Submitted:
19 October 2023
Posted:
20 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Geological Setting and Samples
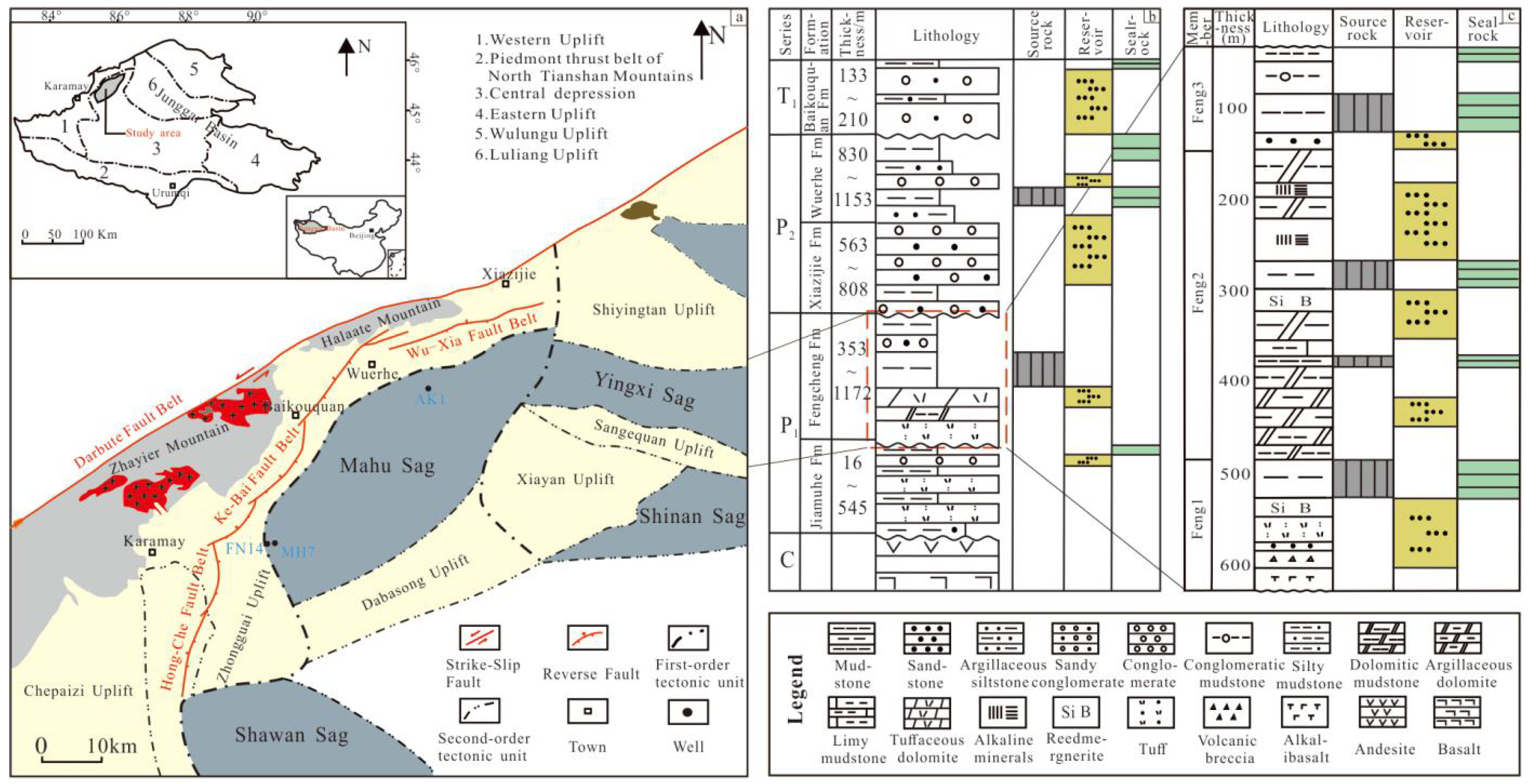
3. Analytical Methods
4. Results
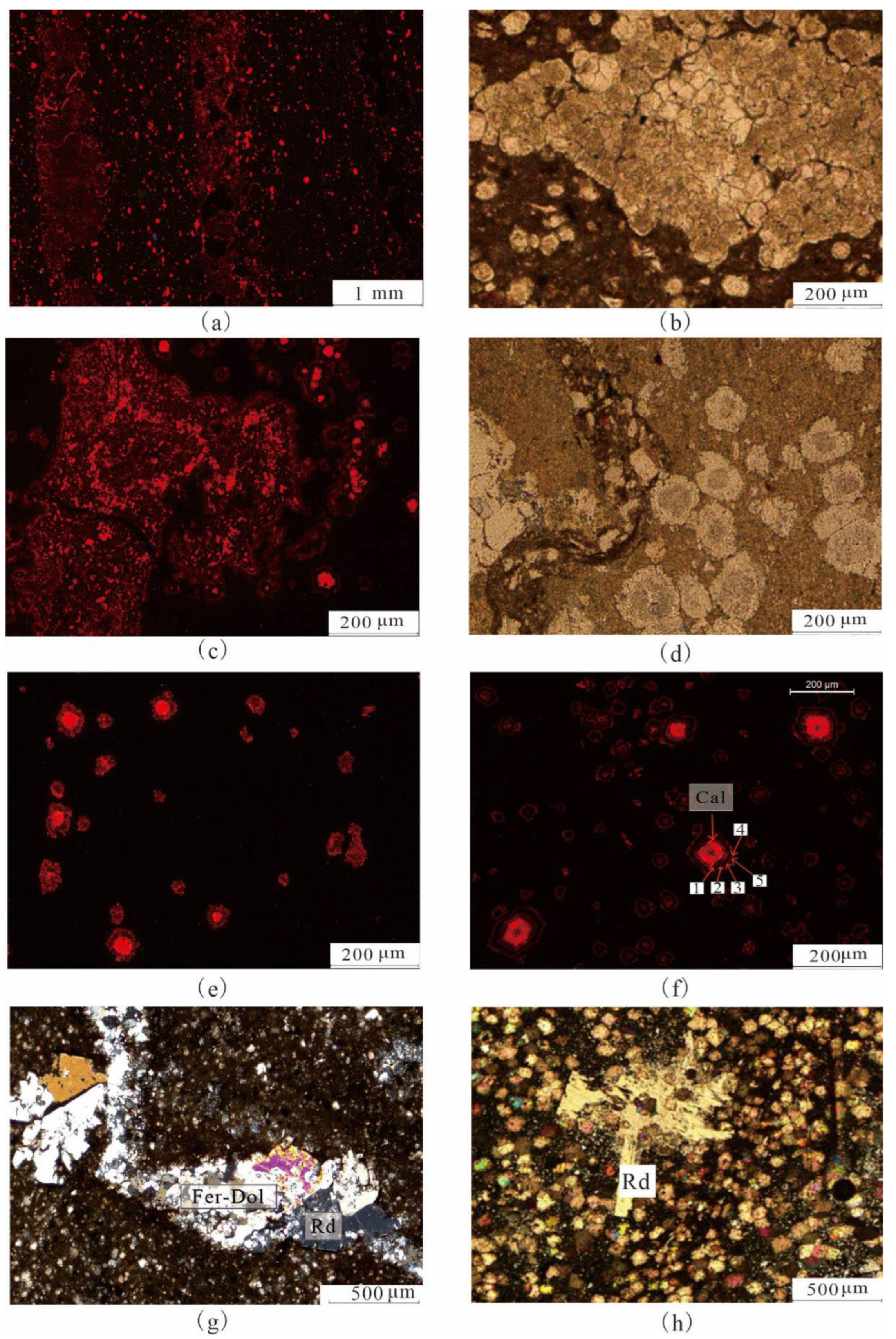
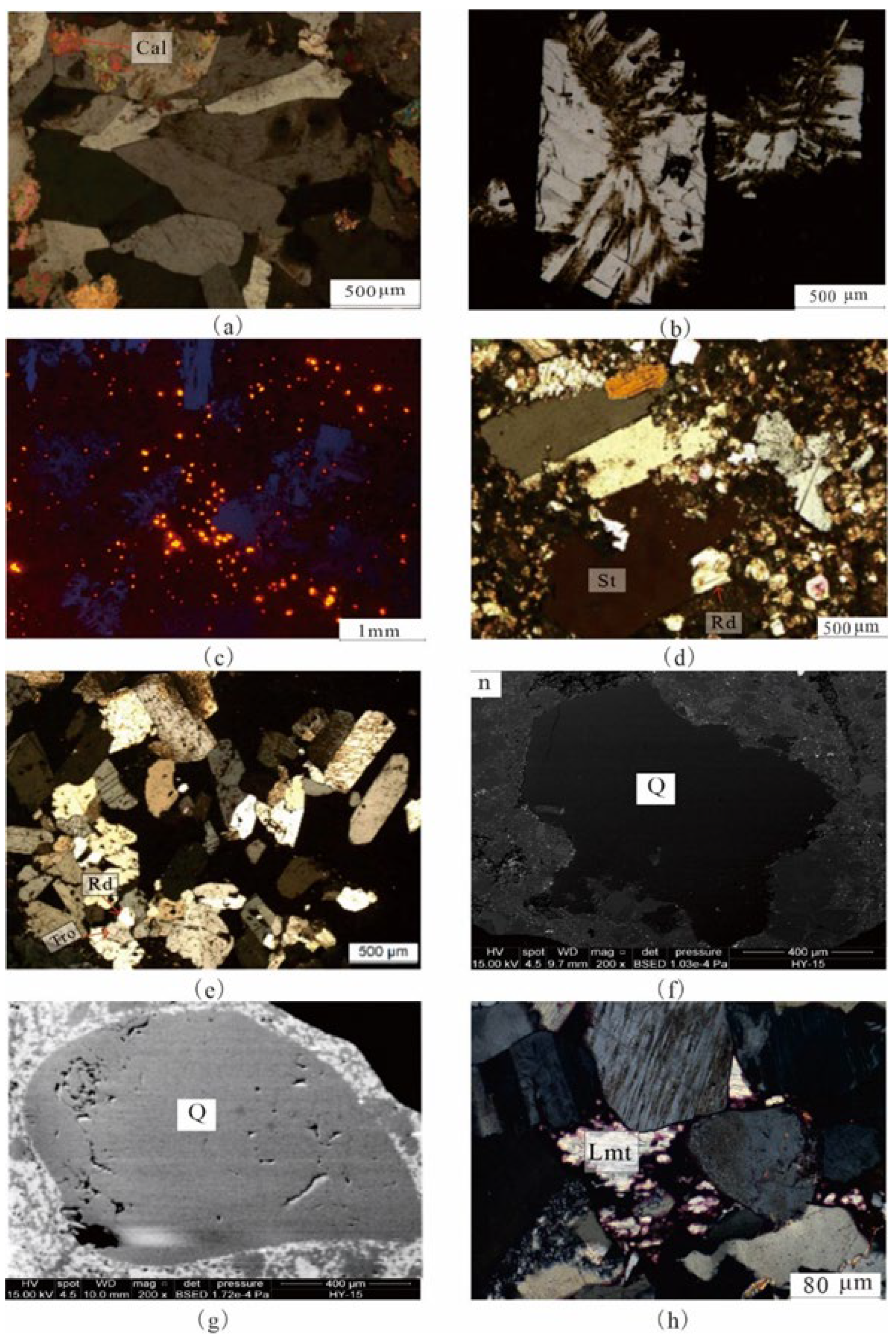
4.1. Petrological Characteristics
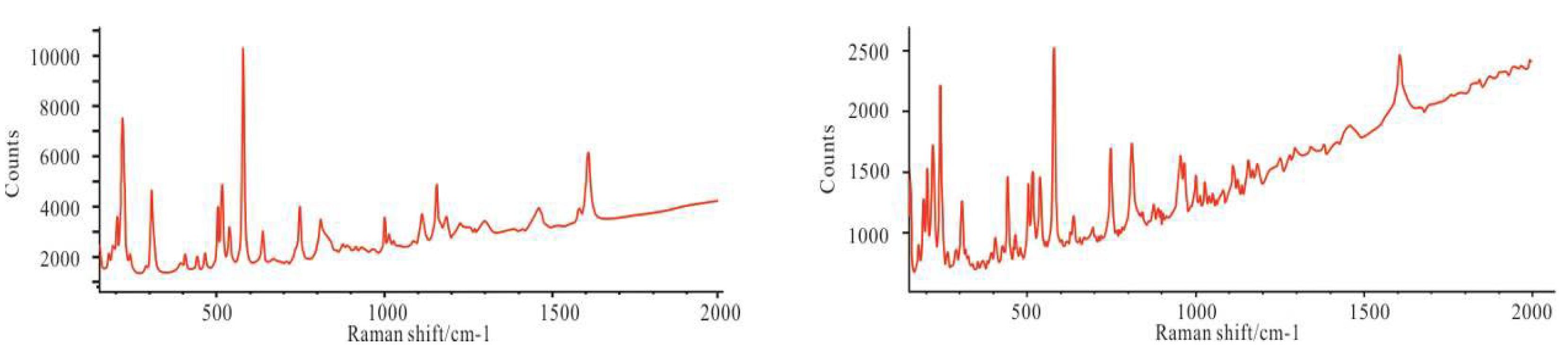
4.2. Mineralogical Characteristics
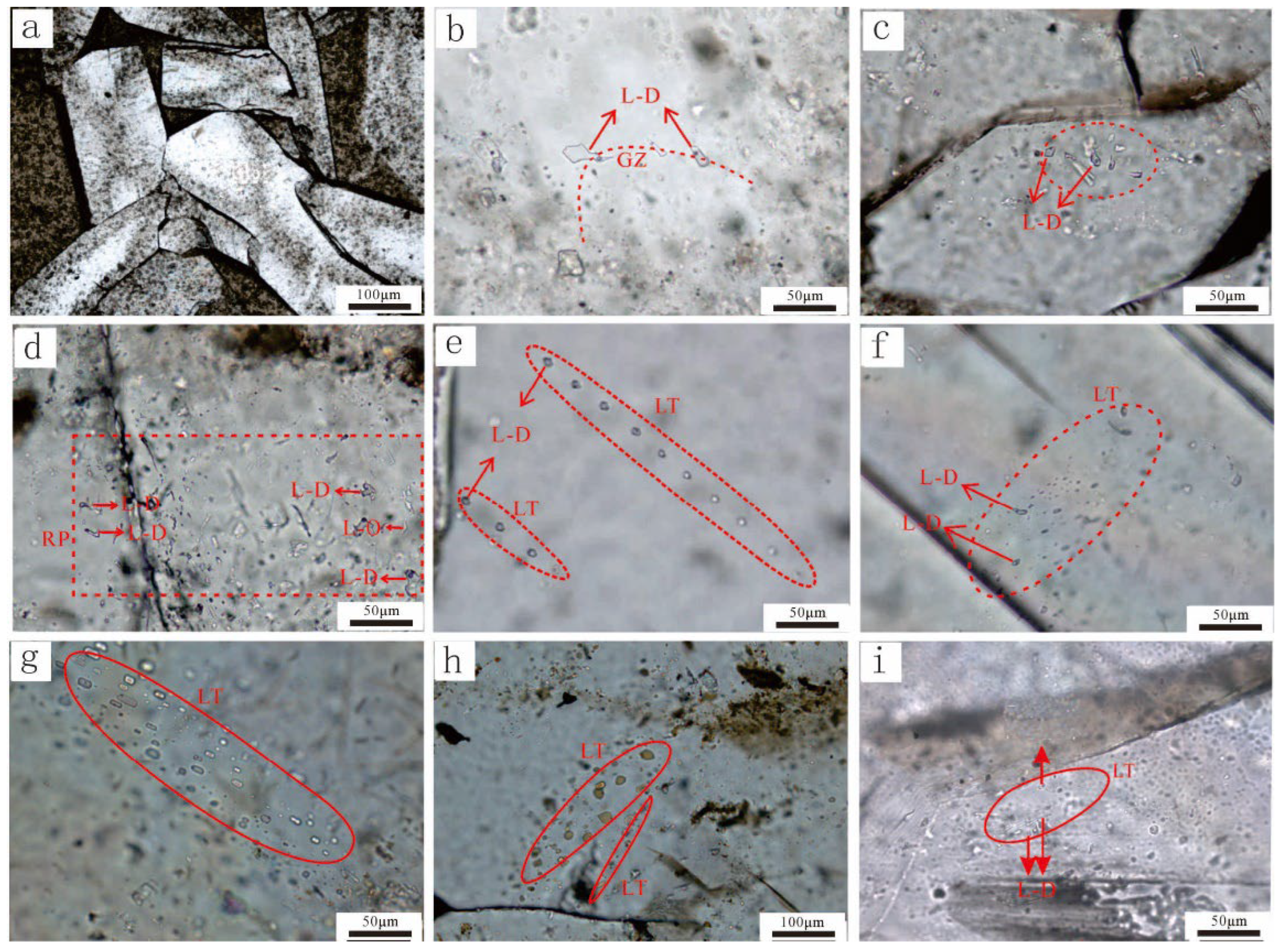
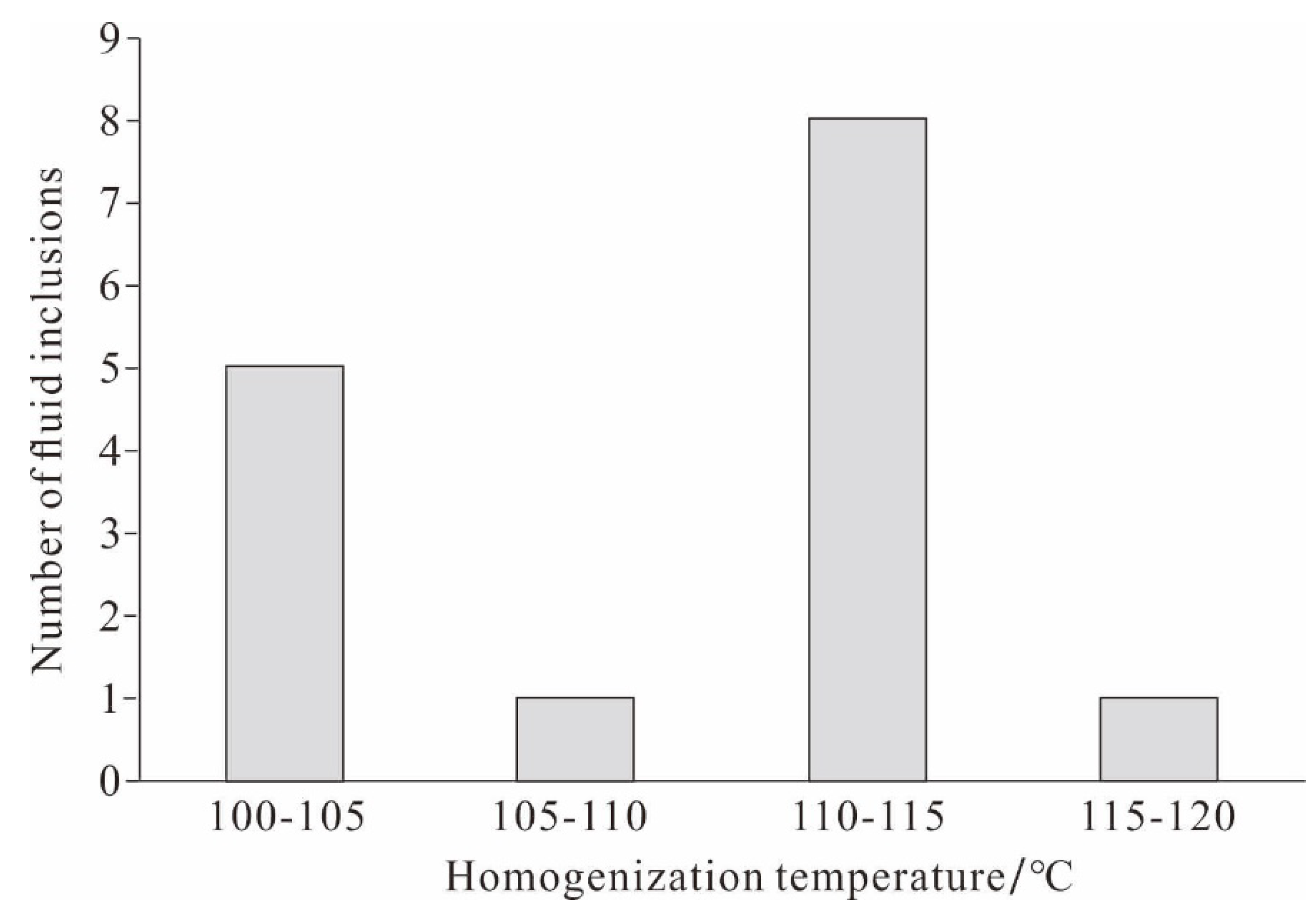
4.3. Homogenization Temperature of Reedmergnerite Primary Fluid Inclusions
5. Discussion
5.1. Types of Alkaline Lake Diagenesis and Their Causes
5.1.1. Indicative Significance of Iron Dolomite Ring
5.1.2. Quartz Dissolution
5.1.3. Cementation of Laumontite
5.1.4. Formation of Reedmergnerite
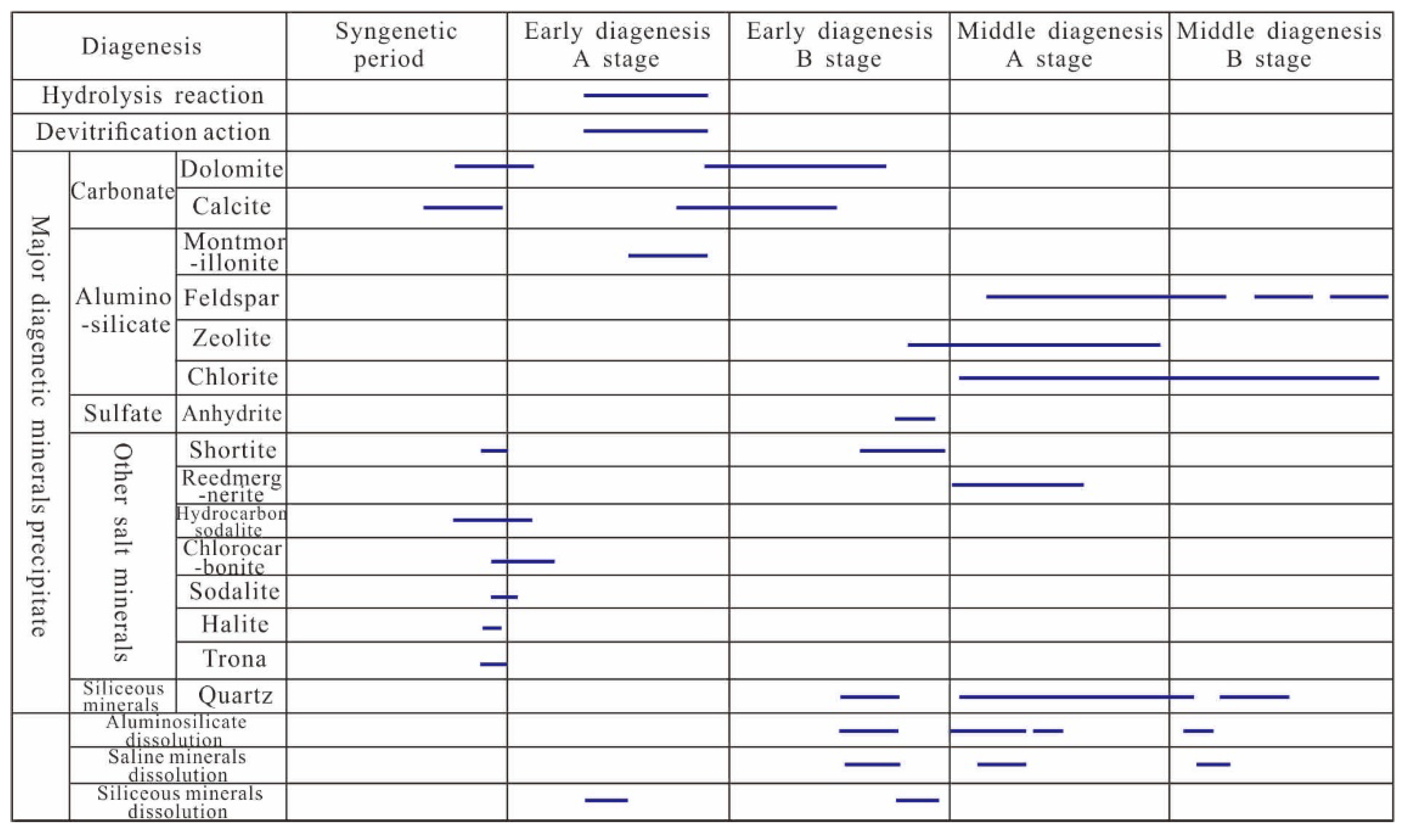
5.2. Diagenetic Evolutionary Sequence of Fengcheng Formation Shale Series in Mahu Sag
5.2.1. Devitrification and Hydrolysis
5.2.2. Diagenetic Mineral Precipitation Sequence
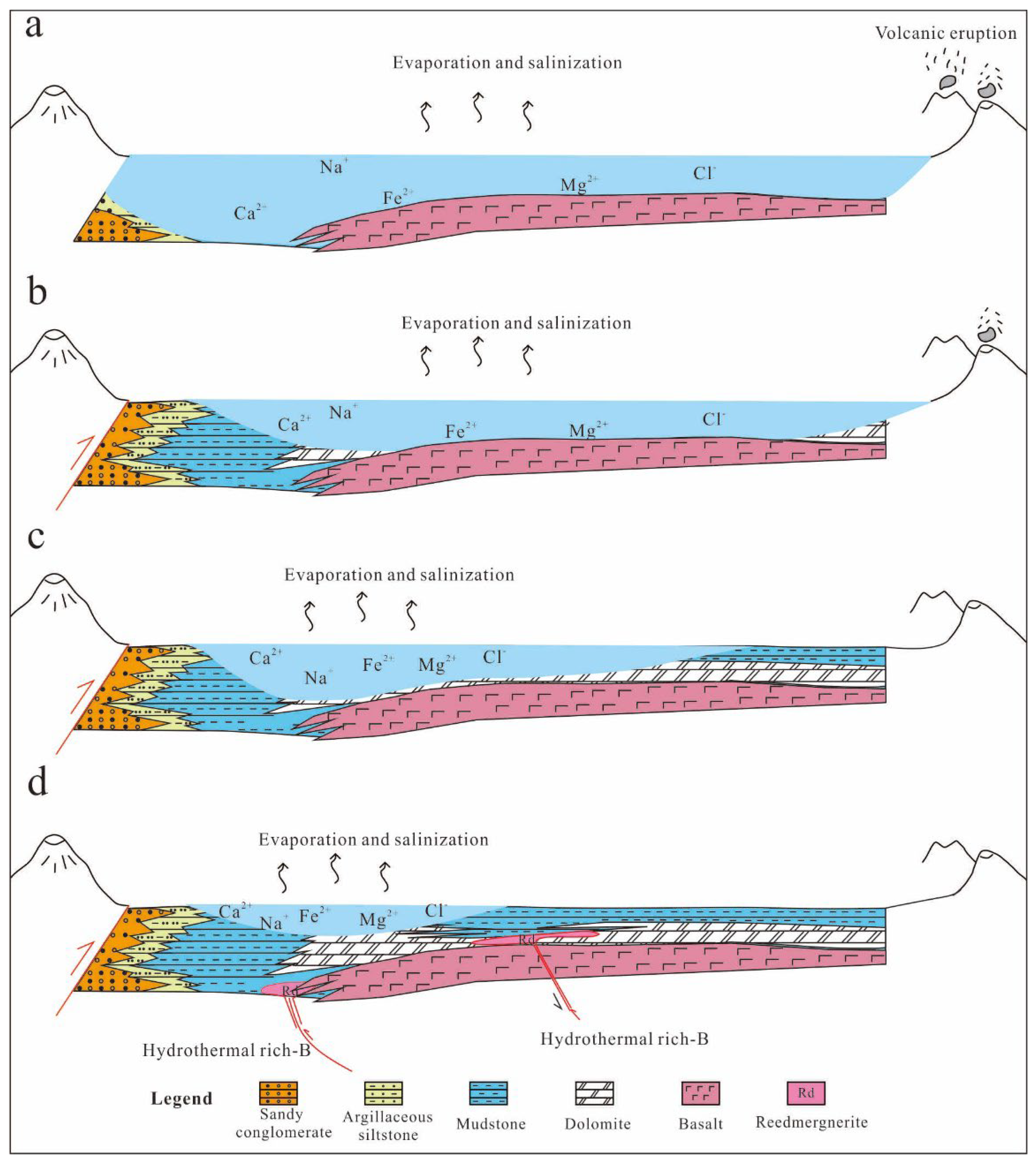
5.3. Alkaline Sedimentary Evolution and Diagenetic Model of Fengcheng Formation Shale Series in Mahu Sag
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EPMA | electron probe microanalysis |
| SEM | scanning electron microscope |
| CL | cathode luminescence |
| LR | laser Raman |
| Cal | calcite |
| Fer-DOL | ferric dolomite |
| Rd | reedmergnerite |
| Tro | trona |
| St | shortite |
| Lmt | Laumonite cemented between particles |
References
- Kuang, L. C.; Tang, Y.; Lei, D. W.; Chang, Q. S.; Ouyang, M.; Hou, L. H.; Liu, D. G. Formation conditions and exploration potential of tight oil in the Permian saline lacustrine dolomitic rock, Junggar Basin, NW China. Petroleum Exploration and Development. 2012. 39, 657–667. [CrossRef]
- Kuang, L. C.; Cui, X. K.; Zou, C. N.; Hou, L. H. Oil accumulation and concentration regularity of volcanic lithostratigraphic oil reservoir: A case from upper-plate Carboniferous of KA-BAI fracture zone, Junggar Basin. Petroleum Exploration and Development 2007, 34, 285–290. [Google Scholar]
- Yu, K.; Cao, Y.; Qiu, L.; Sun, P. The hydrocarbon generation potential and migration in an alkaline evaporate basin: The Early Permian Fengcheng Formation in the Junggar Basin, northwestern China. Marine and Petroleum Geology. 2018, 98, 12–32. [Google Scholar] [CrossRef]
- Jagniecki, E. A.; Jenkins, D. M.; Lowenstein, T. K.; Carroll, A. R. Experimental Study of Shortite (Na2Ca2(CO3)3) Formation and Application to the Burial History of the Wilkins Peak Member, Green River Basin, Wyoming, USA. Geochimica et Cosmochimica Acta. 2013. 115, 31–45. [CrossRef]
