Submitted:
07 October 2023
Posted:
08 October 2023
You are already at the latest version
Abstract
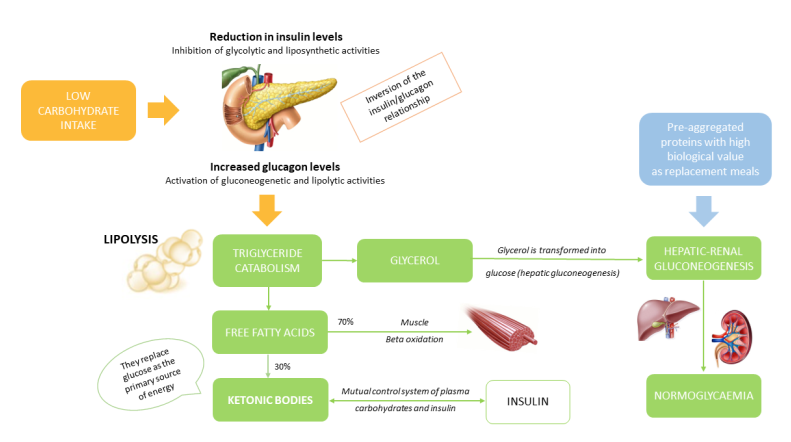
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study population
2.2. Study design
2.3. Assays
2.4. Statistical analysis
3. Results
| Patients with Cushing’s disease (N=15) |
|||||
|---|---|---|---|---|---|
| Baseline Mean (± SD) |
Week 1 Mean (± SD) |
Week 3 Mean (± SD) |
Week 5 Mean (± SD) |
p |
|
| Clinical parameters | |||||
| BMI (Kg/m2) | 35.7 ± 6.54 | 34.4 ± 6.15 | 33.2 ± 5.91 | 32.3 ± 5.53 | 0.002 |
| Waist circumference (cm) | 114.4 ± 12.7 | 111.6 ± 11.1 | 109 ± 10.7 | 107.8 ± 8.16 | 0.024 |
| Systolic blood pressure (mmHg) | 125.8 ± 15.6 | 120.4 ± 11.8 | 118.4 ± 10.3 | 113.8 ± 9.6 | 0.015 |
| Diastolic blood pressure (mmHg) | 88.2 ± 7.8 | 85.4 ± 5.8 | 84.2 ± 6.1 | 82.1 ± 5.1 | 0.005 |
| Hormonal parameters | |||||
| Fasting serum cortisol (mcg/dL) | 11.4 ± 2.22 | 12.7 ± 3.33 | 14.2 ± 3.08 | 14.7 ± 2.53 | 0.122 |
| Fasting serum ACTH (ng/L) | 77.8 ± 40.4 | 60.3 ± 29.5 | 58.2 ± 27.3 | 41.8 ± 26.9 | 0.026 |
| Urinary free cortisol (mcg/24h) | 56.8 ± 30.9 | 67.9 ± 43.3 | 55.6 ± 43.7 | 52.3 ± 37.6 | 0.472 |
| Late-night salivary cortisol | 6.67 ± 4.76 | 7.38 ± 3.32 | 4.76 ± 3.73 | 2.67 ± 2.33 | 0.160 |
| Cortisol after low dose suppression test (mcg/dL) | 4.14 ± 3.55 | 3.89 ± 3.03 | 3.88 ± 3.01 | 3.06 ± 2.85 | 0.139 |
| 17OHP (mcg/L) | 0.53 ± 0.22 | 0.63 ± 0.21 | 0.85 ± 0.18 | 0.76 ± 0.14 | 0.897 |
| Androstenedione (mcg/L) | 2.86 ± 2.31 | 2.5 ± 2.11 | 2.36 ± 1.47 | 3.05 ± 1.98 | 0.558 |
| DHEAS (mcg/L) | 1743 ± 1110 | 1892.5 ± 165.6 | 1871.7 ± 333.5 | 1656.7 ± 323.5 | 0.125 |
| Cortisone (mcgd/L) | 15.1 ± 3.9 | 16.6 ± 4.01 | 17.8 ± 3.85 | 19.8 ± 4.51 | 0.025 |
| Metabolic parameters | |||||
| Glycaemia (mmol/L) | 4.91 ± 0.83 | 4.88 ± 1.11 | 4.94 ± 0.55 | 4.46 ± 0.7 | 0.356 |
| Insulinemia (microU/mL) | 6.7 ± 4.02 | 5.8 ± 3.11 | 4.15 ± 3.33 | 5.63 ± 5.02 | 0.241 |
| Total cholesterol (mmol/L) | 4.96 ± 0.6 | 4.92 ± 0.83 | 4.16 ± 0.75 | 4.06 ± 0.81 | 0.006 |
| HDL cholesterol (mmol/L) | 1.36 ± 0.28 | 1.43 ± 0.28 | 1.46 ± 0.35 | 1.63 ± 0.3 | 0.017 |
| Triglycerides (mmol/L) | 1.2 ± 0.5 | 0.9 ± 0.23 | 0.89 ± 0.2 | 0.75 ± 0.15 | 0.040 |
| LDL cholesterol (mmol/L) | 2.63 ± 0.58 | 2.78 ± 0.74 | 2.22 ± 0.75 | 2.25 ± 0.78 | 0.017 |
| GOT (U/L) | 17.5 ± 5.06 | 20.1 ± 11.3 | 16.7 ± 8.99 | 15.2 ± 8.77 | 0.075 |
| GPT (U/L) | 22.2 ± 10.8 | 24.6 ± 15.7 | 24.6 ± 12.3 | 27 ± 11.5 | 0.166 |
| Alkaline phosphatase (U/L) | 71.7 ± 21 | 68 ± 19 | 64.2 ± 21.2 | 57.2 ± 20.2 | 0.008 |
