Submitted:
03 November 2023
Posted:
06 November 2023
You are already at the latest version
Abstract
Keywords:
Introduction
- Overview of Atrial Fibrillation (AF):
- Role of SGLT2 Inhibitors in Cardiovascular Disease Management
- Mechanisms Underlying the Relationship:
- Hemodynamic Effects:
- 1.Blood Pressure Modulation:
- 2.Reduction in Extracellular Volume:
- Electrophysiological Changes:
- 1. Atrial Repolarization:
- 2. Intracellular Calcium Handling:
- Metabolic and Renal Factors:
- 1. Glucose Control:
- 2. Lipid Profile Modification:
- 3. Renal Hemodynamics:
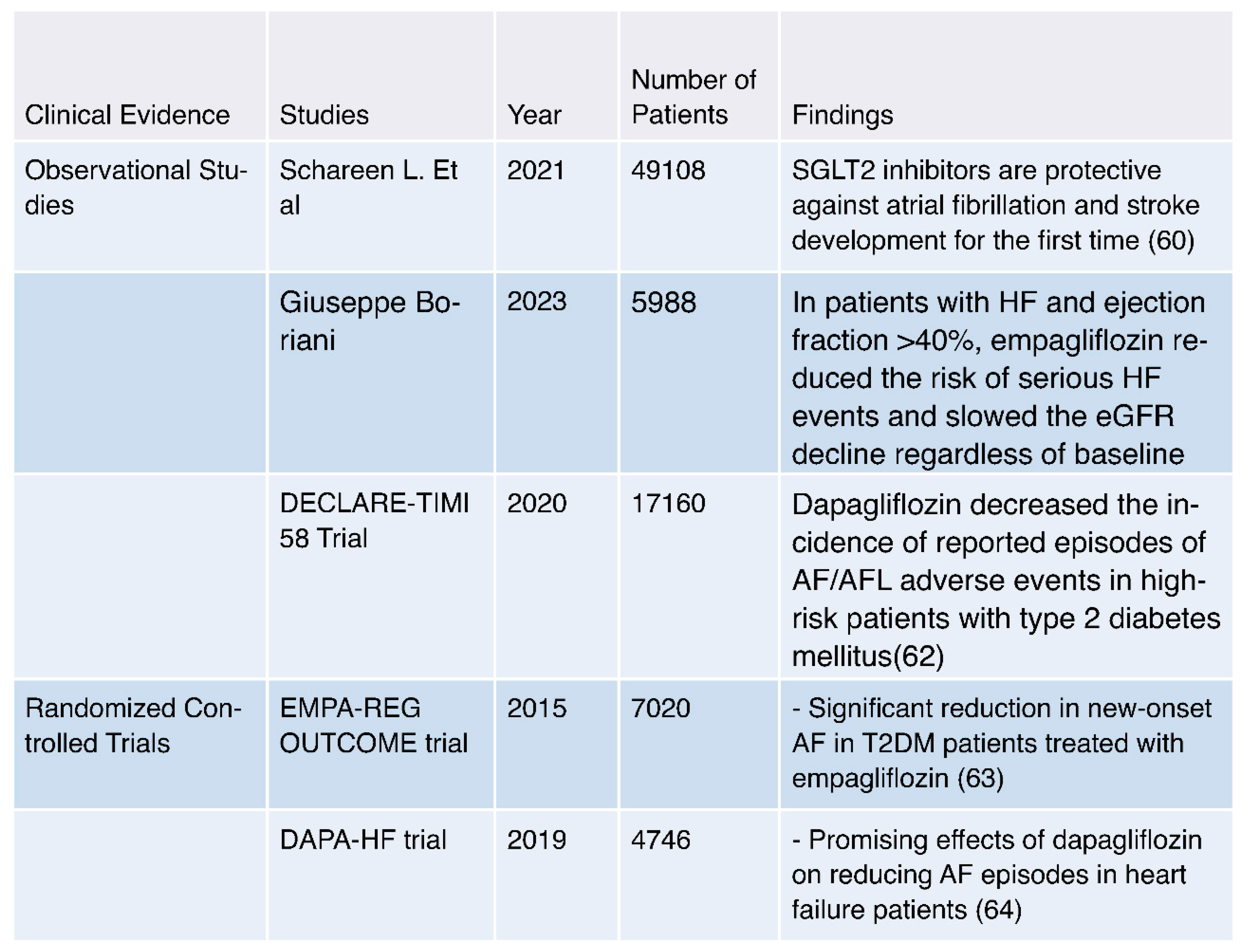
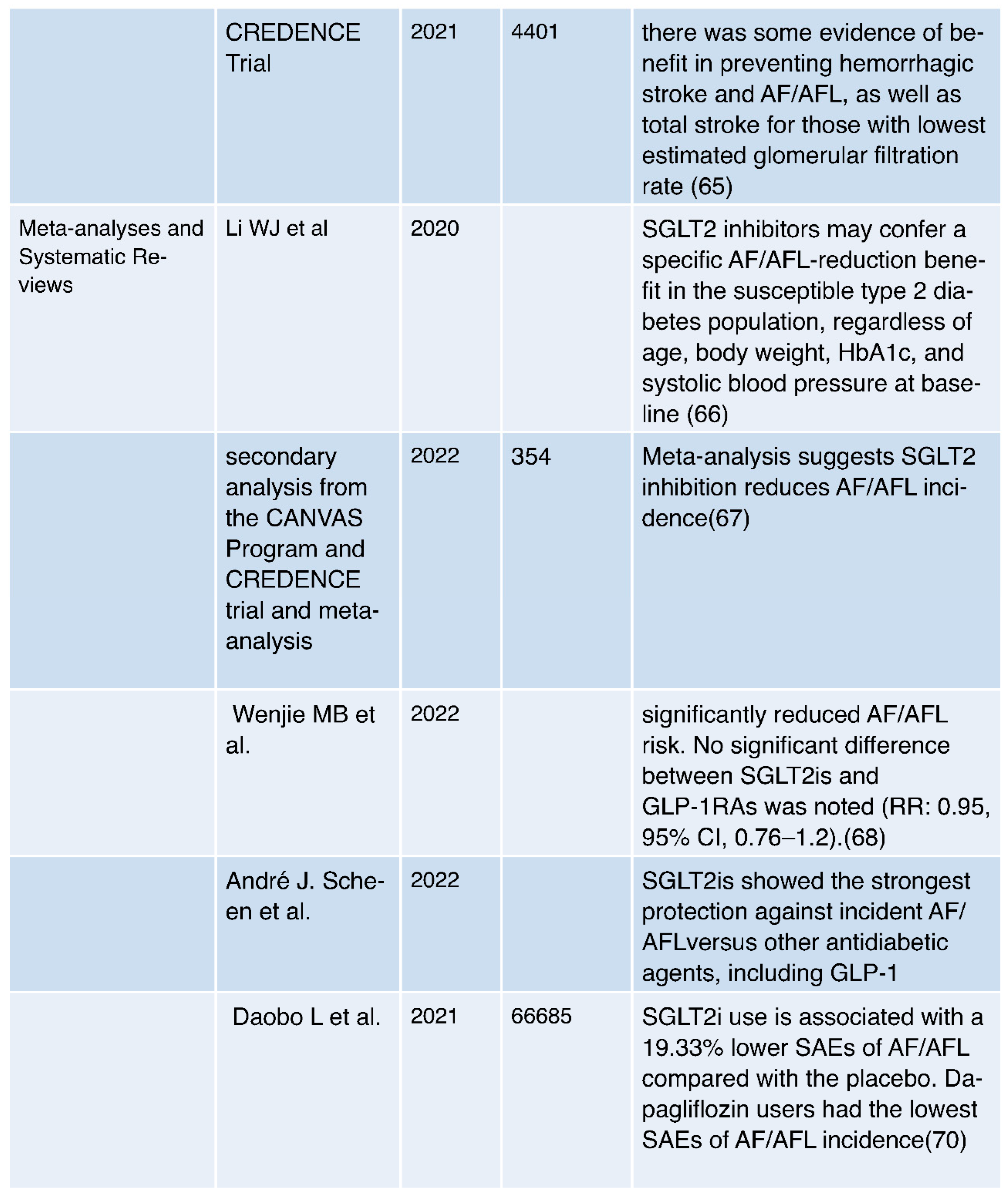
- Clinical Evidence:
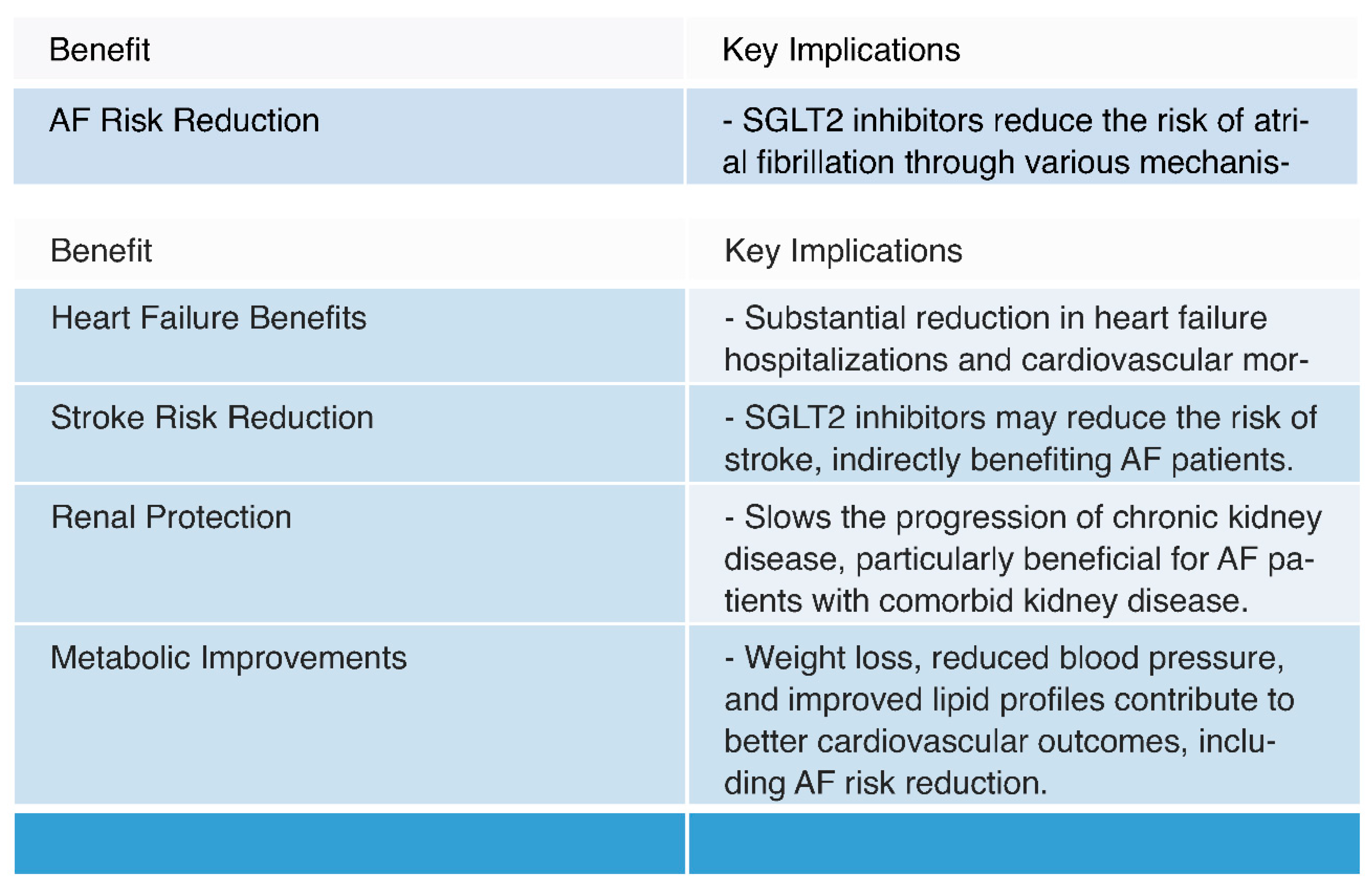
- Potential Benefits:
- 1. AF Risk Reduction:
- Mechanisms: SGLT2 inhibitors have been shown to reduce the risk of atrial fibrillation (AF) through various mechanisms. These include improved glycemic control, diuresis leading to reduced plasma volume and blood pressure, reduction in oxidative stress and inflammation, and potential modulation of atrial electrophysiology [23,71,72].
- Observational Evidence: Multiple observational studies have reported a consistent association between SGLT2 inhibitor use and a reduced risk of AF in individuals with type 2 diabetes mellitus (T2DM) and heart failure. This suggests a potential protective effect of these medications against AF [60,61,62].
- Clinical Trials: Randomized controlled trials, such as the EMPA-REG OUTCOME trial and the DECLARE-TIMI 58 Trial, have provided clinical evidence supporting the reduction of AF risk in patients treated with specific SGLT2 inhibitors, such as empagliflozin and dapagliflozin. These trials demonstrate the real-world applicability of AF risk reduction [62,63].
