Submitted:
03 November 2023
Posted:
06 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
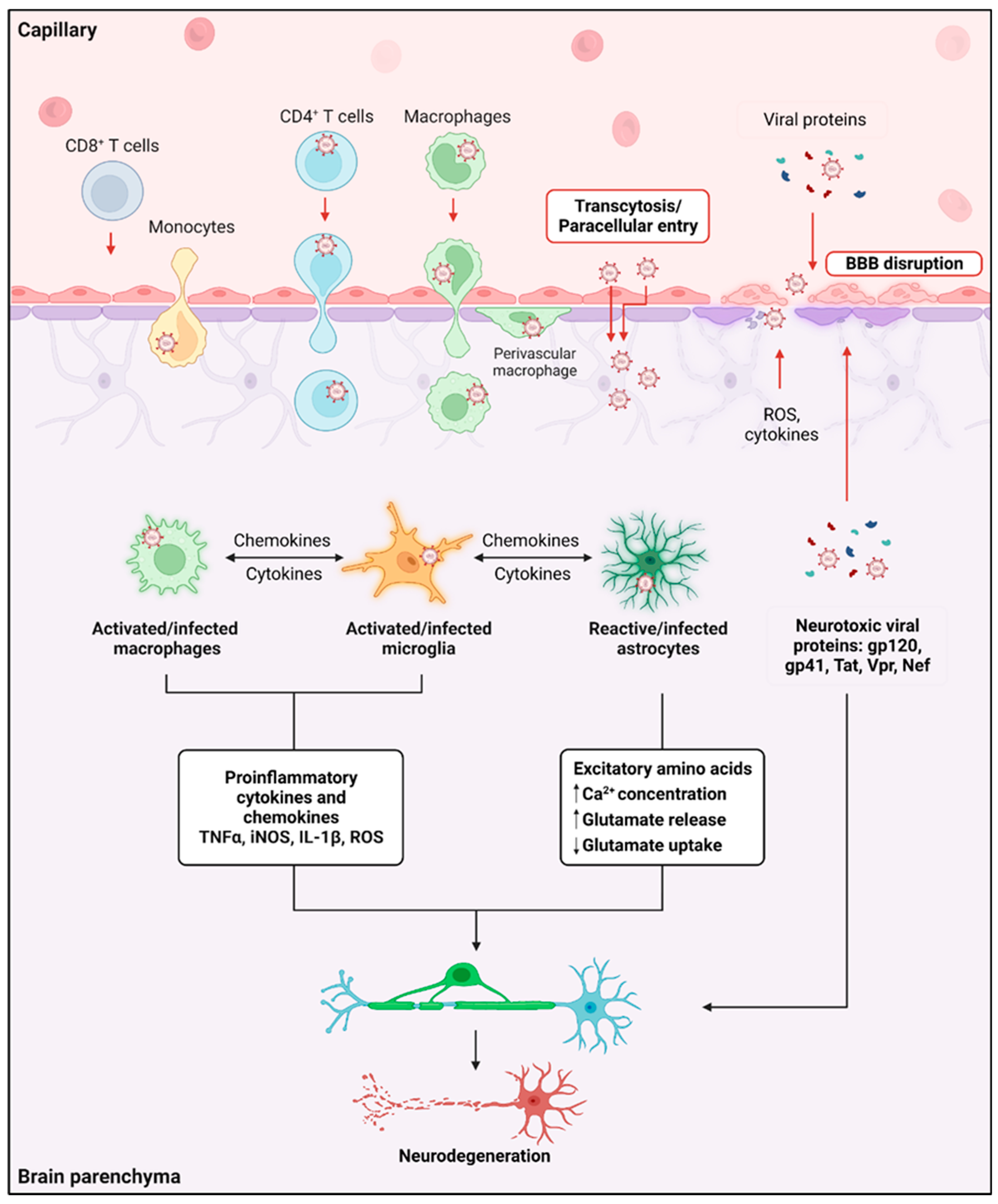
2. Consequences of HIV Infection in the Brain
3. HIV Infection of Brain-Associated Cell Types
4. Consequences of Microglia/Macrophage Infection by HIV
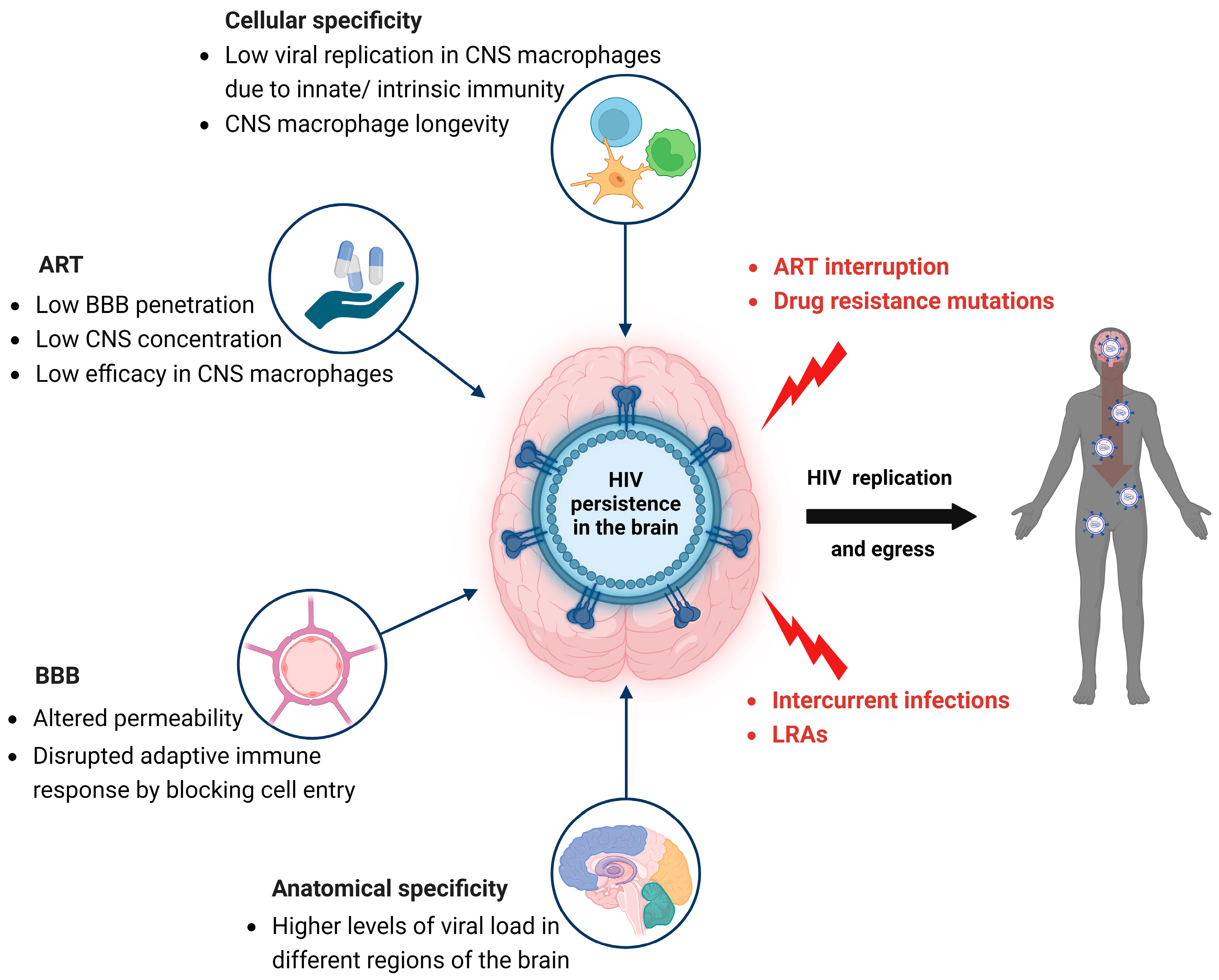
5. HIV Persistence in the CNS Despite ART
6. HIV Compartmentalization in the CNS and Drug Resistance Mutations
7. CSF Viral Escape Dynamics
8. Impact of ART on HIV Persistence in the Brain
9. Discussion
Acknowledgments
References
- HIV/AIDS. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed on 28 September 2021).