| GammaGT (U/L) | 15.2 ± 8.01 | 16.5 ± 6.24 | 14.2 ± 3.86 | 14.5 ± 5.01 | 0.431 |
| Creatinine (mg/dL) | 0.72 ± 0.08 | 0.82 ± 0.13 | 0.78 ± 0.13 | 0.78 ± 0.12 | 0.055 |
| Na (mmol/L) | 140.2 ± 2.16 | 139.6 ± 1.67 | 139.6 ± 1.14 | 139.8 ± 1.30 | 0.824 |
| K (mmol/L) | 4.44 ± 0.42 | 4.32 ± 0.31 | 4.12 ± 0.27 | 4.08 ± 0.35 | 0.063 |
| Calcium (mg/dL) | 9.36 ± 0.25 | 9.68 ± 0.23 | 9.58 ± 0.24 | 9.34 ± 0.36 | 0.246 |
| Phosphorus (mg/dL) | 3.28 ± 0.29 | 3.36 ± 0.34 | 3.32 ± 0.24 | 3.14 ± 0.27 | 0.952 |
| Vitamin D (mcg/L) | 21.4 ± 5.223. | 27.2 ± 6.53 | 30.2 ± 7.69 | 32 ± 14.03 | 0.015 |
| PTH (ng/L) | 52.7 ± 17.5 | 44.7 ± 14.7 | 50.5 ± 21.8 | 38.5 ± 27.7 | 0.188 |
| C-reactive protein (mg/L) | 2.44 ± 1.92 | 2.34 ± 2.28 | 1.13 ± 1.02 | 1.39 ± 1.35 | 0.682 |

4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbot, M.; Zilio, M.; Scaroni, C. Cushing’s Syndrome: Overview of Clinical Presentation, Diagnostic Tools and Complications. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101380. [CrossRef]
- Guarnotta, V.; Prinzi, A.; Pitrone, M.; Pizzolanti, G.; Giordano, C. Circulating Irisin Levels as a Marker of Osteosarcopenic-Obesity in Cushing’s Disease. DMSO 2020, 13, 1565–1574. [CrossRef]
- Nieman, L.K. Hypertension and Cardiovascular Mortality in Patients with Cushing Syndrome. Endocrinol. Metab. Clin. North Am. 2019, 48, 717–725. [CrossRef]
- Kirkpatrick, C.F.; Bolick, J.P.; Kris-Etherton, P.M.; Sikand, G.; Aspry, K.E.; Soffer, D.E.; Willard, K.-E.; Maki, K.C. Review of Current Evidence and Clinical Recommendations on the Effects of Low-Carbohydrate and Very-Low-Carbohydrate (Including Ketogenic) Diets for the Management of Body Weight and Other Cardiometabolic Risk Factors: A Scientific Statement from the National Lipid Association Nutrition and Lifestyle Task Force. J. Clin. Lipidol. 2019, 13, 689-711.e1. [CrossRef]
- Paoli, A.; Rubini, A.; Volek, J.S.; Grimaldi, K.A. Beyond Weight Loss: A Review of the Therapeutic Uses of Very-Low-Carbohydrate (Ketogenic) Diets. Eur J Clin Nutr 2013, 67, 789–796. [CrossRef]
- Cicero, A.F.G.; Benelli, M.; Brancaleoni, M.; Dainelli, G.; Merlini, D.; Negri, R. Middle and Long-Term Impact of a Very Low-Carbohydrate Ketogenic Diet on Cardiometabolic Factors: A Multi-Center, Cross-Sectional, Clinical Study. High Blood Press Cardiovasc Prev 2015, 22, 389–394. [CrossRef]