- 2. Cardiovascular Outcomes:
- Heart Failure Benefits: SGLT2 inhibitors have consistently demonstrated substantial benefits in reducing heart failure hospitalizations and cardiovascular mortality. Given the frequent coexistence of AF and heart failure, improved heart failure outcomes indirectly contribute to better AF management [7,25,73,74].
- Renal Protection: SGLT2 inhibitors have shown renal benefits, including a reduction in albuminuria and slowing the progression of chronic kidney disease [26,76,77]. Improved renal function can have a positive impact on overall cardiovascular health, potentially benefiting AF patients, especially those with comorbid kidney disease [26].
Conclusions
- Future Research and Clinical Practice:
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Song, S., Ko, J. S., Lee, H. A., et al. (2022). Clinical Implications of Heart Rate Control in Heart Failure With Atrial Fibrillation: Multi-Center Prospective Observation Registry (CODE-AF Registry). Frontiers in Cardiovascular Medicine, 9. [CrossRef]
- Thorén, E., Wernroth, ML., Christersson, C. et al. Compared with matched controls, patients with postoperative atrial fibrillation (POAF) have increased long-term AF after CABG, and POAF is further associated with increased ischemic stroke, heart failure and mortality even after adjustment for AF. Clin Res Cardiol 109, 1232–1242 (2020). [CrossRef]
