Submitted:
27 October 2023
Posted:
27 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction:
2. Methods
2.1. Study population
2.2. Inclusion criteria
2.3. Exclusion Criteria
2.4. Inclusion criteria for controls
2.5. Exclusion criteria for controls
2.6. Data collection
2.7. Blood specimen collection from T2DM patients
2.8. Blood specimen collection from healthy controls
2.9. Estimation of Biochemical parameters
3. Results
3.1. Anthropometric indices and biochemical parameters of male T2DM patients
3.2. Anthropometric indices and biochemical parameters of female T2DM patients
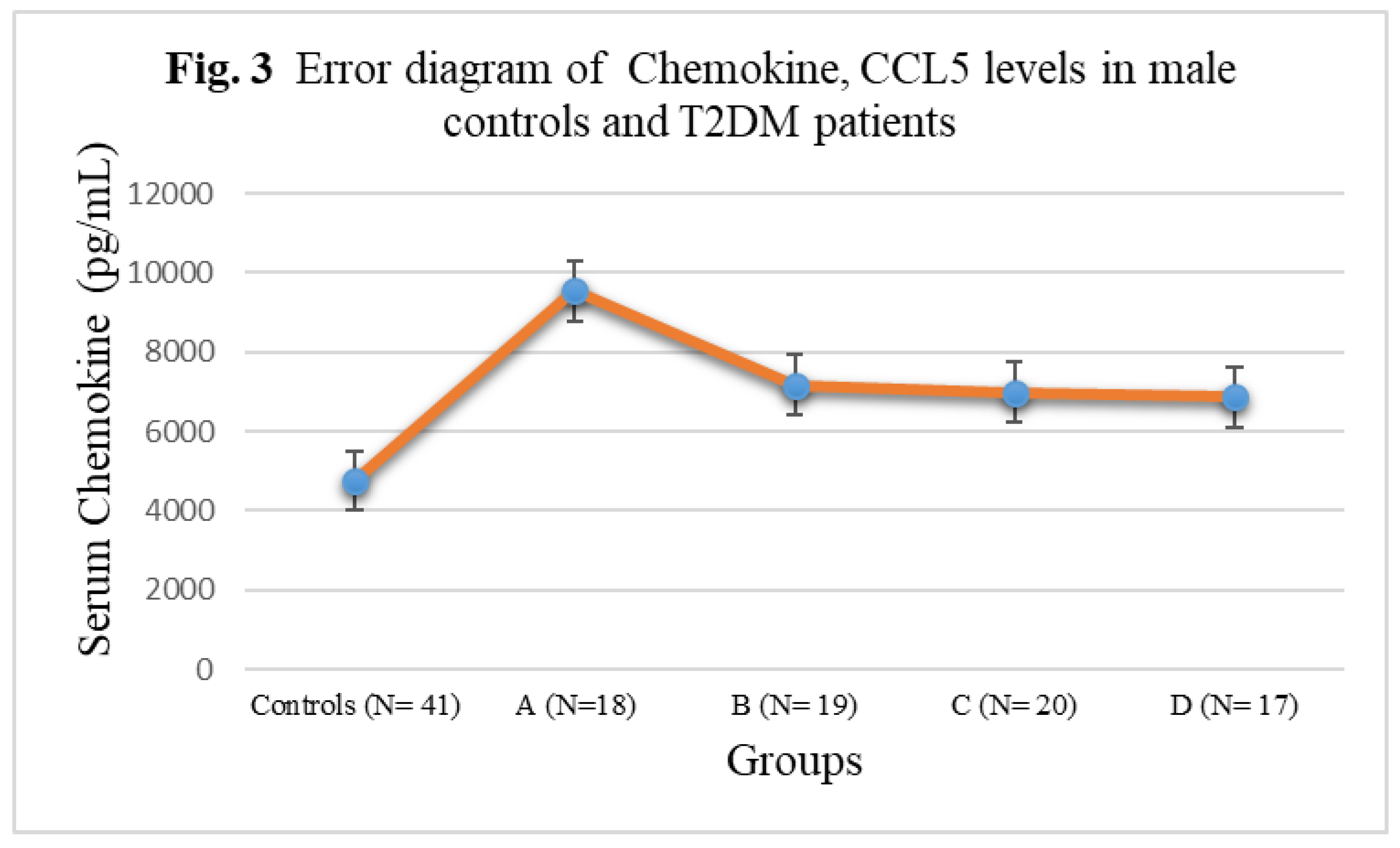
3.3. Chemokine Concentrations in male T2DM patients and controls
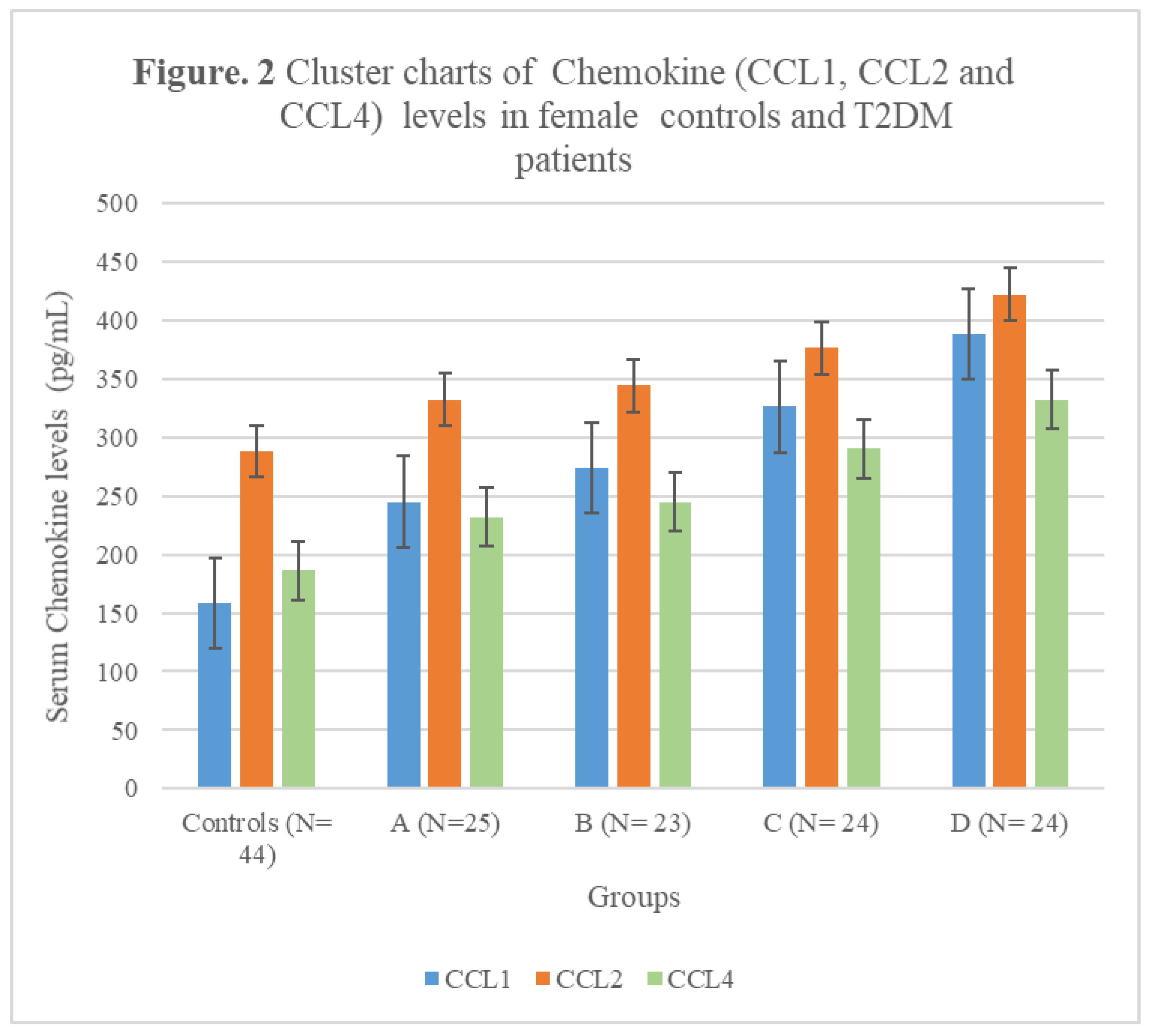
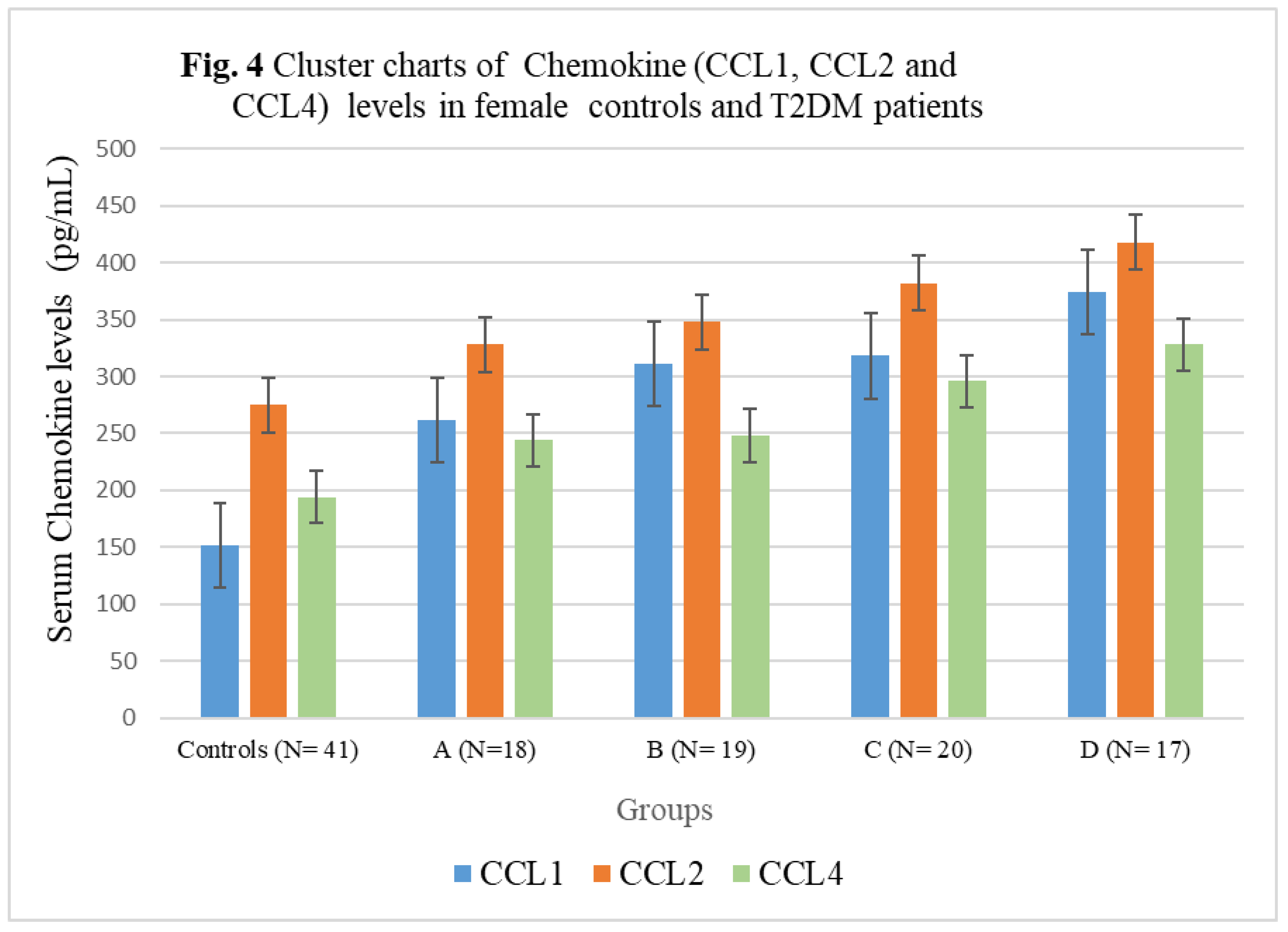
3.4. Chemokine Concentrations in female T2DM patients and controls
4. Discussion
5. Limitations
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [CrossRef]
- Yan Y, Wu T, Zhang M, Li C, Liu Q, Li F. Prevalence, awareness and control of type 2 diabetes mellitus and risk factors in Chinese elderly population. BMC Public Health. 2022;22(1):1382. Published 2022 Jul 19. [CrossRef]
- Mir MM, Mir R, Alghamdi MAA, et al. Differential Association of Selected Adipocytokines, Adiponectin, Leptin, Resistin, Visfatin and Chemerin, with the Pathogenesis and Progression of Type 2 Diabetes Mellitus (T2DM) in the Asir Region of Saudi Arabia: A Case Control Study. J Pers Med. 2022;12(5):735. Published 2022 May 1. [CrossRef]
- Mir MM, Mir R, Alghamdi MAA, et al. Potential impact of GCK, MIR-196A-2 and MIR-423 gene abnormalities on the development and progression of type 2 diabetes mellitus in Asir and Tabuk regions of Saudi Arabia. Mol Med Rep. 2022;25(5):162. [CrossRef]
