1. Introduction
B-Cell Chronic Lymphocytic Leukemia (CLL) is a mature B-cell neoplasm characterized by the progressive accumulation of a rare population of mature B-lymphocytes (classified as CD19+CD5+) in the blood and lymphoid organs [
1]. It is the most prevalent type of leukemia in the Western world with an overall incidence rate of 4.2 per 100,000 [
2,
3]. CLL presents a gender disparity of approximately 2:1, with a higher risk associated with the male faction (6.1 per 100,000 in males compared to 3.1 in females) [
4,
5]. In the United States alone, approximately 18,740 new cases of CLL are diagnosed each year (American Cancer Society). This comprises roughly 25-30% of all newly diagnosed leukemia cases and accounts for about 1.1% of all new cancer diagnoses. CLL primarily affects older individuals, with the typical age of diagnosis hovering at approximately 70 years [
2]. The incidence of CLL rises notably after the age of 50 and continues to increase with advancing age [
6].
While increasing age is one of the most significant risk factors for CLL, certain genetic abnormalities have been associated with an increased risk of developing the disease. Cytogenetic studies reveal recurrent chromosomal alterations, including deletions at loci 11q, 13q, 17p, and trisomy 12 [
7]. Moreover, CLL patients with an unmutated IGHV status have a less favorable prognosis. Unmutated IGHV is associated with a more rapid disease progression, a shorter time for treatment, and reduced overall survival rate. In these patients, the IGHV gene has not undergone somatic hypermutation, suggesting that the precursor of the leukemic cells is a pre-germinal center (GC) B cell less terminally differentiated than that of CLL with mutated IGHV [
7]. Although CLL has been extensively studied, the molecular mechanisms underlying disease development are still to be fully elucidated. Genes deregulated and/or altered in CLL have been identified and singled out as possible driver genes. This includes genes such as TP53, ATM, MYD88, NRAS, and KRAS, which are involved in fundamental cellular processes such as DNA repair, cell-cycle control, Notch, Wnt, and B-cell receptor (BCR) signalling [
8].
RAS proteins are a family of small guanosine triphosphate hydrolases (GTPases) that include well-known oncogenic players such as aforementioned K-RAS, N-RAS, alongside H-RAS. R-RAS2 belongs to the RAS-related subfamily of RAS proteins, which share approximately 60% amino acid identity with classic RAS. Seemingly, R-RAS2 also shares associated proteins that regulate it’s activation-inactivation cycles with its classical counterparts including guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) [
9,
10]. R-RAS2 has been identified as a key player in immune system homeostasis. It interacts with both the B- and the T-cell receptors (BCR and TCR, respectively), facilitating the generation of tonic survival signals from these receptors [
11]. In B cells, R-RAS2 has been shown to be crucial for the effective establishment of a proper GC reaction by regulating B cell metabolism [
12].
Although early studies from the 1990s show that oncogenic mutations in RRAS2 (G23V, Q72L) have higher transformation potential than analogous mutations in classical RAS genes (G12V, Q61L), RRAS2 is mainly found overexpressed in its wild-type form rather than mutated [
9,
10]. It has been described that R-RAS2 protein is overexpressed in different types of human malignancies, including skin cancers [
13], oral cancers [
14], and esophageal tumors [
15]. We have recently demonstrated a driver role for RRAS2 bearing the activating mutation Q72L, which causes T-cell acute lymphocytic leukemia, a leukemia of immature B cells, a Harderian gland adenoma, and an ovarian cystadenocarcinoma in 100% of mice, among other malignancies [
16]. However, those mice do not develop leukemias or lymphomas of mature T cell or B cell origin. In contrast, in another recent publication, we demonstrated that overexpression of wild-type human RRAS2 bearing no mutations causes the development of B-cell chronic lymphocytic leukemia in 100% of mice, thus demonstrating that wild-type RRAS2 is an oncogene driver if overexpressed [
17]. The driver role in mice was paralleled by the finding of wild-type RRAS2 mRNA overexpression in 82% of human CLL blood samples. Moreover, RRAS2 overexpression was found to be higher in patients with full-blown CLL than in patients with the pre-malignant stage monoclonal B-cell lymphocytosis (MBL). In addition, higher RRAS2 overexpression was correlated with higher lymphocytosis, a higher percentage of CD19+CD5+ malignant cells in the blood, and a reduced number of platelets, all data indicating that RRAS2 mRNA expression levels are associated with a more aggressive disease and worse prognosis.
In addition to the association between CLL aggressiveness and RRAS2 expression in patients, we found another powerful correlation in human disease that sustains our hypothesis suggesting
RRAS2 overexpression as one of the key driver oncogenes in CLL. L. We found the existence of a single nucleotide polymorphism (SNP) (rs8570) that catalogues the change of the canonical G nucleotide at position +124 after the STOP codon in the RRAS2 3′UTR for a C [
17]. The presence of a C allele at the SNP position, whether in homozygosis or heterozygosis, was strongly linked to higher RRAS2 mRNA expression and various indicators of worse prognosis.
In this paper, we present a comprehensive investigation into the mutations occurring within the 3′UTR of RRAS2, revealing a striking pattern of recurrent mutations at positions +26, +53, and +180 after the STOP codon. We show how these somatic mutations, distinct from the SNP rs8570, associate with RRAS2 mRNA expression and markers of CLL aggressiveness.
2. Materials and Methods
2.1. Human Cells
Human blood samples were obtained from volunteer CLL patients from the Hematology Unit of the Salamanca University Hospital after providing written informed consent, with authorization number PI 2019 03217. Fresh human peripheral blood mononuclear cells (PBMCs) were obtained by density centrifugation in a Lymphoprep™ (StemCell technologies) gradient of whole blood for flow cytometry, RT-qPCR analysis and gDNA extraction.
