Submitted:
09 October 2023
Posted:
09 October 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Methodology
2.1. Data Collection
2.2. Specimen Sampling
2.3. Testing and Identification
2.4. Determining Hospital Associated Transmission
2.5. Statistical Analysis
3. Results
3.1. Prevalence: Age
3.2. Prevalence: Patient Characteristics
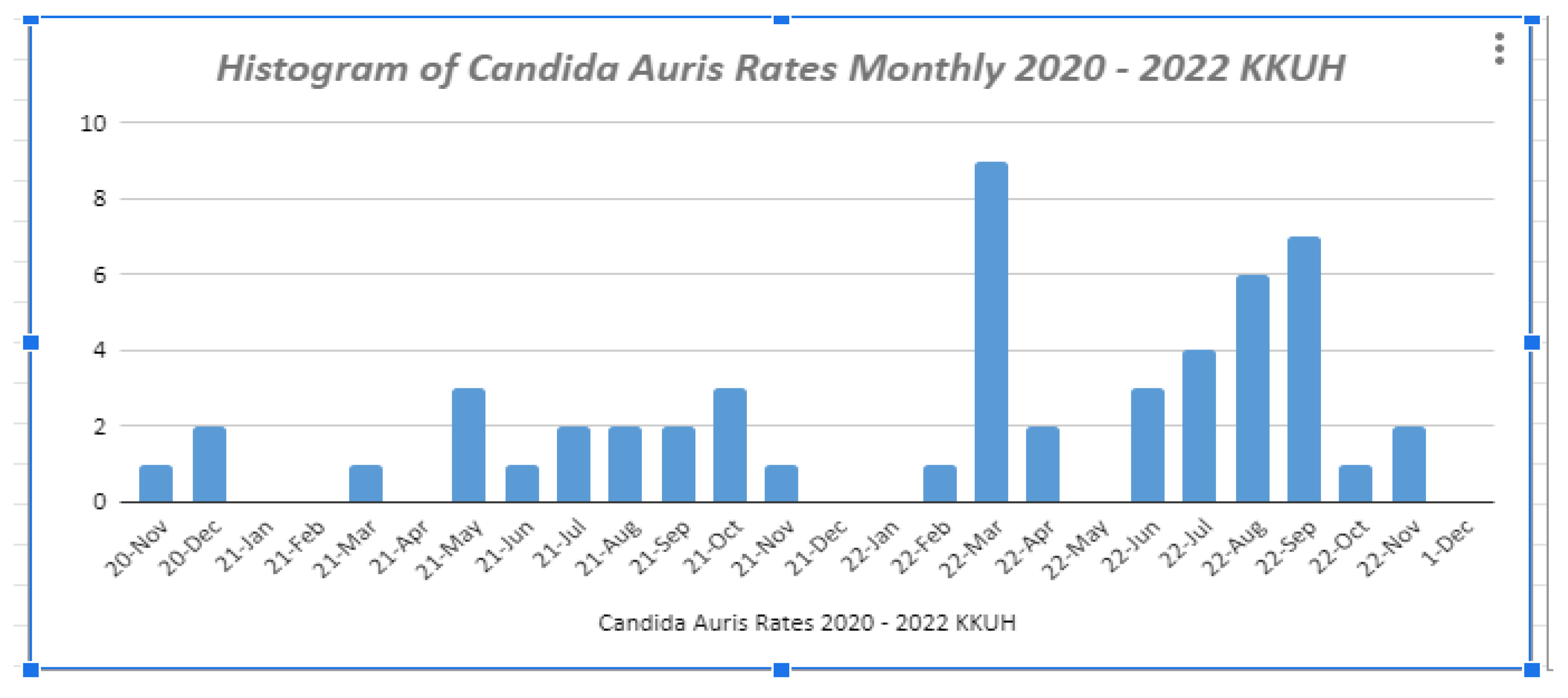
3.3. Prevalence: Hospital versus Community-Acquired C. auris
3.4. Prevalence: Infection versus Colonization
3.5. Prevalence: Clinical versus Surveillance samples
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- S. Vallabhaneni et al., “Investigation of the First Seven Reported Cases of Candida auris, a Globally Emerging Invasive, Multidrug-Resistant Fungus—United States, May 2013–August 2016,” American Journal of Transplantation, vol. 17, no. 1. Blackwell Publishing Ltd, pp. 296–299, Jan. 01, 2017. [CrossRef]
- K. Satoh, K. Makimura, Y. Hasumi, Y. Nishiyama, K. Uchida, and H. Yamaguchi, “Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital,” Microbiol Immunol, vol. 53, no. 1, pp. 41–44, Jan. 2009. [CrossRef]
- S. R. Lockhart et al., “Simultaneous emergence of multidrug-resistant candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses,” Clinical Infectious Diseases, vol. 64, no. 2, pp. 134–140, 2017. [CrossRef]
- M. Biswal et al., “Controlling a possible outbreak of Candida auris infection: lessons learnt from multiple interventions,” Journal of Hospital Infection, vol. 97, no. 4, pp. 363–370, Dec. 2017. [CrossRef]
- M. A. Sayeed, J. Farooqi, K. Jabeen, S. Awan, and S. F. Mahmood, “Clinical spectrum and factors impacting outcome of Candida auris: A single center study from Pakistan,” BMC Infect Dis, vol. 19, no. 1, p. 384, Dec. 2019. [CrossRef]
- R. E. Magobo, C. Corcoran, S. Seetharam, and N. P. Govender, “Candida auris –Associated Candidemia, South Africa,” Emerg Infect Dis, vol. 20, no. 7, pp. 1250–1251, Jul. 2014. [CrossRef]
