Submitted:
06 October 2023
Posted:
10 October 2023
You are already at the latest version
Abstract
Keywords:
Highlights
- Solid fuel processing methods are discussed including extensive analysis of kinetics and thermodynamics
- Emission of pollutants from solid fuels is examined
- Mechanism for release of carbon monoxide (CO) is outlined
- Detailed analysis of systems used for minimizing human exposure to CO
- Recommendations for better methods used to process solid fuels especially those used for cooking are discussed
1. Introduction
2. Methodology
- To succinctly delineate the solid fuel processing technologies including combustion, thermochemical and biochemical processing; give an account on the solid fuel combustion kinetics; and elucidate on the burden associated with combustion emissions with focus on carbon monoxide,
- To list the major systems that have been used to minimize human exposure to carbon monoxide emissions from solid fuel combustion
- To briefly justify the chemical changes imparted by use of chemical impregnation methods on solid fuel combustion
- The solid fuel processing technologies, combustion kinetics and the challenge brought by CO poisoning was delineated. The authors carefully studied the relevant literature in relation to solid fuel combustion (i.e. smoldering, flaming), thermochemical conversion (torrefaction, flash carbonization, pyrolysis, gasification, hydrothermal, and liquefaction), and biochemical conversion technologies. They further collected literature on solid fuel combustion kinetics including the mechanisms for release of emissions, and kinetic parameters. Finally, the authors enumerated the challenges brought by CO poisoning including statistics on death by year in England and Wales.
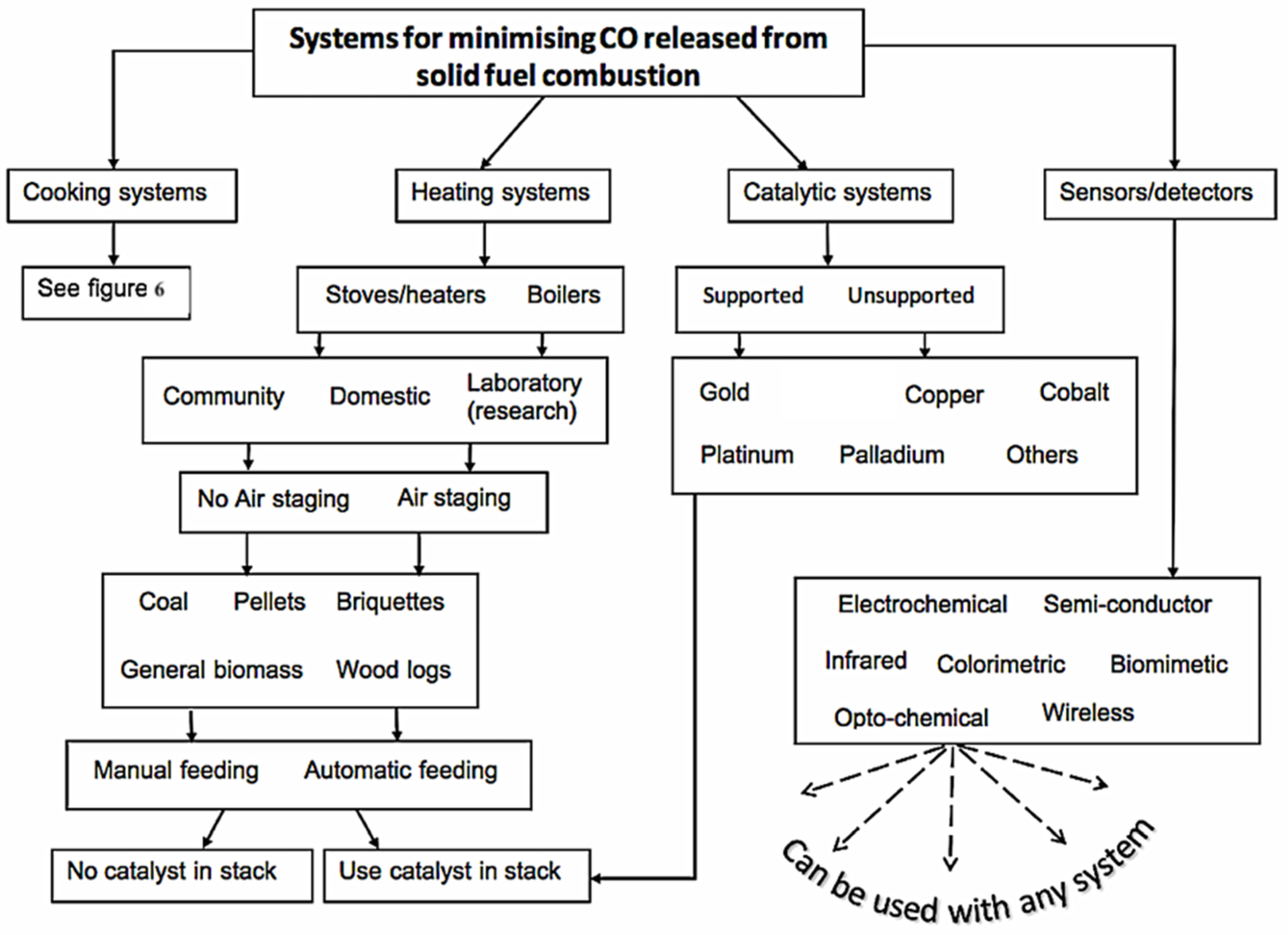
- The major systems used to minimize human exposure to CO are listed and briefly explained. These included, improved cookstoves, heating systems, catalytic oxidation systems and CO detection systems
- The authors also briefly highlight the importance of using chemical catalysts as additives to solid fuels to be used for various applications.
3. Solid fuel processing, kinetics, and CO emissions
3.1. Solid fuel processing technologies
3.1.1. Combustion
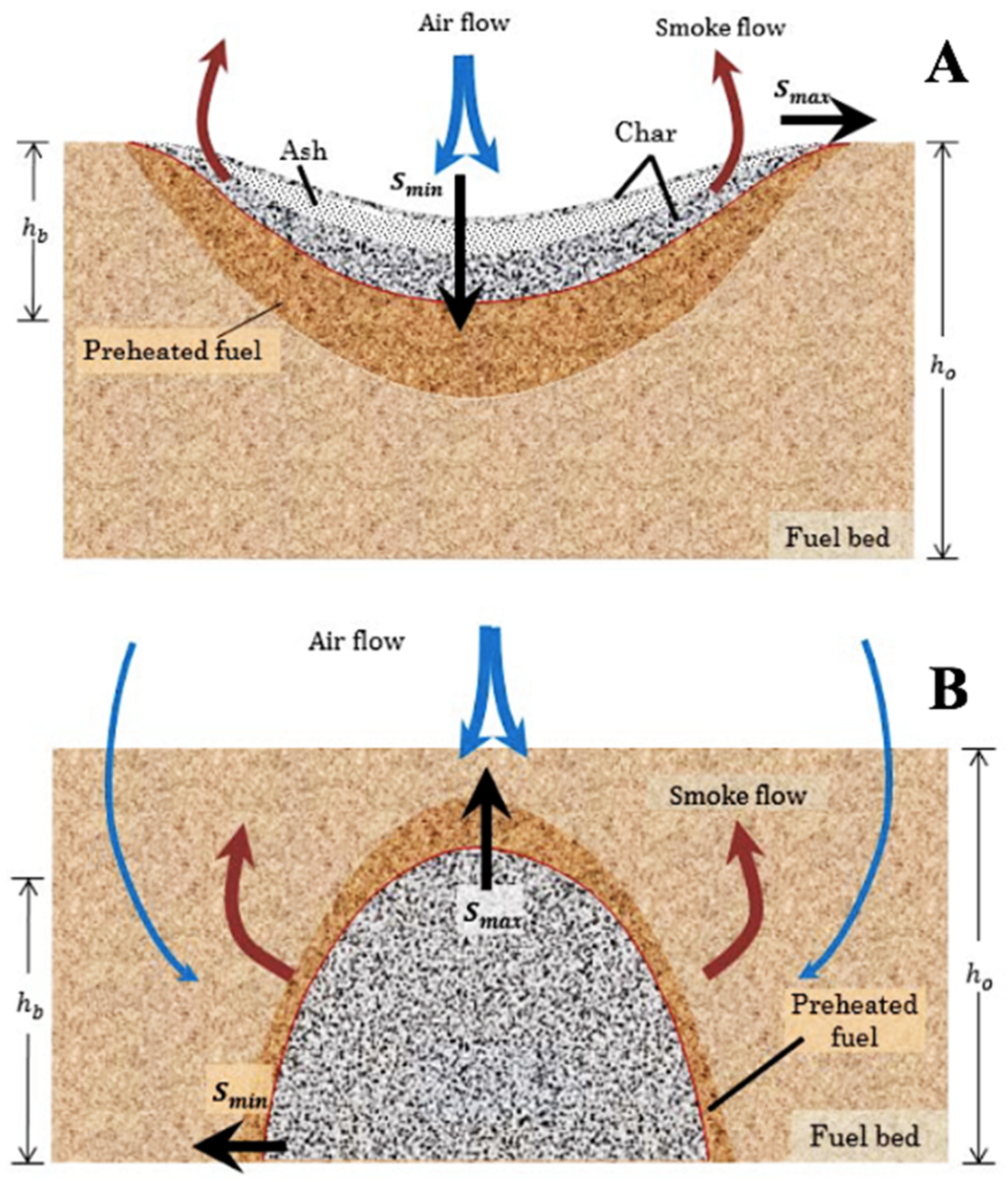
Smoldering
Flaming
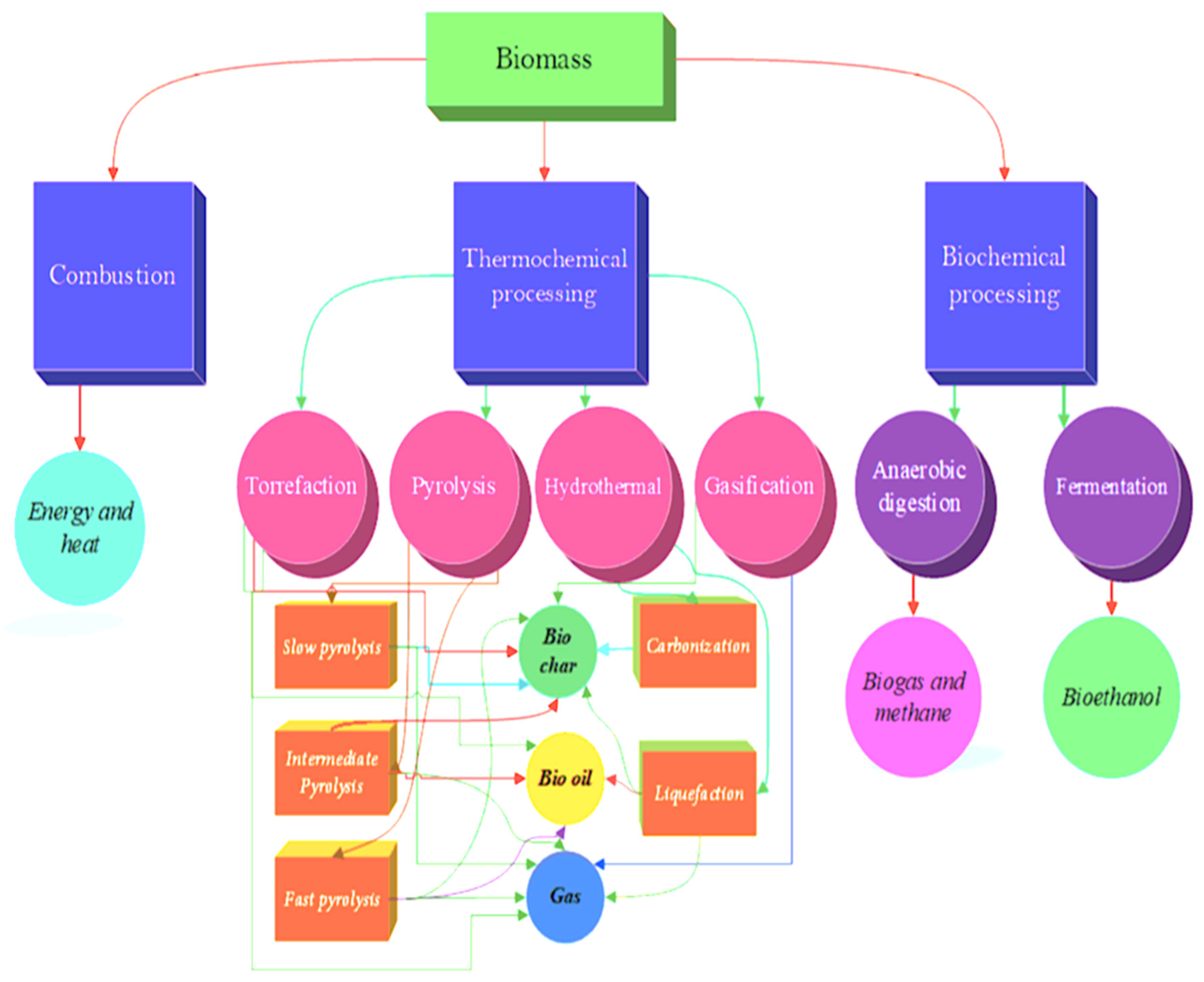
3.1.2. Thermochemical processing
Torrefaction
Flash carbonization
Pyrolysis
Gasification
Hydrothermal
Liquefaction
3.1.3. Biochemical processing
3.2. Solid fuel combustion kinetics
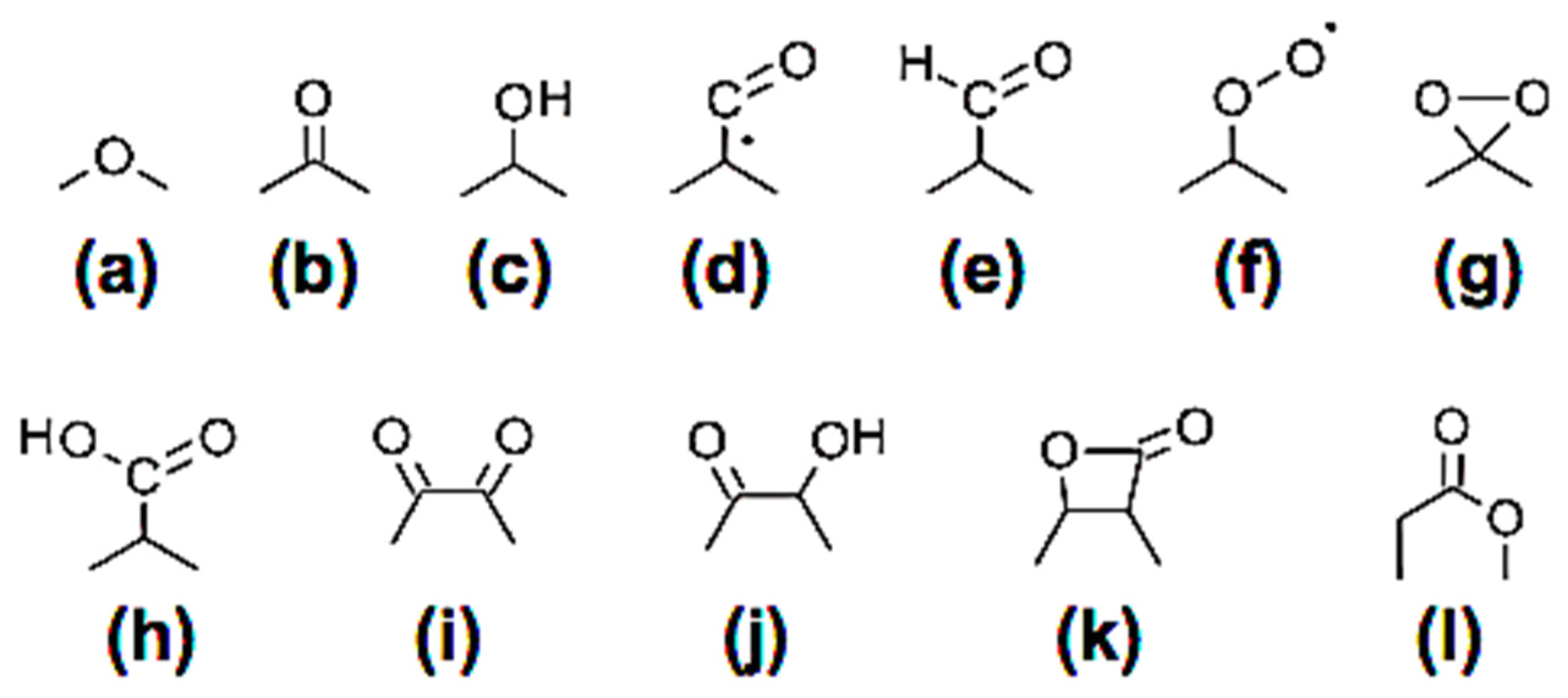
3.2.1. General mechanism for release of emissions
3.2.2. Mechanisms for release of CO
3.2.3. Kinetic parameters for pyrolysis or oxidation of solid fuels
3.3. Carbon monoxide from solid fuels: a persistent challenge
4. Systems for minimising CO released from solid fuels
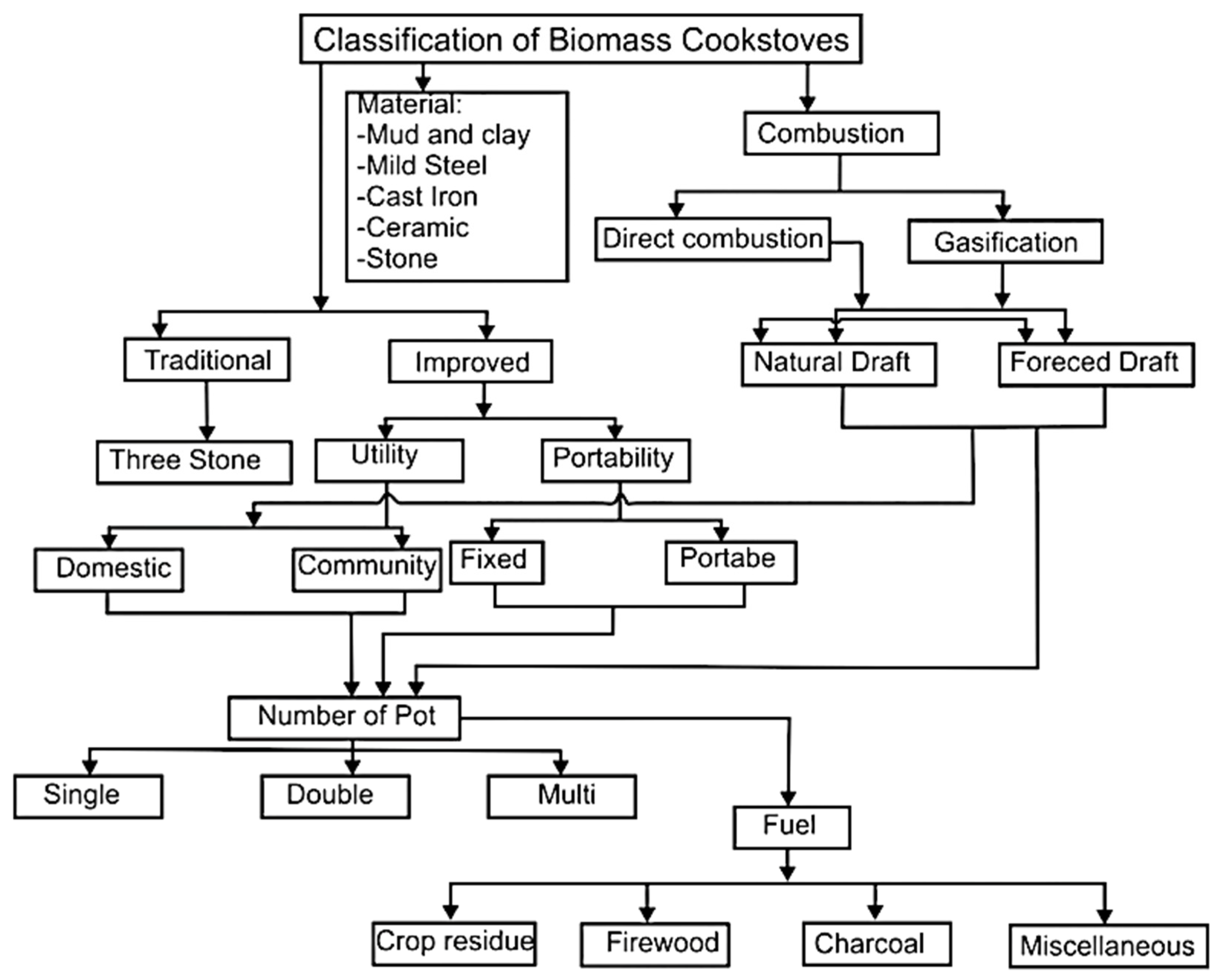
4.1. Improved cookstoves
4.2. Heating systems
4.2.1. Air staging/two-stage combustion
4.2.2. Improved boiler systems
