2. Materials and Methods
The research was conducted at the Clinic for Gynecology and Obstetrics of the University Clinical Center of Serbia in Belgrade, approved by the expert board of the Clinic for Gynecology and Obstetrics of the University Clinical Center of Serbia and the Ethics Committee of the Clinical Center of Serbia. A retrospective cohort study was designed for our study, involving 60 patients over a period of 12 years, from 2010 to 2022.
The study included pregnant women in term pregnancies referred from primary and secondary healthcare institutions to the Clinic for Gynecology and Obstetrics of the University Clinical Center of Serbia, in whom intrauterine fetal death was diagnosed as well as the patients in whom this diagnosis was established during examination or hospitalization at our clinic. Patient data were obtained by reviewing medical documentation (medical histories, pathology service protocols of the Clinic for Gynecology and Obstetrics of the University Clinical Center of Serbia in Belgrade). The analysis included the number of deliveries conducted at the Clinic for Gynecology and Obstetrics and the number of live births and stillbirths. For the study, data about the demographic and socio-epidemiological characteristics of patients, parity, previous pregnancy losses, antenatal screening, comorbidities, and any therapy used were collected, as well as information on the mode of delivery, pathohistological findings of the placenta, and autopsy findings of the fetus. A database was formed based on the obtained data, recording relevant information for the study. All patients provided informed consent to participate in the study. Multiple pregnancies, pregnancies with diagnosed major fetal anomalies, pregnancies where intrauterine fetal death occurred before the 37th week of gestation, as well as cases of intrapartum fetal death and postpartum death, were excluded from the research.
Statistical data analysis was conducted using the Statistical Package for Social Sciences (SPSS) software, employing both descriptive and analytical statistical methods. Descriptive statistical methods included frequencies and measures of central tendency (mean and median), while standard deviation was used as a measure of variability.
3. Results