- Qadir MI, Ahmed Z. lep Expression and Its Role in Obesity and Type-2 Diabetes. Crit Rev Eukaryot Gene Expr. 2017;27(1):47-51. [CrossRef]
- Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. [CrossRef]
- Zhou X, Guan H, Zheng L, et al. Prevalence and awareness of diabetes mellitus among a rural population in China: results from Liaoning Province. Diabet Med. 2015;32(3):332-342. [CrossRef]
- Moin ASM, Butler AE. Alterations in Beta Cell Identity in Type 1 and Type 2 Diabetes. Curr Diab Rep. 2019;19(9):83. Published 2019 Aug 10. [CrossRef]
- Yin J, Yeung R, Luk A, et al. Gender, diabetes education, and psychosocial factors are associated with persistent poor glycemic control in patients with type 2 diabetes in the Joint Asia Diabetes Evaluation (JADE) program. J Diabetes. 2016;8(1):109-119. [CrossRef]
- Liu M, Lv X, Li Y, Li J, He Y. Prevalence and Control Status of Diabetes and Related Risk Factors Among 4196 Chinese Male Older Elderly Aged ≥80 Years. Int J Gerontol. 2018;12(2):122–126. [CrossRef]
- Wang H, Yao J, Yin X, et al. Organisational and individual characteristics associated with glycaemic control among patients with type 2 diabetes: cross-sectional study in China. BMJ Open. 2020;10(4):e036331. Published 2020 Apr 6. [CrossRef]
- Irazola V, Rubinstein A, Bazzano L, et al. Prevalence, awareness, treatment and control of diabetes and impaired fasting glucose in the Southern Cone of Latin America. PLoS One. 2017;12(9):e0183953. Published 2017 Sep 6. [CrossRef]
