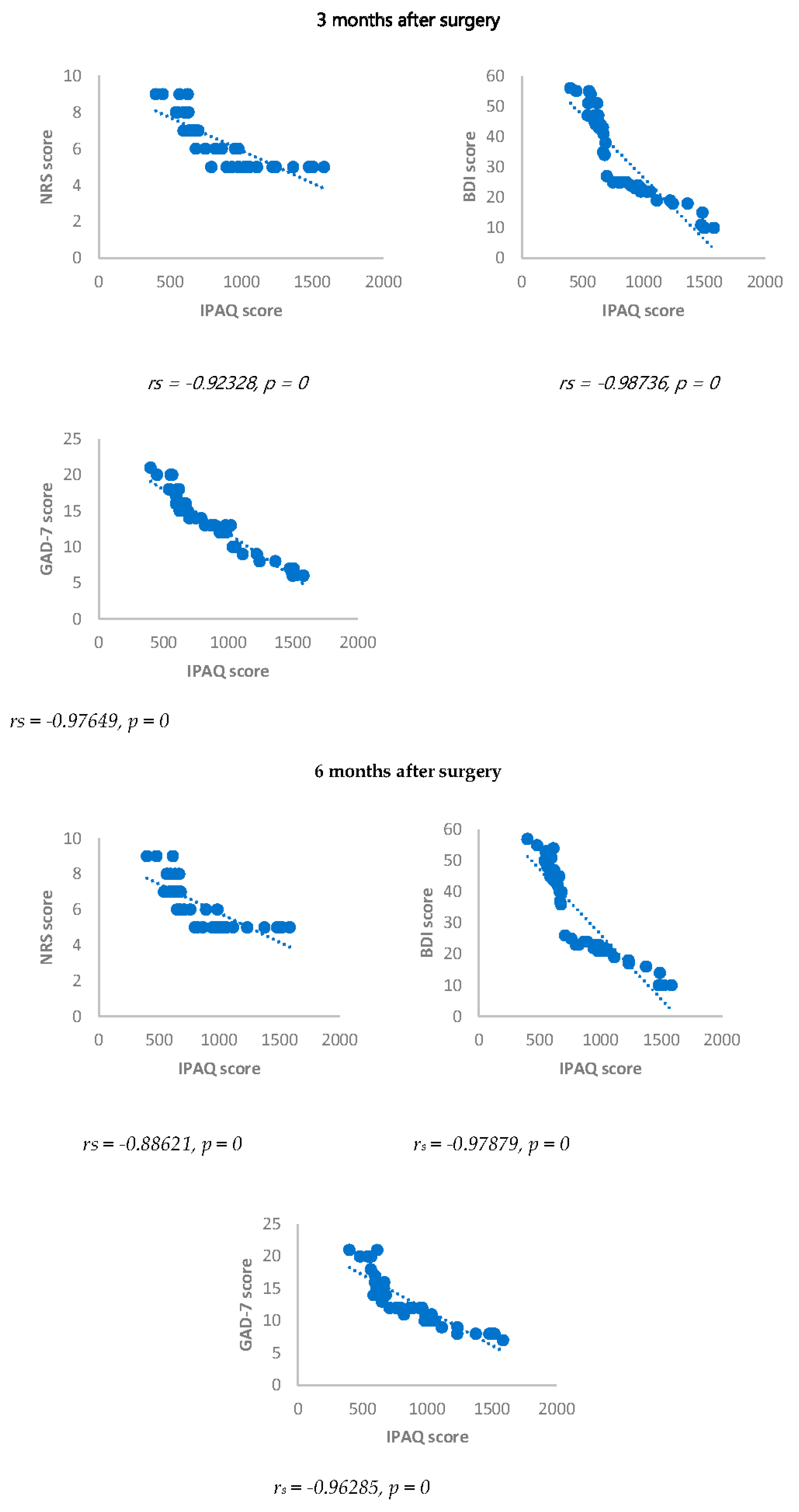
Spearman’s correlation was performed to analyze the relationship between scores obtained with questionnaires NRS, BDI, GAD-7 and biomarkers investigated (IL17, IL1-β, cortisol and ACTH). Results showed a statistically significant positive correlation between NRS and the biomarkers considered and between BDI, GAD-7, NRS and biomarkers considered either 3 or 6 months after surgery (
Figure 1,
Figure 2,
Figure 3,
Figure 4,
Figure 5 and
Figure 6).
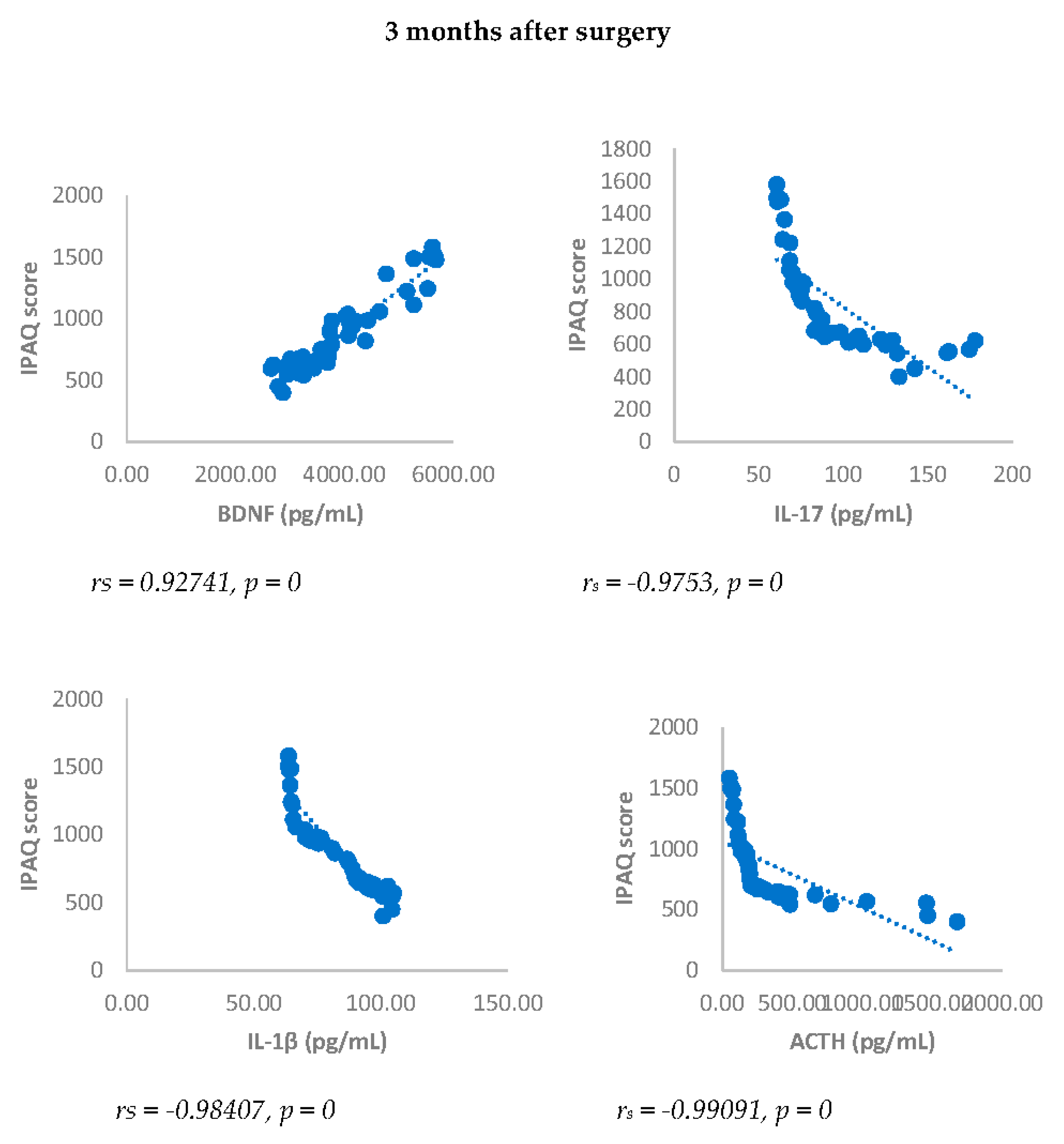
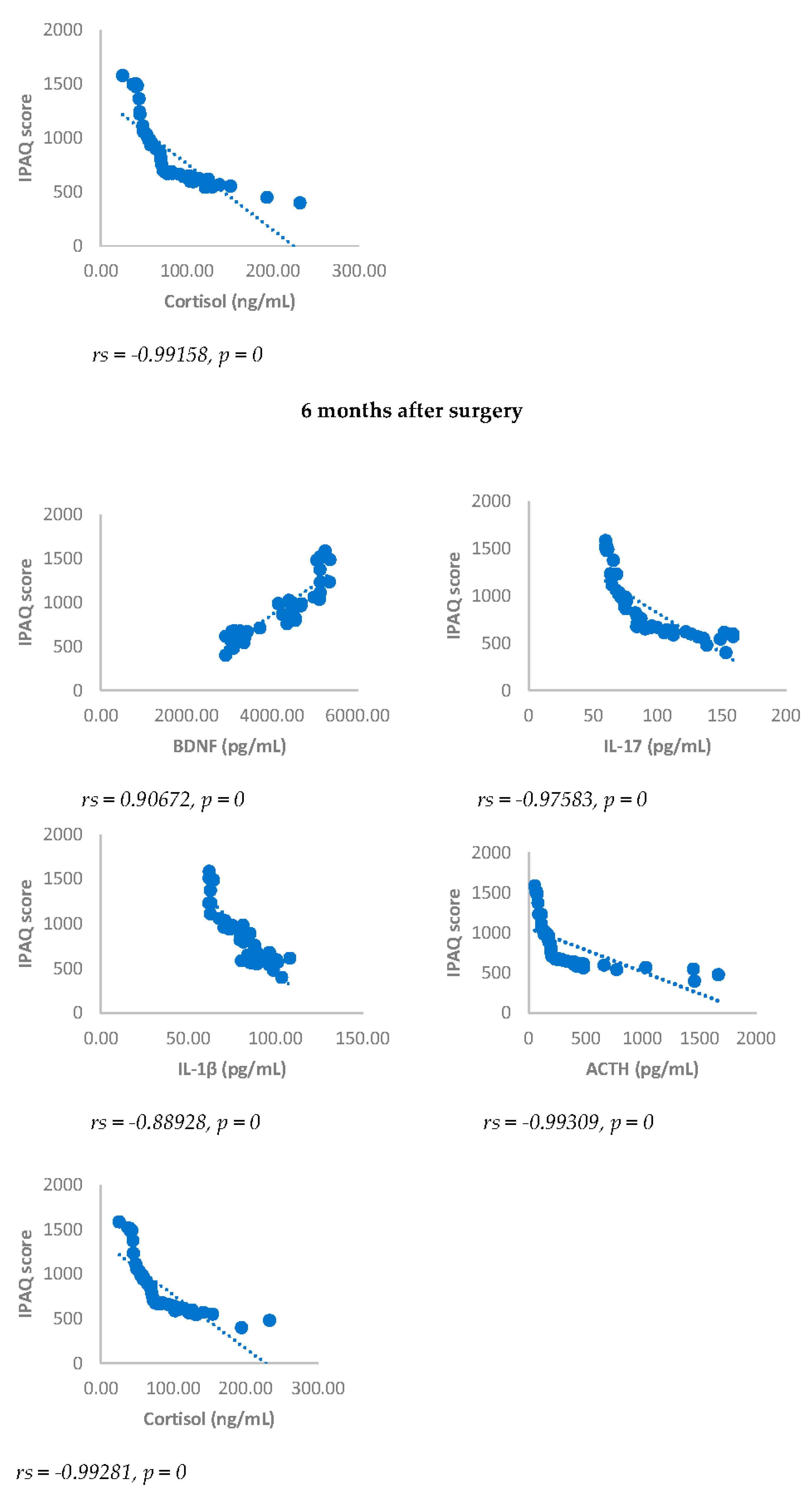
IPAQ score showed a statistically significant negative correlation with NRS, BDI, GAD-7, IL-17, IL-1-β, cortisol and ACTH. On the contrary, IPAQ score showed a statistically significant positive correlation with BDNF either 3 or 6 months after surgery (
Figure 7 and
Figure 8).
4. Discussion
As defined from World Health Organization (WHO), physical activity is considered any bodily movement produced by skeletal muscles that requires energy expenditure (WHO, 2019). Physical activity is referred to all movements including during leisure time, for transport to get to and from places, or daily activities. Both moderate- and vigorous-intensity physical activity improve mental health, and reduces the risk of developing noncommunicable diseases such as heart disease, stroke, diabetes, and cancers [
27,
28]. Physical activity has also been found to improve memory and concentration thus favoring the protection of cognitive function in elderly [
39], through stimulation of blood-transported neurotrophic factors secretion, including BDNF, that is also produced in working skeletal muscles [
40].