4.3. Catalytic oxidation of CO
4.3.1. Carbon monoxide and oxygen chemisorption on metals
Carbon monoxide
Oxygen
4.3.2. Laboratory oxidation of CO over metals
Supported metal catalysts
CO oxidation over simple oxide catalysts
Gold-based catalysts
Copper-based catalysts
Cobalt catalysts
Platinum and Palladium catalysts
Ceria based catalysts
Other catalysts
4.4. CO detection technologies/sensors
4.4.1. Sensors
Colorimetric CO sensors
Electrochemical
Semiconductor
Infrared sensors
4.4.2. Wireless systems
5. Catalyst impregnation on to solid fuels
5.1. Enhancing pyrolysis and char gasification
5.2. Other applications of catalyst impregnated solid fuels
5.2.1. Improving char properties and removal of pollutants
5.2.2. Fuel cell performance enhancement
6. Conclusions and ideas for future work
- o
- Solid fuel processing may be achieved by combustion (smoldering or flaming), thermochemical (torrefaction, flash carbonization, pyrolysis, gasification, hydrothermal or liquefaction), and biochemical (anaerobic digestion, fermentation and photobiological processing),
- o
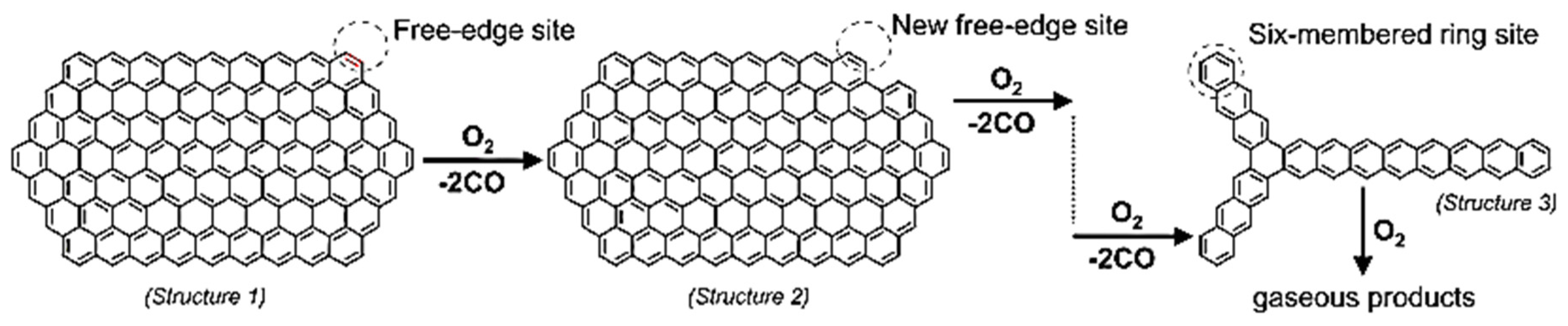
- The general emission products from combustion of solid fuels may be organic and could include more than 15 inorganic elements. Carbon monoxide is released from oxygenated surface functional groups followed by free edge and zig-zag site reactions on PAHs molecules reacting with oxygen until the entire structure is oxidized.
- o
- The methods used to determine kinetic parameters include among others, Friedman, Gupita, KAS, FWO, Starink, Boswel, Coats and Redfern, ASTM methods, Karaosmanoglu and Cif, isothermal methods, iterative methods and Kissinger. There are also methods for determining entropy (ΔS), pre-exponential factor (A), Gibbs free energy (ΔG), and enthalpy change (ΔH).
- o
- Carbon monoxide has been a silent killer since the paleolithic era and has continued to threaten human lives until today.
- o
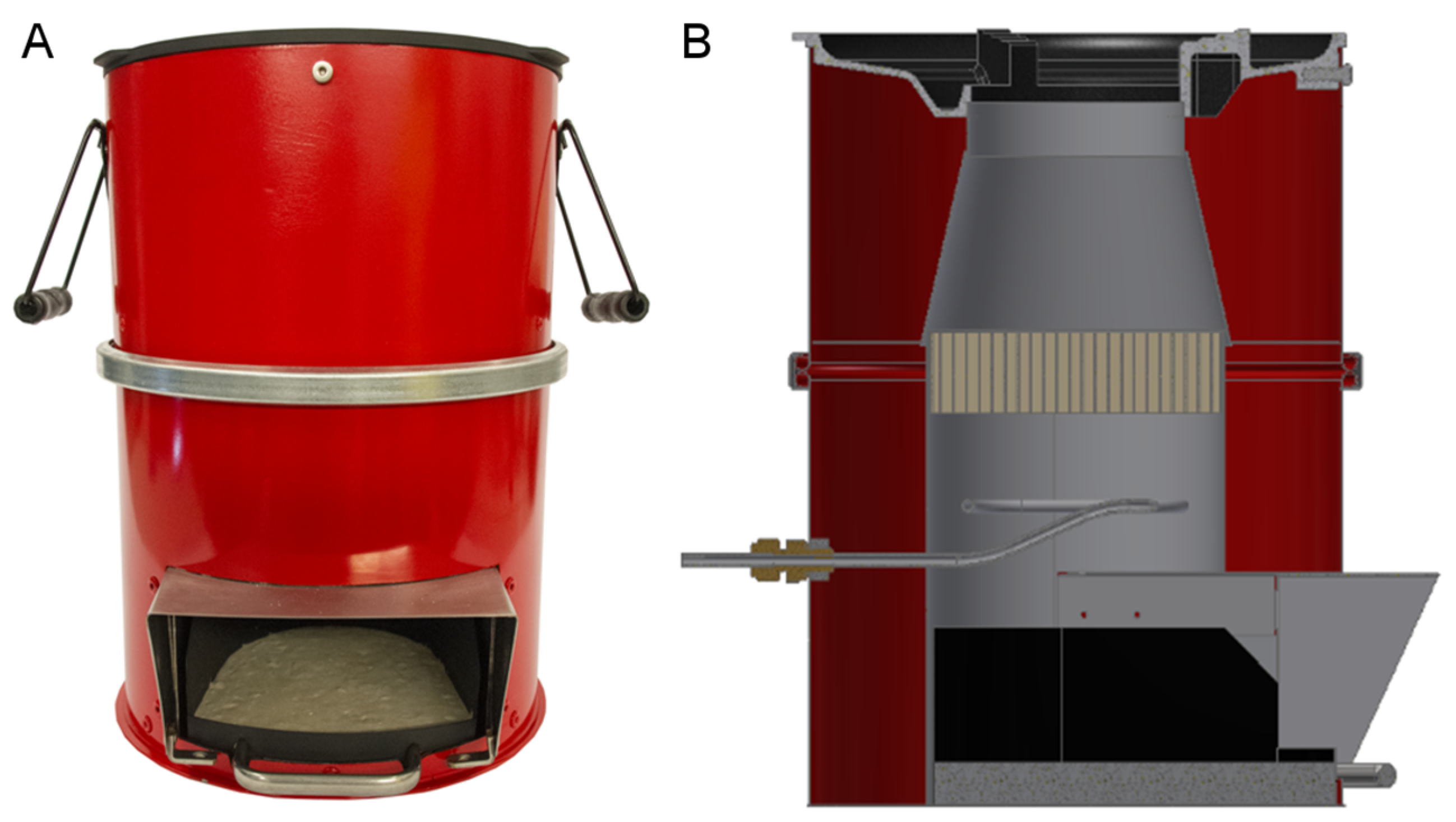
- Improved cook-stoves offer high heat conservation with less or no smoke at all. They are highly efficient with natural and forced draft air systems and some employ catalyst layers hence ensuring the safety of users from toxic combustion emissions
- o
- Heating systems with two-stage combustion and improved biomass boilers with/without catalysts have also been very crucial in reducing CO exposure. They achieve up to 80% CO conversion and offer multi-solid-fuel usage designs. They have low energy requirements and could be operated as domestic or for district heating systems
- o
- Direct catalytic oxidation of post-combustion pollutants are highly efficient and can achieve 100% CO conversion at very low catalyst loading and ambient temperature. They can be used in conjunction with other systems like cooking and heating. There are various combinations of catalysts available commercially and many more to be designed in the future. Their success is attributed to their high affinity for oxygen and toxic pollutants onto their surfaces.
