Submitted:
21 November 2023
Posted:
22 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
3. Conclusion
4. Materials and Methods
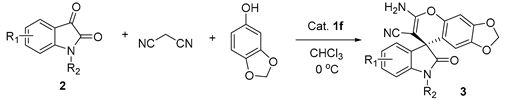
4.1. Chemistry
4.2. General Procedure for the Enantioselective Knoevenagel/Michael/cyclization reaction of isatins, malononitrile and sesamol
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xiao, Z.-P.; Peng, Z.-Y.; Dong, J.-J.; He, J.; Ouyang, H.; Feng, Y.-T.; Lu, C.-L.; Lin, W.-Q.; Wang, J.-X.; Xiang, Y.-P.; Zhu, H.-L. Synthesis, Structure−Activity Relationship Analysis and Kinetics Study of Reductive Derivatives of Flavonoids as Helicobacter Pylori Urease Inhibitors. Eur. J. Med. Chem. 2013, 63, 685–695. [Google Scholar] [CrossRef]
- Yee, E. M. H.; Pasquier, E.; Iskander, G.; Wood, K.; Black, D. S.; Kumar, N. Synthesis of Novel Isoflavene- Propranolol Hybrids as Anti-Tumor Agents. Bioorg. Med. Chem. 2013, 21, 1652–1660. [Google Scholar] [CrossRef]
- Eiffe, E.; Pasquier, E.; Kavallaris, M.; Herbert, C.; Black, D. S.; Kumar, N. Synthesis, Anticancer and Anti- Inflammatory Activity of Novel 2-Substituted Isoflavenes. Bioorg. Med. Chem, 2014; 22, 5182–5193. [Google Scholar] [CrossRef]
- Chen, Y.; Cass, S. L.; Kutty, S. K.; Yee, E. M. H.; Chan, D. S. H.; Gardner, C. R.; Vittorio, O.; Pasquier, E.; Black, D. S.; Kumar, N. Synthesis, Biological Evaluation and Structure−Activity Relationship Studies of Isoflavene Based Mannich Bases with Potent Anti-Cancer Activity. Bioorg. Med. Chem. Lett. 2015, 25, 5377–5383. [Google Scholar] [CrossRef]
- Renko, D.; Provot, O.; Rasolofonjatovo, E.; Bignon, J.; Rodrigo, J.; Dubois, J.; Brion, J. D.; Hamze, A.; Alami, M. Rapid Synthesis of 4- Arylchromenes from Ortho-Substituted Alkynols: A Versatile Access to Restricted Isocombretastatin A-4 Analogues as Antitumor Agents. Eur. J. Med. Chem. 2015, 90, 834–844. [Google Scholar] [CrossRef]
