1. Introduction
Immunotherapy currently serves as the cornerstone of new drug treatments for various types of cancer. In the case of breast cancer, immunotherapy has become a standard treatment for both advanced and early stages of the disease, specifically targeting certain patient subgroups, like the triple-negative subtype. [
1] However, existing biomarkers have limitations in accurately predicting treatment response. In fact, the efficacy of immune checkpoint inhibitors in the neoadjuvant setting remains unaffected by PDL-1 expression, which is the most common marker used to define the activity of these agents in different tumor models. [
2,
3]
Tertiary lymphoid structures (TLS) are ectopic lymphoid formations that can arise after prolonged exposure to inflammatory signals mediated by chemokines and cytokines. These structures bear resemblance to secondary lymphoid structures (SLS), and usually include germinal centers surrounded by T lymphocytes, dendritic cells and high endothelial venules (HEV) in close proximity. [
1,
2] (4) TLSs, unlike SLSs, lack a surrounding capsule and are typically observed in the context of chronic non-neoplastic inflammatory conditions. However, TLSs have been identified in the microenvironment of different tumor models, including melanoma, lung, colorectal, and breast cancer. [
5,
6,
7]
Remarkably, the presence of TLSs has been shown to be independent of tumor mutational burden, which can influence the immune response and TILs in several tumor entities. [
4]
2. Materials and Methods
A comprehensive literature assessment was performed by querying the following databases: PubMed/Medline, Scopus, and EMBASE. The databases were systematically searched from their inception to August 2023.
3. Results
3.1. Organization of TLS
The mechanisms involved in the induction of TLS remain a subject of debate. Some aspects of TLS organization are inherently linked to the development of lymph nodes and secondary lymphoid structures (SLS) during embryogenesis. Pimienta et al. described in their analysis that specific chemokines, such as CCL19, CCL21, and CXCL13, secreted by the surrounding mesenchyme, also play a role in attracting Lymphoid tissue-inducing (LTi) cells and lymphocyte subsets that populate the developing lymph node. [
8] Then, CCR7, which is a receptor present on subsets of T cells and dendritic cells (DCs), leads to their activation through CCL19 and CCL21 signaling. CCR7 activation is crucial for recruiting memory T cells and DCs during immune responses. CXCL13 facilitates the influx of migratory B cells that express the CXCR5 receptor. [
9] Also, circulating immune cells such as B, T, or dendritic cells can act as LTi cells in response to chemokines secreted by injured tissue. LTα promotes HEV growth and activation of follicular helper T cells (Tfh), which might represent circulating counterparts of follicular dendritic cells (FDCs). Furthermore, the architecture of TLS typically features a zone of T cells adjacent to a B cell follicle, resembling the structure of SLS and mucosa-associated lymphoid tissues. [
8] TLS are characterized by loosely organized structures containing HEV-like structures, which differ from the classic HEVs typically found in peripheral lymph nodes. [
10] These specialized blood vessels contribute to the migration dynamics of central and naïve memory T cells, as well as naïve B cells, by offering structural support for chemotactic cytokines, including CCL19, CCL21, CXCL12, and CXCL13. [
10,
11]
TLS can adopt diverse histoarchitectural patterns, comprising a B-cell compartment, that incorporates CD23+ germinal center B cells (GC-B) and peripheral naïve, plasma (PC), and memory B cells. In close proximity, Tfh cells are the predominant cell type in the T-cell compartment of TLS, suggesting their role as regulators of the B lymphocyte lineage. Other essential cell subpopulations found within TLS include fibroblastic reticular cells (FRCs), which play a specific regulatory role in T-cell development, and dendritic cell (DC) subpopulations that may vary within TLS compartments. These DC subpopulations include dendritic cell-lysosomal associated membrane protein (DC-LAMP) and CD21+ follicular dendritic cells (FDCs), which are found in the T and B-cell regions, respectively. [
12] One characteristic of TLSs is their plasticity, as they can form temporarily and then disappear when the antigen is removed. The location of lymphoid aggregates is predetermined by the expression of the lymphotoxin β receptor (LTβR) on endothelial cells. [
13,
14] Meylan et al. showed that in TLS-associated tumors, IgG- and IgA-producing plasma cells (PCs) spread to tumor beds along fibroblastic pathways. Tumors that express TLS, in turn, exhibit IgG-producing PCs and IgG-stained apoptotic malignant cells, indicating potential antitumor activity. [
15] It has also been evidenced that the subtype of antibody produced by B lymphocytes is an important factor associated with immunity response. . Importantly, IgG+ and IgA+ PCs are found within TLS and are also present at a distance in tumors along CXCL-12 and COL1 positive fibroblasts. Additionally, tumors exhibiting TLS are coated with IgG antibodies and have augmented proapoptotic signaling. [