- Hu D, Fu P, Xie J, et al. Increasing prevalence and low awareness, treatment and control of diabetes mellitus among Chinese adults: the InterASIA study. Diabetes Res Clin Pract. 2008;81(2):250-257. [CrossRef]
- Galicia-Garcia U, Benito-Vicente A, Jebari S, et al. Pathophysiology of Type 2 Diabetes Mellitus. Int J Mol Sci. 2020;21(17):6275. Published 2020 Aug 30. [CrossRef]
- Abdul-Ghani MA, Jayyousi A, DeFronzo RA, Asaad N, Al-Suwaidi J. Insulin Resistance the Link between T2DM and CVD: Basic Mechanisms and Clinical Implications. Curr Vasc Pharmacol. 2019;17(2):153-163. [CrossRef]
- Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137-188. [CrossRef]
- Niu G, Li J, Wang H, Ren Y, Bai J. Associations of A-FABP with Anthropometric and Metabolic Indices and Inflammatory Cytokines in Obese Patients with Newly Diagnosed Type 2 Diabetes. Biomed Res Int. 2016;2016:9382092. [CrossRef]
- Urbanavičius V, Abalikšta T, Brimas G, Abraitienė A, Gogelienė L, Strupas K. Comparison of changes in blood glucose, insulin resistance indices, and adipokine levels in diabetic and nondiabetic subjects with morbid obesity after laparoscopic adjustable gastric banding. Medicina (Kaunas). 2013;49(1):9-14. [CrossRef]
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006;116:1793-801. [CrossRef]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity linked insulin resistance. Science 1993;259:87-91. [CrossRef]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112:1821-30. [CrossRef]
- Pan X, Kaminga AC, Wen SW, Liu A. Chemokines in Prediabetes and Type 2 Diabetes: A Meta-Analysis. Front Immunol. 2021;12:622438. Published 2021 May 13. [CrossRef]