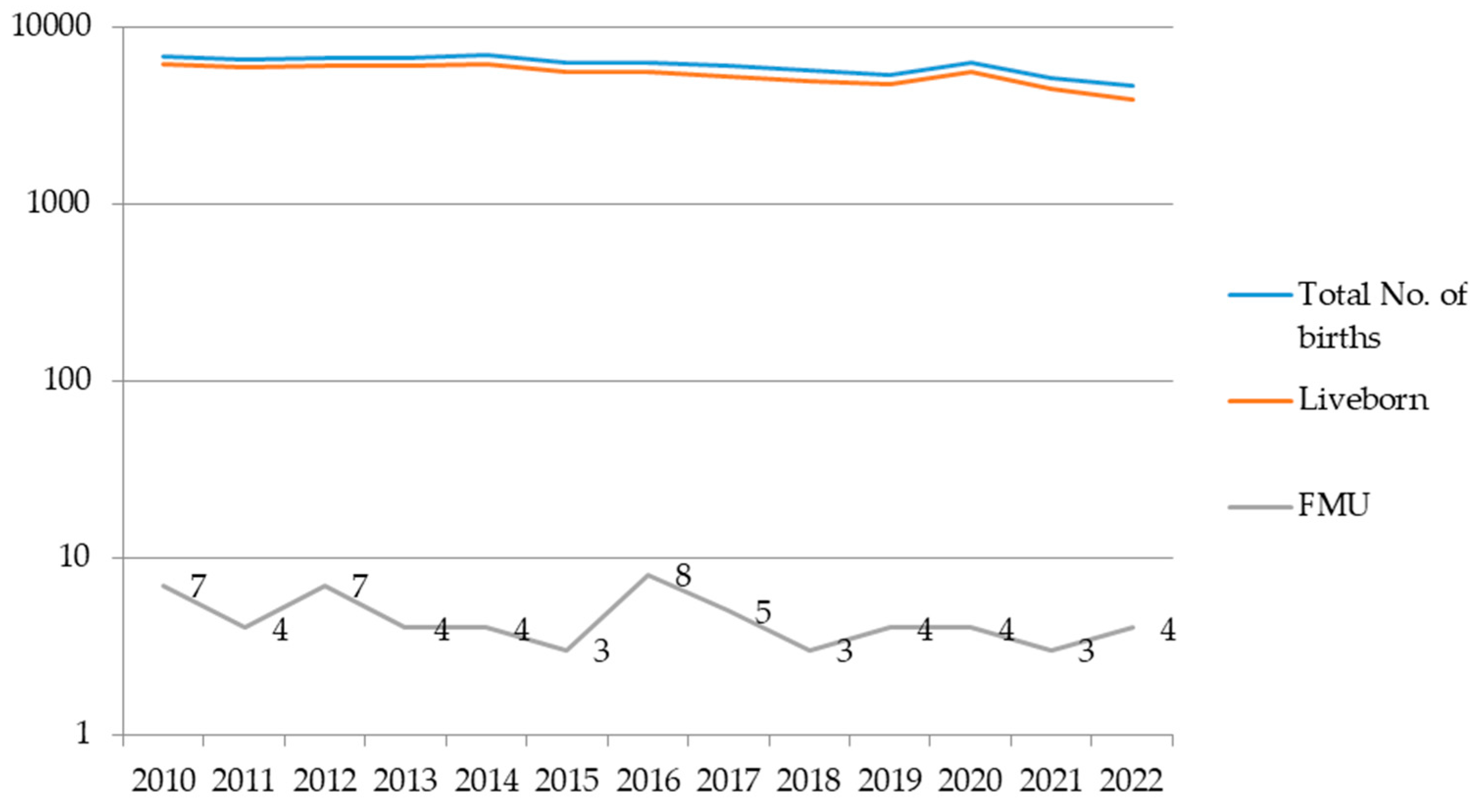
During the examined period, the total number of births in the Clinic for Gynecology and Obstetrics of the University Clinical Center of Serbia was 79.148, with 60 cases of intrauterine fetal death in patients at term pregnancy. The incidence of intrauterine fetal death in our Clinic, as one of the two gynecological tertiary institutions in Belgrade, was 0.1% in 2010 and 0.08% in 2022 (
Figure 1).
Analysis of demographic data revealed that the average age of our patients was 30 years (30.6 ± 6.38), with no patients younger than 18 years, while two patients (3.34%) were older than 40 years. There were 13 patients aged over 35 years, which represents slightly more than one-fifth (21.67%) of the examined patients. The majority of patients (47, or 78.33%) were between 18 and 35 years old. In most cases, the patients had a secondary (43.33%) or high level of education (46.67%), which represents 90% of our respondents. Pregnancy was regularly monitored in only one-third of the patients (33.33%). According to the Women’s Health Care Protocol of the Ministry of Health of Serbia, regular pregnancy monitoring implies a pregnancy during which at least three ultrasound examinations and three to four gynecological examinations were performed during the pregnancy [
7]. In our study, 70% of patients fulfilled the above-mentioned criteria for pregnancy monitoring, while 25% of pregnancies were unmonitored. For 5%, it was not possible to obtain sufficient data to reach a conclusive result. Analyzing the parity of the respondents, it is shown that our patients were mainly primiparous (53.33%), and their pregnancies occurred spontaneously. The given data are presented in
Table 1.
When analyzing the gestational age of pregnancies, it was found that intrauterine death occurred on average at the 39th week of gestation (38.5 ± 1.14), with more than half occurring during the early term pregnancy period (58.33%), between 37 and 38 weeks and 6 days. There were no post-term intrauterine fetal deaths. There was no observed difference in gender among the fetuses; 33 (55%) were male, and 27 (45%) were female (
Table 2). The average birth weight of the fetus was approximately 3000g (3035.71±704.28), with 18.33% weighing less than 2500g(
Table 2).