- Smith, M. E.; Carroll, A. R.; Singe, B. S. Synoptic Reconstruction of a Major Ancient Lake System:Eocene Green River Formation, Western United States. Geological Society of America Bulletin. 2008, 1-2, 54–84. [Google Scholar]
- Javier, G. V.; Ibrahim, G.; Cahit, H.; Eva, P. Genetic Model for Na-Carbonate Mineral Precipitation in the Miocene Beypazarı Trona Deposit, Ankara Province, Turkey. Sedimentary Geology. 2013, 294, 315–327. [Google Scholar]
- Meng, T.; Liu, P.; Qiu, L. W.; Wang, Y. S.; Liu, Y. L.; Lin, H. M.; Cheng, F. Q.; Qu, C. S. Formation and distribution of the high quality reservoirs in a deep saline lacustrine basin: A case study from the upper part of the 4th member of Paleogene Shahejie Formation in Bonan sag, Jiyang depression, Bohai Bay Basin, East China. Petroleum Exploration and Development. 2017, 44, 896–906. [Google Scholar] [CrossRef]
- Tang, Y.; Lyu, Z. X.; He, W. J.; Qing, Y. H.; Li, X.; Song, X. Z.; Yang, S.; Cao, Q. M.; Qian, Y. X.; Zhao, X. M. Origin of dolomites in the Permian dolomitic reservoirs of Fengcheng Formation in Mahu Sag, Junggar Basin, NW China. Petroleum Exploration and Development. 2023. 50, 38–50. [CrossRef]
- Jiang, F. J.; Hu, M. L.; Hu, T.; Lyu, J. H.; Huang, L. L.; Liu, C. L.; Jiang, Z. X.; Huang, R. D.; Zhang, C. X.; Wu, G. Y.; Wu, Y. P. Controlling factors and models of shale oil enrichment in Lower Permian Fengcheng Formation, Mahu Sag, Junggar Basin, NW China. Petroleum Exploration and Development. 2023. 50, 706–718. [CrossRef]
- Tang, Y.; Cao, J.; He, W. J.; Guo, X. G.; Zhao, K. B.; Li, W. W. Discovery of shale oil in alkaline lacustrine basins: The Late Paleozoic Fengcheng Formation, Mahu Sag, Junggar Basin, China. Petroleum Science. 2021. 18, 1281–1293. [CrossRef]
- Qiu, L.W.; Jang, Z.X.; Cao, Y.C.; Qiu, R.H.; CHEN, W.X.; TU, Y.F. Alkaline diagenesis and its influence on a reservoir in the Biyang depression. Science in China (Series D). 2002, 45, 643–653. [Google Scholar] [CrossRef]
- Li, S.Y.; Tian, J.C.; Lin, X.B.; Zuo, Y.H.; Kang, H.; Yang, D.D. Effect of alkaline diagenesis on sandstone reservoir quality: Insights from the Lower Cretaceous Erlian Basin, China. Energy Exploration & Exploitation. 2002, 38, 434–453. [Google Scholar]
- Zhang, S.B.; Liu, Z.; Liu, H.J.; Song, Y.H.; Li, X.M.; Xu, T. Alkali Diagenesis of Xujiahe Sandstone in Hebaochang Block in Sichuan Basin. Xingjiang Petroleum Geology. 2011, 32, 464–468. [Google Scholar]
- Cao, T.Y.; Zhong, D.K.; Niu, S.L.; Sun, H.T.; Cao, X.; Wang, F. Alkaline Diagenesis and Porosity Evolution of Zhu hai Formation Reservoirs in Eastern Huizhou Sag. Acta Sedimentologica Sinica. 2020, 38, 1327–1337. [Google Scholar]