- D. A. Solomon, A. K. Nyerere, A. Kanyua, and C. W. Ngugi, “Prevalence, Species Distribution and Antifungal Susceptibility Profile of <i>Candida</i> Species Isolated from Bloodstream of Critical Care Unit Patients in a Tertiary Care Hospital in Kenya,” Open J Med Microbiol, vol. 11, no. 01, 2021. [CrossRef]
- H. F. Garcia-Jeldes et al., “Prevalence of Candida auris in Canadian acute care hospitals among at-risk patients, 2018,” Antimicrob Resist Infect Control, vol. 9, no. 1, 2020. [CrossRef]
- A. Sanyaolu et al., “Candida auris : An Overview of the Emerging Drug-Resistant Fungal Infection,” Infect Chemother, vol. 54, no. 2, p. 236, 2022. [CrossRef]
- Abdalhamid, R. Almaghrabi, S. Althawadi, and A. Omrani, “First report of Candida auris infections from Saudi Arabia,” Journal of Infection and Public Health, vol. 11, no. 4. Elsevier Ltd, pp. 598–599, Jul. 01, 2018. [CrossRef]
- R. Kaki, “Risk factors and mortality of the newly emerging Candida auris in a university hospital in Saudi Arabia,” Mycology, pp. 1–8, Jun. 2023. [CrossRef]
- M. B. Alashqar, L. Alabdan, M. Khan, A. H. Almakadma, and S. Almustanyir, “A Case Report of a Candida auris Infection in Saudi Arabia,” Cureus, May 2021,. [CrossRef]
- R. Sabino, C. Veríssimo, Á. A. Pereira, and F. Antunes, “Candida auris, an agent of hospital-associated outbreaks: Which challenging issues do we need to have in mind?,” Microorganisms, vol. 8, no. 2. 2020. [CrossRef]
- H. Du, J. Bing, T. Hu, C. L. Ennis, C. J. Nobile, and G. Huang, “Candida auris: Epidemiology, biology, antifungal resistance, and virulence,” PLoS Pathog, vol. 16, no. 10, 2020. [CrossRef]
- R. S. Almaghrabi et al., “Molecular characterisation and clinical outcomes of Candida auris infection: Singlecentre experience in Saudi Arabia,” Mycoses, vol. 63, no. 5, pp. 452–460, May 2020. [CrossRef]
- S. S. Ilan and Tanis C. Dingle, “Candida auris,” CMAJ, vol. 191, no. E865, Aug. 2019, Accessed: Jul. 02, 2023. [Online]. Available: https://www.cmaj.ca/content/cmaj/191/31/E865.full.pdf.