- Yee, E. M. H.; Brandl, M. B.; Black, D. S.; Vittorio, O.; Kumar, N. Synthesis of Isoflavene- Thiosemicarbazone Hybrids and Evaluation of Their Anti-Tumor Activity. Bioorg. Med. Chem. Lett. 2017, 27, 2454–2458. [Google Scholar] [CrossRef]
- Malik, N.; Zhang, Z.; Erhardt, P. Total Synthesis of (±)-Glyceollin II and a Dihydro Derivative. J. Nat. Prod. 2015, 78, 2940–2947. [Google Scholar] [CrossRef]
- Muharini, R.; Díaz, A.; Ebrahim, W.; Mandi, A.; Kurtan, T.; Rehberg, N.; Kalscheuer, R.; Hartmann, R.; Orfali, R. S.; Lin, W.; Liu, Z.; Proksch, P. Antibacterial and Cytotoxic Phenolic Metabolites from the Fruits of Amorpha Ruticosa. J. Nat. Prod. 2017, 80, 169–180. [Google Scholar] [CrossRef]
- Rueda-Zubiaurre, A.; Yahiya, S.; Fischer, O. J.; Hu, X.; Saunders, C. N.; Sharma, S.; Straschil, U.; Shen, J.; Tate, E. W.; Delves, M. J.; Baum, J.; Barnard, A.; Fuchter, M. J. Structure−Activity Relationship Studies of a Novel Class of Transmission Blocking Antimalarials Targeting Male Gametes. J. Med. Chem. 2020, 63, 2240–2262. [Google Scholar] [CrossRef]
- Chen, M,-S.; Yu, S.-J. Characterization of Lipophilized Monomeric and Oligomeric Grape Seed Flavan-3-ol Derivatives. J. Agric. Food. Chem. 2017, 65, 8875-8883. [CrossRef]
- Hu, B.; Liu, X.; Zhang, C.; Zeng, X. Food Macromolecule Based Nanodelivery Systems for Enhancing the Bioavailability of Polyphenols. J. Food. Drug. Anal. 2017, 25, 3–15. [Google Scholar] [CrossRef]
- Cheenpracha, S.; Karalai, C.; Ponglimanont, C.; Kanjana-Opas, A. Candenatenins, A. Phenolic Compounds from the Heartwood of Dalbergia Candenatensis. J. Nat. Prod. 2009, 72, 1395–1398. [Google Scholar] [CrossRef]