2.2. Flow Cytometry
Human single-cell suspensions were incubated for 15 minutes with Ghost Dye™ 540 (Tonbo) in PBS to label and discard dead cells from analysis. Cells were washed with PBS + 2% FBS and incubated with fluorescently labelled antibodies for 30 min at 4 °C after. Afterwards, cells were washed in PBS + 2% FBS and data were collected on a FACS Canto II (Becton Dickinson) cytometer. A minimum of 50,000 and a maximum of 200,000 events was acquired in every measurement. Analyses were performed using FlowJo software (TreeStar).
2.3. gDNA Extraction
10^6 cells were harvested per sample. 500μl of lysis buffer (Tris-HCl pH8 50 mM, NaCl 200 mM, EDTA 10 mM, SDS 1% and fresh proteinase K 0.2 mg/ml) were added to each sample and incubated overnight (ON) at 55ºC. The next day, gDNA was purified using phenol chloroform and resuspended in 100μl of 10mM Tris-HCl pH 8.5 depending on the pellet size obtained after the last centrifugation step.
2.4. Sequencing Strategy for Patients’ Samples
10^6 cells per patient sample were used for RNA extraction from PBMCs of CLL patients. RNA was isolated using the RNAeasy Plus Mini Kit (QIAGEN). cDNA was synthesized with SuperScript III (Invitrogen) using Oligo-dT primers. cDNA was used as template to sequence the 3′UTR region of RRAS2. Specific oligonucleotides were used to detect the presence of the canonical or alternative allele in each position (26, 53, 124, 180;
Table 1) by quantitative real-time PCR (qPCR). All qPCR readings were performed in triplicate using 100 ng cDNA, SYBR Green PCR Master Mix and gene-specific primers (
Table 1) in an ABI 7300 Real Time PCR System. The oligonucleotides in
Table 1 were designed as described in [
18]. As loading reference, specific oligonucleotides aligning to constitutive
RRAS2 exons 3 and 4 were used (Forward: GCA GGA CAA GAA GAG TTT GGA; Reverse: TCA TTG GGA ACT CAT CAC GA). The obtained cycle threshold (Ct) values were used to normalize RRAS2 3′UTR mRNA expression in each individual patient relative to their respective exons 3 and 4 expression. This approach accounted for variability in gene expression across samples, ensuring accurate comparisons.
2.5. RRAS2 Expression Measurement
A set of primers that expand constitutive exons 3 and 4 was used to measure mRNA expression of RRAS2 in patient PBMCs (Forward: GCA GGA CAA GAA GAG TTT GGA; Reverse: TCA TTG GGA ACT CAT CAC GA). Obtained cycle threshold (Ct) values were used to calculate mRNA levels relative to 18S rRNA expression using the 2^^(−ΔΔCt) method. Outliers for RRAS2 expression were identified using ROUT model at Q=0.1% to remove definite outliers from analysis.
2.6. Fluorescent Probes Method to Sequence rs8570 SNP in CLL Patients’ Samples
Fluorescent dual-labelled probe technology, pre-developed by Applied Biosystems (Foster City, CA, USA) and custom made to target rs8570 SNP was used to sequence our CLL and healthy patients. A detailed description of the methodology employed by the custom-made TaqMan technology is available at [
19]. All qPCR reactions were performed using an initial cycle of 10 min at 95ºC for polymerase activation, followed by 40 cycles of 15s at 95ºC and 1min at 60ºC. rs8570 allele distribution was assessed by plotting the relative fluorescent units (RFU) of both fluorophores in a scatter-plot representation.
2.7. Statistical Analysis
Statistical parameters including the exact value of n, the mean +/− S.D. or S.E.M. are described in the Figures and Figure legends. Parametric and non-parametric tests were carried out after assessing the normal distribution of the data. Two-tailed Student’s t test, ANOVA tests, Mann-Whitney test, Chi-squared test with or without Yates’ correction and Dunn’s multiple comparisons tests were used as indicated in the Figure legends to assess the significance of mean differences. All data was analysed using the GraphPad Prism 9.5.1 software.
4. Discussion
In this study, we describe three somatic mutations in the 3′-UTR of RRAS2 mRNA in CLL patients. In contrast to the rarity of missense mutations in the RRAS2 gene, the 3′UTR harbours mutational hotspots at specific positions: +26 (G>T), +53 (T>C), and +180 (T>C) after the stop codon. Mutations within the 3′UTR of genes have historically been overshadowed by their counterparts within coding regions or canonical splice sites. However, the importance of these non-coding mutations is becoming increasingly evident, as they can disrupt RNA-protein interactions, microRNA binding sites, or other regulatory elements, thereby impacting gene expression and cellular phenotypes [
23].