- Saylor, D.; Dickens, A.M.; Sacktor, N.; Haughey, N.; Slusher, B.; Pletnikov, M.; Mankowski, J.L.; Brown, A.; Volsky, D.J.; McArthur, J.C. HIV-Associated Neurocognitive Disorder--Pathogenesis and Prospects for Treatment. Nat Rev Neurol 2016, 12, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, S.; Ances, B.; Cinque, P.; Dravid, A.; Dreyer, A.J.; Gisslén, M.; Joska, J.A.; Kwasa, J.; Meyer, A.-C.; Mpongo, N.; et al. Cognitive Impairment in People Living with HIV: Consensus Recommendations for a New Approach. Nat Rev Neurol 2023, 19, 424–433. [Google Scholar] [CrossRef]
- WHO WHO UNAIDS. Global Update on HIV Treatmnt 2013: Results, Impact and Opportunities. Geneva: WHO (2013).
- Sáez-Cirión, A.; Bacchus, C.; Hocqueloux, L.; Avettand-Fenoel, V.; Girault, I.; Lecuroux, C.; Potard, V.; Versmisse, P.; Melard, A.; Prazuck, T.; et al. Post-Treatment HIV-1 Controllers with a Long-Term Virological Remission after the Interruption of Early Initiated Antiretroviral Therapy ANRS VISCONTI Study. PLoS Pathog 2013, 9, e1003211. [Google Scholar] [CrossRef]
- Chun, T.W.; Stuyver, L.; Mizell, S.B.; Ehler, L.A.; Mican, J.A.; Baseler, M.; Lloyd, A.L.; Nowak, M.A.; Fauci, A.S. Presence of an Inducible HIV-1 Latent Reservoir during Highly Active Antiretroviral Therapy. Proc Natl Acad Sci U S A 1997, 94, 13193–13197. [Google Scholar] [CrossRef]
- Blankson, J.N.; Persaud, D.; Siliciano, R.F. The Challenge of Viral Reservoirs in HIV-1 Infection. Annu Rev Med 2002, 53, 557–593. [Google Scholar] [CrossRef]
- Chaillon, A.; Gianella, S.; Dellicour, S.; Rawlings, S.A.; Schlub, T.E.; Oliveira, M.F.D.; Ignacio, C.; Porrachia, M.; Vrancken, B.; Smith, D.M. HIV Persists throughout Deep Tissues with Repopulation from Multiple Anatomical Sources. J Clin Invest 2020, 130, 1699–1712. [Google Scholar] [CrossRef]
- Wong, J.K.; Yukl, S.A. Tissue Reservoirs of HIV. Curr Opin HIV AIDS 2016, 11, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Dufour, C.; Ruiz, M.J.; Pagliuzza, A.; Richard, C.; Shahid, A.; Fromentin, R.; Ponte, R.; Cattin, A.; Wiche Salinas, T.R.; Salahuddin, S.; et al. Near Full-Length HIV Sequencing in Multiple Tissues Collected Postmortem Reveals Shared Clonal Expansions across Distinct Reservoirs during ART. Cell Rep 2023, 42, 113053. [Google Scholar] [CrossRef]
- Valcour, V.; Chalermchai, T.; Sailasuta, N.; Marovich, M.; Lerdlum, S.; Suttichom, D.; Suwanwela, N.C.; Jagodzinski, L.; Michael, N.; Spudich, S.; et al. Central Nervous System Viral Invasion and Inflammation During Acute HIV Infection. The Journal of Infectious Diseases 2012, 206, 275–282. [Google Scholar] [CrossRef]
- Georgsson, G. Neuropathologic Aspects of Lentiviral Infections. Ann N Y Acad Sci 1994, 724, 50–67. [Google Scholar] [CrossRef]
- Bomsel, M. Transcytosis of Infectious Human Immunodeficiency Virus across a Tight Human Epithelial Cell Line Barrier. Nat Med 1997, 3, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.Q.; Lossinsky, A.S.; Popik, W.; Li, X.; Gujuluva, C.; Kriederman, B.; Roberts, J.; Pushkarsky, T.; Bukrinsky, M.; Witte, M.; et al. Human Immunodeficiency Virus Type 1 Enters Brain Microvascular Endothelia by Macropinocytosis Dependent on Lipid Rafts and the Mitogen-Activated Protein Kinase Signaling Pathway. J Virol 2002, 76, 6689–6700. [Google Scholar] [CrossRef]
- Tugizov, S. Human Immunodeficiency Virus-Associated Disruption of Mucosal Barriers and Its Role in HIV Transmission and Pathogenesis of HIV/AIDS Disease. Tissue Barriers 2016, 4, e1159276. [Google Scholar] [CrossRef]
- González-Scarano, F.; Martín-García, J. The Neuropathogenesis of AIDS. Nat Rev Immunol 2005, 5, 69–81. [Google Scholar] [CrossRef] [PubMed]
- León-Rivera, R.; Veenstra, M.; Donoso, M.; Tell, E.; Eugenin, E.A.; Morgello, S.; Berman, J.W. Central Nervous System (CNS) Viral Seeding by Mature Monocytes and Potential Therapies To Reduce CNS Viral Reservoirs in the cART Era. mBio 2021, 12, e03633-20. [Google Scholar] [CrossRef] [PubMed]
- Kincer, L.P.; Schnell, G.; Swanstrom, R.; Miller, M.B.; Spudich, S.; Eron, J.J.; Price, R.W.; Joseph, S.B. HIV-1 Is Transported into the Central Nervous System by Trafficking Infected Cells. Pathog Immun 2022, 7, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, N.; Roda, W.; Branton, W.G.; Clain, J.; Rabezanahary, H.; Zghidi-Abouzid, O.; Gelman, B.B.; Angel, J.B.; Cohen, E.A.; Gill, M.J.; et al. Lentiviral Infections Persist in Brain despite Effective Antiretroviral Therapy and Neuroimmune Activation. mBio 12 e02784-21. [CrossRef] [PubMed]
- Smail, R.C.; Brew, B.J. Chapter 7 - HIV-Associated Neurocognitive Disorder. In Handbook of Clinical Neurology; Brew, B.J., Ed.; The Neurology of HIV Infection; Elsevier, 2018; Vol. 152, pp. 75–97.
- Heaton, R.K.; Clifford, D.B.; Franklin, D.R.; Woods, S.P.; Ake, C.; Vaida, F.; Ellis, R.J.; Letendre, S.L.; Marcotte, T.D.; Atkinson, J.H.; et al. HIV-Associated Neurocognitive Disorders Persist in the Era of Potent Antiretroviral Therapy: CHARTER Study. Neurology 2010, 75, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Zenebe, Y.; Necho, M.; Yimam, W.; Akele, B. Worldwide Prevalence of HIV Associated Neurocognitive Disorders (HAND) and Its Associated Factors: A Systematic Review and Meta- Analysis 2021.
