Submitted:
20 November 2023
Posted:
20 November 2023
You are already at the latest version
Abstract
Keywords:
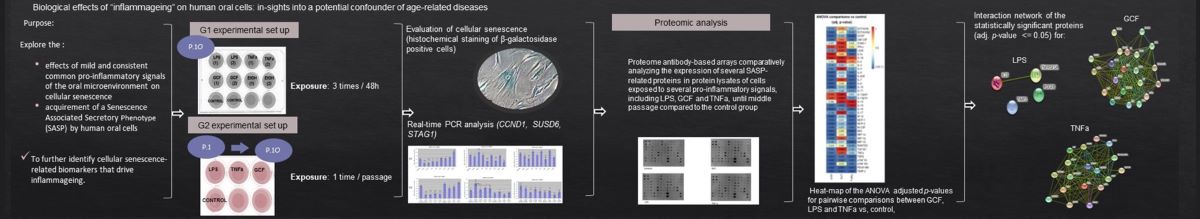
1. Introduction
2. Results
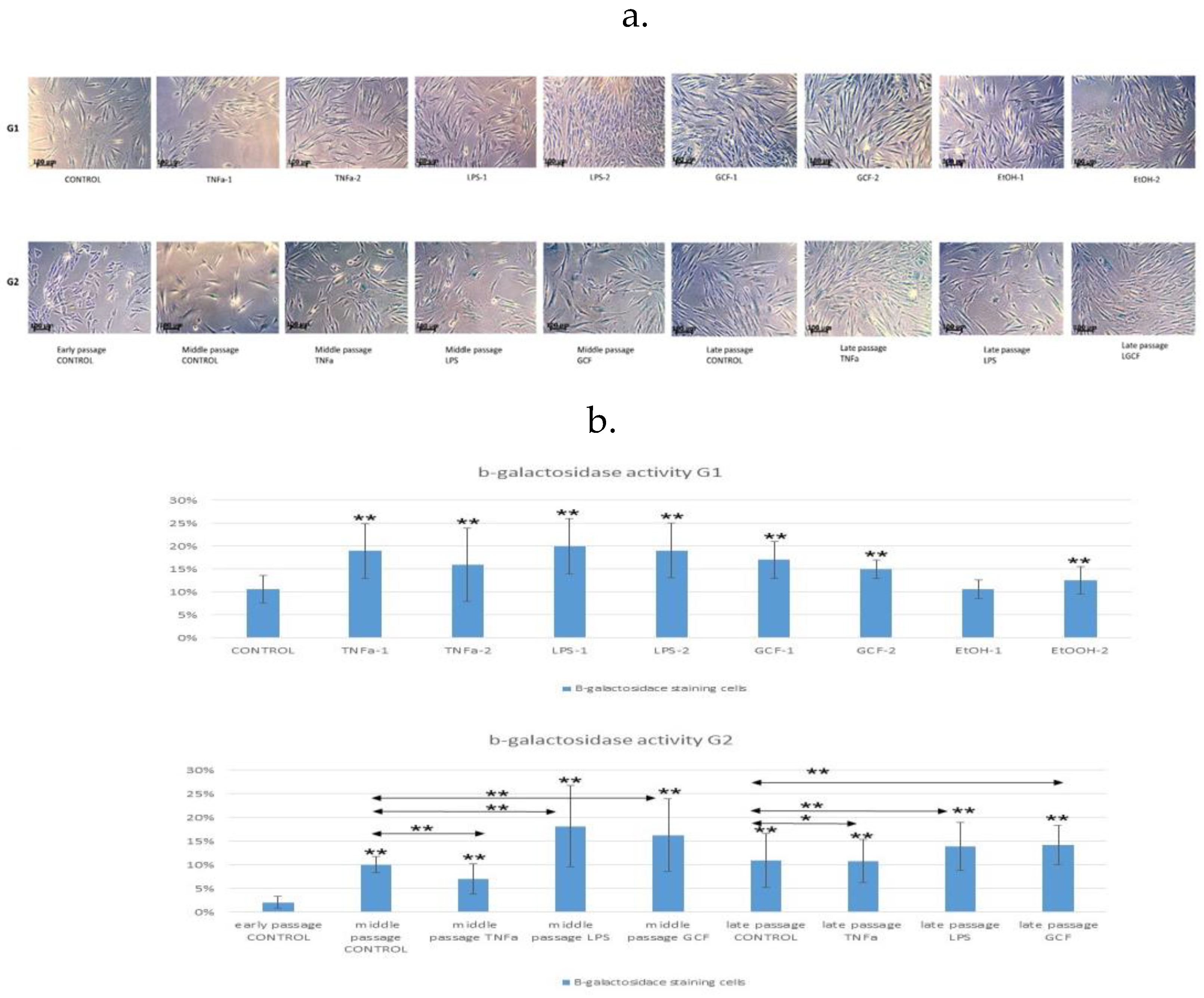
2.1. Senescence-related beta-galactosidase activity
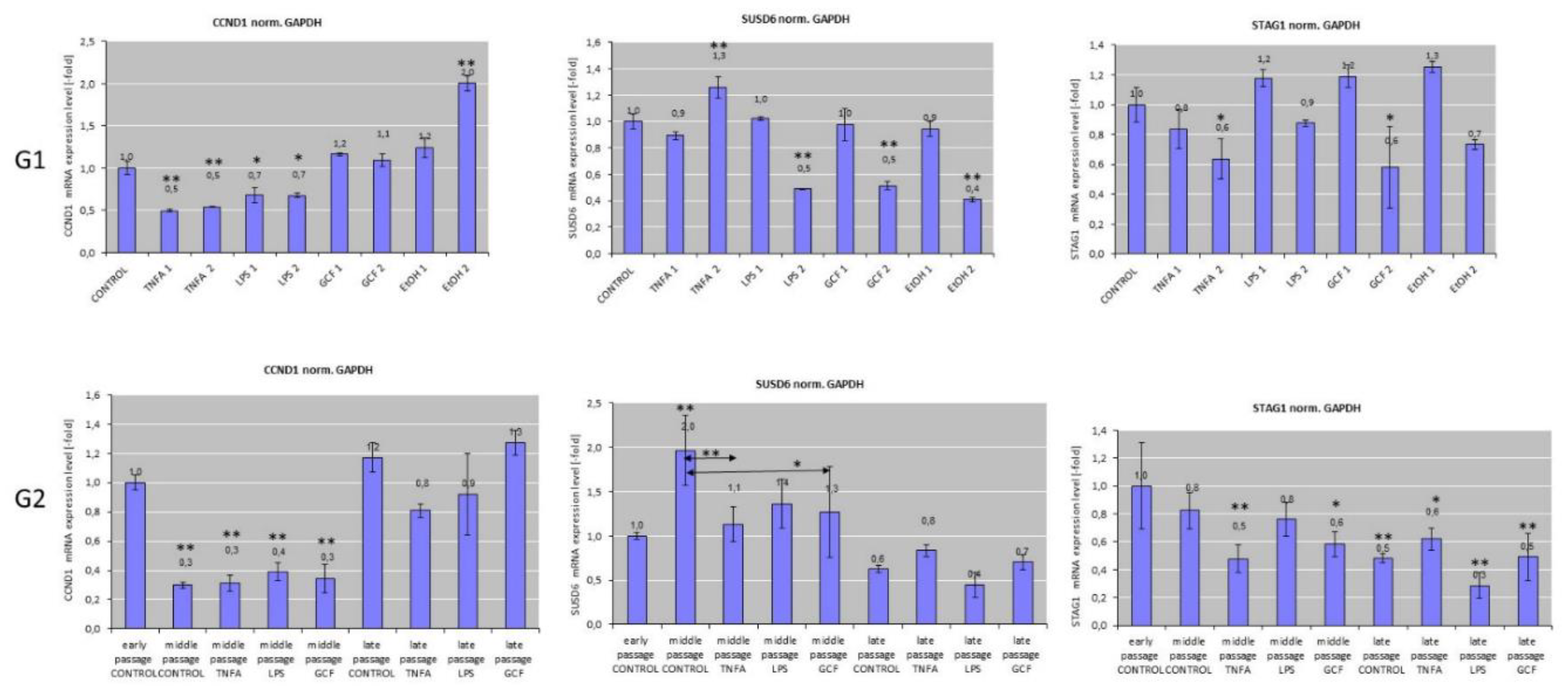
2.2. Senescence-related gene expression patterns
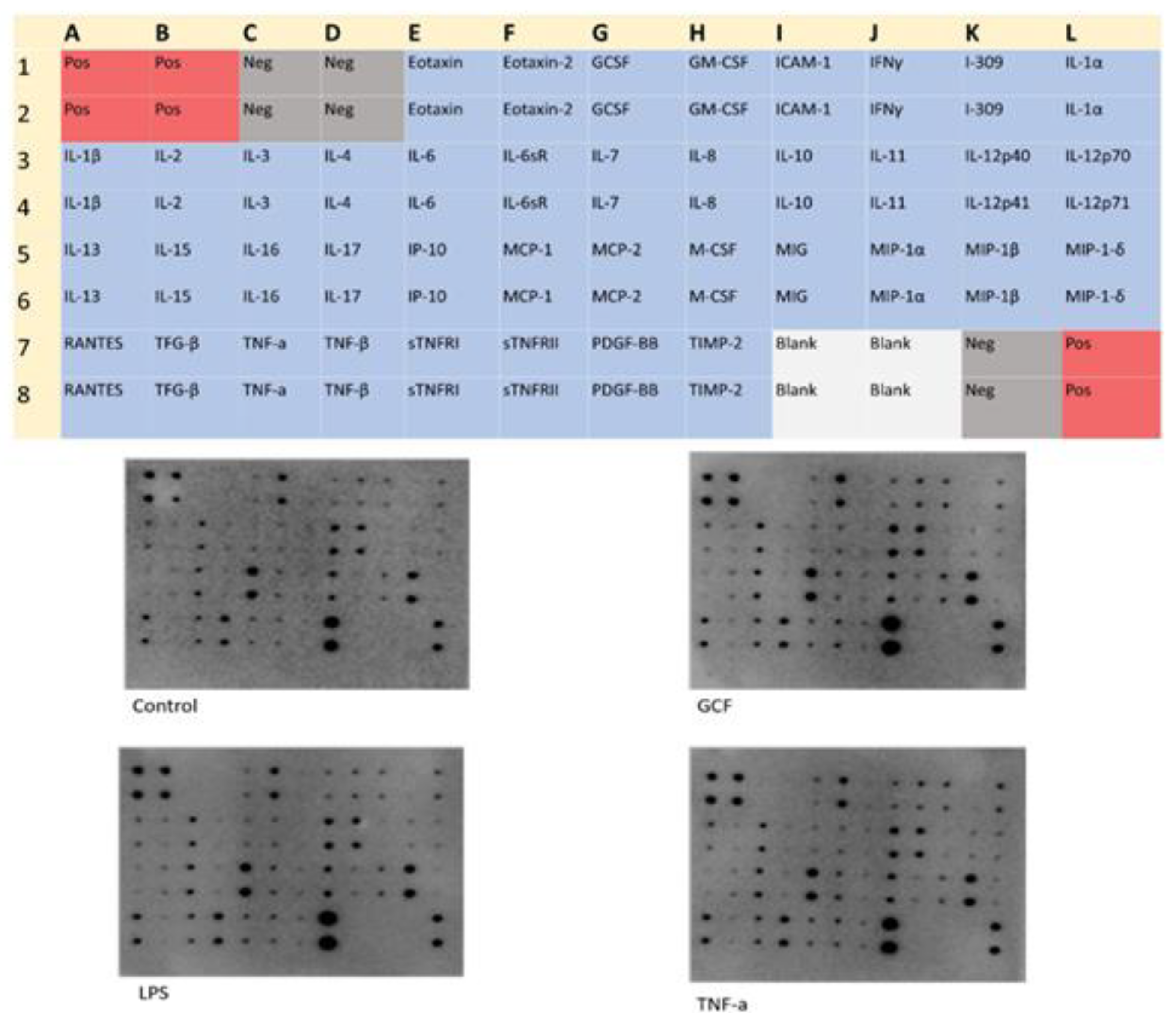
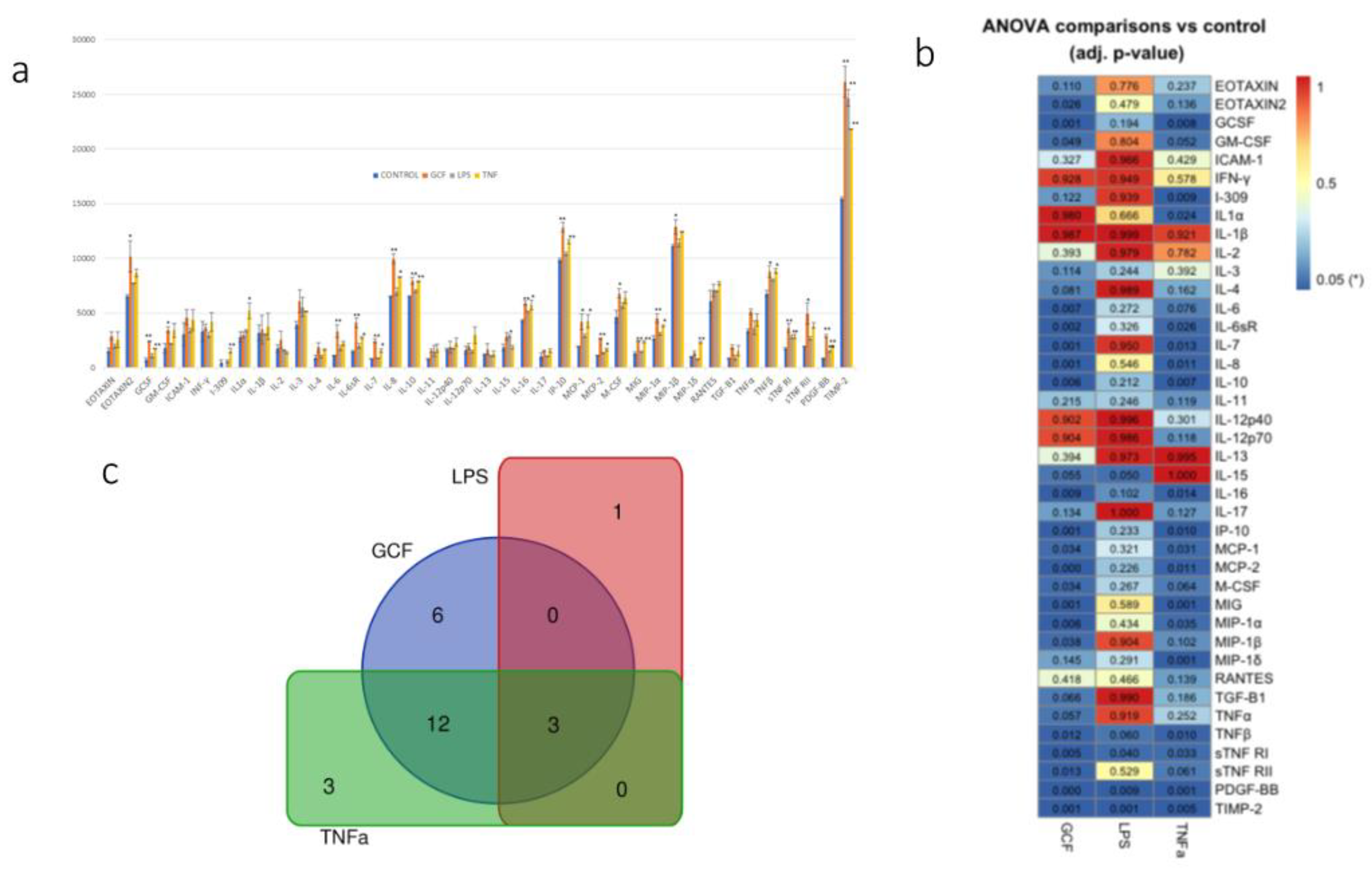
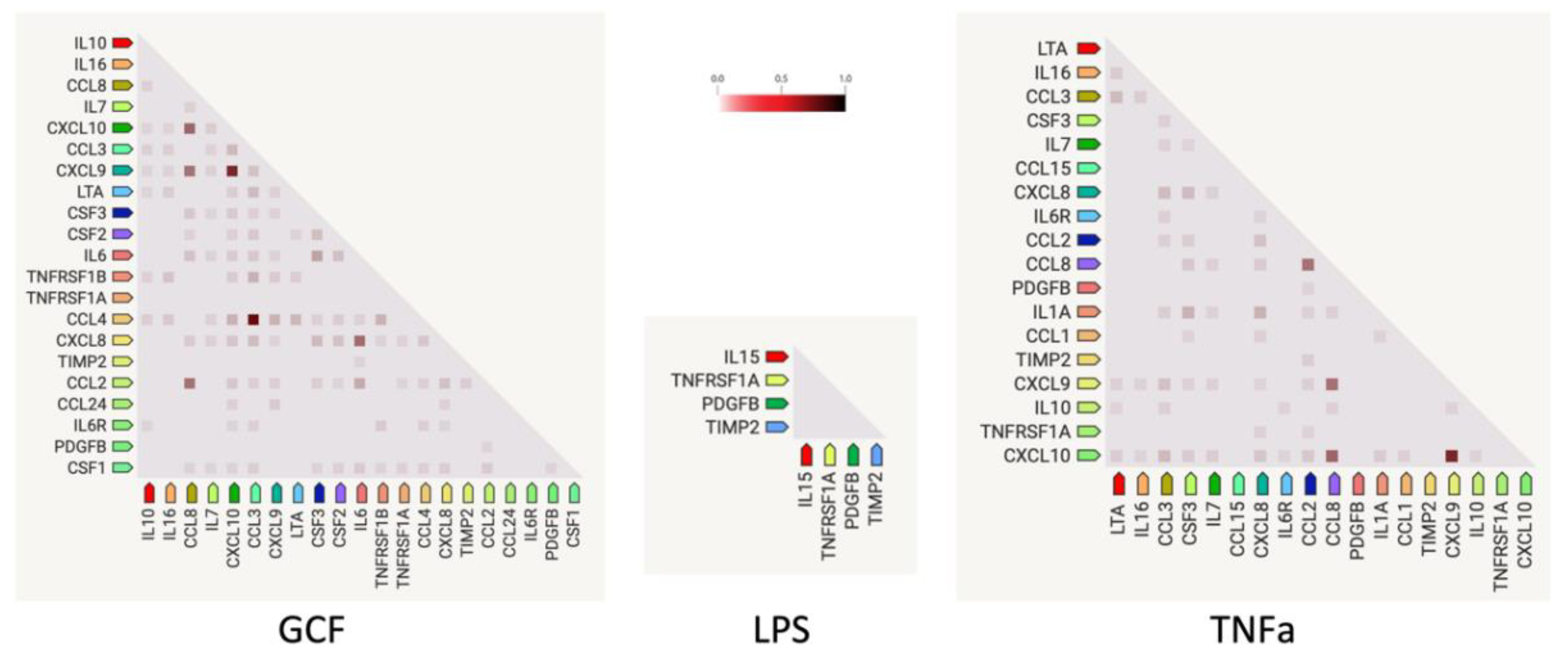
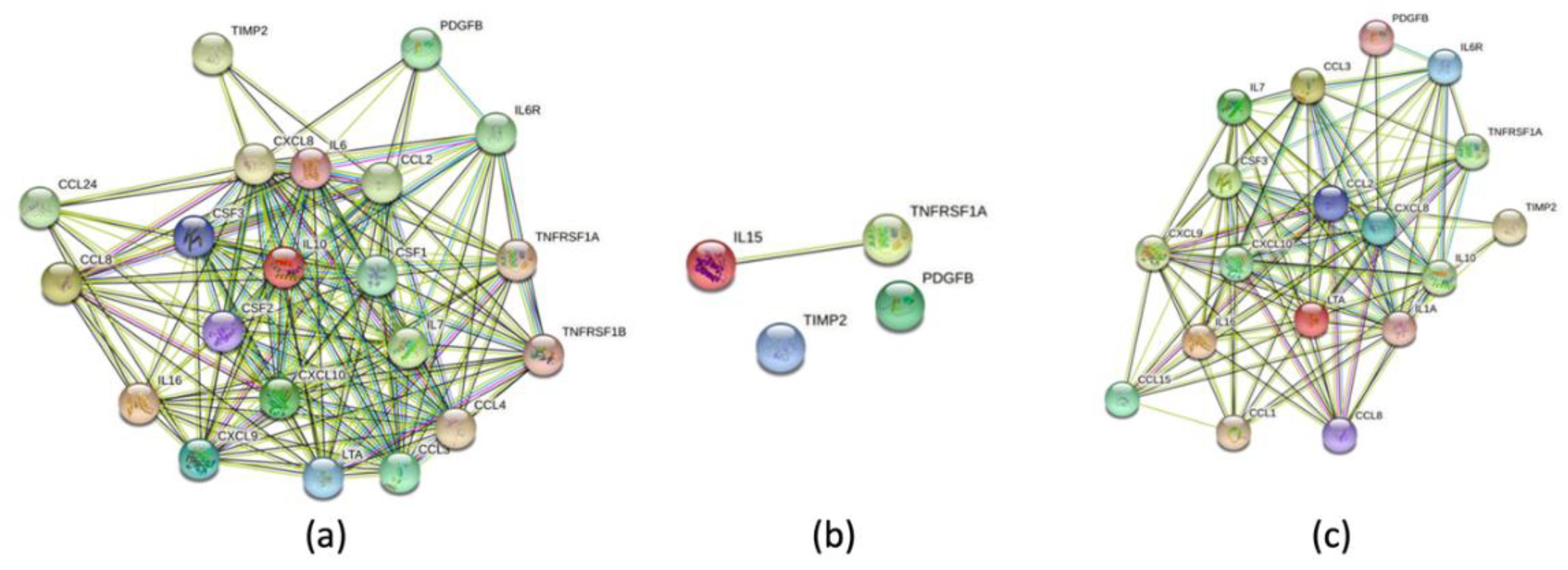
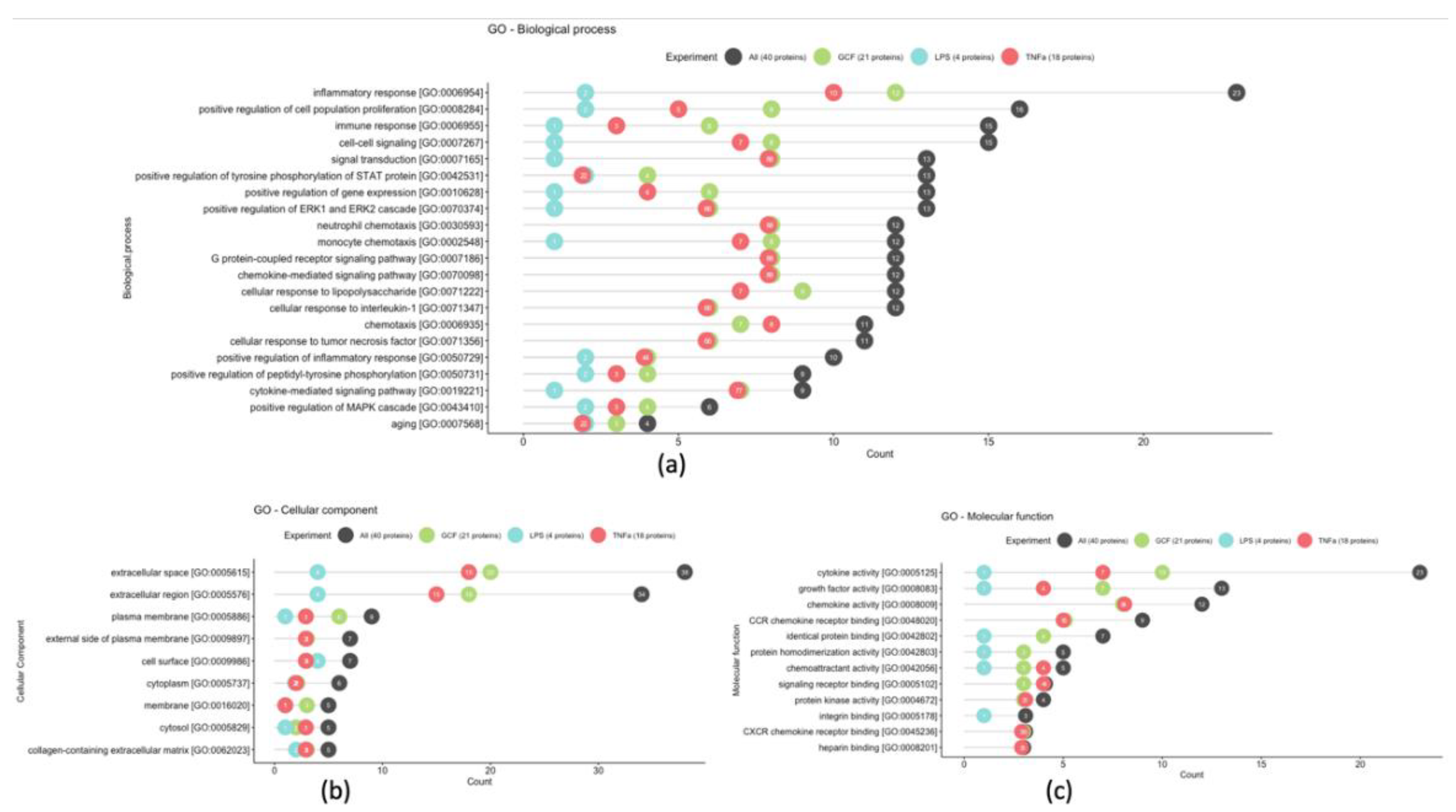
2.3. SASP-related marker expression analysis
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, Shijin, Xinyan Zhang, Songbai Zheng, Ramin Khanabdali, Bill Kalionis, Junzhen Wu, Wenbin Wan, and Xiantao Tai. “An Update on Inflamm-Aging: Mechanisms, Prevention, and Treatment.” J Immunol Res. 2016;2016:8426874. Epub 2016 Jul 14. PMID: 27493973; PMCID: PMC4963991. [CrossRef] [PubMed]
- Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000 Jun;908:244-54. doi: 10.1111/j.1749-6632.2000.tb06651.x. PMID: 10911963. [CrossRef] [PubMed]
- Franceschi, Claudio, Miriam Capri, Daniela Monti, Sergio Giunta, Fabiola Olivieri, Federica Sevini, Maria Panagiota Panourgia, et al. 2007. “Inflammaging and Anti-Inflammaging: A Systemic Perspective on Aging and Longevity Emerged from Studies in Humans.” Mech Ageing Dev. 2007 Jan;128(1):92-105. Epub 2006 Nov 20. PMID: 17116321. [CrossRef] [PubMed]
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013 Mar 2;381(9868):752-62. doi: 10.1016/S0140-6736(12)62167-9. Epub 2013 Feb 8. Erratum in: Lancet. 2013 Oct 19;382(9901):1328. PMID: 23395245; PMCID: PMC4098658. [CrossRef] [PubMed]
- Doyle R, Sadlier DM, Godson C. Pro-resolving lipid mediators: Agents of anti-ageing? Semin Immunol. 2018 Dec;40:36-48. doi: 10.1016/j.smim.2018.09.002. Epub 2018 Oct 4. PMID: 30293857. [CrossRef] [PubMed]
- Anastassiadou V. Older people profile, Chapter e-book. «Management of Complex Conditions in Geriatric Dentistry. Integrated Dental Care for the Elderly» Principal author: Prof. Vasiliki Anastassiadou. Electronic repository of the Action «Hellenic Academic E-Books / Kallipos». 2015 (ISBN: 978-960-603- 244-8). https://repository.kallipos.gr/handle/11419/3356.