Our results reveal an intriguing relationship between RRAS2 mRNA expression and the three 3′-UTR somatic mutations. The newly identified 3′-UTR mutations present an inverse relationship with RRAS2 mRNA expression levels and correlate with more positive prognostic or less severe clinical factors (reduced lymphocytosis and improved platelet count). The reduction in RRAS2 mRNA expression levels could be behind the improved clinical factors observed in CLL patients with these mutations. Thus, the effect of the three somatic mutations at the 3′-UTR seems to exert the opposite effect to the expression of the alternate C allele at the SNP rs8570 [
17]. Overall, our findings hint at a complex relationship between RRAS2 mRNA expression, the rs8570 SNP, and the three somatic mutations identified in the gene’s 3′UTR. Higher RRAS2 expression is linked to the dose of the C allele: CC homozygous leukemias express more mRNA than GC heterozygous, and those than GG homozygous. Comparing RRAS2 mRNA expression between mutated and unmutated samples at any of the three 3′-UTR positions for the three SNP genotypes, we show that mutation reduces RRAS2 expression, but the reduction is especially significant for GC SNP heterozygotes. In terms of frequency, the presence of either one of the three somatic mutations or their combinations is also associated with GC heterozygosity at the SNP position. It can be hypothesized that the three somatic mutations in the 3′-UTR are selected to limit the effect of the SNP C allele on mRNA expression, and that such effect is in cis. This could be the reason that the mutations are preferentially selected in rs8570 heterozygote (GC) patients because they have just one chromosome to be modified. In fact, after sequencing the entire 3′-UTR region expanding the first 200 nucleotides after the STOP codon, we find a preferential enrichment in the three mutations in the chromosome bearing the C allele compared to the one bearing the G allele. This conclusion is preliminary, given the relatively small number of GC heterozygotes at the SNP position that we have been able to sequence. If confirmed, the association in cis could indicate that mutations in positions +26, +53, and +180 could interfere with microRNAs or other regulatory mechanisms associated with the SNP in position +124.
Our leading theory consists of a compensatory mechanism at play. The 124 C-allele (rs8570) has been associated with higher RRAS2 expression and, consequently, a worse disease prognosis [
17]. We propose that the presence of three newly identified 3′UTR mutations compensates for the negative attributes of the 124 C-allele and could potentially reduce the adverse effects of elevated RRAS2 expression. In addition to describing three somatic mutations in the 3′-UTR sequence of the RRAS2 gene, we reinforce the idea that the SNP rs8570, and overall RRAS2 overexpression in its wild-type form, is linked to CLL development. The major finding, in addition to linking the alternate C allele to higher mRNA expression, was that the distribution of GG, GC, and CC genotypes was tilted towards a higher abundance of CC homozygotes in the CLL patient cohort [
17]. Here, we have increased the number of patient samples in the CLL cohort and compared it with a cohort of healthy blood donors. Thus, we confirm the disproportion in favour of CC homozygotes within the CLL cohort and show that such disproportion is not found in the healthy population cohort, which is in Hardy-Weinberg equilibrium. However, we still do not know if the increased frequency of CC homozygotes within the cohort of CLL leukemic cells is originated from somatic G>C mutation at the SNP position or it is due to a higher propensity of the human population with CC homozygosity at the SNP position to develop CLL.
In the mouse model of RRAS2 overexpression driving CLL development, we found that with time there is a selection in favor of leukemic cells with even higher expression of RRAS2 mRNA [
17]. Therefore, a higher proportion of leukemias bearing the SNP rs8570 in CC homozygosity is understandable due to its association with higher expression. By contrast, what could be the reason for the emergence of the three somatic mutations described here, that result in lower RRAS2 expression and lower lymphocytosis? Our running hypothesis is that during the biological evolution of the leukemic CLL clones, excessive RRAS2 overexpression could lead to senescence or to a dead-end differentiation of the pre-malignant B cell clones. Overall, the results shown in this paper further link wild-type RRAS2 overexpression to CLL development and clinical progression.
Figure 1.
The C allele at rs8570 SNP in the 3′-UTR of RRAS2 is overrepresented in CLL patients compared to the normal population. (A) Pie chart representation of the Observed versus the Expected distribution of the GG, GC and CC genotypes at the rs8570 SNP in blood samples from our cohort of CLL patients (n= 203). The Chi-squared value (χ2) is greater than 3.84 such that the hypothesis that the Observed and Expected distributions are equivalent is rejected at p<0.001. (B) Pie chart representation of the Observed versus the Expected distribution of the GG, GC and CC genotypes at the rs8570 SNP in blood samples from our cohorts of healthy volunteers (n= 235). The Chi-squared value (χ2) is lower than 3.84 such that the hypothesis that the Observed and Expected distributions are different is rejected at p>0.05. (C) Stacked bar plot showing a comparison of the number of blood samples with GG, GC and CC genotypes at the rs8570 SNP within the patient and healthy donor cohorts. Contingency tests were carried to compare two-by-two the frequencies of genotypes between the patient and the healthy volunteer cohorts. A Chi-squared test was used to compute the p values. ns, not significant (p>0.05).
Figure 1.
The C allele at rs8570 SNP in the 3′-UTR of RRAS2 is overrepresented in CLL patients compared to the normal population. (A) Pie chart representation of the Observed versus the Expected distribution of the GG, GC and CC genotypes at the rs8570 SNP in blood samples from our cohort of CLL patients (n= 203). The Chi-squared value (χ2) is greater than 3.84 such that the hypothesis that the Observed and Expected distributions are equivalent is rejected at p<0.001. (B) Pie chart representation of the Observed versus the Expected distribution of the GG, GC and CC genotypes at the rs8570 SNP in blood samples from our cohorts of healthy volunteers (n= 235). The Chi-squared value (χ2) is lower than 3.84 such that the hypothesis that the Observed and Expected distributions are different is rejected at p>0.05. (C) Stacked bar plot showing a comparison of the number of blood samples with GG, GC and CC genotypes at the rs8570 SNP within the patient and healthy donor cohorts. Contingency tests were carried to compare two-by-two the frequencies of genotypes between the patient and the healthy volunteer cohorts. A Chi-squared test was used to compute the p values. ns, not significant (p>0.05).