16]. Furthermore, Harris et al. recently demonstrated in their study that clonal expansion towards IgG isotype production is a favorable prognostic factor in breast cancer for desease free survival in TNBC. [
17]
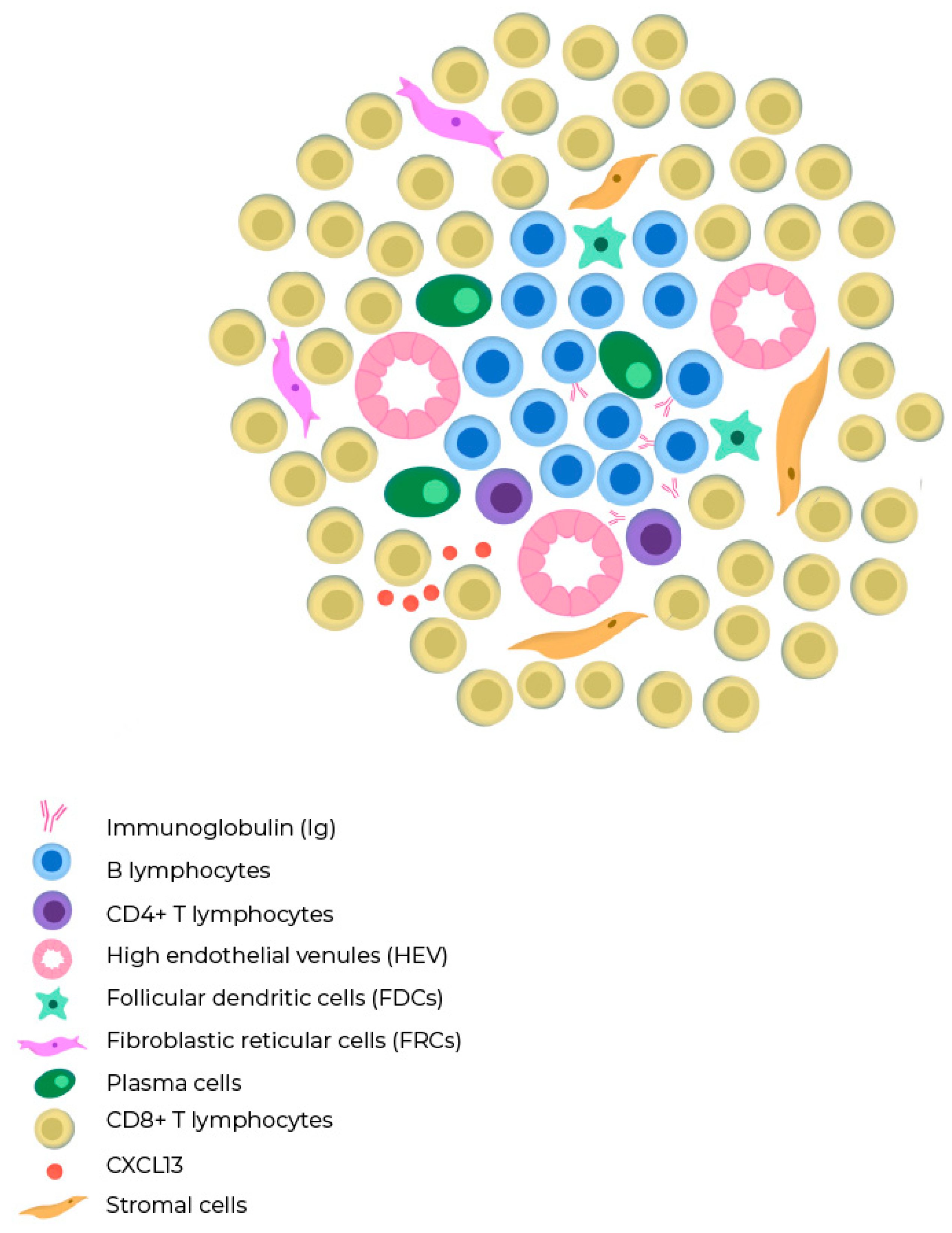
Figure 1.
Composition of a tertiary lymphoid structure. B cells are mostly intermingled with each other, but occasionally, they form distinct B cell and T cell compartments similar to those found in lymph nodes. TLSs lack a capsule and exhibit high endothelial venules (HEV) in their architecture. The T cell zone contains mature dendritic cells and fibroblastic reticular cells, while the B cell zone has a germinal center with plasma cells, macrophages, and follicular dendritic cells.
Figure 1.
Composition of a tertiary lymphoid structure. B cells are mostly intermingled with each other, but occasionally, they form distinct B cell and T cell compartments similar to those found in lymph nodes. TLSs lack a capsule and exhibit high endothelial venules (HEV) in their architecture. The T cell zone contains mature dendritic cells and fibroblastic reticular cells, while the B cell zone has a germinal center with plasma cells, macrophages, and follicular dendritic cells.
3.2. Role in other tumor models
TLSs have been predominantly linked to a favorable clinical outcome in patients with various types of solid tumors, such as hepatocarcinoma and colon cancer, where they have demonstrated an improvement in recurrence-free survival (RFS) [
18,
19,
20]. Similarly, in melanoma, TLSs have been associated with improved overall survival and recurrence-free survival in patients treated with ICIs . One possible explanation for these findings is that TLSs are associated with a higher density of CD8+ T lymphocytes that infiltrate the tumor with an activated and cytotoxic immune signature [
21,
22]
In
Table 1 we summarize selected studies studying the prognostic role of TLSs in different tumor models
The case of soft tissue sarcomas is particularly noteworthy due to their typical classification as non-immune responsive tumors. However, in recent exploratory analyses, an association was established between intratumoral plasma cell (PC) abundance and progression-free survival in patients who were treated with pembrolizumab and cyclophosphamide. [
23,
24]
3.3. TLS in breast cancer
Immunological profiling of breast tumors has provided valuable insights into potential immune evasion mechanisms in breast cancer and has revealed unique aspects of the tumor microenvironment (TME). [
8]
A significant area of interest in breast cancer research involves targeting immune checkpoint molecules, particularly in the triple-negative subtype, where treatment options remain limited. The expression of immune checkpoint signals has been associated with the presence of TILs and TLS, although there is notable heterogeneity, even within the same biological tumor subtypes. TLSs are predominantly found in the stromal region of approximately 60% of breast cancer cases. [
25] The infiltration of immune cells in breast tumors has proven to be both predictive and prognostic. Tumors with higher levels of TILs were described to respond more favorably to various treatments, such as ICIs, chemotherapy, and radiation, compared to tumors with low TILs. [
26] Despite the growing interest in immune checkpoint blockade in breast cancer, the response rates achieved with single-agent therapy have been relatively low in the metastatic setting, indicating the presence of intrinsic or innate resistance mechanisms. [
24] GuTrantien et al. devised an 8-gene signature to reflect the presence of TLS. [
27] This signature was found to be prognostic in breast cancer patients undergoing surgical resection with or without neoadjuvant chemotherapy. Particularly, CXCL13 expression was found to have the closest association with prognostic signature in this cohort. Moreover, this cytokine has also been found to allow identification of TLSs in colorectal cancer and soft tissue sarcoma.
Another genomic platform evaluated the prognostic role of 12 genes which encode chemokines (12-CK) associated with immunity and inflammation in different tumor models [
28]. In the breast cancer subgroup, authors described that basal and HER2-enriched tumors, and a high calculated score of chemokine-gene expression were associated with higher DFS and OS. By molecular subtypes, patients with the basal subtype and HER2 obtained a survival benefit by having a high expression of the 12-CK gene.
Wang et al. [
29] conducted a meta-analysis involving 15 studies with 3,898 patients. Their combined analysis revealed that the presence of TLSs was associated with improved disease-free survival and overall survival. Additionally, TLS presence was positively correlated with early tumor TNM stage and high tumor-infiltrating lymphocytes, as well as with human epidermal growth factor receptor 2 and Ki-67 but inversely correlated with the status of estrogen and progesterone receptors. The study also found that the tumor immune microenvironment was more favorable in the high-TLS signature group, leading to better survival outcomes for breast cancer patients.