- Barnes VM, Kennedy AD, Panagakos F, Devizio W, Trivedi HM, Jönsson T, Guo L, Cervi S, Scannapieco FA. Global metabolomic analysis of human saliva and plasma from healthy and diabetic subjects, with and without periodontal disease. PLoS One. 2014 Aug 18;9(8):e105181. Erratum in: PLoS One. 2014;9(11):e114091. PMID: 25133529; PMCID: PMC4136819. [CrossRef] [PubMed]
- Panagiotakos DB, Milias GA, Pitsavos C, Stefanadis C. MedDietScore: a computer program that evaluates the adherence to the Mediterranean dietary pattern and its relation to cardiovascular disease risk. Comput Methods Programs Biomed. 2006 Jul;83(1):73-7. Epub 2006 Jun 27. PMID: 16806570. [CrossRef] [PubMed]
- Maoyang Lu, Songyu Xuan, Zhao Wang,Oral microbiota: A new view of body health,Food Science and Human Wellness, Issue 1,2019,Volume 8, Pages 8-15,ISSN 2213-4530. [CrossRef]
- Chrysohoou C, Pitsavos C, Panagiotakos DB, Skoumas J, Stefanadis C. Association between prehypertension status and inflammatory markers related to atherosclerotic disease: The ATTICA Study. Am J Hypertens. 2004 Jul;17(7):568-73. PMID: 15233975. [CrossRef] [PubMed]
- Scannapieco FA, Cantos A. Oral inflammation and infection, and chronic medical diseases: implications for the elderly. Periodontol 2000. 2016 Oct;72(1):153-75. [CrossRef] [PubMed]
- Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018 Oct;14(10):576-590. [CrossRef] [PubMed]
- Fulop, T., Larbi, A., Witkowski, J.M. et al. Aging, frailty and age-related diseases. Biogerontology 11, 547–563 (2010). [CrossRef]
- da Costa JP, Vitorino R, Silva GM, Vogel C, Duarte AC, Rocha-Santos T. A synopsis on aging-Theories, mechanisms and future prospects. Ageing Res Rev. 2016 Aug;29:90-112. Epub 2016 Jun 25. PMID: 27353257; PMCID: PMC5991498. [CrossRef] [PubMed]
- Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD, Kirkland JL. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013 May 7;17(5):644-656. PMCID: PMC3942783. [CrossRef] [PubMed]
- Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010 May;16(5):238-46. Epub 2010 May 3. PMID: 20444648; PMCID: PMC2879478. [CrossRef] [PubMed]
- Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007 Feb 8;445(7128):656-60. Epub 2007 Jan 24. Erratum in: Nature. 2011 May 26;473(7348):544. PMID: 17251933; PMCID: PMC4601097. [CrossRef] [PubMed]