- Liang W, Qi Y, Yi H, et al. The Roles of Adipose Tissue Macrophages in Human Disease. Front Immunol. 2022;13:908749. Published 2022 Jun 9. [CrossRef]
- Xu L, Kitade H, Ni Y, Ota T. Roles of Chemokines and Chemokine Receptors in Obesity-Associated Insulin Resistance and Nonalcoholic Fatty Liver Disease. Biomolecules. 2015;5(3):1563-1579. Published 2015 Jul 21. [CrossRef]
- Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol 2001;2:108-15. [CrossRef]
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity 2000;12:121-7. [CrossRef]
- Hughes CE, Nibbs RJB. A guide to chemokines and their receptors. FEBS J. 2018;285(16):2944-2971. [CrossRef]
- Chait A, den Hartigh LJ. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front Cardiovasc Med. 2020;7:22. Published 2020 Feb 25. [CrossRef]
- CHEN, Hai-jun, et al. "Association between serum levels of interleukin-12, Chemokine cytokines ligand 1 and occurrence of Type 2 diabetes mellitus complicated with pulmonary tuberculosis." Chinese Journal of Antituberculosis 39.12 (2017): 1278. [CrossRef]
- Ooms, Mark, et al. "Increased spontaneous CCL2 (MCP-1) and CCL5 (RANTES) secretion in vitro in LADA compared to type 1 diabetes and type 2 diabetes: Action LADA 14." Diabetes/metabolism research and reviews 37.7 (2021): e3431. [CrossRef]
- Guan, Ruili, et al. "Chemokine (CC motif) ligand 2 (CCL2) in sera of patients with type 1 diabetes and diabetic complications." PloS one 6.4 (2011): e17822. [CrossRef]
- Chang TT, Chen JW. The Role of Chemokines and Chemokine Receptors in Diabetic Nephropathy. Int J Mol Sci. 2020;21(9):3172. Published 2020 Apr 30. [CrossRef]