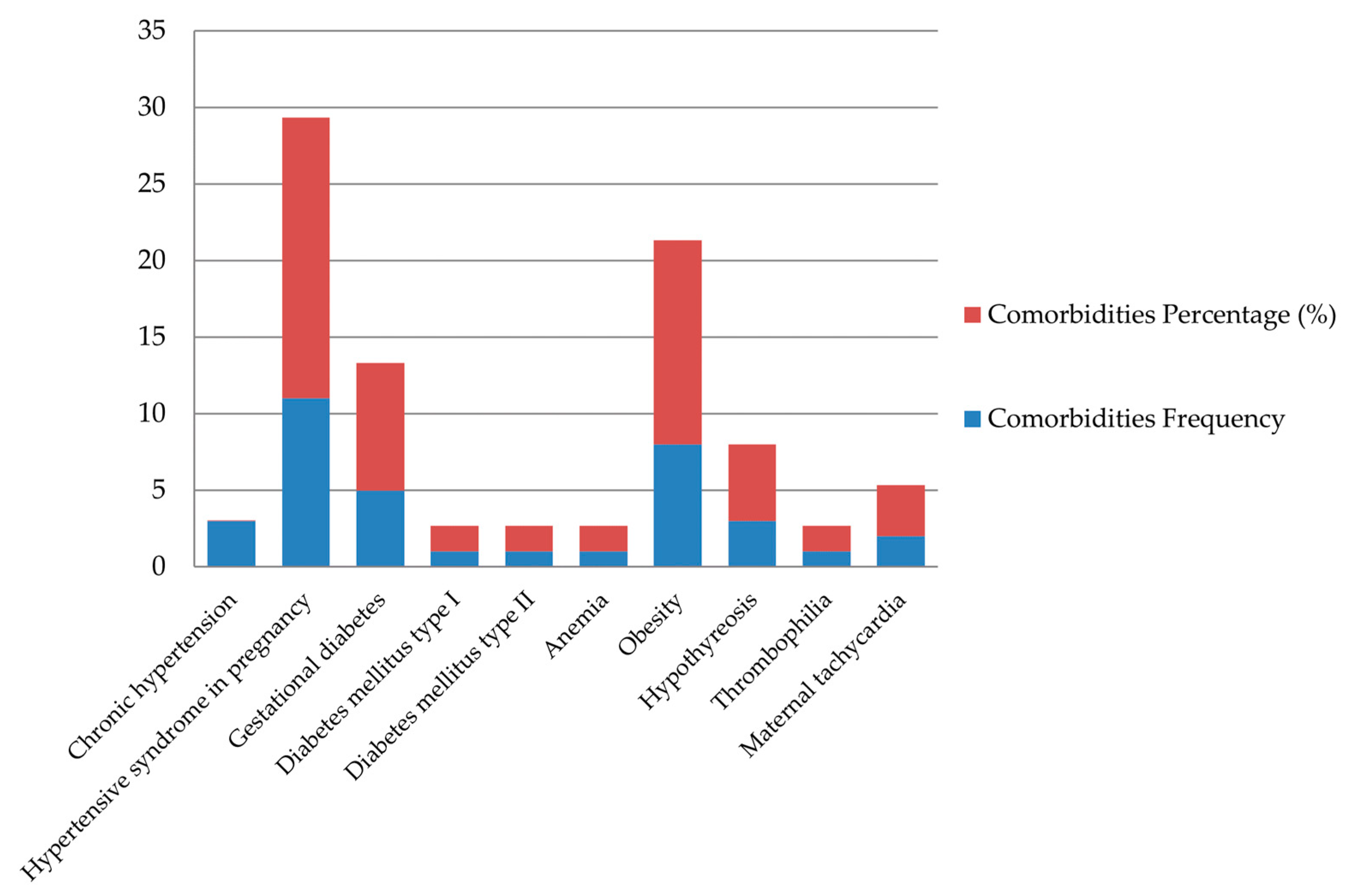
In 78.33% of cases, pregnancies ended with vaginal delivery, while in 21.67% of cases, a cesarean section was performed. While analyzing the comorbidities of our patients, it was determined that 38.3% of patients had one to two associated illnesses, more than three were found in 5% of patients, while 56.7% of women had no comorbidities. The most common pathology among our patients was one of the disorders of hypertensive syndrome in pregnancy (pregnancy-induced hypertension, preeclampsia), which was present in 11 patients (18.33%). Obesity was the second most frequent comorbidity, occurring in 8 patients (13.33%). Data about maternal comorbidities is shown in
Figure 2.
The recurrence of intrauterine fetal death in our patient population was 10%, while 8.33% of women had a history of miscarriage (
Table 3). Histopathological examination of the placenta, as shown in
Table 4. revealed that in the majority of cases, infarctions of the placenta were present (36.67%), while signs of infection in the placental tissue or fetus were observed in 23.33% of cases. Signs of placental abruption were present in 18.33% of cases, while a normal histopathological finding was found in one-fifth of our patients (20%). There was no statistically significant difference in the appearance of the umbilical cord - in 28% of patients, the umbilical cord was normal, while in 28% of patients, there were some anatomical variations (short or long umbilical cord), true knots, velamentous cord insertion, and others (marginal cord insertion, single umbilical artery syndrome, and spiralization disorders).