Immune checkpoint blockade has yielded remarkable and enduring responses across various cancer types. Nevertheless, not all patients exhibit favorable responses, and the currently available biomarkers (such as PDL1 expression, tumor-infiltrating lymphocytes, and tumor mutational burden) do not consistently provide reliable predictions of treatment outcomes. Consequently, there exists an urgent need for novel and more specific biomarkers to guide the clinical practice [
30]. Tertiary lymphoid structures (TLSs) have long been acknowledged as prognostic markers for enhancing patient outcomes. Recently, mature TLSs have been identified as predictors of successful outcomes in patients treated with immune checkpoint inhibitors. This predictive capability likely arises from their role in eliciting immunogenic cell death, leading to the release of neoantigens [
31]. The findings from Solinas et al.'s study (8) revealed that TLSs in breast cancer were infiltrated with cells expressing PD-L1, PD-L2, LAG3, and TIM3, suggesting that TLSs represent important sites of immune activation and regulation. However, it is important to note that while information from preclinical trials suggests that TLSs could serve as valuable predictors of the effectiveness of immunotherapy, this matter still warrants further clinical and translational investigation [
32,
33].
In
Table 2 we summarize selected studies studying the prognostic and predictive role of TLSs in breast cancer with favorable outcomes en DFS, OS and pCR.
3.4. Her2 positive tumors
In Her2+ tumors, the presence of macrophage infiltration has been associated with the presence of TLSs. Additionally, a strong correlation was reported between the extension of the of the ductal carcinoma in situ (DCIS) component of an invasive lesion and the identification of TLSs. [
11] According to the findings of the retrospective study by Xia Liu, which involved 248 samples of both HER2-positive and HER2-negative breast cancer, it was demonstrated that TLSs were associated with a prolonged PFS. Importantly, the authors described that TLSs were not associated with the presence of TILs, which may support the characterization of TLSs as a independent prognostic biomarker. [
2] Based on these findings, it is possible to speculate that increased HER2 protein expression may contribute to a pro-immunogenic environment, attracting lymphocytes to the tissue and promoting TLS formation. Another potential explanation could be related to the frequent presence of comedonecrosis in HER2+ DCIS, which may lead to an increased infiltration of macrophages. Macrophages serve as antigen-presenting cells in antitumor immune response, potentially contributing to the development of TLS.
Gu-Trantian and colleagues characterized that CXCL13-producing follicular helper T (TFH) cells were associated with better disease-free survival (DFS) and complete pathological response in patients with localized HER2-positive tumors. [
27,
34]. Xia Liu and colleagues also showed an association with TLSs presence and better prognosis in a cohort of 55 patients with HER2+ breast cancer. This was not associated with other ICI-associated predictive biomarkers, such as the level of TILs. [
2]
Indeed, the HER2DX assay recently developed and validated by Prat et al. for predicting pathological response in HER2-positive tumors demonstrated that tumors with a higher immune profile exhibit better rates of complete pathological response following neoadjuvant therapy. HER2DX is based on four distinct gene signatures encompassing 27 genes, capturing diverse biological processes, including immune infiltration, tumor cell proliferation, luminal differentiation, and HER2 amplicon expression. It was observed that tumors with increased levels of CD4+, CD8+, CD20+ s-TILs, and CD20+ intratumoral TILs were independently associated with a higher probability of achieving a pathological complete response (P=0.03). [
35]
3.5. HR positive breast tumors
Classically, luminal subtype tumors are considered to have the "coolest" immune microenvironment when compared to their triple-negative or HER2-positive counterparts. Luminal subtype tumors are characterized by high levels of FOXP1 expression and low levels of TLSs [
36]. One explanation for this tumor microenvironment is that increased lymphocytic infiltration in tumors is inversely correlated with the expression of estrogen receptor (ER) and/or progesterone receptor (PR) [
37]. FOXP1 expression is higher in ER-positive breast cancers compared to ER-negative ones. Of note, FOXP1-high tumors are significantly associated with fewer TILs and TLSs, and a higher expression of IL10 and TGFβ, resulting in an immunosuppressive environment [
38]. However, the initial outcomes of the first prespecified interim analysis of the KEYNOTE-756 randomized and double-blind phase III trial have recently been disclosed. The association of ICI treatment with higher pCR in a high-risk ER+ subgroup supports the necessity of adequately identifying which biomarkers can better explain treatment response to these agents. [
39].
Another intriguing aspect concerning HR-positive tumors is that prior treatment with CDK inhibitors (CDKi) in the metastatic setting has shown the potential to stimulate heightened immune responses. In preclinical models, CDK4/6 inhibitors were associated with enhanced tumor antigen presentation, reduced proliferation of regulatory T cells (Tregs), and a lower expression of immune inhibitory receptors, such as PD-1. [
40]
3.6. Triple negative breast tumors
Tumors classified within the triple-negative breast cancer (TNBC) subgroup exhibit the presence of TLS in 83.7% of cases [
41]. Within this subgroup, the level of FOXP1 is of significance as it regulates the chemokine CXCL13, a B-cell chemoattractant. Comparable findings were reported by Song et al. analyzed a cohort of 108 TNBC patients who underwent neoadjuvant chemotherapy.TLS density, as well as CD8+ and CD20+ and CXCL13 expression were found to be correlated with pCR [
25]. In the metastatic setting, f PD-L1 expression is a predictive factor for ICI response in this patient subgroup [
42]. Moreover, we are aware that neoadjuvant immunotherapy is now considered the standard treatment approach for TNBC. Nevertheless, further research is still needed within this subgroup to assess the prognostic value of TLS to understand its role as an independent biomarker. According to the results from the study conducted by Vanhersecke and colleagues [
22], which evaluated the role of TLS in samples with positive and negative PD-L1 tumors, according to a cutoff value of 1% for tumor proportion score. Patients with TLS-positive tumors had response rates of 69.2% and 40.3%, considering PD-L1 positive and negative tumors, respectively. In turn, patients with TLS-negative tumors had significantly lower response rates, of 35.6% and 14.1%, respectively. In this study, the proportion of PD-L1-positive tumors was similar between tumors with a high density of TLS (22.4%) and those with low TLS density or no TLS (21.1%). Regardless of the PD-L1 expression status, patients with TLS-positive tumors exhibited superior outcomes.