- Singh, G. S.; Desta, Z. Y. Isatins as Privileged Molecules in Design and Synthesis of Spiro Fused Cyclic Frameworks. Chem. Rev. 2012, 112, 6104–6155. [Google Scholar] [CrossRef]
- Williams, R. M.; Cox, R. J. Paraherquamides, Brevianamides, and Asperparalines:Laboratory Synthesis and Biosynthesis. An Interim Report. Acc. Chem. Res. 2003, 36, 127–139. [Google Scholar] [CrossRef]
- Yang, Y.-T.; Zhu, J.-F.; Liao, G.; Xu, H.-J.; Yu, B. The Development of Biologically Important Spirooxindoles as New Antimicrobial Agents. Curr. Med. Chem. 2018, 25, 2233–2244. [Google Scholar] [CrossRef]
- Panda, S. S.; Jones, R. A.; Bachawala, P.; Mohapatra, P. P. Spirooxindoles as Potential Pharmacophores. Mini. Rev. Med. Chem. 2017, 17, 1515–1536. [Google Scholar] [CrossRef]
- Chen, W.-B.; Wu, Z.-J.; Pei, Q.-L.; Cun, L.-F.; Zhang, X.-M.; Yuan, W.-C. Highly Enantioselective Construction Of Spiro, [4H-Pyran-3.30 -Oxindoles] Through a Domino Knoevenagel/Michael/Cyclization Sequence Catalyzed by Cupreine. Org. Lett. 2010, 12, 3132–3135. [Google Scholar] [CrossRef]
- Pellissier, H.; Recent Developments in Asymmetric Organocatalytic Domino Reactions. Recent Developments in Asymmetric Organocatalytic Domino Reactions. Adv. Synth. Catal. 2012, 354, 237–294. [CrossRef]
- Cheng, D.; Ishihara, Y.; Tan, B.; Barbas, C. F. Organocatalytic Asymmetric Assembly Reactions: Synthesis of Spirooxindoles via Organocascade Strategies. ACS Catal. 2014, 4, 743–762. [Google Scholar] [CrossRef]
- Santos, M. M. M. Recent Advances in the Synthesis of Biologically Active Spirooxindoles. Tetrahedron 2014, 70, 9735–9757. [Google Scholar] [CrossRef]
- Chauhan, P.; Mahajan, S.; Kaya, U.; Hack, D.; Enders, D. Bifunctional AmineSquaramides: Powerful Hydrogen-Bonding Organocatalysts for Asymmetric Domino/Cascade Reactions. Adv. Synth. Catal. 2015, 357, 253–281. [Google Scholar] [CrossRef]