- Zhu, H.H.; Zhong, D.K.; Yao, J.L.; Sun, H.T.; Niu, X.B.; Liang, X.W.; You, Y.; Li, X. Alkaline diagenecs and its effects on reservoir porosity: A case study of Upper Triassic Chang 7 Member tight sandstone in Ordos Basin, NW China. Petroleum Exploration and Development. 2015, 42, 56–65. [Google Scholar] [CrossRef]
- Hu, T.; Pang, X.Q.; Jiang, S.; Wang, Q.F.; Xu, T.W.; Lu, K.; Huang, C.; Chen, Y.Y.; Zheng, X.W. Impact of Paleosalinity, Dilution, Redox, and Paleoproductivity on Organic Matter Enrichment in a Saline Lacustrine Rift Basin: A Case Study of Paleogene Organic-Rich Shale in Dongpu Depression, Bohai Bay Basin, Eastern China. Energy & Fuels. 2008, 32, 5045–5061. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Chen, J.F.; Zhong, N.N.; Fei, W.W.; Dong, Q.W.; Chen, J.; Wang, Y.Y. The geneses of sedimentary organic matter with anomalous 13C-enriched isotopic composition in saline and freshwater lakes: A case study of lacustrine source rocks from Dongpu and Qikou sags, Bohai Bay Basin, eastern China. Marine and Petroleum Geology. 2020, 118, 104434. [Google Scholar] [CrossRef]
- Wang, K.; Pang, X.Q.; Zhang, H.G.; Zhao, Z.F.; Su, S.C.; Hui, S.S. Characteristics and genetic types of natural gas in the northern Dongpu Depression, Bohai Bay Basin, China. Journal of Petroleum Science and Engineering. 2018, 170, 453–466. [Google Scholar] [CrossRef]
- Tan, X.F.; Tian, J.C.; Li, Z.B.; Zhang, S.P.; Wang, W.Q. Diagenesis evolution of fragmental reservoir in alkali sediment environment-taking the Member 4 of Shahejie Formation of steep-slope zone in Dongying sag, Shandong, China for example. Geological Bulletin of China. 2010, 4, 535–543. [Google Scholar]
- Cao, J.; Lei, D.W.; Li, Y.W.; Tang, Y.; Abulimiti. ; Chang, Q.S.; Wang, T.T. Ancient high-quality alkaline lacustrine source rocks discovered in the Lower Permian Fengcheng Formation, Junggar Basin. Acta Petrolei Sinica. 2015, 36, 781–790. [Google Scholar]
- Cao, J.; Xia, L.W.; Wang, T.T.; Zhi, D.M. An alkaline lake in the Late Paleozoic Ice Age (LPIA): A review and new insights into paleoenvironment and petroleum geology. Earth-Science Reviews. 2020, 202, 103091. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Yuan, X.J.; Wang, M.S.; Zhou, C.M.; Tang, Y.; Chen, X.Y.; Lin, M.J.; Cheng, D.W. Alkaline-lacustrine deposition and paleoenvironmental evolution in permian fengcheng formation at the mahu sag, junggar basin, nw china. Petroleum Exploration and Development. 2018, 45, 1036–1049. [Google Scholar] [CrossRef]