- Gumprecht, J., Domek, M., Lip, G.Y.H. et al. Invited review: hypertension and atrial fibrillation: epidemiology, pathophysiology, and implications for management. J Hum Hypertens 33, 824–836 (2019). [CrossRef]
- Guo, Xue-Yuan; Ma, Chang-Sheng. Atrial Fibrillation Ablation: Indications, Outcomes, Complications, and Future Directions. Chinese Medical Journal 130(16):p 1891-1893, August 20, 2017. |. [CrossRef]
- Kochhar, S. (2016). Atrial fibrillation: An update. Independent Nurse, 2016(2), 30. Published Online:2 Feb 2016. [CrossRef]
- Stroo, J. F., van Steenbergen, G. J., van Straten, A. H. M., Houterman, S., & Soliman-Hamad, M. A. (2023). Long-term Outcome of Reexploration for Bleeding After Coronary Artery Bypass Grafting. Journal of Cardiothoracic and Vascular Anesthesia, Published online June 09, 2023. [CrossRef]
- Khiali, S., Taban-Sadeghi, M., Sarbakhsh, P., et al. (2023). SGLT2 Inhibitors’ Cardiovascular Benefits in Individuals Without Diabetes, Heart Failure, and/or Chronic Kidney Disease: A Systematic Review. Journal of Clinical Pharmacy and Therapeutics, Published online July 16, 2023. [CrossRef]
- Pawlos, Agnieszka, Marlena Broncel, Ewelina Woźniak, and Paulina Gorzelak-Pabiś. 2021. "Neuroprotective Effect of SGLT2 Inhibitors" Molecules 26, no. 23: 7213. [CrossRef]
- Kaneto, Hideaki, Atsushi Obata, Tomohiko Kimura, Masashi Shimoda, Tome Kinoshita, Taka-aki Matsuoka, and Kohei Kaku. 2021. "Unexpected Pleiotropic Effects of SGLT2 Inhibitors: Pearls and Pitfalls of This Novel Antidiabetic Class" International Journal of Molecular Sciences 22, no. 6: 3062. [CrossRef]
- Vallon V, Verma S. Effects of SGLT2 Inhibitors on Kidney and Cardiovascular Function. Annu Rev Physiol. 2021 Feb 10;83:503-528.