- o
- The CO sensor systems are various and offer the portability advantage. They have very low detection limits and quick response times. They operate at ambient temperatures enabling usage in various environments. Lately, they can be incorporated with wireless systems allowing CO detection remotely.
Acknowledgments
Declaration of interest
Appendix A
Appendix A - Table 1: Semi-conductor sensors
| Working/sensing material | CO conc. (ppm) | Response time (s) | Operating temp (oC) | Ref. |
| Ag-Co3O4 | 5–1500 | 10-30 | 50–200 | (Molavi and Sheikhi, 2018) |
| Cobalt oxide nanosheet and carbon nanotube film | 200 | 23 | Room temp. | (Dai et al., 2010) |
| Au-doped CoOOH | 1000 | 40 | 60 – 110 | (Zhuiykov, 2008) |
| Cobalt oxide (CoOOH) | 1000 | 60 | 80 | (Wu et al., 2006) |
| Ni and Zn doped SnO2 | 500 | 5-7 | 280 | (Tang et al., 2017) |
| Pd on gallia: tin oxide | 30 | 10 | 300–500 | (Kundu et al., 2018) |
| n-type Zn2SnO4 | 200 | Quick response | 50 | (Chen et al., 2018) |
| SnO2/CMOS | 200 | Quick response | 375 | (Lackner et al., 2017) |
| Ca-SnO2 | 1 | 10-12 | 350 | (Ghosh et al., 2014) |
| Pd2+/SnO2/CNT | 500 | 2 | 100 | (Hu et al., 2014) |
| hydroxypropyl cellulose with Pd/SnO2 | 6-18 | Quick response | 60 | (Kim et al., 2013) |
| V–SnO2 and Au/V–SnO2 | 50-1000 | 5-20 | 125 – 175 | (C. T. Wang et al., 2013) |
| V–SnO2 | 50-500 | 14-19 | 175 | (Wang and Chen, 2010) |
| Si–B–C–N-coated SnO2 | 10 – 120 | 20-60 | 350 – 530 | (Prasad et al., 2010) |
| ultrathin SnO2-films | 300 | 20-30 LOD <5ppm |
250-400 | (Tischner et al., 2008) |
| Pt/SnO,/i- diamond/p+-diamond CAIS (Catalyst/Adsorptive Oxide/lnsulator/Semiconductor)diode | 0.4 – 5.4 torr | 24-28 | 50-500 | (Gurbuz et al., 1998) |
| CaO/Nb2O5/SnO2 | 30-2800 | Quick response | 100-230 | (Tsai et al., 1995) |
| Si/SnO2, Pd/SnO2, Borosilicate glass/SnO2 | 40-200 | Quick response | 200-400 | (Van Geloven et al., 1991) |
| SnO2 thin film | 1-100 | Quick response | 350 | (Windischmann, 2006) |
| SnO2 /La2O3, SnO2/Sb2O5+La2O3 and SnO2/Pt+Pd | 200 | Quick response | 100-600 | (Malyshev and Pislyakov, 2008) |
| SnO2 thin film | 1000 | 5-20 | 50-250 | (Salehi, 2003) |
| Ti-doped SnO2 | 300 | 18-20 | 150-450 | (Z. Wang et al., 2013) |
| CoxOy/SnO2 | 125-2500 | 4.9-40.5 | Room temp. | (Oleksenko et al., 2013) |
| Bismuth ferrite (BiFeO3) | 5-30 | 25 | 270-450 | (Chakraborty and Pal, 2018) |
| Prism/Au/ZnO | 0.5-100 | Quick response | Room temp. | (Paliwal et al., 2017) |
| SnO2/ZnO | 100-1000 | 120-240 | 470-510 | (Zaikin et al., 2002) |
| pyridyl-functionalized single-walled carbon nanotubes (F-SWCNTs) and iron porphyrin (Fe(tpp)ClO4) |
50-200 | 30-60 | Room temp. | (Savagatrup et al., 2017) |
| ZnO nanoparticles onto 3D graphene Oxide |
1000 | 7 | 200 | (Phuong et al., 2017) |
| TiO2 (Au- TiO2) thin films | 60-125 | 20 | 230-320 | (Joy et al., 2006) |
| TiO2 thin films | 250 | Quick response | 550 | (Dutta et al., 2005) |
| Nb–TiO2 | 1000 | Quick response | 550 – 850 | (Anukunprasert et al., 2005) |
| TiO2, TiO2/La2O3, TiO2/La2O3/CuO and TiO2/CuO | 500 | Quick response | 600 | (Savage et al., 2001) |
| (La2-x A’x Cu1-y B’y O4; A’ = Sr, Ba, Ce; B’ = Zr, x, y = 0–0.2) | 50-600 | Quick response | 300–600 | (Shimizu et al., 2017) |
| (x)NiFe2O4 (spinel)(1−x) La0.8Pb0.2Fe0.8Co0.2O3 (0≤x≤1) | 10-500 | 60-360 | 125 – 175 | (Maity et al., 2016) |
| NdFeO3 | 0-50,000 | Responsive | Room temp. | (Ho et al., 2011) |
| LaCoO3–In2O3, LaCoO3–Bi2O3 and LaCoO3–PdO | 10-40 | ~ 60-360 | 200-300 | (Salker et al., 2005) |
| Pt/La9.0Si5.8Mn0.2O27-δ | 500 | 10-20 | 30-160 | (Hosoya et al., 2016) |
| LaCoO3 | 500 | 181 | 100 - 550 | (Ding et al., 2015) |
| La0.87Co1.13O3-loaded Ce0.67Zr0.18Sn0.15O2.0 | 500 | 20-40 | 130 | (Hosoya et al., 2015) |
| Pt/Ce4La6O17 | 1-80 | 0.5-1 | 80-180 | (Yang et al., 2019) |
| Pd/SiO2/Si | 10,000 | 10-60 | 150 | (Jelley and Maclay, 1987) |
| MoO3, MoO3/ZrO2 and MoO3/Pd | 0-5000 | 180-1020 | 350-500 | (Azad, 2006) |
| BaSnO3 | 0-50,000 | <120 | 550 – 950 | (Lampe et al., 1995) |
| LuFe2O4 | 20-30 | 10-60 | 300-600 | (Ghosh et al., 2016) |
| MgSb2O6 | 0-500 | Quick response | 23-300 | (Guillén-Bonilla et al., 2016) |
| TiO2/Cu under UV illumination | 100 | 1.5-32 | 120-290 | (Nikfarjam and Salehifar, 2015) |
| Cu doped cryptomelane octahedral molecular sieves (Cu-OMS-2) | 10-8000 | 55 | Room temp | (R. Kumar et al., 2018) |
| Au, Pd, and Pt on CoO and Co3O4 | Up to 10000 | <300 | 50-250 | (Nagai et al., 2013) |
| Nano-SnO2 powder | 5-100 | 8 | 200-500 | (Chen et al., 2012) |
| Pt/SnO2 | 600 | Quick response | 25–350 | (Kocemba and Rynkowski, 2011) |
| Pt/SiO2SiC transistor sensor | 0-1250 | Quick response | 100-400 | (Becker et al., 2011) |
| Co-Ce oxide | 3vol% | 72 | 90-125 | (Xu et al., 2008) |
| Ag-doped SnO2 | 100-500 | 10-17 | 200 | (Petruk and Kravets, 2007) |
References
- A. Nyombi, M. Williams, R.W., 2019. Reactivity and Free Radical Chemistry of Lilac (Syringa) Charcoal. Energy & Fuels 33, 1227–1235. [CrossRef]
- Adrian, B., 2011. Coal fire emissions curb children’s growth. Environ. Health Perspect. 119, A245. [CrossRef]
- Ahmad, M.S., Mehmood, M.A., Ye, G., Al-Ayed, O.S., Ibrahim, M., Rashid, U., Luo, H., Qadir, G., Nehdi, I.A., 2017. Thermogravimetric analyses revealed the bioenergy potential of Eulaliopsis binata. Therm. Anal. Calorim. 130, 1237–1247. [CrossRef]
- Ahmad, W., Noor, T., Zeeshan, M., 2017. Effect of synthesis route on catalytic properties and performance of Co3O4/TiO2 for carbon monoxide and hydrocarbon oxidation under real engine operating conditions. Catal. Commun. [CrossRef]
- Akhtar, A., Krepl, V., Ivanova, T., 2018. A Combined Overview of Combustion, Pyrolysis, and Gasification of Biomass. Energy & Fuels 32, 7294−7318. [CrossRef]
- Alayan, H.M., Alsaadi, M.A., Das, R., Abo-Hamad, A., Ibrahim, R.K., AlOmar, M.K., Hashim, M.A., 2018. The formation of hybrid carbon nanomaterial by chemical vapor deposition: An efficient adsorbent for enhanced removal of methylene blue from aqueous solution. [CrossRef]
- Anastasescu, M., Stan, I., Moldovan, C., Gartner, M., Mihaiu, S., Chesler, P., Muscalu, G., Vladut, C., Calderon Moreno, J.M., Hornoiu, C., Firtat, B., Brasoveanu, C., 2016. Nanostructured SnO2–ZnO composite gas sensors for selective detection of carbon monoxide. Beilstein J. Nanotechnol. 7, 2045–2056. [CrossRef]
- Anca-Couce, A., Dieguez-Alonso, A., Zobel, N., Berger, A., Kienzl, N., Behrendt, F., 2017. Influence of Heterogeneous Secondary Reactions during Slow Pyrolysis on Char Oxidation Reactivity of Woody Biomass. Energy and Fuels 31, 2335-2344. [CrossRef]
- Anderson, G.Q.A., Fergusson, M.J., 2006. Energy from biomass in the UK : sources, processes and biodiversity implications, in: Lbis. pp. 180–183. [CrossRef]
- Anukunprasert, T., Saiwan, C., Traversa, E., 2005. The development of gas sensor for carbon monoxide monitoring using nanostructure of Nb-TiO2. Sci. Technol. Adv. Mater. 6, 359–363. [CrossRef]
- Azad, A.M., 2006. Behavior of a New ZrO2-MoO3 Sensor for Carbon Monoxide Detection. J. Electrochem. Soc. 139, 2913. [CrossRef]
- Bai, X., Chai, S., Liu, C., Ma, K., Cheng, Q., Tian, Y., Ding, T., Jiang, Z., Zhang, J., Zheng, L., Li, X., 2017. Insight into Copper Oxide-Tin Oxide Catalysts for the Catalytic Oxidation of Carbon Monoxide: Identification of Active Copper Species and a Reaction Mechanism. ChemCatChem. [CrossRef]
- Bailis, R., Ezzati, M., Kammen, D.M., 2003. Greenhouse gas implications of household energy technology in Kenya. Environ. Sci. Technol. 37, 2051-2059. [CrossRef]
- Barron-Jimenez, R., Caton, J.A., Anderson, T.N., Lucht, R.P., Walther, T., Roy, S., Brown, M.S., Gord, J.R., 2006. Application of a difference-frequency-mixing based diode-laser sensor for carbon monoxide detection in the 4.4-4.8 μm spectral region. Appl. Phys. B Lasers Opt. 85, 185–197. [CrossRef]
- Becker, E., Andersson, M., Eriksson, M., Spetz, A.L., Skoglundh, M., 2011. Study of the sensing mechanism towards carbon monoxide of platinum-based field effect sensors. IEEE Sens. J. 11, 1527–1534. [CrossRef]
- Bicelli, S., Depari, A., Faglia, G., Flammini, A., Fort, A., Mugnaini, M., Ponzoni, A., Vignoli, V., Rocchi, S., 2009. Model and experimental characterization of the dynamic behavior of low-power carbon monoxide MOX sensors operated with pulsed temperature profiles, in: IEEE Transactions on Instrumentation and Measurement. IEEE, pp. 1324–1332. [CrossRef]
- Biemelt, T., Wegner, K., Teichert, J., Lohe, M.R., Martin, J., Grothe, J., Kaskel, S., 2016. Hopcalite nanoparticle catalysts with high water vapour stability for catalytic oxidation of carbon monoxide. [CrossRef]
- Bignal, K.L., Langridge, S., Zhou, J.L., 2008. Release of polycyclic aromatic hydrocarbons, carbon monoxide and particulate matter from biomass combustion in a wood-fired boiler under varying boiler conditions. Atmos. Environ. 42, 8863–8871. [CrossRef]
- Blaine, R.L., Kissinger, H.E., 2012. Homer Kissinger and the Kissinger equation. Thermochim. Acta 540, 1–6. [CrossRef]
- Blondeau-Patissier, V., Vanotti, M., Rabus, D., Ballandras, S., Chkounda, M., Barbe, J.M., Ballandras, S., Rauch, J.Y., 2011. Development of accurate system of gas detection based on love wave sensors functionalized with cobalt corroles applied to the detection of carbon monoxide. Proc. IEEE Sensors 1078–1081. [CrossRef]
- Bornand, E., 1983. Influence of the annealing temperature of non-doped sintered tin dioxide sensors on their sensitivity and response time to carbon monoxide. Sensors and Actuators 4, 613–620. [CrossRef]
- Borrego, A.G., Garavaglia, L., Kalkreuth, W.D., 2009. Characteristics of high heating rate biomass chars prepared under N2 and CO2 atmospheres. Int. J. Coal Geol. 77, 409-415. [CrossRef]
- Borsos, E., Makra, L., Beczib, R., Vitanyi, B., Szentpeteri, M., 2003. Anthropogenic Air pollution in ancient times. Acta Climatol. Chorol. 36–37, 5–15.