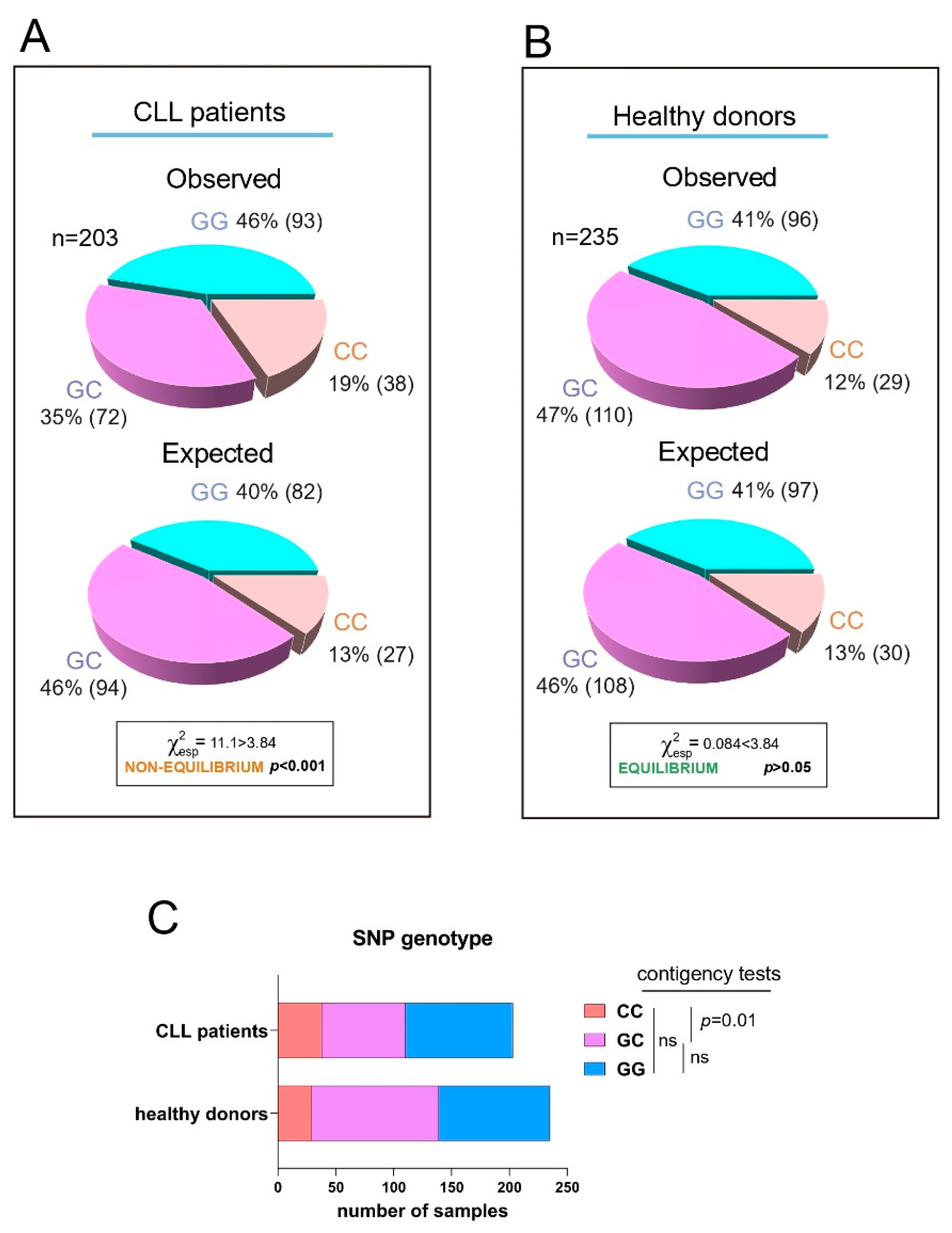
Figure 2.
The 3′-UTR of RRAS2 frequently bears somatic mutations at positions +26, +53 and +180 from the STOP codon. (A) Schematic representation of the site of rs8570 in the 3′UTR of RRAS2 mRNA, 124 nucleotides downstream of the stop codon. The SNP position is indicated in a box and the canonical nucleotide (G) at that position is in red. Other sites in the 3′-UTR (+26, +53 and +180) at which somatic mutations are found in our cohort of CLL patients are indicated with the canonical nucleotides. (B-D) Dedicated qPCRs were used to determine the ratio between the alternate and canonical nucleotides for each of the three sites found mutated in the cohort (n=270) of CLL blood samples and represented in the y-axis. The sample number is indicated in the x-axis. The mean value of all ratios is indicated with a solid green line and the median with a broken green line. Samples with ratios above the median were considered as bearing the alternate allele at least in heterozygosity. Numbers beside the red dots point out to the samples containing simultaneously the three mutations. (E) Stacked bar plot showing the distribution of CLL blood samples according to the presence of somatic mutations at positions +26, +53 and +180 in isolation or in combinations.
Figure 2.
The 3′-UTR of RRAS2 frequently bears somatic mutations at positions +26, +53 and +180 from the STOP codon. (A) Schematic representation of the site of rs8570 in the 3′UTR of RRAS2 mRNA, 124 nucleotides downstream of the stop codon. The SNP position is indicated in a box and the canonical nucleotide (G) at that position is in red. Other sites in the 3′-UTR (+26, +53 and +180) at which somatic mutations are found in our cohort of CLL patients are indicated with the canonical nucleotides. (B-D) Dedicated qPCRs were used to determine the ratio between the alternate and canonical nucleotides for each of the three sites found mutated in the cohort (n=270) of CLL blood samples and represented in the y-axis. The sample number is indicated in the x-axis. The mean value of all ratios is indicated with a solid green line and the median with a broken green line. Samples with ratios above the median were considered as bearing the alternate allele at least in heterozygosity. Numbers beside the red dots point out to the samples containing simultaneously the three mutations. (E) Stacked bar plot showing the distribution of CLL blood samples according to the presence of somatic mutations at positions +26, +53 and +180 in isolation or in combinations.