- J. de Cássia Orlandi Sardi, D. R. Silva, M. J. Soares Mendes-Giannini, and P. L. Rosalen, “Candida auris: Epidemiology, risk factors, virulence, resistance, and therapeutic options,” Microbial Pathogenesis, vol. 125. Academic Press, pp. 116–121, Dec. 01, 2018. [CrossRef]
- K. Vinayagamoorthy, K. C. Pentapati, and H. Prakash, “Prevalence, risk factors, treatment and outcome of multidrug resistance Candida auris infections in Coronavirus disease (COVID-19) patients: A systematic review,” Mycoses, vol. 65, no. 6. 2022. [CrossRef]
- S. Mahmoudi, K. Ahmadikia, M. Kord, A. Ahmadi, and S. Khodavaisy, “Candida auris, an emerging fungal pathogen,” Journal of Mazandaran University of Medical Sciences, vol. 29, no. 172, 2019. [CrossRef]
- M. Kordalewska and D. S. Perlin, “Identification of drug resistant candida auris,” Frontiers in Microbiology, vol. 10, no. AUG. Frontiers Media S.A., 2019. [CrossRef]
- M. G. Frías-De-león et al., “Antifungal resistance in Candida auris: Molecular determinants,” Antibiotics, vol. 9, no. 9. 2020. [CrossRef]
- Narayanan, P. Selvakumar, R. Siddharthan, and K. Sanyal, “ClaID: a Rapid Method of Clade-Level Identification of the Multidrug Resistant Human Fungal Pathogen Candida auris,” Microbiol Spectr, vol. 10, no. 2, Apr. 2022. [Google Scholar] [CrossRef]
- N. A. Chow, T. de Groot, H. Badali, M. Abastabar, T. M. Chiller, and J. F. Meis, “Potential Fifth Clade of Candida auris, Iran, 2018,” Emerg Infect Dis, vol. 25, no. 9, pp. 1780–1781, Sep. 2019. [CrossRef]
- D. G. De Luca et al., “Four genomic clades of Candida auris identified in Canada, 2012–2019,” Med Mycol, vol. 60, no. 1, Jan. 2022. [CrossRef]
- S. Tsay, A. Kallen, B. R. Jackson, T. M. Chiller, and S. Vallabhaneni, “Approach to the Investigation and Management of Patients with Candida auris, an Emerging Multidrug-Resistant Yeast,” Clinical Infectious Diseases, vol. 66, no. 2, pp. 306–311, Jan. 2018. [CrossRef]
- A. Cortegiani, G. Misseri, T. Fasciana, A. Giammanco, A. Giarratano, and A. Chowdhary, “Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris,” Journal of Intensive Care, vol. 6, no. 1. BioMed Central Ltd., p. 69, Oct. 29, 2018. [CrossRef]
- H. Du, J. Bing, T. Hu, C. L. Ennis, C. J. Nobile, and G. Huang, “Candida auris: Epidemiology, biology, antifungal resistance, and virulence,” PLoS Pathog, vol. 16, no. 10, p. e1008921, Oct. 2020. [CrossRef]
- W. Alfouzan, R. Dhar, A. Albarrag, and H. Al-Abdely, “The emerging pathogen Candida auris: A focus on the Middle-Eastern countries,” Journal of Infection and Public Health, vol. 12, no. 4. Elsevier Ltd, pp. 451–459, Jul. 01, 2019. [CrossRef]
- M. Snayd, F. Dias, R. W. Ryan, D. Clout, and D. B. Banach, “Misidentification of Candida auris by RapID yeast plus, a commercial, biochemical enzyme-based manual rapid identification system,” Journal of Clinical Microbiology, vol. 56, no. 5. 2018. [CrossRef]
- R. M. Welsh et al., “Survival, Persistence, and Isolation of the Emerging Multidrug-Resistant Pathogenic Yeast Candida auris on a Plastic Health Care Surface,” J Clin Microbiol, vol. 55, no. 10, p. 2996, Oct. 2017. [CrossRef]
- W. Alfouzan, R. Dhar, A. Albarrag, and H. Al-Abdely, “The emerging pathogen Candida auris: A focus on the Middle-Eastern countries,” Journal of Infection and Public Health, vol. 12, no. 4. Elsevier Ltd, pp. 451–459, Jul. 01, 2019. [CrossRef]
- S. Chatterjee, S. V. Alampalli, R. K. Nageshan, S. T. Chettiar, S. Joshi, and U. S. Tatu, “Draft genome of a commonly misdiagnosed multidrug resistant pathogen Candida auris,” BMC Genomics, vol. 16, no. 1, 2015. [CrossRef]
- “Antifungal Susceptibility Testing and Interpretation | Candida auris | Fungal Diseases | CDC.” Accessed: May 31, 2023. [Online]. Available: https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html.