- Ardkhean, R.; Caputo, D. F. J.; Morrow, S. M.; Shi, H.; Xiong, Y.; Anderson, E. A. Cascade Polycyclizations in Natural Product Synthesis. Chem. Soc. Rev. 2016, 45, 1557–1569. [Google Scholar] [CrossRef]
- Mei, G.-J.; Shi, F. Catalytic Asymmetric Synthesis of Spirooxindoles: Recent Developments. Chem. Commun. 2018, 54, 6607–6621. [Google Scholar] [CrossRef]
- Chanda, T.; Zhao, J. C.-G. Recent Progress in Organocatalytic Asymmetric Domino Transformations. Adv. Synth. Catal. 2018, 360, 2–79. [Google Scholar] [CrossRef]
- Mohammadi Ziarani, G.; Moradi, R.; Lashgari, N. Asymmetric Synthesis of Chiral Oxindoles Using Isatin as Starting Material. Tetrahedron 2018, 74, 1323–1353. [Google Scholar] [CrossRef]
- Konda, S.; Jakkampudi, S.; Arman, H. D.; Zhao, J.C.-G. Enantioselective Synthesis of Spiro[4H- pyran-3,3-oxindole] Derivatives Catalyzed by Cinchona Alkaloid Thioureas: Significant Water Effects on the Enantioselectivity. Synthetic Commun. 2019, 49, 2971–2982. [Google Scholar] [CrossRef]
- Tan, F.; Lu, L.-Q.; Yang, Q.-Q.; Guo, W.; Bian, Q.; Chen, J.-R.; Xiao, W.-J. Enantioselective Cascade Michael Addition/ Cyclization Reactions of 3-Nitro-2H-Chromenes with 3-Isothiocyanato Oxindoles: Efficient Synthesis of Functionalized Polycyclic Spirooxindoles. Chem. Eur. J. 2014, 20, 3415–3420. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, Y.; Yao, J.; Cao, Y.; Kai, M.; He, N.; Zhang, X.; Wang, Y.; Wang, R. Core Scaffold-Inspired Concise Synthesis of Chiral Spirooxindole-Pyranopyrimidines with Broad- Spectrum Anticancer Potency. Adv. Synth. Catal. 2012, 354, 917–925. [Google Scholar] [CrossRef]