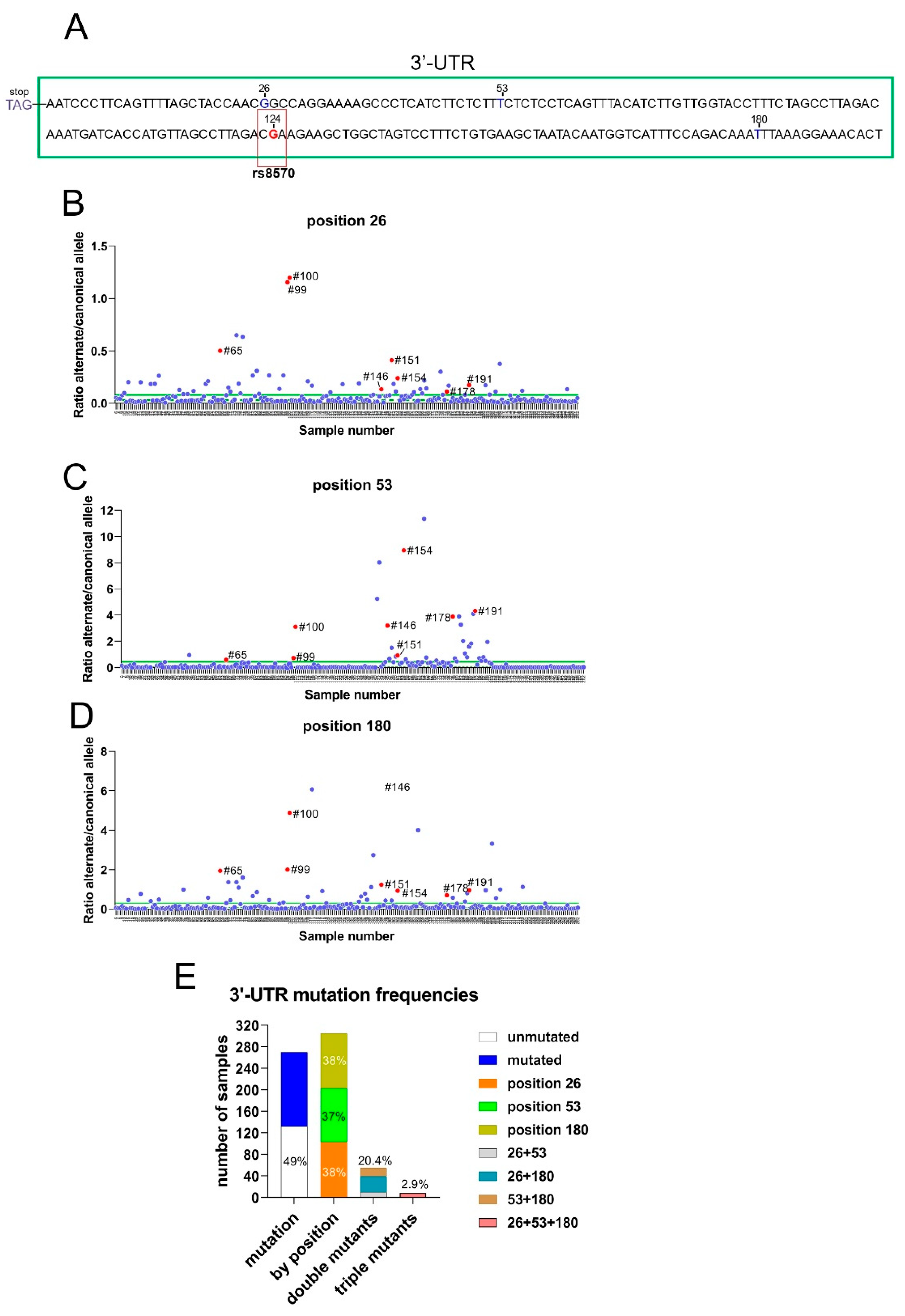
Figure 3.
The three somatic mutations in the 3′-UTR of RRAS2 are associated to the SNP rs8570 in heterozygosity and to lower mRNA expression. (A) Box and whisker plots showing all the points, the median and the mean (cross, +) values of the alternate/canonical frequency ratios for each of the 3′-UTR mutated positions and classified according to the SNP rs8570 genotype. Significance was assessed using a multiple comparison One-way ordinary ANOVA test. ns, not significant (p>0.05). (B) Stacked bar plot showing the total number of mutations at the three 3′-UTR positions in the patient CLL cohort classified according to the SNP rs8570 genotype. Inserted numbers indicate the average number of mutations detected per CLL sample. (C) Stacked bar plot showing the percentage of SNP rs8570 genotypes in CLL patients classified according to the presence of somatic mutations at the indicated 3′-UTR positions. Contingency tests were carried out to compare two-by-two the frequencies of genotypes between the different mutant populations. A Chi-squared test with Yates’ correction was used to compute the p values. **, p=0.005; *, p=0.016; ns, not significant (p>0.05). (D-E) Pie chart representation of the Observed distribution of the GG, GC and CC genotypes at the rs8570 SNP in blood samples of the cohorts of CLL patients bearing no mutations in the 3′-UTR and bearing mutation at the +26 position. The Chi-squared value (χ2) is greater than 3.84 for both groups such that the hypothesis that genotypes are at Hardy-Weinberg equilibrium is rejected with p<0.025. (F) Violin plot showing all experimental points, the median and the upper and lower quartiles for RRAS2 expression in all CLL patients in our cohort classified according to the presence or not of any mutation in the 3′-UTR. Significance was assessed using a non-parametric unpaired Mann-Whitney rank test (****, p<0.0001). (G) Violin plot showing all experimental points, the median and the upper and lower quartiles for RRAS2 expression in all CLL patients in our cohort classified according to the presence or not of mutations at the indicated positions of the 3′-UTR. Significance was assessed using a non-parametric unpaired Dunn’s multiple comparisons test (****, p<0.0001; ***, p=0.0002; **, p=0.0016). (H) Violin plot showing all experimental points, the median and the upper and lower quartiles for RRAS2 expression in all CLL patients in our cohort classified according to the SNP rs8570 genotype and to the presence or not of mutations at the three positions of the 3′-UTR. Significance was assessed using a using a non-parametric unpaired Mann-Whitney rank test (**, p=0.0067; *, p=0.017). ns, not significant (p>0.05).