3.7. Diagnosis of TLS
Diagnosing TLS in the tumor microenvironment is a complex task due to different reasons, including the heterogeneity of TLS and tumoral microenvironment For instance, the assessment of PD-L1 expression, a commonly used biomarker, is prone to high inter-observer variability due to the use of different antibodies, platforms, and scoring systems. [
43,
44] An important consideration when selecting the type of tissue for TLS diagnosis is to recognize that these structures display spatial variations within tumor tissues and may not be fully represented when examined through small-needle biopsy samples or tissue microarrays. This challenge can be particularly significant in breast cancer patients, as they frequently undergo neoadjuvant systemic therapy following an initial diagnosis with a core needle biopsy, limiting the availability of tissue samples for adequately assessing the presence of TLSs. [
2,
45]
TLS identification methods can vary depending on the tumor type and the specific research objectives. Some commonly employed techniques for quantifying TLSs include the recognition of immune cell markers and the immunohistochemical labeling of components such as high endothelial venules. Staining of CD21 and CD23, which indicate the presence of follicular dendritic cells (FDCs), are frequently utilized for TLS identification. Additionally, the co-localization of CD3+ T cells and CD20+ B cells represents another reported technique [
46].
Another way to recognize TLSs is using gene or chemokine signatures by immunohistochemistry. TLSs can be detected by analyzing the expression of specific genes or chemokines associated with its development [
47].
Lastly, digital and computational pathology, integrating deep learning and artificial intelligence, can be instrumental in both identifying and quantifying TLSs [
48]. Multiplex fluorescent immunohistochemistry/immunofluorescence (mIHC/IF) has demonstrated superior performance compared to immunohistochemistry. These multiplex methods allow for the simultaneous assessment of multiple targets, detection of cell densities and subpopulations, estimation of the functional states of the immune infiltrate, and characterization of spatial organization by analyzing cell-cell interactions and distribution in various regions of interest and tissue compartments. [
49] Digital imaging facilitates reproducibility in the analysis of these complex spatial protein profiles in tumor tissues.
Immunological signatures, although informative, lack spatial information and may be dominated by the most abundant cell population, limiting their accuracy. Additionally, assessing tumor-infiltrating lymphocytes (TILs) with hematoxylin and eosin staining can be challenging, as it represents a mass measurement of stromal lymphocytic infiltration and is subject to high interobserver variability. [
50] The limitation of this diagnostic approach includes that not all tumor microenvironment components can be easily detected by immunohistochemistry, such as intracellular cytokines, which are indicative of the functional state of the tumor milieu. Thus, there are techniques that combine tissue biomarker characterization and in situ transcriptional profiling. [
51,
52]. As demonstrated by the results of the PEMBROSARC tria [
23,
53], it is hypothesized that the spatial distribution, rather than the density of B cells within tumors, may provide a better tool to predict treatment response, with a better prognosis for tumors that have a greater dispersion of lymphoid structures and a higher density of CD8-T cells. It is important to note that not only the inflammatory component of the primary tumor is significant, but also the presence of TLS in metastases. In particular, lung metastases, an association with improved overall survival has been reported [
54,
55].
3.8. TLS manipulation as therapeutic opportunity
TLSs are not merely a surrogate indicator of an active immune response; rather, they are believed to actively modulate the anti-tumor immune activity. In addition to serving as markers of responses to immunotherapy, TLSs have the potential to act as therapeutic agents in promoting anti-tumor immune responses. This is achieved through the recruitment of lymphocytes, control of tumor growth, and the induction of a long-lasting humoral anti-tumor immune response [
47,
57], akin to therapies involving chimeric antigen receptor T (CAR-T) cells. [
58]
Furthermore, in mouse models, it has been demonstrated that the administration of CCL21, CXCL13, or LTα alone can induce the formation of TLSs [
8]. The induction of de novo TLS formation holds significant therapeutic promise in overcoming inherent immune inhibitory mechanisms within the tumor microenvironment. This approach can potentially convert immunologically "cold" tumors, which do not respond to immunotherapy, into "hot" tumors that are susceptible to treatment [
59]. Nonetheless, these findings should be considered only as hypothesis-generating and further research is strongly needed to determine whether this biomarker may be evaluated for microenvironment-modulating therapies.
4. Discussion
Authors should discuss the results and how they can be interpreted from the perspective of previous studies and of the working hypotheses. The findings and their implications should be discussed in the broadest context possible. Future research directions may also be highlighted.
5. Conclusions
In conclusion, the landscape of cancer immunotherapy response prediction is witnessing a transformative shift, with TLS emerging as a compelling and potentially more robust biomarker compared to the extensively studied PD-L1 expression and TILs. The ongoing investigation of TLS as a treatment response predictor represents a dynamic frontier in precision immuno-oncology, brimming with the promise of enhancing patient outcomes.
The evidence thus far underscores TLS's remarkable ability to discern responders to anti-PD1/PDL1 therapy, presenting a pivotal mechanism for leveraging cancer immunotherapies to modulate the tumor microenvironment effectively. Moreover, TLS induction unveils an exciting new therapeutic avenue in the battle against cancer, calling for the implementation of standardized pathology methodologies to accurately detect and diagnose this intriguing biomarker. While the retrospective evidence showcases TLSs with better predictive value than PD-L1 in early breast breast cancer patients treated with ICIs, it also underscores the need for prospective studies to validate and fully comprehend the impact of spatial distribution in prognosis. As we traverse the frontier of precision medicine, continued research in this realm promises to revolutionize cancer care, paving the way for tailored therapies and unparalleled advancements in immuno-oncology, the exploration of TLSs as a promising avenue necessitates collaborative endeavors within the scientific community to determine if it is possible to finally find a better biomarker than PD-L1.