- Clifford, D.B.; Ances, B.M. HIV-Associated Neurocognitive Disorder (HAND). Lancet Infect Dis 2013, 13, 976–986. [Google Scholar] [CrossRef]
- Wiley, C.A.; Masliah, E.; Morey, M.; Lemere, C.; DeTeresa, R.; Grafe, M.; Hansen, L.; Terry, R. Neocortical Damage during HIV Infection. Ann Neurol 1991, 29, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Masliah, E.; Ge, N.; Achim, C.L.; Hansen, L.A.; Wiley, C.A. Selective Neuronal Vulnerability in HIV Encephalitis. J Neuropathol Exp Neurol 1992, 51, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Raja, F.; Sherriff, F.E.; Morris, C.S.; Bridges, L.R.; Esiri, M.M. Cerebral White Matter Damage in HIV Infection Demonstrated Using Beta-Amyloid Precursor Protein Immunoreactivity. Acta Neuropathol 1997, 93, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Timtim, S.H.; Simmons, A.N.; Hays, C.; Strigo, I.; Sorg, S.; Ellis, R.; Keltner, J.R. HIV Peripheral Neuropathy-Related Degeneration of White Matter Tracts to Sensorimotor Cortex. J Neurovirol 2022, 28, 505–513. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Li, H.; Zhang, H.; Shi, Y.; Wei, F.; Liu, D.; Liu, K.; Chen, D. Accumulation of Nuclear and Mitochondrial DNA Damage in the Frontal Cortex Cells of Patients with HIV-Associated Neurocognitive Disorders. Brain Res 2012, 1458, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Khodr, C.E.; Chen, L.; Al-Harthi, L.; Hu, X.-T. HIV-Induced Hyperactivity of Striatal Neurons Is Associated with Dysfunction of Voltage-Gated Calcium and Potassium Channels at Middle Age. Membranes (Basel) 2022, 12, 737. [Google Scholar] [CrossRef]
- Mitra, P.; Sharman, T. HIV Neurocognitive Disorders; StatPearls Publishing, 2022. [Google Scholar]
- Minagar, A.; Commins, D.; Alexander, J.S.; Hoque, R.; Chiappelli, F.; Singer, E.J.; Nikbin, B.; Shapshak, P. NeuroAIDS: Characteristics and Diagnosis of the Neurological Complications of AIDS. Mol Diagn Ther 2008, 12, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Power, C.; Zhang, K.; van Marle, G. Comparative Neurovirulence in Lentiviral Infections: The Roles of Viral Molecular Diversity and Select Proteases. J Neurovirol 2004, 10 Suppl 1, 113–117. [Google Scholar] [CrossRef]
- Lenz, K.M.; Nelson, L.H. Microglia and Beyond: Innate Immune Cells As Regulators of Brain Development and Behavioral Function. Frontiers in Immunology 2018, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Wake, H.; Moorhouse, A.J.; Nabekura, J. Functions of Microglia in the Central Nervous System--beyond the Immune Response. Neuron Glia Biol 2011, 7, 47–53. [Google Scholar] [CrossRef]
- Bachiller, S.; Jiménez-Ferrer, I.; Paulus, A.; Yang, Y.; Swanberg, M.; Deierborg, T.; Boza-Serrano, A. Microglia in Neurological Diseases: A Road Map to Brain-Disease Dependent-Inflammatory Response. Frontiers in Cellular Neuroscience 2018, 12, 488. [Google Scholar] [CrossRef]
- Herbein, G.; Varin, A. The Macrophage in HIV-1 Infection: From Activation to Deactivation? Retrovirology 2010, 7, 33. [Google Scholar] [CrossRef]
- Porcheray, F.; Samah, B.; Léone, C.; Dereuddre-Bosquet, N.; Gras, G. Macrophage Activation and Human Immunodeficiency Virus Infection: HIV Replication Directs Macrophages towards a pro-Inflammatory Phenotype While Previous Activation Modulates Macrophage Susceptibility to Infection and Viral Production. Virology 2006, 349, 112–120. [Google Scholar] [CrossRef]
- Abreu, C.M.; Veenhuis, R.T.; Avalos, C.R.; Graham, S.; Parrilla, D.R.; Ferreira, E.A.; Queen, S.E.; Shirk, E.N.; Bullock, B.T.; Li, M.; et al. Myeloid and CD4 T Cells Comprise the Latent Reservoir in Antiretroviral Therapy-Suppressed SIVmac251-Infected Macaques. mBio 10, e01659-19. [CrossRef]
- Abreu, C.; Shirk, E.N.; Queen, S.E.; Beck, S.E.; Mangus, L.M.; Pate, K.A.M.; Mankowski, J.L.; Gama, L.; Clements, J.E. Brain Macrophages Harbor Latent, Infectious SIV. AIDS 2019, 33, S181–S188. [Google Scholar] [CrossRef]
- Kumar, A.; Abbas, W.; Herbein, G. HIV-1 Latency in Monocytes/Macrophages. Viruses 2014, 6, 1837–1860. [Google Scholar] [CrossRef]
- Le Douce, V.; Herbein, G.; Rohr, O.; Schwartz, C. Molecular Mechanisms of HIV-1 Persistence in the Monocyte-Macrophage Lineage. Retrovirology 2010, 7, 32. [Google Scholar] [CrossRef]
- Kincer, L.P.; Joseph, S.B.; Gilleece, M.M.; Hauser, B.M.; Sizemore, S.; Zhou, S.; Di Germanio, C.; Zetterberg, H.; Fuchs, D.; Deeks, S.G.; et al. Rebound HIV-1 in Cerebrospinal Fluid after Antiviral Therapy Interruption Is Mainly Clonally Amplified R5 T Cell-Tropic Virus. Nat Microbiol 2023, 8, 260–271. [Google Scholar] [CrossRef]
- Lutgen, V.; Narasipura, S.D.; Barbian, H.J.; Richards, M.; Wallace, J.; Razmpour, R.; Buzhdygan, T.; Ramirez, S.H.; Prevedel, L.; Eugenin, E.A.; et al. HIV Infects Astrocytes in Vivo and Egresses from the Brain to the Periphery. PLoS Pathog 2020, 16, e1008381. [Google Scholar] [CrossRef]
- Luo, X.; He, J.J. Cell-Cell Contact Viral Transfer Contributes to HIV Infection and Persistence in Astrocytes. J Neurovirol 2015, 21, 66–80. [Google Scholar] [CrossRef]
- Gorry, P.R.; Ong, C.; Thorpe, J.; Bannwarth, S.; Thompson, K.A.; Gatignol, A.; Vesselingh, S.L.; Purcell, D.F.J. Astrocyte Infection by HIV-1: Mechanisms of Restricted Virus Replication, and Role in the Pathogenesis of HIV-1-Associated Dementia. Curr HIV Res 2003, 1, 463–473. [Google Scholar] [CrossRef]
- Secretion of Neurotoxins by Mononuclear Phagocytes Infected with HIV-1. Available online: https://www-science-org.login.ezproxy.library.ualberta.ca/doi/abs/10.1126/science.2148832 (accessed on 28 September 2021).