- Yu, K.H.; Cao, Y.C. , Qiu, L.W., Sun, P.P.; Jia, X.Y.; Wan, M. Geochemical characteristics and origin of sodium carbonates in a closed alkaline basin: The Lower Permian Fengcheng Formation in the Mahu Sag, northwestern Junggar Basin, China. Palaeogeography, Palaeoclimatology, Palaeoecology. 2018, 511, 506–531. [Google Scholar] [CrossRef]
- Tang, W.B.; Zhang, Y.Y.; Georgia, P.P.; David, J.W.P.; Guo, Z.J.; Li, W. Permian to early Triassic tectono-sedimentary evolution of the Mahu sag, Junggar Basin, western China: sedimentological implications of the transition from rifting to tectonic inversion. Marine and Petroleum Geology. 2021, 123, 104730. [Google Scholar] [CrossRef]
- He, D.F.; Li, D.; Fan, C.; Yang, X.F. Geochronology, geochemistry and tectonostratigraphy of Carboniferous strata of the deepest Well Moshen-1 in the Junggar Basin, northwest China: Insights into the continental growth of Central Asia. Gondwana Research. 2013, 24, 560–577. [Google Scholar] [CrossRef]
- He, D.F.; Zhang, L.; Wu, S.T.; Li, D.; Zhen, Y. Tectonic evolution stages and features of the Junggar Basin. Oil & Gas Geology. 2018, 39, 845–861. [Google Scholar]
- He, D.F.; Yin, C.; Du, S.K.; Shi, X.; Ma, H.S. Characteristics of structural segmentation of foreland thrust belts -A case study of the fault belts in the northwestern margin of Junggar Basin. Earth Science Frontiers. 2004, 3, 91–101. [Google Scholar]
- Cao, J.; Yao, S.P.; Jin, Z.J.; Hu, W.X.; Zhang, Y.J.; Wang, X.L.; Zhang, Y.Q.; Tang, Y. Petroleum migration and mixing in the northwestern Junggar Basin (NW China): constraints from oil-bearing fluid inclusion analyses. Organic Geochemistry. 2006, 37, 827–846. [Google Scholar] [CrossRef]
- Xu, Z.H.; Hu, S.Y.; Wang, L.; Zhao, W.Z.; Cao, Z.L.; Wang, R.J.; Shi, S.Y.; Jiang, L. Seismic sedimentologic study of facies and reservoir in middle Triassic Karamay Formation of the Mahu Sag, Junggar Basin, China. Marine and Petroleum Geology. 2019, 107, 222–236. [Google Scholar] [CrossRef]
- Li, D.; He, D.F.; Santosh, M.; Ma, D.L.; Tang, J.Y. Tectonic framework of the northern Junggar Basin part I: The eastern Luliang Uplift and its link with the East Junggar terrane. Gondwana Research. 2015, 27, 1089–1109. [Google Scholar] [CrossRef]
- Zhu, S.F.; Zhu, X.M.; Niu, H.P.; Han, X.F.; Zhang, Y.Q. Genetic Mechanism of Dolomitization in Fengcheng Formation in the Wu-Xia area of Junggar Basin, China. Acta Geologica Sinica-english Edition. 2012, 86, 447–461. [Google Scholar] [CrossRef]
- Hou, L.H.; Zou, C.N.; Liu, L.; Wen, B.H.; Wu, X.Z.; Wei, Y.Z.; Mao, Z.G. Geologic essential elements for hydrocarbon accumulation with in Carboniferous volcanic weathered crusts in Xinjiang, China. Acta Petrolei Sinica. 2012, 33, 533–540. [Google Scholar]
