Submitted:
03 November 2023
Posted:
06 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Genetic heterogeneity
2.1. Key driver genes
2.2. Chromosomal instability (CIN)
2.3. Microsatellite instability (MSI) status
3. Transcriptomic heterogeneity
3.1. MicroRNAs (miRNAs)
3.3. LncRNAs
3.4. Transcription factors
4. Protein heterogeneity
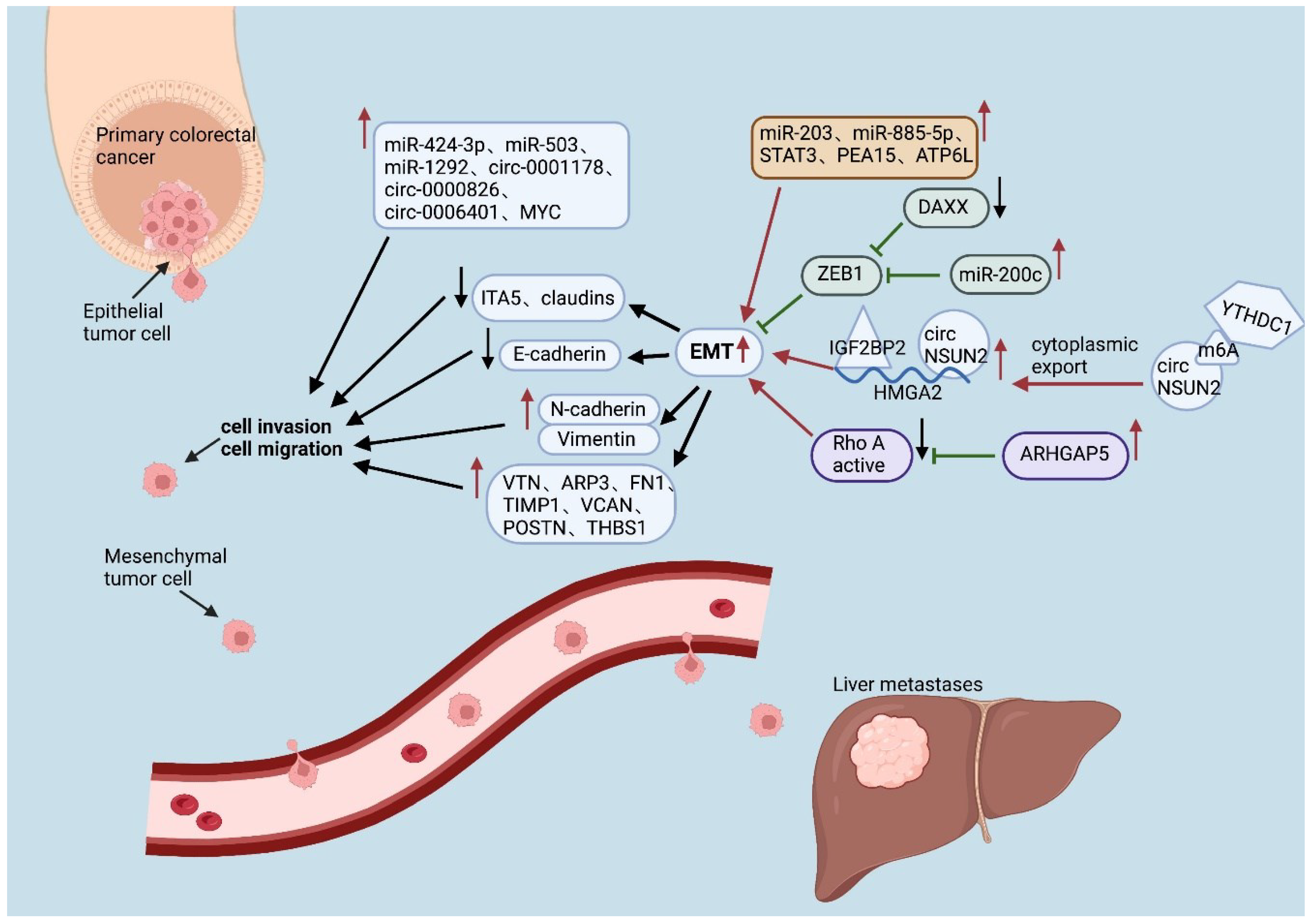
4.1. EMT-related proteins
4.2. Other proteins
5. Metabolic heterogeneity
6. Immune heterogeneity
6.1. Macrophages
6.2. T cells
6.3. Dendritic cells
7. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Assenov, Y. , Brocks, D. & Gerhauser, C. Intratumor heterogeneity in epigenetic patterns. Semin Cancer Biol 2018, 51, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y. et al. Spatiotemporal Immune Landscape of Colorectal Cancer Liver Metastasis at Single-Cell Level. Cancer Discov 2022, 12, 134–153. [Google Scholar] [CrossRef] [PubMed]
- Yu, X. et al. Emerging Role of Immunotherapy for Colorectal Cancer with Liver Metastasis. Onco Targets Ther 2020, 13, 11645–11658. [Google Scholar] [CrossRef] [PubMed]
- Xu, J. et al. Chinese guidelines for the diagnosis and comprehensive treatment of colorectal liver metastases (version 2018). J Cancer Res Clin Oncol 2019, 145, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K. et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol 2019, 16, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Gelsomino, F. , Barbolini, M., Spallanzani, A., Pugliese, G. & Cascinu, S. The evolving role of microsatellite instability in colorectal cancer: A review. Cancer Treat Rev 2016, 51, 19–26. [Google Scholar] [CrossRef]
- Willis, J. A. , Reyes-Uribe, L., Chang, K., Lipkin, S. M. & Vilar, E. Immune Activation in Mismatch Repair-Deficient Carcinogenesis: More Than Just Mutational Rate. Clinical Cancer Research : an Official Journal of the American Association For Cancer Research 2020, 26, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Tamiya, M. et al. Metastatic site as a predictor of nivolumab efficacy in patients with advanced non-small cell lung cancer: A retrospective multicenter trial. PLoS One 2018, 13, e0192227. [Google Scholar] [CrossRef]
- Dagogo-Jack, I. & Shaw, A. T. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Hou, Y. et al. Single-cell triple omics sequencing reveals genetic, epigenetic, and transcriptomic heterogeneity in hepatocellular carcinomas. Cell Res 2016, 26, 304–319. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, S. D. & Bertagnolli, M. M. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med 2009, 361, 2449–2460. [Google Scholar] [CrossRef] [PubMed]
- Sagaert, X. , Vanstapel, A. & Verbeek, S. Tumor Heterogeneity in Colorectal Cancer: What Do We Know So Far? Pathobiology 2018, 85, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Koulouridi, A. et al. Prognostic Value of Mutations in Colorectal Cancer Patients. Cancers (Basel) 2022, 14, 3320. [Google Scholar] [CrossRef] [PubMed]
- Lièvre, A. et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol 2008, 26, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Santos, C. et al. Phase II study of high-sensitivity genotyping of KRAS, NRAS, BRAF and PIK3CA to ultra-select metastatic colorectal cancer patients for panitumumab plus FOLFIRI: the ULTRA trial. Ann Oncol 2019, 30, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Di Nicolantonio, F. et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 2008, 26, 5705–5712. [Google Scholar] [CrossRef] [PubMed]
- Brunsell, T. H. et al. High Concordance and Negative Prognostic Impact of RAS/BRAF/PIK3CA Mutations in Multiple Resected Colorectal Liver Metastases. Clin Colorectal Cancer 2020, 19, e26–e47. [Google Scholar] [CrossRef] [PubMed]