- Brabers, N. a. C.H.; Nottet, H.S.L.M. Role of the Pro-Inflammatory Cytokines TNF-Alpha and IL-1beta in HIV-Associated Dementia. Eur J Clin Invest 2006, 36, 447–458. [Google Scholar] [CrossRef]
- Tyor, W.R.; Glass, J.D.; Griffin, J.W.; Becker, P.S.; McArthur, J.C.; Bezman, L.; Griffin, D.E. Cytokine Expression in the Brain during the Acquired Immunodeficiency Syndrome. Ann Neurol 1992, 31, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Garvey, L.J.; Pavese, N.; Politis, M.; Ramlackhansingh, A.; Brooks, D.J.; Taylor-Robinson, S.D.; Winston, A. Increased Microglia Activation in Neurologically Asymptomatic HIV-Infected Patients Receiving Effective ART. AIDS 2014, 28, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.P.; Morgan, E.E.; Marquie-Beck, J.; Carey, C.L.; Grant, I.; Letendre, S.L. HIV Neurobehavioral Research Center (HNRC) Group Markers of Macrophage Activation and Axonal Injury Are Associated with Prospective Memory in HIV-1 Disease. Cogn Behav Neurol 2006, 19, 217–221. [Google Scholar] [CrossRef] [PubMed]
- D’Aversa, T.G.; Yu, K.O.A.; Berman, J.W. Expression of Chemokines by Human Fetal Microglia after Treatment with the Human Immunodeficiency Virus Type 1 Protein Tat. J Neurovirol 2004, 10, 86–97. [Google Scholar] [CrossRef]
- Chivero, E.T.; Guo, M.-L.; Periyasamy, P.; Liao, K.; Callen, S.E.; Buch, S. HIV-1 Tat Primes and Activates Microglial NLRP3 Inflammasome-Mediated Neuroinflammation. J. Neurosci. 2017, 37, 3599–3609. [Google Scholar] [CrossRef]
- Xu, C.; Liu, J.; Chen, L.; Liang, S.; Fujii, N.; Tamamura, H.; Xiong, H. HIV-1 Gp120 Enhances Outward Potassium Current via CXCR4 and cAMP-Dependent Protein Kinase A Signaling in Cultured Rat Microglia. Glia 2011, 59, 997–1007. [Google Scholar] [CrossRef]
- Mamik, M.K.; Hui, E.; Branton, W.G.; McKenzie, B.A.; Chisholm, J.; Cohen, E.A.; Power, C. HIV-1 Viral Protein R Activates NLRP3 Inflammasome in Microglia: Implications for HIV-1 Associated Neuroinflammation. J Neuroimmune Pharmacol 2017, 12, 233–248. [Google Scholar] [CrossRef]
- Yarandi, S.S.; Duggan, M.R.; Sariyer, I.K. Emerging Role of Nef in the Development of HIV Associated Neurological Disorders. J Neuroimmune Pharmacol 2021, 16, 238–250. [Google Scholar] [CrossRef]
- Adamson, D.C.; Kopnisky, K.L.; Dawson, T.M.; Dawson, V.L. Mechanisms and Structural Determinants of HIV-1 Coat Protein, Gp41-Induced Neurotoxicity. J Neurosci 1999, 19, 64–71. [Google Scholar] [CrossRef]
- Nolting, T.; Lindecke, A.; Hartung, H.-P.; Koutsilieri, E.; Maschke, M.; Husstedt, I.-W.; Sopper, S.; Stüve, O.; Arendt, G. German Competence Network HIV/AIDS Cytokine Levels in CSF and Neuropsychological Performance in HIV Patients. J Neurovirol 2012, 18, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tang, H.; Xiong, H. Chemokine CCL2 Enhances NMDA Receptor-Mediated Excitatory Postsynaptic Current in Rat Hippocampal Slices-a Potential Mechanism for HIV-1-Associated Neuropathy? J Neuroimmune Pharmacol 2016, 11, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Abassi, M.; Morawski, B.M.; Nakigozi, G.; Nakasujja, N.; Kong, X.; Meya, D.B.; Robertson, K.; Gray, R.; Wawer, M.J.; Sacktor, N.; et al. Cerebrospinal Fluid Biomarkers and HIV-Associated Neurocognitive Disorders in HIV- Infected Individuals in Rakai, Uganda. J Neurovirol 2017, 23, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Knight, A.; Gupta, S.; Knapp, P.; Hauser, K.; Keller, J.; Bruce-Keller, A. HIV Tat Elicits Microglial Glutamate Release: Role of NAPDH Oxidase and the Cystine-Glutamate Antiporter. Neuroscience letters 2010, 485, 233–236. [Google Scholar] [CrossRef]
- Achim, C.L.; Heyes, M.P.; Wiley, C.A. Quantitation of Human Immunodeficiency Virus, Immune Activation Factors, and Quinolinic Acid in AIDS Brains. J Clin Invest 1993, 91, 2769–2775. [Google Scholar] [CrossRef]
- Zhang, K.; McQuibban, G.A.; Silva, C.; Butler, G.S.; Johnston, J.B.; Holden, J.; Clark-Lewis, I.; Overall, C.M.; Power, C. HIV-Induced Metalloproteinase Processing of the Chemokine Stromal Cell Derived Factor-1 Causes Neurodegeneration. Nat Neurosci 2003, 6, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Michael, H.; Mpofana, T.; Ramlall, S.; Oosthuizen, F. The Role of Brain Derived Neurotrophic Factor in HIV-Associated Neurocognitive Disorder: From the Bench-Top to the Bedside. Neuropsychiatr Dis Treat 2020, 16, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Alirezaei, M.; Kiosses, W.B.; Fox, H.S. Decreased Neuronal Autophagy in HIV Dementia: A Mechanism of Indirect Neurotoxicity. Autophagy 2008, 4, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Bonfoco, E.; Krainc, D.; Ankarcrona, M.; Nicotera, P.; Lipton, S.A. Apoptosis and Necrosis: Two Distinct Events Induced, Respectively, by Mild and Intense Insults with N-Methyl-D-Aspartate or Nitric Oxide/Superoxide in Cortical Cell Cultures. Proc Natl Acad Sci U S A 1995, 92, 7162–7166. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, J.; Bao, J.; Bai, Q.; Wang, G. Interaction of Microglia and Astrocytes in the Neurovascular Unit. Frontiers in Immunology 2020, 11, 1024. [Google Scholar] [CrossRef]
- Hammer, S.M.; Squires, K.E.; Hughes, M.D.; Grimes, J.M.; Demeter, L.M.; Currier, J.S.; Eron, J.J.; Feinberg, J.E.; Balfour, H.H.; Deyton, L.R.; et al. A Controlled Trial of Two Nucleoside Analogues plus Indinavir in Persons with Human Immunodeficiency Virus Infection and CD4 Cell Counts of 200 per Cubic Millimeter or Less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med 1997, 337, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.L.; Hanson, D.L.; Dworkin, M.S.; Alderton, D.L.; Fleming, P.L.; Kaplan, J.E.; Ward, J. Surveillance for AIDS-Defining Opportunistic Illnesses, 1992-1997. MMWR CDC Surveill Summ 1999, 48, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Palella, F.J.; Delaney, K.M.; Moorman, A.C.; Loveless, M.O.; Fuhrer, J.; Satten, G.A.; Aschman, D.J.; Holmberg, S.D. Declining Morbidity and Mortality among Patients with Advanced Human Immunodeficiency Virus Infection. HIV Outpatient Study Investigators. N Engl J Med 1998, 338, 853–860. [Google Scholar] [CrossRef]