As recommended, adults (18-64 years) should perform weekly at least 150–300 min of moderate-intensity aerobic physical activity or at least 75–150 min of vigorous-intensity aerobic physical activity; alternatively, an equivalent combination of moderate- and vigorous-intensity activity throughout the week. This recommendation is particularly suggested for people affected by chronic diseases such as hypertension, type 2 diabetes, HIV [
41].
It is evaluated that 25-60% of patients who underwent breast cancer surgical resection suffer are affected by PMP syndrome [
42] and psychosocial factors such as anxiety, depression, sleep disturbance and catastrophizing have proven to be important contributors to the development of persistent pain. A link between pain and depression is well known and it has been suggested that
30%-45% of patients affected by chronic pain, experience depression [
43,
44,
45]
. Several studies have suggested a bidirectional relationship between depression and pain, suggesting that depression is a positive predictor of the development of chronic pain and chronic pain can increase the risk of developing depression. Moreover, depression is considered a moderator of the relationship between pain severity and physical functioning. In this view, pain and depression create a vicious cycle in which pain worsens symptoms of depression, and then the resulting depression negatively influences feelings of pain [
46]. It is known that depressive disorders can occur together with anxiety disorders [
47,
48], anxiety symptoms are among the diagnostic criteria for major depressive disorder included in the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5) [
49]. Results obtained from case-control studies suggest that inflammation could be involved in generalized anxiety disorder, indicating that inflammation could increase subsequent to the development of anxiety disorders [
50,
51].
In this study, 45.6% of a sample of women who underwent surgery needed for breast cancer manifested PMP after the surgical intervention. A similar proportion was found by other authors, reporting a percentage of 43% of women with PMP still three years after breast tumor resection [
52]. The 60.2% of PMP woman reported symptoms of anxiety and depression (
Table 3). In this subgroup, IL-17, IL-1-β, cortisol and ACTH levels, evaluated either 3 or 6 months after surgery, were significantly enhanced in DA-PMP women in comparison to not DA-PMP women (
Table 3). BDNF level was significantly reduced in DA-PMP women
vs to not DA-PMP women (
Table 3) both at three and six months after surgery. Moreover, at the same timepoints, DA-PMP inactive women showed a statistically significant increase of pain intensity (p < 0.01) and an increase of anxiety and depression signs compared to DA-PMP adequate active women (
Table 3).
Physical activity was evaluated among DA-PMP patients by IPAQ and results showed that 55% of this resulted classified as physically inactive while 65.5% of this group was adequately active. As stated by analysis of the answers obtained from IPAQ, no fully active woman was detected in this group. Six months after surgery results were overlapping with those obtained 3 months after surgery.
Our results showed a decrease of IL-17, IL-1β, cortisol and ACTH in the group of DA-PMP adequate active women, compared with DA-PMP inactive women, and an increase of BDNF levels in the group of PMP-AD adequate active women, compared with DA-PMP inactive women.
There is a great deal of correlational evidence that patients who suffer from anxiety and depression show elevations in circulating levels of cytokines that are pro-inflammatory in nature, such as Tumor necrosis factor alpha, IL-1β and IL-6 and IL-17. Furthermore, these patients have elevated levels of leukocytes, which may be the source of these increased inflammatory cytokines [
53,
54,
55]. It has been hypothesized a neuroimmune inflammatory role in the pathogenetic mechanism for psychiatric disorders such as depression and bipolar disorders. This hypothesis correlates psychiatric disorders with the activation of the immune-inflammatory response system resulting in an increase in pro-inflammatory factors, and the activation of the compensatory immunoregulatory response system, playing a negative immunoregulatory effect through T helper activation and T regulatory mechanism and suppression of immune-inflammatory response system hyperreaction [
21].
Moreover, several studies agree that one of the important findings in biological psychiatry is the hyper activity of the hypothalamic–pituitary–Adrenocortical axis observed in patients with major depression [
56]
in fact, it has been suggested that prolonged exposure to stress causes metabolic changes such as the hypothalamic–pituitary–adrenal axis activation, with consequent increase of cortisol release [
57].
It is known that regular physical activity improves bodily functions reducing the risk of developing of chronic diseases [
27,
28]. It has also been suggested as even limited amounts of routine daily activities can reduce the risk of falling [
58] or developing neurologic diseases and has positive effects on brain health and cognitive function [
59,
60].
The beneficial effects of physical activity on brain disorders have been widely studied [
61,
62,
63]. It has been hypothesized that it could reduce the risk of neurodegenerative diseases to inhibit cognitive decline and to produce a positive effect on stress, anxiety, and depression [
63,
64],
with consequent amelioration of several biomarkers associated with depressive symptoms such as hypothalamic-pituitary-adrenal (HPA) axis homeostasis, anti-neurodegenerative effects, monoamine metabolism regulation and neuroimmune system functioning [
64]
. Acute or chronic exercise programs were found to increase BDNF levels [
65,
66] thus indicating that physical activity could be helpful in treating depression and anxiety in adults. Finally, it has been also suggested that performing an individual chronic exercise can be positively associated to increase of neurogenesis and positively related with mental health.
In conclusion, our results agree with other studies that suggest a positive effects of physical activity on anxiety and depression. In our sample, in women affected by post mastectomy pain syndrome, physical activity reduces signs of anxiety and depression and this effect is associated with reduction of pain and level of inflammatory citokines, corstisol, ACTH and, increase of BDNF. These results suggest that exercise could be effective in reducing inflammatory markers and consequently to prevent the development of chronic post mastectomy pain syndrome pain and psychiatric disorders associated with it such as anxiety and depression.