- Wu, W.; Li, Q.; Pei, J.X.; Ning, S.Y.; Tong, L.Q.; Liu, W.Q.; Feng, Z.D. Seismic sedimentology, facies analyses, and high-quality reservoir predictions in fan deltas: A case study of the Triassic Baikouquan Formation on the western slope of the Mahu Sag in China's Junggar Basin. Marine and Petroleum Geology. 2020, 120, 104546. [Google Scholar] [CrossRef]
- Chen, Z.L.; Liu, G.D.; Wang, X.L.; Gao, G.; Xiang, B.L.; Ren, J.L.; Ma, W.Y.; Zhang, Q. Origin and mixing of crude oils in Triassic reservoirs of Mahu slope area in Junggar Basin, NW China: Implication for control on oil distribution in basin having multiple source rocks. Marine and Petroleum Geology. 2016, 78, 373–389. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Zhang, Y.Y.; Chen, S.; Guo, Z.J.; Tang. W.B. Controls of a strike-slip fault system on the tectonic inversion of the Mahu depression at the northwestern margin of the Junggar Basin, NW China. Journal of Asian Earth Sciences. 2020, 198, 104229. [Google Scholar] [CrossRef]
- Lei, W.D.; Chen, G.Q.; Liu, H.L.; Li, X.; Tao, K.Y.; Cao, J.; Abulimiti. Study on the forming conditions and exploration fields of the Mahu Giant Oil (Gas) Province, Junggar Basin. Acta Geologic Sinica. 2017, 91, 1604–1619. [Google Scholar]
- Zhang, G.Y.; Wang, Z.Z.; Guo, X.G.; Sun, Y.Y.; Sun, L.; Pan, L. Characteristics of lacustrine dolomitic rock reservoir and accumulation of tight oil in the Permian Fengcheng Formation, the western slope of the Mahu Sag, Junggar Basin, NW China. Journal of Asian Earth Sciences. 2019, 178, 64–80. [Google Scholar] [CrossRef]
- Chen, Y.B.; Cheng, X.G.; Zhang, H.; Li, C.Y.; Ma, Y.P.; Wang, G.D. Fault characteristics and control on hydrocarbon accumulation of middle-shallow layers in the slope zone of Mahu sag, Junggar Basin, NW China. Petroleum Exploration and Development. 2018, 6, 1050–1060. [Google Scholar] [CrossRef]
- Feng, C.; Li, T.; He, W.J.; Zheng, M.L. Organic geochemical traits and paleo-depositional conditions of source rocks from the Carboniferous to Permian sediments of the northern Mahu Sag, Junggar Basin, China. Journal of Petroleum Science and Engineering. 2020, 191, 107117. [Google Scholar] [CrossRef]
- Li, J.; Tang, Y.; Wu, Tao. Zhao, J.Z.; Wu, H.Y.; Wu, W.T.; Bai, Y.B. Overpressure origin and its effects on petroleum accumulation in the conglomerate oil province in Mahu Sag, Junggar Basin, NW China. Petroleum Exploration and Development. 2020; 47, 726–739. [CrossRef]
- Yang, M.Z.; Wang, F.Z.; Zheng, J.P. Geochemistry and tectonic setting of basic volcanic rocks in Ke-Xia region, northwestern Junggar Basin. Acta Petrologica Et Mineralogica. 2006, 20, 165–174. [Google Scholar]