Author Contributions
Conceptualization, Dana Narvaez. and Federico Waisberg; methodology, Federico Waisberg; investigation, Dana Narvaez.; data curation, Dana Narvaez and Federico Waisberg; writing—original draft preparation, Dana Narvaez.; writing—review and editing, Danilo Aguirre, Alexis Ostinelli, Fernando Petracci and Federico Waisberg; visualization Matias Chacon.; supervision, Victoria Costanzo. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med. 2020, 382, 810–21. [Google Scholar] [CrossRef]
- Liu, X.; Tsang, J.Y.S.; Hlaing, T.; Hu, J.; Ni, Y.B.; Chan, S.K. Distinct Tertiary Lymphoid Structure Associations and Their Prognostic Relevance in HER2 Positive and Negative Breast Cancers. Oncologist 2017, 22, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, P.; Gandini, S.; Trapani, D.; Criscitiello, C.; Curigliano, G. Immunotherapy addition to neoadjuvant chemotherapy for early triple negative breast cancer: A systematic review and meta-analysis of randomized clinical trials. Crit Rev Oncol Hematol. 2021, 159, 103223. [Google Scholar] [CrossRef] [PubMed]
- Trüb, M.; Zippelius, A. Tertiary Lymphoid Structures as a Predictive Biomarker of Response to Cancer Immunotherapies. Front Immunol. 2021, 12, 674565. [Google Scholar] [CrossRef] [PubMed]
- Wakasu, S.; Tagawa, T.; Haratake, N.; Kinoshita, F.; Oku, Y.; Ono, Y.; et al. Preventive effect of tertiary lymphoid structures on lymph node metastasis of lung adenocarcinoma. Cancer Immunol Immunother CII. 2023, 72, 1823–34. [Google Scholar] [CrossRef] [PubMed]
- Horeweg, N.; Workel, H.H.; Loiero, D.; Church, D.N.; Vermij, L.; Léon-Castillo, A.; et al. Tertiary lymphoid structures critical for prognosis in endometrial cancer patients. Nat Commun. 2022, 13, 1373. [Google Scholar] [CrossRef] [PubMed]
- Di Caro, G.; Bergomas, F.; Grizzi, F.; Doni, A.; Bianchi, P.; Malesci, A.; et al. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin Cancer Res Off J Am Assoc Cancer Res. 2014, 20, 2147–58. [Google Scholar] [CrossRef] [PubMed]
- Solinas, C.; Garaud, S.; De Silva, P.; Boisson, A.; Van den Eynden, G.; de Wind, A.; et al. Immune Checkpoint Molecules on Tumor-Infiltrating Lymphocytes and Their Association with Tertiary Lymphoid Structures in Human Breast Cancer. Front Immunol. 2017, 8, 1412. [Google Scholar] [CrossRef] [PubMed]
- Brandum, E.P.; Jørgensen, A.S.; Rosenkilde, M.M.; Hjortø, G.M. Dendritic Cells and CCR7 Expression: An Important Factor for Autoimmune Diseases, Chronic Inflammation, and Cancer. Int J Mol Sci. 2021, 22, 8340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vaghjiani, R.G.; Skitzki, J.J. Tertiary Lymphoid Structures as Mediators of Immunotherapy Response. Cancers. 2022, 14, 3748. [Google Scholar] [CrossRef]
- Blanchard, L.; Girard, J.P. High endothelial venules (HEVs) in immunity, inflammation and cancer. Angiogenesis. 2021, 24, 719–753. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, N.; Wang, D.; Hu, X.; Zhang, G.; Li, Z.; Zhao, Y.; et al. Analysis of immune status in gastric adenocarcinoma with different infiltrating patterns and origin sites. Front Immunol. 2022, 13, 978715. [Google Scholar] [CrossRef]
- Colbeck, E.J.; Ager, A.; Gallimore, A.; Jones, G.W. Tertiary Lymphoid Structures in Cancer: Drivers of Antitumor Immunity, Immunosuppression, or Bystander Sentinels in Disease? Front Immunol. 2017, 8, 1830. [Google Scholar] [CrossRef]
- Tang, H.; Zhu, M.; Qiao, J.; Fu, Y.X. Lymphotoxin signalling in tertiary lymphoid structures and immunotherapy. Cell Mol Immunol. 2017, 14, 809–818. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meylan, M.; Petitprez, F.; Becht, E.; Bougoüin, A.; Pupier, G.; Calvez, A.; et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity. 2022, 55, 527–541e5. [Google Scholar] [CrossRef]
- Weiner, L.M.; Surana, R.; Wang, S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010, 10, 317–327. [Google Scholar] [CrossRef]
- Harris, R.J.; Cheung, A.; Ng, J.C.F.; et al. Tumor-Infiltrating B Lymphocyte Profiling Identifies IgG-Biased, Clonally Expanded Prognostic Phenotypes in Triple-Negative Breast Cancer. Cancer Res. 2021, 81, 4290–4304. [Google Scholar] [CrossRef]
- Calderaro, J.; Petitprez, F.; Becht, E.; Laurent, A.; Hirsch, T.Z.; Rousseau, B.; et al. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma. J Hepatol. 2019, 70, 58–65. [Google Scholar] [CrossRef]
- Posch, F.; Silina, K.; Leibl, S.; Mündlein, A.; Moch, H.; Siebenhüner, A.; et al. Maturation of tertiary lymphoid structures and recurrence of stage II and III colorectal cancer. Oncoimmunology. 2018, 7, e1378844. [Google Scholar] [CrossRef]
- Meshcheryakova, A.; Tamandl, D.; Bajna, E.; Stift, J.; Mittlboeck, M.; Svoboda, M.; et al. B cells and ectopic follicular structures: novel players in anti-tumor programming with prognostic power for patients with metastatic colorectal cancer. PloS One. 2014, 9, e99008. [Google Scholar] [CrossRef]
- Sautès-Fridman, C.; Petitprez, F.; Calderaro, J.; Fridman, W.H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019, 19, 307–25. [Google Scholar] [CrossRef]
- Vanhersecke, L.; Brunet, M.; Guégan, J.P.; Rey, C.; Bougouin, A.; Cousin, S.; et al. Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression. Nat Cancer. 2021, 2, 794–802. [Google Scholar] [CrossRef]
- Italiano, A.; Bessede, A.; Pulido, M.; Bompas, E.; Piperno-Neumann, S.; Chevreau, C.; et al. Pembrolizumab in soft-tissue sarcomas with tertiary lymphoid structures: a phase 2 PEMBROSARC trial cohort. Nat Med. 2022, 28, 1199–206. [Google Scholar] [CrossRef]
- Gatti-Mays, M.E.; Balko, J.M.; Gameiro, S.R.; Bear, H.D.; Prabhakaran, S.; Fukui, J.; et al. If we build it they will come: targeting the immune response to breast cancer. NPJ Breast Cancer. 2019, 5, 37. [Google Scholar] [CrossRef]
- Song, I.H.; Heo, S.H.; Bang, W.S.; Park, H.S.; Park, I.A.; Kim, Y.A.; et al. Predictive Value of Tertiary Lymphoid Structures Assessed by High Endothelial Venule Counts in the Neoadjuvant Setting of Triple-Negative Breast Cancer. Cancer Res Treat. 2017, 49, 399–407. [Google Scholar] [CrossRef]