Author Contributions
Conceptualization, M.C., C.M., F.C., I.A., L.C.; methodology, M.C., C.M., F.C., I.A., L.C., G.C..; surgery, M.C., L.P., D.A.V., G.B., A.B., R.B., software, I.A., F.C., L.C.; validation, M.C., C.M., F.C., I.A., M.C., G.F, L.C., G.C., E.E., D.D., F.T.; formal analysis, C.M., I.A., F.C., L.C.; investigation, C.M., F.C., I.A., L.C., M C., G.F, F.T., D.D.; resources, M.C., G.C., L.P.; data curation, I.A., L.C., F.C., C.M.; writing—original draft preparation, M.C., C.M., F.C., I.A., L.C., E.E., G.C., F.T., D.D., G.B., A.B.; writing—review and editing, C.M., M.C., G.C.; supervision, C.M., M.C., G.C.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of University of AOU Policlinico “G. Martino”: Approval Number: Prot. 70-23, 18/04/2023, Board Name: Comitato Etico Interaziendale Messina.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; Giamberardino, M.A.; Kaasa, S.; Korwisi, B.; Kosek, E.; Lavand’homme, P.; Nicholas, M.; Perrot, S.; Scholz, J.; Schug, S.; Smith, B.H.; Svensson, P:, Vlaeyen, J.W.S.; Wang, S.J. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). Pain 2019, 160(1), 19–27. [CrossRef]
- Thapa, P.; Euasobhon, P. Chronic postsurgical pain: current evidence for prevention and management. Korean J Pain 2018, 31(3), 155-173. [CrossRef]
- Werner, M.U.; Kongsgaard, U.E.I. Defining persistent postsurgical pain: is an update required? Br J Anaesth. 2014, 113, 1–4. [CrossRef]
- Andersen, K.G.; Kehlet, H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain. 2011, 12, 725–746. [CrossRef]
- Katz, J.; Poleshuck, E.L.; Andrus, C.H.; Hogan, L.A.; Jung, B.F.; Kulick, D.I.; Dworkin, R.H. Risk factors for acute pain and its persistence following breast cancer surgery. PAIN® 2005, 119, 16–25. [CrossRef]
- Vilholm, O.J.; Cold, S.; Rasmussen, L.; Sindrup, S.H. The postmastectomy pain syndrome: an epidemiological study on the prevalence of chronic pain after surgery for breast cancer. Br J Cancer 2008, 99, 604–610. [CrossRef]
- Jung, B.F.; Ahrendt, G.M.; Oaklander, A.L.; Dworkin, R.H. Neuropathic pain following breast cancer surgery: proposed classification and research update. PAIN® 2003, 104, 1–13. [CrossRef]
- Gartner, R.; Jensen, M.B.; Nielsen, J.; Ewertz, M.; Kroman, N.; Kehlet, H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA 2009, 302, 1985–1992. [CrossRef]
- Kudel, I.; Edwards, R.R.; Kozachik, S.; Block, B.M.; Agarwal, S.; Heinberg, L.J.; Haythornthwaite, J.; Raja, S.N. Predictors and consequences of multiple persistent postmastectomy pains. J Pain Symptom Manage 2007, 34, 619–627. [CrossRef]
- Steegers, M.A.; Wolters, B.; Evers, A.W.; Strobbe, L.; Wilder-Smith, O.H. Effect of axillary lymph node dissection on prevalence and intensity of chronic and phantom pain after breast cancer surgery. J Pain 2008, 9, 813–822. [CrossRef]
- Varela, A.J.; Van Asselt, K.W. The relationship between psychosocial factors and reported disability: the role of pain self-efficacy. BMC Musculoskelet Disord 2022, 23(1), 21. [CrossRef]
- Skidmore, J.R.; Koenig, A.L.; Dyson, S.J.; Kupper, A.E.; Garner, M.J.; Keller, C.J. Pain self-efficacy mediates the relationship between depressive symptoms and pain severity. Clin J Pain. 2015, 31, 137–144. [CrossRef]
- Pearce, M.; Garcia, L.; Abbas, A.; Strain, T.; Schuch, F.B.; Golubic, R.; Kelly, P.; Khan, S.; Utukuri, M.; Laird, Y.; Mok, A.; Smith, A.; Tainio, M.; Brage, S.; Woodcock, J. Association Between Physical Activity and Risk of Depression: A Systematic Review and Meta-analysis. JAMA Psychiatry 2022, 79(6), 550-559. [CrossRef]
- Finlay, A.; Wittert, G.; Short, C.E. A systematic review of physical activity-based behaviour change interventions reaching men with prostate cancer. Journal of Cancer Survivorship 2018, 12(4), 571–591. [CrossRef]
- Peterson, L.L.; Ligibel, J.A. Physical activity and breast cancer: an opportunity to improve outcomes. Curr Oncol Rep 2018, 20(7), 1–10. [CrossRef]
- Hadi, M.A.; McHugh, G.A.; Closs, S.J. Impact of Chronic Pain on Patients’ Quality of Life: A Comparative Mixed-Methods Study. J. Patient Exp. 2019, 6, 133–141. [CrossRef]
- Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012 Jul-Aug;62(4):275-6. Epub 2012 May 7. PMID: 22570061. [CrossRef]
- Giusti, E.M.; Lacerenza, M.; Manzoni, G.M.; Castelnuovo, G. Psychological and psychosocial predictors of chronic postsurgical pain: a systematic review and meta-analysis. Pain 2021, 162(1), 10-30. [CrossRef]
- Ji. R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 2018, 129(2), 343–366. [CrossRef]
- Matsuda, M.; Huh, Y.; Ji, R.R. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J Anesth 2019, 33(1), 131–139. [CrossRef]
- Hodes, G.E.; Kana, V.; Menard, C.; Merad, M.; Russo, S.J. Neuroimmune mechanisms of depression. Nat Neurosci 2015, 18(10), 1386-1393. [CrossRef]
- Lu, L.; Hu, X.; Jin, X. IL-4 as a potential biomarker for differentiating major depressive disorder from bipolar depression. Medicine (Baltimore) 2023, 102(15):e33439. [CrossRef]
- Maes, M.; Abe, Y.; Sirichokchatchawan, W.; Suwimonteerabutr, J.; Sangkomkamhangd, U.; Almulla, A.F.; Satthapisit, S. The Cytokine, Chemokine, and Growth Factor Network of Prenatal Depression. Brain Sci 2023, 13(5) 727. [CrossRef]
- World-Cancer-Research-Fund/American-Institute-for-Cancer-Research. Continuous Update Project Report: Diet, Nutrition, Physical Activity, and Breast Cancer Survivors (2014).