- Teeraananchai, S.; Kerr, S.J.; Amin, J.; Ruxrungtham, K.; Law, M.G. Life Expectancy of HIV-Positive People after Starting Combination Antiretroviral Therapy: A Meta-Analysis. HIV Med 2017, 18, 256–266. [Google Scholar] [CrossRef]
- Lanman, T.; Letendre, S.; Ma, Q.; Bang, A.; Ellis, R. CNS Neurotoxicity of Antiretrovirals. J Neuroimmune Pharmacol 2021, 16, 130–143. [Google Scholar] [CrossRef]
- Amusan, P.; Power, C.; Gill, M.J.; Gomez, D.; Johnson, E.; Rubin, L.H.; Fujiwara, E. Lifetime Antiretroviral Exposure and Neurocognitive Impairment in HIV. J Neurovirol 2020, 26, 743–753. [Google Scholar] [CrossRef]
- De Benedetto, I.; Trunfio, M.; Guastamacchia, G.; Bonora, S.; Calcagno, A. A Review of the Potential Mechanisms of Neuronal Toxicity Associated with Antiretroviral Drugs. J Neurovirol 2020, 26, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Heaton, R.K.; Franklin, D.R.; Ellis, R.J.; McCutchan, J.A.; Letendre, S.L.; Leblanc, S.; Corkran, S.H.; Duarte, N.A.; Clifford, D.B.; Woods, S.P.; et al. HIV-Associated Neurocognitive Disorders before and during the Era of Combination Antiretroviral Therapy: Differences in Rates, Nature, and Predictors. J Neurovirol 2011, 17, 3–16. [Google Scholar] [CrossRef]
- Gelman, B.B. Neuropathology of HAND With Suppressive Antiretroviral Therapy: Encephalitis and Neurodegeneration Reconsidered. Curr HIV/AIDS Rep 2015, 12, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Heaton, R.K.; Franklin, D.R.; Deutsch, R.; Letendre, S.; Ellis, R.J.; Casaletto, K.; Marquine, M.J.; Woods, S.P.; Vaida, F.; Atkinson, J.H.; et al. Neurocognitive Change in the Era of HIV Combination Antiretroviral Therapy: The Longitudinal CHARTER Study. Clin Infect Dis 2015, 60, 473–480. [Google Scholar] [CrossRef]
- Caruana, G.; Vidili, G.; Serra, P.A.; Bagella, P.; Spanu, A.; Fiore, V.; Calvisi, D.F.; Manetti, R.; Rocchitta, G.; Nuvoli, S.; et al. The Burden of HIV-Associated Neurocognitive Disorder (HAND) in Post-HAART Era: A Multidisciplinary Review of the Literature. Eur Rev Med Pharmacol Sci 2017, 21, 2290–2301. [Google Scholar] [PubMed]
- Estes, J.D.; Kityo, C.; Ssali, F.; Swainson, L.; Makamdop, K.N.; Del Prete, G.Q.; Deeks, S.G.; Luciw, P.A.; Chipman, J.G.; Beilman, G.J.; et al. Defining Total-Body AIDS-Virus Burden with Implications for Curative Strategies. Nat Med 2017, 23, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Lamers, S.L.; Rose, R.; Maidji, E.; Agsalda-Garcia, M.; Nolan, D.J.; Fogel, G.B.; Salemi, M.; Garcia, D.L.; Bracci, P.; Yong, W.; et al. HIV DNA Is Frequently Present within Pathologic Tissues Evaluated at Autopsy from Combined Antiretroviral Therapy-Treated Patients with Undetectable Viral Loads. J Virol 2016, 90, 8968–8983. [Google Scholar] [CrossRef] [PubMed]
- Avalos, C.R.; Abreu, C.M.; Queen, S.E.; Li, M.; Price, S.; Shirk, E.N.; Engle, E.L.; Forsyth, E.; Bullock, B.T.; Mac Gabhann, F.; et al. Brain Macrophages in Simian Immunodeficiency Virus-Infected, Antiretroviral-Suppressed Macaques: A Functional Latent Reservoir. mBio 2017, 8, e01186-17. [Google Scholar] [CrossRef]
- Gandhi, R.T.; Cyktor, J.C.; Bosch, R.J.; Mar, H.; Laird, G.M.; Martin, A.; Collier, A.C.; Riddler, S.A.; Macatangay, B.J.; Rinaldo, C.R.; et al. Selective Decay of Intact HIV-1 Proviral DNA on Antiretroviral Therapy. J Infect Dis 2021, 223, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Gabuzda, D.; Yin, J.; Misra, V.; Chettimada, S.; Gelman, B.B. Intact Proviral DNA Analysis of the Brain Viral Reservoir and Relationship to Neuroinflammation in People with HIV on Suppressive Antiretroviral Therapy. Viruses 2023, 15, 1009. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, C.R.; Angelovich, T.A.; Byrnes, S.J.; Waring, E.; Guanizo, A.C.; Trollope, G.S.; Zhou, J.; Vue, J.; Senior, L.; Wanicek, E.; et al. Intact HIV Proviruses Persist in the Brain Despite Viral Suppression with ART. Ann Neurol 2022, 92, 532–544. [Google Scholar] [CrossRef]
- Abreu, C.M.; Veenhuis, R.T.; Avalos, C.R.; Graham, S.; Parrilla, D.R.; Ferreira, E.A.; Queen, S.E.; Shirk, E.N.; Bullock, B.T.; Li, M.; et al. Myeloid and CD4 T Cells Comprise the Latent Reservoir in Antiretroviral Therapy-Suppressed SIVmac251-Infected Macaques. mBio 2019, 10, e01659-19. [Google Scholar] [CrossRef]
- Angelovich, T.A.; Cochrane, C.R.; Zhou, J.; Tumpach, C.; Byrnes, S.J.; Jamal Eddine, J.; Waring, E.; Busman-Sahay, K.; Deleage, C.; Jenkins, T.A.; et al. Regional Analysis of Intact and Defective HIV Proviruses in the Brain of Viremic and Virally Suppressed People with HIV. Annals of Neurology 2023, 94, 798–802. [Google Scholar] [CrossRef]
- Gama, L.; Abreu, C.M.; Shirk, E.N.; Price, S.L.; Li, M.; Laird, G.M.; Pate, K.A.M.; Wietgrefe, S.W.; O’Connor, S.L.; Pianowski, L.; et al. Reactivation of Simian Immunodeficiency Virus Reservoirs in the Brain of Virally Suppressed Macaques. AIDS 2017, 31, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chaillon, A.; Gianella, S.; Wong, L.M.; Li, D.; Simermeyer, T.L.; Porrachia, M.; Ignacio, C.; Woodworth, B.; Zhong, D.; et al. Brain Microglia Serve as a Persistent HIV Reservoir despite Durable Antiretroviral Therapy. J Clin Invest 2023, 133, e167417. [Google Scholar] [CrossRef]
- Einkauf, K.B.; Osborn, M.R.; Gao, C.; Sun, W.; Sun, X.; Lian, X.; Parsons, E.M.; Gladkov, G.T.; Seiger, K.W.; Blackmer, J.E.; et al. Parallel Analysis of Transcription, Integration, and Sequence of Single HIV-1 Proviruses. Cell 2022, 185, 266–282. [Google Scholar] [CrossRef]
- Huang, A.S.; Ramos, V.; Oliveira, T.Y.; Gaebler, C.; Jankovic, M.; Nussenzweig, M.C.; Cohn, L.B. Integration Features of Intact Latent HIV-1 in CD4+ T Cell Clones Contribute to Viral Persistence. J Exp Med 2021, 218, e20211427. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Lian, X.; Gao, C.; Sun, X.; Einkauf, K.B.; Chevalier, J.M.; Chen, S.M.Y.; Hua, S.; Rhee, B.; Chang, K.; et al. Distinct Viral Reservoirs in Individuals with Spontaneous Control of HIV-1. Nature 2020, 585, 261–267. [Google Scholar] [CrossRef]
