Background
A recent study published in Nature Medicine (Mautner et al., Nat Med 2023 Nov 2. doi: 10.1038/s41591-023-02632-w. Epub ahead of print) reported findings regarding a four-arm parallel, multicenter, single-blind, randomized, controlled clinical trial with 480 patients, aimed at comparing the therapeutic effectiveness of orthobiologic interventions against corticosteroid injections for the treatment of knee osteoarthritis (OA). The study concluded that, one-year post-treatment, all orthobiologic therapies examined were equivalent in effectiveness to each other and to corticosteroid injections. While we commend the scope and methodological rigor of the trial, the publication could be enhanced by a more comprehensive presentation of data to support the conclusions drawn. Particularly, the omission of baseline data for primary outcomes, the missing context to evaluate absolute values, the lack of discussion regarding interindividual variability, and the unexpectedly favorable results for corticosteroids that contrast the established literature, are areas that merit additional examination and insight. Here we aim to highlight these points for consideration, and advocate for the release of supplementary data to facilitate a comprehensive understanding and utilization of this rich dataset.
On 02 November 2023 Mautner et al.1 reported in Nature Medicine the results of a four-arm parallel, multicenter, single-blind, randomized, controlled clinical trial with 480 patients with a diagnosis of knee osteoarthritis (OA) (Kellgren-Lawrence (KL) grade II-IV). Patients were treated with a single injection of respectively autologous bone marrow aspirate concentrate (BMAC arm), autologous adipose stromal vascular fraction (SVF arm), allogeneic human umbilical cord tissue-derived mesenchymal stromal cells (hUC-MSCs arm) or corticosteroid (CSI arm). The co-primary endpoints were the Visual Analog Scale (VAS) Pain score and Knee injury and Osteoarthritis Outcome Score (KOOS) Pain score at 12 months post-treatment (M12) versus baseline. With respect to efficacy the key outcome of the study by Mautner et al.1 was that at M12, there was no superior orthobiologic as compared to CSI for knee osteoarthritis.
As outlined in the following, the manner in which Mautner et al.1 presented and discussed their results raises more questions than provides answers regarding the true value of the investigated treatments for management of knee OA.
First, the authors did not provide baseline data of the co-primary endpoints (i.e., baseline VAS Pain scores and baseline KOOS Pain scores). However, next to age, gender, and BMI (shown in Table 1 in Mautner et al.1) global knee pain, function of knee and duration since onset of symptoms indicating knee OA are considered relevant baseline characteristics for describing patients with knee OA2.
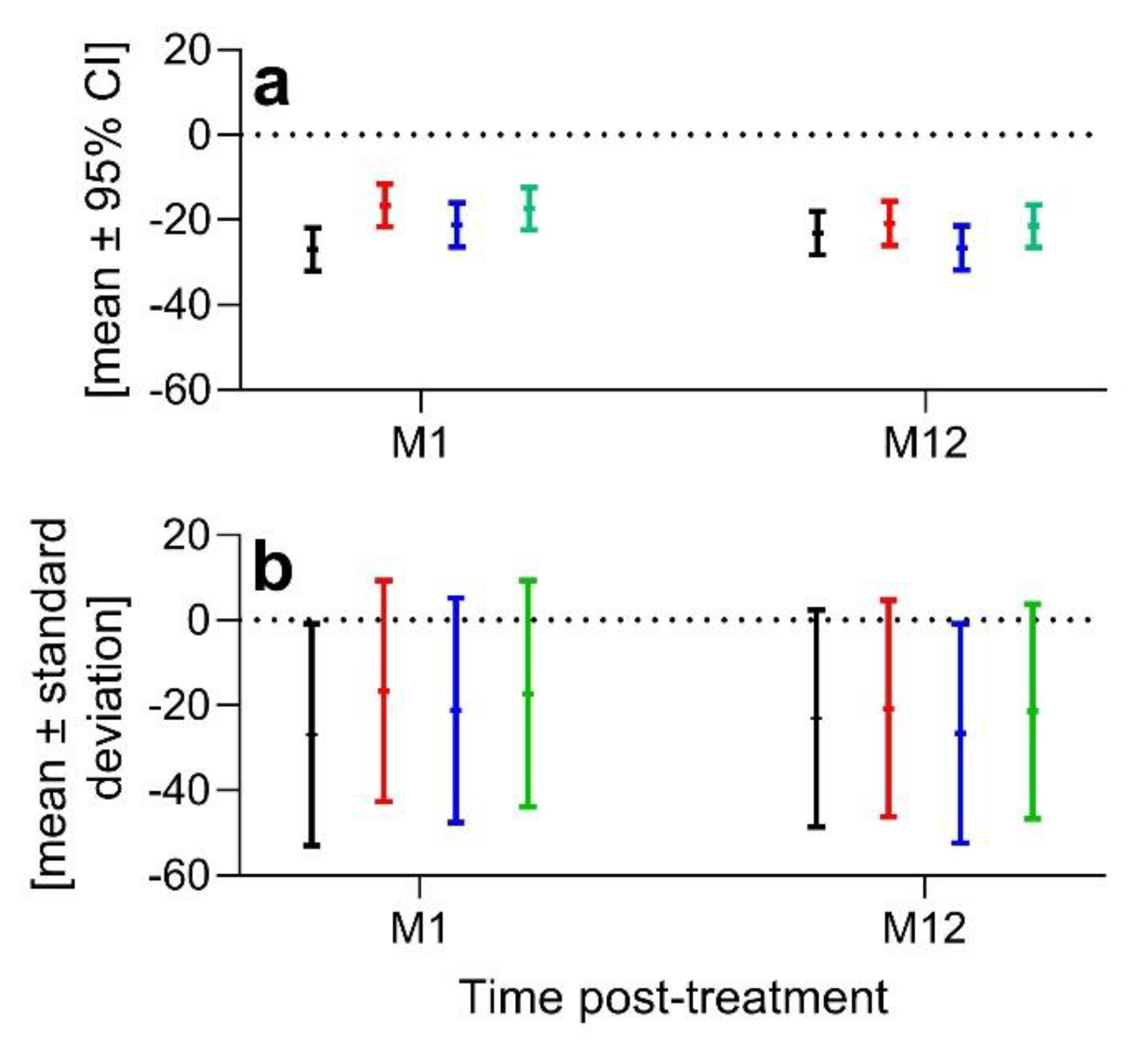