- Dieli-Conwright, C.M.; Lee, K.; Kiwata, J.L. Reducing the risk of breast cancer recurrence: an evaluation of the effects and mechanisms of diet and exercise. Curr Breast Cancer Rep 2016, 8(3), 139–150. [CrossRef]
- Cannioto, R.A.; Hutson, A.; Dighe, S.; McCann, W.; McCann, S.E.; Zirpoli, G.R., Barlow, W.; Kelly,: K.M.; DeNysschen, C.A.; Hershman, D.L.; Unger, J.M. Moore, H.C.F.; Stewart, J.A.; Isaacs, C.; Hobday, T.J.; Salim, M.; Hortobagyi, G.N.; Gralow, J.R.; Albain, K.S.; Budd GT, Ambrosone CB. Physical Activity Before, During, and After Chemotherapy for High-Risk Breast Cancer: Relationships With Survival. J Natl Cancer Inst 2021, 113(1), 54-63. [CrossRef]
- Segal, R.; Zwaal, C.; Green, E.; Tomasone, J.R.; Loblaw, A.; Petrella, T. Exercise for People with Cancer Guideline Development Group. Exercise for people with cancer: a systematic review. Curr Oncol 2017, 24(4):e290-e315. [CrossRef]
- Misiąg, W.; Piszczyk, A.; Szymańska-Chabowska, A.; Chabowski, M. Physical Activity and Cancer Care-A Review. Cancers (Basel) 2022, 14(17), 4154. [CrossRef]
- Calapai, M.; Puzzo, L.; Bova, G.; Vecchio, D.A.; Blandino, R.; Barbagallo, A.; Ammendolia, I.; Cardia, L.; De Pasquale, M.; Calapai, F.; Esposito, E.; Trimarchi, F.; Di Mauro, D.; Calapai, G.; Mannucci, C. Effects of Physical Exercise and Motor Activity on Oxidative Stress and Inflammation in Post-Mastectomy Pain Syndrome. Antioxidants (Basel) 2023, 12(3), 643. [CrossRef]
- Ballard-Barbash, R.; Friedenreich, C.M.; Courneya, K.S.; Siddiqi, S.M.; McTiernan, A.; Alfano, C.M. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst 2012, 104(11), 815-40. [CrossRef]
- Simpson, R.J.; Kunz, H.; Agha, N.; Graff, R. Exercise and the Regulation of Immune Functions. Prog Mol Biol Transl Sci 2015, 135, 355-380. [CrossRef]
- Alderman, B.L.; Olson, R.L.; Brush, C.J.; Shors, T.J. MAP training: combining meditation and aerobic exercise reduces depression and rumination while enhancing synchronized brain activity. Transl. Psychiatry 2016, 6, e726. [CrossRef]
- Archer, T.; Josefsson, T.; Lindwall, M. Effects of physical exercise on depressive symptoms and biomarkers in depression. CNS Neurol Disord Drug Targets 2014, 13, 1640–1653. [CrossRef]
- Breivik, H.; Borchgrevink, P.C.; Allen, S.M.; Rosseland, L.A.; Romundstad, L.; Hals, E.K.; Kvarstein, G.; Stubhaug, A. Assesment of pain. Br J Anaesth 2008, 101(1), 17-24. [CrossRef]
- Farrar, J.T.; Young, J.P. Jr; LaMoreaux, L.; Werth, J.L.; Poole, M.R. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001, 94(2), 149-158. [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch Gen Psychiatry 1961, 4, 561–571. [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.; Lowe, B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sj.str.m, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; Oja, P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003, 35(8), 1381-1395. [CrossRef]
- Yang, J.L.; Lin, Y.T.; Chuang, P.C.; Bohr, V.A.; Mattson, M.P. BDNF and exercise enhance neuronal DNA repair by stimulating CREB-mediated production of apurinic/apyrimidinic endonuclease 1. Neuromol Med 2014, 16(1), 161–174. [CrossRef]
- Tari, A.R.; Norevik, C.S.; Scrimgeour, N.R.; Kobro-Flatmoen, A.; Storm-Mathisen J, Bergersen, L.H.; Wrann, C.D.; Selbæk G, Kivipelto, M.; Moreira, J.B.N.; Wisløff, U. Are the neuroprotective effects of exercise training systemically mediated? Prog Cardiovasc Dis. 2019, 62(2), 94-101. [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; Dempsey, P.C.; DiPietro, L.; Ekelund, U.; Firth, J.; Friedenreich, C.M.; Garcia, L.; Gichu, M.; Jago, R.; Katzmarzyk, P.T.; Lambert, E.; Leitzmann, M.; Milton, K.; Ortega, F.B.; Ranasinghe, C.; Stamatakis, E.; Tiedemann, A.; Troiano, R.P.; van der Ploeg, H.P.; Wari, V.; Willumsen, J.F. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 2020, 54(24), 1451-1462. [CrossRef]
- Gartner, R.; Jensen, M.B.; Nielsen, J.; Ewertz, M.; Kroman, N.; Kehlet, H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA 2009, 302, 1985–1992. [CrossRef]
- Törer, N.; Nursal, T.Z.; Calişkan, K.; Ezer, A.; Colakoğlu, T.; Moray, G.; Haberal, M. The effect of the psychological status of breast cancer patients on the short-term clinical outcome after mastectomy. Acta Chir Belg 2010, 110(4), 467-470. [CrossRef]
- Vase, L.; Nikolajsen, L.; Christensen, B.; Egsgaard, L.L.; Arendt-Nielsen, L.; Svensson, P.; Jensen, T.S. Cognitive-emotional sensitization contributes to wind-up-like pain in phantom limb pain patients. Pain 2011, 152(1), 157-162. [CrossRef]
- Weissman-Fogel, I.; Sprecher, E.; Pud, D. Effects of catastrophizing on pain perception and pain modulation. Exp Brain Res 2008, 186(1), 79-85. [CrossRef]
- Lépine, J.P.; Briley, M . The epidemiology of pain in depression. Hum Psychopharmacol 2004, 19 Suppl 1, S3- S7. [CrossRef]
- Kendler, K.S. Major depression and generalised anxiety disorder. Br J Psychiatry 1996, 168, 68–75. [CrossRef]
- Wray, N.R.; Ripke, S.; Mattheisen, M. et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet 2018, 50, 668–681. [CrossRef]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5). Washington, DC: American Psychiatric Pub; 2013.