Figure 3.
The three somatic mutations in the 3′-UTR of RRAS2 are associated to the SNP rs8570 in heterozygosity and to lower mRNA expression. (A) Box and whisker plots showing all the points, the median and the mean (cross, +) values of the alternate/canonical frequency ratios for each of the 3′-UTR mutated positions and classified according to the SNP rs8570 genotype. Significance was assessed using a multiple comparison One-way ordinary ANOVA test. ns, not significant (p>0.05). (B) Stacked bar plot showing the total number of mutations at the three 3′-UTR positions in the patient CLL cohort classified according to the SNP rs8570 genotype. Inserted numbers indicate the average number of mutations detected per CLL sample. (C) Stacked bar plot showing the percentage of SNP rs8570 genotypes in CLL patients classified according to the presence of somatic mutations at the indicated 3′-UTR positions. Contingency tests were carried out to compare two-by-two the frequencies of genotypes between the different mutant populations. A Chi-squared test with Yates’ correction was used to compute the p values. **, p=0.005; *, p=0.016; ns, not significant (p>0.05). (D-E) Pie chart representation of the Observed distribution of the GG, GC and CC genotypes at the rs8570 SNP in blood samples of the cohorts of CLL patients bearing no mutations in the 3′-UTR and bearing mutation at the +26 position. The Chi-squared value (χ2) is greater than 3.84 for both groups such that the hypothesis that genotypes are at Hardy-Weinberg equilibrium is rejected with p<0.025. (F) Violin plot showing all experimental points, the median and the upper and lower quartiles for RRAS2 expression in all CLL patients in our cohort classified according to the presence or not of any mutation in the 3′-UTR. Significance was assessed using a non-parametric unpaired Mann-Whitney rank test (****, p<0.0001). (G) Violin plot showing all experimental points, the median and the upper and lower quartiles for RRAS2 expression in all CLL patients in our cohort classified according to the presence or not of mutations at the indicated positions of the 3′-UTR. Significance was assessed using a non-parametric unpaired Dunn’s multiple comparisons test (****, p<0.0001; ***, p=0.0002; **, p=0.0016). (H) Violin plot showing all experimental points, the median and the upper and lower quartiles for RRAS2 expression in all CLL patients in our cohort classified according to the SNP rs8570 genotype and to the presence or not of mutations at the three positions of the 3′-UTR. Significance was assessed using a using a non-parametric unpaired Mann-Whitney rank test (**, p=0.0067; *, p=0.017). ns, not significant (p>0.05).
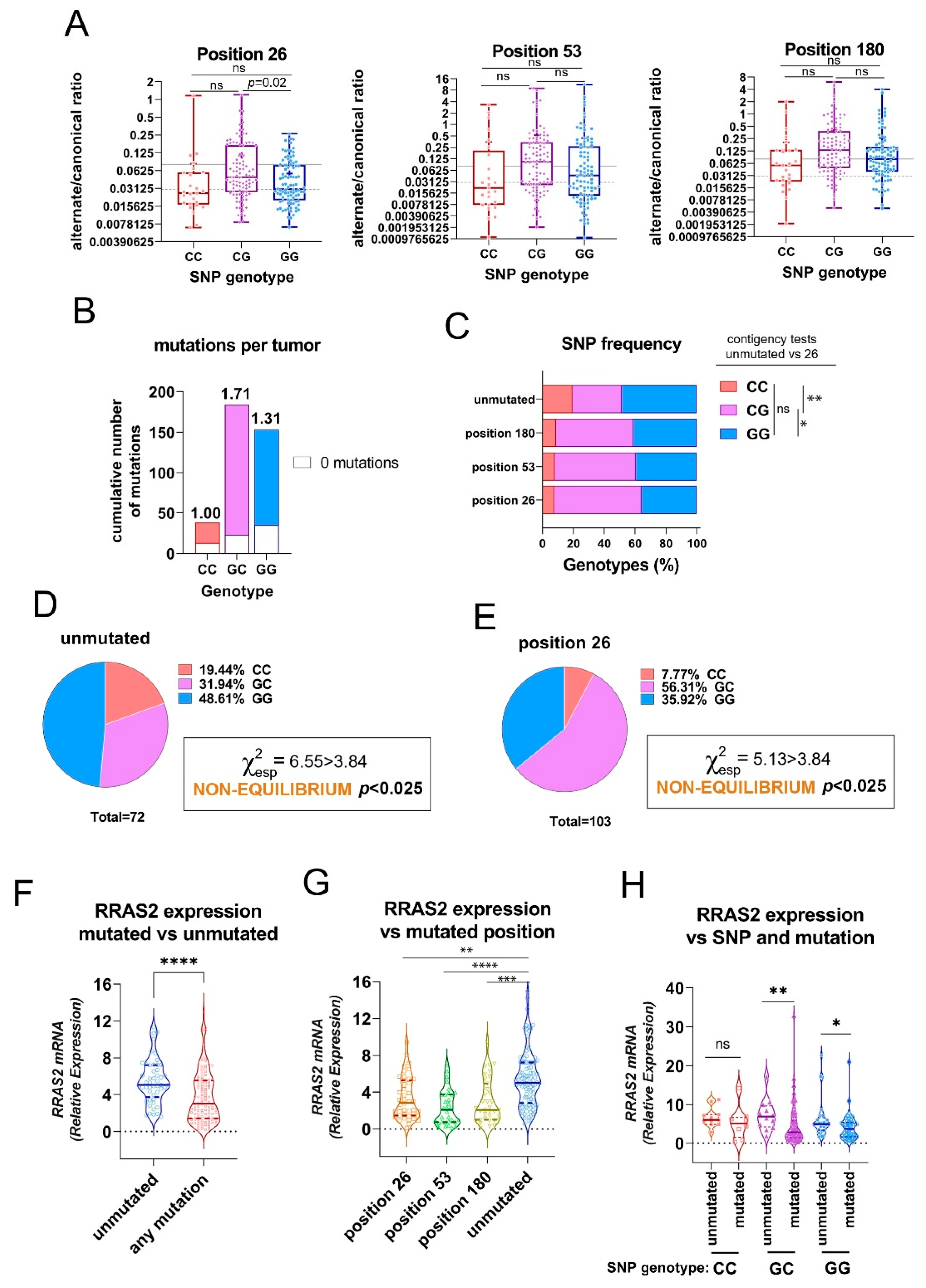
Figure 4.