- Boswell, P.G., 1980. On the calculation of activation energies using a modified Kissinger method. J. Therm. Anal. 18, 353–358. [CrossRef]
- Bourahla, S., Harrats, C., Belayachi, H., Nemchi, F., Belhakem, M., 2018. Grape marc activated carbon/TiO2 hybrid degradation of RB5 azo dye: FT-IR and UV-visible analysis. [CrossRef]
- Brennan, L., Owende, P., 2010. Biofuels from microalgae — A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 14, 557–577. [CrossRef]
- Byul, S., Kyu, K., Choi, S., Hee, H., Han, L.G., 2018. Analysis of Emission Characteristics and Emission Factors of Carbon Monoxide and Nitrogen Oxide Emitted from Wood Pellet Combustion in Industrial Wood Pellet Boilers Supplied According to the Subsidy Program of Korea Forest Service. J. Korean Wood Sci. Technol. 46, 597–609. [CrossRef]
- Calvo, A.I., Martins, V., Nunes, T., Duarte, M., Hillamo, R., Teinilä, K., Pont, V., Castro, A., Fraile, R., Tarelho, L., Alves, C., 2015. Residential wood combustion in two domestic devices: Relationship of different parameters throughout the combustion cycle. Atmos. Environ. 116, 72–82. [CrossRef]
- Cantalini, C., Faccio, M., Ferri, G., Pelino, M., 1994. The influence of water vapour on carbon monoxide sensitivity of α-Fe2O3 microporous ceramic sensors. Sensors Actuators B. Chem. 19, 437–442. [CrossRef]
- Cao, J.-L., Li, G.-J., Wang, Y., Sun, G., Wang, X.-D., Hari, B., Zhang, Z.-Y., 2014. Mesoporous Co-Fe-O nanocatalysts: Preparation, characterization and catalytic carbon monoxide oxidation. [CrossRef]
- Caposciutti, G., Antonelli, M., 2018. Experimental investigation on air displacement and air excess effect on CO, CO2 and NOx emissions of a small size fixed bed biomass boiler. Renew. Energy 116, 795–804. [CrossRef]
- Capros, P., Paroussos, L., Fragkos, P., Tsani, S., Boitier, B., Wagner, F., Busch, S., Resch, G., Blesl, M., Bollen, J., 2014. European decarbonisation pathways under alternative technological and policy choices : A multi-model analysis. Energy Strateg. Rev. 2, 231–245. [CrossRef]
- Carltonbird, M., Eaimsumang, S., Pongstabodee, S., Boonyuen, S., Smith, S.M., Luengnaruemitchai, A., 2018. Effect of the exposed ceria morphology on the catalytic activity of gold/ceria catalysts for the preferential oxidation of carbon monoxide. Chem. Eng. J. [CrossRef]
- Carroll, J.P., Finnan, J.M., Biedermann, F., Brunner, T., Obernberger, I., 2015. Air staging to reduce emissions from energy crop combustion in small scale applications. Fuel 155, 37–43. [CrossRef]
- Chakraborty, S., Pal, M., 2018. Highly efficient novel carbon monoxide gas sensor based on bismuth ferrite nanoparticles for environmental monitoring. New J. Chem. 42, 7188–7196. [CrossRef]
- Char, C., Graphite, N., 2017. Experimental Model Development of Oxygen-Enriched Combustion Kinetics on Porous Coal Char and Non-Porous Graphite. [CrossRef]
- Chen, C.C., Sung, G.N., Chen, W.C., Kuo, C.T., Chue, J.J., Wu, C.M., Huang, C.M., 2016. A wireless and batteryless intelligent carbon monoxide sensor. Sensors (Switzerland) 16, 1–11. [CrossRef]
- Chen, D., Zheng, Z., Fu, K., Zeng, Z., Wang, J., Lu, M., 2015. Torrefaction of biomass stalk and its effect on the yield and quality of pyrolysis products. Fuel 159, 27–32. [CrossRef]
- Chen, W., Zhou, Q., Wan, F., Gao, T., 2012. Gas Sensing Properties and Mechanism of Nano-SnO2-Based Sensor for Hydrogen and Carbon Monoxide. J. Nanomater. 2012, 1–9. [CrossRef]
- Chen, Y.C., Shen, Y.R., Hsiao, C.L., 2018. A carbon monoxide interdigitated-capacitor gas sensor based upon a n-type Zn2SnO4 thin film. J. Mater. Sci. Mater. Electron. 29, 1658–1663. [CrossRef]
- Chen, Z., Shi, Z., Guo, Q., 2013. Design of wireless sensor network node for carbon monoxide monitoring. Telecommun. Syst. 53, 47–53. [CrossRef]
- Clark, M.L., Peel, J.L., Burch, J.B., Nelson, T.L., Robinson, M., Conway, S., Bachand, A.M., Reynolds, S.J., Clark, M.L., Peel, J.L., Burch, J.B., Nelson, T.L., Robinson, M.M., Conway, S., Bachand, A.M., Reynolds, S.J., 2009. Impact of improved cookstoves on indoor air pollution and adverse health effects among Honduran women. Int. J. Environ. Heal. Res. 19, 357–368. [CrossRef]
- CO-Gas-Safety, 2015a. Fuel type relating to UK Deaths from unintentional carbon monoxide & appliance type relating to UK Deaths from unintentional carbon monoxide.
- CO-Gas-Safety, 2015b. Carbon monoxide - Death and injuries.
- Coffey, E.R., Muvandimwe, D., Hagar, Y., Wiedinmyer, C., Kanyomse, E., Piedrahita, R., Dickinson, K.L., Oduro, A., Hannigan, M.P., 2017. New Emission Factors and Efficiencies from in-Field Measurements of Traditional and Improved Cookstoves and Their Potential Implications. Environ. Sci. Technol. 51, 12508-12517. [CrossRef]
- Collard, F.-X., Bensakhria, A., Drobek, M., Volle, G., Blin, J., 2015. Influence of impregnated iron and nickel on the pyrolysis of cellulose. [CrossRef]
- Combustor, B., 2016. Effect of Air Staging Ratios on the Burning Rate and Emissions in an Underfeed Fixed-Bed. Energies 940, 1–16. [CrossRef]
- Dai, C.L., Chen, Y.C., Wu, C.C., Kuo, C.F., 2010. Cobalt oxide nanosheet and CNT micro carbon monoxide sensor integrated with readout circuit on chip. Sensors 10, 1753–1764. [CrossRef]
- Dai, G., Chen, L., Zhao, X., 2018. Catalytic oxidation mechanisms of carbon monoxide over single and double vacancy Cr-embedded graphene. [CrossRef]
- Dang, J., Yu, H., Zheng, C., Wang, L., Sui, Y., Wang, Y., 2018. Development a low-cost carbon monoxide sensor using homemade CW-DFB QCL and board-level electronics. Opt. Laser Technol. 101, 57–67. [CrossRef]
- Danvirutai, C., Noisong, P., 2015. Combined facile methods of the DSC and origin lab program to study the dehydration kinetics of KMnPO4.H2O. Therm. Anal. Calorim. 2249–2255. [CrossRef]
- Davis, S.R., Chadwick, A. V., Wright, J.D., 1998. The effects of crystallite growth and dopant migration on the carbon monoxide sensing characteristics of nanocrystalline tin oxide based sensor materials. J. Mater. Chem. A 8, 2065–2071. [CrossRef]
- Ding, J.C., Li, H.Y., Cai, Z.X., Zhang, X.D., Guo, X., 2015. LaCoO3-based sensors with high sensitivity to carbon monoxide. RSC Adv. 5, 65668–65673. [CrossRef]
- Dong, M., Zheng, C., Miao, S., Song, F., Wang, Y., 2017. A mid-infrared carbon monoxide sensor system using wideband absorption spectroscopy and a single-reflection spherical optical chamber. Infrared Phys. Technol. 85, 450–456. [CrossRef]
- dos Santos Xavier, L.P., Rico-Pérez, V., Hernández-Giménez, A.M., Lozano-Castelló, D., Bueno-López, A., 2015. Simultaneous catalytic oxidation of carbon monoxide, hydrocarbons and soot with Ce-Zr-Nd mixed oxides in simulated diesel exhaust conditions. [CrossRef]
- Du, P.-P., Hu, X.-C., Wang, X., Ma, C., Du, M., Zeng, J., Jia, C.-J., Huang, Y.-Y., Si, R., 2017. Synthesis and metal-support interaction of subnanometer copper-palladium bimetallic oxide clusters for catalytic oxidation of carbon monoxide. Inorg. Chem. Front. [CrossRef]
- Dutta, P.K., Frank, M., Hunter, G.W., George, M., 2005. Reactively sputtered titania films as high temperature carbon monoxide sensors. Sensors Actuators, B Chem. 106, 810–815. [CrossRef]
- Ezzati, M., Mbinda, B.M.B.M., Kammen, D.M.D.M., 2000. Comparison of emissions and residential exposure from traditional and improved cookstoves in Kenya. Environ. Sci. Technol. 34, 578-583. [CrossRef]
- Fan, S., Sheng, C., 2016. Impact of Inorganic Matter on the Low-Temperature Oxidation of Cornstalk and Cellulose Chars. Energy and Fuels 30, 1783–1791. [CrossRef]
- Fedunik-hofman, L., Bayon, A., Hinkley, J., Lipin, W., 2019. Friedman method kinetic analysis of CaO-based sorbent for high-temperature thermochemical energy storage. Chem. Eng. Sci. 200, 236–247. [CrossRef]
- Fernandez, A., Mazza, G., Rodriguez, R., 2017. Thermal decomposition under oxidative atmosphere of lignocellulosic wastes : Different kinetic methods application. J. Environ. Chem. Eng. 6, 404–415. [CrossRef]
- Firdaus, Ahriman, N., Yulianto, A., Kusriyanto, M., 2016. Wireless sensor network application for carbon monoxide monitoring, in: Proceeding of the 2015 9th International Conference on Telecommunication Systems Services and Applications, TSSA 2015. IEEE, pp. 1–4. [CrossRef]
- Formosa, M., Jelley, K.W., Nowroozi-esfahani, S., 1988. The response of MOS sensors with ultrathin palladium gates to carbon monoxide and methane. Sensors and Actuators 14, 331–348. [CrossRef]
- Gas Safety Trust - UK, 2019. Carbon monoxide Gas Safety Statistics on death and Injuries - 2019.
- Gas Safety Trust - UK, 2018. Notes relating to the compilation of CO-Gas Safety statistics and graphic representations: Fuel Type relating to UK deaths from unintentional CO poisoning.
- Gas Safety Trust - UK, 2017. UK deaths caused by accidental Carbon Monoxide (CO) poisoning. Isle of Wight.
- Ghosh, S., Chowdhury, U., Roy, S., Bandyopadhyay, R., 2016. Detection of low ppm carbon monoxide with charge ordered LuFe 2 O 4 gas sensor – A novel sensing mechanism. Ceram. Int. 42, 14944–14948. [CrossRef]
- Ghosh, S., Narjinary, M., Sen, A., Bandyopadhyay, R., Roy, S., 2014. Fast detection of low concentration carbon monoxide using calcium-loaded tin oxide sensors. Sensors Actuators, B Chem. 203, 490–496. [CrossRef]
- Guillén-Bonilla, H., Flores-Martínez, M., Rodríguez-Betancourtt, V.M., Guillen-Bonilla, A., Reyes-Gómez, J., Gildo-Ortiz, L., de la Luz Olvera Amador, M., Santoyo-Salazar, J., 2016. A novel gas sensor based on MgSb2O6 nanorods to indicate variations in carbon monoxide and propane concentrations. Sensors (Switzerland) 16, 1–12. [CrossRef]
- Gunnell, D., Coope, C., Fearn, V., Wells, C., Chang, S.-S., Hawton, K., Kapur, N., 2015. Suicide by gases in England and Wales 2001-2011: Evidence of the emergence of new methods of suicide. Affect. Disord. 170, 190-195. [CrossRef]
- Gupta, A.K., Jena, A.K., Chaturvedi, M.C., 1988. A differential technique for the determination of the activation energy of precipitation reactions from differential scanning calorimetric data. Scr. Metall. 22, 369–371.
- Gurbuz, Y., Kang, W.P., Davidson, J.L., Zhou, Q., Kerns, D. V., Henderson, T., 1998. A new high temperature solid-state microelectronic carbon monoxide gas sensor, in: 1998 4th International High Temperature Electronics Conference, HITEC 1998. pp. 230–233. [CrossRef]
- Hadden, R.M., Rein, G., Belcher, C.M., 2013. Study of the competing chemical reactions in the initiation and spread of smouldering combustion in peat. Proc. Combust. Inst. 34, 2547–2553. [CrossRef]
- Hangauer, A., Chen, J., Strzoda, R., Fleischer, M., Amann, M.-C., 2014. Performance of a fire detector based on a compact laser spectroscopic carbon monoxide sensor. Opt. Express 22, 13680. [CrossRef]
- He, F., Yi, W., Li, Y., Zha, J., Luo, B., 2014. Effects of fuel properties on the natural downward smoldering of piled biomass powder: Experimental investigation. Biomass and Bioenergy 67, 288–296. [CrossRef]
- Hernik, B., Jagodzi´nska, K., Matuszek, D., 2018. Numerical studies on the influence of air staging on the temperature of flue gas and emission of gases in the combustion chamber of OP 230 boiler. Chem. Process Eng. 39, 59–74. [CrossRef]
- Ho, T.G., Ha, T.D., Pham, Q.N., Giang, H.T., Do, T.A.T., Nguyen, N.T., 2011. Nanosized perovskite oxide NdFeO3 as material for a carbon-monoxide catalytic gas sensor. Adv. Nat. Sci. Nanosci. Nanotechnol. 2. [CrossRef]
- Hong, X., Sun, Y., 2016. Effect of Preparation Methods on the Performance of Pt/CeO2 Catalysts for the Catalytic Oxidation of Carbon Monoxide. Catal. Letters. [CrossRef]
- Hosoya, A., Tamura, S., Imanaka, N., 2016. A Catalytic Combustion-type Carbon Monoxide Gas Sensor Incorporating an Apatite-type Oxide. ISIJ Int. 56, 1634–1637. [CrossRef]
- Hosoya, A., Tamura, S., Imanaka, N., 2015. A New Catalytic Combustion-type Carbon Monoxide Gas Sensor Employing Precious Metal-free CO Oxidizing Catalyst. ISIJ Int. 55, 1699–1701. [CrossRef]
- Hu, Q., Liu, S., Lian, Y., 2014. Sensors for carbon monoxide based on Pd/SnO2/CNT nanocomposites. Phys. Status Solidi Appl. Mater. Sci. 211, 2729–2734. [CrossRef]
- Hu, Z.-P., Zhu, Y.-P., Gao, Z.-M., Wang, G., Liu, Y., Liu, X., Yuan, Z.-Y., 2016. CuO catalysts supported on activated red mud for efficient catalytic carbon monoxide oxidation. Chem. Eng. J. [CrossRef]
- Izu, N., Matsubara, I., Itoh, T., Shin, W., 2016. Performance of a carbon monoxide sensor based on zirconia-doped ceria. J. Asian Ceram. Soc. 4, 205–208. [CrossRef]
- Jamali, T., Fatmi, Z., Shahid, A., Khoso, A., 2017. Evaluation of short-term health effects among rural women and reduction in household air pollution due to improved cooking stoves : quasi experimental study. Air Qual. Atmos. Heal. 10, 809–819. [CrossRef]
- Jeguirim, M., Said, R., Trouvé, G., El may, Y., Dorge, S., 2012. Experimental investigation on gaseous emissions from the combustion of date palm residues in laboratory scale furnace. Bioresour. Technol. 131, 94–100. [CrossRef]
- Jelley, K.W., Maclay, G.J., 1987. A Dual-Mechanism Carbon-Monoxide and Hydrogen Sensor Utilising an Ultrathin Layer of Palladium. IEEE Trans. Electron Devices 34, 2086–2097. [CrossRef]
- Jenkins, B.M., Baxter, L.L., Jr, T.R.M., Miles, T.R., 1998. Combustion properties of biomass. Fuel Process. Technol. 54, 17–46. [CrossRef]
- Jerez, A., 1983. A modification to the freeman and carroll method for the analysis of the kinetics of non-isothermal processes. J. Therm. Anal. 26, 315–318.