- Costello, H.; Gould, R.L.; Abrol, E.; Howard, R. Systematic review and meta-analysis of the association between peripheral inflammatory cytokines and generalised anxiety disorder. BMJ Open 2019, 9, e027925. [CrossRef]
- Glaus, J.; von Känel, R.; Lasserre, A.M.; Strippoli, M.F.; Vandeleur, C.L.; Castelao, E.; Gholam-Rezaee, M.; Marangoni, C.; Wagner, E.N.; Marques-Vidal, P.; Waeber, G.; Vollenweider, P.; Preisig, M, Merikangas, K.R. The bidirectional relationship between anxiety disorders and circulating levels of inflammatory markers: Results from a large longitudinal population-based study. Depress Anxiety 2018, 35(4), 360-371. [CrossRef]
- Bruce, J.; Poobalan, A.S.; Smith, W.C.; Chambers, W.A. Quantitative assessment of chronic postsurgical pain using the McGill Pain Questionnaire. Clin J Pain. 2004, 20(2), 70-5. [CrossRef]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A meta-analysis of cytokines in major depression. Biol Psychiatry 2010, 67(5), 446-457. [CrossRef]
- Hodes, G.E.; Pfau, M.L.; Leboeuf, M.; Golden, S.A.; Christoffel, D.J.; Bregman, D.; Rebusi, N.; Heshmati, M.; Aleyasin, H.; Warren, B.L.; Lebonté, B.; Horn, S.; Lapidus, K.A.; Stelzhammer, V.; Wong, E.H.; Bahn, S.; Krishnan, V, Bolaños-Guzman CA, Murrough JW, Merad M, Russo SJ. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci U S A. 2014 Nov 11;111(45):16136-41. Epub 2014 Oct 20. PMID: 25331895; PMCID: PMC4234602. [CrossRef]
- Jiang, X.; Zhou, R.; Zhang, Y.; Zhu, T.; Li, Q.; Zhang, W. Interleukin-17 as a potential therapeutic target for chronic pain. Front Immunol 2022, 13, 999407. [CrossRef]
- Nemeroff, C.B.; Neigh, G.N. Neuroendocrinology. In: Gelder M, Andreasen NA, Lopez-Ibor JJ, Geddes JR, editors. New oxford textbook of psychiatry. 2nd ed. New York, NY: Oxford UniversityPress, 2009, 160–168.
- Dziurkowska, E.; Wesolowski, M. Cortisol as a Biomarker of Mental Disorder Severity. J Clin Med 2021, 10(21), 5204. [CrossRef]
- Martínez-Hernández BM, Rosas-Carrasco O, López-Teros M, González-Rocha A, Muñoz-Aguirre P, Palazuelos-González R, Ortíz-Rodríguez A, Luna-López A, Denova-Gutiérrez E. Association between physical activity and physical and functional performance in non-institutionalized Mexican older adults: a cohort study. BMC Geriatr. 2022 May 3;22(1):388. PMID: 35505279; PMCID: PMC9066903. [CrossRef]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci 2007, 30(9), 464-472. Erratum in: Trends Neurosci. 2007, 30(10), 489. [CrossRef]
- De la Rosa, A.; Olaso-Gonzalez, G.; Arc-Chagnaud, C.; Millan, F.; Salvador-Pascual, A.; García-Lucerga, C.; Blasco-Lafarga, C.; Garcia-Dominguez, E.; Carretero, A.; Correas, A.G.; Viña, J.; Gomez-Cabrera, M.C. Physical exercise in the prevention and treatment of Alzheimer’s disease. J Sport Health Sci 2020, 9(5), 394-404. [CrossRef]
- Aarsland, D.; Sardahaee, F.S.; Anderssen, S.; Ballard, C.; Alzheimer’s Society Systematic Review group. Is physical activity a potential preventive factor for vascular dementia? A systematic review. Aging Ment Health 2010, 14(4), 386-395. [CrossRef]
- Lee, B.C.; Choe, Y.M.; Suh, G.H.; Choi, I.G.; Kim, H.S.; Hwang, J.; Yi, D.; Kim, J.W. Association between physical activity and episodic memory and the moderating effects of the apolipoprotein E ε4 allele and age. Front Aging Neurosci 2023, 15, 1184609. [CrossRef]
- Yamasaki, T. Preventive Strategies for Cognitive Decline and Dementia: Benefits of Aerobic Physical Activity, Especially Open-Skill Exercise. Brain Sci 2023, 13(3), 521. [CrossRef]
- Archer, T.; Josefsson, T.; Lindwall, M. Effects of physical exercise on depressive symptoms and biomarkers in depression. CNS Neurol Disord Drug Targets 2014, 13(10), 1640-1653. [CrossRef]
- Bağlan Yentur S.; Ercan, Z.; Deniz, G.; Karataş, A.; Gür, M.; Alkan, G.; Koca, S.S. Effects of acute aerobic exercise on brain-derived neurotrophic factor level in rheumatoid arthritis patients. Arch Rheumatol 2022, 38(2), 209-216. [CrossRef]
- Håkansson. K.; Ledreux, A.; Daffner, K.; Terjestam, Y.; Bergman, P.; Carlsson, R.; Kivipelto, M.; Winblad, B.; Granholm, A.C.; Mohammed, A.K. BDNF Responses in Healthy Older Persons to 35 Minutes of Physical Exercise, Cognitive Training, and Mindfulness: Associations with Working Memory Function. J Alzheimers Dis 2017, 55(2), 645-657. [CrossRef]
Figure 1.
Numerical rating Scale (NRS) score and BDNF, IL-17, IL-1β, ACTH, cortisol, BDI and GAD-7, in DA-PMP group, 3 months after surgery. DA-PMP = women reporting BDI score ≥ 10 and GAD-7 score ≥ 5; BDI = Beck’s Depression Inventory; GAD-7 = Generalized Anxiety Disorders-7; BDNF = brain-derived neurotrophic factor; ACTH = adrenocorticotropic hormone; IL-17 = Interleukin 17; IL-1β = Interleukin-1beta.
Figure 1.
Numerical rating Scale (NRS) score and BDNF, IL-17, IL-1β, ACTH, cortisol, BDI and GAD-7, in DA-PMP group, 3 months after surgery. DA-PMP = women reporting BDI score ≥ 10 and GAD-7 score ≥ 5; BDI = Beck’s Depression Inventory; GAD-7 = Generalized Anxiety Disorders-7; BDNF = brain-derived neurotrophic factor; ACTH = adrenocorticotropic hormone; IL-17 = Interleukin 17; IL-1β = Interleukin-1beta.
Figure 2.
Spearman’s correlation between Numerical rating Scale (NRS) score and BDNF, IL-17, IL-1β, ACTH, cortisol, BDI and GAD-7, in DA-PMP group, 6 months after surgery. DA-PMP = women reporting BDI score ≥ 10 and GAD-7 score ≥ 5; BDI = Beck’s Depression Inventory; GAD-7 = Generalized Anxiety Disorders-7; BDNF = brain-derived neurotrophic factor; ACTH = adrenocorticotropic hormone; IL-17 = Interleukin 17; IL-1β = Interleukin-1beta.
Figure 2.
Spearman’s correlation between Numerical rating Scale (NRS) score and BDNF, IL-17, IL-1β, ACTH, cortisol, BDI and GAD-7, in DA-PMP group, 6 months after surgery. DA-PMP = women reporting BDI score ≥ 10 and GAD-7 score ≥ 5; BDI = Beck’s Depression Inventory; GAD-7 = Generalized Anxiety Disorders-7; BDNF = brain-derived neurotrophic factor; ACTH = adrenocorticotropic hormone; IL-17 = Interleukin 17; IL-1β = Interleukin-1beta.