- Baldus, S. E. et al. Prevalence and heterogeneity of KRAS, BRAF, and PIK3CA mutations in primary colorectal adenocarcinomas and their corresponding metastases. Clin Cancer Res 2010, 16, 790–799. [Google Scholar] [CrossRef]
- Knijn, N. et al. KRAS mutation analysis: a comparison between primary tumours and matched liver metastases in 305 colorectal cancer patients. Br J Cancer 2011, 104, 1020–1026. [Google Scholar] [CrossRef]
- Brannon, A. R. et al. Comparative sequencing analysis reveals high genomic concordance between matched primary and metastatic colorectal cancer lesions. Genome Biol 2014, 15, 454. [Google Scholar] [CrossRef] [PubMed]
- Vakiani, E. et al. Comparative genomic analysis of primary versus metastatic colorectal carcinomas. J Clin Oncol 2012, 30, 2956–2962. [Google Scholar] [CrossRef] [PubMed]
- Hou, J. , Zhang, Y. & Zhu, Z. Gene heterogeneity in metastasis of colorectal cancer to the lung. Semin Cell Dev Biol 2017, 64, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Tie, J. et al. KRAS mutation is associated with lung metastasis in patients with curatively resected colorectal cancer. Clin Cancer Res 2011, 17, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Sansregret, L. , Vanhaesebroeck, B. & Swanton, C. Determinants and clinical implications of chromosomal instability in cancer. Nature Reviews. Clinical Oncology 2018, 15, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Rondón, N. , Villegas, V. E. & Rondón-Lagos, M. The Role of Chromosomal Instability in Cancer and Therapeutic Responses. Cancers (Basel) 2017, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Bakhoum, S. F. et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 2018, 553, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Sveen, A. et al. Intra-patient Inter-metastatic Genetic Heterogeneity in Colorectal Cancer as a Key Determinant of Survival after Curative Liver Resection. PLoS Genet 2016, 12, e1006225. [Google Scholar] [CrossRef] [PubMed]
- Gambaro, K. et al. Copy number and transcriptome alterations associated with metastatic lesion response to treatment in colorectal cancer. Clin Transl Med 2021, 11, e401. [Google Scholar] [CrossRef]
- Mogensen, M. B. et al. Genomic alterations accompanying tumour evolution in colorectal cancer: tracking the differences between primary tumours and synchronous liver metastases by whole-exome sequencing. BMC Cancer 2018, 18, 752. [Google Scholar] [CrossRef]
- Mamlouk, S. et al. DNA copy number changes define spatial patterns of heterogeneity in colorectal cancer. Nat Commun 2017, 8, 14093. [Google Scholar] [CrossRef] [PubMed]
- Egeblad, M. & Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002, 2, 161–174. [Google Scholar] [PubMed]
- Saad, R. S. , Ghorab, Z., Khalifa, M. A. & Xu, M. CDX2 as a marker for intestinal differentiation: Its utility and limitations. World J Gastrointest Surg 2011, 3, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Mekenkamp, L. J. M. et al. Chromosomal copy number aberrations in colorectal metastases resemble their primary counterparts and differences are typically non-recurrent. PLoS One 2014, 9, e86833. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S. et al. Identification of chromosomal aberrations of metastatic potential in colorectal carcinoma. Genes Chromosomes Cancer 2010, 49, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Jones, S. et al. Comparative lesion sequencing provides insights into tumor evolution. Proc Natl Acad Sci U S A 2008, 105, 4283–4288. [Google Scholar] [CrossRef] [PubMed]
- Jass, J. R. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology 2007, 50, 113–130. [Google Scholar] [CrossRef] [PubMed]