- Szekeres, Zsolt, Kalman Toth, and Eszter Szabados. 2021. "The Effects of SGLT2 Inhibitors on Lipid Metabolism" Metabolites 11, no. 2: 87.
- Durante, William, Ghazaleh Behnammanesh, and Kelly J. Peyton. 2021. "Effects of Sodium-Glucose Co-Transporter 2 Inhibitors on Vascular Cell Function and Arterial Remodeling" International Journal of Molecular Sciences 22, no. 16: 8786. [CrossRef]
- Lymperopoulos, Anastasios, Jordana I. Borges, Natalie Cora, and Anastasiya Sizova. 2021. "Sympatholytic Mechanisms for the Beneficial Cardiovascular Effects of SGLT2 Inhibitors: A Research Hypothesis for Dapagliflozin’s Effects in the Adrenal Gland" International Journal of Molecular Sciences 22, no. 14: 7684. [CrossRef]
- Gailin, Ye., Shuai, Wang., Daoquan, Peng. (2021). Effects of SGLT2 Inhibitor on Ischemic Events Stemming From Atherosclerotic Coronary Diseases: A Systematic Review and Meta-analysis With Trial Sequential Analysis of Randomized Controlled Trials.. Journal of Cardiovascular Pharmacology. [CrossRef]
- Fernand, Bonnet., André, Scheen. (2018). Effects of SGLT2 inhibitors on systemic and tissue low-grade inflammation: The potential contribution to diabetes complications and cardiovascular disease.. Diabetes & Metabolism. [CrossRef]
- Anja, Schork., Janine, Saynisch., Andreas, Vosseler., Benjamin, Assad, Jaghutriz., Nils, Heyne., Andreas, Peter., Hans-Ulrich, Häring., Norbert, Stefan., Andreas, Fritsche., Ferruh, Artunc. (2019). Effect of SGLT2 inhibitors on body composition, fluid status and renin-angiotensin-aldosterone system in type 2 diabetes: a prospective study using bioimpedance spectroscopy.. Cardiovascular Diabetology. [CrossRef]
- Asadur, Rahman., Hirofumi, Hitomi., Akira, Nishiyama. (2017). Cardioprotective effects of SGLT2 inhibitors are possibly associated with normalization of the circadian rhythm of blood pressure.. Hypertension Research. [CrossRef]
- Abdullah, Kaplan., Emna, Abidi., Ahmed, F., El-Yazbi., Ahmed, F., El-Yazbi., Ali, H., Eid., Ali, H., Eid., George, W., Booz., Fouad, A., Zouein. (2018). Direct cardiovascular impact of SGLT2 inhibitors: mechanisms and effects.. Heart Failure Reviews. [CrossRef]
- Kazuomi, Kario., Keith, C., Ferdinand., James, H., O'Keefe. (2020). Control of 24-hour blood pressure with SGLT2 inhibitors to prevent cardiovascular disease. Progress in Cardiovascular Diseases. [CrossRef]
- Steven, G., Chrysant. (2017). Promising cardiovascular and blood pressure effects of the SGLT2 inhibitors: a new class of antidiabetic drugs.. Drugs of Today. [CrossRef]
- Samar, A., Nasser., Neha, Arora., Keith, C., Ferdinand. (2022). Addressing Cardiovascular Disparities in Racial/Ethnic Populations: The Blood Pressure-Lowering Effects of SGLT2 Inhibitors. Reviews in Cardiovascular Medicine. [CrossRef]
- Jamie, L., Benham., Jane, E., Booth., Ronald, J., Sigal., Stella, S., Daskalopoulou., Alexander, A., Leung., Doreen, M., Rabi. (2021). Systematic review and meta-analysis: SGLT2 inhibitors, blood pressure and cardiovascular outcomes. IJC Heart & Vasculature. [CrossRef]
- V., K., Kurashin., N., Yu., Borovkova., V., A., Kurashina., T., E., Bakka. (2021). Possibilities of cardio- and nephroprotective eff ects of drugs of the SGLT2 inhibitor group. [CrossRef]
- Monda, V.M., Gentile, S., Porcellati, F. et al. Heart Failure with Preserved Ejection Fraction and Obstructive Sleep Apnea: A Novel Paradigm for Additional Cardiovascular Benefit of SGLT2 Inhibitors in Subjects With or Without Type 2 Diabetes. Adv Ther 39, 4837–4846 (2022). [CrossRef]
- Vincenzo, De, Marzo., Gianluigi, Savarese., Italo, Porto., Marco, Metra., Pietro, Ameri. (2023). Efficacy of SGLT2-inhibitors across different definitions of heart failure with preserved ejection fraction. Journal of Cardiovascular Medicine. [CrossRef]
- Sajad, Khiali., Mohammad, Reza, Sadeghi., Parvin, Sarbakhsh., Naser, Khezerlouy, Aghdam., Afra, Rezagholizadeh., Hila, Asham., Taher, Entezari, Maleki. (2023). SGLT2 Inhibitors' Cardiovascular Benefits In Individuals Without Diabetes, Heart Failure, And/Or Chronic Kidney Disease: A Systematic Review. The Journal of Clinical Pharmacology. [CrossRef]