Figure 3.
Spearman’s correlation between Beck’s Depression Inventory (BDI) score and BDNF, IL-17, IL-1β, ACTH, cortisol and GAD-7, in DA-PMP group, 3 months after surgery. DA-PMP = women reporting BDI score ≥ 10 and GAD-7 score ≥ 5; GAD-7 = Generalized Anxiety Disorders-7; BDNF = brain-derived neurotrophic factor; ACTH = adrenocorticotropic hormone; IL-17 = Interleukin 17; IL-1β = Interleukin-1beta.
Figure 3.
Spearman’s correlation between Beck’s Depression Inventory (BDI) score and BDNF, IL-17, IL-1β, ACTH, cortisol and GAD-7, in DA-PMP group, 3 months after surgery. DA-PMP = women reporting BDI score ≥ 10 and GAD-7 score ≥ 5; GAD-7 = Generalized Anxiety Disorders-7; BDNF = brain-derived neurotrophic factor; ACTH = adrenocorticotropic hormone; IL-17 = Interleukin 17; IL-1β = Interleukin-1beta.
Figure 4.
Spearman’s correlation between Beck’s Depression Inventory (BDI) score and BDNF, IL-17, IL-1β, ACTH, cortisol and GAD-7, in DA-PMP group, 6 months after surgery. DA-PMP = women reporting BDI score ≥ 10 and GAD-7 score ≥ 5; GAD-7 = Generalized Anxiety Disorders-7; BDNF = brain-derived neurotrophic factor; ACTH = adrenocorticotropic hormone; IL-17 = Interleukin 17; IL-1β = Interleukin-1beta.
Figure 4.
Spearman’s correlation between Beck’s Depression Inventory (BDI) score and BDNF, IL-17, IL-1β, ACTH, cortisol and GAD-7, in DA-PMP group, 6 months after surgery. DA-PMP = women reporting BDI score ≥ 10 and GAD-7 score ≥ 5; GAD-7 = Generalized Anxiety Disorders-7; BDNF = brain-derived neurotrophic factor; ACTH = adrenocorticotropic hormone; IL-17 = Interleukin 17; IL-1β = Interleukin-1beta.
Figure 5.
Spearman’s correlation between Generalized Anxiety Disorders-7 (GAD-7) score and BDNF, IL-17, IL-1β, ACTH and cortisol in DA-PMP group, 3 months after surgery. DA-PMP = women reporting BDI score ≥ 10 and GAD-7 score ≥ 5; BDNF = brain-derived neurotrophic factor; ACTH = adrenocorticotropic hormone; IL-17 = Interleukin 17; IL-1β = Interleukin-1beta.
Figure 5.
Spearman’s correlation between Generalized Anxiety Disorders-7 (GAD-7) score and BDNF, IL-17, IL-1β, ACTH and cortisol in DA-PMP group, 3 months after surgery. DA-PMP = women reporting BDI score ≥ 10 and GAD-7 score ≥ 5; BDNF = brain-derived neurotrophic factor; ACTH = adrenocorticotropic hormone; IL-17 = Interleukin 17; IL-1β = Interleukin-1beta.
Figure 6.
Spearman’s correlation between Generalized Anxiety Disorders-7 (GAD-7) score and BDNF, IL-17, IL-1β, ACTH and cortisol in DA-PMP group, 6 months after surgery. DA-PMP = women reporting BDI score ≥ 10 and GAD-7 score ≥ 5; BDNF = brain-derived neurotrophic factor; ACTH = adrenocorticotropic hormone; IL-17 = Interleukin 17; IL-1β = Interleukin-1beta.
Figure 6.
Spearman’s correlation between Generalized Anxiety Disorders-7 (GAD-7) score and BDNF, IL-17, IL-1β, ACTH and cortisol in DA-PMP group, 6 months after surgery. DA-PMP = women reporting BDI score ≥ 10 and GAD-7 score ≥ 5; BDNF = brain-derived neurotrophic factor; ACTH = adrenocorticotropic hormone; IL-17 = Interleukin 17; IL-1β = Interleukin-1beta.
Figure 7.
Sperman’s correlation between IPAQ/NRS, IPAQ/BDI and IPAQ/GAD-7 in in DA-PMP group, 3 and 6 months after surgery. IPAQ = International Physical Activity Questionnaire; NRS = Numerical Rating Scale; DA-PMP = women reporting BDI score ≥ 10 and GAD-7 score ≥ 5; BDI = Beck’s Depression Inventory; GAD-7 = Generalized Anxiety Disorders-7.
Figure 7.
Sperman’s correlation between IPAQ/NRS, IPAQ/BDI and IPAQ/GAD-7 in in DA-PMP group, 3 and 6 months after surgery. IPAQ = International Physical Activity Questionnaire; NRS = Numerical Rating Scale; DA-PMP = women reporting BDI score ≥ 10 and GAD-7 score ≥ 5; BDI = Beck’s Depression Inventory; GAD-7 = Generalized Anxiety Disorders-7.
Figure 8.
Sperman’s correlation between IPAQ and BDNF, IL-17, IL-1β, ACTH and cortisol in DA-PMP group, 3 and 6 months after surgery. IPAQ = International Physical Activity Questionnaire; DA-PMP = women reporting BDI score ≥ 10 and GAD-7 score ≥ 5; BDNF = brain-derived neurotrophic factor; ACTH = adrenocorticotropic hormone; IL-17 = Interleukin 17; IL-1β = Interleukin-1beta.
Figure 8.
Sperman’s correlation between IPAQ and BDNF, IL-17, IL-1β, ACTH and cortisol in DA-PMP group, 3 and 6 months after surgery. IPAQ = International Physical Activity Questionnaire; DA-PMP = women reporting BDI score ≥ 10 and GAD-7 score ≥ 5; BDNF = brain-derived neurotrophic factor; ACTH = adrenocorticotropic hormone; IL-17 = Interleukin 17; IL-1β = Interleukin-1beta.
Table 1.
Age, education level, lymph nodes dissection and marital status in PMP and non-PMP groups.
Table 1.