- He, W.-Z. et al. Comparison of Mismatch Repair Status Between Primary and Matched Metastatic Sites in Patients With Colorectal Cancer. J Natl Compr Canc Netw 2019, 17, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Evrard, C. et al. Heterogeneity of Mismatch Repair Status and Microsatellite Instability between Primary Tumour and Metastasis and Its Implications for Immunotherapy in Colorectal Cancers. International Journal of Molecular Sciences 2022, 23, 4427. [Google Scholar] [CrossRef]
- Jung, J. et al. Comparison of the Mismatch Repair System between Primary and Metastatic Colorectal Cancers Using Immunohistochemistry. J Pathol Transl Med 2017, 51, 129–136. [Google Scholar] [CrossRef]
- Haraldsdottir, S. et al. Mismatch repair deficiency concordance between primary colorectal cancer and corresponding metastasis. Fam Cancer 2016, 15, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Lim, L. P. et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005, 433, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Lu, T. X. & Rothenberg, M. E. MicroRNA. J Allergy Clin Immunol 2018, 141, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Imaoka, H. et al. Circulating microRNA-1290 as a novel diagnostic and prognostic biomarker in human colorectal cancer. Ann Oncol 2016, 27, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Nassar, F. J. et al. Circulating miRNA as Biomarkers for Colorectal Cancer Diagnosis and Liver Metastasis. Diagnostics (Basel) 2021, 11, 341. [Google Scholar] [CrossRef] [PubMed]
- Vychytilova-Faltejskova, P. et al. Genome-wide microRNA Expression Profiling in Primary Tumors and Matched Liver Metastasis of Patients with Colorectal Cancer. Cancer Genomics Proteomics 2016, 13, 311–316. [Google Scholar] [PubMed]
- Hur, K. et al. Identification of a metastasis-specific MicroRNA signature in human colorectal cancer. J Natl Cancer Inst 2015, 107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T. et al. Identifying the key genes and microRNAs in colorectal cancer liver metastasis by bioinformatics analysis and in vitro experiments. Oncol Rep 2019, 41, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-C. et al. MicroRNA-122, a tumor suppressor microRNA that regulates intrahepatic metastasis of hepatocellular carcinoma. Hepatology 2009, 49, 1571–1582. [Google Scholar] [CrossRef]
- Thakral, S. & Ghoshal, K. miR-122 is a unique molecule with great potential in diagnosis, prognosis of liver disease, and therapy both as miRNA mimic and antimir. Curr Gene Ther 2015, 15, 142–150. [Google Scholar]
- Lam, C. S.-C. et al. Identification of microRNA 885-5p as a novel regulator of tumor metastasis by targeting CPEB2 in colorectal cancer. Oncotarget 2017, 8, 26858–26870. [Google Scholar] [CrossRef] [PubMed]
- Su, M. , Qin, B., Liu, F., Chen, Y. & Zhang, R. miR-885-5p upregulation promotes colorectal cancer cell proliferation and migration by targeting suppressor of cytokine signaling. Oncol Lett 2018, 16, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Pizzini, S. et al. Impact of microRNAs on regulatory networks and pathways in human colorectal carcinogenesis and development of metastasis. BMC Genomics 2013, 14, 589. [Google Scholar] [CrossRef] [PubMed]
- Song, J. J. & Li, W. MiR-10b suppresses the growth and metastasis of colorectal cancer cell by targeting FGF13. Eur Rev Med Pharmacol Sci 2019, 23, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Hur, K. et al. Circulating microRNA-203 predicts prognosis and metastasis in human colorectal cancer. Gut 2017, 66, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Hur, K. et al. MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT) in human colorectal cancer metastasis. Gut 2013, 62, 1315–1326. [Google Scholar] [CrossRef]
- Furuta, M. et al. miR-124 and miR-203 are epigenetically silenced tumor-suppressive microRNAs in hepatocellular carcinoma. Carcinogenesis 2010, 31, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y. et al. miR-203, a tumor suppressor frequently down-regulated by promoter hypermethylation in rhabdomyosarcoma. J Biol Chem 2014, 289, 529–539. [Google Scholar] [CrossRef]