- Jetter, J., Zhao, Y., Smith, K.R., Khan, B., Decarlo, P., Hays, M.D.M.D., Yelverton, T., Decarlo, P., Hays, M.D.M.D., 2012. Pollutant emissions and energy efficiency under controlled conditions for household biomass cookstoves and implications for metrics useful in setting international test standards. Environ. Sci. Technol. 46, 10827-10834. [CrossRef]
- Jiao, Y., Tian, W., Chen, H., Shi, H., Yang, B., Li, C., Shao, Z., Zhu, Z., Li, S.-D., 2015. In situ catalyzed Boudouard reaction of coal char for solid oxide-based carbon fuel cells with improved performance. [CrossRef]
- Johansson, L.S., Leckner, B., Gustavsson, L., Cooper, D., Tullin, C., Potter, A., 2004. Emission characteristics of modern and old-type residential boilers fired with wood logs and wood pellets. Atmos. Environ. 38, 4183–4195. [CrossRef]
- Joseph, F.H., Leo, W.A., 1966. A quick, direct method for the determination of activation energy from thermogravimetric data. Polym. Lett. 4, 323–328. [CrossRef]
- Joy, T., Wojtek, W., Kourosh, K.-Z., Peter, L., 2006. Carbon monoxide gas sensor based on titanium dioxide nanocrystalline with a langasite substrate. Proc. IEEE Sensors 228–231. [CrossRef]
- Juszczak, M., 2014. Concentrations of carbon monoxide and nitrogen oxides from a 25 kw boiler supplied periodically and continuously with wood pellets. Chem. Process Eng. 35, 163–172. [CrossRef]
- Juszczak, M., Cichy, W., Pałaszyńska, K., 2016. Usefulness assessment of automatic air flow control system with oxygen sensor in a 20 kW boiler with periodic wood pellet supply. Drewno 59. [CrossRef]
- Juszczak, M., Lossy, K., 2012. Pollutant emission from a heat station supplied with agriculture biomass and wood pellet mixture. Chem. Process Eng. 33, 231–242. [CrossRef]
- Khodaei, H., Guzzomi, F., Patiño, D., Rashidian, B., Yeoh, G.H., 2017. Air staging strategies in biomass combustion-gaseous and particulate emission reduction potentials. Fuel Process. Technol. 157, 29–41. [CrossRef]
- Kim, B., Lu, Y., Hannon, A., Meyyappan, M., Li, J., 2013. Low temperature Pd/SnO2 sensor for carbon monoxide detection. Sensors Actuators, B Chem. 177, 770–775. [CrossRef]
- Kirtania, K., Axelsson, J., Matsakas, L., Christakopoulos, P., Umeki, K., Furusjö, E., 2017. Kinetic study of catalytic gasification of wood char impregnated with different alkali salts. [CrossRef]
- Kissinger, H.E., 1956. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. (1934). 57, 217. [CrossRef]
- Kocemba, I., Rynkowski, J., 2011. The influence of catalytic activity on the response of Pt/SnO2 gas sensors to carbon monoxide and hydrogen. Sensors Actuators, B Chem. 155, 659–666. [CrossRef]
- Koizumi, K., Nobusada, K., Boero, M., 2015. Reaction Pathway and Free Energy Landscape of Catalytic Oxidation of Carbon Monoxide Operated by a Novel Supported Gold-Copper Alloy Cluster. [CrossRef]
- Kucharczyk, B., 2015. Catalytic Oxidation of Carbon Monoxide on Pd-Containing LaMnO3 Perovskites. [CrossRef]
- Kumar, P., Samiksha, S., Gill, R., 2018. Carbon Monoxide Gas Sensor Based on Fe-ZnO Thin Film. Asian J. Chem. 30, 2737–2742. [CrossRef]
- Kumar, R., Mittal, J., Kushwaha, N., Rao, B. V., Pandey, S., Liu, C.P., 2018. Room temperature carbon monoxide gas sensor using Cu doped OMS-2 nanofibers. Sensors Actuators, B Chem. 266, 751–760. [CrossRef]
- Kundu, S., Sudharson, R., Narjinary, M., 2018. Pd impregnated gallia: tin oxide nanocomposite—An excellent high temperature carbon monoxide sensor. Sensors Actuators, B Chem. 254, 437–447. [CrossRef]
- Lackner, E., Krainer, J., Wimmer-Teubenbacher, R., Sosada, F., Deluca, M., Gspan, C., Rohracher, K., Wachmann, E., Köck, A., 2017. Carbon monoxide detection with CMOS integrated thin film SnO2 gas sensor. Mater. Today Proc. 4, 7128–7131. [CrossRef]
- Lamberg, H., Sippula, O., Tissari, J., Jokiniemi, J., 2011. Effects of Air Staging and Load on Fine-Particle and Gaseous Emissions from a Small-Scale Pellet Boiler. Energy & Fuels 25, 4952–4960. [CrossRef]
- Lamberg, H., Sippula, O., Tissari, J., Virén, A., Kaivosoja, T., Aarinen, A., Salminen, V., Jokiniemi, J., 2017. Operation and Emissions of a Hybrid Stove Fueled by Pellets and Log Wood. Energy & Fuels 31, 1961–1968. [CrossRef]
- Lampe, U., Gerblinger, J., Meixner, H., 1995. Carbon-monoxide sensors based on thin films of BaSnO3. "Sensors Actuators, B Chem. 24–25, 657–660. [CrossRef]
- Lara-García, H.A., Vera, E., Mendoza-Nieto, J.A., Gómez-García, J.F., Duan, Y., Pfeiffer, H., 2017. Bifunctional application of lithium ferrites (Li5FeO4 and LiFeO2) during carbon monoxide (CO) oxidation and chemisorption processes. A catalytic, thermogravimetric and theoretical analysis. Chem. Eng. J. [CrossRef]
- Lask, K., Gadgil, A., 2017. Performance and emissions characteristics of a lighting cone for charcoal stoves. Energy Sustain. Dev. 36, 64-67. [CrossRef]
- Launhardt, T., Thoma, H., 2000. Investigation on organic pollutants from a domestic heating system using various solid biofuels. Chemosphere 40, 1149–1157. [CrossRef]
- Lee, I., Zaera, F., 2014. Catalytic oxidation of carbon monoxide at cryogenic temperatures. [CrossRef]
- Lee, X.J., Lee, L.Y., Gan, S., Thangalazhy-Gopakumar, S., Ng, H.K., 2017. Biochar potential evaluation of palm oil wastes through slow pyrolysis: Thermochemical characterization and pyrolytic kinetic studies. Bioresour. Technol. 236, 236, pp. 155–163. [CrossRef]
- Li, L., Deng, D., Huang, S., Song, H., Xu, K., Zhang, L., Lv, Y., 2018. UV-Assisted Cataluminescent Sensor for Carbon Monoxide Based on Oxygen-Functionalized g-C 3 N 4 Nanomaterials. Anal. Chem. 90, 9598–9605. [CrossRef]
- Li, Z., Nan, D., Yuhan, G., 2012. Research on Infrared Carbon Monoxide Monitoring System Based on Least Squares Support Vector Machine, in: International Workshop on Information and Electronics Engineering (IWIEE). pp. 1926–1931. [CrossRef]
- Liao, Y., Jia, L., Chen, R., Gu, O., Sakurai, M., Kameyama, H., Zhou, L., Ma, H., Guo, Y., 2016. Charcoal-supported catalyst with enhanced thermal-stability for the catalytic combustion of volatile organic compounds. [CrossRef]
- Lin, C., Xian, X., Qin, X., Wang, D., Tsow, F., Forzani, E., Tao, N., 2018. High Performance Colorimetric Carbon Monoxide Sensor for Continuous Personal Exposure Monitoring. ACS Sensors 3, 327–333. [CrossRef]
- Lisandy, K.Y., Kim, Gyeong-min, Kim, J., Kim, Gyu-bo, Jeon, C., 2017. Enhanced Accuracy of the Reaction Rate Prediction Model for Carbonaceous Solid Fuel Combustion. [CrossRef]
- Lucarelli, K., Wyne, K., Svenson, J.E., Lucarelli, K., Wyne, K., Svenson, J.E., 2018. Improved cookstoves and their effect on carbon monoxide levels in San Lucas Tolimán, Guatemala. Int. J. Environ. Health Res. 3123, 1–7. [CrossRef]
- MacCarty, N., Still, D., Ogle, D., 2010. Fuel use and emissions performance of fifty cooking stoves in the laboratory and related benchmarks of performance. Energy Sustain. Dev. 14, 161-171. [CrossRef]
- Maity, A., Ghosh, A., Majumder, S.B., 2016. Engineered spinel-perovskite composite sensor for selective carbon monoxide gas sensing. Sensors Actuators, B Chem. 225, 128–140. [CrossRef]
- Malika, A., Jacques, N., Jaafar, E.F., Fatima, B., Mohammed, A., 2016. Pyrolysis investigation of food wastes by TG-MS-DSC technique. Biomass Convers. Biorefinery 6, 161–172. [CrossRef]
- Malyshev, V. V., Pislyakov, A. V., 2008. Investigation of gas-sensitivity of sensor structures to hydrogen in a wide range of temperature, concentration and humidity of gas medium. Sensors Actuators, B Chem. 134, 913–921. [CrossRef]
- Mantananont, N., Patumsawad, S., 2016. Particulate matter and gaseous emission rate from combustion of Thai lignite and agricultural residues in a fixed-bed combustor. Energy Sources, Part A Recover. Util. Environ. Eff. 38, 478–484. [CrossRef]
- Martín-Lara, M.A., Blázquez, G., Ronda, A., Calero, M., 2016. Kinetic study of the pyrolysis of pine cone shell through non-isothermal thermogravimetry: Effect of heavy metals incorporated by biosorption. Renew. Energy 96, 613–624. [CrossRef]
- Martín-Lara, M.A., Ronda, A., Blázquez, G., Pérez, A., Calero, M., 2018. Pyrolysis kinetics of the lead-impregnated olive stone by non-isothermal thermogravimetry. Process Saf. Environ. Prot. 113, 448–458. [CrossRef]
- Mehetre, S.A., Panwar, N.L., Sharma, D., Kumar, H., 2017. Improved biomass cookstoves for sustainable development : A review. Renew. Sustain. Energy Rev. 73, 672–687. [CrossRef]
- Ministry of New and Renewable Energy - India, 2015. Approved Models of Portable Improved Biomass Cookstoves [WWW Document]. Manuf. Improv. Biomass Cookstoves (As 08.07.2015). URL http://mnre.gov.in/file-manager/UserFiles/approved-models-of-portable-improved-biomass-cookstove-manufactures.pdf (accessed 2.13.19).
- Moghtaderi, B., Fletcher, D.F., 1998. Flaming Combustion Characteristics of Wood-Based Materials, in: International Association for Fire Safety Science. pp. 209–219.
- Mohajeri, A., Hassani, N., 2018. Catalytic activity of corrole complexes with post-transition elements for the oxidation of carbon monoxide: A first-principles study. New J. Chem. [CrossRef]
- Mokoena, L., Pattrick, G., Scurrell, M.S., 2016. Catalytic activity of gold-perovskite catalysts in the oxidation of carbon monoxide. Gold Bull. [CrossRef]
- Molavi, R., Sheikhi, M.H., 2018. Low temperature carbon monoxide gas sensor based on Ag-Co3O4 thick film nanocomposite. Mater. Lett. 233, 74–77. [CrossRef]
- Moragues, M.E., Toscani, A., Sancenón, F., Martínez-Máñez, R., White, A.J.P.P., Wilton-Ely, J.D.E.T.E.T., 2014. A chromo-fluorogenic synthetic “canary” for CO detection based on a pyrenylvinyl ruthenium (II) complex. J. Am. Chem. Soc. 136, 11930–11933. [CrossRef]
- Mukhopadhyay, R., Sambandam, S., Pillarisetti, A., Jack, D., Mukhopadhyay, K., Balakrishnan, K., Vaswani, M., Bates, N., Kinney, P., Arora, N., Smith, K., Mukhopadhyay, R., Sambandam, S., Pillarisetti, A., Jack, D., Mukhopadhyay, R., Sambandam, S., Pillarisetti, A., Jack, D., Mukhopadhyay, K., Balakrishnan, K., Vaswani, M., Bates, M.N., Kinney, P.L., Arora, N., Smith, K.R., 2012. Cooking practices, air quality, and the acceptability of advanced cookstoves in Haryana, India : an exploratory study to inform large-scale interventions. Glob. Health Action 5, 1–13. [CrossRef]
- Muktham, R., Ball, A.S., Bhargava, S.K., Bankupalli, S., 2016. Study of thermal behavior of deoiled karanja seed cake biomass: Thermogravimetric analysis and pyrolysis kinetics. Energy Sci. Eng. 4, 86–95. [CrossRef]
- Mulrooney, J., Clifford, J., Fitzpatrick, C., Chambers, P., Lewis, E., 2008. A mid-infrared optical fibre sensor for the detection of carbon monoxide exhaust emissions. Sensors Actuators, A Phys. 144, 13–17. [CrossRef]
- Nagai, D., Nakashima, T., Nishibori, M., Itoh, T., Izu, N., Shin, W., 2013. Thermoelectric gas sensor with CO combustion catalyst for ppm level carbon monoxide detection. Sensors Actuators, B Chem. 182, 789–794. [CrossRef]
- Nandy, T., Ronald A. Coutu, J., Ababei, C., 2018. Carbon Monoxide Sensing Technologies for Next-Generation Cyber-Physical Systems. Sensors 18, 1–29. [CrossRef]
- Nikfarjam, A., Salehifar, N., 2015. UV Enhancement in Carbon Monoxide Detection Using Cu Thin Film on TiO2 Nanofiber Sensor. Sensors Lett. 13, 599-604(6). [CrossRef]
- Njenga, M., Iiyama, M., J., Amnadass, R., Helander, H., Larsson, L., De Leeuw, J., Neufeldt, H., Röing De Nowina, K., Sundberg, C., Iiyama, M., Jamnadass, R., Helander, H., Larsson, L., Leeuw, J. De, 2016. Gasifier as a cleaner cooking system in rural Kenya. Clean. Prod. 121, 208-217. [CrossRef]
- Njenga, M., Mahmoud, Y., Mendum, R., Iiyama, M., Jamnadass, R., De Nowina, K.R., Sundberg, C., 2017. Quality of charcoal produced using micro gasification and how the new cook stove works in rural Kenya. Environ. Res. Lett. 12, art. no. 095001,. [CrossRef]
- Nussbaumer, T., 2003. Combustion and Co-combus tion of Biomass: Fundamentals, Technologie s, and Primary Measures for Emission Reduction. Energy & Fuels 17, 1510–1521. [CrossRef]
- Nuutinen, K., Jokiniemi, J., Sippula, O., Lamberg, H., Sutinen, J., 2014. Effect of air staging on fine particle, dust and gaseous emissions from masonry heaters. Biomass and Bioenergy 67, 167–178. [CrossRef]
- Nyombi, A., Williams, M.R., Wessling, R., 2019. Toxic emissions from smouldering combustion of woody biomass and derived char with a case study of CO build-up in an ISO container. Energy Sources, Part A Recover. Util. Environ. Eff. [CrossRef]
- Office-for-National-Statistics-UK, 2016. Number of deaths from accidental poisoning by carbon monoxide, England and Wales, deaths registered in 2011-2015. London.