- Ping-Fan, Lu. (2023). Efficacy of sacubitril-valsartan and SGLT2 inhibitors in heart failure with reduced ejection fraction: A systematic review and meta-analysis. Clinical Cardiology. [CrossRef]
- Atsushi, Tanaka., Takumi, Imai., Shigeru, Toyoda., Kazuhiro, Sugimoto., Ruka, Yoshida., Machi, Furuta., Koichi, Node. (2023). Long-term observation of estimated fluid volume reduction after the initiation of ipragliflozin in patients with type 2 diabetes mellitus: a sub-analysis from a randomized controlled trial (PROTECT). Diabetology & Metabolic Syndrome. [CrossRef]
- Niels, H, Brandt-Jacobsen., Mikkel, Jürgens., Phillip, Hasbak., Peter, Gæde., Peter, Rossing., Jon, J, Rasmussen., Camillla, Fuchs, Andersen., Julie, Lyng, Forman., Jens, Faber., Silvio, E., Inzucchi., Finn, Gustafsson., Morten, Schou., Caroline, Kistorp. (2022). ODP154 Reduction of Cardiac Adipose Tissue Volume with Short-Term Empagliflozin in Patients with Type 2 Diabetes: Results from the SIMPLE Randomized Clinical Trial. Journal of the Endocrine Society. [CrossRef]
- Kristina, Johnsson., Eva, Johnsson., TA, Mansfield., Yshai, Yavin., Agata, Ptaszynska., Shamik, Parikh. (2016). Osmotic diuresis with SGLT2 inhibition: analysis of events related to volume reduction in dapagliflozin clinical trials. Postgraduate Medicine. [CrossRef]
- Marianna, Fontana., Ana, Martinez-Naharro., Liza, Chacko., Dorota, Rowczenio., Janet, A., Gilbertson., Carol, J., Whelan., Svetla, G., Strehina., Thirusha, Lane., James, C., Moon., David, F., Hutt., Peter, Kellman., Aviva, Petrie., Philip, N., Hawkins., Julian, D., Gillmore. (2021). Reduction in CMR Derived Extracellular Volume With Patisiran Indicates Cardiac Amyloid Regression.. Jacccardiovascular Imaging. [CrossRef]
- Giuseppe, Cannella., S., Ghielmi., Massimo, Sandrini., Claudio, Poiesi., Mario, Gaggiotti., A., Rodella., Rosario, Maiorca. (1988). Effect of reduction of blood volume on plasma immunoreactive atrial natriuretic factor concentrations in normal man.. Nephrology Dialysis Transplantation. [CrossRef]
- Tamique, Mason., Otavio, R., Coelho-Filho., Subodh, Verma., Biswajit, Chowdhury., Fei, Zuo., Adrian, Quan., Kevin, E., Thorpe., Christopher, Bonneau., Hwee, Teoh., Richard, E., Gilbert., Lawrence, A., Leiter., Peter, Jüni., Bernard, Zinman., Michael, Jerosch-Herold., C., David, Mazer., Andrew, T., Yan., Kim, A., Connelly., Kim, A., Connelly. (2021). Empagliflozin Reduces Myocardial Extracellular Volume in Patients With Type 2 Diabetes and Coronary Artery Disease.. Jacc-cardiovascular Imaging. [CrossRef]
- Mads, Ersbøll., Mikkel, Jürgens., Philip, Hasbak., Andreas, Kjaer., Emil, Wolsk., Bo, Zerahn., Niels, H, Brandt-Jacobsen., Peter, Gæde., Peter, Rossing., Peter, Rossing., Jens, Faber., Silvio, E., Inzucchi., Finn, Gustafsson., Morten, Schou., Caroline, Kistorp., Caroline, Kistorp. (2021). Effect of empagliflozin on myocardial structure and function in patients with type 2 diabetes at high cardiovascular risk: the SIMPLE randomized clinical trial. International Journal of Cardiovascular Imaging. [CrossRef]
- Murat, Ziyrek., Mustafa, Duran. (2021). Effects of SGLT2 Inhibitors as an Addon Therapy to Metformin on Electrocardiographic Indices of Ventricular Repolarization. Acta Cardiologica Sinica. [CrossRef]
- Edoardo, Gronda., Gary, D., Lopaschuk., Arduino, Arduini., Antonio, Santoro., Giuditta, Benincasa., Alberto, Palazzuoli., Domenico, Gabrielli., Claudio, Napoli. (2022). Mechanisms of action of SGLT2 inhibitors and their beneficial effects on the cardiorenal axis.. Canadian Journal of Physiology and Pharmacology. [CrossRef]
- Subodh, Verma., John, J.V., McMurray. (2018). SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. [CrossRef]
- Jason, R.B., Samuel, Sossalla., Nazha, Hamdani., Ruben, Coronel., Nina, C., Weber., Peter, E., Light., Coert, J., Zuurbier. (2022). Cardiac mechanisms of the beneficial effects of SGLT2 inhibitors in heart failure: Evidence for potential off-target effects.. Journal of Molecular and Cellular Cardiology. [CrossRef]
- T, Sato., Takayuki, Miki., Hirofumi, Ohnishi., T., Yamashita., A., Takada., Toshiyuki, Yano., Masaya, Tanno., Akihito, Tsuchida., Tetsuji, Miura. (2017). Effect of sodium-glucose co-transporter-2 inhibitors on impaired ventricular repolarization in people with Type 2 diabetes. Diabetic Medicine. [CrossRef]