- Torres, S. et al. Combined miRNA profiling and proteomics demonstrates that different miRNAs target a common set of proteins to promote colorectal cancer metastasis. J Pathol 2017, 242, 39–51. [Google Scholar] [CrossRef]
- Vo, J. N. et al. The Landscape of Circular RNA in Cancer. Cell 2019, 176. [Google Scholar] [CrossRef]
- Capel, B. et al. Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell 1993, 73, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Greene, J. et al. Circular RNAs: Biogenesis, Function and Role in Human Diseases. Front Mol Biosci 2017, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L. S. et al. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet 2019, 20, 675–691. [Google Scholar] [CrossRef]
- Guo, Y. et al. Circ3823 contributes to growth, metastasis and angiogenesis of colorectal cancer: involvement of miR-30c-5p/TCF7 axis. Mol Cancer 2021, 20, 93. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. et al. The circular RNA 001971/miR-29c-3p axis modulates colorectal cancer growth, metastasis, and angiogenesis through VEGFA. J Exp Clin Cancer Res 2020, 39, 91. [Google Scholar] [CrossRef] [PubMed]
- Long, F. et al. Comprehensive landscape and future perspectives of circular RNAs in colorectal cancer. Mol Cancer 2021, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Xu, H. , Wang, C., Song, H., Xu, Y. & Ji, G. RNA-Seq profiling of circular RNAs in human colorectal Cancer liver metastasis and the potential biomarkers. Mol Cancer 2019, 18, 8. [Google Scholar] [CrossRef]
- Chen, R.-X. et al. N-methyladenosine modification of circNSUN2 facilitates cytoplasmic export and stabilizes HMGA2 to promote colorectal liver metastasis. Nat Commun 2019, 10, 4695. [Google Scholar] [CrossRef]
- Zhang, C. et al. Circular RNA hsa_circ_0006401 promotes proliferation and metastasis in colorectal carcinoma. Cell Death Dis 2021, 12, 443. [Google Scholar] [CrossRef]
- Li, Y. et al. HMGA2 induces transcription factor Slug expression to promote epithelial-to-mesenchymal transition and contributes to colon cancer progression. Cancer Lett 2014, 355, 130–140. [Google Scholar] [CrossRef]
- Zhao, S. et al. LncRNA MIR17HG promotes colorectal cancer liver metastasis by mediating a glycolysis-associated positive feedback circuit. Oncogene 2021, 40, 4709–4724. [Google Scholar] [CrossRef]
- Li, B. et al. LncRNA GAL promotes colorectal cancer liver metastasis through stabilizing GLUT1. Oncogene 2022, 41, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S. A. et al. The Human Transcription Factors. Cell 2018, 172, 650–665. [Google Scholar] [CrossRef] [PubMed]
- Bushweller, J. H. Targeting transcription factors in cancer - from undruggable to reality. Nat Rev Cancer 2019, 19, 611–624. [Google Scholar] [CrossRef]
- Chi, P. et al. ETV1 is a lineage survival factor that cooperates with KIT in gastrointestinal stromal tumours. Nature 2010, 467, 849–853. [Google Scholar] [CrossRef]
- Jané-Valbuena, J. et al. An oncogenic role for ETV1 in melanoma. Cancer Res 2010, 70, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. et al. Death Domain-Associated Protein Promotes Colon Cancer Metastasis through Direct Interaction with ZEB1. J Cancer 2020, 11, 750–758. [Google Scholar] [CrossRef]
- Klupp, F. et al. Expressional STAT3/STAT5 Ratio is an Independent Prognostic Marker in Colon Carcinoma. Ann Surg Oncol, 2015, 22 Suppl 3, S1548-S1555. [CrossRef]
- Malilas, W. et al. Cancer upregulated gene 2, a novel oncogene, enhances migration and drug resistance of colon cancer cells via STAT1 activation. Int J Oncol 2013, 43, 1111–1116. [Google Scholar] [CrossRef]
- Colomiere, M. et al. Cross talk of signals between EGFR and IL-6R through JAK2/STAT3 mediate epithelial-mesenchymal transition in ovarian carcinomas. Br J Cancer 2009, 100, 134–144. [Google Scholar] [CrossRef]