4. Discussion
The incidence of stillbirth worldwide is 18.9 per 1000 live births, with significant variations depending on the country that is being observed. In highly developed countries, the incidence ranges from 2, as in Finland, to 7, as in the United States, per 1000 live births. In contrast, in less developed countries, the incidence can rise up to 47, as in Pakistan, per 1000 live births [
3]. Progress in reducing stillbirths globally has been slower than expected, primarily due to significant disparities in healthcare accessibility in many socioeconomically developed countries [
3,
8]. Based on data from our institution, it is noticeable that the trend in the incidence of intrauterine fetal death has remained relatively stable over the past twelve years (0.1% in 2010, 0.08% in 2022), with a drop below 0.1% being consistent since 2016. This trend can be explained by improved antenatal care during this period.
In the scientific literature on intrauterine fetal death, maternal age, especially over 40 years of age, stands out as a significant risk factor for this event at any gestational age, including term pregnancy [
4,
8]. It is expected that in the population of patients over 40 years, there is a higher prevalence of comorbidities such as hypertension and diabetes, as well as higher parity, all of which are recognized risk factors. However, despite the presence of these conditions, maternal age is considered an independent risk factor for intrauterine fetal death. On the other hand, Fretts and colleagues [
9]. reported that the risk of intrauterine fetal death nearly doubles in patients over 35 years, which is not consistent with our results - around one-fifth (21.67%) of the patients in our study were over 35 years old. In our study, there were only 2 (3.34%) patients over 40 years old - one was an uncontrolled pregnancy in a grand multipara (this being her thirteenth delivery), complicated by preeclampsia and diabetes, and the other in a primiparous woman with a normal pregnancy course. These two patients represent two extremes often seen in cases of intrauterine fetal death: in one case, there are multiple risk factors with combined effects in an uncontrolled pregnancy, while in the other case, there is an isolated factor - maternal age in an adequately controlled pregnancy. The majority of patients (78.33%) were between 18 and 35 years old, which is in line with the profile of pregnant women in our country.
It is well known that the level of education of patients is associated with socioeconomic status, accessibility of healthcare, and awareness of the necessity of regular prenatal check-ups [
10,
11,
12]. Although healthcare is accessible to all pregnant women in Serbia, pregnancies in patients with lower levels of education and socioeconomic status are often inadequately monitored. In our patient population, 10% had only primary or incomplete primary education, which is significant considering that primary education is compulsory for all citizens according to the Constitution of the Republic of Serbia. It is important to note that there were no underage patients among our population. Although the percentage of our patients without completed primary education is high, it is worth mentioning that only 25% of these pregnancies were uncontrolled.
Patient parity is one of the risk factors for intrauterine fetal death at term often mentioned in the literature but conclusions on this topic vary. Some sources state that primiparous women are at higher risk [
1,
8], while others indicate that the risk increases after the second or even the fifth delivery [
1,
13]. As shown in our results, most of our patients (83.33%) were multiparous, and importantly, 50% of them had more than 5 deliveries, consistent with the results of Sharma and colleagues [
13].
Through a systematic review of the literature, Mondal and colleagues concluded that the risk of intrauterine fetal death is even 10% higher in male fetuses compared to female fetuses. However, an explanation for such a significant gender difference has not yet been found [
14]. In our study, there was a slightly higher number of male fetuses compared to female fetuses, although this difference was not statistically significant.
Several studies have highlighted that adverse outcomes in previous pregnancies are predictive factors for subsequent pregnancies. In the studied population, 8.33% of patients reported previous miscarriages, and 10% had a history of previous stillbirth, consistent with the data reported in the literature [
15,
16]. While some sources state that the risk of recurrence of intrauterine fetal death in the next pregnancy is nearly six times higher than in the general population [
16,
17,
18]. more recent sources report an increased but statistically insignificant risk (4.6 and 6.8/1000), which is not a sufficient reason to introduce changes in antepartum monitoring protocols [
19].