- Zhang, N., Gong, M., Tse, G., Zhang, Z., Meng, L., Yan, B. P.,... & Liu, T. (2018). Prolonged corrected QT interval in predicting atrial fibrillation: a systematic review and meta-analysis. Pacing and Clinical Electrophysiology, 41(3), 321-327. [CrossRef]
- Cho, M. S., Nam, G. B., Kim, Y. N., Kim, J., Choi, K. J., & Kim, Y. H. (2020). Clinical Implications of Ventricular Repolarization Parameters on Long-Term Risk of Atrial Fibrillation―Longitudinal Follow-up Data From a General Ambulatory Korean Population―. Circulation Journal, 84(7), 1067-1074. [CrossRef]
- Dobromir, Dobrev., Stanley, Nattel. (2008). Calcium Handling Abnormalities in Atrial Fibrillation as a Target for Innovative Therapeutics. Journal of Cardiovascular Pharmacology. [CrossRef]
- Chung-Chuan, Chou., Peng, Sheng, Chen., Delon, Wu., Delon, Wu. (2008). Intracellular Calcium Dynamics and Autonomic Stimulation in Atrial Fibrillation: Mechanisms and Implications. Journal of Arrhythmia. [CrossRef]
- Gary, L., Aistrup., Rishi, Arora., Søren, Grubb., Shin, Yoo., Benjamin, Toren., Manvinder, Kumar., Aaron, Kunamalla., William, Marszalec., Tej, Motiwala., Shannon, Tai., Sean, Yamakawa., Satya, Yerrabolu., Francisco, J., Alvarado., Héctor, H., Valdivia., Jonathan, M., Cordeiro., Yohannes, Shiferaw., J., A., Wasserstrom. (2017). Triggered intracellular calcium waves in dog and human left atrial myocytes from normal and failing hearts. Cardiovascular Research. [CrossRef]
- Funsho, E, Fakuade., Vanessa, Steckmeister., F., Seibertz., Judith, Gronwald., Stefanie, Kestel., Julia, Menzel., Julius, Ryan, D., Pronto., Karim, Taha., Fereshteh, Haghighi., George, Kensah., Charles, M., Pearman., Felix, Wiedmann., Arco, J., Teske., Constanze, Schmidt., Katharine, M., Dibb., Aschraf, El-Essawi., Bernhard, C., Danner., Hassina, Baraki., Blanche, Schwappach., Ingo, Kutschka., Fleur, E., Mason., Niels, Voigt. (2021). Altered atrial cytosolic calcium handling contributes to the development of postoperative atrial fibrillation. Cardiovascular Research. [CrossRef]
- Dobromir, Dobrev., Xander, H.T., Wehrens. (2017). Calcium-mediated cellular triggered activity in atrial fibrillation.. The Journal of Physiology. [CrossRef]
- Qing, Wang., Qing, Wang., Jing, Wang., Pei, Wang., Liaoyuan, Wang., Lanting, Jia., Xinyu, Ling., Wang, Xi., Jie, Min., Hua, Shen., Jian, Xiao., Jinxiang, Yuan., Zhinong, Wang. (2019). Glycemic control is associated with atrial structural remodeling in patients with type 2 diabetes. BMC Cardiovascular Disorders. [CrossRef]
- Shruti, S, Joshi., Trisha, Singh., David, E., Newby., Jagdeep, Singh. (2021). Sodium-glucose co-transporter 2 inhibitor therapy: mechanisms of action in heart failure. Heart. [CrossRef]
- David, Z.I., Cherney., Ayodele, Odutayo., Ronnie, Aronson., Justin, A., Ezekowitz., John, D., Parker. (2019). Sodium Glucose Cotransporter-2 Inhibition and Cardiorenal Protection: JACC Review Topic of the Week. Journal of the American College of Cardiology. [CrossRef]
- Silvio, E., Inzucchi., Bernard, Zinman., Christoph, Wanner., Roberto, Ferrari., David, Fitchett., Stefan, Hantel., Rosa-Maria, Espadero., Hans-Juergen, Woerle., Uli, C., Broedl., Odd, Erik, Johansen. (2015). SGLT-2 inhibitors and cardiovascular risk: Proposed pathways and review of ongoing outcome trials. Diabetes and Vascular Disease Research. [CrossRef]
- Christopher, Redford., Laura, Doherty., Jamie, Smith. (2015). SGLT2 inhibitors and the risk of diabetic ketoacidosis. Practical Diabetes. [CrossRef]
- Julieta, Lazarte., Tharsan, Kanagalingam., Robert, A., Hegele. (2021). Lipid effects of sodium-glucose cotransporter 2 inhibitors.. Current Opinion in Lipidology. [CrossRef]
- Zsolt, Szekeres., Kalman, Toth., Eszter, Szabados. (2021). The Effects of SGLT2 Inhibitors on Lipid Metabolism.. Metabolites. [CrossRef]
- Adriana, Sánchez-García., Mario, Simental-Mendía., Juan, Manuel, Millán-Alanís., Luis, E., Simental-Mendía. (2020). Effect of sodium-glucose co-transporter 2 inhibitors on lipid profile: A systematic review and meta-analysis of 48 randomized controlled trials. Pharmacological Research.