Second, without reporting the baseline data of the co-primary endpoints, the relevance of the primary outcome measures over time shown in Figure 2 in Mautner et al.1 cannot be assessed. We assume that the data shown in Figure 2 in Mautner et al.1 represents absolute values, as it is stated in the Statistical Analysis section that absolute change from baseline in VAS pain score and KOOS pain score were derived from imputed scores, however this was not explicitly stated in the legend of Figure 2 in Mautner et al.1. This information provides necessary context, because a mean improvement of the VAS Pain score by 25 (on a scale of 0 to 100, where 0 indicates no pain and 100 indicates maximum pain) has different relevance depending on whether the baseline mean VAS Pain score was 30 or 80. Furthermore, comparison of absolute values of primary outcome measures over time as shown in Figure 2 in Mautner et al.1 would only make sense if the baseline data of the different treatment arms were comparable.
Third, Mautner et al.
1 did not discuss the substantial interindividual variability of the VAS Pain score and KOOS Pain score data of the patients enrolled and the possible impact of this interindividual variability on the outcome of their study. The data shown in Figure 2 in Mautner et al.
1 (model-based means and 95% confidence intervals (95% CIs) of the primary outcome measures over time) do not allow assessment of the interindividual variability of the baseline co-primary outcome measures, nor do they allow any conclusions to be drawn about patient-specific differences in individual time courses post-treatment. Of note, in the main text of Mautner et al.
1 the changes in the co-primary outcome measures are described as e.g. "-24.3 ± standard error of the mean (s.e.m.)" at 12 months post-treatment (M12) in the change in VAS Pain score from baseline for BMAC, "-19.4 ± s.e.m." at M12 in the change in VAS Pain score from baseline for SVF, etc. (i.e. the standard error of the mean data were not reported in the study by Mautner et al.
1). Based on the established relationship between the mean, upper limit (UL) and lower limit (LL) of the 95% CI of the mean and the standard deviation (SD) of a given variable, as well as the number of investigated subjects, (n) (
;
) we calculated mean ± SD of the VAS Pain score data reported in the study by Mautner et al.
1 at M1 and M12 post-treatment (
Figure 1).
These data indicate substantial interindividual variability of the VAS Pain score of the patients enrolled and establish the need to evaluate distinct patient groups. This raises the question whether select patient sub-populations experienced different outcomes. Additional sub-group analyses would have provided important information about potential follow-up studies, enhancing its value to other researchers. Although the authors allude to future analyses, the omission of such critical evaluations in the present work could inadvertently create a chilling effect for future research in this field.
