1. Introduction
The classical methods of testing human otolith function using linear acceleration stimuli have now been complemented in the clinic by the use of air conducted sound (ACS) and bone conducted vibration (BCV) as vestibular, and specifically otolithic, stimuli [
1,
2,
3]. Sound and vibration are more clinically practical, and physiological and anatomical evidence shows that the otolith organs are activated by such stimuli (for reviews see [
4,
5]).
ACS or BCV stimuli can activate vestibular receptors resulting in small myogenic potentials called vestibular evoked myogenic potentials (VEMPs). VEMPs are due to synchronized action potentials of vestibular afferents activated at the onset of the stimulus since, as we explain below, prolonging the stimulus rise-time degrades synchronization of action potentials and degrades VEMPs [
6]. VEMPs are being used to identify unilateral utricular and saccular loss [
7,
8,
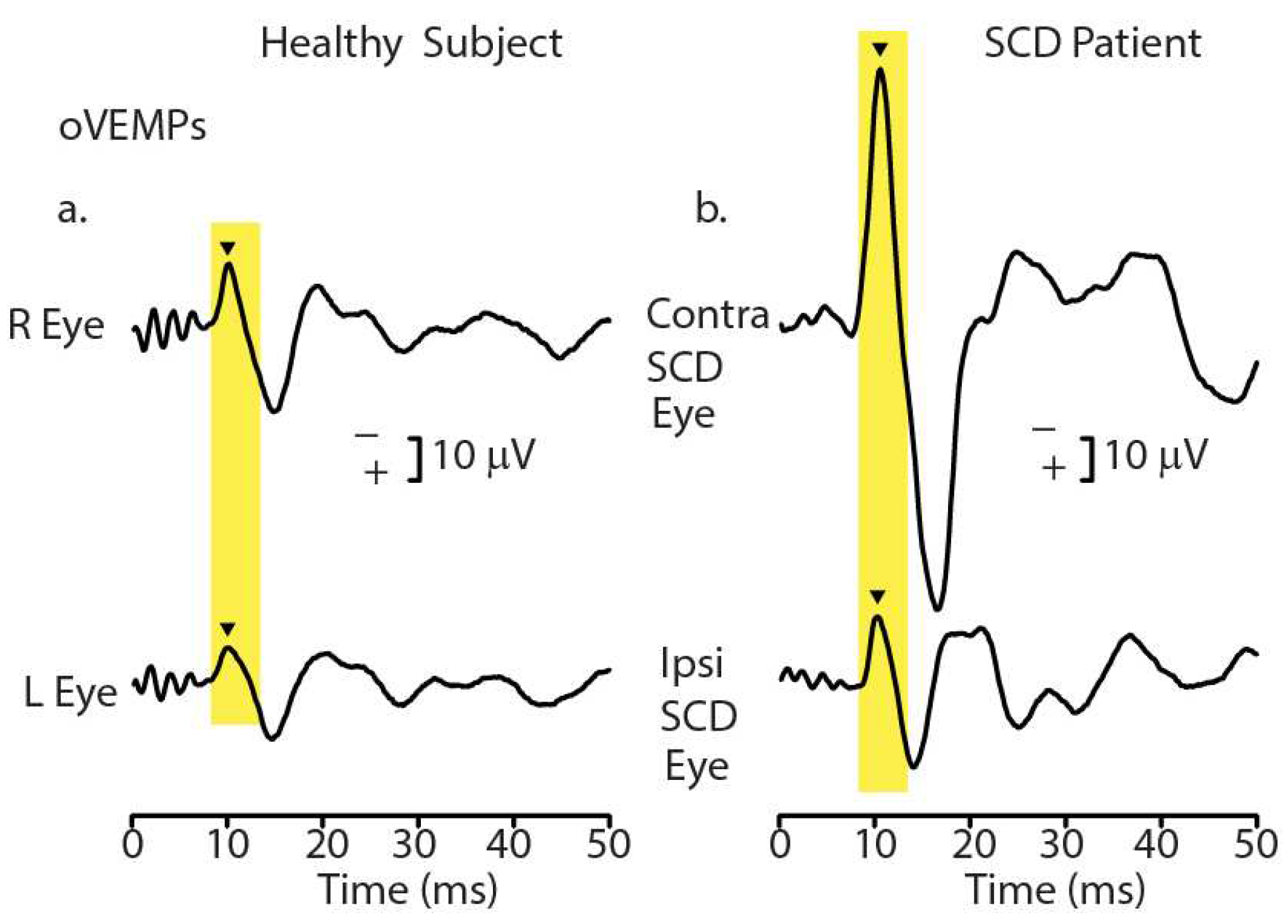
9], semicircular canal dehiscence (see
Figure 1) [
10] and central deficits [
11]
The use of VEMPs as indicators of vestibular function rests on physiological evidence that vestibular receptors and neurons are activated by sound and vibration. Mostly this has come from recording the response of single identified otolithic afferent neurons to sound or vibration in guinea pigs and other species (for reviews see [
4] ). As we show below, new physiological results are clarifying how the initial neural activation by BCV or ACS takes place, and so are validating the use of particular stimuli for clinical VEMP testing. These physiological studies use procedures which allow differentiation of vestibular from cochlear responses, since obviously both cochlear and vestibular receptors and afferent neurons are activated by ACS and BCV. Here we review those procedures, their results, and their interpretation.
2. Physiological Evidence – Overview
In anaesthetized guinea pigs we have measured VIII nerve activation (mainly the compound action potential (CAP)) in response to transient ACS and BCV stimuli (usually click stimuli)[
12,
13,
14]. CAPs are mass neural potentials caused by synchronized action potentials of many individual primary afferent neurons triggered by transient ACS or BCV stimuli such as clicks [
15]. An electrode near the VIII nerve records a response composed of many electrophysiological potentials caused by ACS or BCV stimulation: among them: cochlear compound action potential (cCAP), vestibular compound action potential (vCAP), cochlear and vestibular microphonics (CM and VM) and auditory and vestibular nerve neurophonics (ANN and VNN) [
16,
17]. The largest component is the cochlear compound action potential (cCAP) and for convenience and to relate these data to previous results, we refer to the whole VIII nerve compound action potential as cCAP, recognizing the existence of many components in this potential. We clarify these matters below. These peripheral CAP responses are also the basis for the vestibular evoked potential (VsEP) recorded by scalp electrodes [
18,
19,
20,
21,
22,
23]. The VsEP is a smaller far field correlate of the localized near field response. Our experiments compare vestibular CAPs (vCAPs) to cochlear CAPS (cCAPS) under controlled experimental conditions, and that is the main focus of this review. As we show below, one particularly useful aspect of these physiological recordings is that they provide an objective measure at the vestibular periphery of the optimum stimulus parameters for recording VEMPs in the clinic.
2.1. Independence of Cochlear and Vestibular Labyrinthine Divisions
The vestibular and cochlear divisions of the labyrinth share the same fluids and have receptor cells of superficially similar appearance, so the vestibular and cochlear divisions appear to be interdependent. That appearance is false [
24]. It has been shown that the vestibular system can function independently of the cochlea in humans and guinea pigs: Plontke et al showed that after total surgical ablation of the cochlea in (rare) human patients with intracochlear schwannoma, the semicircular canals and the otoliths continue to function normally [
25]. In 27 consecutive patients there was no significant decline of any of the measured indicators of canal or otolith function after total surgical cochlear ablation. This result shows conclusively that in the human the canals and otoliths can continue to function normally after surgical ablation of the cochlea and, as we show below, that is also the case in guinea pigs. We have measured CAPs in guinea pigs after chemical cochlear ablation (by KCl infusion) which we term that CAP the vCAP identifying it as being of vestibular origin since cochlear function is absent. The vCAP allows us to measure clinically relevant vestibular aspects of transient stimuli which activate vestibular receptors and so optimize the stimulus parameters maximising the vestibular component of VEMPs.
2.2. Differentiating Vestibular and Cochlear Compound Action Potentials
There have been two approaches to differentiating vestibular from cochlear responses in animal experimental studies: either
(a) use stimuli which are accepted as otolithic – transient linear accelerations - and interpret the results (such as the VsEP) as being due to vestibular rather than cochlear activation [
20],
or
(b) use transient BCV or ACS clicks which can activate all labyrinthine sensory regions but use various controls, such as disabling or even removing the cochlea, to identify the vestibular component of the total CAP nerve response [
14,
26].
The problem with using linear acceleration is that the presumably specific otolithic stimulus most likely has aspects that affect the cochlea – it is difficult to deliver a transient linear acceleration without sound or vibration - so it is wise to use broadband masking noise to minimize any possible cochlear contribution [
27]. As we show below, masking by broadband noise has surprisingly little effect on vestibular receptors and afferents. To address these matters, we first briefly review the relevant anatomical and physiological evidence before showing how these physiological results relate to clinical VEMP testing.
3. Anatomy
3.1. Cochlea
Inner hair cells have a fairly uniform appearance throughout the cochlea, although stereocilia height varies along the basilar membrane (see [
28,
29] for reviews). In the cochlea, each individual afferent neuron contacts just one inner hair cell with a bouton synapse (see [
24] for a review), but each inner hair cell receives many afferent bouton contacts from different axons. For every synapse the transmitter is glutamate, released by synaptic ribbons in the inner hair cell [
24,
30,
31,
32]. Ribbons appear to be the main mechanism of glutamatergic synaptic transmission for cochlear primary afferents and are held to be responsible for the temporal precision of cochlear responses to transient stimuli and phase-locking of cochlear afferent action potentials to sinusoidal stimuli [
24,
33,
34,
35].