- Valery Krizhanovsky, Monica Yon, Ross A. Dickins, Stephen Hearn, Janelle Simon, Cornelius Miething, Herman Yee, Lars Zender, Scott W. Lowe,Senescence of Activated Stellate Cells Limits Liver Fibrosis,Cell, 2008, Issue 4,Volume 134,Pages 657-667,ISSN 0092-8674. [CrossRef]
- Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008 Jan;214(2):149-60. PMID: 18161752. [CrossRef]
- Kalliolias GD, Ivashkiv LB. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol. 2016 Jan;12(1):49-62. Epub 2015 Dec 10. PMID: 26656660; PMCID: PMC4809675. [CrossRef] [PubMed]
- Aquino-Martinez R, Rowsey JL, Fraser DG, Eckhardt BA, Khosla S, Farr JN, Monroe DG. LPS-induced premature osteocyte senescence: Implications in inflammatory alveolar bone loss and periodontal disease pathogenesis. Bone. 2020 Mar;132:115220. Epub 2020 Jan 2. PMID: 31904537; PMCID: PMC6990876. [CrossRef] [PubMed]
- Bozkurt, S.B., Hakki, S.S., Hakki, E.E. et al. Porphyromonas gingivalis Lipopolysaccharide Induces a Pro-inflammatory Human Gingival Fibroblast Phenotype. Inflammation 40, (2017) 144–153. [CrossRef]
- Apatzidou DA, Nile C, Bakopoulou A, Konstantinidis A, Lappin DF. Stem cell-like populations and immunoregulatory molecules in periodontal granulation tissue. J Periodontal Res. 2018 Aug;53(4):610-621. Epub 2018 Apr 23. PMID: 29687448. [CrossRef] [PubMed]
- Subbarao KC, Nattuthurai GS, Sundararajan SK, Sujith I, Joseph J, Syedshah YP. Gingival Crevicular Fluid: An Overview. J Pharm Bioallied Sci. 2019 May;11(Suppl 2):S135-S139. PMCID: PMC6555362. [CrossRef] [PubMed]
- Chen JR, Lazarenko OP, Haley RL, Blackburn ML, Badger TM, Ronis MJ. Ethanol impairs estrogen receptor signaling resulting in accelerated activation of senescence pathways, whereas estradiol attenuates the effects of ethanol in osteoblasts. J Bone Miner Res. 2009 Feb;24(2):221-30. PMCID: PMC3276356. [CrossRef] [PubMed]
- Wyganowska-Świątkowska M, Nowak A, Paszyńska E, Grzech-Lesniak K. Ethanol influence on gingival fibroblasts - a real-time in vitro study. Ann Agric Environ Med. 2018 Dec 20;25(4):647-650. Epub 2017 Nov 15. PMID: 30586975. [CrossRef] [PubMed]
- Bae WJ, Park JS, Kang SK, Kwon IK, Kim EC. Effects of Melatonin and Its Underlying Mechanism on Ethanol-Stimulated Senescence and Osteoclastic Differentiation in Human Periodontal Ligament Cells and Cementoblasts. Int J Mol Sci. 2018 Jun 12;19(6):1742. PMCID: PMC6032161. [CrossRef] [PubMed]