- de Jong, V.M.T.; Wang, Y.; Ter Hoeve, N.D.; et al. Prognostic Value of Stromal Tumor-Infiltrating Lymphocytes in Young, Node-Negative, Triple-Negative Breast Cancer Patients Who Did Not Receive (neo)Adjuvant Systemic Therapy. J Clin Oncol. 2022, 40, 2361–2374. [Google Scholar] [CrossRef]
- Gu-Trantien, C.; Loi, S.; Garaud, S.; Equeter, C.; Libin, M.; De Wind, A.; Ravoet, M.; Le Buanec, H.; Sibille, C.; Manfouo-Foutsop, G.; et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J. Clin. Investig. 2013, 123, 2873–2892. [Google Scholar] [CrossRef]
- Li, R.; Berglund, A.; Zemp, L.; Dhillon, J.; Putney, R.; Kim, Y.; et al. The 12-CK Score: Global Measurement of Tertiary Lymphoid Structures. Front Immunol. 2021, 12, 694079. [Google Scholar] [CrossRef]
- Wang, B.; Liu, J.; Han, Y.; Deng, Y.; Li, J.; Jiang, Y. The Presence of Tertiary Lymphoid Structures Provides New Insight Into the Clinicopathological Features and Prognosis of Patients With Breast Cancer. Front Immunol. 2022, 13, 868155. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Feng, Z.; Luo, J.; He, Z.; Liu, J.; Wu, J.; et al. Tertiary Lymphoid Structures in Cancer: The Double-Edged Sword Role in Antitumor Immunity and Potential Therapeutic Induction Strategies. Front Immunol. 2021, 12, 689270. [Google Scholar] [CrossRef] [PubMed]
- Mauldin, I.S.; Mahmutovic, A.; Young, S.J.; Slingluff, C.L., Jr. Multiplex Immunofluorescence Histology for Immune Cell Infiltrates in Melanoma-Associated Tertiary Lymphoid Structures. Methods Mol Biol. 2021, 2265, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, R.; Ng, K.; Monypenny, J.; Ng, T. Insights Into Unveiling a Potential Role of Tertiary Lymphoid Structures in Metastasis. Front Mol Biosci. 2021, 8, 661516. [Google Scholar] [CrossRef] [PubMed]
- NJ; JT; Sl, N. ; Gt, B. Tertiary lymphoid structures and B lymphocytes in cancer prognosis and response to immunotherapies. Oncoimmunology. 2021, 10, 1900508.
- Hsieh, C.H.; Jian, C.Z.; Lin, L.I.; Low, G.S.; Ou, P.Y.; Hsu, C.; et al. Potential Role of CXCL13/CXCR5 Signaling in Immune Checkpoint Inhibitor Treatment in Cancer. Cancers. 2022, 14, 294. [Google Scholar] [CrossRef]
- Prat, A.; Guarneri, V.; Pascual, T.; et al. Development and validation of the new HER2DX assay for predicting pathological response and survival outcome in early-stage HER2-positive breast cancer. EBioMedicine. 2022, 75, 103801. [Google Scholar] [CrossRef]
- Sobottka, B.; Pestalozzi, B.; Fink, D.; Moch, H.; Varga, Z. Similar lymphocytic infiltration pattern in primary breast cancer and their corresponding distant metastases. Oncoimmunology. 2016, 5, e1153208. [Google Scholar] [CrossRef]
- Cardoso et al. KEYNOTE-756: Randomized, double-blind, phase III study of pembrolizumab vs placebo + neoadjuvant chemotherapy (CT) and adjuvant endocrine therapy (ET) for high-risk, early-stage estrogen receptor–positive human epidermal growth factor receptor 2–negative (ER+/HER2−) breast cancer (BC). Annals of Oncology. ABSTRACTS | BREAST CANCER, EARLY STAGE| VOLUME 30, SUPPLEMENT 9, IX7-IX8, NOVEMBER. 2019.
- Zhang, N.N.; Qu, F.J.; Liu, H.; Li, Z.J.; Zhang, Y.C.; Han, X.; et al. Prognostic impact of tertiary lymphoid structures in breast cancer prognosis: a systematic review and meta-analysis. Cancer Cell Int. 2021, 21, 536. [Google Scholar] [CrossRef]
- Cardoso et al. KEYNOTE-756: Randomized, double-blind, phase III study of pembrolizumab vs placebo + neoadjuvant chemotherapy (CT) and adjuvant endocrine therapy (ET) for high-risk, early-stage estrogen receptor–positive human epidermal growth factor receptor 2–negative (ER+/HER2−) breast cancer (BC). Annals of Oncology. ABSTRACTS | BREAST CANCER, EARLY STAGE| VOLUME 30, SUPPLEMENT 9, IX7-IX8, NOVEMBER. 2019.
- Deng, J.; Wang, E.S.; Jenkins, R.W.; et al. CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-cell Activation. Cancer Discov. 2018, 8, 216–233. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, B.; Liu, Y.; Wang, Z. Tertiary lymphoid structure signatures are associated with survival and immunotherapy response in muscle-invasive bladder cancer. Oncoimmunology. 2021, 10, 1915574. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020, 396, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Alhesa, A.; Awad, H.; Bloukh, S.; et al. PD-L1 expression in breast invasive ductal carcinoma with incomplete pathological response to neoadjuvant chemotherapy. Int J Immunopathol Pharmacol. 2022, 36, 3946320221078433. [Google Scholar] [CrossRef] [PubMed]
- Badve, S.S. Determining PD-L1 Status in Patients With Triple-Negative Breast Cancer: Lessons Learned From IMpassion130. Journal of the National Cancer Institute 2022, 114, 664–675. [Google Scholar] [CrossRef]
- Buisseret, L.; Desmedt, C.; Garaud, S.; Fornili, M.; Wang, X.; Van den Eyden, G.; et al. Reliability of tumor-infiltrating lymphocyte and tertiary lymphoid structure assessment in human breast cancer. Mod Pathol Off J U S Can Acad Pathol Inc. 2017, 30, 1204–12. [Google Scholar] [CrossRef] [PubMed]
- Massa, D.; Tosi, A.; Rosato, A.; Guarneri, V.; Dieci, M.V. Multiplexed In Situ Spatial Protein Profiling in the Pursuit of Precision Immuno-Oncology for Patients with Breast Cancer. Cancers. 2022, 14, 4885. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Qiao, S.; Li, M.; et al. The gene signature of tertiary lymphoid structures within ovarian cancer predicts the prognosis and immunotherapy benefit. Front Genet. 2023, 13, 1090640. [Google Scholar] [CrossRef] [PubMed]
- Rakaee, M.; Adib, E. Biagio Ricciuti Artificial intelligence in digital pathology approach identifies the predictive impact of tertiary lymphoid structures with immune-checkpoints therapy in NSCLC, Meeting Abstract | 2022 ASCO Annual Meeting, DOI: 10.1200/JCO.2022.40.16_suppl.9065 Journal of Clinical Oncology 40, no. 16_suppl (June 01, 2022) 9065-9065. [CrossRef]
- Mauldin, I.S.; Mahmutovic, A.; Young, S.J.; Slingluff, C.L., Jr. Multiplex Immunofluorescence Histology for Immune Cell Infiltrates in Melanoma-Associated Tertiary Lymphoid Structures. Methods Mol Biol. 2021, 2265, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Domblides, C.; Rochefort, J.; Riffard, C.; Panouillot, M.; Lescaille, G.; Teillaud, J.L.; et al. Tumor-Associated Tertiary Lymphoid Structures: From Basic and Clinical Knowledge to Therapeutic Manipulation. Front Immunol. 2021, 12, 698604. [Google Scholar] [CrossRef] [PubMed]