- He, G. & Karin, M. NF-κB and STAT3 - key players in liver inflammation and cancer. Cell Res 2011, 21, 159–168. [Google Scholar] [CrossRef]
- Kamal, Y. , Schmit, S. L., Hoehn, H. J., Amos, C. I. & Frost, H. R. Transcriptomic Differences between Primary Colorectal Adenocarcinomas and Distant Metastases Reveal Metastatic Colorectal Cancer Subtypes. Cancer Res 2019, 79, 4227–4241. [Google Scholar] [CrossRef] [PubMed]
- Masoud, G. N. & Li, W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B 2015, 5, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S. , Xu, J. & Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Yin, X. et al. Large scale systematic proteomic quantification from non-metastatic to metastatic colorectal cancer. Sci Rep 2015, 5, 12120. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. et al. THBS1 facilitates colorectal liver metastasis through enhancing epithelial-mesenchymal transition. Clin Transl Oncol 2020, 22, 1730–1740. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y. K. et al. Fibronectin expression in carcinoma cells correlates with tumor aggressiveness and poor clinical outcome in patients with invasive breast cancer. Hum Pathol 2013, 44, 2028–2037. [Google Scholar] [CrossRef] [PubMed]
- Hope, C. et al. Versican-Derived Matrikines Regulate Batf3-Dendritic Cell Differentiation and Promote T Cell Infiltration in Colorectal Cancer. J Immunol 2017, 199, 1933–1941. [Google Scholar] [CrossRef] [PubMed]
- Song, G. et al. TIMP1 is a prognostic marker for the progression and metastasis of colon cancer through FAK-PI3K/AKT and MAPK pathway. J Exp Clin Cancer Res 2016, 35, 148. [Google Scholar] [CrossRef] [PubMed]
- Li, Z. et al. Periostin expression and its prognostic value for colorectal cancer. International Journal of Molecular Sciences 2015, 16, 12108–12118. [Google Scholar] [CrossRef]
- Yu, J. et al. Novel recurrently mutated genes and a prognostic mutation signature in colorectal cancer. Gut 2015, 64, 636–645. [Google Scholar] [CrossRef]
- Li, Y. et al. Downregulated IGFBP7 facilitates liver metastasis by modulating epithelial-mesenchymal transition in colon cancer. Oncol Rep 2019, 42, 1935–1945. [Google Scholar] [CrossRef]
- Hamidi, H. & Ivaska, J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, M. B. , Dhawan, P. & Baumert, T. F. Tight junction proteins in gastrointestinal and liver disease. Gut 2019, 68, 547–561. [Google Scholar] [CrossRef]
- Wang, K. et al. Claudin-7 downregulation induces metastasis and invasion in colorectal cancer via the promotion of epithelial-mesenchymal transition. Biochem Biophys Res Commun 2019, 508, 797–804. [Google Scholar] [CrossRef]
- Georges, R. et al. Sequential biphasic changes in claudin1 and claudin4 expression are correlated to colorectal cancer progression and liver metastasis. J Cell Mol Med 2012, 16, 260–272. [Google Scholar] [CrossRef]
- Tian, T. et al. Investigation of the role and mechanism of ARHGAP5-mediated colorectal cancer metastasis. Theranostics 2020, 10, 5998–6010. [Google Scholar] [CrossRef]
- Wang, B. et al. MYH9 Promotes Growth and Metastasis via Activation of MAPK/AKT Signaling in Colorectal Cancer. J Cancer 2019, 10, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Li, Q. et al. RUNX1 promotes tumour metastasis by activating the Wnt/β-catenin signalling pathway and EMT in colorectal cancer. J Exp Clin Cancer Res 2019, 38, 334. [Google Scholar] [CrossRef] [PubMed]
- Tang, B. et al. PEA15 promotes liver metastasis of colorectal cancer by upregulating the ERK/MAPK signaling pathway. Oncol Rep 2019, 41, 43–56. [Google Scholar] [CrossRef]
- Cotter, K. et al. Activity of plasma membrane V-ATPases is critical for the invasion of MDA-MB231 breast cancer cells. J Biol Chem 2015, 290, 3680–3692. [Google Scholar] [CrossRef]