In cases of stillbirth at term, the mode of delivery is solely in the interest of the mother. According to the American College of Obstetricians and Gynecologists guidelines, it is recommended to end pregnancies with intrauterine fetal death via vaginal delivery. For patients with a history of previous cesarean section, a trial of labor is recommended, but in cases of increased risk of uterine rupture, ending the pregnancy with a repeat cesarean section is considered justified [
1]. However, it is essential to note that induction of labor in these cases is associated with a significantly higher risk of uterine rupture than in cases where labor is induced after a previous cesarean section with a vital fetus [
20]. In our study, patients who had undergone a cesarean section had indications for the procedure. Eight patients had one or more previous cesarean sections (6.67% had one, and 6.67% had two previous cesarean sections). Two patients underwent emergency cesarean sections due to placental abruption and bleeding, one due to fetomaternal disproportion caused by fetal macrosomia, one due to failed induction of vaginal delivery, and one due to fetal malpresentation.
The term pregnancy is defined as a period from 37.0 to 41.6 weeks, but the risks of maternal and fetal complications are not the same throughout this 6-week period. Fetal complications (stillbirth, the need for admission to the neonatal intensive care unit, and mechanical ventilation) are lowest between 39.0 and 40.6 weeks of gestation [
2,
21]. In our population, more than half (58.33%) of stillbirth cases at term occurred in early-term pregnancies, and almost half as many (38.33%) occurred between 39.0 and 40.6 weeks of pregnancy, confirming the aforementioned data.
Many maternal diseases have been associated with an increased risk of intrauterine fetal death in the literature. Hypertensive disorders, diabetes, and obesity are consistently linked to a higher incidence of this event [
1,
4,
13,
15]. These comorbidities were the most common among the patients included in our study as well as shown in Graph 2. It is known that hypertension in pregnancy increases the risk of uteroplacental circulation disorders and placental abruption [
15,
23]. Diabetes in pregnancy has long been recognized as a risk factor for fetal demise [
23,
24]. If we exclude the risk of congenital anomalies, the pathophysiological mechanism through which poor glycemic control leads to fetal death is mostly represented by metabolic disorders that result in increased oxidative stress, cardiac disturbances, and placental vascular pathology [
4,
23,
24]. Studies have shown that regardless of whether it is pregestational or gestational diabetes in question, fetal demise in these patients most commonly occurs in term pregnancies [
26], with the highest risk in patients with pregestational diabetes and all patients with poor glycemic control during pregnancy, especially in the third trimester [
26,
27]. Obesity represents an independent risk factor for intrauterine fetal death, with the risk increasing with the degree of obesity and gestational weeks, therefore the risk is the highest in term pregnancies [
22,
25]. Considering that these conditions commonly occur together as part of the metabolic syndrome, it is often difficult to determine what was the decisive factor that led to fetal death. It is generally considered that in these cases it is a result of the combined effect of multiple risk factors and pathophysiological mechanisms [
25,
27]. It is of great importance that the mentioned conditions are preventable. Obesity is considered one of the risk factors that can be most effectively influenced. Preconception counseling, appropriate therapy, lifestyle changes, and regular monitoring can prevent complications of diabetes and hypertension or even prevent the development of the diseases in certain cases. Despite this, more than 50% of our patients did not have any associated diseases as risk factors, which is also in line with the literature [
22].
In patients where no maternal or fetal risk factors were recognized, the histopathological findings of the placenta and umbilical cord are of great importance and are increasingly discussed in studies on intrauterine fetal death [
28,
29]. Various studies indicate that pathological changes found in the placenta are considered the cause or at least a contributing factor in fetal death in up to 60% of cases [
29,
30,
31]. In our study, this percentage is even higher - as much as 80% of placentas had some pathological findings. Amir and colleagues stated that as much as 83% of placentas in cases of term intrauterine fetal death show histopathological signs of uteroplacental insufficiency [
32]. This can occur due to parenchymal thrombosis and infarction of the placenta, infections, or vascular occlusions [
31,
32]. In our study, evidence of placental infarction was found in 36.67% of cases, which is significantly higher than the approximately 27% described in the literature [
32]. Despite the use and availability of antibiotics, chorioamnionitis is often cited as one of the most common causes of intrauterine fetal death, even in term pregnancies [
32,
33]. Although the mother's condition may be practically asymptomatic or have very mild clinical symptoms, the consequences for uteroplacental circulation and thus fetal oxygenation are often extremely serious or even fatal [
32,
33]. The use of antibiotics in cases of evident intrauterine infection in most cases has no effect, so preventive measures, regular monitoring, and bacteriological swabs are of great importance in preventing this condition. Data on the frequency of chorioamnionitis in cases of intrauterine fetal death in term pregnancies vary widely in the literature, from 10-15% [
1,
31] to almost 30% [
32]. In our study, evidence of infections was found in 23.33% of cases, which represents another potentially preventable risk factor.