- Chang TT, Lin LY, Chen JW. A Novel Resolution of Diabetes: C-C Chemokine Motif Ligand 4 Is a Common Target in Different Types of Diabetes by Protecting Pancreatic Islet Cell and Modulating Inflammation. Front Immunol. 2021;12:650626. Published 2021 Apr 23. [CrossRef]
- Meagher C, Arreaza G, Peters A, et al. CCL4 protects from type 1 diabetes by altering islet beta-cell-targeted inflammatory responses. Diabetes. 2007;56(3):809-817. [CrossRef]
- Zeng Z, Lan T, Wei Y, Wei X. CCL5/CCR5 axis in human diseases and related treatments. Genes Dis. 2022;9(1):12-27. [CrossRef]
- Bogdanski P, Pupek-Musialik D, Dytfeld J, et al. Influence of insulin therapy on expression of chemokine receptor CCR5 and selected inflammatory markers in patients with type 2 diabetes mellitus. Int J Clin Pharmacol Ther. 2007;45(10):563-567. [CrossRef]
- Ooms M, Strom A, Strassburger K, Menart B, Leslie RD, Schloot NC. Increased spontaneous CCL2 (MCP-1) and CCL5 (RANTES) secretion in vitro in LADA compared to type 1 diabetes and type 2 diabetes: Action LADA 14. Diabetes Metab Res Rev. 2021;37(7):e3431. [CrossRef]
- Chen J, Guo W, Yin H, Ma L, Li S, Li H. Investigation of the Correlation Between the Polymorphism/Expression Level of RANTES and Its Receptor CCR5 Gene Promoter and Type 2 Diabetes Mellitus. Diabetes Metab Syndr Obes. 2023;16:213-223. Published 2023 Jan 24. [CrossRef]
- Mira E, León B, Barber DF, et al. Statins induce regulatory T cell recruitment via a CCL1 dependent pathway. J Immunol. 2008;181(5):3524-3534. [CrossRef]
- Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8(12):923-934. [CrossRef]