- Yang H, Hu C, Li F, Liang L, Liu L. Effect of lipopolysaccharide on the biological characteristics of human skin fibroblasts and hypertrophic scar tissue formation. IUBMB Life. 2013 Jun;65(6):526-32. Epub 2013 May 7. PMID: 23653386. [CrossRef] [PubMed]
- Mountziaris PM, Tzouanas SN, Mikos AG. Dose effect of tumor necrosis factor-alpha on in vitro osteogenic differentiation of mesenchymal stem cells on biodegradable polymeric microfiber scaffolds. Biomaterials. 2010 Mar;31(7):1666-75. PMCID: PMC2813987. [CrossRef] [PubMed]
- HAYFLICK L, MOORHEAD PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961 Dec;25:585-621. [CrossRef] [PubMed]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995 Sep 26;92(20):9363-7. PMCID: PMC40985. [CrossRef] [PubMed]
- Suzuki, K.; Susaki, E.A.; Nagaoka, I. Lipopolysaccharides and Cellular Senescence: Involvement in Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 11148. [CrossRef]
- Nozu A, Hamano S, Tomokiyo A, Hasegawa D, Sugii H, Yoshida S, Mitarai H, Taniguchi S, Wada N, Maeda H. Senescence and odontoblastic differentiation of dental pulp cells. J Cell Physiol. 2018 Jan;234(1):849-859. [CrossRef] [PubMed]
- Liu J, Zeng J, Wang X, Zheng M, Luan Q. P53 mediates lipopolysaccharide-induced inflammation in human gingival fibroblasts. J Periodontol. 2018 Sep;89(9):1142-1151. [CrossRef] [PubMed]
- Feng G, Zheng K, Cao T, Zhang J, Lian M, Huang D, Wei C, Gu Z, Feng X. Repeated stimulation by LPS promotes the senescence of DPSCs via TLR4/MyD88-NF-κB-p53/p21 signaling. Cytotechnology. 2018 Jun;70(3):1023-1035. PMCID: PMC6021280. [CrossRef] [PubMed]
- Aquino-Martinez R, Rowsey JL, Fraser DG, Eckhardt BA, Khosla S, Farr JN, Monroe DG. LPS-induced premature osteocyte senescence: Implications in inflammatory alveolar bone loss and periodontal disease pathogenesis. Bone. 2020 Mar;132:115220. PMCID: PMC6990876. [CrossRef] [PubMed]
- Kim CO, Huh AJ, Han SH, Kim JM. Analysis of cellular senescence induced by lipopolysaccharide in pulmonary alveolar epithelial cells. Arch Gerontol Geriatr. 2012 Mar-Apr;54(2):e35-41. [CrossRef] [PubMed]