- Engelhard, V.H.; Rodriguez, A.B.; Mauldin, I.S.; Woods, A.N.; Peske, J.D.; Slingluff, C.L. Immune Cell Infiltration and Tertiary Lymphoid Structures as Determinants of Antitumor Immunity. J Immunol Baltim Md 1950. 2018, 200, 432–42. [Google Scholar] [CrossRef]
- Tallón de Lara, P.; Castañón, H.; Vermeer, M.; et al. CD39+PD-1+CD8+ T cells mediate metastatic dormancy in breast cancer. Nat Commun. 2021, 12, 769 Published 2021 Feb 3. [Google Scholar] [CrossRef]
- Wortman, J.C.; He, T.F.; Solomon, S.; Zhang, R.Z.; Rosario, A.; Wang, R.; et al. Spatial distribution of B cells and lymphocyte clusters as a predictor of triple-negative breast cancer outcome. NPJ Breast Cancer. 2021, 7, 84. [Google Scholar] [CrossRef]
- Sofopoulos, M.; Fortis, S.P.; Vaxevanis, C.K.; Sotiriadou, N.N.; Arnogiannaki, N.; Ardavanis, A.; et al. The prognostic significance of peritumoral tertiary lymphoid structures in breast cancer. Cancer Immunol Immunother CII. 2019, 68, 1733–45. [Google Scholar] [CrossRef] [PubMed]
- De Silva, P.; Garaud, S.; Solinas, C.; de Wind, A.; Van den Eyden, G.; Jose, V.; et al. FOXP1 negatively regulates tumor infiltrating lymphocyte migration in human breast cancer. EBioMedicine. 2019, 39, 226–38. [Google Scholar] [CrossRef] [PubMed]
- Boman, C.; Zerdes, I.; Mårtensson, K.; Bergh, J.; Foukakis, T.; Valachis, A.; et al. Discordance of PD-L1 status between primary and metastatic breast cancer: A systematic review and meta-analysis. Cancer Treat Rev. 2021, 99, 102257. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Sibéril, S.; Pupier, G.; Soussan, S.; Sautès-Fridman, C. Activation of B cells in Tertiary Lymphoid Structures in cancer: Anti-tumor or anti-self? Semin Immunol. 2023, 65, 101703. [Google Scholar] [CrossRef]
- Nasiri, F.; Kazemi, M.; Mirarefin, S.M.J.; et al. CAR-T cell therapy in triple-negative breast cancer: Hunting the invisible devil. Front Immunol. 2022, 13, 1018786 Published 2022 Nov 22. [Google Scholar] [CrossRef]
- Johansson-Percival, A.; Ganss, R. Therapeutic Induction of Tertiary Lymphoid Structures in Cancer Through Stromal Remodeling. Front Immunol. 2021, 12, 674375. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Huang, X.; Yang, W.; Wang, J.; Liang, Y.; Zhang, T.; et al. Tertiary lymphoid structures favor outcome in resected esophageal squamous cell carcinoma. J Pathol Clin Res. 2022, 8, 422–35. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, X.; Wang, D.; Wang, Y.; Lu, H.; Wen, S.; et al. Prognostic value of tertiary lymphoid structure and tumour infiltrating lymphocytes in oral squamous cell carcinoma. Int J Oral Sci. 15 de septiembre de 2020, 12, 24. [Google Scholar] [CrossRef]
- Wakasu, S.; Tagawa, T.; Haratake, N.; Kinoshita, F.; Oku, Y.; Ono, Y.; et al. Preventive effect of tertiary lymphoid structures on lymph node metastasis of lung adenocarcinoma. Cancer Immunol Immunother CII. 2023, 72, 1823–34. [Google Scholar] [CrossRef]
- Horeweg, N.; Workel, H.H.; Loiero, D.; Church, D.N.; Vermij, L.; Léon-Castillo, A.; et al. Tertiary lymphoid structures critical for prognosis in endometrial cancer patients. Nat Commun. 2022, 13, 1373. [Google Scholar] [CrossRef]
- Di Caro, G.; Bergomas, F.; Grizzi, F.; Doni, A.; Bianchi, P.; Malesci, A.; et al. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin Cancer Res Off J Am Assoc Cancer Res. 2014, 20, 2147–58. [Google Scholar] [CrossRef] [PubMed]
- Posch, F.; Silina, K.; Leibl, S.; Mündlein, A.; Moch, H.; Siebenhüner, A.; et al. Maturation of tertiary lymphoid structures and recurrence of stage II and III colorectal cancer. Oncoimmunology. 2018, 7, e1378844. [Google Scholar] [CrossRef] [PubMed]
- Meshcheryakova, A.; Tamandl, D.; Bajna, E.; Stift, J.; Mittlboeck, M.; Svoboda, M.; et al. B cells and ectopic follicular structures: novel players in anti-tumor programming with prognostic power for patients with metastatic colorectal cancer. PloS One. 2014, 9, e99008. [Google Scholar] [CrossRef] [PubMed]
- Calderaro, J.; Petitprez, F.; Becht, E.; Laurent, A.; Hirsch, T.Z.; Rousseau, B.; et al. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma. J Hepatol. enero de 2019, 70, 58–65. [Google Scholar] [CrossRef]
- Germain, C.; Gnjatic, S.; Tamzalit, F.; Knockaert, S.; Remark, R.; Goc, J.; et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med. 2014, 189, 832–44. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, N.; Gil-Jimenez, A.; Silina, K.; van Montfoort, M.L.; Einerhand, S.; Jonkman, L.; et al. The Tumor Immune Landscape and Architecture of Tertiary Lymphoid Structures in Urothelial Cancer. Front Immunol. 2021, 12, 793964. [Google Scholar] [CrossRef]
- Lynch, K.T.; Young, S.J.; Meneveau, M.O.; Wages, N.A.; Engelhard, V.H.; Slingluff, C.L.; et al. Heterogeneity in tertiary lymphoid structure B-cells correlates with patient survival in metastatic melanoma. J Immunother Cancer. 2021, 9, e002273. [Google Scholar] [CrossRef]
- Cabrita, R.; Lauss, M.; Sanna, A.; Donia, M.; Skaarup Larsen, M.; Mitra, S.; et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020, 577, 561–5. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, I.A.; Song, I.H.; Shin, S.J.; Kim, J.Y.; Yu, J.H.; Gong, G. Tertiary lymphoid structures: prognostic significance and relationship with tumour-infiltrating lymphocytes in triple-negative breast cancer. J Clin Pathol. 2016, 69, 422–30. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Ji, J.; Li, S.; et al. Analysis of the Correlation and Prognostic Significance of Tertiary Lymphoid Structures in Breast Cancer: A Radiomics-Clinical Integration Approach [published online ahead of print, 2023 Aug 1]. J Magn Reson Imaging. 2023;10.1002/jmri.28900. [CrossRef]
- Noël, G.; Fontsa, M.L.; Garaud, S.; et al. Functional Th1-oriented T follicular helper cells that infiltrate human breast cancer promote effective adaptive immunity. J Clin Invest. 2021, 131, e139905. [Google Scholar] [CrossRef]
Table 1.