- Chang, L.H.; Chen, M.Y.; Jin, W.; Li, S.C.; Yu, J.J. Handbook of Identification of transparent mineral slices, 1st ed.; Geological Publishing House: Beijing, China, 2006; pp. 1–259. [Google Scholar]
- Jiang, Y.Q.; Wen, H.G.; Qi, L.Q.; Zhang, X.X.; Li, Y. Salt minerals and their genesis of the permian Fengcheng Formation in urho area, Junggar Basin. Mineralogy and Petrology. 2012, 32, 105–114. [Google Scholar]
- Wang, M.S.; Zhang, Z.J.; Zhou, C.M.; Yuan, X.J.; Lin, M.J.; Liu, Y.H.; Cheng, D.W. Lithological characteristics and origin of alkaline lacustrine of the Lower Permian Fengcheng Formation in Mahu Sag, Junggar Basin. Journal of Paleogeography. 2018, 20, 147–162. [Google Scholar]
- Xu, L.; Chang, Q.S.; Feng, L.L.; Zhang, N.; Liu, H. The reservoir characteristics and control factors of shale oil in Permian Fengcheng Formation of Mahu sag, Junggar Basin. China Petroleum Exploration. 2019, 24, 649–660. [Google Scholar] [CrossRef]
- Yu, K.H.; Cao, Y.C.; Qiu, L.W.; Sun, P.; Yang, Y.Q.; Qu, C.S.; Wan, M. Characteristics of alkaline layer cycles and origin of the Lower Permian Fengcheng Formation in Mahu sag, Junggar Basin. Journal of Paleogeography. 2016, 18, 1012–1029. [Google Scholar]
- You, X.L.; Jia, W.Q.; Xu, F.; Liu, Y. Mineralogical characteristics of ankerite and mechanisms of primary and secondary origins. Earth Science. 2018, 43, 4046–4055. [Google Scholar]
- Pagel, M.; Barbin, V.; Blanc, P.; Ohnenstetter, D. Application of Cathodoluminescence to Carbonate Diagenesis, 1st ed.Springer-Verlag: Berlin, Germary, 2000; pp. 271–301. [Google Scholar] [CrossRef]
- Ma, P.J.; Lin, C.Y.; Jahren, J.; Dong, C.M.; Ren, L.H.; Hellevang, H. Cyclic zoning in authigenic saddle dolomite-ankerite: Indications of a complex interplay between fault-rupturing and diagenetic alteration. Chemical Geology. 2021, 559, 119831. [Google Scholar] [CrossRef]
- Qin, Y.J.; Feng, Y.C.; Yang, X.; Wen, L.Y. A microarea analysis technique of EPMA to probe reedmergnerite. Journal of Chinese Electron Microscopy Society. 2016, 35, 217–222. [Google Scholar]
- Axel, G.; Detlev, K.R; Jan, M.; Rolf, D.N.; Andreas, S. Quantitative high resolution cathodoluminescence spectroscopy of diagenetic and hydrothermal dolomites. Sedimentary Geology. 2001, 140, 191–199. [Google Scholar]
- Braith, J.R. Cathodoluminescence in Quaternary caibonate deposits. Sedimentary Geology. 2016, 337, 29–35. [Google Scholar] [CrossRef]
- Goldstein H, R. Systematics of fluid inclusions in diagenetic minerals. SEPM short course, 31.