- S. Tsay, A. Kallen, B. R. Jackson, T. M. Chiller, and S. Vallabhaneni, “Approach to the Investigation and Management of Patients with Candida auris, an Emerging Multidrug-Resistant Yeast,” Clinical Infectious Diseases, vol. 66, no. 2, pp. 306–311, Jan. 2018. [CrossRef]
- D. H. Caceres et al., “Candida auris: A review of recommendations for detection and control in healthcare settings,” Journal of Fungi, vol. 5, no. 4. MDPI AG, Dec. 01, 2019. [CrossRef]
- T. S. N. Ku, C. J. Walraven, and S. A. Lee, “Candida auris: Disinfectants and Implications for Infection Control,” Front Microbiol, vol. 9, Apr. 2018. [CrossRef]
- P. Arora et al., “Environmental Isolation of Candida auris from the Coastal Wetlands of Andaman Islands, India,” mBio, vol. 12, no. 2, Apr. 2021. [CrossRef]
- F. Visan et al., “ First Candida auris Outbreak Experience in a Tertiary-Care General Hospital in Qatar, 2019,” Infect Control Hosp Epidemiol, vol. 41, no. S1, 2020. [CrossRef]
- M. Theut et al., “The first two cases of Candida auris in Denmark,” Ugeskr Laeger, vol. 184, no. 16, 2022.
- S. Schelenz et al., “First hospital outbreak of the globally emerging Candida auris in a European hospital.,” Antimicrob Resist Infect Control, vol. 5, no. 1, p. 35, Oct. 2016. [CrossRef]
- J. O. KIM, K. H. ST. JOHN, and S. E. COFFIN, “Epidemiology and Infection Prevention and Control,” in Pediatric Infectious Diseases, 2008. [CrossRef]
- S. Hu et al., “Retrospective Analysis of the Clinical Characteristics of Candida auris Infection Worldwide From 2009 to 2020,” Front Microbiol, vol. 12, 2021. [CrossRef]
- CDC, Ncezid, and DHQP, “Identifying Healthcare-associated Infections (HAI) for NHSN Surveillance,” 2023, Accessed: Jun. 01, 2023. [Online]. Available: https://www.cdc.gov/nhsn/pdfs/pscmanual/2psc_identifyinghais_nhsncurrent.pdf.
- S. Ahmad and W. Alfouzan, “Candida auris: Epidemiology, diagnosis, pathogenesis, antifungal susceptibility, and infection control measures to combat the spread of infections in healthcare facilities,” Microorganisms, vol. 9, no. 4. 2021. [CrossRef]
- W. Alfouzan et al., “Molecular epidemiology of candida auris outbreak in a major secondary-care hospital in Kuwait,” Journal of Fungi, vol. 6, no. 4. 2020. [CrossRef]
- A. Al Maani et al., “Ongoing challenges with healthcare-associated candida auris outbreaks in Oman,” Journal of Fungi, vol. 5, no. 4, 2019. [CrossRef]
- J. Mohsin et al., “A Cluster of Candida auris Blood Stream Infections in a Tertiary Care Hospital in Oman from 2016 to 2019.,” Antibiotics (Basel), vol. 9, no. 10, pp. 1–11, Sep. 2020. [CrossRef]
- J. V. Mulet Bayona et al., “Characteristics and management of candidaemia episodes in an established candida auris outbreak,” Antibiotics, vol. 9, no. 9. 2020. [CrossRef]
- H. Villanueva-Lozano et al., “Outbreak of Candida auris infection in a COVID-19 hospital in Mexico,” Clinical Microbiology and Infection, vol. 27, no. 5. 2021. [CrossRef]