Transient stimuli such as clicks deflect cochlear receptor hair bundles causing the mechanoelectrical transduction (MET) channels on the stereocilia to open and depolarize the hair cell and so generate a receptor potential - the cochlear microphonic (CM) - and also action potentials in individual afferents (see [
24,
29,
36] for reviews). The synchronized action potentials of many simultaneously activated cochlear afferents in response to a broadband click stimulus are the major components of the mass neural response – the cochlear action potential (cCAP) - recorded by gross electrodes on, or close to, the auditory nerve. Individual primary cochlear afferents have a remarkable temporal precision as shown by the precision of their phase-locked response to sinusoidal stimuli [
35]. In individual neurons, action potentials are generated at a particular stimulus phase angle (or a narrow band of phase angles) up to high frequencies (>1000Hz) [
33,
35]. Classic examples of phase-locking of individual primary cochlear primary afferents in guinea pig were published by [
37]
3.2. Vestibular
Vestibular receptors have a similar hair bundle appearance as cochlear receptors (see [
29]) but there are systematic anatomical and physiological differences in vestibular receptors across vestibular receptor surfaces [
38,
39,
40,
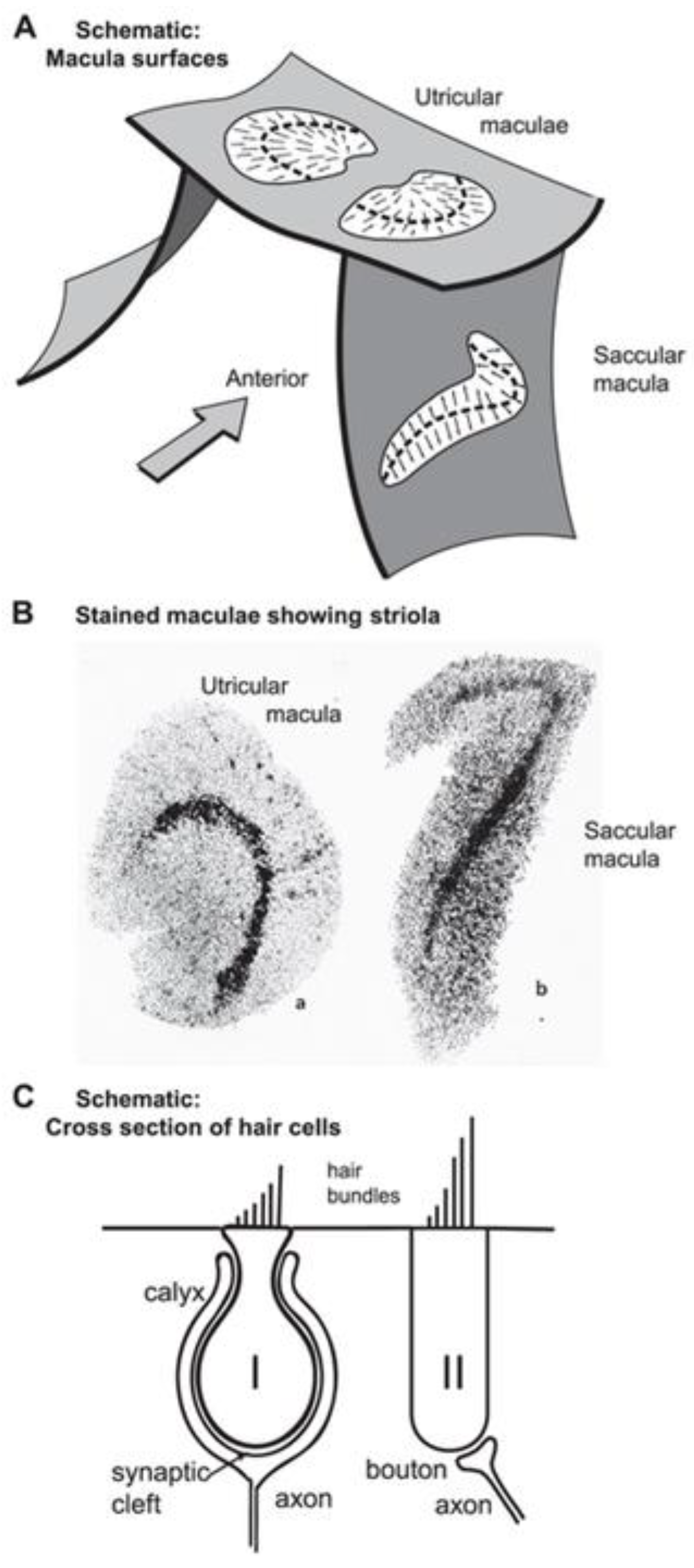
41]. The receptors and afferents from a particular region in each otolithic macula (a stripe called the striola (see
Figure 2A and B) are distinct - it is here that amphora shaped type I receptors, enveloped by a calyx afferent ending are plentiful (
Figure 2C) [
32,
42,
43,
44,
45,
46]. Paralleling cochlear responses, transient vestibular stimuli such as abrupt linear accelerations or ACS or BCV clicks, deflect vestibular hair bundles causing MET channels in the stereocilia to open [
28,
47] resulting in a receptor potential - the vestibular microphonic (VM) [
48] and in action potentials in primary vestibular afferents. Clicks generate vCAPs with the main contributor to the vCAP to click stimuli being from otolithic afferents with irregular resting discharge originating from type I receptors at the striola since it is these otolithic receptors and afferent neurons which are selectively activated by sound or vibration in the guinea pig [
4,
49,
50]. Vestibular afferents with regular resting discharge synapse on type I and II receptors in extrastriolar regions [
51] and have poor or non existent response to ACS or BCV [
41,
49,
52,
50]. Regular afferents are rarely (and then only weakly) activated by sound and vibration at clinically appropriate stimulus levels. In stark contrast, irregular otolithic afferents are activated and phase-lock at low intensities to these stimuli up to high frequencies (>2000Hz ) [
53,
54]. The vestibular type I/calyx afferent synapse is very unusual – the calyx ending of the vestibular afferent fiber envelops the amphora-shaped type I receptor (
Figure 2C), instead of making a punctate synapse as in the cochlea inner hair cells [
46,
55,
56]. However, at this type I/calyx afferent junction the transmitter is glutamate again, as in the cochlea.
The vCAP is identified as being
vestibular since it is present after total cochlear ablation and disappears after vestibular endorgan ablation (
Figure 3). A related potential - the vestibular evoked potential (VsEP), is recorded at the scalp, and the VsEP is also held to reflect the synchronized activity of the vestibular receptors and afferents [
16,
18,
20,
22,
58]. Our simultaneous recording of both vCAP and VsEP in the guinea pig to the same stimuli shows that tight relationship, although the VsEP is smaller than the vCAP by a factor of about 100.
3.3. Specificity
After cochlear ablation the cCAP is abolished, and the remaining CAP is termed the vCAP since it is generated by the remaining vestibular afferents [
59] (see
Figure 3). This is an extremely important control which differentiates the vCAP from the cCAP and allows us to identify optimum vestibular stimuli after eliminating cochlear contributions. Total surgical cochlear ablation involves opening the bony wall of the cochlea and removing the entire cochlear division of the labyrinth, whilst leaving the vestibular division intact. In guinea pigs the vestibular division continues to function, and transient stimuli continue to generate vCAPs after the major surgery on the cochlea. The second method of cochlear ablation, chemical ablation, uses Tasaki’s method of infusing KCl slowly through a small hole in the bony wall of the cochlea at the apex, whilst repeatedly testing the cCAP and CM to verify the decline and eventual absence of cochlear function [
60]. Once cochlear function is lost, the KCl infusion is terminated, and the hole is sealed, so the walls of the bony labyrinth are again intact, and transient stimuli continue to generate vCAPs.
Of the two procedures, chemical ablation is to be preferred since it leaves the bony wall of the labyrinth intact, whereas after surgical ablation of the cochlea, there is a large dehiscence of the bony labyrinth, which acts to change the mechanical operation of the vestibular labyrinth [
61] and so cause enhanced neural responses to ACS and BCV [
62]. Another result of dehiscence is that canal afferents with irregular resting discharge as well as the usual otolithic afferents with irregular resting discharge, will be activated by ACS and BCV stimuli [
3,
62]. This enhanced and broader vestibular activation is unlikely after chemical ablation by KCl since the bony wall of the labyrinth is intact. Importantly after either cochlea ablation procedure, click stimuli can still activate vestibular receptors and generate a vCAP confirming in two different ways that the vCAP is minimally contaminated by a cochlear contribution. The vCAP is probably driven primarily by otolithic afferents, most likely originating from the sound- and vibration- sensitive striolar receptors and irregular afferents, since it is these receptors and afferents which respond to ACS and BCV [
49]. We contend that these different effects of cochlear vs vestibular responses are due to the different synaptic mechanisms.