- Okamoto, H., Obayashi, H., Kudo, T., 1980. Carbon monoxide gas sensor made of stabilised Zirconia. Solid State Ionics 1, 319–326. [CrossRef]
- Oleksenko, L.P., Maksimovich, N.P., Shuvar, L. V, Matushko, I.P., 2013. Nanosized Semiconductor CoxOy/SnO2 Materials for Carbon Monoxide Sensors. Theor. Exp. Chem. 49, 310–314. [CrossRef]
- Ortega, P.P., Rocha, L.S.R., Cortés, J.A., Ramirez, M.A., Buono, C., Ponce, M.A., Simões, A.Z., 2019. Towards carbon monoxide sensors based on europium doped cerium dioxide. Appl. Surf. Sci. 464, 692–699. [CrossRef]
- Osman, A.I., Abdelkader, A., Johnston, C.R., Morgan, K., Rooney, D.W., 2017. Thermal Investigation and Kinetic Modeling of Lignocellulosic Biomass Combustion for Energy Production and Other Applications. Ind. Eng. Chem. Res. 56, 12119–12130. [CrossRef]
- Otagawa, T., Madou, M., Wing, S., Rich-Alexander, J., Kusanagi, S., Fujioka, T., Yasuda, A., 1990. Planar microelectrochemical carbon monoxide sensors. Sensors Actuators B1 1, 319–325. [CrossRef]
- Ots, R., Heal, M.R., Young, D.E., Williams, L.R., Allan, J.D., Nemitz, E., Green, D.C., Kuenen, J.J.P., Reis, S., Vieno, M., 2018. Modelling carbonaceous aerosol from residential solid fuel burning with different assumptions for emissions 4497–4518.
- Ozawa, T., 1965. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 38, 1881–1886. [CrossRef]
- Ozil, F., Tschamber, V., Haas, F., Trouvé, G., 2009. Efficiency of catalytic processes for the reduction of CO and VOC emissions from wood combustion in domestic fireplaces. Fuel Process. Technol. 90, 1053–1061. [CrossRef]
- Özsin, G., Pütün, A.E., 2017. Kinetics and evolved gas analysis for pyrolysis of food processing wastes using TGA/MS/FT-IR. Waste Manag. 64, 315-326. [CrossRef]
- Pałaszynska, K., Juszczak, M., 2018. Gaseous emissions during agricultural biomass combustion in a 50 kW moving step grate boiler. Chem. Process Eng. 39, 197–208. [CrossRef]
- Paliwal, A., Sharma, A., Tomar, M., Gupta, V., 2017. Carbon monoxide (CO) optical gas sensor based on ZnO thin films. Sensors Actuators, B Chem. 250, 679–685. [CrossRef]
- Pannek, C., Tarantik, K.R., Schmitt, K., Wöllenstein, J., 2018. Investigation of gasochromic rhodium complexes towards their reactivity to CO and integration into an optical gas sensor for fire gas detection. Sensors (Switzerland) 18, 1–15. [CrossRef]
- Park, J.-H., Wang, J.J., Xiao, R., Tafti, N., DeLaune, R.D., Seo, D.-C., 2018. Degradation of Orange G by Fenton-like reaction with Fe-impregnated biochar catalyst. [CrossRef]
- Parker, D.S.N., Kaiser, R.I., Troy, T.P., Kostko, O., Ahmed, M., Mebel, A.M., 2015. Toward the Oxidation of the Phenyl Radical and Prevention of PAH Formation in Combustion Systems. [CrossRef]
- Paul, S., Chavan, N.N., Radhakrishnan, S., 2009. Polypyrrole functionalized with ferrocenyl derivative as a rapid carbon monoxide sensor. Synth. Met. 159, 415–418. [CrossRef]
- Paulsen, A.D., Kunsa, T.A., Carpenter, A.L., Amundsen, T.J., Schwartz, N.R., Harrington, J., Reed, J., Alcorn, B., Gattoni, J., Yelvington, P.E., 2018. Gaseous and particulate emissions from a chimneyless biomass cookstove equipped with a potassium catalyst. Appl. Energy 235, 369–378. [CrossRef]
- Pérez-Maqueda, L.A., Criado, J.M., 2000. Accuracy of Senum and Yang’s approximations to the Arrhenius integral. J. Therm. Anal. Calorim. 60, 909–915. [CrossRef]
- Petruk, V.G., Kravets, A.G., 2007. Carbon monoxide sensors based on SnOx nanoparticles. Tech. Phys. 52, 231–234. [CrossRef]
- Phawachalotorn, C., Sanguanruang, O., Ishihara, T., 2012. Highly selective amperometric sensors for carbon monoxide detection in exhaust gas. Sensors Actuators, B Chem. 161, 635–640. [CrossRef]
- Phuong, N.H., Ha, N.H., Thach, P.D., Thinh, D.D., Huong, N.T., Hong, H.S., 2017. Fast response of carbon monoxide gas sensors using a highly porous network of ZnO nanoparticles decorated on 3D reduced graphene oxide. Appl. Surf. Sci. 434, 1048–1054. [CrossRef]
- Poggi, L.A., Singh, K., 2016. Thermal degradation capabilities of modified bio-chars and fluid cracking catalyst (FCC) for acetic acid. [CrossRef]
- Pope, D., Bruce, N., Dherani, M., Jagoe, K., Rehfuess, E., 2017. Real-life effectiveness of ‘improved’ stoves and clean fuels in reducing PM2.5 and CO: Systematic review and meta-analysis. Environ. Int. 101, 7–18. [CrossRef]
- Prasad, R.M., Gurlo, A., Riedel, R., Hübner, M., Barsan, N., Weimar, U., 2010. Microporous ceramic coated SnO2 sensors for hydrogen and carbon monoxide sensing in harsh reducing conditions. Sensors Actuators, B Chem. 149, 105–109. [CrossRef]
- Qiu, X., Wei, Y., Li, N., Guo, A., Zhang, E., Li, C., Peng, Y., Wei, J., Zang, Z., 2019. Development of an early warning fire detection system based on a laser spectroscopic carbon monoxide sensor using a 32-bit system-on-chip. Infrared Phys. Technol. 96, 44–51. [CrossRef]
- Rabaçal, M., Fernandes, U., Costa, M., 2013. Combustion and emission characteristics of a domestic boiler fired with pellets of pine, industrial wood wastes and peach stones. Renew. Energy 51, 220–226. [CrossRef]
- Raj, A., Robert, G., Ho, S., 2012. Reaction mechanism for the free-edge oxidation of soot by O2. Combust. Flame 159, 3423–3436. [CrossRef]
- Raj, A., Yang, S.Y., Cha, D., Tayouo, R., Chung, S.H., 2013. Structural effects on the oxidation of soot particles by O2: Experimental and theoretical study. Combust. Flame 160, 1812–1826. [CrossRef]
- Rakitskaya, T.L., Kiose, T.A., Ennan, A.A., Golubchik, K.O., Oleksenko, L.P., Gerasiova, V.G., 2016. Effect the conditions of the acid–thermal modification of clinoptilolite have on the catalytic properties of palladium–copper complexes anchored on it in the reaction of carbon monoxide oxidation. Russ. J. Phys. Chem. A. [CrossRef]
- Rein, G., 2016. Smoldering Combustion, in: Hurley, M.J., Gottuk, D., Hall, J.R., Harada, K., Kuligowski, E., Puchovsky, M., Torero, J., Watts, J.M., Wieczorek, C. (Eds.), SFPE Handbook of Fire Protection Engineering. Springer New York, New York, NY, pp. 581–603. [CrossRef]
- Rola Mohammad, A.-S., Khaled, M.S., Joydeep, D., 2018. Critical Review of Low-Temperature CO Oxidation and Hysteresis Phenomenon on Heterogeneous Catalysts. Catal. Today 8, 1–19. [CrossRef]
- Ross, A.B., Jones, J.M., Chaiklangmuang, S., Pourkashanian, M., Williams, A., Kubica, K., Andersson, J.T., Kerst, M., Danihelka, P., Bartle, K.D., 2002. Measurement and prediction of the emission of pollutants from the combustion of coal and biomass in a fixed bed furnace. Fuel 81, 571–582. [CrossRef]
- Royer, S., Duprez, D., 2011. Catalytic Oxidation of Carbon Monoxide over Transition Metal Oxides. ChemCatChem 3, 24–65. [CrossRef]
- Russell, J.B., Jeraci, J.L., 1984. Effect of Carbon Monoxide on Fermentation of Fiber, Starch, and Amino Acids by Mixed Rumen Microorganisms In Vitro. Appl. Environ. Microbiol. 48, 211–217.