- Wang, J. et al. ATP6L promotes metastasis of colorectal cancer by inducing epithelial-mesenchymal transition. Cancer Sci 2020, 111, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Ku, X. et al. In-Depth Characterization of Mass Spectrometry-Based Proteomic Profiles Revealed Novel Signature Proteins Associated with Liver Metastatic Colorectal Cancers. Anal Cell Pathol (Amst) 2019, 2019, 7653230. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-K. , Song, M.-J., Jung, Y., Lee, W.-S. & Jang, H. H. Proteomic Analysis of Primary Colon Cancer and Synchronous Solitary Liver Metastasis. Cancer Genomics Proteomics 2019, 16, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C. H. et al. Serpin peptidase inhibitor clade A member 1 is a biomarker of poor prognosis in gastric cancer. Br J Cancer 2014, 111, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Normandin, K. et al. Protease inhibitor SERPINA1 expression in epithelial ovarian cancer. Clin Exp Metastasis 2010, 27, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, B. et al. The effect of combined therapy on activity of cathepsin D and alpha-1-antitrypsin in the blood serum of women with cervical cancer. Eur J Gynaecol Oncol 2008, 29, 617–619. [Google Scholar] [PubMed]
- Kim, S. , Kim, D. H., Jung, W.-H. & Koo, J. S. Succinate dehydrogenase expression in breast cancer. Springerplus 2013, 2, 299. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, C. et al. Germline SDHB mutations and familial renal cell carcinoma. J Natl Cancer Inst 2008, 100, 1260–1262. [Google Scholar] [CrossRef]
- Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Kuo, C.-C. et al. Metastatic Colorectal Cancer Rewrites Metabolic Program Through a Glut3-YAP-dependent Signaling Circuit. Theranostics 2019, 9, 2526–2540. [Google Scholar] [CrossRef]
- Kuo, M.-H. et al. Glucose Transporter 3 is Essential for the Survival of Breast Cancer Cells in the Brain. Cells 2019, 8, 1568. [Google Scholar] [CrossRef] [PubMed]
- Gao, H. et al. Prognostic value of glucose transporter 3 expression in hepatocellular carcinoma. Oncol Lett 2020, 19, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Ali, A. et al. CAV1 - GLUT3 signaling is important for cellular energy and can be targeted by Atorvastatin in Non-Small Cell Lung Cancer. Theranostics 2019, 9, 6157–6174. [Google Scholar] [CrossRef] [PubMed]
- Yang, M. , Guo, Y., Liu, X. & Liu, N. HMGA1 Promotes Hepatic Metastasis of Colorectal Cancer by Inducing Expression of Glucose Transporter 3 (GLUT3). Med Sci Monit 2020, 26, e924975. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-J. et al. JMJD5 regulates PKM2 nuclear translocation and reprograms HIF-1α-mediated glucose metabolism. Proc Natl Acad Sci U S A 2014, 111, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Deng, F. et al. Tumor-secreted dickkopf2 accelerates aerobic glycolysis and promotes angiogenesis in colorectal cancer. Theranostics 2019, 9, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Fahrner, M. , Bronsert, P., Fichtner-Feigl, S., Jud, A. & Schilling, O. Proteome biology of primary colorectal carcinoma and corresponding liver metastases. Neoplasia 2021, 23, 1240–1251. [Google Scholar] [CrossRef]
- Bu, P. et al. Aldolase B-Mediated Fructose Metabolism Drives Metabolic Reprogramming of Colon Cancer Liver Metastasis. Cell Metab 2018, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.-L. et al. Organ-specific cholesterol metabolic aberration fuels liver metastasis of colorectal cancer. Theranostics 2021, 11, 6560–6572. [Google Scholar] [CrossRef]
- Williams, M. D. et al. Characterizing metabolic changes in human colorectal cancer. Anal Bioanal Chem 2015, 407, 4581–4595. [Google Scholar] [CrossRef]