- Eugster, H.P.; Mciver, N.L. Boron analogues of alkali feldspars and related silicates. Bulletin of the Geological Society of America. 1959, 70, 1598. [Google Scholar]
- Mitsuyoshi, K. Synthesis and properties of reedmergnerite. The Journal of the Japanese Association of Mineralogists, Petrologists and Economic Geologists. 1977, 72, 162–172. [Google Scholar]
- Liu, L.H.; Huang, S.J.; Wang, C.L.; Huang, K.K.; Tong, H.P.; Zhong, Q.Q. Cathodoluminescence zonal texture of calcite cement in carbonate rock and its relationship with trace element composition:a case of Ordovician carbonate rock of Tahe oilfield, Tarim basin. Marine Origin Petroleum Geology. 2010, 15, 55–60. [Google Scholar]
- Richter, D.K.; Goette, T.; Niggemann, S.; Wurth, G. REE3+ and Mn2+ activated cathodoluminescence in lateglacial and holocene stalagmites of central europe: evidence for climatic processes? Holocene. 2004, 14, 759–768. [Google Scholar] [CrossRef]
- Lovley, D.R.; Holmes, D.E.; Nevin, K.P. Dissimilatory Fe(III) and Mn(IV) reduction. Advances in Microbial Physiology. 2004, 49, 219–286. [Google Scholar] [PubMed]
- Xue, J.J.; Sun, J.; Zhu, X.M.; Liu, W.; Zhu, S.F. Characteristics and Formation Mechanism for Dolomite Reservoir of Permian Fengcheng Formation in Junggar Basin. Geoscience. 2012, 26, 755–761. [Google Scholar]
- Qiu, L.W.; Jiang, Z.X.; Chen, W.X. A new type of secondary porosity--quartz dissolution porosity. Acta Sedimentologica Sinica. 2002, 4, 621–627. [Google Scholar]
- Qu, X.Y.; Chen, X.; Qiu, L.W.; Zhang, M.L.; Zhang, X.J. Genesis of secondary pore of quartz dissolution type and its influences on reservoir: Taking the tight sandstone reservoir in the Upper Paleozoic of Daniudi gas field as an example. Oil & Gas Geology. 2015, 36, 804–813. [Google Scholar]
- Liu, J.K.; Peng, J.; Shi, Y.; Bao, Z.F.; Sun, Y.L.; Liu, X.M.; Zhang, Z. The genesis of quartz dissolution in tight sand reservoirs and its impact on pore development: a case study of Xujiahe Formation in the transitional zone of Central-Southern Basin. Acta Petrolei Sinica. 2015, 2015. 36, 1090–1097. [Google Scholar]
- Rimstidt, J.D.; Zhang, Y.L.; Zhu, C. Rate equations for sodium catalyzed amorphous silica dissolution. Geochimica Et Cosmochimica Acta. 2016, 2016. 195, 120–125. [Google Scholar] [CrossRef]
- Zhang, S.T.; Liu, Y. Molecular-level mechanisms of quartz dissolution under neutral and alkaline conditions in the presence of electrolytes. Geochemical Journal. 2014, 2014. 48, 189–205. [Google Scholar] [CrossRef]
- Yu, K.H.; Cao, Y.C.; Qiu, L.W.; Sun, P.P.; Su, Y.G. Brine evolution of ancient lake and mechanism of carbonate minerals during the sedimentation of Early Permian Fengcheng Formation in Mahu Depression, Junggar Basin, China. Natural Gas Geoscience. 2016, 2016. 27, 1248–1263. [Google Scholar]
- Zhao, Y.; Guo, P.; Lu, Z.Y.; Zheng, R.C.; Chang, H.L.; Wang, G.Z.; Wei, Y.; Wen, H.G. Genesis of Reedmergnerite in the Lower Permian Fengcheng Formation of the Junggar Basin, NE China. Acta Sedimentologica Sinica. 2020, 2020. 38, 966–979. [Google Scholar]
- Zhang, H.; Cheng, L.; Fan, H.T. Formation overpressure and its influence on physical properties in Mahu sag, Junggar Basin. Progress in Geophysics, 2021; 1–8. [Google Scholar]
- Zhang, S.T.; Liu, Y. Progress Review of Quartz Dissolution Models. Bulletin of Mineralogy, Petrology and Geochemistry, 2009; 28, 294–300. [Google Scholar]
- Tian, X.R.; Zhang, Y.Y.; Zhuo, Q.G.; Yu, Z.C.; Guo, Z.J. Tight oil charging characteristics of the Lower Permian Fengcheng Formation in Mahu sag, Junggar Basin: evidence from fluid in alkaline minerals. Acta Petrolei Sinica. 2019, 40, 646–659. [Google Scholar]
- Rao, S.; Zhu, Y.K.; Hu, S.B.; Wang, Q. The Thermal History of Junggar Basin: Constraints on the Tectonic Attribute of the Early-Middle Permian Basin. Acta Geologica Sinica. 2018, 92, 1176–1195. [Google Scholar]
- Zhu, S.F.; Zhu, X.M.; Wu, D.; Liu, Y.H.; Li, P.P.; Jiang, S.X.; Liu, X.C. Alteration of volcanics and its controlling factors in the Lower Permian reservoirs at northwestern margin of Junggar Basin. Oil & Gas Geology, 2014; 35, 77–85. [Google Scholar]
- Guo, M.Z.; Shou, J.F.; Xu, Y.; Guo, H.J.; Zou, Z.W.; Han, S.H. Distribution and controlling factors of Permian zeolite cements in Zhongguai—Northwest margin of Junggar Basin. Acta Petrolei Sinica 2016, 37, 695–705. [Google Scholar]
- Chipera, S.J.; Goff, F.; Goff, C.J.; Fittipaldo, M. Zeolitization of intracaldera sediments and rhyolitic rocks in the 1.25 Ma lake of Valles caldera, New Mexico, USA. Journal of Volcanology & Geothermal Research 2008, 178, 317–330. [Google Scholar]
- Zhu, S.F.; Zhu, X.M. Liu, X.C.; Li, C., Wang, X.X., Tan, M.X., Geng, M.Y., Eds.; Li, Y.P. Alteration products of volcanic materials and their influence on reservoir space in hydrocarbon reservoirs: evidence from Lower Permian strata in Ke-Xia region, Junggar Basin. Acta Petrolei Sinica. 2014. [Google Scholar]
- Zhu, S.F.; Zhu, X.M.; Wang, X.L.; Liu, Z.Y. Zeolite diagenesis and its control on petroleum reservoir quality of Permian in northwestern margin of Junggar Basin, China. Science China Earth Sciences. 2012, 55, 386–396. [Google Scholar] [CrossRef]