- Ryan, T.J., Arnold, K.J., 2011. Residential Carbon Monoxide Detector Failure Rates in the United States. Am. J. Public Health 101, 15–17. [CrossRef]
- Sadaka, S., Liechty, H., Pelkki, M., Blazier, M., 2015. Pyrolysis and combustion kinetics of raw and carbonized cottonwood and switchgrass agroforest. [CrossRef]
- Sajjad, M., Aamer, M., Taha, S., Taqvi, H., 2017. Pyrolysis, kinetics analysis, thermodynamics parameters and reaction mechanism of Typha latifolia to evaluate its bioenergy potential Bioresource Technology. Bioresour. Technol. 245, 491–501. [CrossRef]
- Salehi, A., 2003. A highly sensitive self heated SnO2 carbon monoxide sensor. Sensors Actuators, B Chem. 96, 88–93. [CrossRef]
- Salker, A. V., Choi, N.J., Kwak, J.H., Joo, B.S., Lee, D.D., 2005. Thick films of In, Bi and Pd metal oxides impregnated in LaCoO3 perovskite as carbon monoxide sensor. Sensors Actuators, B Chem. 106, 461–467. [CrossRef]
- Sambandam, S., Balakrishnan, K., Ghosh, S., Sadasivam, A., Madhav, S., Ramasamy, R., Samanta, M., Mukhopadhyay, K., Rehman, H., Ramanathan, V., 2015. Can Currently Available Advanced Combustion Biomass Cook-Stoves Provide Health Relevant Exposure Reductions? Results from Initial Assessment of Select Commercial Models in India. Ecohealth 12, 25–41. [CrossRef]
- Santhosh, P., Manesh, K.M., Gopalan, A., Lee, K.P., 2007. Novel amperometric carbon monoxide sensor based on multi-wall carbon nanotubes grafted with polydiphenylamine-Fabrication and performance. Sensors Actuators, B Chem. 125, 92–99. [CrossRef]
- Savagatrup, S., Schroeder, V., He, X., Lin, S., He, M., Yassine, O., Salama, K.N., Zhang, X.-X., Swager, T.., 2017. Bio-Inspired Carbon Monoxide Sensors with Voltage-Activated Sensitivity. J. Ger. Chem. Soc. 56, 14066–14070. [CrossRef]
- Savage, N.O., Akbar, S.A., Dutta, P.K., 2001. Titanium dioxide based high temperature carbon monoxide selective sensor. Sensors Actuators, B Chem. 72, 239–248. [CrossRef]
- Schmidl, C., Luisser, M., Padouvas, E., Lasselsberger, L., Rzaca, M., Ramirez-Santa Cruz, C., Handler, M., Peng, G., Bauer, H., Puxbaum, H., 2011. Particulate and gaseous emissions from manually and automatically fired small scale combustion systems. [CrossRef]
- Senum, G.I., Yang, R.., 1977. Rational approximations of the intergral of the Arrhenius function. Therm. Anal. 445–447. [CrossRef]
- Shanying, Z., Youping, C., Gang, Z., Jiming, S., 2010. A near-infrared optical fiber sensor for carbon monoxide concentration monitoring. Microw. Opt. Technol. Lett. 55, 363–366. [CrossRef]
- Shen, G., Xue, M., Chen, Y., Yang, C., Li, W., Shen, H., Huang, Y., Zhang, Y., Chen, H., Zhu, Y., Wu, H., Ding, A., Tao, S., 2014. Comparison of carbonaceous particulate matter emission factors among different solid fuels burned in residential stoves. Atmos. Environ. 89, 337–345. [CrossRef]
- Sher, F., Pans, M.A., Daniel, T.A., Sun, C., Liu, H., 2017. Experimental investigation of woody and non-woody biomass combustion in a bubbling fluidised bed combustor focusing on gaseous emissions and temperature profiles. Energy 141, 2069–2080. [CrossRef]
- Shimizu, Y., Kaneyasu, K., Ueda, T., Goto, T., Hyodo, T., 2016. Potentiometric Carbon Monoxide Sensors Using an Anion-Conducting Polymer Electrolyte and Au-Loaded SnO2 Electrodes. J. Electrochem. Soc. 163, B300–B308. [CrossRef]
- Shimizu, Y., Yamamoto, S., Takase, S., 2017. A thick-film impedancemetric carbon monoxide sensor using layered perovskite-type cuprate. Sensors Actuators, B Chem. 249, 667–672. [CrossRef]
- Sikarwar, V.S., Zhao, M., Fennell, P.S., Shah, N., Anthony, E.J., 2017. Progress in biofuel production from gasification. Prog. Energy Combust. Sci. 61, 189!248. [CrossRef]
- Sin, J.K.., Sharma, R.K., Chan, P.C.., Tang, Z., Yan, G., Hsing, I.-M., 2002. Sensitive, selective and stable tin dioxide thin-films for carbon monoxide and hydrogen sensing in integrated gas sensor array applications. Sensors Actuators B Chem. 72, 160–166. [CrossRef]
- Song, Y., Chu, X., Lin, Y., Yang, X., 2017. Pyrrolidone Modifying Gold Nanocatalysts for Enhanced Catalytic Activities in Aerobic Oxidation of Alcohols and Carbon Monoxide. J. Chem. [CrossRef]
- Starink, M.J., 2003. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta 404, 163–176. [CrossRef]
- Still, D., Bentson, S., Li, H., 2015. Results of Laboratory Testing of 15 Cookstove Designs in Accordance with the ISO/IWA Tiers of Performance. Ecohealth 12, 12–24. [CrossRef]
- Sudarsanam, P., Selvakannan, P.R., Soni, S.K., Bhargava, S.K., Reddy, B.M., 2014. Structural evaluation and catalytic performance of nano-Au supported on nanocrystalline Ce0.9Fe0.1O2-δ solid solution for oxidation of carbon monoxide and benzylamine. [CrossRef]
- Sun, J., Hoon, S., Jung, S., Ryu, C., Jeon, J., Shin, M., Park, Y., 2016. Production and utilization of biochar : A review. J. Ind. Eng. Chem. 40, 1–15. [CrossRef]
- Suryono, S., Saputra, R., Surarso, B., Bardadi, A., 2017. A web-based wireless sensor system to measure carbon monoxide concentration, in: International Conference on Electrical Engineering, Computer Science and Informatics (EECSI). pp. 19–21. [CrossRef]
- Svintsitskiy, D.A., Pakharukov, I.Y., Slavinskaya, E.M., Kardash, T.Y., Parmon, V.N., Boronin, A.I., 2016. Influence of the Copper(II) Oxide Dispersion on its Catalytic Properties in Carbon Monoxide Oxidation: A Comparative Study by Using Two Types of Catalytic Reactors. ChemCatChem. [CrossRef]
- Tagle, M., Smith, K.R., Pillarisetti, A., Teresa, M., Karin, H., Soares, A., Torres, R., Galeano, A., Oyola, P., Balmes, J., 2018. Monitoring and modeling of household air quality related to use of different Cookfuels in Paraguay. Indoor Air 1–11. [CrossRef]
- Tang, C., Zhou, Q., Zhu, S., Zhao, Z., Xu, L., Chen, W., Kumar, R., Gui, Y., 2017. Highly sensitive carbon monoxide (CO) gas sensors based on Ni and Zn doped SnO 2 nanomaterials. Ceram. Int. 44, 4392–4399. [CrossRef]
- Tang, J.-Y., Shen, J.-S., Chen, L., Jiang, J.-W., Lu, J., Zhao, X., Dai, G.-L., 2018. Investigation of carbon monoxide catalytic oxidation on vanadium-embedded graphene. Monatshefte fur Chemie. [CrossRef]
- Tess, M.E., Cox, J.A., 1998. Humidity-Independent Solid-State Amperometric Sensor for Carbon Monoxide Based on an Electrolyte Prepared by Sol-Gel Chemistry. Anal. Chem. 70, 187–190. [CrossRef]
- Thomazy, D., So, S., Kosterev, A., Lewicki, R., Dong, L., Sani, A.A., Tittel, F.K., 2010. Low-power laser-based carbon monoxide sensor for fire and post-fire detection using a compact Herriott multipass cell, in: Quantum Sensing and Nanophotonic Devices VII. pp. 76080C1-76080C6. [CrossRef]
- Thurmond, K., Loparo, Z., Partridge, W., Vasu, S.S., 2016. A light-emitting diode- (LED-) based absorption sensor for simultaneous detection of carbon monoxide and carbon dioxide. Appl. Spectrosc. 70, 962–971. [CrossRef]
- Tischner, A., Maier, T., Stepper, C., Köck, A., 2008. Ultrathin SnO2 gas sensors fabricated by spray pyrolysis for the detection of humidity and carbon monoxide. Sensors Actuators, B Chem. 134, 796–802. [CrossRef]
- Toscani, A., Mar, Ì., Moragues, Ì.E., Dingwall, P., Brown, N.J., Mart, Û., Çez, Ì., White, A.J.P., Wilton-ely, J.D.E.T., 2015. Ruthenium (II) and Osmium (II) Vinyl Complexes as Highly Sensitive and Selective Chromogenic and Fluorogenic Probes for the Sensing of Carbon Monoxide in Air. Chem. Eur. J. 21, 14529–14538. [CrossRef]
- Trubetskaya, A., Jensen, P.A., Jensen, A.D., Steibel, M., Spliethoff, H., Glarborg, P., Larsen, F.H., 2016. Comparison of high temperature chars of wheat straw and rice husk with respect to chemistry, morphology and reactivity. [CrossRef]
- Tsai, P.P., Chen, I.C., Tzeng, M.H., 1995. Tin oxide (SnOX) carbon monoxide sensor fabricated by thick-film methods. Sensors Actuators B. Chem. 25, 537–539. [CrossRef]
- Tschamber, V., Trouvé, G., Leyssens, G., Le-Dreff-Lorimier, C., Jaffrezo, J.L.J.-L., Genevray, P., Dewaele, D., Cazier, F., Labbé, S., Postel, S., 2016. Domestic Wood Heating Appliances with Environmental High Performance: Chemical Composition of Emission and Correlations between Emission Factors and Operating Conditions. Energy and Fuels 30, 7241–7255. [CrossRef]
- Umegaki, T., Inoue, T., Kojima, Y., 2016. Fabrication of hollow spheres of Co3O4 for catalytic oxidation of carbon monoxide. [CrossRef]
- Van Geloven, P., Honore, M., Roggen, J., Leppavuori, S., Rantala, T., 1991. The influence of relative humidity on the response of tin oxide gas sensors to carbon monoxide. Sensors Actuators B. Chem. 4, 185–188. [CrossRef]
- Vanderover, J., Oehlschlaeger, M.A., 2010. A mid-infrared scanned-wavelength laser absorption sensor for carbon monoxide and temperature measurements from 900 to 4000 K. Appl. Phys. B Lasers Opt. 99, 353–362. [CrossRef]
- Vanderover, J., Wang, W., Oehlschlaeger, M.A., 2011. A carbon monoxide and thermometry sensor based on mid-IR quantum-cascade laser wavelength-modulation absorption spectroscopy. Appl. Phys. B Lasers Opt. 103, 959–966. [CrossRef]
- Varjani, S., Kumar, G., Rene, E.R., 2019. Developments in biochar application for pesticide remediation : Current knowledge and future research directions. J. Environ. Manage. 232, 505–513. [CrossRef]
- Vlaev, L., Nedelchev, N., Gyurova, K., Zagorcheva, M., 2008. A comparative study of non-isothermal kinetics of decomposition of calcium oxalate monohydrate. J. Anal. Appl. Pyrolysis 81, 253–262. [CrossRef]
- Vyazovkin, S., 2006. Model-free Kinetics: Staying free of multiplying entities without necessity. J. Therm. Anal. Calorim. 83, 45–51.
- Vyazovkin, S., Wight, A, C., 1998. Isothermal and non-isotherm al kinetics of therm ally stimulated reactions of solids. Int. Rev. s Phys. Chem. 17, 407–433.
- Wan, S., Wu, J., Zhou, S., Wang, R., Gao, B., He, F., 2018. Enhanced lead and cadmium removal using biochar-supported hydrated manganese oxide (HMO) nanoparticles: Behavior and mechanism. [CrossRef]
- Wang, C.T., Chen, H.Y., Chen, Y.C., 2013. Gold/vanadium-tin oxide nanocomposites prepared by co-precipitation method for carbon monoxide gas sensors. Sensors Actuators, B Chem. 176, 945–951. [CrossRef]
- Wang, C.T., Chen, M.T., 2010. Vanadium-promoted tin oxide semiconductor carbon monoxide gas sensors. Sensors Actuators, B Chem. 150, 360–366. [CrossRef]
- Wang, G., Zhang, J., Shao, J., Sun, H., Zuo, H., 2014. Thermogravimetric Analysis of Coal Char Combustion Kinetics. J. Iron Steel Res. Int. 21, 897–904. [CrossRef]
- Wang, P., Howard, B.H., 2018. Impact of thermal pretreatment temperatures on woody biomass chemical composition, physical properties and microstructure. Energies 11, 25. [CrossRef]
- Wang, S., Zhao, Y., Huang, J., Wang, Y., Wu, S., Zhang, S., Huang, W., 2006. Low-temperature carbon monoxide gas sensors based gold/tin dioxide. Solid. State. Electron. 50, 1728–1731. [CrossRef]
- Wang, Z., Lin, L., Li, Y., Miao, B., Zeng, W., 2013. Recognition of carbon monoxide with SnO2/Ti thick-film sensor and its gas-sensing mechanism. Sensors Actuators B Chem. 191, 1–8. [CrossRef]
- Weber, K., Quicker, P., 2017. Properties of biochar. Fuel 217, 240–261. [CrossRef]
- Wielgosi!nski, G., Łechta!nska, P., Namiecinska, O., 2017. Emission of some pollutants from biomass combustion in comparison to hard coal combustion. J. Energy Inst. 90, 787e796. [CrossRef]
- Wilklow-Marnell, M., Jones, W.D., 2017. Catalytic oxidation of carbon monoxide by A-alumina supported 3 nm cerium dioxide nanoparticles. Mol. Catal. [CrossRef]
- Win, K.M., Persson, T., 2014. Emissions from residential wood pellet boilers and stove characterized into start-up, steady operation, and stop emissions. Energy & Fuels 28, 2496–2505. [CrossRef]
- Win, K.M., Persson, T., Bales, C., 2012. Particles and gaseous emissions from realistic operation of residential wood pellet heating systems. Atmos. Environ. 59, 320–327. [CrossRef]
- Windischmann, H., 2006. A Model for the Operation of a Thin-Film SnO2 Conductance-Modulation Carbon Monoxide Sensor. J. Electrochem. Soc. 126, 627. [CrossRef]
- Windischmann, H., Mark, P., 1979. A Model for the Operation of a Thin-Film SnOx Conductance-Modulation Carbon Monoxide Sensor. J. Electrochem. Soc. 126, 627–633. [CrossRef]
- Wu, R.J., Wu, J.G., Tsai, T.K., Yeh, C.T., 2006. Use of cobalt oxide CoOOH in a carbon monoxide sensor operating at low temperatures. Sensors Actuators, B Chem. 120, 104–109. [CrossRef]
- Xie, S., Dai, H., Deng, J., Yang, H., Han, W., Arandiyan, H., Guo, G., 2014. Preparation and high catalytic performance of Au/3DOM Mn2O3 for the oxidation of carbon monoxide and toluene. [CrossRef]
- Xu, T., Huang, H., Luan, W., Qi, Y., Tu, S. tung, 2008. Thermoelectric carbon monoxide sensor using Co-Ce catalyst. Sensors Actuators, B Chem. 133, 70–77. [CrossRef]
- Yang, F., Gu, C., Liu, B., Hou, C., Zhou, K., 2019. Pt-activated Ce4La6O17 nanocomposites for formaldehyde and carbon monoxide sensor at low operating temperature. J. Alloys Compd. 787, 173–179. [CrossRef]
- Yip, F., Christensen, B., Sircar, K., Naeher, L., Bruce, N., Pennise, D., Lozier, M., Pilishvili, T., Loo, J., Stanistreet, D., Nyagol, R., Muoki, J., Beer, L. De, Sage, M., Kapil, V., 2017. Assessment of traditional and improved stove use on household air pollution and personal exposures in rural western Kenya. Environ. Int. 99, 185–191. [CrossRef]
- Yu, I.K.M., Tsang, D.C.W., Yip, A.C.K., Chen, S.S., Wang, L., Ok, Y.S., Poon, C.S., 2017. Catalytic valorization of starch-rich food waste into hydroxymethylfurfural (HMF): Controlling relative kinetics for high productivity. [CrossRef]
- Zaikin, V.A., Chakhunashvili, G.B., Koltypin, E.A., Eryshkin, A.V., Vasiliev, A.A., Buturlin, A.I., Malyshev, V.V., Shubin, Y.I., 2002. Gas sensitivity of SnO2 and ZnO thin-film resistive sensors to hydrocarbons, carbon monoxide and hydrogen. Sensors Actuators B Chem. 10, 11–14. [CrossRef]
- Zajac, G., Szyszlak-Barglowicz, J., Slowik, T., Wasilewski, J., Kuranc, A., 2017. Emission characteristics of biomass combustion in a domestic heating boiler fed with wood and Virginia mallow pellets. Fresenius Environ. Bull. 26, 4663–4670.