3.4. Transmitter
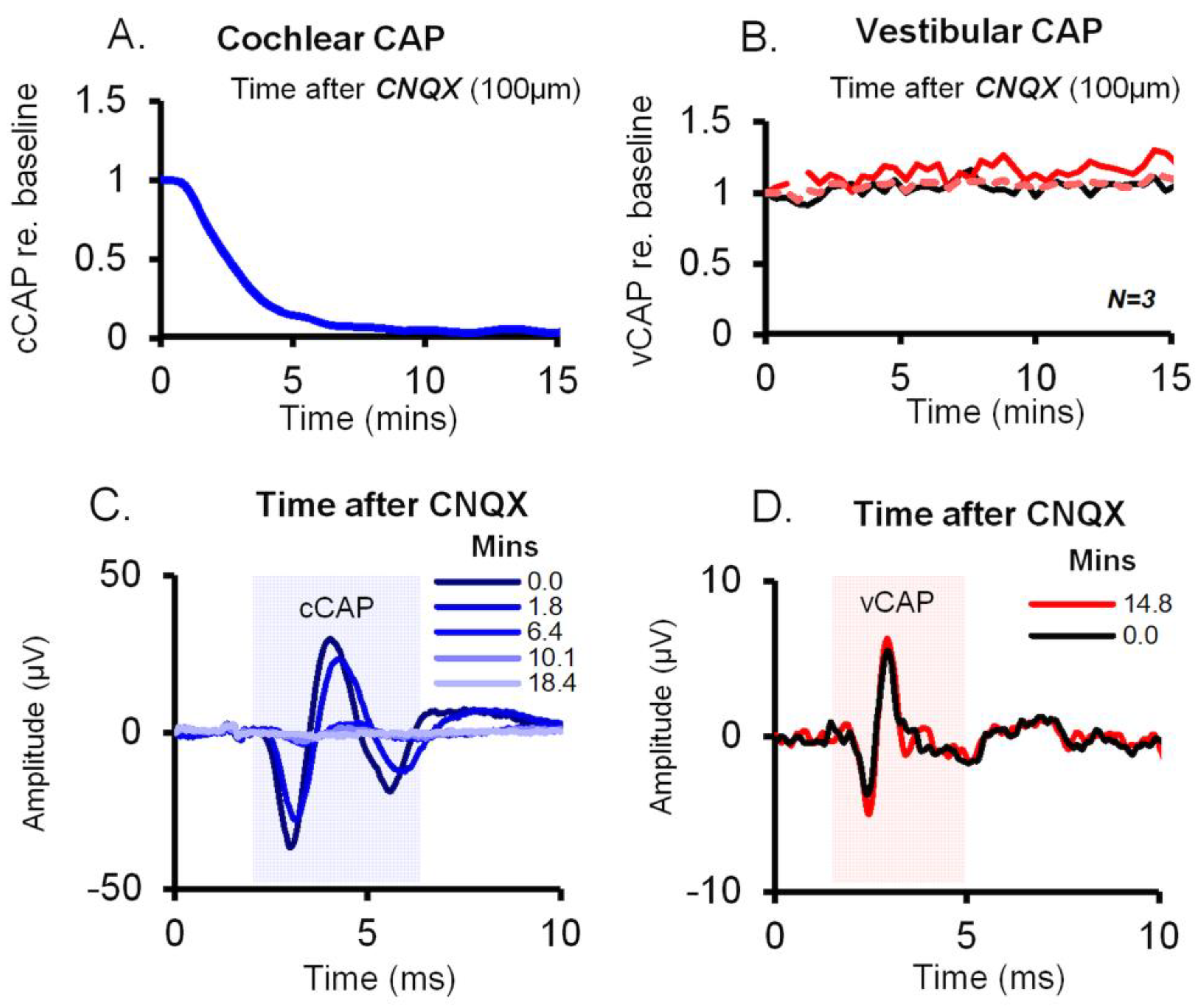
The neurotransmitter between receptors and primary afferents in both cochlear and vestibular system is the same – glutamate (see [
29] for a review). However blocking of glutamate transmission by the AMPA receptor antagonist, CNQX, has very different effects in the two systems :
in the cochlea, CNQX blocks cochlear afferent responses to sound - the cCAP is greatly reduced and almost completely disappears (Littman et al., 1989; Pastras et al., 2023a; Rutherford et al., 2021; Doi et al., 1990) (see
Figure 4) whereas
in the vestibular system CNQX does not block vestibular activation - CNQX has very little effect on the vCAP response to BCV or ACS transient stimuli (Pastras et al., 2017; Pastras et al., 2023) (
Figure 4) – compare A and B.
Contini et al have shown that synaptic transmission at the type I-calyx synapse is very unusual [
63,
64]. By means of simultaneous patch-clamp recordings of a type I receptor and its own calyx afferent, Contini et al have shown there are three factors governing transmission at this synapse:
glutamate release (quantal transmission)
the level of potassium in the synaptic cleft
resistive coupling between the type I receptor and the enveloping calyx (non-quantal transmission).
Addressing each factor :
a) Glutamate release is apparently similar to that in the cochlea – vestibular type I receptors contain ribbon synapses which release glutamate probably by relatively slow (sustained ) stimuli. This is quantal glutamate transmission. Activation of the post-synaptic neuron by glutamatergic transmission is relatively slow [
65].
b) Vestibular stimulation causes the deflection of the stereocilia and so opens the mechanoelectrical transduction (MET) channels on the stereocilia of type I receptors, so potassium enters the type I receptor from the potassium-rich endolymph and is released by the receptor into the narrow (femtolitre) synaptic cleft between the type I receptor and its enveloping calyx. Potassium levels in the narrow synaptic cleft act to modulate the membrane potential of the receptor and the calyx [
64,
66,
67].
c) Most importantly, the simultaneous patch clamp recording of a type I receptor and its enveloping calyx afferent showed a form of synaptic transmission which is not dependent on glutamate reception on the post-synaptic membrane – it is called resistive coupling. This is a form of ultrafast electrical coupling between the type I receptor and calyx afferent: channels on the type I receptor membrane and the facing membrane of its calyx afferent are both open near resting potential allowing ultrafast depolarization of the irregular primary afferent [
63,
68]. We contend that this ultrafast non-quantal resistive coupling explains the precision of vestibular phase-locking and the extremely short latency of some irregular primary afferents to ACS clicks (0.5ms) [
69] and is likely the key neural event in the generation of the vCAP and so the generation of VEMPs.
One consequence of blocking glutamate transmission by CNQX is that after CNQX blockade a stimulus will still open the MET channels on the stereocilia of the type I receptors and so will still cause receptor membrane depolarization (and so vestibular microphonics). The receptor membrane depolarization allows ultrafast resistive coupling between the type I receptor and its calyx to take place independently of the (relatively slow) quantal glutamatergic synaptic transmission [
65,
70]. This explains why the vestibular microphonic and the vCAP will be relatively unaffected by CNQX blockade of glutamatergic transmission.
3.5. Temporal Precision of Irregular Vestibular Afferents. 1. Latency
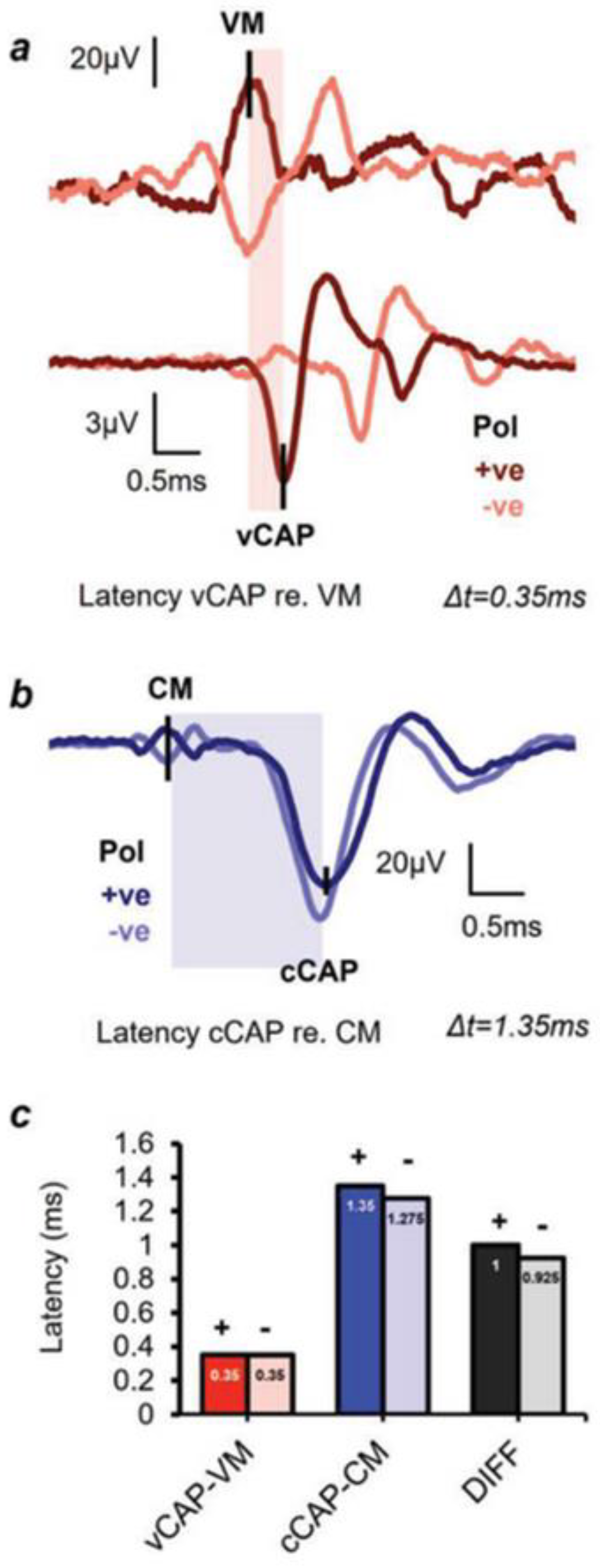
By using the peaks of the cochlear and vestibular microphonics as the benchmark for comparing the latencies of the vCAP and the cCAP, we have shown that the vCAP has a shorter latency than the cCAP (see
Figure 5). That result corresponds to the short latency of primary vestibular afferents to ACS clicks, with latencies of some primary vestibular afferents to air conducted clicks being as short as 0.5ms [
69].