- Chang, H.L.; Zheng, R.C.; Guo, C.L.; Wen, H.G. Characteristics of Rare Earth Elements of Exhalative Rock in Fengcheng Formation, Northwestern Margin of Jungger Basin. Geological Review 2016, 62, 550–568. [Google Scholar]
- Jia, B.; Wen, H.G.; Li, Y.B.; Liu, Y.P.; Wang, T. Fluid inclusions in the salt minerals from the Permian Fengcheng Formation in the Urho region, Junggar Basin, Xinjiang. Sedimentary Geology and Tethyan Geology. 2015, 35, 33–42. [Google Scholar]
- Broxton, D.E. Chemical changes associated with zeolitization of the tuffaceous beds of Calico Hills, Yucca Mountain, Nevada, USA. Proceedings - International Symposium on Water-Rock Interaction 1992, 7, 699–703. [Google Scholar]
- Lijima, A. Zeolites in petroleum and natural gas reservoirs. Reviews in Mineralogy and Geochemistry. 2001, 45, 347–402. [Google Scholar]
- Zhu, S.F.; Zhu, X.M.; Wu, D.; Liu, Y. Volcanic material alteration and its controlling factors in Lower Permian oil and gas reservoirs in the northwestern margin of Junggar Basin. Oil & Gas Geology 2014, 35, 77–85. [Google Scholar]
- Zhu, S.F.; Zhu, X.M.; Liu, Y.H.; Chen, X.Y.; Wang, J.H.; Wang, X.J.; Ma, A.Y. Petrological and Geochemical Features of Dolomitic Rocks in the Lower Permian Fengcheng Formation in Wuerhe-Xiazijie Area, Junggar Basin. Geological Review 2014, 60, 1113–1122. [Google Scholar]
- Jin, Q.; Wang, J.; Song, G.Q.; Wang, L.; Lin, L.M.; Bai, S.P. Interaction between source rock and evaporite: a case study of natural gas generation in the northern dongying depression. Chinese Journal of Geochemistry. 2010, 29, 75–81. [Google Scholar] [CrossRef]
- Zhi, D.M.; Tang, Y.; Zheng, M.L.; Xu, Y.; Cao, J.; Ding, J.; Zhao, C.Y. Geological characteristics and accumulation controlling factors of shale reservoirs in Fengcheng Formation, Mahu sag, Junggar Basin. China Petroleum Exploration. 2019, 24, 615–623. [Google Scholar]
- Hips, K.; Haas, J.; Poros, Z.; Kele, S.; Budai, T. Dolomitization of Triassic microbial mat deposits (Hungary): Origin of microcrystalline dolomite. Sedimentary Geology. 2015, 318, 113–129. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, Y.J.; Hu, W.X.; Yao, S.P.; Wang, X.L.; Zhang, Y.Q.; Tang, Y. The Permian hybrid petroleum system in the northwest margin of the Junggar Basin, northwest China. Marine and Petroleum Geology. 2005, 22, 331–349. [Google Scholar] [CrossRef]
| Na2O | TiO2 | SO3 | SiO2 | FeO | P2O5 | Al2O3 | MnO | K2O | MgO | BaO | CaO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.024 | 20.276 | 0.056 | 37.513 | 8.687 | 0.051 | 6.745 | 0.122 | 0.116 | 2.243 | 0.073 | 18.156 |
| Na2O | TiO2 | SO3 | SiO2 | FeO | P2O5 | Al2O3 | MnO | K2O | MgO | BaO | CaO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.07 | 0.22 | - | - | 9.39 | - | 0.01 | 0.46 | 0.01 | 15.49 | 0.09 | 27.91 |
| - | - | - | 0.03 | 9.38 | 0.06 | 0.02 | 0.62 | 0.02 | 15.01 | - | 27.85 |
| - | - | 0.06 | - | 8.01 | - | - | 0.11 | 0.01 | 17.58 | - | 30.84 |
| - | - | - | - | 6.44 | 0.02 | 0.02 | 0.37 | - | 17.99 | 0.09 | 32.02 |
| - | 0.04 | 0.04 | 0.01 | 12.99 | - | 0.05 | 0.33 | - | 13.96 | - | 29.45 |
| - | - | 0.04 | - | 10.98 | 0.04 | 0.01 | 0.54 | 0.01 | 13.74 | 0.07 | 28.03 |
| - | - | - | 0.16 | 6.69 | - | 0.08 | 0.18 | 0.03 | 17.85 | 0.04 | 31.10 |
| - | - | 0.04 | - | 8.41 | 0.01 | - | 1.21 | 0.04 | 18.25 | - | 32.05 |
| - | 0.05 | - | - | 7.45 | 0.01 | - | 0.86 | - | 17.49 | 0.13 | 30.46 |
| 0.07 | 0.08 | 0.25 | 8.54 | 5.43 | 0.08 | 1.86 | 0.16 | 0.54 | 15.58 | - | 23.58 |
| Na2O | TiO2 | SO3 | SiO2 | FeO | P2O5 | Al2O3 | MnO | K2O | MgO | BaO | CaO | B2O5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10.000 | - | - | 70.517 | - | - | - | - | 0.001 | 0.010 | 0.025 | - | 19.447 |
| 10.000 | - | 0.010 | 69.767 | 0.028 | - | 0.043 | 0.012 | - | - | 20.118 | ||
| 13.794 | - | - | 71.165 | 0.009 | 0.025 | 0.047 | - | - | 0.007 | - | 0.005 | 14.948 |
| 11.936 | 0.065 | - | 69.377 | 0.017 | 0.017 | 0.068 | - | 0.062 | 0.014 | 0.016 | 0.013 | 18.415 |
| 10.012 | - | 0.162 | 69.697 | 0.020 | 0.017 | 0.086 | - | 0.088 | 0.027 | - | - | 19.891 |
| 10.000 | 0.258 | - | 70.632 | - | - | 0.094 | 0.009 | 0.008 | 0.028 | 0.007 | - | 18.964 |
| 10.063 | 0.080 | 0.031 | 70.288 | - | - | 0.010 | - | 0.015 | - | - | - | 19.513 |
| 10.042 | - | 0.005 | 70.015 | 0.004 | - | 0.003 | 0.025 | 0.024 | - | - | 0.004 | 19.878 |
| 10.072 | 0.032 | - | 64.868 | 0.009 | 0.017 | 0.004 | 0.024 | 0.021 | 0.018 | - | - | 20.935 |
| Borehole | Deep/m | Fluid inclusions occurrence | Associations of fluid inclusions | Type | Size/μm | Homogenization temperature/℃ |
|---|---|---|---|---|---|---|
| Fengnan2 | 4100.58 | growth zone | FIA⁃1 | gas & liquid | 20 | 112 |
| liquid | 10 | 100 | ||||
| liquid | 15 | 111 | ||||
| Fengnan2 | 4100.58 | growth zone | FIA⁃2 | gas & liquid | 5 | 112 |
| gas & liquid | 4 | 100 | ||||
| liquid | 4 | 111 | ||||
| liquid | 5 | 112 | ||||
| liquid | 4 | 100 | ||||
| Fengnan2 | 4100.58 | growth zone | FIA⁃3 | gas & liquid | 4 | 111 |
| gas & liquid | 6 | 104 | ||||
| gas & liquid | 5 | 103 | ||||
| liquid | 7 | 109 | ||||
| liquid | 5 | 110 | ||||
| liquid | 7 | 116 | ||||
| liquid | 6 | 112 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