- Zhao, H.-W.; Li, B.; Tian, T.; Meng, W.; Yang, Z.; Song, X.-Q.; Chen, X.-Q.; Pang, H.-L. Highly Enantioselective Synthesis of Chiral Pyranonaphthoquinone-Fused Spirooxindoles through Organocatalytic Three-Component Cascade Reactions. Eur. J. Org. Chem. 2015, 2015, 3320–3326. [Google Scholar] [CrossRef]
- Kumari, P.; Nandi, S.; Kumar, G.; Khan,N. H.; Kureshy,R. I.; Abdi, S. H. R.; Eringathodi, S.; Bajaj, H. C. Construction of Highly Enantioselective Spiro-oxindole Derivatives with Fused Chromene via Organocascade Catalysis. RSC Adv. 2016, 6, 52384–52390. [CrossRef]
- Yang, C.; Yang, J.; Guo F. -M.; Zhao, Z.; Feng, T.-T.; Liu X. -L.; Zhou Y. Synthesis of Novel Spiro-Fused Pyranyl Benzo[d] [1,3]dioxol Oxindoles. Chin. J. Synth. Chem. 2015, 23, 599–602. [CrossRef]
- Abdolmohammadi, S.; Rasouli Nasrabadi, S. R.; Dabiri, M. R.; Banihashemi Jozdani, S. M. TiO2 Nanoparticles Immobilized on Carbon Nanotubes: An Efficient Heterogeneous Catalyst in Cyclocondensation Reaction of Isatins with Malononitrile and 4-Hydroxycoumarin or 3, 4- Methylenedioxyphenol under Mild Reaction Conditions. Appl Organometal Chem. 2020, e5462. [Google Scholar] [CrossRef]
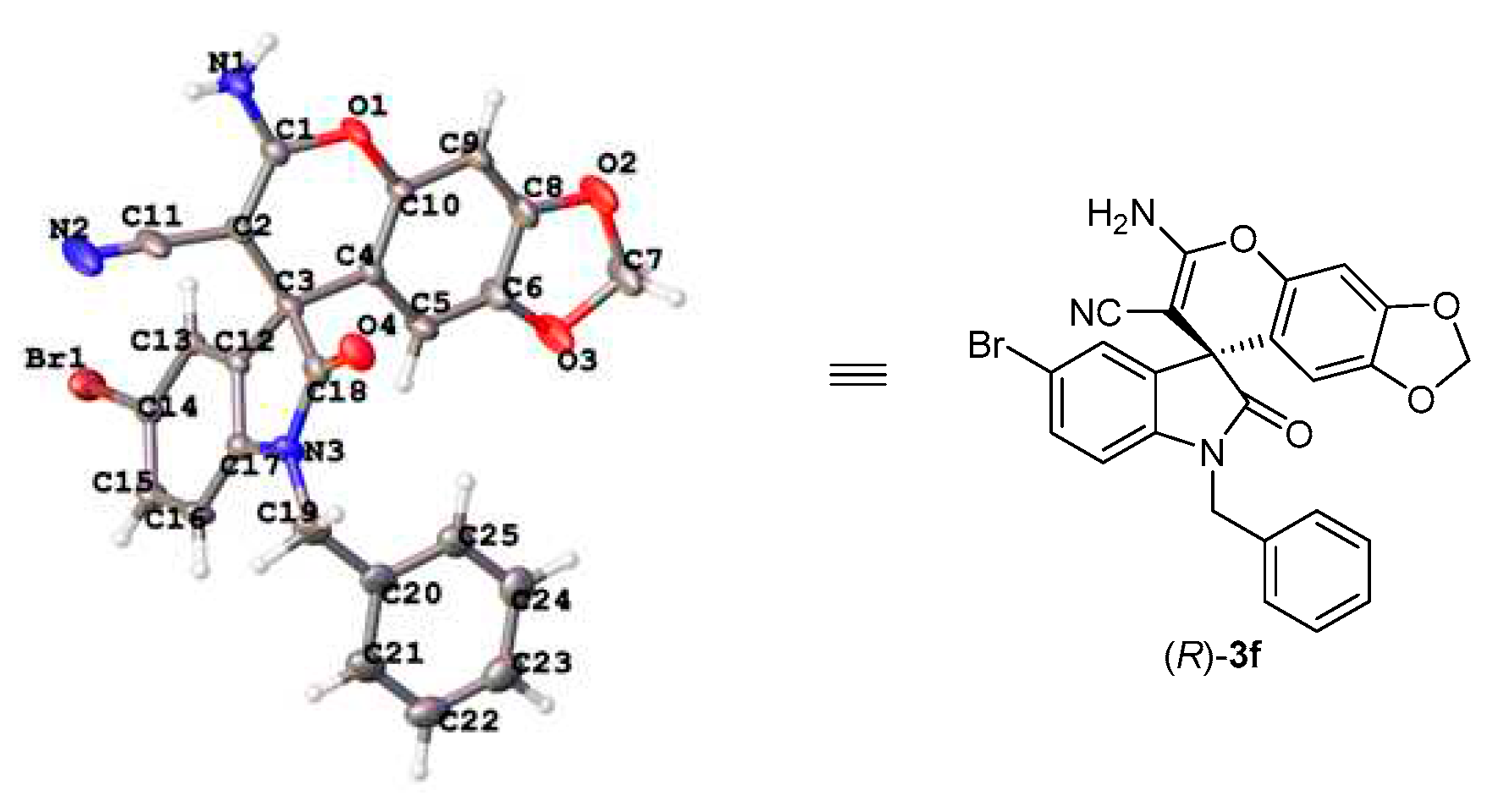
- CCDC-2291478 (3f) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via. www.ccdc.cam.ac.uk/data_request/cif.
- Prathima, P. S.; Rajesh, P.; Rao, J. V.; Kailash, U. S.; Sridhar, B.; Rao, M. M. “On Water” Expedient Synthesis of 3-Indolyl- 3- Hydroxy Oxindole Derivatives and Their Anticancer Activity in Vitro. Eur. J. Med. Chem. 2014, 84, 155–159. [Google Scholar] [CrossRef]
- Huong, T. T. L.; Dung,D.T. M.; Huan,N. V.; Cuong, L. V.; Hai, P. T.; Huong, L.T. T.; Kim, J.; Kim, Y. G.; Han,S. B.; Nam, N. H. Novel N-Hydroxybenzamides Incorporating 2-Oxoindoline with Unexpected Potent Histone Deacetylase Inhibitory Effects and Antitumor Cytotoxicity. Bioorg. Chem. 2017, 71, 160- 169. [CrossRef] [PubMed]