- Elsheikh, M.M.; Tang, Y.; Li, D.; Jiang, G. Deep Latency: A New Insight into a Functional HIV Cure. EBioMedicine 2019, 45, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.J.; Bacchetti, P.; Ritter, K.D.; Beg, S.; Lai, J.; Martin, J.N.; Hunt, P.W.; Henrich, T.J.; Siliciano, J.D.; Siliciano, R.F.; et al. Differential Decay of Intact and Defective Proviral DNA in HIV-1-Infected Individuals on Suppressive Antiretroviral Therapy. JCI Insight 2020, 5, e132997. [Google Scholar] [CrossRef] [PubMed]
- Petit, F.; Arnoult, D.; Lelièvre, J.-D.; Moutouh-de Parseval, L.; Hance, A.J.; Schneider, P.; Corbeil, J.; Ameisen, J.C.; Estaquier, J. Productive HIV-1 Infection of Primary CD4+ T Cells Induces Mitochondrial Membrane Permeabilization Leading to a Caspase-Independent Cell Death. J Biol Chem 2002, 277, 1477–1487. [Google Scholar] [CrossRef]
- Ho, D.D.; Neumann, A.U.; Perelson, A.S.; Chen, W.; Leonard, J.M.; Markowitz, M. Rapid Turnover of Plasma Virions and CD4 Lymphocytes in HIV-1 Infection. Nature 1995, 373, 123–126. [Google Scholar] [CrossRef]
- McMyn, N.F.; Varriale, J.; Fray, E.J.; Zitzmann, C.; MacLeod, H.; Lai, J.; Singhal, A.; Moskovljevic, M.; Garcia, M.A.; Lopez, B.M.; et al. The Latent Reservoir of Inducible, Infectious HIV-1 Does Not Decrease despite Decades of Antiretroviral Therapy. J Clin Invest 133, e171554. [CrossRef]
- Roda, W.C.; Li, M.Y.; Akinwumi, M.S.; Asahchop, E.L.; Gelman, B.B.; Witwer, K.W.; Power, C. Modeling Brain Lentiviral Infections during Antiretroviral Therapy in AIDS. J. Neurovirol. 2017, 23, 577–586. [Google Scholar] [CrossRef]
- Stefic, K.; Chaillon, A.; Bouvin-Pley, M.; Moreau, A.; Braibant, M.; Bastides, F.; Gras, G.; Bernard, L.; Barin, F. Probing the Compartmentalization of HIV-1 in the Central Nervous System through Its Neutralization Properties. PLOS ONE 2017, 12, e0181680. [Google Scholar] [CrossRef]
- Brese, R.L.; Gonzalez-Perez, M.P.; Koch, M.; O’Connell, O.; Luzuriaga, K.; Somasundaran, M.; Clapham, P.R.; Dollar, J.J.; Nolan, D.J.; Rose, R.; et al. Ultradeep Single-Molecule Real-Time Sequencing of HIV Envelope Reveals Complete Compartmentalization of Highly Macrophage-Tropic R5 Proviral Variants in Brain and CXCR4-Using Variants in Immune and Peripheral Tissues. J. Neurovirol. 2018, 24, 439–453. [Google Scholar] [CrossRef]
- Ritola, K.; Robertson, K.; Fiscus, S.A.; Hall, C.; Swanstrom, R. Increased Human Immunodeficiency Virus Type 1 (HIV-1) Env Compartmentalization in the Presence of HIV-1-Associated Dementia. Journal of Virology 2005, 79, 10830–10834. [Google Scholar] [CrossRef]
- Gianella, S.; Kosakovsky Pond, S.L.; Oliveira, M.F.; Scheffler, K.; Strain, M.C.; De la Torre, A.; Letendre, S.; Smith, D.M.; Ellis, R.J. Compartmentalized HIV Rebound in the Central Nervous System after Interruption of Antiretroviral Therapy. Virus Evol 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Power, C.; McArthur, J.C.; Johnson, R.T.; Griffin, D.E.; Glass, J.D.; Perryman, S.; Chesebro, B. Demented and Nondemented Patients with AIDS Differ in Brain-Derived Human Immunodeficiency Virus Type 1 Envelope Sequences. J Virol 1994, 68, 4643–4649. [Google Scholar] [CrossRef] [PubMed]
- Harrington, P.R.; Schnell, G.; Letendre, S.L.; Ritola, K.; Robertson, K.; Hall, C.; Burch, C.L.; Jabara, C.B.; Moore, D.T.; Ellis, R.J.; et al. Cross-Sectional Characterization of HIV-1 Env Compartmentalization in Cerebrospinal Fluid over the Full Disease Course. AIDS 2009, 23, 907–915. [Google Scholar] [CrossRef]
- Schnell, G.; Joseph, S.; Spudich, S.; Price, R.W.; Swanstrom, R. HIV-1 Replication in the Central Nervous System Occurs in Two Distinct Cell Types. PLoS Pathog 2011, 7, e1002286. [Google Scholar] [CrossRef]
- Dunfee, R.L.; Thomas, E.R.; Gorry, P.R.; Wang, J.; Taylor, J.; Kunstman, K.; Wolinsky, S.M.; Gabuzda, D. The HIV Env Variant N283 Enhances Macrophage Tropism and Is Associated with Brain Infection and Dementia. PNAS 2006, 103, 15160–15165. [Google Scholar] [CrossRef] [PubMed]
- Coffin, J.; Swanstrom, R. HIV Pathogenesis: Dynamics and Genetics of Viral Populations and Infected Cells. Cold Spring Harb Perspect Med 2013, 3, a012526. [Google Scholar] [CrossRef] [PubMed]
- Mansky, L.M.; Temin, H.M. Lower in Vivo Mutation Rate of Human Immunodeficiency Virus Type 1 than That Predicted from the Fidelity of Purified Reverse Transcriptase. J Virol 1995, 69, 5087–5094. [Google Scholar] [CrossRef]
- Redd, A.D.; Quinn, T.C.; Tobian, A.A.R. Frequency and Implications of HIV Superinfection. Lancet Infect Dis 2013, 13, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Koning, F.A.; Badhan, A.; Shaw, S.; Fisher, M.; Mbisa, J.L.; Cane, P.A. Dynamics of HIV Type 1 Recombination Following Superinfection. AIDS Res Hum Retroviruses 2013, 29, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Simon-Loriere, E.; Holmes, E.C. Why Do RNA Viruses Recombine? Nat Rev Microbiol 2011, 9, 617–626. [Google Scholar] [CrossRef]
- Feng, Y.; Baig, T.T.; Love, R.P.; Chelico, L. Suppression of APOBEC3-Mediated Restriction of HIV-1 by Vif. Front Microbiol 2014, 5, 450. [Google Scholar] [CrossRef] [PubMed]
- Mulder, L.C.F.; Harari, A.; Simon, V. Cytidine Deamination Induced HIV-1 Drug Resistance. Proc Natl Acad Sci U S A 2008, 105, 5501–5506. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, N.; Zhang, N.; Branton, W.G.; Zghidi-Abouzid, O.; Cohen, E.A.; Gelman, B.B.; Estaquier, J.; Kong, L.; Power, C. The HIV Restriction Factor Profile in the Brain Is Associated with the Clinical Status and Viral Quantities. Viruses 2023, 15, 316. [Google Scholar] [CrossRef]
- Smit, T.K.; Brew, B.J.; Tourtellotte, W.; Morgello, S.; Gelman, B.B.; Saksena, N.K. Independent Evolution of Human Immunodeficiency Virus (HIV) Drug Resistance Mutations in Diverse Areas of the Brain in HIV-Infected Patients, with and without Dementia, on Antiretroviral Treatment. J Virol 2004, 78, 10133–10148. [Google Scholar] [CrossRef] [PubMed]
