Submitted:
14 November 2023
Posted:
15 November 2023
You are already at the latest version
Abstract
Keywords:
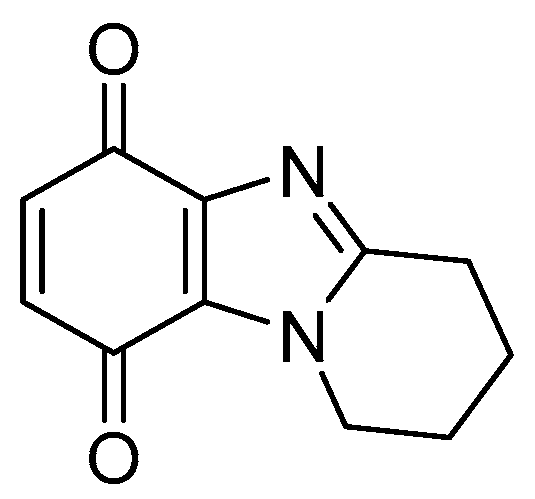
1. Introduction
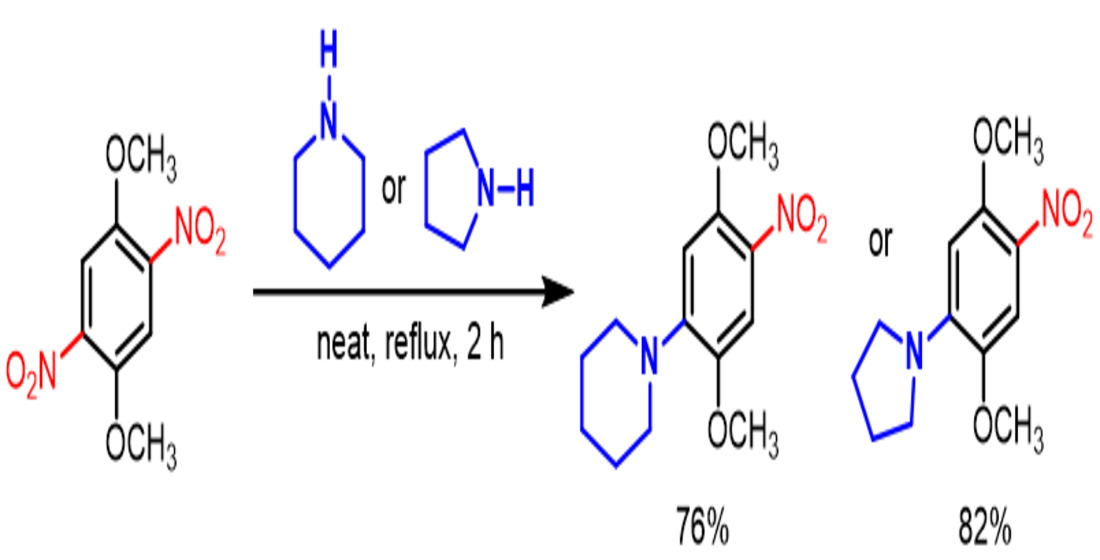
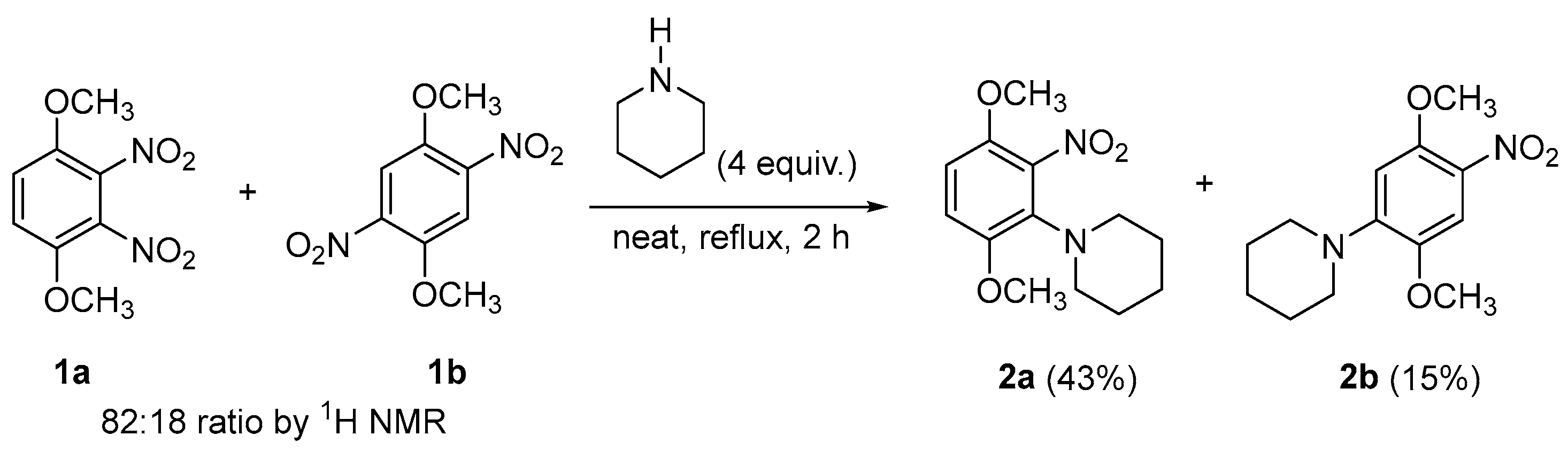
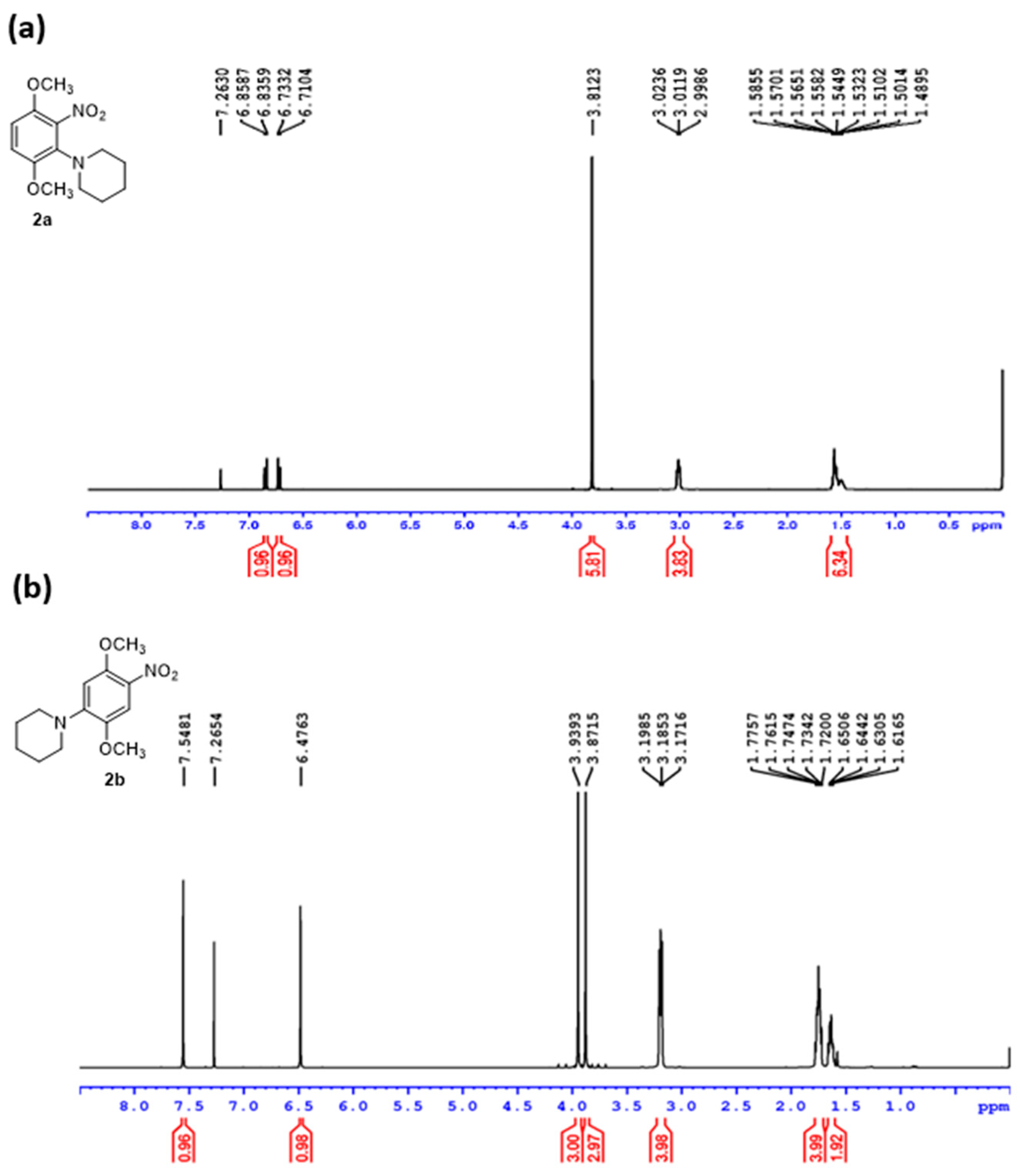
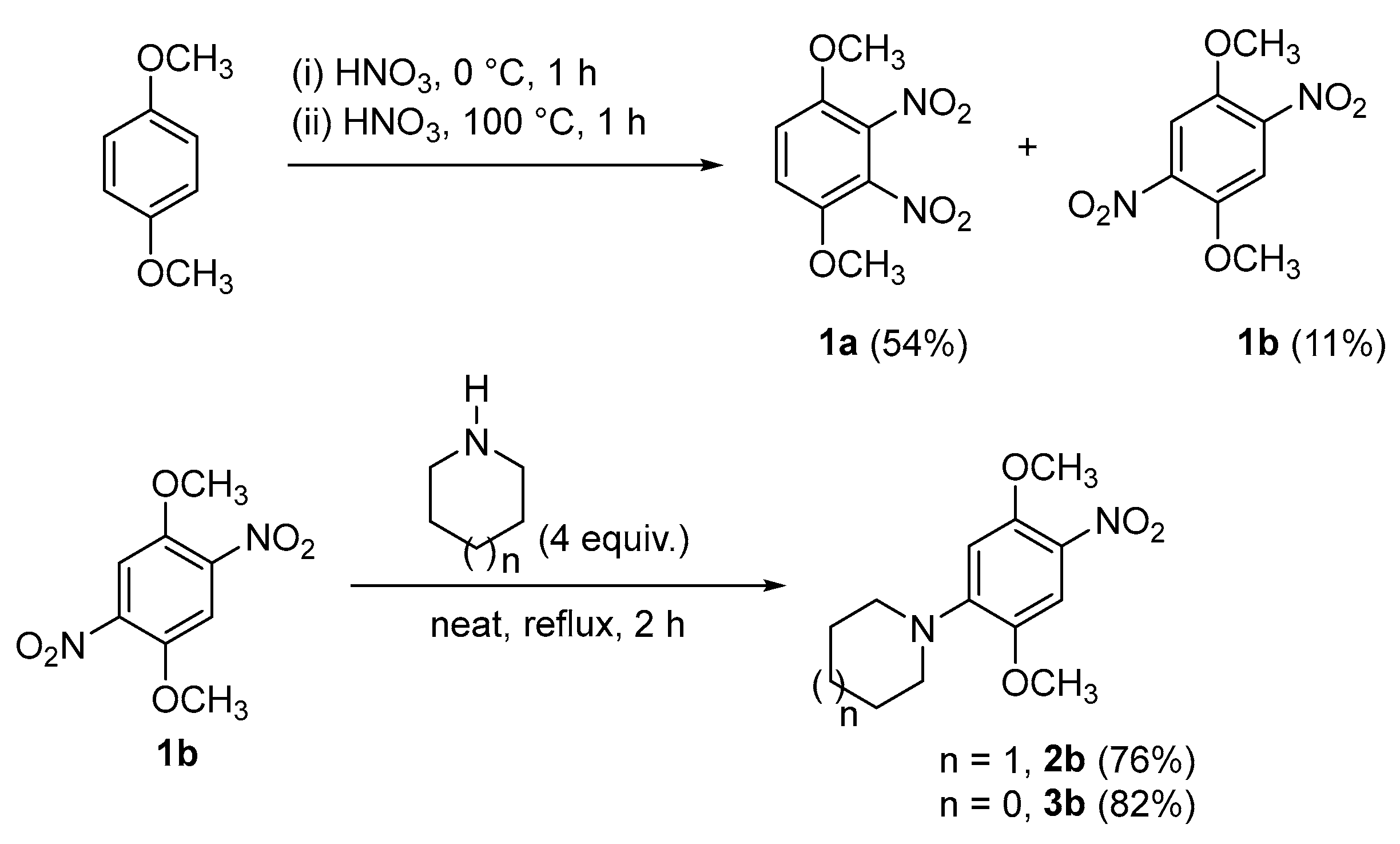
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Measurements
3.2. Synthesis of 1-(2,5-dimethoxy-4-nitrophenyl)piperidine (2b)
3.3. Synthesis of 1-(2,5-dimethoxy-4-nitrophenyl)pyrrolidine (3b)
Supplementary Materials
Author Contributions
Acknowledgements
Conflicts of Interest
References
- Haji, N.; Faizi, M.; Koutentis, P. A.; Carty, M. P.; Aldabbagh, F. Heterocyclic iminoquinones and quinones from the National Cancer Institute (NCI, USA) COMPARE Analysis. Molecules 2023, 28, 5202. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.; Conboy, D.; Mirallai, S. I.; Aldabbagh, F. Advances in the synthesis of ring-fused benzimidazoles and imidazobenzimidazoles. Molecules 2021, 26, 2684. [Google Scholar] [CrossRef] [PubMed]
- Gellis, A.; Kovacic, H.; Boufatah, N.; Vanelle, P. Synthesis and cytotoxicity evaluation of some benzimidazole-4,7-diones as bioreductive anticancer agents. Eur. J. Med. Chem. 2008, 43, 1858–1864. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, L.; Carty, M. P.; Aldabbagh, F. First synthesis of N-[(aziridin-2-yl)methyl]benzimidazolequinone and analysis of toxicity towards normal and Fanconi anemia cells. Chem. Commun. 2008, 5592–5594. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.; Hehir, S.; Kavanagh, P.; Leech, D.; O'Shaughnessy, J.; Carty, M. P.; Aldabbagh, F. Synthesis by radical cyclization and cytotoxicity of highly potent bioreductive alicyclic ring fused [1,2-a]benzimidazolequinones. Chem. Eur.J. 2007, 13, 3218–3226. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, E.; Carr, M.; Bonham, S.; Carty, M. P.; Aldabbagh, F. Synthesis and toxicity towards normal and cancer cell lines of benzimidazolequinones containing fused aromatic rings and 2-aromatic ring substituents. Eur. J. Med. Chem. 2010, 45, 3762–3769. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.; Gurry, M.; Keane, L. -A. J.; Aldabbagh, F. Greener synthesis using hydrogen peroxide in ethyl acetate of alicyclic ring-fused benzimidazoles and anti-tumour benzimidazolequinones. Tetrahedron Lett. 2017, 58, 3565–3567. [Google Scholar] [CrossRef]
- Shopsowitz, K.; Lelj, J.; MacLachlan, M. J. Regioselectivity in the nitration of dialkoxybenzenes. J. Org. Chem. 2011, 76, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Hammershøj, P.; Reenberg, T. K.; Pittelkow, M.; Nielsen, C. B.; Hammerich, O.; Christensen, J. B. Synthesis and properties of 2,3-dialkynyl-1,4-benzoquinones. Eur. J. Org. Chem. 2006, 2786–2794. [Google Scholar] [CrossRef]
- CAS Registry Number: 71230-77-8, Catalogue Number: CA0784117. Chemieliva Pharmaceutical, Product List, Jiang Bei Chongqing, China. https://www.chemieliva.com/new_content.html?casno=71230-77-8 (accessed 16/10/2023).
- CAS Registry Number: 71230-77-8, Catalogue Number: PR-518937. Atomax Chemicals, Product List, Shenzhen, Guangdong, China. http://en.atomaxchem.com/71230-77-8.html (accessed 16/10/2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).