- Merra, G.; Miranda, R.; Barrucco, S.; Gualtieri, P.; Mazza, M.; Moriconi, E.; Marchetti, M.; Chang, T.F.M.; De Lorenzo, A.; Di Renzo, L. Very-Low-Calorie Ketogenic Diet with Aminoacid Supplement versus Very Low Restricted-Calorie Diet for Preserving Muscle Mass during Weight Loss: A Pilot Double-Blind Study. Eur Rev Med Pharmacol Sci 2016, 20, 2613–2621.
- Antonio, J.; Ellerbroek, A.; Silver, T.; Vargas, L.; Tamayo, A.; Buehn, R.; Peacock, C.A. A High Protein Diet Has No Harmful Effects: A One-Year Crossover Study in Resistance-Trained Males. J. Nutr. Metab. 2016, 2016, 1–5. [CrossRef]
- Guarnotta, V.; Emanuele, F.; Amodei, R.; Giordano, C. Very Low-Calorie Ketogenic Diet: A Potential Application in the Treatment of Hypercortisolism Comorbidities. Nutrients 2022, 14, 2388. [CrossRef]
- Lemmens, S.G.; Born, J.M.; Martens, E.A.; Martens, M.J.; Westerterp-Plantenga, M.S. Influence of Consumption of a High-Protein vs. High-Carbohydrate Meal on the Physiological Cortisol and Psychological Mood Response in Men and Women. PLoS ONE 2011, 6, e16826. [CrossRef]
- Galvão-Teles, A.; Graves, L.; Burke, C.W.; Fotherby, K.; Fraser, R. FREE CORTISOL IN OBESITY; EFFECT OF FASTING. Acta Endocrinol. 1976, 81, 321–329. [CrossRef]
- Effects of Weight Loss on the Dexamethasone Suppression Test. AJP 1983, 140, 338–341. [CrossRef]
- Tomiyama, A.J.; Mann, T.; Vinas, D.; Hunger, J.M.; DeJager, J.; Taylor, S.E. Low Calorie Dieting Increases Cortisol. Psychosom. Med. 2010, 72, 357–364. [CrossRef]
- Peeters, F.; Nicholson, N.A.; Berkhof, J. Cortisol Responses to Daily Events in Major Depressive Disorder. Psychosom. Med. 2003, 65, 836–841. [CrossRef]
- Martens, M.J.I.; Rutters, F.; Lemmens, S.G.T.; Born, J.M.; Westerterp-Plantenga, M.S. Effects of Single Macronutrients on Serum Cortisol Concentrations in Normal Weight Men. Physiol. Behav. 2010, 101, 563–567. [CrossRef]
- Bray, G.A.; Most, M.; Rood, J.; Redmann, S.; Smith, S.R. Hormonal Responses to a Fast-Food Meal Compared with Nutritionally Comparable Meals of Different Composition. Ann Nutr Metab 2007, 51, 163–171. [CrossRef]
- Berger, M. Influence of Weight Loss on the Dexamethasone Suppression Test. Arch Gen Psychiatry 1983, 40, 585. [CrossRef]
- Abbasi, J. Interest in the Ketogenic Diet Grows for Weight Loss and Type 2 Diabetes. JAMA 2018, 319, 215. [CrossRef]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of Fats and Carbohydrate Intake with Cardiovascular Disease and Mortality in 18 Countries from Five Continents (PURE): A Prospective Cohort Study. Lancet 2017, 390, 2050–2062. [CrossRef]