(b) The latency difference between the onset of the CM and the onset of the cCAP is 1.35 ms. So the cochlear afferent response to clicks is around 1ms slower than the vestibular afferent response. (c) The averaged latencies and latency differences of the vCAP and cCAP (across 5 animals). Both vestibular and cochlear responses in a) and b) were evoked by an identical 0.6ms air-conducted sound (ACS) pulse, with a 0.3ms rise-fall time at a stimulus intensity 20dB above threshold.
3.6. Temporal Precision of Irregular Vestibular Afferents. 2. Phase-Locking
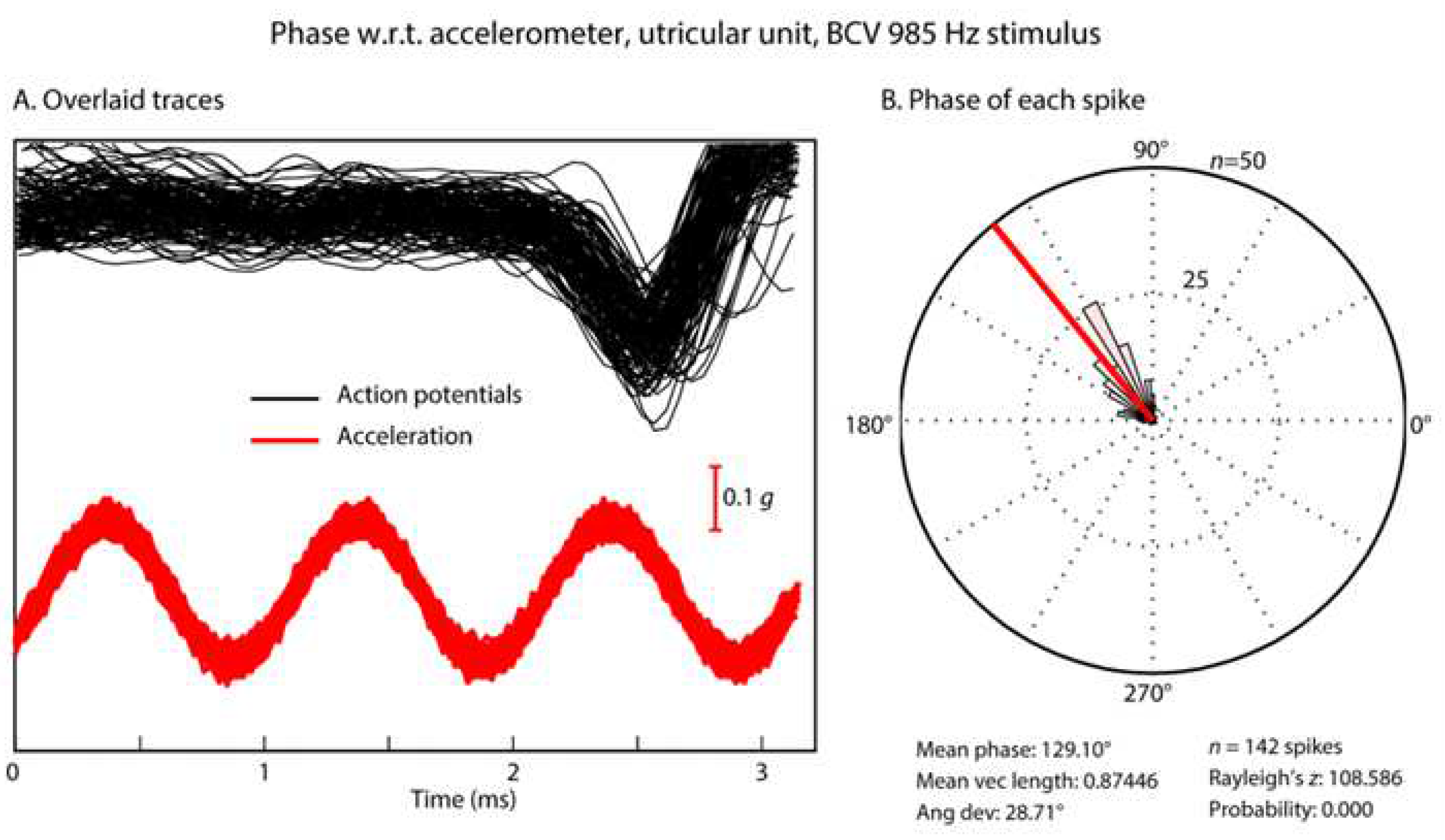
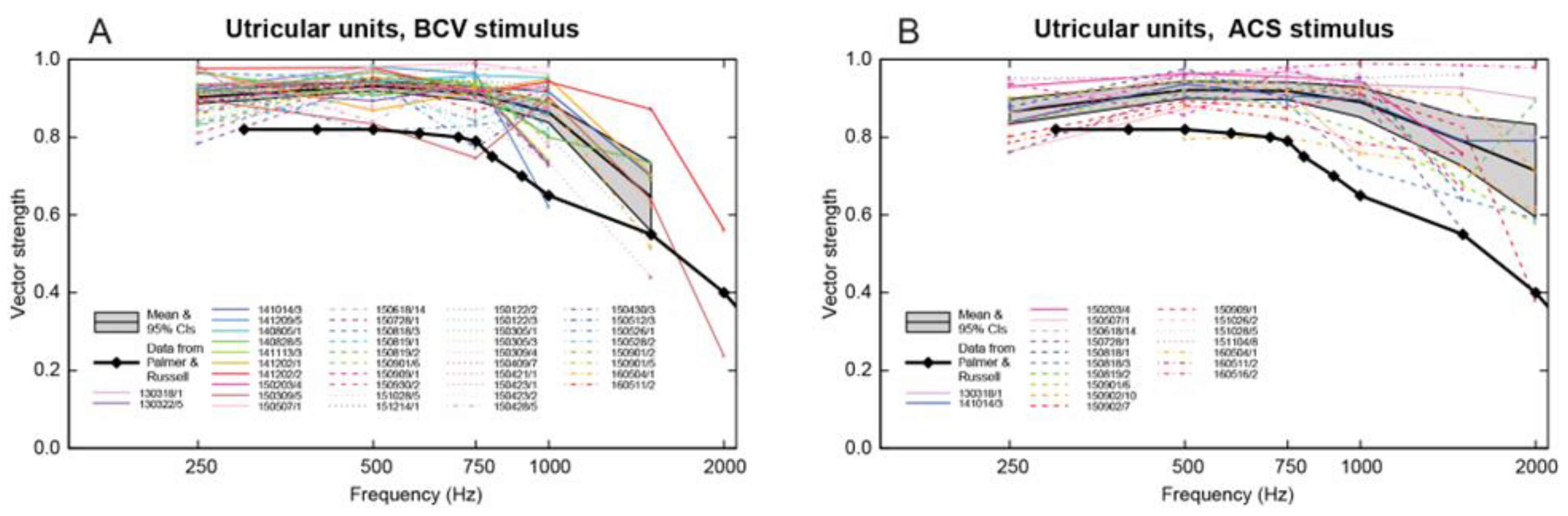
In response to sinusoidal stimuli, individual cochlear and vestibular afferents with irregular resting activity show precise phase-locking: action potentials are generated at a particular phase angle (or a narrow band of phase angles) of the stimulus with a high precision [
24,
35,
53,
71]. This does not mean that the neuron fires on every cycle of the stimulus, indeed it may miss many cycles, but the moment when the neuron fires is locked to a particular phase angle of the sinusoidal stimulus [
24] (see
Figure 6). Phase-locking in vestibular afferents is similar to that shown for cochlear afferent neurons, however the precision of phase-locking in irregular primary vestibular afferents to sound and vibration, as measured by vector strength, is as least as good (and apparently superior to) to that reported for cochlear afferents [
37,
53] (see
Figure 7).
Further evidence of the temporal precision of irregular vestibular afferents comes from examining the frequency limits of phase locking (
Figure 7). The upper frequency of phase-locking and average vector strength of vestibular primary afferents in the anaesthetized guinea pig appears to be higher (3000Hz) than reported for cochlear primary afferents by [
37] (
Figure 7). We suggest that the precise vestibular phase-locking at high frequencies is most likely primarily due to resistive coupling, whereas recent evidence has shown that for cochlear afferents the high precision of phase-locking is most likely due to multiple ribbon synapses [
24,
34,
72]. Tight vestibular phase-locking may be due to a different mechanisms in the cochlear and vestibular systems.
4. Applications of Physiological Results to Clinical Vestibular (VEMP) Testing
In light of the above evidence we consider how the cochlear and vestibular systems respond to various stimulus manipulations of relevance for clinical vestibular testing. In some experiments these manipulations are carried out using the kind of procedures outlined above to eliminate or minimize cochlear contributions to the measured vestibular response.