- Venturi, G.; Catucci, M.; Romano, L.; Corsi, P.; Leoncini, F.; Valensin, P.E.; Zazzi, M. Antiretroviral Resistance Mutations in Human Immunodeficiency Virus Type 1 Reverse Transcriptase and Protease from Paired Cerebrospinal Fluid and Plasma Samples. J Infect Dis 2000, 181, 740–745. [Google Scholar] [CrossRef]
- Edén, A.; Fuchs, D.; Hagberg, L.; Nilsson, S.; Spudich, S.; Svennerholm, B.; Price, R.W.; Gisslén, M. HIV-1 Viral Escape in Cerebrospinal Fluid of Subjects on Suppressive Antiretroviral Treatment. J Infect Dis 2010, 202, 1819–1825. [Google Scholar] [CrossRef]
- Pérez-Valero, I.; Ellis, R.; Heaton, R.; Deutsch, R.; Franklin, D.; Clifford, D.B.; Collier, A.; Gelman, B.; Marra, C.; McCutchan, J.A.; et al. Cerebrospinal Fluid Viral Escape in Aviremic HIV-Infected Patients Receiving Antiretroviral Therapy: Prevalence, Risk Factors and Neurocognitive Effects. AIDS 2019, 33, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Henderson, M.; Pepper, N.; Bawa, M.; Muir, D.; Everitt, A.; Mackie, N.E.; Winston, A. Cerebrospinal Fluid Virology in People with HIV. HIV Med 2023, 24, 838–844. [Google Scholar] [CrossRef]
- Filippidis, P.; Damas, J.; Viala, B.; Assal, F.; Nawej Tshikung, O.; Tarr, P.; Derfuss, T.; Oberholzer, M.; Jelcic, I.; Hundsberger, T.; et al. Cerebrospinal Fluid HIV-1 Escape in Patients With Neurocognitive Symptoms: Pooled Data From a Neuro-HIV Platform and the NAMACO Study. J Acquir Immune Defic Syndr 2023, 93, 219–228. [Google Scholar] [CrossRef]
- Manesh, A.; Barnabas, R.; Mani, S.; Karthik, R.; Abraham, O.C.; Chacko, G.; Kannangai, R.; Varghese, G.M. Symptomatic HIV CNS Viral Escape among Patients on Effective cART. Int J Infect Dis 2019, 84, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, C.D.; Reed-Walker, K.; Sheth, A.N.; Acosta, E.P.; Vunnava, A.; Ofotokun, I. Cerebrospinal Fluid Concentrations of Tenofovir and Emtricitabine in the Setting of HIV-1 Protease Inhibitor-Based Regimens. J Clin Pharmacol 2016, 56, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Mukerji, S.S.; Misra, V.; Lorenz, D.; Cervantes-Arslanian, A.M.; Lyons, J.; Chalkias, S.; Wurcel, A.; Burke, D.; Venna, N.; Morgello, S.; et al. Temporal Patterns and Drug Resistance in CSF Viral Escape Among ART-Experienced HIV-1 Infected Adults. J Acquir Immune Defic Syndr 2017, 75, 246–255. [Google Scholar] [CrossRef]
- Dravid, A.N.; Natrajan, K.; Kulkarni, M.M.; Saraf, C.K.; Mahajan, U.S.; Kore, S.D.; Rathod, N.M.; Mahajan, U.S.; Wadia, R.S. Discordant CSF/Plasma HIV-1 RNA in Individuals on Virologically Suppressive Antiretroviral Therapy in Western India. Medicine (Baltimore) 2018, 97, e9969. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, Y.; Wei, F.; Zhao, Q.; Wang, X.; Yuan, L.; Li, N.; Chen, D. Discordant Genotypic Resistance and HIV-1 Genetic Diversity from Paired Plasma and Cerebrospinal Fluid Samples in Chinese Settings. J Neurovirol 2013, 19, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Bavaro, D.F.; Calamo, A.; Lepore, L.; Fabrizio, C.; Saracino, A.; Angarano, G.; Monno, L. Cerebrospinal Fluid Compartmentalization of HIV-1 and Correlation with Plasma Viral Load and Blood-Brain Barrier Damage. Infection 2019, 47, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Canestri, A.; Lescure, F.-X.; Jaureguiberry, S.; Moulignier, A.; Amiel, C.; Marcelin, A.G.; Peytavin, G.; Tubiana, R.; Pialoux, G.; Katlama, C. Discordance between Cerebral Spinal Fluid and Plasma HIV Replication in Patients with Neurological Symptoms Who Are Receiving Suppressive Antiretroviral Therapy. Clin Infect Dis 2010, 50, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Blood-Brain Barrier Drug Targeting: The Future of Brain Drug Development. Mol Interv 2003, 3, 90–105. [Google Scholar] [CrossRef]
- Nath, A. Eradication of Human Immunodeficiency Virus from Brain Reservoirs. J Neurovirol 2015, 21, 227–234. [Google Scholar] [CrossRef]
- Asahchop, E.L.; Meziane, O.; Mamik, M.K.; Chan, W.F.; Branton, W.G.; Resch, L.; Gill, M.J.; Haddad, E.; Guimond, J.V.; Wainberg, M.A.; et al. Reduced Antiretroviral Drug Efficacy and Concentration in HIV-Infected Microglia Contributes to Viral Persistence in Brain. Retrovirology 2017, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Gates, T.M.; Cysique, L.A.; Siefried, K.J.; Chaganti, J.; Moffat, K.J.; Brew, B.J. Maraviroc-Intensified Combined Antiretroviral Therapy Improves Cognition in Virally Suppressed HIV-Associated Neurocognitive Disorder. AIDS 2016, 30, 591–600. [Google Scholar] [CrossRef]
- Letendre, S.; Marquie-Beck, J.; Capparelli, E.; Best, B.; Clifford, D.; Collier, A.C.; Gelman, B.B.; McArthur, J.C.; McCutchan, J.A.; Morgello, S.; et al. Validation of the CNS Penetration-Effectiveness Rank for Quantifying Antiretroviral Penetration into the Central Nervous System. Arch Neurol 2008, 65, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Letendre, S.L.; Ellis, R.J.; Ances, B.M.; McCutchan, J.A. Neurologic Complications of HIV Disease and Their Treatment. Top HIV Med 2010, 18, 45–55. [Google Scholar] [PubMed]
- Arentoft, A.; Troxell, K.; Alvarez, K.; Aghvinian, M.; Rivera Mindt, M.; Cherner, M.; Van Dyk, K.; Razani, J.; Roxas, M.; Gavilanes, M. HIV Antiretroviral Medication Neuropenetrance and Neurocognitive Outcomes in HIV+ Adults: A Review of the Literature Examining the Central Nervous System Penetration Effectiveness Score. Viruses 2022, 14, 1151. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.J.; Borrelli, E.P.; Vyas, A.; Taylor, L.E.; Buchanan, A.L. The Effect of Antiretroviral Therapy with High Central Nervous System Penetration on HIV-Related Cognitive Impairment: A Systematic Review and Meta-Analysis. AIDS Care 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.M.A.; Locatelli, I.; Métral, M.; Calmy, A.; Lecompte, T.D.; Nadin, I.; Hauser, C.; Cusini, A.; Hasse, B.; Kovari, H.; et al. Cross-Sectional and Cumulative Longitudinal Central Nervous System Penetration Effectiveness Scores Are Not Associated With Neurocognitive Impairment in a Well Treated Aging Human Immunodeficiency Virus-Positive Population in Switzerland. Open Forum Infect Dis 2019, 6, ofz277. [Google Scholar] [CrossRef] [PubMed]