- Jager J, Grémeaux T, Cormont M, Le Marchand-Brustel Y, Tanti JF. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology. 2007;148(1):241-251. [CrossRef]
- Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280(5):E745-E751. [CrossRef]
- Harakeh S, Kalamegam G, Pushparaj PN, et al. Chemokines and their association with body mass index among healthy Saudis. Saudi J Biol Sci. 2020;27(1):6-11. [CrossRef]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860-867. [CrossRef]
- Xu L, Kitade H, Ni Y, Ota T. Roles of Chemokines and Chemokine Receptors in Obesity-Associated Insulin Resistance and Nonalcoholic Fatty Liver Disease. Biomolecules. 2015;5(3):1563-1579. Published 2015 Jul 21. [CrossRef]
- Feghali CA, Wright TM. Cytokines in acute and chronic inflammation. Front Biosci. 1997;2:d12-d26. Published 1997 Jan 1. [CrossRef]
- Purohit S, Sharma A, Hopkins D, et al. Large-Scale Discovery and Validation Studies Demonstrate Significant Reductions in Circulating Levels of IL8, IL-1Ra, MCP-1, and MIP-1β in Patients With Type 1 Diabetes. J Clin Endocrinol Metab. 2015;100(9):E1179-E1187. [CrossRef]
- Harakeh S, Kalamegam G, Pushparaj PN, et al. Chemokines and their association with body mass index among healthy Saudis. Saudi J Biol Sci. 2020;27(1):6-11. [CrossRef]
- Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding [published correction appears in J Clin Invest. 2006 May;116(5):1457]. J Clin Invest. 2006;116(1):115-124. [CrossRef]
- Tamura Y, Sugimoto M, Murayama T, et al. Inhibition of CCR2 ameliorates insulin resistance and hepatic steatosis in db/db mice. Arterioscler Thromb Vasc Biol. 2008;28(12):2195-2201. [CrossRef]