4.1. Effect of Rise-Time
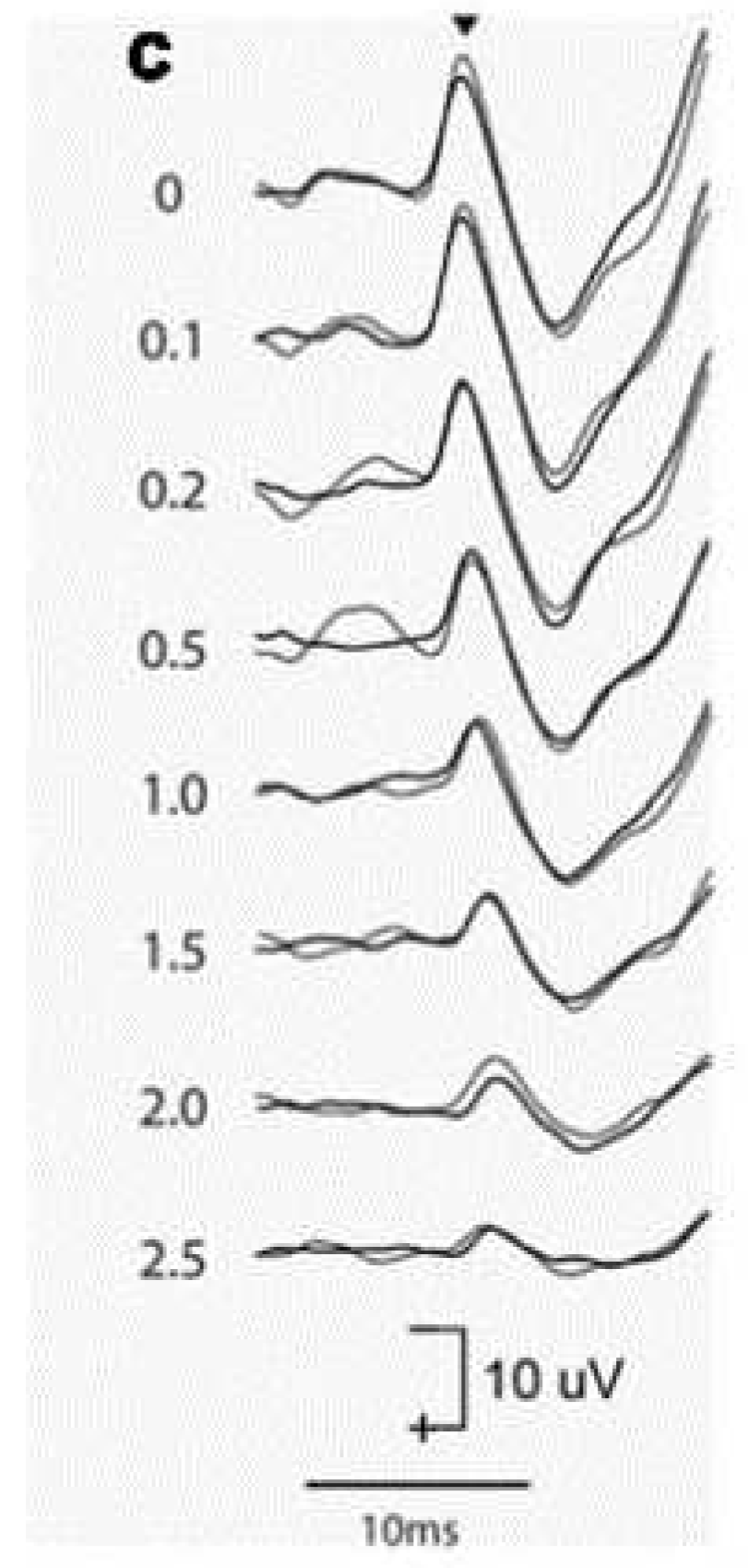
The importance of stimulus onset in VEMP testing of human subjects is shown by a study which systematically decreased the total duration of a 500Hz tone burst stimulus from 10 ms to 2ms with the result that the oVEMP n10 for a 2ms stimulus had the same amplitude as the response amplitude to a stimulus of 10ms duration [
73]. We have confirmed that result which shows that it is the
onset of the stimulus which is the key parameter in the generation of VEMPs.
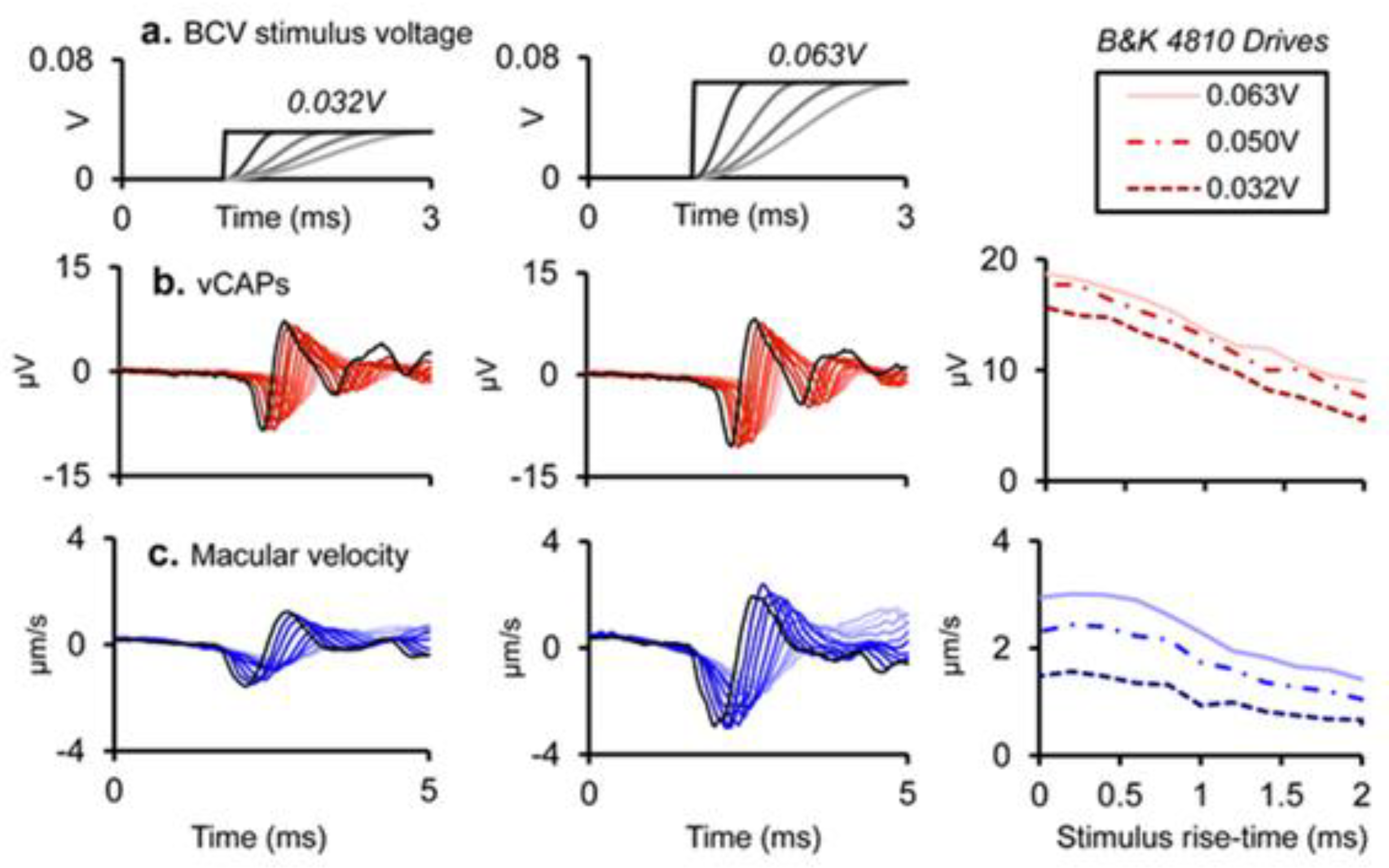
Systematic variation of the rise-time of short 500Hz BCV tone bursts shows the importance of stimulus rise-time in generating VEMPs in human subjects: increasing the rise-time of 7ms 500Hz tone bursts decreased VEMP amplitude (Burgess et al 2013), with the largest VEMPs being to stimuli with zero ms rise-time (see
Figure 8).
These clinical data correspond to the results from primary vestibular afferents. We have shown that as rise-time of a click is increased the amplitude of the vCAP decreased. This is expected because increasing rise-time will decrease the synchronisation of action potentials across neurons [
15] (see
Figure 8). The optimum stimulus for maximum vCAPs (and so large VEMPs) is one with zero rise-time [
6,
14] (see
Figure 9).
Evidence from single neuron recordings shows the excellent temporal precision of irregular vestibular afferents, which respond with short latencies to transient stimuli [
75] and phase-lock with small variability to high frequencies [
53]. However some clinical studies continue to use long (2ms or even longer) rise-times for testing VEMPs. Such stimuli generate modest VEMPs, but they are much less than optimum stimuli. The use of such long rise-times for VEMP testing is an inappropriate carryover from audiological testing of perceptual thresholds to pure tone stimuli where it is necessary to use a long rise-time in order to eliminate audible clicks at stimulus onset. However in testing VEMPs the situation is
exactly opposite to that in audiometric testing of pure tone thresholds: for VEMPs the most effective VEMP stimulus is one with a very short rise-time which synchronises vestibular action potentials in primary afferent neurons. Unfortunately some audiometers do not allow rise-times less than 2ms, and some audiologists do not realize how important it is to minimise the rise-time for VEMP testing as opposed to auditory threshold testing.
4.2. Masking
The amplitude of the cCAP to ACS or BCV is reduced by masking – both simultaneous broadband masking or forward masking [
59] whereas the amplitude of the vCAP is minimally affected by masking. On the other hand we have confirmed that in the guinea pig, vCAPs to ACS or BCV click stimuli are minimally affected by simultaneous broadband noise or forward masking by noise stimuli at levels which effectively mask cCAPs [
20].