- Hanny, Sawaf., Moarij, Qaz., Jeeda, Ismail., Ali, Mehdi. (2022). The Renal Effects of SGLT2 Inhibitors. [CrossRef]
- Volker, Vallon., Subodh, Verma. (2021). Effects of SGLT2 Inhibitors on Kidney and Cardiovascular Function.. Annual Review of Physiology. [CrossRef]
- Rosalie, A., Scholtes., Michaël, J.B., van, Baar., Megan, D., Kok., Petter, Bjornstad., David, Z.I., Cherney., Jaap, A., Joles., Daniël, H., van, Raalte., Daniël, H., van, Raalte. (2021). Renal haemodynamic and protective effects of renoactive drugs in type 2 diabetes: Interaction with SGLT2 inhibitors. Nephrology. [CrossRef]
- Shan-Jiang, Chen., Ruben, Coronel., Markus, W., Hollmann., Nina, C., Weber., Coert, J., Zuurbier. (2022). Direct cardiac effects of SGLT2 inhibitors. Cardiovascular Diabetology. [CrossRef]
- Josselin, Nespoux., Volker, Vallon. (2020). Renal effects of SGLT2 inhibitors: an update.. Current Opinion in Nephrology and Hypertension. [CrossRef]
- Lee, S., Zhou, J., Chang, C., Liu, T., Chang, D., Wong, W. T., Leung, K. S. K., Wai, A. K. C., Cheung, B. M. Y., Tse, G., & Zhang, Q. (2021). Comparative effects of sodium glucose cotransporter 2 (SGLT2) inhibitors and dipeptidyl peptidase-4 (DPP4) inhibitors on new-onset atrial fibrillation and stroke outcomes. medRxiv 2021.01.04.21249211. [CrossRef]
- Boriani G, Mei DA, Imberti JF. Therapeutic effects of sodium-glucose cotransporter 2 inhibitors in patients with heart failure with preserved ejection fraction: From outcome improvement to potentially favourable influences on atrial fibrillation burden, atrial fibrillation progression and atrial cardiomyopathy. Eur J Heart Fail. 2023 Jul;25(7):978-980. [CrossRef]
- Zelniker TA, Bonaca MP, Furtado RHM, Mosenzon O, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Budaj A, Kiss RG, Padilla F, Gause-Nilsson I, Langkilde AM, Raz I, Sabatine MS, Wiviott SD. Effect of Dapagliflozin on Atrial Fibrillation in Patients With Type 2 Diabetes Mellitus: Insights From the DECLARE-TIMI 58 Trial. Circulation. 2020 Apr 14;141(15):1227-1234. [CrossRef]
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015 Nov 26;373(22):2117-28. [CrossRef]
- McMurray JJV, DeMets DL, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, Sjöstrand M, Solomon SD; DAPA-HF Committees and Investigators. A trial to evaluate the effect of the sodium-glucose co-transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA-HF). Eur J Heart Fail. 2019 May;21(5):665-675. [CrossRef]
- Zhou Z, Jardine MJ, Li Q, Neuen BL, Cannon CP, de Zeeuw D, Edwards R, Levin A, Mahaffey KW, Perkovic V, Neal B, Lindley RI; CREDENCE Trial Investigators*. Effect of SGLT2 Inhibitors on Stroke and Atrial Fibrillation in Diabetic Kidney Disease: Results From the CREDENCE Trial and Meta-Analysis. Stroke. 2021 May;52(5):1545-1556. [CrossRef]
- Li WJ, Chen XQ, Xu LL, Li YQ, Luo BH. SGLT2 inhibitors and atrial fibrillation in type 2 diabetes: a systematic review with meta-analysis of 16 randomized controlled trials. Cardiovasc Diabetol. 2020 Aug 26;19(1):130. [CrossRef]
- Li C, Yu J, Hockham C, Perkovic V, Neuen BL, Badve SV, Houston L, Lee VYJ, Barraclough JY, Fletcher RA, Mahaffey KW, Heerspink HJL, Cannon CP, Neal B, Arnott C. Canagliflozin and atrial fibrillation in type 2 diabetes mellitus: A secondary analysis from the CANVAS Program and CREDENCE trial and meta-analysis. Diabetes Obes Metab. 2022 Oct;24(10):1927-1938. [CrossRef]
- Wenjie: Journal of Cardiovascular Pharmacology 79(3):p 281-288, March 2022. |. [CrossRef]