- Fleseriu, M.; Auchus, R.; Bancos, I.; Ben-Shlomo, A.; Bertherat, J.; Biermasz, N.R.; Boguszewski, C.L.; Bronstein, M.D.; Buchfelder, M.; Carmichael, J.D.; et al. Consensus on Diagnosis and Management of Cushing’s Disease: A Guideline Update. Lancet Diabetes Endocrinol. 2021, 9, 847–875. [CrossRef]
- Barbot, M.; Guarnotta, V.; Zilio, M.; Ceccato, F.; Ciresi, A.; Daniele, A.; Pizzolanti, G.; Campello, E.; Frigo, A.C.; Giordano, C.; et al. Effects of Pasireotide Treatment on Coagulative Profile: A Prospective Study in Patients with Cushing’s Disease. Endocrine 2018, 62, 207–214. [CrossRef]
- Perticone, M.; Maio, R.; Sciacqua, A.; Suraci, E.; Pinto, A.; Pujia, R.; Zito, R.; Gigliotti, S.; Sesti, G.; Perticone, F. Ketogenic Diet-Induced Weight Loss Is Associated with an Increase in Vitamin D Levels in Obese Adults. Molecules 2019, 24, 2499. [CrossRef]
- Dubuc, G.R.; Phinney, S.D.; Stern, J.S.; Havel, P.J. Changes of Serum Leptin and Endocrine and Metabolic Parameters after 7 Days of Energy Restriction in Men and Women. Metabolism 1998, 47, 429–434. [CrossRef]
- Tomlinson, J.W.; Walker, E.A.; Bujalska, I.J.; Draper, N.; Lavery, G.G.; Cooper, M.S.; Hewison, M.; Stewart, P.M. 11beta-Hydroxysteroid Dehydrogenase Type 1: A Tissue-Specific Regulator of Glucocorticoid Response. Endocr Rev 2004, 25, 831–866. [CrossRef]
- Purnell, J.Q.; Kahn, S.E.; Samuels, M.H.; Brandon, D.; Loriaux, D.L.; Brunzell, J.D. Enhanced Cortisol Production Rates, Free Cortisol, and 11beta-HSD-1 Expression Correlate with Visceral Fat and Insulin Resistance in Men: Effect of Weight Loss. Am J Physiol Endocrinol Metab 2009, 296, E351-357. [CrossRef]
- Stimson, R.H.; Johnstone, A.M.; Homer, N.Z.M.; Wake, D.J.; Morton, N.M.; Andrew, R.; Lobley, G.E.; Walker, B.R. Dietary Macronutrient Content Alters Cortisol Metabolism Independently of Body Weight Changes in Obese Men. J Clin Endocrinol Metab 2007, 92, 4480–4484. [CrossRef]
- Stimson, R.H.; Mohd-Shukri, N.A.; Bolton, J.L.; Andrew, R.; Reynolds, R.M.; Walker, B.R. The Postprandial Rise in Plasma Cortisol in Men Is Mediated by Macronutrient-Specific Stimulation of Adrenal and Extra-Adrenal Cortisol Production. J. Clin. Endocrinol. Metab. 2014, 99, 160–168. [CrossRef]
- Stomby, A.; Simonyte, K.; Mellberg, C.; Ryberg, M.; Stimson, R.H.; Larsson, C.; Lindahl, B.; Andrew, R.; Walker, B.R.; Olsson, T. Diet-Induced Weight Loss Has Chronic Tissue-Specific Effects on Glucocorticoid Metabolism in Overweight Postmenopausal Women. Int J Obes (Lond) 2015, 39, 814–819. [CrossRef]
- Engeli, S.; Böhnke, J.; Feldpausch, M.; Gorzelniak, K.; Heintze, U.; Janke, J.; Luft, F.C.; Sharma, A.M. Regulation of 11beta-HSD Genes in Human Adipose Tissue: Influence of Central Obesity and Weight Loss. Obes Res 2004, 12, 9–17. [CrossRef]