- P. S. Shastri, S. A. Shankarnarayan, J. Oberoi, S. M. Rudramurthy, C. Wattal, and A. Chakrabarti, “Candida auris candidaemia in an intensive care unit – Prospective observational study to evaluate epidemiology, risk factors, and outcome,” J Crit Care, vol. 57, 2020,. [CrossRef]
- K. Arensman et al., “Clinical Outcomes of Patients Treated for Candida auris Infections in a Multisite Health System, Illinois, USA,” Emerg Infect Dis, vol. 26, no. 5. 2020. [CrossRef]
- C. Prestel et al., “ Candida auris Outbreak in a COVID-19 Specialty Care Unit — Florida, July–August 2020 ,” MMWR Morb Mortal Wkly Rep, vol. 70, no. 2, 2021,. [CrossRef]
- K. Etienne et al., “Epidemiology and Whole Genome Sequence Typing of Globally Emerging, Multidrug-Resistant Candida auris,” Open Forum Infect Dis, vol. 3, no. suppl_1, Dec. 2016. [CrossRef]
- B. Abdalhamid, R. Almaghrabi, S. Althawadi, and A. Omrani, “First report of Candida auris infections from Saudi Arabia,” Journal of Infection and Public Health, vol. 11, no. 4. Elsevier Ltd, pp. 598–599, Jul. 01, 2018. [CrossRef]
- A. Elsawy, K. Alquthami, N. Alkhutani, D. Marwan, and A. Abbas, “The second confirmed case of Candida auris from Saudi Arabia,” Journal of Infection and Public Health, vol. 12, no. 6. Elsevier Ltd, pp. 907–908, Nov. 01, 2019. [CrossRef]
- R. AlJindan, D. M. AlEraky, N. Mahmoud, S. AbdulAzeez, and J. F. Borgio, “Emergence of multi-drug resistance Candida auris in Saudi Arabia,” Jun. 2020. [CrossRef]
- M. M. Alshamrani et al., “Management of Candida auris outbreak in a tertiary-care setting in Saudi Arabia,” Infect Control Hosp Epidemiol, 2020. [CrossRef]
- A. Alatoom et al., “Persistent candidemia despite appropriate fungal therapy: First case of Candida auris from the United Arab Emirates,” International Journal of Infectious Diseases, vol. 70, pp. 36–37, May 2018. [CrossRef]
- “WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020.” Accessed: Jul. 03, 2023. [Online]. Available: https://www.who.int/director-general/speeches/detail/who-directorgeneral- s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.
- H. Najeeb et al., “The Menace of Candida auris Epidemic Amidst the COVID-19 Pandemic: A Systematic Review,” Diseases, vol. 10, no. 3, p. 58, Aug. 2022. [CrossRef]
- D. Lin et al., “Co-infections of SARS-CoV-2 with multiple common respiratory pathogens in infected patients,” Sci China Life Sci, vol. 63, no. 4, pp. 606–609, Apr. 2020. [CrossRef]
- A. Chowdhary, B. Tarai, A. Singh, and A. Sharma, “Multidrug-resistant candida auris infections in critically Ill Coronavirus disease patients, India, April–July 2020,” Emerg Infect Dis, vol. 26, no. 11, pp. 2694–2696, Nov. 2020. [CrossRef]
- A. Chowdhary and A. Sharma, “The lurking scourge of multidrug resistant Candida auris in times of COVID-19 pandemic,” J Glob Antimicrob Resist, vol. 22, pp. 175–176, Sep. 2020. [CrossRef]
- N. Pandya et al., “International Multicentre Study of Candida auris Infections,” Journal of Fungi, vol. 7, no. 10, p. 878, Oct. 2021. [CrossRef]
- K. Southwick et al., “A description of the first Candida auris-colonized individuals in New York State, 2016-2017,” Am J Infect Control, vol. 50, no. 3, pp. 358–360, Mar. 2022. [CrossRef]