- Aquino-Martinez R, Rowsey JL, Fraser DG, Eckhardt BA, Khosla S, Farr JN, Monroe DG. LPS-induced premature osteocyte senescence: Implications in inflammatory alveolar bone loss and periodontal disease pathogenesis. Bone. 2020 Mar;132:115220. PMCID: PMC6990876. [CrossRef] [PubMed]
- Wang H, Fu H, Zhu R, Wu X, Ji X, Li X, Jiang H, Lin Z, Tang X, Sun S, Chen J, Wang X, Li Q, Ji Y, Chen H. BRD4 contributes to LPS-induced macrophage senescence and promotes progression of atherosclerosis-associated lipid uptake. Aging (Albany NY). 2020 May 11;12(10):9240-9259. PMCID: PMC7288959. [CrossRef] [PubMed]
- Dumont P, Balbeur L, Remacle J, Toussaint O. Appearance of biomarkers of in vitro ageing after successive stimulation of WI-38 fibroblasts with IL-1alpha and TNFalpha: senescence associated beta-galactosidase activity and morphotype transition. J Anat. 2000 Nov;197 Pt 4(Pt 4):529-37. PMCID: PMC1468167. [CrossRef] [PubMed]
- Mavrogonatou E, Konstantinou A, Kletsas D. Long-term exposure to TNF-α leads human skin fibroblasts to a p38 MAPK- and ROS-mediated premature senescence. Biogerontology. 2018 Jul;19(3-4):237-249. [CrossRef] [PubMed]
- Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, et al. (2008) Replicative Senescence of Mesenchymal Stem Cells: A Continuous and Organized Process. PLoS ONE 3(5): e2213. [CrossRef]
- Wagner W, Bork S, Horn P, Krunic D, Walenda T, Diehlmann A, et al. (2009) Aging and Replicative Senescence Have Related Effects on Human Stem and Progenitor Cells. PLoS ONE 4(6): e5846. https://doi.org/10.1371/journal.pone.0005846. [CrossRef]
- Noren Hooten N, Evans MK. Techniques to Induce and Quantify Cellular Senescence. J Vis Exp. 2017 May 1;(123):55533. PMCID: PMC5565152. [CrossRef] [PubMed]
- Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc. 2009;4(12):1798-806. [CrossRef] [PubMed]
- Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007 Sep;8(9):729-40. [CrossRef] [PubMed]
- GeneCards: The Human Gene Database. https://www.genecards.org/.
- Hernandez-Segura A, de Jong TV, Melov S, Guryev V, Campisi J, Demaria M. Unmasking Transcriptional Heterogeneity in Senescent Cells. Curr Biol. 2017 Sep 11;27(17):2652-2660.e4. PMCID: PMC5788810. [CrossRef] [PubMed]
- Hernandez-Segura A, Rubingh R, Demaria M. Identification of stable senescence-associated reference genes. Aging Cell. 2019 Apr;18(2):e12911. PMCID: PMC6413663. [CrossRef] [PubMed]