Fourth, Mautner et al.
1 did not discuss the unusually positive outcomes observed for corticosteroid treatment (-23.6% at M6 and -20.9 at M12 in the change in mean VAS Pain score from baseline), which substantially deviates from prior research focusing on management of knee OA with corticosteroid injections. For example, Wang et al.
3 reported in 2022 for n= 66 patients with KL score I-IV and a duration of knee OA of 3.6 ± 3.6 years a reduction in mean VAS Pain score from 6.6 ± 1.4 (on a scale of 0 to 10) at baseline to 5.0 ± 2.5 at 12 weeks post-treatment (W12) and 5.6 ± 2.3 at W24 (all data given in mean ± SD). Pretorius et al.
4 found (also in 2022) for n= 29 patients with KL score II-III a reduction in mean VAS Pain score from 6.69 ± 2.17 at baseline to 4.86 ± 3.2 at W13 and 5.97 ± 2.66 at W26 (all data given in mean ± SD). Tschopp et al.
5 reported in 2023 for n= 30 patients with KL score I-III and VAS Pain score of 39.50 [17.75, 47.75] (median [interquartile range] on a scale of 0 to 100) at baseline an increased mean VAS Pain score of approximately +5 on the VAS scale at M3, approximately +2 at M6 and approximately +0.5 at M12. In a systematic review published in 2023, Chang et al.
6 concluded overall negative outcome for the management of knee OA with corticosteroid injection, which is in line with a publication by da Costa et al.
7 in 2016 who reported that intra-articular corticosteroid injection may be associated with moderate improvement in pain and a small improvement in physical function up to 6 weeks after injection. Of note, (i) the interindividual variability in the primary outcome measures reported by Mautner et al.
1 after corticosteroid injection (
Figure 1) appears to be much higher than the interindividual variability reported by Wang et al.
3, Pretorius et al.
4, and Tschopp et al.
5, although this can only be conclusively assessed by re-analyzing the raw data of the study by Mautner et al.
1; (ii) the discrepancy between the results by Mautner et al.
1 and the findings by Wang et al.
3, Pretorius et al.
4 and Tschopp et al.
5 cannot be explained by different corticosteroid doses (Mautner et al.
1: 1 mL of 40mg/mL methylprednisolone acetate; approximate hydrocortisone equivalent (HE;
https://clincalc.com/Corticosteroids/) = 5; Wang et al.
3: 40 mg of triamcinolone (HE = 5); Pretorius et al.
4: 80 mg (2 mL) of methylprednisolone acetate; Tschopp et al.
5: 1 ml of 40 mg/mL triamcinolone); and (iii) the dose “1mL of depomedrol (40mg/dL)” reported in Supplementary Information of the study by Mautner et al.
1 is probably incorrect and was corrected here. Depo-Medrol
® is a registered trademark of Pfizer, Inc. (New York City, NY, USA), is a methylprednisolone acetate injectable suspension, and is available in three strengths: 20 mg/mL, 40 mg/mL and 80 mg/mL (
https://labeling.pfizer.com/ShowLabeling.aspx?id =551). A dose of “1mL of depomedrol (40mg/dL),” as stated by Mautner et al.
1, would imply that the authors would have applied a hydrocortisone equivalent that would have been 100 times smaller than what was applied by Wang et al.
3 and Tschopp et al.
5, and 50 times smaller than what was applied by Pretorius et al.
4. Considering the recent literature, it is logically consistent to discuss concomitant factors that could have played a role in the unexpectedly favorable outcomes for corticosteroid treatment in the study by Mautner et al.
1. Specifically, approximately one third of the patients who received corticosteroid injection were subjected to a bone marrow aspiration. In this regard a recent pilot study found that transverse distraction osteogenesis can augment healing of distally located wounds
8. It is currently unknown whether the procedure of bone marrow aspiration itself has a positive effect on pain perception related to knee OA. However, by reporting subgroup-specific mean and SD at baseline and the different follow-up time points of the three subgroups of patients that were treated with corticosteroid injection (CSI patients) in the study by Mautner et al.
1 , the authors could have demonstrated that such potential concomitant factors did not play a role in their findings, justifying the pooling of three subgroups of CSI patients into a single CSI arm. With in-depth insights into the study data and study procedures, the authors could have shed light on these surprising findings. The lack of discussion of the study's findings in the context of the related, published body of work deprives the reader of important context for the interpretation of the study by Mautner et al.
1.