- A. Al-Rashdi, A. Al-Maani, A. Al-Wahaibi, A. Alqayoudhi, A. Al-Jardani, and S. Al-Abri, “Characteristics, risk factors, and survival analysis of candida auris cases: Results of one-year national surveillance data from oman,” Journal of Fungi, vol. 7, no. 1. 2021. [Google Scholar] [CrossRef]
- E. van Schalkwyk et al., “Epidemiologic Shift in Candidemia Driven by Candida auris, South Africa, 2016–20171,” Emerg Infect Dis, vol. 25, no. 9, pp. 1698–1707, Sep. 2019. [CrossRef]
- X. Wang et al., “The first isolate of Candida auris in China: Clinical and biological aspects article,” Emerg Microbes Infect, vol. 7, no. 1. 2018. [CrossRef]
- M. V. Moreno et al., “First isolation de Candida auris in Chile,” Revista Chilena de Infectologia, vol. 36, no. 6. 2019. [CrossRef]
- F. Allaw et al., “First candida auris outbreak during a covid-19 pandemic in a tertiary-care center in Lebanon,” Pathogens, vol. 10, no. 2021. [CrossRef]
- E. J. Zasowski et al., “ 289. International Validation of a Methicillin-Resistant Staphylococcus aureus (MRSA) Risk Assessment Tool for Acute Bacterial Skin and Skin Structure Infections (ABSSSI) ,” Open Forum Infect Dis, vol. 5, no. suppl_1, 2018. [CrossRef]
- B. T. Helfand, C. A. Conran, J. Xu, and W. J. Catalona, “A multiparametric approach to improve upon existing prostate cancer screening and biopsy recommendations,” Curr Opin Urol, vol. 27, no. 5, pp. 475– 480, Sep. 2017. [CrossRef]
| Bundle Element/Risk Factor | Score |
| History of Admission from other hospital, | 3 |
| Has any of these: Septicaemia + CKD, DM, or chronic lung disease | 1 |
| Previous history of MDRO infection or colonization | 1 |
| History of admission in hospital outside the KSA (within the past 12 months) | 1 |
| Presence of wounds or indwelling devices, | 1 |
| Admission to high risk units (ICU, HDU, Oncology etc) | 1 |
| Contact of MDRO / ASC | 1 |
| Previous surgery < 3 months | 1 |
| N | % | |
| Age (years) | ||
| ≤20 | 2 | 3.8 |
| 21-30 | 4 | 7.5 |
| 31-40 | 3 | 5.7 |
| 41-50 | 2 | 3.8 |
| 51-60 | 13 | 24.5 |
| 61-70 | 14 | 26.4 |
| ≥71 | 15 | 28.3 |
| Gender | ||
| Male | 33 | 62.3 |
| Female | 20 | 37.7 |
| Specimen | ||
| Urine | 16 | 30.2 |
| Axilla | 6 | 11.3 |
| Thigh | 5 | 9.4 |
| Anus | 5 | 9.4 |
| Arm | 4 | 7.5 |
| Swab | 3 | 5.7 |
| Penis | 3 | 5.7 |
| Hip | 3 | 5.7 |
| Nose | 2 | 3.8 |
| Buttock | 2 | 3.8 |
| Leg | 2 | 3.8 |
| Neck | 2 | 3.8 |
| Nasal | 2 | 3.8 |
| Tissue | 1 | 1.9 |
| Wound | 1 | 1.9 |
| Nail | 1 | 1.9 |
| Rectal | 1 | 1.9 |
| Blood | 1 | 1.9 |
| Foot | 1 | 1.9 |
| N | % | 95% CI of rate | |
| Comorbidities | 44 | 83 | 60.3 to 111.5 |
| Admission to other hospital | 27 | 50.9 | 33.6 to 74.1 |
| High Risk Areas | 19 | 35.8 | 21.6 to 56 |
| Wounds | 18 | 34.0 | 20.1 to 53.7 |
| Devices | 17 | 32.1 | 18.7 to 51.4 |
| Antimicrobials | 12 | 22.6 | 11.7 to 39.6 |
| ASC | 11 | 20.8 | 10.4 to 37.1 |
| Surgeries | 7 | 13.2 | 5.3 to 27.2 |
| MDRO | 1 | 1.9 | 0.0 to 10.5 |
| Outside KSA | 0 | 0.0 | --- |
| Contact of MDRO | 0 | 0.0 | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