Placental abruption complicates about 1% of all pregnancies [
15,
35] but despite this, it is described as the cause of fetal death in 10-20% of cases [
1,
15]. Although placental abruption is primarily a clinical diagnosis, in a certain number of cases, the diagnosis is made only through histopathological analysis of the placenta due to the subclinical presentation of this condition [
35]. In our patient population, histopathological signs of placental abruption were present in around 18% of cases. Although some sources suggest that timely diagnosis and appropriate response could reduce the frequency of placental abruption as a cause of fetal death in term pregnancies [
15], this condition can occur in patients without any risk factors as an acute event that poses a life-threatening risk to both the mother and the fetus in a short period [
37].
The pathology of the umbilical cord is considered a potential cause of intrauterine fetal death in up to 15% of cases [
1,
4,
15,
35]. In a small number of studies, the sole length of the umbilical cord is considered a risk factor for adverse pregnancy outcomes. More often it is discussed in the context of the risk it poses due to wrapping around the neck or body of the fetus, leading to subsequent strangulation or terminal compression of the umbilical cord, the formation of true knots of the umbilical cord, and umbilical cord prolapse. In our study, as many as 25% of umbilical cords were classified as long. Other conditions related to the umbilical cord (such as velamentous and marginal cord insertion, single umbilical artery syndrome, and disorders of spiralization of the umbilical cord) as well as cord entanglement around the neck or other body parts were present in 16.67% of cases. The presence of nuchal cord occurs in almost 24% of live-born term infants and in 3.7% of cases of term intrauterine fetal demise [
1] . Although not pathological in itself, if it becomes constricted or compressed, it can lead to increased resistance or stasis in umbilical circulation resulting in thrombosis, or even strangulation of the fetus. Interruption of circulation can also occur when there is a true knot in the umbilical cord. In our study, a true knot was present in 6.67% of cases, consistent with literature data [
13,
15]. This is why a detailed examination of the fetus's body during autopsy is necessary to find any signs of the umbilical cord being constricted around parts of the body or evidence of tight true knots [
35]. By comparing risk factors for intrauterine fetal death in preterm and term pregnancies, Ohana and colleagues concluded that in term deliveries intrauterine fetal death is more often caused by acute and unpredictable events related to placental and umbilical cord accidents that are mostly impossible to prevent, unlike chronic processes that are more prevalent in cases of intrauterine fetal death in preterm pregnancies [
15,
38].
5. Conclusions
The rate of intrauterine fetal death in term pregnancies at the Clinic of Gynecology and Obstetrics, University Clinical Center of Serbia in Belgrade, during the studied period, shows a trend of stability, dropping below 0.1% from what it was in 2010, continuing through 2016. and beyond.
The most significant risk factors for intrauterine fetal death in term pregnancies in our population during the study period were: pregnancy-induced hypertensive disorders in 23.33% of cases, obesity in 13.33%, and diabetes in 11.67%. Less prevalent risk factors included hypothyroidism in 5%, maternal tachycardia in 3.33%, and anemia in 1.67%.
In over 80% of placentas in our study, histopathological examination revealed some pathology. Most commonly, these were infarctions in 36.67% of cases, followed by infections (23.33%) and signs of placental abruption (8.33%). Regarding umbilical cord findings, 46.67% of patients had a normal cord, 25% had a long cord, 11.67% had a short cord, 6.67% had a true knot of the cord, and less common anatomical variations occurred in a total of 16.67% of cases (single umbilical artery syndrome, marginal and velamentous cord insertion, and disorders of spiralization).
Although many risk factors for intrauterine fetal death in term pregnancies have been identified, nearly half of these cases remain unexplained. For patients with recognized preventable risk factors, preconception counseling and appropriate therapy are necessary to minimize the risk in subsequent pregnancies. Further, a more detailed analysis and recognition of new risk factors for this complex phenomenon are essential to develop strategies for identifying fetuses at higher risk and to devise protocols for monitoring these pregnancies, aiming to further reduce the incidence of intrauterine fetal death.