- Kim YM, Byun HO, Jee BA, Cho H, Seo YH, Kim YS, Park MH, Chung HY, Woo HG, Yoon G. Implications of time-series gene expression profiles of replicative senescence. Aging Cell. 2013 Aug;12(4):622-34. Epub 2013 May 7. Erratum in: Aging Cell. 2020 May;19(5):e13165. PMID: 23590226. [CrossRef] [PubMed]
- Marthandan S, Priebe S, Baumgart M, Groth M, Cellerino A, Guthke R, Hemmerich P, Diekmann S. Similarities in Gene Expression Profiles during In Vitro Aging of Primary Human Embryonic Lung and Foreskin Fibroblasts. Biomed Res Int. 2015;2015:731938. PMCID: PMC4538583. [CrossRef] [PubMed]
- Marthandan S, Baumgart M, Priebe S, Groth M, Schaer J, Kaether C, et al. (2016) Conserved Senescence Associated Genes and Pathways in Primary Human Fibroblasts Detected by RNA-Seq. PLoS ONE 11(5): e0154531. [CrossRef]
- Fukami J, Anno K, Ueda K, Takahashi T, Ide T. Enhanced expression of cyclin D1 in senescent human fibroblasts. Mech Ageing Dev. 1995 Jul 14;81(2-3):139-57. [CrossRef] [PubMed]
- Leontieva OV, Demidenko ZN, Blagosklonny MV. MEK drives cyclin D1 hyperelevation during geroconversion. Cell Death Differ. 2013 Sep;20(9):1241-9. PMCID: PMC3741510. [CrossRef] [PubMed]
- Huang CJ, Yang SH, Huang SM, Lin CM, Chien CC, Chen YC, Lee CL, Wu HH, Chang CC. A predicted protein, KIAA0247, is a cell cycle modulator in colorectal cancer cells under 5-FU treatment. J Transl Med. 2011 May 28;9:82. PMCID: PMC3126726. [CrossRef] [PubMed]
- Polato F, Rusconi P, Zangrossi S, Morelli F, Boeri M, Musi A, Marchini S, Castiglioni V, Scanziani E, Torri V, Broggini M. DRAGO (KIAA0247), a new DNA damage-responsive, p53-inducible gene that cooperates with p53 as oncosuppressor. [Corrected]. J Natl Cancer Inst. 2014 Apr;106(4):dju053. Epub 2014 Mar 20. Erratum in: J Natl Cancer Inst. 2014 Aug;106(8). PMID: 24652652; PMCID: PMC3988459. [CrossRef] [PubMed]
- Chen Z, Dodig-Crnković T, Schwenk JM, Tao SC. Current applications of antibody microarrays. Clin Proteomics. 2018 Feb 28;15:7. [CrossRef] [PubMed]
- Bostanci, Nagihan & Belibasakis, Georgios. Gingival crevicular fluid and its immune mediators in the proteomic era. Periodontology 2000. (2017). 76. [CrossRef]
- Subbarao KC, Nattuthurai GS, Sundararajan SK, Sujith I, Joseph J, Syedshah YP. Gingival Crevicular Fluid: An Overview. J Pharm Bioallied Sci. 2019 May;11(Suppl 2):S135-S139. PMCID: PMC6555362. [CrossRef] [PubMed]
- Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006 Jun;61(6):575-84. PMCID: PMC2645627. [CrossRef] [PubMed]
- Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front Immunol. 2018 Apr 9;9:586. PMCID: PMC5900450. [CrossRef] [PubMed]
- John E. Morley, Richard N. Baumgartner, Cytokine-Related Aging Process, The Journals of Gerontology:September 2004, Series A, Volume 59, Issue 9,Pages M924–M929. [CrossRef]
- Gong X, Gong W, Kuhns DB, Ben-Baruch A, Howard OM, Wang JM. Monocyte chemotactic protein-2 (MCP-2) uses CCR1 and CCR2B as its functional receptors. J Biol Chem. 1997 May 2;272(18):11682-5. [CrossRef] [PubMed]
- Yousefzadeh MJ, Schafer MJ, Noren Hooten N, Atkinson EJ, Evans MK, Baker DJ, Quarles EK, Robbins PD, Ladiges WC, LeBrasseur NK, Niedernhofer LJ. Circulating levels of monocyte chemoattractant protein-1 as a potential measure of biological age in mice and frailty in humans. Aging Cell. 2018 Apr;17(2):e12706. PMCID: PMC5847863. [CrossRef] [PubMed]
- Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, Yamauchi T, Ueki K, Oishi Y, Nishimura S, Manabe I, Hashimoto H, Ohnishi Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Nagai R, Kadowaki T. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006 Sep 8;281(36):26602-14. Epub 2006 Jun 29. PMID: 16809344. [CrossRef] [PubMed]
- El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007 Apr;13(4):432-8. [CrossRef] [PubMed]
- Singh S, Anshita D, Ravichandiran V. MCP-1: Function, regulation, and involvement in disease. Int Immunopharmacol. 2021 Dec;101(Pt B):107598. PMCID: PMC8135227. [CrossRef] [PubMed]
- Bettcher BM, Neuhaus J, Wynn MJ, Elahi FM, Casaletto KB, Saloner R, Fitch R, Karydas A, Kramer JH. Increases in a Pro-inflammatory Chemokine, MCP-1, Are Related to Decreases in Memory Over Time. Front Aging Neurosci. 2019 Feb 13;11:25. PMCID: PMC6381047. [CrossRef] [PubMed]
- Zhou C, Gao Y, Ding P, Wu T, Ji G. The role of CXCL family members in different diseases. Cell Death Discov. 2023 Jul 1;9(1):212. PMCID: PMC10314943. [CrossRef] [PubMed]
- Silva TA, Garlet GP, Fukada SY, Silva JS, Cunha FQ. Chemokines in oral inflammatory diseases: apical periodontitis and periodontal disease. J Dent Res. 2007 Apr;86(4):306-19. [CrossRef] [PubMed]