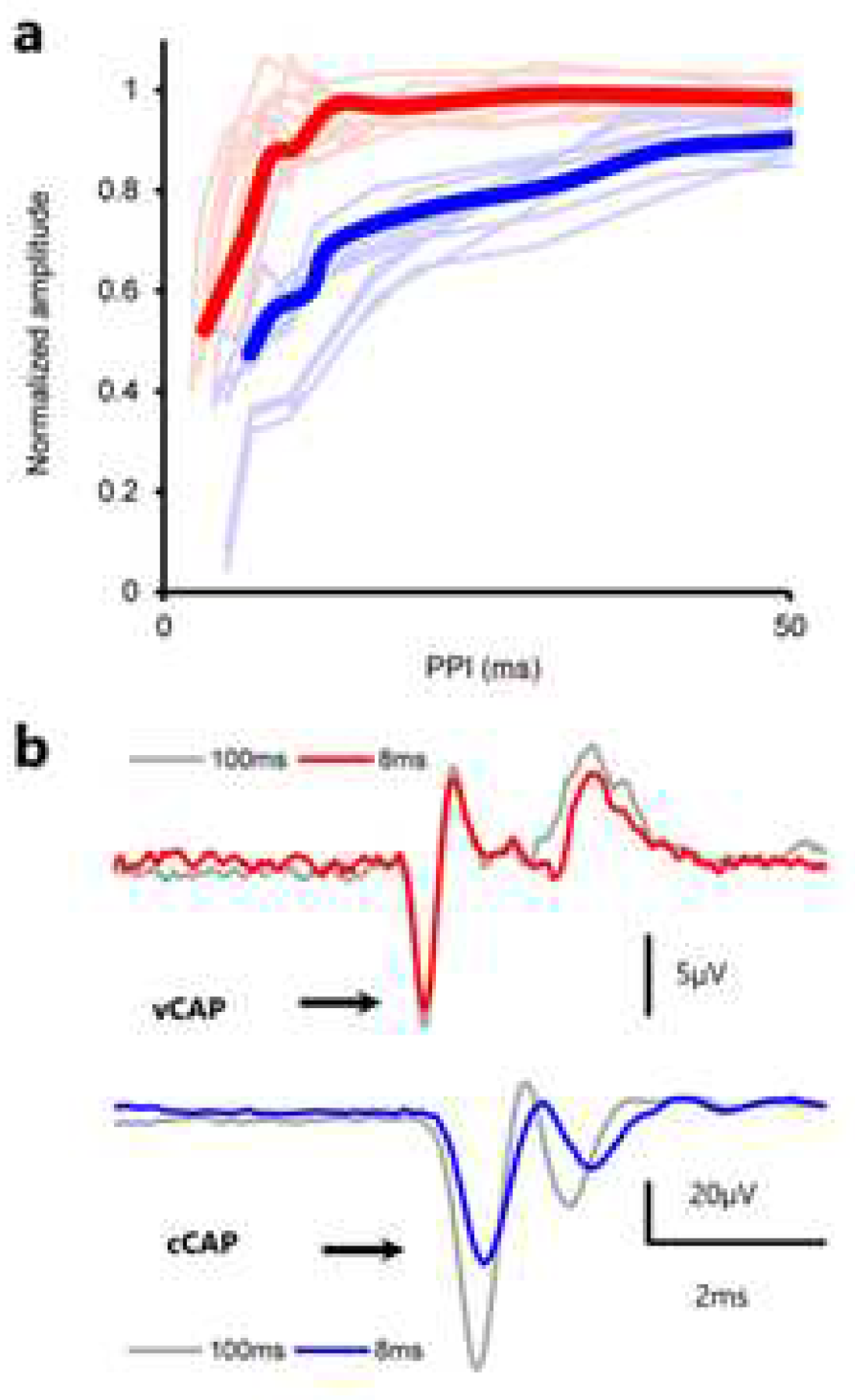
4.3. Paired Pulse Stimuli
Reducing the interval between successive pulses reduces the cCAP to the second pulse, with reductions in cCAP amplitude beginning at an interstimulus interval of about 50ms, and reaching a 50% response suppression at an interstimulus interval of ~10ms [
76,
77](se
Figure 10). However in the same paradigm the vCAP to the second pulse is minimally reduced at short interstimulus intervals [
59] (see
Figure 10).
Vestibular afferents are resistant to masking, whereas with cochlea afferents it is relatively easy to mask their response. We contend that the differences in synaptic transmission, cochlea vs vestibular, are responsible for this differential effect of masking stimuli.
5. Other Stimuli
For both auditory and cochlear systems there are other components and variants of these labyrinthine electrophysiological potentials to stimuli other than transients (see [
16], but these are beyond the scope of this review , e.g.
6. Conclusion
One outcome of measuring vCAPs is that they allow us to evaluate which particular stimulus parameters, at the level of otolithic receptors, are likely optimum for clinical testing of VEMPs. So for example the results have confirmed the importance of short rise-times of optimizing VEMPs stimuli. A simple way of reducing cochlear contributions to vestibular responses is to use simultaneous masking noise as confirmed by the physiological results here. Another preliminary result is that the CE chirp stimulus (500-4000Hz sweeps (also called narrow band chirps)) are particularly clinically effective stimuli for oVEMPs [
79,
80]. Our recordings of the vCAP to chirp stimuli confirm that the chirp stimulus with zero rise-time is particularly effective in generating vCAPs at the level of primary afferents in comparison to simple clicks [
12].
7. Summary
1. It is otolithic receptors and their afferents with irregular resting discharge originating from the striola which are activated by sound and vibration (for reviews see [
4,
49,
54] and so are responsible for the vCAP.
2. Vestibular receptors and afferents can function independently of the cochlea, both in humans and guinea pigs.
3. It is possible to differentiate between the vestibular and cochlear responses to transient stimuli. Such differentiation provides further support of the basis of present vestibular testing using to sound and vibration stimuli and may allow new clinical tests of dynamic vestibular function. For future studies of putative vestibular responses to clinically realistic transient stimuli, we suggest that it is advisable to have continuous broadband masking present simultaneously to minimize cochlear contributions to the response [
27].
4. In clinical testing of VEMPs the situation is exactly opposite to that in audiometric testing of pure tone thresholds: the effective VEMP stimulus is one with an abrupt rise-time which synchronises vestibular action potentials in primary afferent neurons. In contrast, for audiometric testing of thresholds, a long rise-time is mandatory. Unfortunately some audiometers do not allow rise-times less than 2ms and some audiologists do not realize how important it is to minimise the rise-time for VEMP testing as opposed to auditory threshold testing. Our recordings of the vCAP to chirp stimuli confirm that the chirp stimulus is particularly effective in generating vCAPs at the level of primary afferents in comparison to simple clicks.
Dedication. This paper is a tribute to Wally Grant, a great friend, a brilliant scientist and engineer whose insights and contributions advanced the understanding of so many areas of vestibular function.
Author Contributions
IC wrote the paper. CJP reviewed and edited the paper. Both authors have read and agreed to the published version of the manuscript.
Funding
his work was supported by a Macquarie University Research Fellowship, MQRF0001126.
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable
Data Availability Statement
Not applicable
Acknowledgments
The work reported here has also been supported by Garnett Passe and Rodney Williams Memorial Foundation, and we are very grateful for their continued support of this research over many years.
Conflicts of Interest
The authors have no conflicts of interest.