- Scheen: https://doi.org/10.1016/j.diabet.2022.101390, 2022 Nov;48(6):101390. [CrossRef]
- Li D, Liu Y, Hidru TH, Yang X, Wang Y, Chen C, Li KHC, Tang Y, Wei Y, Tse G, Xia Y. Protective Effects of Sodium-Glucose Transporter 2 Inhibitors on Atrial Fibrillation and Atrial Flutter: A Systematic Review and Meta- Analysis of Randomized Placebo-Controlled Trials. Front Endocrinol (Lausanne). 2021 Mar 19;12:619586. [CrossRef]
- Alex, Ali, Sayour., Mihály, Ruppert., Attila, Oláh., Kálmán, Benke., Bálint, András, Barta., Eszter, Zsáry., Béla, Merkely., Tamás, Radovits. (2021). Effects of SGLT2 Inhibitors beyond Glycemic Control—Focus on Myocardial SGLT1. International Journal of Molecular Sciences. [CrossRef]
- Ashish, Verma., Ankit, B., Patel., Sushrut, S., Waikar. (2020). SGLT2 Inhibitor: Not a Traditional Diuretic for Heart Failure.. Cell Metabolism. [CrossRef]
- Omar, Mourad., Shabana, Vohra., Sara, S., Tunes. (2023). SGLT2 transcriptomic expression atlas supports a kidney-centric role for empagliflozin’s benefits in heart failure. bioRxiv. [CrossRef]
- Sara, Albalushi. (2023). Meta-analysis on the effectiveness of SGLT2 inhibitors in heart failure. European heart journal. Acute cardiovascular care. [CrossRef]
- Fatima, Alzahra, Al, Hamed., Hazem, Elewa. (2020). Potential Therapeutic Effects of Sodium Glucose-linked Cotransporter 2 Inhibitors in Stroke.. Clinical Therapeutics. [CrossRef]
- Brendon, L., Neuen., Min, Jun., James, Wick., S., Kotwal., Sunil, Badve., Meg, Jardine., M., Gallagher., John, Chalmers., Kellie, Nallaiah., Vlado, Perkovic., D., Peiris., A., Rodgers., Mark, Woodward., Paul, E., Ronksley. (2023). Estimating the population-level kidney benefits of improved uptake of SGLT2 inhibitors in patients with chronic kidney disease in Australian primary care. med-Rxiv. [CrossRef]
- Pasquale, Paolisso., Luca, Bergamaschi., Arturo, Cesaro., Emanuele, Gallinoro., Felice, Gragnano., Celestino, Sardu., Niya, Mileva., Alberto, Foà., Matteo, Armillotta., Angelo, Sansonetti., S., Amicone., A., Impellizzeri., Marta, Belmonte., Giuseppe, Esposito., Nuccia, Morici., Gianni, Casella., Ciro, Mauro., Dobrin, Vassilev., Nazzareno, Galiè., Gaetano, Santulli., Paolo, Calabrò., Emanuele, Barbato., Raffaele, Marfella., Carmine, Pizzi. (2023). Impact of SGLT2-inhibitors on contrast-induced acute kidney injury in Diabetic patients with Acute Myocardial Infarction with and without chronic kidney disease: Insight from SGLT2-I AMI PROTECT Registry.. Diabetes Research and Clinical Practice. [CrossRef]
- Francisco, García-Carrizo., Sebastià, Galmés., Catalina, Picó., Andreu, Palou., Ana, M., Rodríguez. (2022). Supplementation with the Prebiotic High-Esterified Pectin Improves Blood Pressure and Cardiovascular Risk Biomarker Profile, Counteracting Metabolic Malprogramming. Journal of Agricultural and Food Chemistry. [CrossRef]
- J., Blose., P., Stickles., A., Battaglino., A., Trumbetti., J., James., K., Cooper., A., Schade., Melissa, A., Reed., Selen, Razon., Melissa, A., Whidden. (2020). The Effects of a Six-Week Weight Loss Program on Blood Lipid Profiles and Cardiovascular Health.
- Yukiko, Hasegawa., Tomoko, Nakagami., Junko, Oya., Kanako, Takahashi., Chisato, Isago., Moritoshi, Kurita., Yuki, Tanaka., Arata, Ito., Tadasu, Kasahara., Yasuko, Uchigata. (2019). Body Weight Reduction of 5% Improved Blood Pressure and Lipid Profiles in Obese Men and Blood Glucose in Obese Women: A Four-Year Follow-up Observational Study. Metabolic Syndrome and Related Disorders. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