- Ndhlovu, L.C.; Umaki, T.; Chew, G.M.; Chow, D.C.; Agsalda, M.; Kallianpur, K.J.; Paul, R.; Zhang, G.; Ho, E.; Hanks, N.; et al. Treatment Intensification with Maraviroc (CCR5 Antagonist) Leads to Declines in CD16-Expressing Monocytes in cART-Suppressed Chronic HIV-Infected Subjects and Is Associated with Improvements in Neurocognitive Test Performance: Implications for HIV-Associated Neurocognitive Disease (HAND). J Neurovirol 2014, 20, 571–582. [Google Scholar] [CrossRef]
- Barber, T.J.; Imaz, A.; Boffito, M.; Niubó, J.; Pozniak, A.; Fortuny, R.; Alonso, J.; Davies, N.; Mandalia, S.; Podzamczer, D.; et al. CSF Inflammatory Markers and Neurocognitive Function after Addition of Maraviroc to Monotherapy Darunavir/Ritonavir in Stable HIV Patients: The CINAMMON Study. J Neurovirol 2018, 24, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Berruti, M.; Riccardi, N.; Canetti, D.; Lo Caputo, S.; Taramasso, L.; Di Biagio, A. Injectable Antiretroviral Drugs: Back to the Future. Viruses 2021, 13, 228. [Google Scholar] [CrossRef]
- Venkatesan, P. Long-Acting Injectable ART for HIV: A (Cautious) Step Forward. Lancet Microbe 2022, 3, e94. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, W.; Sun, M.; Li, T. Broadly Neutralizing Antibodies for HIV-1: Efficacies, Challenges and Opportunities. Emerg Microbes Infect 9. [CrossRef]
- Hsu, D.C.; Mellors, J.W.; Vasan, S. Can Broadly Neutralizing HIV-1 Antibodies Help Achieve an ART-Free Remission? Front Immunol 2021, 12, 710044. [Google Scholar] [CrossRef]
- Spencer, D.A.; Shapiro, M.B.; Haigwood, N.L.; Hessell, A.J. Advancing HIV Broadly Neutralizing Antibodies: From Discovery to the Clinic. Front Public Health 2021, 9, 690017. [Google Scholar] [CrossRef] [PubMed]
- Haynes, B.F.; Wiehe, K.; Borrow, P.; Saunders, K.O.; Korber, B.; Wagh, K.; McMichael, A.J.; Kelsoe, G.; Hahn, B.H.; Alt, F.; et al. Strategies for HIV-1 Vaccines That Induce Broadly Neutralizing Antibodies. Nat Rev Immunol 2023, 23, 142–158. [Google Scholar] [CrossRef]
- Rubenstein, J.L.; Combs, D.; Rosenberg, J.; Levy, A.; McDermott, M.; Damon, L.; Ignoffo, R.; Aldape, K.; Shen, A.; Lee, D.; et al. Rituximab Therapy for CNS Lymphomas: Targeting the Leptomeningeal Compartment. Blood 2003, 101, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Sengsayadeth, S.; Savani, B.N.; Oluwole, O.; Dholaria, B. Overview of Approved CAR-T Therapies, Ongoing Clinical Trials, and Its Impact on Clinical Practice. EJHaem 2021, 3, 6–10. [Google Scholar] [CrossRef]
- Sun, M.-Y.; Li, W.; Chen, W. Chimeric Antigen Receptor T Cell and Regulatory T Cell Therapy in Non-Oncology Diseases: A Narrative Review of Studies from 2017 to 2023. Hum Vaccin Immunother 2023, 19, 2251839. [Google Scholar] [CrossRef] [PubMed]
- Seif, M.; Einsele, H.; Löffler, J. CAR T Cells Beyond Cancer: Hope for Immunomodulatory Therapy of Infectious Diseases. Front Immunol 2019, 10, 2711. [Google Scholar] [CrossRef]
- Ali, A.; Kitchen, S.G.; Chen, I.S.Y.; Ng, H.L.; Zack, J.A.; Yang, O.O. HIV-1-Specific Chimeric Antigen Receptors Based on Broadly Neutralizing Antibodies. J Virol 2016, 90, 6999–7006. [Google Scholar] [CrossRef] [PubMed]
- Hale, M.; Mesojednik, T.; Romano Ibarra, G.S.; Sahni, J.; Bernard, A.; Sommer, K.; Scharenberg, A.M.; Rawlings, D.J.; Wagner, T.A. Engineering HIV-Resistant, Anti-HIV Chimeric Antigen Receptor T Cells. Mol Ther 2017, 25, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Buck, A.M.; Deveau, T.-M.; Henrich, T.J.; Deitchman, A.N. Challenges in HIV-1 Latent Reservoir and Target Cell Quantification in CAR-T Cell and Other Lentiviral Gene Modifying HIV Cure Strategies. Viruses 2023, 15, 1126. [Google Scholar] [CrossRef] [PubMed]
- Prator, C.A.; Donatelli, J.; Henrich, T.J. From Berlin to London: HIV-1 Reservoir Reduction Following Stem Cell Transplantation. Curr HIV/AIDS Rep 2020, 17, 385–393. [Google Scholar] [CrossRef]
- Jensen, B.-E.O.; Knops, E.; Cords, L.; Lübke, N.; Salgado, M.; Busman-Sahay, K.; Estes, J.D.; Huyveneers, L.E.P.; Perdomo-Celis, F.; Wittner, M.; et al. In-Depth Virological and Immunological Characterization of HIV-1 Cure after CCR5Δ32/Δ32 Allogeneic Hematopoietic Stem Cell Transplantation. Nat Med 2023, 29, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Henrich, T.J.; Hanhauser, E.; Marty, F.M.; Sirignano, M.N.; Keating, S.; Lee, T.-H.; Robles, Y.P.; Davis, B.T.; Li, J.Z.; Heisey, A.; et al. Antiretroviral-Free HIV-1 Remission and Viral Rebound Following Allogeneic Stem Cell Transplantation: A Report of Two Cases. Ann Intern Med 2014, 161, 319–327. [Google Scholar] [CrossRef]
- Hoare, J.; Stein, D.J.; Heany, S.J.; Fouche, J.-P.; Phillips, N.; Er, S.; Myer, L.; Zar, H.J.; Horvath, S.; Levine, A.J. Accelerated Epigenetic Aging in Adolescents Living with HIV Is Associated with Altered Development of Brain Structures. J Neurovirol 2022, 28, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S.; Stein, D.J.; Phillips, N.; Heany, S.J.; Kobor, M.S.; Lin, D.T.S.; Myer, L.; Zar, H.J.; Levine, A.J.; Hoare, J. Perinatally Acquired HIV Infection Accelerates Epigenetic Aging in South African Adolescents. AIDS 2018, 32, 1465–1474. [Google Scholar] [CrossRef]
- Chahroudi, A.; Wagner, T.A.; Persaud, D. CNS Persistence of HIV-1 in Children: The Untapped Reservoir. Curr HIV/AIDS Rep 2018, 15, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Hauser, K.F.; Ohene-Nyako, M.; Knapp, P.E. Accelerated Brain Aging with Opioid Misuse and HIV: New Insights on the Role of Glially Derived pro-Inflammation Mediators and Neuronal Chloride Homeostasis. Curr Opin Neurobiol 2023, 78, 102653. [Google Scholar] [CrossRef]
- Fitting, S.; McRae, M.; Hauser, K.F. Opioid and neuroHIV Comorbidity - Current and Future Perspectives. J Neuroimmune Pharmacol 2020, 15, 584–627. [Google Scholar] [CrossRef]
- Trunfio, M.; Chaillon, A.; Beliakova-Bethell, N.; Deiss, R.; Letendre, S.L.; Riggs, P.K.; Higgins, N.; Gianella, S. Beyond the Syndemic of Opioid Use Disorders and HIV: The Impact of Opioids on Viral Reservoirs. Viruses 2023, 15, 1712. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