Age, education level, lymph nodes dissection and marital status in PMP and non-PMP groups.
| Group |
Age
(years) |
Education level |
Lymph nodes
dissection |
Marital status |
NRS |
Non-PMP
(n = 87) |
49.83 ± 10.94 |
Secondary = 10.5%
Post-secondary = 30.33%
Graduation = 12.22% |
31.42% |
41.72% |
1.25 ± 1.67 |
PMP
(n = 73) |
50.98 ± 12.23 |
Secondary = 9.8%
Post-secondary = 25.10%
Graduation = 13.82% |
35.15% |
42.15% |
5.93 ± 1.25* |
Table 2.
Evaluation of intensity of pain, and BDNF, IL-17, IL1-β, ACTH and cortisol in post-mastectomy pain (PMP) and non-post-mastectomy pain (non-PMP) groups at 3 and 6 months after surgery.
Table 2.
Evaluation of intensity of pain, and BDNF, IL-17, IL1-β, ACTH and cortisol in post-mastectomy pain (PMP) and non-post-mastectomy pain (non-PMP) groups at 3 and 6 months after surgery.
| |
Non-PMP
n = 87 |
PMP
n = 73 |
| |
3 months |
6 months |
3 months |
6 months |
NRS score
(intensity of pain) |
1.25 ± 1.67 |
1.43 ± 1.71 |
5.93 ± 1.25 * |
5.81 ± 1.11 * |
| BDNF (pg/mL) |
6112.14 ± 30.18 |
6114.36 ± 30.12 |
4607.46 ± 1014.91 * |
4552.85 ± 1027.35 * |
| IL-17 (pg/mL) |
13.26 ± 2.11 |
12.32 ± 2.06 |
82.92 ± 29.14 * |
83.074 ± 27.17 * |
| IL1-β (pg/mL) |
11.26 ± 6.11 |
11.81± 5.98 |
76.54± 14.79* |
75.24± 13.84 * |
| ACTH (pg/mL) |
12.44 ± 2.32 |
12.77 ± 2.31 |
222.18 ± 335.28 * |
221.54 ± 333.78 * |
| Cortisol (ng/mL) |
5.80 ± 2.22 |
5.6 ± 2.20 |
62.36 ± 40.95 * |
62.72 ± 41.29 * |
Table 3.
Evaluation of intensity of pain, and biomarkers of BDNF, IL-17, IL1-β, ACTH and cortisol I DA-PM subgroup and non DA-PMP subgroup at 3 and 6 months after surgery.
Table 3.
Evaluation of intensity of pain, and biomarkers of BDNF, IL-17, IL1-β, ACTH and cortisol I DA-PM subgroup and non DA-PMP subgroup at 3 and 6 months after surgery.
| |
DA-PMP
n = 44 |
non DA-PMP
n = 29 |
| |
3 months |
6 months |
3 months |
6 months |
| BDI score |
31.90 ± 14.21* |
31.75 ± 14.72* |
1.55 ± 0.63 |
1.48 ± 0.68 |
| GAD-7 score |
13.45 ± 4.0* |
13.09 ± 3.98* |
1.41 ± 0.63 |
1.31 ± 0.47 |
NRS score
(intensity of pain) |
6.38 ± 1.40* |
6.22 ± 1.34* |
5.24 ± 0.45 |
5.31 ± 0.47 |
| BDNF (pg/mL) |
3914.33 ± 906.06* |
4031.75 ± 850.88* |
5512 ± 214.36 |
5504.46 ± 169.41 |
| IL-17 (pg/mL) |
94.29 ± 32.66* |
93.80 ± 30.69* |
65.68 ± 5.87 |
68,48 ± 7.21 |
| IL1-β (pg/mL) |
83.87 ± 14.33* |
81.91 ± 13.58* |
65.41 ± 5.88 |
65.09 ± 5.67 |
| ACTH (pg/mL) |
349.84 ± 382.43* |
347.58 ± 381.52* |
28.48 ± 1.44 |
28.03 ± 1.57 |
| Cortisol (ng/mL) |
82.33 ± 41.85* |
82.61 ± 42.45* |
32.06 ± 6.70 |
32.54 ± 7.16 |
Table 4.
Evaluation of physical activity by IPAQ (International Physical Activity Questionnaire) in DA-PMP subgroup 3 and 6 months after surgery.
Table 4.
Evaluation of physical activity by IPAQ (International Physical Activity Questionnaire) in DA-PMP subgroup 3 and 6 months after surgery.
| IPAQ score |
|---|
| METs |
DA-PMP
(n = 44) |
| |
3 months after surgery |
6 months after surgery |
< 700 (Inactive)
(N = 20) |
602.55 ± 75.55 |
600.95 ± 70.42 |
700–2509 (Adequate active)
(N = 24) |
1093.70 ± 265.46 * |
1098.25 ± 266.52 *° |
> 2510 (Active)
(N = 0) |
N.D |
N.D |
Table 5.
Evaluation of intensity of pain, and BDNF, IL-17, IL1-β; ACTH and cortisol levels in DA-PMP subgroup at 3 and 6 months after surgery, according to physical activity score.
Table 5.
Evaluation of intensity of pain, and BDNF, IL-17, IL1-β; ACTH and cortisol levels in DA-PMP subgroup at 3 and 6 months after surgery, according to physical activity score.
| |
IPAQ score |
|
| |
Adequate Active DA-PMP
n = 24 |
Inactive DA-PMP
n = 20 |
| |
3 months |
6 months |
3 months |
6 months |
NRS score
(intensity of pain) |
5.29 ± 0.55 |
5.17 ± 0.38 |
7.75 ± 0.85 * |
7.50 ± 0.88 * |
| BDI score |
20.16 ± 5.28 |
19.82 ± 5.13 ° |
46.0 ± 6.36 * |
46.55 ± 6.02 * |
| GAD-7 score. |
10.58 ± 2.74 |
10.21 ± 1.71 |
16.9 ± 2.07 * |
16.7 ± 2.69 * |
| BDNF (pg/mL) |
4554.06 ± 725.31 |
4735.25 ± 445.92 |
3146.67 ± 29.93 * |
3187.56 ± 150.07 * |
| IL-17 (pg/mL) |
71.50 ± 7.92 |
71.60 ± 2.55 |
121.62 ± 14.37 * |
121.03 ± 12.88 * |
| IL1-β (pg/mL) |
72.77 ± 8.91 |
72.37 ± 8.86 |
97.15 ± 5.15 * |
93.88 ± 6.79 * |
| ACTH (pg/mL) |
132.56 ± 49.55 |
136.20 ± 49.24 |
610.58 ± 443.64 * |
608.70 ± 440.82 * |
| Cortisol (ng/mL) |
53.64 ± 12.19 |
54.77 ± 12.19 |
116.75 ± 38.70 * |
117.48 ± 39.39 * |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).