The three somatic mutations in the 3′-UTR of RRAS2 are associated with lower lymphocytosis in blood of CLL patients. (A) Stacked bar plot showing the percentage of patients with full-blown CLL or pre-malignant MBL at diagnosis classified according to the presence or not of mutations at the indicated positions of the 3′-UTR. Differences were not significant (p>0.05) using an unpaired t-test. (B) Stacked bar plot showing the percentage of patients with leukemia bearing either mutated or unmutated IgHV classified according to the presence or not of mutations at the indicated positions of the 3′-UTR. Differences were not significant (p>0.05) using an unpaired t-test. (C) Stacked bar plot showing the percentage of patients with leukemia bearing the indicated chromosomal anomalies and classified according to the presence or not of mutations at the indicated positions of the 3′-UTR. Differences were not significant (p>0.05) using a Two-way ANOVA test. (D) Stacked bar plot showing the percentage of patients with leukemia classified according to their sex and to the presence or not of mutations at the indicated positions of the 3′-UTR. Differences were not significant (p>0.05) using contingency and Chi-squared tests. (E) Box and whisker plots showing all the points, the median and the mean (cross, +) values of the age at diagnosis for CLL patients bearing leukemias with mutations at the indicated positions. Significance was assessed using a non-parametric unpaired Dunn’s multiple comparisons test. ns, not significant (p>0.05). (F) Scatter plot with bars showing all the points and the mean±SEM values of the platelet concentration in blood of CLL patients Significance was assessed using an ordinary One-way ANOVA test. ns, not significant (p>0.05). (G-H) Scatter plots with bars showing all the points and the mean±SEM values of the platelet concentration (G) and total lymphocytes (H) in blood of CLL patients. Significance was assessed using a non-parametric unpaired Dunn’s multiple comparisons test. (****, p<0.0001; ***, p=0.0006; **, p=0.0047)- (I) Scatter plots with bars showing all the points and the mean±SEM values of the percentage of leukemic CD19+CD5+ within blood lymphocytes in CLL patients. Significance was assessed using a non-parametric unpaired Dunn’s multiple comparisons test. (****, p<0.0001; ***, p=0.0007; **, p=0.0025).
Figure 4.
The three somatic mutations in the 3′-UTR of RRAS2 are associated with lower lymphocytosis in blood of CLL patients. (A) Stacked bar plot showing the percentage of patients with full-blown CLL or pre-malignant MBL at diagnosis classified according to the presence or not of mutations at the indicated positions of the 3′-UTR. Differences were not significant (p>0.05) using an unpaired t-test. (B) Stacked bar plot showing the percentage of patients with leukemia bearing either mutated or unmutated IgHV classified according to the presence or not of mutations at the indicated positions of the 3′-UTR. Differences were not significant (p>0.05) using an unpaired t-test. (C) Stacked bar plot showing the percentage of patients with leukemia bearing the indicated chromosomal anomalies and classified according to the presence or not of mutations at the indicated positions of the 3′-UTR. Differences were not significant (p>0.05) using a Two-way ANOVA test. (D) Stacked bar plot showing the percentage of patients with leukemia classified according to their sex and to the presence or not of mutations at the indicated positions of the 3′-UTR. Differences were not significant (p>0.05) using contingency and Chi-squared tests. (E) Box and whisker plots showing all the points, the median and the mean (cross, +) values of the age at diagnosis for CLL patients bearing leukemias with mutations at the indicated positions. Significance was assessed using a non-parametric unpaired Dunn’s multiple comparisons test. ns, not significant (p>0.05). (F) Scatter plot with bars showing all the points and the mean±SEM values of the platelet concentration in blood of CLL patients Significance was assessed using an ordinary One-way ANOVA test. ns, not significant (p>0.05). (G-H) Scatter plots with bars showing all the points and the mean±SEM values of the platelet concentration (G) and total lymphocytes (H) in blood of CLL patients. Significance was assessed using a non-parametric unpaired Dunn’s multiple comparisons test. (****, p<0.0001; ***, p=0.0006; **, p=0.0047)- (I) Scatter plots with bars showing all the points and the mean±SEM values of the percentage of leukemic CD19+CD5+ within blood lymphocytes in CLL patients. Significance was assessed using a non-parametric unpaired Dunn’s multiple comparisons test. (****, p<0.0001; ***, p=0.0007; **, p=0.0025).