- Halama, N. et al. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res 2011, 71, 5670–5677. [Google Scholar] [CrossRef] [PubMed]
- Wculek, S. K. & Malanchi, I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 2015, 528, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Vitale, I. , Shema, E., Loi, S. & Galluzzi, L. Intratumoral heterogeneity in cancer progression and response to immunotherapy. Nat Med 2021, 27, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H. et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer 2017, 17, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Qi, J. et al. Single-cell and spatial analysis reveal interaction of FAP fibroblasts and SPP1 macrophages in colorectal cancer. Nat Commun 2022, 13, 1742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. et al. Single-cell transcriptome analysis reveals tumor immune microenvironment heterogenicity and granulocytes enrichment in colorectal cancer liver metastases. Cancer Letters 2020, 470, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. et al. Immune phenotypic linkage between colorectal cancer and liver metastasis. Cancer Cell 2022, 40. [Google Scholar] [CrossRef] [PubMed]
- Zilionis, R. et al. Single-Cell Transcriptomics of Human and Mouse Lung Cancers Reveals Conserved Myeloid Populations across Individuals and Species. Immunity 2019, 50. [Google Scholar] [CrossRef] [PubMed]
- Tu, W. , Gong, J., Zhou, Z., Tian, D. & Wang, Z. TCF4 enhances hepatic metastasis of colorectal cancer by regulating tumor-associated macrophage via CCL2/CCR2 signaling. Cell Death Dis 2021, 12, 882. [Google Scholar] [CrossRef]
- Ballotta, V. , Driessen-Mol, A., Bouten, C. V. C. & Baaijens, F. P. T. Strain-dependent modulation of macrophage polarization within scaffolds. Biomaterials 2014, 35, 4919–4928. [Google Scholar] [CrossRef]
- Waldo, S. W. et al. Heterogeneity of human macrophages in culture and in atherosclerotic plaques. Am J Pathol 2008, 172, 1112–1126. [Google Scholar] [CrossRef]
- Geissmann, F. , Gordon, S., Hume, D. A., Mowat, A. M. & Randolph, G. J. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol 2010, 10, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Yona, S. & Gordon, S. From the Reticuloendothelial to Mononuclear Phagocyte System - The Unaccounted Years. Front Immunol 2015, 6, 328. [Google Scholar] [CrossRef] [PubMed]
- Donadon, M. et al. Macrophage morphology correlates with single-cell diversity and prognosis in colorectal liver metastasis. J Exp Med 2020, 217. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y. & Nishikawa, H. Roles of regulatory T cells in cancer immunity. Int Immunol 2016, 28, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Huang, X. et al. Changes of T cells and cytokines TGF-β1 and IL-10 in mice during liver metastasis of colon carcinoma: implications for liver anti-tumor immunity. J Gastrointest Surg 2013, 17, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Huang, X. et al. Increase in CD4FOXP3 regulatory T cell number and upregulation of the HGF/c-Met signaling pathway during the liver metastasis of colorectal cancer. Oncol Lett 2020, 20, 2113–2118. [Google Scholar] [CrossRef] [PubMed]
- Oft, M. IL-10: master switch from tumor-promoting inflammation to antitumor immunity. Cancer Immunol Res 2014, 2, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Mannino, M. H. et al. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett 2015, 367, 103–107. [Google Scholar] [CrossRef]
- Neurath, M. F. IL-23 in inflammatory bowel diseases and colon cancer. Cytokine Growth Factor Rev 2019, 45, 1–8. [Google Scholar] [CrossRef]