- Kumar, S.; Saha, S. T.; Gu, L.; Palma, G.; Perumal, S.; Singh-Pillay, A.; Singh, P.; Anand, A.; Kaur, M. ; Kumar, V 1H-1,2,3-Triazoletethered Nitroimidazole-Isatin Conjugates: Synthesis, Docking, and Anti-Proliferative Evaluation against Breast Cancer. ACS Omega, 2018, 3, 12106–12113. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M. R.; Alagumuthu, M.; Dhayabaran, V. V. N-Substituted Hydroxynaphthalene Imino-oxindole Derivatives as New Class of PI3-Kinase Inhibitor and Breast Cancer Drug: Molecular Validation and Structure-Activity Relationship Studies. Chem. Biol. Drug Des. [CrossRef]
- Diao, Q. -P.; Guo, H.; Wang, G. -Q. Design, Synthesis, and in Vitro Anticancer Activities of Diethylene Glycoltethered Isatin -1,2,3-Triazole-Coumarin Hybrids. J Heterocycl Chem. 2019, 56, 1667 -1671. [CrossRef]
- Shirai, F.; Mizutani, A.; Yashiroda, Y.; Tsumura, T.; Kano, Y.; Muramatsu, Y.; Chikada, T.; Yuki, H.; Niwa, H.; Sato, S.; Washizuka, K.; Koda, Y.; Mazaki, Y.; Jang, M. K.; Yoshida, H.; Nagamori, A.; Okue, M.; Watanabe, T.; Kitamura, K.; Shitara, E.; Honma, T.; Umehara,T.; Shirouzu, M.; Fukami, T.; Seimiya, H.; Yoshida, M.; Koyama, H. Design and Discovery of an Orally Efficacious Spiroindolinone-Based Tankyrase Inhibitor for the Treatment of Colon Cancer. J. Med. Chem. 2020, 63, 4183 -4204. [CrossRef]

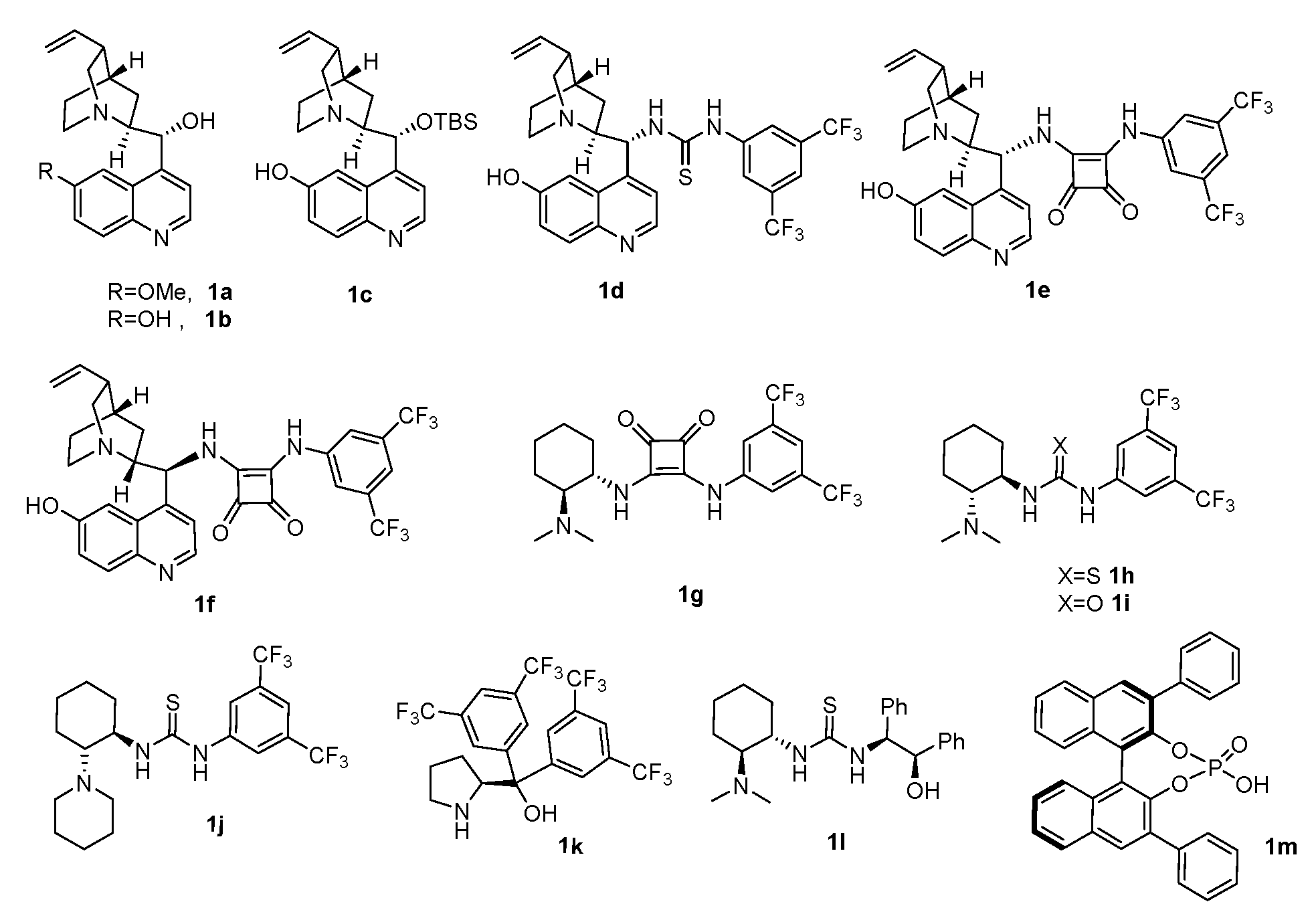

 | |||
|---|---|---|---|
| Entry | Catalyst | Yield (%)b | %eec |
| 1 | 1a | 78 | 10 |
| 2 | 1b | 76 | 12 |
| 3 | 1c | 80 | 10 |
| 4 | 1d | 81 | 29 |
| 5 | 1e | 83 | 38 |
| 6 | 1f | 85 | 40 |
| 7 | 1g | 82 | 26 |
| 8 | 1h | 80 | 20 |
| 9 | 1i | 68 | 5 |
| 10 | 1j | 73 | 12 |
| 11 | 1k | 74 | 12 |