- Pham MN, Hawa MI, Roden M, et al. Increased serum concentrations of adhesion molecules but not of chemokines in patients with Type 2 diabetes compared with patients with Type 1 diabetes and latent autoimmune diabetes in adult age: action LADA 5. Diabet Med. 2012;29(4):470-478. [CrossRef]
| Anthropometric and biochemical indices | Controls | Group A | Group B | Group C | Group D |
| N= 44 | N=96 | ||||
| 25 | 23 | 24 | 24 | ||
| Age | 46(28-62) | 43(27-51) | 47(29-55) | 49(30-66) | 47(32-67) |
| WHR | 0.86 (0.84-0.95) |
0.92 (0.84-0.1.05) |
0.94 (0.83-1.06) |
1.07**T (0.97-1.11) |
1.14*S (1.02-1.19) |
| BMI (kg/m2) | 21.78 ± 1.78 | 21.80 ± 2.14 | 27.44 ± 2.29 | 34.94 ± 3.18*T | 47.22± 5.70*WX |
| Fasting Glucose (mg/dL) | 92(78-116) | 115(88-130) | 114(92-138) | 116(92-144) | 124(103-152) |
| HbA1c (g/dL) | 4.9±0.88 | 7.4 ±1.02* | 8.1±1.2* | 8.7±1.48*S | 7.6±0.78* |
| Cholesterol-T (mg/dL) | 186 (135-224) | 204**(154-230) | 214*(148-232) | 215*(142-240) | 226*(158-258) |
| HDL-C (mg/dL) | 53(38- 63) | 46(36—60) | 48(38-56) | 45(33-58) | 44(28-54) |
| LDL-C (mg/dL) | 94±32.20 | 98 (62-131) | 118** (80-136) | 120** (80-152) | 136*w (95-162) |
| TG (mg/dL) | 116(86-132) | 122(96-136) | 146(98-178) | 232*(162-256) | 242*w(162-286) |
| Anthropometric and biochemical indices | Controls | Group A | Group B | Group C | Group D |
| N= 41 | N=74 | ||||
| 18 | 19 | 20 | 17 | ||
| Age | 48(27-64) | 44(26-55) | 47(27-57) | 46(28-62) | 48(30-69) |
| WHR | 0.85 (0.78-0.96) |
0.91 (0.82-0.1.04) |
0.92 (0.84-1.05) |
1.06**T (0.97-1.13) |
1.10*S (1.02-1.22) |
| BMI (kg/m2) | 21.2 ± 1.66 | 21.41 ± 2.14 | 28.22 ± 2.26 | 33.88± 3.20*T | 46.48± 5.20*WX |