Abbreviations
| CAP |
compound action potential |
| vCAP |
vestibular compound action potential |
| cCAP |
cochlear compound action potential |
| CM |
cochlear microphonic |
| VM |
vestibular microphonic |
| ANN |
auditory nerve neurophonic |
| VNN |
vestibular nerve neurophonic |
| ACS |
air conducted sound |
| BCV |
bone conducted vibration |
| SCD |
semicircular canal dehiscence |
| VEMP |
vestibular evoked myogenic potential |
| VsEP |
vestibular evoked potential |
| oVEMP |
ocular vestibular evoked myogenic potential |
| CNQX |
6-cyano-7-nitroquinoxaline-2,3-dione |
| MET |
mechanoelectrical transduction |
| KCl |
potassium chloride |
| PPI |
paired pulse interval |
| LDV |
laser doppler vibrometer |
References
- Curthoys, I.S.; Grant, J.W.; Burgess, A.M.; Pastras, C.J.; Brown, D.J.; Manzari, L. Otolithic Receptor Mechanisms for Vestibular-Evoked Myogenic Potentials: A Review. Frontiers in Neurology 2018, 9, 366. [Google Scholar] [CrossRef]
- Rosengren, S.M.; Colebatch, J.G. The contributions of vestibular evoked myogenic potentials and acoustic vestibular stimulation to our understanding of the vestibular system. Frontiers in Neurology 2018, 9, 481. [Google Scholar] [CrossRef] [PubMed]
- Curthoys, I.S. The new vestibular stimuli: sound and vibration-anatomical, physiological and clinical evidence. Exp. Brain Res. 2017, 235, 957–972. [Google Scholar] [CrossRef]
- Curthoys, I.S.; Vulovic, V.; Burgess, A.M.; Manzari, L.; Sokolic, L.; Pogson, J.; Robins, M.; Mezey, L.E.; Goonetilleke, S.; Cornell, E.D.; et al. Neural basis of new clinical vestibular tests: otolithic neural responses to sound and vibration. Clin. Exp. Pharmacol. Physiol. 2014, 41, 371–380. [Google Scholar] [CrossRef]
- Curthoys, I.S.; Grant, J.W.; Pastras, C.J.; Brown, D.J.; Burgess, A.M.; Brichta, A.M.; Lim, R. A review of mechanical and synaptic processes in otolith transduction of sound and vibration for clinical VEMP testing. Journal of Neurophysiology (Bethesda) 2019, 122, 259–276. [Google Scholar] [CrossRef]
- Burgess, A.M.; Mezey, L.E.; Manzari, L.; Macdougall, H.G.; McGarvie, L.A.; Curthoys, I.S. Effect of Stimulus Rise-Time on the Ocular Vestibular-Evoked Myogenic Potential to Bone-Conducted Vibration. Ear Hear. 2013, 34, 799–805. [Google Scholar] [CrossRef]
- Curthoys, I.S. A critical review of the neurophysiological evidence underlying clinical vestibular testing using sound, vibration and galvanic stimuli. Clin. Neurophysiol. 2010, 121, 132–144. [Google Scholar] [CrossRef]
- Colebatch, J.G.; Rosengren, S.M. Investigating short latency subcortical vestibular projections in humans: what have we learned? J. Neurophysiol. 2019, 122, 2000–2015. [Google Scholar] [CrossRef]
- Rosengren, S.M.; Colebatch, J.G.; Young, A.S.; Govender, S.; Welgampola, M.S. Vestibular evoked myogenic potentials in practice: Methods, pitfalls and clinical applications. Clinical neurophysiology practice 2019, 4, 47–68. [Google Scholar] [CrossRef]
- Manzari, L.; Burgess, A.M.; McGarvie, L.A.; Curthoys, I.S. Ocular and cervical vestibular evoked myogenic potentials to 500 Hz fz bone-conducted vibration in superior semicircular canal dehiscence. Ear Hear. 2012, 33, 508–520. [Google Scholar] [CrossRef]
- Oh, S.Y.; Kim, H.J.; Kim, J.S. Vestibular-evoked myogenic potentials in central vestibular disorders. J. Neurol. 2016, 263, 210–220. [Google Scholar] [CrossRef]
- Pastras, C.J.; Curthoys, I.S.; Rabbitt, R.D.; Brown, D.J. Using macular velocity measurements to relate parameters of bone conduction to vestibular compound action potential responses. Scientific Reports 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Pastras, C.J.; Gholami, N.; Jennings, S.; Zhu, H.; Zhou, W.; Brown, D.J.; Curthoys, I.S.; Rabbitt, R.D. A mathematical model for mechanical activation and compound action potential generation by the utricle in response to sound and vibration. Frontiers in Neurology 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Pastras, C.J.; S., C.I.; Rabbitt, R.D.; Brown, D.J. Vestibular compound action potentials and macular velocity evoked by sound and vibration in the guinea pig. J. Neurosci. 2023, in press. [Google Scholar]
- Goldstein, M.H.; Kiang, N.Y.S. Synchrony of neural activity in electric responses evoked by transient acoustic stimuli. J. Acoust. Soc. Am. 1958, 30, 107–114. [Google Scholar] [CrossRef]
- Brown, D.J.; Pastras, C.J.; Curthoys, I.S. Electrophysiological measurements of peripheral vestibular function-a review of electrovestibulography. Frontiers in Systems Neuroscience 2017, 11. [Google Scholar] [CrossRef]
- Pastras, C.J.; Stefani, S.P.; Camp, A.J.; Curthoys, I.S.; Brown, D.J. Summating potentials from the utricular macula of anaesthetized guinea pigs. Hear. Res. 2021, 406. [Google Scholar] [CrossRef]
- Bohmer, A.; Hoffman, L.F.; Honrubia, V. CHARACTERIZATION OF VESTIBULAR POTENTIALS-EVOKED BY LINEAR ACCELERATION PULSES IN THE CHINCHILLA. Am. J. Otol. 1995, 16, 498–504. [Google Scholar]
- Jones, T.A. Vestibular short latency responses to pulsed linear acceleration in unanesthetized animals. Electroencephalogr. Clin. Neurophysiol. 1992, 82, 377–386. [Google Scholar] [CrossRef]
- Jones, T.A.; Jones, S.M. Short latency compound action potentials from mammalian gravity receptor organs. Hear. Res. 1999, 136, 75–85. [Google Scholar] [CrossRef]
- Nazareth, A.M.; Jones, T.A. Central and peripheral components of short latency vestibular responses in the chicken. Journal of Vestibular Research-Equilibrium & Orientation 1998, 8, 233–252. [Google Scholar]
- Jones, T.A.; Pedersen, T.L. Short latency vestibular responses to pulsed linear acceleration. Am. J. Otolaryngol. 1989, 10, 327–335. [Google Scholar] [CrossRef]
- Elidan, J.; Langhofer, L.; Honrubia, V. Recording of short-latency vestibular evoked-potentials induced by acceleration impulses in experimental-animals - current status of the method and its applications. Electroencephalogr. Clin. Neurophysiol. 1987, 68, 58–69. [Google Scholar] [CrossRef]
- Rutherford, M.A.; von Gersdorff, H.; Goutman, J.D. Encoding sound in the cochlea: from receptor potential to afferent discharge. Journal of Physiology-London 2021, 599, 2527–2557. [Google Scholar] [CrossRef] [PubMed]
- Plontke, S.K.; Rahne, T.; Curthoys, I.S.; Håkansson, B.; Fröhlich, L. A case series shows independent vestibular labyrinthine function after major surgical trauma to the human cochlea. Commun Med (Lond) 2021, 1, 37. [Google Scholar] [CrossRef]
- Jones, T.A.; Jones, S.M.; Vijayakumar, S.; Brugeaud, A.; Bothwell, M.; Chabbert, C. The adequate stimulus for mammalian linear vestibular evoked potentials (VsEPs). Hear. Res. 2011, 280, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.M.; Robertson, N.G.; Given, S.; Giersch, A.B.S.; Liberman, M.C.; Morton, C.C. Hearing and vestibular deficits in the Coch(-/-) null mouse model: Comparison to the Coch(G88E/G88E) mouse and to DFNA9 hearing and balance disorder. Hear. Res. 2011, 272, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Hudspeth, A.J. Mechanical Frequency Tuning by Sensory Hair Cells, the Receptors and Amplifiers of the Inner Ear. In Annual Review of Condensed Matter Physics, Vol 12, 2021, Mackenzie, A.P., Marchetti, M.C., Eds.; Annual Review of Condensed Matter Physics; 2021; Volume 12, pp. 29-49.
- Caprara, G.A.; Peng, A.W. Mechanotransduction in mammalian sensory hair cells. Molecular and Cellular Neuroscience 2022, 120. [Google Scholar] [CrossRef] [PubMed]
- Littman, T.; Bobbin, R.P.; Fallon, M.; Puel, J.L. THE QUINOXALINEDIONES DNQX, CNQX AND 2 RELATED CONGENERS SUPPRESS HAIR CELL TO AUDITORY NERVE TRANSMISSION. Hear. Res. 1989, 40, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Mori, N.; Matsunaga, T.; Tsumoto, T. BLOCKADE OF SYNAPTIC TRANSMISSION FROM HAIR-CELLS TO AUDITORY AFFERENT NERVES BY 6-CYANO-2,3-DIHYDROXY-7-NITROQUINOXALINE, A SELECTIVE NON-NMDA RECEPTOR ANTAGONIST. Eur. Arch. Otorhinolaryngol. 1990, 248, 25–30. [Google Scholar] [CrossRef]
- Eatock, R.A.; Songer, J.E. Vestibular hair cells and afferents: two channels for head motion signals. Annu. Rev. Neurosci. 2011, 34, 501–534. [Google Scholar] [CrossRef]
- Heil, P.; Peterson, A.J. Spike timing in auditory-nerve fibers during spontaneous activity and phase locking. Synapse 2017, 71, 5–36. [Google Scholar] [CrossRef]
- Li, G.L.; Cho, S.; von Gersdorff, H. Phase-Locking Precision Is Enhanced by Multiquantal Release at an Auditory Hair Cell Ribbon Synapse. Neuron 2014, 83, 1404–1417. [Google Scholar] [CrossRef]
- Rose, J.E.; Brugge, J.F.; Anderson, D.J.; Hind, J.E. Phase-locked response to low-frequency tones in single auditory nerve fibers of squirrel monkey. J. Neurophysiol. 1967, 30, 769. [Google Scholar] [CrossRef]
- Fettiplace, R. Hair Cell Transduction, Tuning, and Synaptic Transmission in the Mammalian Cochlea. Comprehensive Physiology 2017, 7, 1197–1227. [Google Scholar] [CrossRef]
- Palmer, A.R.; Russell, I.J. Phase-locking in the cochlear nerve of the guinea-pig and its relation to the receptor potential of inner hair-cells. Hear. Res. 1986, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.M. Afferent diversity and the organization of central vestibular pathways. Exp. Brain Res. 2000, 130, 277–297. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Xue, J.; Peterson, E.H. Architecture of the mouse utricle: macular organization and hair bundle heights. J. Neurophysiol. 2008, 99, 718–733. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.H.; Cotton, J.R.; Peterson, E.H.; Grant, W. Mechanical properties and consequences of stereocilia and extracellular links in vestibular hair bundles. Biophys. J. 2006, 90, 2786–2795. [Google Scholar] [CrossRef]
- Goldberg, J.M.; Desmadryl, G.; Baird, R.A.; Fernandez, C. The vestibular nerve of the chinchilla. V. Relation between afferent discharge properties and peripheral innervation patterns in the utricular macula. J. Neurophysiol. 1990, 63, 791–804. [Google Scholar] [CrossRef]
- Watanuki, K.; Meyer zum Gottesberge, A. Morphological observations of sensory epithelium of macula sacculi and utriculi in guinea pig. Archiv Fur Klinische Und Experimentelle Ohren-Nasen-Und Kehlkopfheilkunde 1971, 200, 136. [Google Scholar] [CrossRef] [PubMed]
- Watanuki, K.; Schuknecht, H.F. Morphological-study of human vestibular sensory epithelia. Archives of Otolaryngology-Head & Neck Surgery 1976, 102, 583–588. [Google Scholar]
- Eatock, R.A. Adaptation in hair cells. Annu. Rev. Neurosci. 2000, 23, 285–314. [Google Scholar] [CrossRef] [PubMed]
- Lysakowski, A. Synaptic organization of the crista ampullaris in vertebrates. In New Directions in Vestibular Research, Highstein, S.M., Cohen, B., ButtnerEnnever, J.A., Eds.; Annals of the New York Academy of Sciences; 1996; Volume 781, pp. 164-182.