- Zhang, G., Tai, H., Xie, G., Jiang, Y., Zhou, Y., 2013. A carbon monoxide sensor based on single-walled carbon nanotubes doped with copper chloride. Sci. China Technol. Sci. 56, 2576–2580. [CrossRef]
- Zhang, R., Lu, K., Zong, L., Tong, S., Wang, X., Zhou, J., Lu, Z.-H., Feng, G., 2017. Control synthesis of CeO2 nanomaterials supported gold for catalytic oxidation of carbon monoxide. Mol. Catal. [CrossRef]
- Zhang, W., Wu, F., Li, J., You, Z., 2017. Dispersion–precipitation synthesis of highly active nanosized Co3O4 for catalytic oxidation of carbon monoxide and propane. [CrossRef]
- Zhi, M., Koneru, A., Yang, F., Manivannan, A., Li, J., Wu, N., 2012. Electrospun La0.8Sr0.2MnO3 nanofibers for a high-temperature electrochemical carbon monoxide sensor. Nanotechnology 23. [CrossRef]
- Zhou, B., Yu, L., Song, H., Li, Y., Zhang, P., Guo, B., Duan, E., 2015. Adsorption and oxidation of SO2 in a fixed-bed reactor using activated carbon produced from oxytetracycline bacterial residue and impregnated with copper. [CrossRef]
- Zhu, L., Zheng, Y., Jian, J., 2015. Effect of palladium oxide electrode on potentiometric sensor response to carbon monoxide. Ionics (Kiel). 21, 2919–2926. [CrossRef]
- Zhu, S., Chen, Y., Zhang, G., Sa, J., 2010. An optical fiber sensor based on absorption spectroscopy for carbon monoxide detection, in: 2010 International Conference on Computer Design and Applications, ICCDA 2010. pp. 1–4. [CrossRef]
- Zhuiykov, S., 2008. Carbon monoxide detection at low temperatures by semiconductor sensor with nanostructured Au-doped CoOOH films. Sensors Actuators, B Chem. 129, 431–441. [CrossRef]
| Element | Coefficient | Hybrid poplar | Rice straw | Rice/Poplar |
|---|---|---|---|---|
| C | x1 | 4.1916 | 3.2072 | 0.77 |
| H | x2 | 6.0322 | 5.1973 | 0.86 |
| O | x3 | 2.5828 | 2.8148 | 1.09 |
| N | x4 | 0.0430 | 0.0625 | 1.45 |
| S | x5 | 0.0006 | 0.0057 | 9.50 |
| Cl | x6 | 0.0003 | 0.0165 | 55.00 |
| Si | x7 | 0.0057 | 0.5000 | 87.72 |
| K | x8 | 0.0067 | 0.0592 | 8.84 |
| Ca | x9 | 0.0337 | 0.0141 | 0.42 |
| Mg | x10 | 0.0205 | 0.0135 | 0.66 |
| Na | x11 | 0.0002 | 0.0079 | 39.50 |
| P | x12 | 0.0012 | 0.0086 | 7.17 |
| Fe | x13 | 0.0007 | 0.0029 | 4.14 |
| Al | x14 | 0.0008 | 0.0073 | 9.13 |
| Ti | x15 | 0.0002 | 0.0004 | 2.00 |
| Method | Equation | Reference |
|---|---|---|
| Friedman | (Starink, 2003), (Fedunik-hofman et al., 2019) | |
| Gupita | (Gupta et al., 1988) | |
| Freeman & Car-roll |
where, and |
(Jerez, 1983) |
| Kissinger-Akahira-Sanose (KAS) | (Danvirutai and Noisong, 2015) | |
| Flyn-Wall-Onzawa (FWO) | (Ozawa, 1965), (Joseph and Leo, 1966) | |
| Starink | (Starink, 2003) | |
| Boswel | (Starink, 2003), (Boswell, 1980) | |
| Coats and Redfern | (Sajjad et al., 2017) | |
| ASTM-E698 | (Osman et al., 2017) | |
| Karaosmanoglu & Cif | (Fernandez et al., 2017) | |
| Isothermal method | (Wang et al., 2014) | |
| FWO and KAS Iterative methods |
where and which is the 4th degree Senum and Yang approximation that gives an accuracy better than 10-5 % for x = E/RT ≥20. |
(Senum and Yang, 1977), (Pérez-Maqueda and Criado, 2000) |
| Vyazovkin |
Where the time integral: where, T(t) is the actual sample temperature, J is the integral with respect to T(t) and Ti(t) is the temperature programs |
(Vyazovkin and Wight, A, 1998), (Vyazovkin, 2006) |
| Kissinger | (Kissinger, 1956), (Blaine and Kissinger, 2012) |
| No. | Symbol | Name of the Function | g(α) | f(α) | Rate-determining mechanism |
|---|---|---|---|---|---|
| 1. Chemical process or mechanism non-invoking equations | |||||
| 1 | F1/3 | One-third order | 1-(1-α)2/3 | (3/2)(1-α)1/3 | Chemical reaction |
| 2 | F3/4 | Three-quarters order | 1-(1- α)1/4 | 4(1-α)3/4 | Chemical reaction |
| 3 | F3/2 | one and a half order | [(1- α)-1/2-1] | 2(1-α)3/2 | Chemical reaction |
| 4 | F2 | Second order | (1- α)-1-1 | (1-α)2 | Chemical reaction |
| 5 | F3 | Third order | (1- α)-2-1 | (1/2)(1-α)3 | Chemical reaction |
| 2. Acceleratory rate equations | |||||
| 6 | P3/2 | Mampel power law | α3/2 | (2/3)α-1/2 | Nucleation |
| 7 | P1/2 | Mampel power law | α1/2 | 2α1/2 | Nucleation |
| 8 | P1/3 | Mampel power law | α1/3 | 3α2/3 | Nucleation |
| 9 | P1/4 | Mampel power law | α1/4 | 4α3/4 | Nucleation |
| 10 | E1 | Exponential law | lnα | α | Nucleation |
| 3. Sigmoidal rate equations or random nucleation and subsequent growth | |||||
| 11 | A1, F1 | Avrami-Erofeev equation | -ln(1- α) | (1-α) | Assumed random nucleation and its subsequent growth, n=1 |
| 12 | A3/2 | Avrami-Erofeev equation | [-ln(1-α)]2/3 | (3/2)(1-α)[-In(1-α)]1/3 | Assumed random nucleation and its subsequent growth, n=1.5 |
| 13 | A2 | Avrami-Erofeev equation | [-ln(1-α)]1/2 | 2(1-α)[-In(1-α)]1/2 | Assumed random nucleation and its subsequent growth, n=2 |
| 14 | A3 | Avrami-Erofeev equation | [-ln(1-α)]1/3 | 3(1-α)[-In(1-α)]2/3 | Assumed random nucleation and its subsequent growth, n=3 |
| 15 | A4 | Avrami-Erofeev equation | [-ln(1-α)]1/4 | 4(1-α)[-In(1-α)]3/4 | Assumed random nucleation and its subsequent growth, n=4 |
| 16 | Au | Prout-Tomkins equation | Ln[ɑ/(1-α)] | α (1-α) | Branching nuclei |
| 4. Deceleratory rate equations 4.1 Phase boundary reactions | |||||
| 17 | R1, F0, P1 | Power law | α | (1-α)0 | Contracting disk |
| 18 | R2, F1/2 | Power law | 1-(1-α)1/2 | 2(1-α)1/2 | Contracting cylinder (Cylindrical symmetry) |
| 19 | R3, F2/3 | Power law | 1-(1-α)1/3 | 3(1-α)2/3 | Contracting sphere (spherical symmetry) |
| 4.2 Based on the diffusion mechanism | |||||
| 20 | D1 | Parabola low | α2 | 1/2α | One-dimensional diffusion |
| 21 | D2 | Valensi equation | α+(1- α)ln(1-α) | [-In(1-α)]-1 | Two-dimension diffusion |
| 22 | D3 | Jander equation | [1-(1-α)1/3]2 | (3/2)(1-α)2/3[1-(1-α)1/3]-1 | Three-dimensional diffusion, Spherical symmetry |
| 23 | D4 | Ginstling-Brounstein equation | [1-2α/3-(1-α)2/3 | (3/2)[(1-α)-1/3-1]-1 | Three-dimensional diffusion, Cylindrical symmetry |
| 44 | D5 | Zhuravlev, Lesokin, Tempelman equation | [(1-α)-1/3-1]2 | (3/2)(1-α)4/3[(1-α)-1/3-1]-1 | Three-dimensional diffusion |
| 25 | D6 | Anti-Jander equation | [(1+α)1/3-1]2 | (3/2)(1+α)2/3[(1+α)1/3-1]-1 | Three-dimensional diffusion |
| 26 | D7 | Anti-Ginstling-Brounstein equation | 1+2ɑ/3-(1+ɑ)2/3 | (3/2)[(1+α)-1/3-1]-1 | Three-dimensional diffusion |
| 27 | D8 | Anti-Zhuravlev, Lesokin, Tempelman equation | [(1+α)-1/3-1]2 | (3/2)(1+α4/3[(1+α)-1/3-1]-1 | Three-dimensional diffusion |
| 5. Another Kinetic equation with unjustified mechanism | |||||
| 28 | G1 | 1-(1-α)2 | ½(1-α) | ||
| 29 | G2 | 1-(1-α)3 | 1/3(1-α)2 | ||
| 30 | G3 | 1-(1-α)4 | 1/4(1-α)3 | ||
| 31 | G4 | [-In(1-α)2 | (1/2)(1-α)[1In(1-α)]-1 | ||
| 32 | G5 | [-In(1-α)3 | (1/3)(1-α)[1In(1-α)]-2 | ||
| 33 | G6 | [-In(1-α)4 | (1/4)(1-α)[1In(1-α)]-3 | ||
| 34 | G7 | [1-(1-α)1/2]1/2 | 4{(1-α)[1-(1-α)1/2}1/2 | ||
| 35 | G8 | [1-(1-α)1/3]1/2 | 6{(1-α)2/3[1-(1-α)1/3}1/2 | ||
| ICD 10 Code | Underlying cause | England | Wales | ||||||||
| 2011 | 2012 | 2013 | 2014 | 2015 | 2011 | 2012 | 2013 | 2014 | 2015 | ||
| V00-X59 | All accidental carbon monoxide poisonings | 75 | 58 | 57 | 52 | 48 | 4 | 7 | 3 | 3 | 5 |
| X47 | Accidental poisoning by other gases and vapours | 33 | 23 | 22 | 25 | 24 | 1 | 2 | 2 | 1 | 1 |
| X47.0 | Occurrence at home | 28 | 17 | 14 | 18 | 23 | 1 | 1 | 2 | 0 | 1 |
| X47.1 | Occurrence in residential institution | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| X47.2 | Occurrence at school other institution/public admin area | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| X47.3 | Occurrence at sports/athletics area | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| X47.4 | Occurrence on street/highway | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| X47.5 | Occurrence at trade/service area | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| X47.6 | Occurrence at industrial/construction area | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| X47.7 | Occurrence on farm | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| X47.8 | Occurrence at other specified place | 3 | 4 | 6 | 4 | 1 | 0 | 1 | 0 | 1 | 0 |
| X47.9 | Occurrence at unspecified place | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| V01-V99 | Transport accident | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| X00-X09 | Accidental exposure to smoke, fire and flames | 42 | 35 | 34 | 27 | 23 | 3 | 5 | 1 | 2 | 4 |
| Description of traditional stoves | Description of improved stoves | CO from traditional stove | CO from improved stove | % CO reduction | Reference |
|---|---|---|---|---|---|
| Three-brick stove, a cooking pot placed over the bricks uses fire wood | Made of clay and husk, two cooking pots, and a stack | 10 ppm | 2.5 ppm | 75 | (Jamali et al., 2017) |
| stoves with chimneys or smoke hoods | 11.9 ppma | 5.1 ppmb | 57.1 | (Pope et al., 2017) | |
| stoves without chimneys or smoke hoods | 10.8 ppma | 6.6 ppmb | 38.7 | (Pope et al., 2017) | |
| Charcoal stoves | 27.4 ppma | 9.6 ppmb | 64.9 | (Pope et al., 2017) | |
| Advanced combustion stoves | 11.3 ppma | 5.7 ppmb | 49.7 | (Pope et al., 2017) | |
| The Hifadhi stove has an air entrance and a combustion chamber. | Galvanised Gasifier cook stove | 42 ppm | 20 ppm | 52.0 | (Njenga et al., 2016) |
| Three stone cook stove - burning prunings, maize cobs, and coconut shells | Galvanised Gasifier cook stove | 36.5 ppm | 20 ppm | 45 | (Njenga et al., 2016) |
| Three-stone open fires for burning wood | Metal braziers for burning charcoal | 19.4 ppm | 7.6 ppm | 60.8 | (Tagle et al., 2018) |
| Open in the home, or in an annexed kitchen or outside the home, or under a ledge along outer house wall | Stove with chimney, in good condition; little to no visible damage | 5.0% | 4.6 % | 8.0 | (Lucarelli et al., 2018) |
| Open fire or poorly designed combustion chambers– burning wood | Improved stove: with a chimney and a better combustion system – burning wood | 14.3 ppm | 1.8 ppm | 87.4 | (Clark et al., 2009) |
| The traditional stationary hearth and the portable hearth. All burn biomass | Philips advanced biomass combustion stoves, with two-stage combustion & forced air | 30 ppm | 7.4 ppm | 75.3 | (Mukhopadhyay et al., 2012) |
| Traditional three-stone open fires for burning wood | Envirofit B1200-Natural Draft (rocket stove) | 9.6 ppm | 6.4 ppm | 33.3 | (Sambandam et al., 2015) |
| Envirofit G3300 Natural Draft rocket stove | 10.2 ppm | 7.5 ppm | 26.5 | (Sambandam et al., 2015) | |
| Prakti Leo-Natural Draft (rocket stove) | 11.6 ppm | 4.7 ppm | 59.5 | (Sambandam et al., 2015) | |
| Philips-Natural Draft (micro gasifier) | 3.6 ppm | 3.2 ppm | 11.1 | (Sambandam et al., 2015) | |
| Philips-Forced Draft (micro gasifier) | 29 ppm | 9.6 ppm | 66.9 | (Sambandam et al., 2015) | |
| Oorja forced draft micro gasifier using pellets | 4.3 ppm | 2.7 ppm | 37.2 | (Sambandam et al., 2015) | |
| Traditional 3 stone | Eco Chula - Electric fan-assisted gasifier | 6.5 ppm | 5.4 ppm | 16.9 | (Yip et al., 2017) |
| EcoZoom - Improved rocket | 6.5 ppm | 6.7 ppm | -3.1 | (Yip et al., 2017) | |
| Envirofit - Improved rocket | 6.5 ppm | 4.9 ppm | 24.6 | (Yip et al., 2017) | |
| Philips - Electric fan-assisted gasifier | 6.5 ppm | 3.8 ppm | 40.0 | (Yip et al., 2017) | |
| Prakti - Double pot rocket with chimney | 6.5 ppm | 4.5 ppm | 30.8 | (Yip et al., 2017) | |
| Built-in rocket stove | 6.5 ppm | 4.4 ppm | 32.3 | (Yip et al., 2017) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