- Prasad G, McCullough M. Chemokines and cytokines as salivary biomarkers for the early diagnosis of oral cancer. Int J Dent. 2013;2013:813756. PMCID: PMC3860143. [CrossRef] [PubMed]
- Sahingur SE, Yeudall WA. Chemokine function in periodontal disease and oral cavity cancer. Front Immunol. 2015 May 5;6:214. PMCID: PMC4419853. [CrossRef]
- Sarode, Gargi & Sarode, Sachin & Patil, Shankargauda & Yadahalli, Roopa. Significance of CC Group of Chemokines in Oral Squamous Cell Carcinoma and Oral Potential Malignant Disorders: A Review. World Journal of Dentistry. (2021). 12. 160-165. [CrossRef]
- Bakopoulou A, Leyhausen G, Volk J, Tsiftsoglou A, Garefis P, Koidis P, Geurtsen W. Assessment of the impact of two different isolation methods on the osteo/odontogenic differentiation potential of human dental stem cells derived from deciduous teeth. Calcif Tissue Int. 2011 Feb;88(2):130-41. [CrossRef] [PubMed]
- Bakopoulou A, Apatzidou D, Aggelidou E, Gousopoulou E, Leyhausen G, Volk J, Kritis A, Koidis P, Geurtsen W. Isolation and prolonged expansion of oral mesenchymal stem cells under clinical-grade, GMP-compliant conditions differentially affects "stemness" properties. Stem Cell Res Ther. 2017 Nov 2;8(1):247. PMCID: PMC5667471. [CrossRef] [PubMed]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012 Jul;9(7):671-5; PMCID: PMC5554542. [CrossRef] [PubMed]
- UniProt Consortium. UniProt: the Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023 Jan 6;51(D1):D523-D531. PMCID: PMC9825514. [CrossRef] [PubMed]
- Szklarczyk D, Gable AL, Nastou KC, Lyon D, Kirsch R, Pyysalo S, Doncheva NT, Legeay M, Fang T, Bork P, Jensen LJ, von Mering C. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021 Jan 8;49(D1):D605-D612. Erratum in: Nucleic Acids Res. 2021 Oct 11;49(18):10800. PMID: 33237311; PMCID: PMC7779004. [CrossRef] [PubMed]
|
Gene
symbol |
Forward (5’-3’) | Reverse (5’-3’) |
Amplicon size (bp) |
|---|---|---|---|
| CCND1 | AGCTGTGCATCTACACCGAC | GAAATCGTGCGGGGTCATTG | 113 |
| SUSD6 | TTAGCTGCCGTCTCAACGAG | CTGGTCACGCCTGCTATGAT | 170 |
| STAG1 | GATTGCAGCTCCGTTGAAGG | GCCGACCATCGACCTAGTTT | 125 |
| GAPDH | GACAGTCAGCCGCATCTTCT | GCGCCCAATACGACCAAATC | 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