- Lysakowski, A.; Goldberg, J.M. A regional ultrastructural analysis of the cellular and synaptic architecture in the chinchilla cristae ampullares. J. Comp. Neurol. 1997, 389, 419–443. [Google Scholar] [CrossRef]
- Hudspeth, A.J. Micromechanics of Hearing. In Mechanics of Hearing: Protein to Perception, Karavitaki, K.D., Corey, D.P., Eds.; AIP Conference Proceedings; 2015; Volume 1703.
- Pastras, C.J.; Curthoys, I.S.; Brown, D.J. In vivo recording of the vestibular microphonic in mammals. Hear. Res. 2017, 354, 38–47. [Google Scholar] [CrossRef]
- Curthoys, I.S.; Vulovic, V.; Sokolic, L.; Pogson, J.; Burgess, A.M. Irregular primary otolith afferents from the guinea pig utricular and saccular maculae respond to both bone conducted vibration and to air conducted sound. Brain Res. Bull. 2012, 89, 16–21. [Google Scholar] [CrossRef]
- Zhu, H.; Tang, X.; Wei, W.; Mustain, W.; Xu, Y.; Zhou, W. Click-evoked responses in vestibular afferents in rats. J. Neurophysiol. 2011, 106, 754–763. [Google Scholar] [CrossRef]
- Goldberg, J.M.; Desmadryl, G.; Baird, R.A.; Fernandez, C. The vestibular nerve of the chinchilla. IV. Discharge properties of utricular afferents. J. Neurophysiol. 1990, 63, 781–790. [Google Scholar] [CrossRef]
- Fernandez, C.; Baird, R.A.; Goldberg, J.M. The vestibular nerve of the chinchilla. I. Peripheral innervation patterns in the horizontal and superior semicircular canals. J. Neurophysiol. 1988, 60, 167–181. [Google Scholar] [CrossRef]
- Curthoys, I.S.; Burgess, A.M.; Goonetilleke, S.C. Phase-locking of irregular guinea pig primary vestibular afferents to high frequency (> 250 Hz) sound and vibration. Hear. Res. 2019, 373, 59–70. [Google Scholar] [CrossRef]
- Curthoys, I.S.; Vulovic, V.; Burgess, A.M.; Sokolic, L.; Goonetilleke, S.C. The response of guinea pig primary utricular and saccular irregular neurons to bone-conducted vibration (BCV) and air-conducted, sound (ACS). Hear. Res. 2016, 331, 131–143. [Google Scholar] [CrossRef]
- Goldberg, J.M.; Lysakowski, A.; Fernandez, C. Structure and function of vestibular nerve fibers in the chinchilla and squirrel monkey. Ann. N. Y. Acad. Sci. 1992, 656, 92–107. [Google Scholar] [CrossRef]
- Mukhopadhyay, M.; Pangrsic, T. Synaptic transmission at the vestibular hair cells of amniotes. Molecular and Cellular Neuroscience 2022, 121. [Google Scholar] [CrossRef] [PubMed]
- Watanuki, K.; Meyer zum Gottesberge, A. Morphological study of sensory epithelium of vestibular organs. Tohoku J. Exp. Med. 1971, 104, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Pastras, C.J.; Curthoys, I.S.; Brown, D.J. Dynamic response to sound and vibration of the guinea pig utricular macula, measured in vivo using Laser Doppler Vibrometry. Hear. Res. 2018, 370, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Pastras, C.J.; Curthoys, I.S.; Asadnia, M.; McAlpine, D.; Rabbitt, R.D.; Broen, D.J. Evidence that ultrafast non-quantal transmission underlies synchronized vestibular action potential generation. Journal of Neuroscience in press 2023. [Google Scholar] [CrossRef] [PubMed]
- Tasaki, I. Nerve impulses in individual auditory nerve fibers of guinea pig. J. Neurophysiol. 1954, 17, 97–122. [Google Scholar] [CrossRef] [PubMed]
- Rosowski, J.J.; Songer, J.E.; Nakajima, H.H.; Brinsko, K.M.; Merchant, S.N. Clinical, experimental, and theoretical investigations of the effect of superior semicircular canal dehiscence on hearing mechanisms. Otol. Neurotol. 2004, 25, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Dlugaiczyk, J.; Burgess, A.M.; Curthoys, I.S. Activation of Guinea Pig Irregular Semicircular Canal Afferents by 100 Hz Vibration: Clinical Implications for Vibration-induced Nystagmus and Vestibular-evoked Myogenic Potentials. Otol. Neurotol. 2020, 41, E961–E970. [Google Scholar] [CrossRef]
- Contini, D.; Holstein, G.R.; Art, J.J. Synaptic cleft microenvironment influences potassium permeation and synaptic transmission in hair cells surrounded by calyx afferents in the turtle. Journal of Physiology-London 2020, 598, 853–889. [Google Scholar] [CrossRef]
- Contini, D.; Holstein, G.R.; Art, J.J. Simultaneous Dual Recordings From Vestibular Hair Cells and Their Calyx Afferents Demonstrate Multiple Modes of Transmission at These Specialized Endings. Frontiers in Neurology 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Songer, J.E.; Eatock, R.A. Tuning and Timing in Mammalian Type I Hair Cells and Calyceal Synapses. J. Neurosci. 2013, 33, 3706–3724. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Kindig, A.E.; Donne, S.W.; Callister, R.J.; Brichta, A.M. Potassium accumulation between type I hair cells and calyx terminals in mouse crista. Exp. Brain Res. 2011, 210, 607–621. [Google Scholar] [CrossRef]
- Contini, D.; Price, S.D.; Art, J.J. Accumulation of K+ in the synaptic cleft modulates activity by influencing both vestibular hair cell and calyx afferent in the turtle. Journal of Physiology-London 2017, 595, 777–803. [Google Scholar] [CrossRef]
- Govindaraju, A.C.; Quraishi, I.H.; Lysakowski, A.; Eatock, R.A.; Raphael, R.M. Nonquantal transmission at the vestibular hair cell-calyx synapse: KLV currents modulate fast electrical and slow K+ potentials. Proc. Natl. Acad. Sci. U. S. A. 2023, 120. [Google Scholar] [CrossRef]
- Murofushi, T.; Curthoys, I.S.; Topple, A.N.; Colebatch, J.G.; Halmagyi, G.M. Responses of guinea pig primary vestibular neurons to clicks. Exp. Brain Res. 1995, 103, 174–178. [Google Scholar] [CrossRef]
- Spaiardi, P.; Tavazzani, E.; Manca, M.; Russo, G.; Prigioni, I.; Biella, G.; Giunta, R.; Johnson, S.L.; Marcotti, W.; Masetto, S. K(+)Accumulation and Clearance in the Calyx Synaptic Cleft of Type I Mouse Vestibular Hair Cells. Neuroscience 2020, 426, 69–86. [Google Scholar] [CrossRef]
- Young, E.D.; Fernandez, C.; Goldberg, J.M. Responses of squirrel monkey vestibular neurons to audio-frequency sound and head vibration. Acta Otolaryngol 1977, 84, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.J.; Heil, P. Phase Locking of Auditory Nerve Fibers: The Role of Lowpass Filtering by Hair Cells. J. Neurosci. 2020, 40, 4700–4714. [Google Scholar] [CrossRef]
- Lim, L.J.Z.; Dennis, D.L.; Govender, S.; Colebatch, J.G. Differential effects of duration for ocular and cervical vestibular evoked myogenic potentials evoked by air- and bone-conducted stimuli. Exp. Brain Res. 2013, 224, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Curthoys, I.S.; Grant, J.W.; Pastras, C.J.; Frohlich, L.; Brown, D.J. Similarities and differences between vestibular and cochlear systems – a review of clinical and physiological evidence. Front. Neurosci. 2021. [Google Scholar] [CrossRef]
- Curthoys, I.S.; Kim, J.; McPhedran, S.K.; Camp, A.J. Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Exp. Brain Res. 2006, 175, 256–267. [Google Scholar] [CrossRef]
- Parham, K.; Zhao, H.B.; Kim, D.O. Responses of auditory nerve fibers of the unanesthetized decerebrate cat to click pairs as simulated echoes. J. Neurophysiol. 1996, 76, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Wickesberg, R.E.; Stevens, H.E. Responses of auditory nerve fibers to trains of clicks. J. Acoust. Soc. Am. 1998, 103, 1990–1999. [Google Scholar] [CrossRef] [PubMed]
- Henry, K.R. AUDITORY-NERVE NEUROPHONIC RECORDED FROM THE ROUND WINDOW OF THE MONGOLIAN GERBIL. Hear. Res. 1995, 90, 176–184. [Google Scholar] [CrossRef]
- Walther, L.E.; Cebulla, M. Band limited chirp stimulation in vestibular evoked myogenic potentials. Eur. Arch. Otorhinolaryngol. 2016, 273, 2983–2991. [Google Scholar] [CrossRef] [PubMed]
- Reddy, T.M.; Heinze, B.; Biagio-de Jager, L.; Maes, L. Cervical and ocular vestibular evoked myogenic potential: A comparison of narrowband chirp, broadband chirp, tone burst and click stimulation. Int. J. Audiol. 2023, 62, 579–586. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).