- Tian, Y. , Guo, X., Wu, T., Fei, K. & Wu, L. Identification of a novel cDC2-committed progenitor within mouse common dendritic cell progenitor population. Protein Cell 2022, 13, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y. S. et al. Human CD141 dendritic cells (cDC1) are impaired in patients with advanced melanoma but can be targeted to enhance anti-PD-1 in a humanized mouse model. J Immunother Cancer 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Cytlak, U. et al. Differential IRF8 Transcription Factor Requirement Defines Two Pathways of Dendritic Cell Development in Humans. Immunity 2020, 53. [Google Scholar] [CrossRef] [PubMed]
- Bourdely, P. et al. Transcriptional and Functional Analysis of CD1c Human Dendritic Cells Identifies a CD163 Subset Priming CD8CD103 T Cells. Immunity 2020, 53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N. et al. Novel therapeutic strategies: targeting epithelial-mesenchymal transition in colorectal cancer. Lancet Oncol 2021, 22, e358–e368. [Google Scholar] [CrossRef]
- Stine, Z. E. , Schug, Z. T., Salvino, J. M. & Dang, C. V. Targeting cancer metabolism in the era of precision oncology. Nat Rev Drug Discov 2022, 21, 141–162. [Google Scholar] [CrossRef]
- Bansal, A. & Simon, M. C. Glutathione metabolism in cancer progression and treatment resistance. J Cell Biol 2018, 217, 2291–2298. [Google Scholar] [CrossRef] [PubMed]
- Traverso, N. et al. Role of glutathione in cancer progression and chemoresistance. Oxid Med Cell Longev 2013, 2013, 972913. [Google Scholar] [CrossRef]
- Niu, B. et al. Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials 2021, 277, 121110. [Google Scholar] [CrossRef]
- Li, X. et al. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat Rev Cancer 2021, 21, 541–557. [Google Scholar] [CrossRef]
- Lee, J. C. et al. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci Immunol 2020, 5. [Google Scholar] [CrossRef] [PubMed]

| levels | items | factors | change | references |
|---|---|---|---|---|
| Genetic level | APC, RAS, BRAF, PIK3CA, TP53, MSI status | - | [18,19,20,21,22,38,39,40,41] | |
| DNA copy numbers | -/↑ | [28,29,30,31,34,35] | ||
| levels | items | factors | change | references |
|---|---|---|---|---|
| Transcriptomic level | MiRNAs | MiR-122, MiR-122*, MiR-885-5p ,MiR-203, MiR-200c, MiR-424-3p, MiR-503, MiR-1292 | ↑ | [46,47,48,55,56,59] |
| MiR-143, MiR-10b, MiR-28-5p | ↓ | [46,47,48] | ||
| CircRNAs | Circ0001178, Circ0000826, CircNSUN2, Circ0006401 | ↑ | [67,68,69] | |
| LncRNAs | LncRNA GAL, LncRNA MIR17HG | ↑ | [71,72] | |
| Transcription factors | STAT1, STAT3, MYC, HIF1α | ↑ | [78,82] | |
| DAXX, STAT4, STAT5 | ↓ | [77,78] |
| levels | items | factors | change | references |
|---|---|---|---|---|
| Protein level | EMT-related proteins | Migration-associated proteins: VTN, ARP3, FN1, TIMP1, VCAN, POSTN, THBS1, IGFBP7 | ↑ | [85,86,92] |
| Adhesion protein: claudins, ITA5 | ↓ | [85,95,96] | ||
| ARHGAP5, PEA15, ATP6L, FILIP1L | ↑ | [97,100,103] | ||
| Other proteins | PLG, Serpin A1, APOA1A, CA1, SDHA | ↑ | [103,104] | |
| the mitochondrial matrix, the mitochondrial intermembrane space, the proteasome complex, and the actin cytoskeleton | ↓ | [104] |
| levels | items | factors | change | references |
|---|---|---|---|---|
| Metabolic level | Aerobic glycolysis | GLUT3, HMGA1, PKM2, DKK2, Pyruvate carboxylase, Fructose-bisphosphate aldolase B, Fructose-1,6-bisphosphatase 1 | ↑ | [111,115,116,117,118] |
| Fructose metabolism | ALDOB | ↑ | [119] | |
| Cholesterol metabolism | SREBP2, LDLR, SRB1 | ↑ | [120] | |
| Fatty acids, acylcarnitines, oxidative compounds, polyamines | GSH, putrescine | ↑ | [121] |
| levels | items | factors | change | references |
|---|---|---|---|---|
| Immune cells level | TAMs | MRC1+ CCL18+ TAMs, SPP1+ TAMs | ↑ | [3,128,130] |
| T cells | Treg-IL10, Treg-CTLA4, CD4+FOXP3+ Tregs | ↑ | [137,138] | |
| CD4+ T cells, CD8+ T cells | ↓ | [137,138] | ||
| DCs | cDC2-TIMP1 | ↑ | [128] | |
| cDC2-C1QC(DC3s) | ↓ | [128] | ||
| Neutrophils | ↑ | [3] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