| Fasting Glucose(mg/dL) | 96(82-114 | 118**(90-141) | 114**(92-138) | 116**(92-144) | 124*(103-152) |
| HbA1c (g/dL) | 4.5±0.43 | 7.8±1.02* | 8.1±1.2* | 8.5±1.48*S | 8.4±0.98* |
| Cholesterol-T(mg/dL) | 168 (132-224) | 196**(145-227) | 204**(144-238) | 215**(155-235) | 228**(162-262) |
| HDL-C (mg/dL) | 56(41- 66) | 54 (39—62) | 52(40-63) | 46(35-64) | 44(35-62) |
| LDL-C (mg/dL) | 96±28.22 | 92±28.28 | 111**±33.08 | 108**±30.30 | 132*±32.12 |
| TG (mg/dL) | 96(88-124) | 126**(94-168) | 142**(88-222) | 152**(99- 223) | 195*wx(98-245) |
| Subject Groups | CCL1 (pg/mL) | CCL2 (pg/mL) | CCL4 (pg/mL) | CCL5 (pg/mL) |
|---|---|---|---|---|
| Controls (N= 44) | 158± 21.02 | 288 ± 33.12 | 186± 22.56 | 4880± 348.98 |
| A (N=25) | 245± 27.46** | 332 ± 35.76*** | 232± 25.44*** | 9620± 523.22* |
| B (N= 23) | 274± 23.12** | 344 ± 35.87*** | 245± 25.72*** | 7266± 387.55**N |
| C (N= 24) | 326± 35.44*N | 376 ± 36.92*N | 290± 24.14*O | 7056± 382.34**N |
| D (N= 24) | 388± 37.12*LM | 422 ± 44.25*LM | 332± 29.90*LM | 6892± 377.16***NQ |
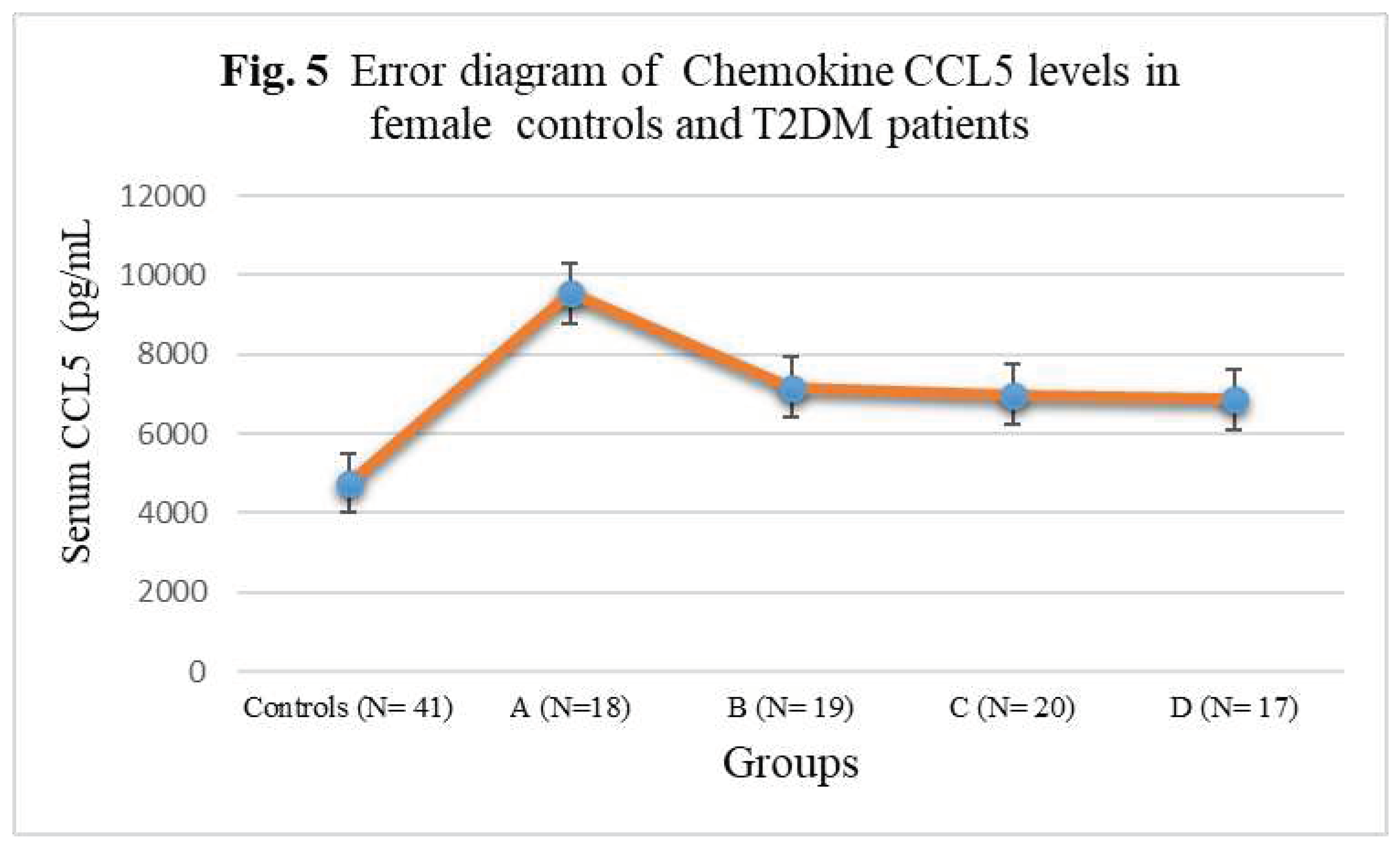
| Subject Groups | CCL1 (pg/mL) | CCL2 (pg/mL) | CCL4 (pg/mL) | CCL5 (pg/mL) |
|---|---|---|---|---|
| Controls (N= 41) | 152± 20.88 | 275 ± 32.14 | 194± 22.54 | 4756± 342.56 |
| A (N=18) | 262± 27.52** | 328± 35.66*** | 244± 25.38*** | 9546± 522.44* |
| B (N= 19) | 311± 25.10** | 348 ± 35.58*** | 248± 25.24*** | 7168± 388.24**N |
| C (N= 20) | 318± 35.08*N | 382± 36.82*N | 296± 26.08*O | 6978± 384.74**N |
| D (N= 17) | 374± 37.04*LM | 418 ± 44.16*LM | 328± 29.88*LM | 6866± 376.12***NQ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