.
| Author |
Year |
Tumor model |
Sample Size |
Tipo |
Recognition of TLS |
Findings |
| Rutao Li [60] |
2022 |
Esophaugus |
185 |
Retrospective |
IHC for CD45+, CD20+ B, CD4+ CD8+ T cells, CD11c+ DC |
Better DFS
Better OS |
| Nana Zhang [33] |
2020 |
Gastric adenocarcinoma |
180 |
Retrospective |
IHC staining
and MECA-79 + (HEV) |
Better OS |
| Qunxing Li [61] |
2020 |
Orac Squamous cell carcinoma |
168 |
Retrospective |
Multiplex IHC (CD3+ T cells, CD20+ B cells, PNAd+ HEV DC-LAMP + (LAMP3) |
Better 5-year OS rate
Better RFS |
| Sho Wakasu[62] |
2022 |
Lung adenocarcinoma |
218 |
Retrospective |
The overlap of T-cell zone and B-cell zone |
Better OS
Better DFS |
| Nanda Horeweg [63] |
2022 |
Endometrial adenocarcinoma |
All included patients from the PORTEC-3 study |
Retrospective |
scRNA-seq of B-cells to establish the presence of cycling/germinal center B-cells and antibody-secreting B-cells |
Better RFS |
| Di Caro et al [64] |
2014 |
Colorectal |
351 stage II and III colorectal cancer |
Retrospective |
IHC (CD3, CD20, PNAd , Lyve-1, CD21, α-smooth muscle actin and CXCL13 and CCL21 |
Better RFS |
| Posch et al [65] |
2018 |
Colorrectal |
109 patients with stage II/III nmCRC |
Retrospective |
NR |
Better RR of recurrence |
| Meshcheryakova et al [66] |
2014 |
Colorectal |
65 metastatic colorec al cancer in the liver |
Retrospective |
IHQ (CD45, CD20, AID, IgM, CD138, and CD68) |
Better RFS |
| Julien Calderaro [67] |
2019 |
HCC |
273 patients with HCC treated by surgical resection |
Retrsopective |
NR |
Better risk of early relapse |
| Germain C et al [68] |
2014 |
Lung Cancer |
74 untreated patients with early-stage NSCLC |
Retrospective |
Follicular CD20+ B-cell |
Better OS
Better DSS |
| Van Dijk [69] |
2021 |
Urothelial cancer |
31 cystectomy specimens obtained from NABUCCO |
Retrospective |
Multiplex immunofluorescence (CD3, CD8, FoxP3, CD68, CD20, PanCK, DAPI) |
Better response to anti-PD-1/CTLA-4 immunotherapy |
| Lynch et al [70] |
2021 |
Melanoma (metastases) |
64 patients |
Retrospective |
Multiplex immunofluorescence. |
Better RFS
Better OS |
| Cabrita el al [71] |
2020 |
Melanoma |
177 |
Retrospective |
IHC
Anti-CD20
AntiCXCR5 and Anti-CXCL13 |
Better OS |
| Italiano et al [72] |
2022 |
Sarcomas |
PEMBROSARC |
Multicohort phase 2 study of pembrolizumab combined with low-dose cyclophosphamide (30 patients) |
|
Better 6-month NPR |
| Lin Zhou [73] |
2021 |
Bladder |
168
bladder tissue of the IMvigor210 cohort |
|
Transcriptome RNA sequencing (RNA-seq) |
Better OS
Better response rate to PD1 blockade. |
| Maxime Meylan [74] |
2022 |
Renal cell cancer |
51 patients treated by ICI, either Nivolumab alone, or NI |
|
Visium 10X spatial transcriptomics technique that allowed both quantification and localization of B cell-specific gene expression. |
Better PFS |
Table 2.
.
| Author |
BREAST CANCER SUBTYPE |
Type |
N |
year of publication |
Recognition of TLS |
Findings |
| Lee et all [70] |
TNBC localized |
Retrospective |
769 |
2016 |
IHC for MECA-79 and CD31 |
Better DFS
Better OS |
| Xia Liu [2] |
Her2 |
Restrospective |
248 |
2017 |
IHC CD3, CD20, and CD23 |
Better DFS |
| Song [25] |
TNBC localized |
Retrospective |
108 |
2017 |
IHC for MECA79, CD3, CD8, and CD20 and Nanostring analysis of CXCL13 |
Better pCR
Better DFS |
| Bin Wang [29] |
BC |
Systematic Review and Meta-Analysis (PRISMA) criteria |
15 studies with a total of 3,898 patients |
2022 |
NR |
Better DFS
Better OS |
| Kezhen Li [73] |
BC |
Retrospective |
242 |
2023 |
NR |
Better DFS |
| Noel et al. [74] |
TNBC (27) & Her2+ (21) |
Retrospective |
48 |
2021 |
IHC CD3/CD20 |
Better DFS |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).