| 12 | 1l | 72 | 9 |
| 13 | 1m | - | - |
| Entry. | Solvent | Temperature | Catalyst. Amount (% mmol) |
Yield (%)b | %eec |
|---|---|---|---|---|---|
| 1 | CH2Cl2 | rt | 10 | 85 | 40 |
| 2 | CHCl3 | rt | 10 | 84 | 42 |
| 3 | (CH2)2Cl2 | rt | 10 | 76 | 28 |
| 4 | Et2O | rt | 10 | 78 | 13 |
| 5 | THF | rt | 10 | 78 | 16 |
| 6 | PhMe | rt | 10 | 81 | 16 |
| 7 | EtOAc | rt | 10 | 75 | 10 |
| 8 | CHCl3 | rt | 5 | 71 | 35 |
| 9 | CHCl3 | rt | 20 | 88 | 36 |
| 10 | CHCl3 | 0 | 10 | 83 | 58 |
| 11 | CHCl3 | -20 | 10 | 68 | 40 |
| 12d | CHCl3 | 0 | 10 | 83 | 52 |
| 13e | CHCl3 | 0 | 10 | 85 | 61 |
| 14f | CHCl3 | 0 | 10 | 87 | 60 |
 | ||||
|---|---|---|---|---|
| Entry | R1, R2 | Product | Yield (%)b | %eec |
| 1 | H, Bn(2a) | 3a | 85 | 61 |
| 2 | 4-Cl, Bn(2b) | 3b | 83 | 65 |
| 3 | 4-Br, Bn(2c) | 3c | 77 | 50 |
| 4 | 5-F, Bn(2d) | 3d | 81 | 60 |
| 5 | 5-Cl, Bn(2e) | 3e | 85 | 50 |
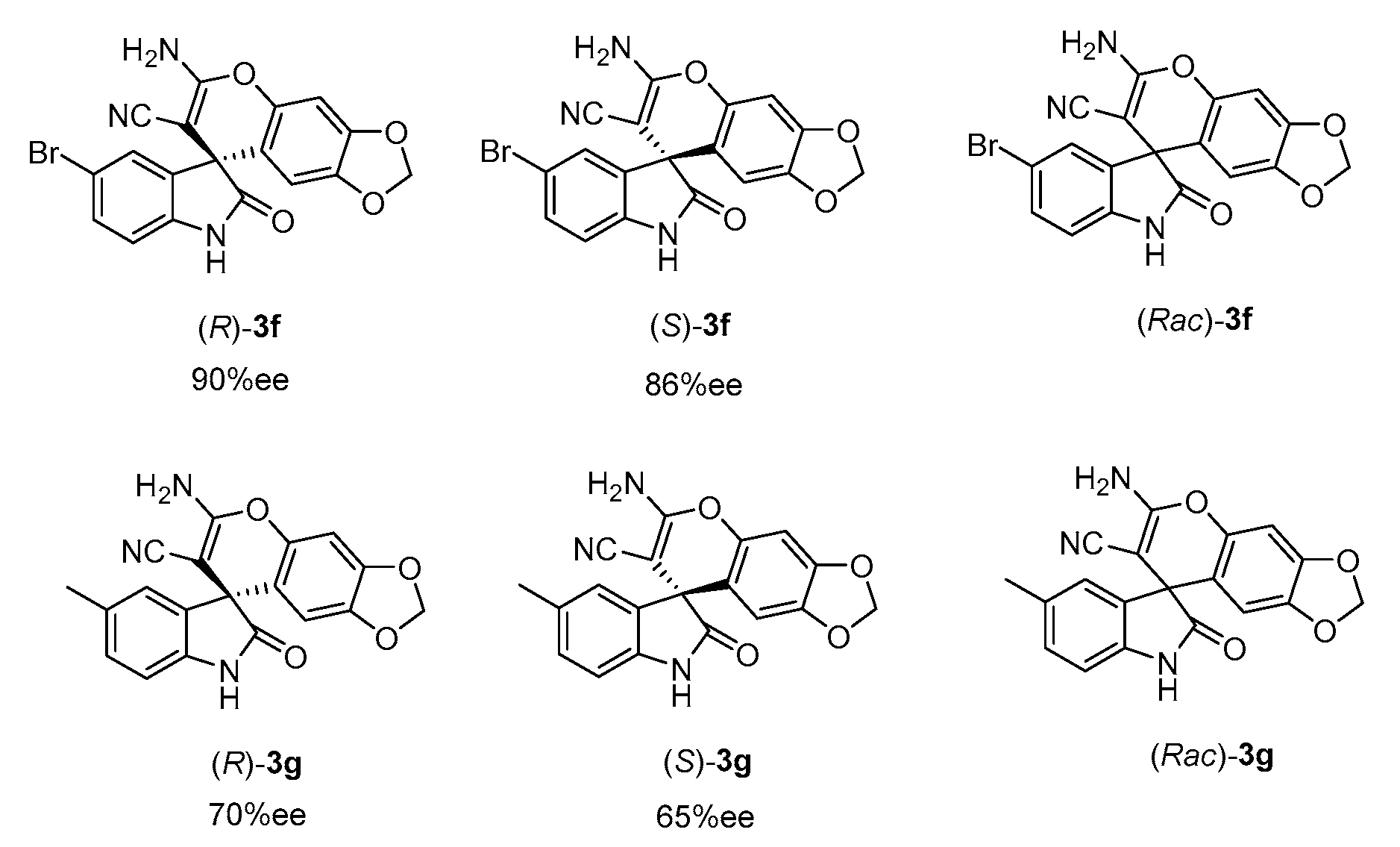
| 6 | 5-Br, Bn(2f) | 3f | 87(82) d | 90(85)d |
| 7 | 5-Me, Bn(2g) | 3g | 81 | 70 |
| 8 | 5-OMe, Bn(2h) | 3h | 79 | 57 |
| 9 | 5-NO2, Bn(2i) | 3i | 84 | 10 |
| 10 | 6-Cl, Bn(2j) | 3j | 82 | 55 |
| 11 | 7-Cl, Bn(2k) | 3k | 75 | 55 |
| 12 | 7-Br, Bn(2l) | 3l | 80 | 67 |
| 13 | H, H(2m) | 3m | 79 | 45 |
| 14 | H, Me(2n) | 3n | 78 | 50 |
| 15 | 5-Br, H(2o) | 3o | 83 | 72 |
| 16 | 5-Me, H(2p) | 3p | 81 | 59 |
| Entry | compounds | IC50 valuesa (μg/mL) | ||
|---|---|---|---|---|
| C42 | HeLa | U2OS | ||
| 1 | R-3f | 11.57 | 16.32 | 6.73 |
| 2 | S-3f | 23.83 | 74.69 | 62.31 |
| 3 | Racemic-3f | 15.32 | 55.52 | 26.39 |
| 4 | R-3g | 12.22 | 47.97 | 17.98 |
| 5 | S-3g | 31.88 | 225.62 | 73.10 |
| 6 | Racemic-3g | 18.48 | 171.91 | 44.26 |
| 7 | Adriamycin | 6.02 | 1.48 | 4.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