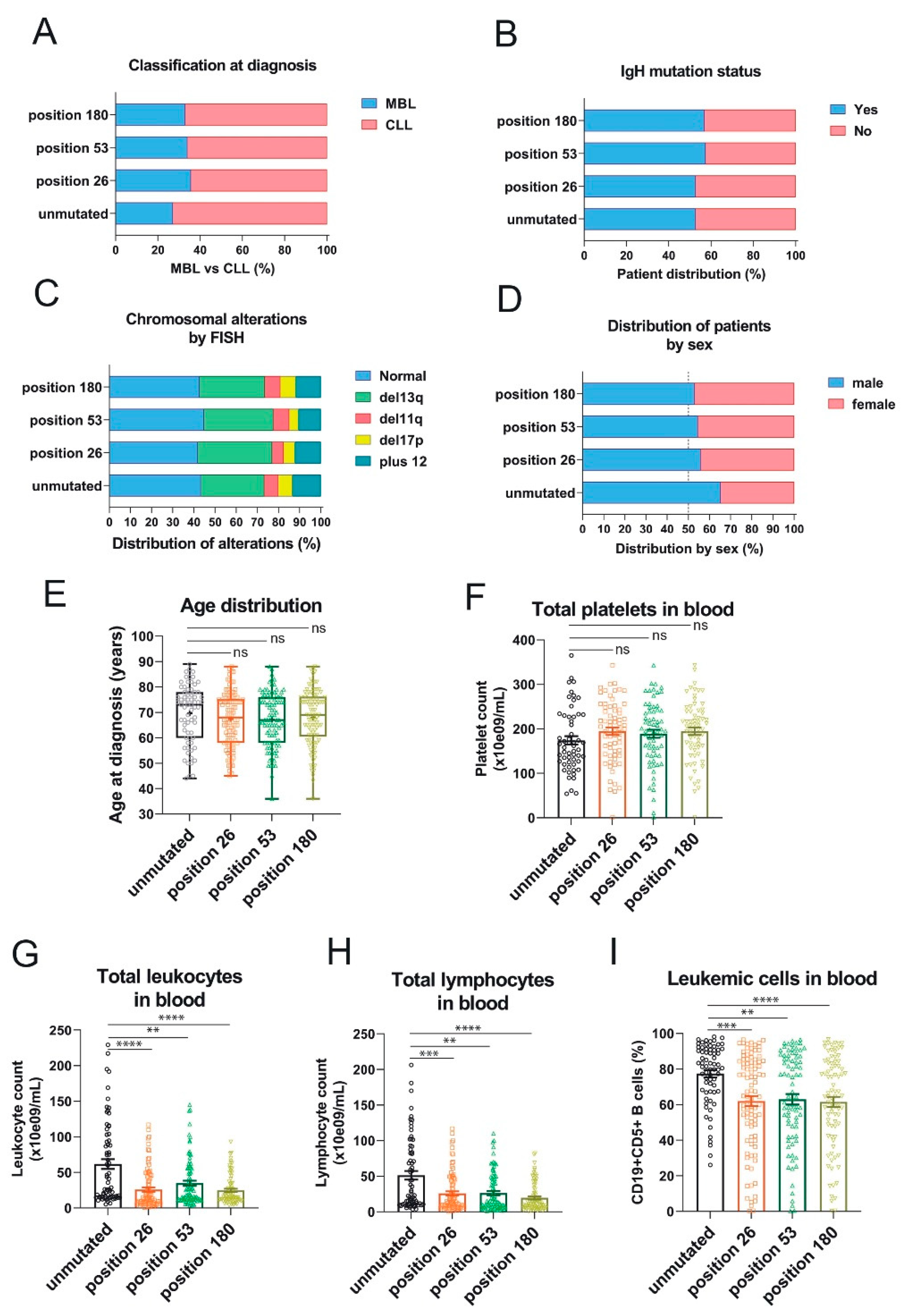
Table 1.
provides a detailed overview of the specific oligonucleotides utilized in the qPCR analysis to characterize mutations within RRAS2 3′UTR. Notably, the nucleotide in blue type represents the mutation site in the somatic mutations 26, 53 and 180 as well as the SNP (rs8570) at 124. The red nucleotides differ from the native gene sequence to enhance the correct separation of the allele populations according to results in [
18] (Lefever et al., 2019).
Table 1.
provides a detailed overview of the specific oligonucleotides utilized in the qPCR analysis to characterize mutations within RRAS2 3′UTR. Notably, the nucleotide in blue type represents the mutation site in the somatic mutations 26, 53 and 180 as well as the SNP (rs8570) at 124. The red nucleotides differ from the native gene sequence to enhance the correct separation of the allele populations according to results in [
18] (Lefever et al., 2019).
| 3′UTR Mutation |
Allele |
Forward primer sequence |
Reverse primer sequence |
| 26 |
G (canonical) |
CCCTTCAGTTTTAGCTACCCACG
|
GCTTCACAGAAAGGACTAGCC |
|
T (alternative) |
CCCTTCAGTTTTAGCTACCCACT
|
| 53 |
T (canonical) |
GGAAAAGCCCTCATCTTCTATTT
|
AGCAGCCTTAGTGTTTCCTT |
|
C (alternative) |
GGAAAAGCCCTCATCTTCTATTC
|
| 124 |
G (canonical) |
GATCACCATGTTAGCCTTATACC
|
AGCAGCCTTAGTGTTTCCTT |
|
C (alternative) |
GATCACCATGTTAGCCTTATACG
|
| 180 |
T (canonical) |
CTACCAACGGCCAGGAAAAG |
GCAGCCTTAGTGTTTCCTTGAAA
|
|
C (alternative) |
GCAGCCTTAGTGTTTCCTTGAAG
|