- Woods, C.P.; Corrigan, M.; Gathercole, L.; Taylor, A.; Hughes, B.; Gaoatswe, G.; Manolopoulos, K.; Hogan, A.E.; O’Connell, J.; Stewart, P.M.; et al. Tissue Specific Regulation of Glucocorticoids in Severe Obesity and the Response to Significant Weight Loss Following Bariatric Surgery (BARICORT). J Clin Endocrinol Metab 2015, 100, 1434–1444. [CrossRef]
- Nakamura, Y.; Walker, B.R.; Ikuta, T. Systematic Review and Meta-Analysis Reveals Acutely Elevated Plasma Cortisol Following Fasting but Not Less Severe Calorie Restriction. Stress 2016, 19, 151–157. [CrossRef]
- Capello, A.E.M.; Markus, C.R. Effect of Sub Chronic Tryptophan Supplementation on Stress-Induced Cortisol and Appetite in Subjects Differing in 5-HTTLPR Genotype and Trait Neuroticism. Psychoneuroendocrinology 2014, 45, 96–107. [CrossRef]
- Starks, M.A.; Starks, S.L.; Kingsley, M.; Purpura, M.; Jäger, R. The Effects of Phosphatidylserine on Endocrine Response to Moderate Intensity Exercise. J. Int. Soc. Sports Nutr. 2008, 5, 11. [CrossRef]
- Miklós, I.H.; Kovács, K.J. GABAergic Innervation of Corticotropin-Releasing Hormone (CRH)-Secreting Parvocellular Neurons and Its Plasticity as Demonstrated by Quantitative Immunoelectron Microscopy. Neuroscience 2002, 113, 581–592. [CrossRef]
- Song, Z. Taurine and the Control of Basal Hormone Release from Rat Neurohypophysis. Exp. Neurol. 2003, 183, 330–337. [CrossRef]
- Dugandzic, M.K.; Pierre-Michel, E.-C.; Kalayjian, T. Ketogenic Diet Initially Masks Symptoms of Hypercortisolism in Cushing’s Disease. Metabolites 2022, 12, 1033. [CrossRef]
- Yang, Z.; Mi, J.; Wang, Y.; Xue, L.; Liu, J.; Fan, M.; Zhang, D.; Wang, L.; Qian, H.; Li, Y. Effects of Low-Carbohydrate Diet and Ketogenic Diet on Glucose and Lipid Metabolism in Type 2 Diabetic Mice. Nutrition 2021, 89, 111230. [CrossRef]
- Ma, S.; Huang, Q.; Tominaga, T.; Liu, C.; Suzuki, K. An 8-Week Ketogenic Diet Alternated Interleukin-6, Ketolytic and Lipolytic Gene Expression, and Enhanced Exercise Capacity in Mice. Nutrients 2018, 10, 1696. [CrossRef]
- Xu, Y.; Qiao, J. Association of Insulin Resistance and Elevated Androgen Levels with Polycystic Ovarian Syndrome (PCOS): A Review of Literature. J. Healthc. Eng. 2022, 2022, 1–13. [CrossRef]
- Mavropoulos, J.C.; Yancy, W.S.; Hepburn, J.; Westman, E.C. The Effects of a Low-Carbohydrate, Ketogenic Diet on the Polycystic Ovary Syndrome: A Pilot Study. Nutr Metab (Lond) 2005, 2, 35. [CrossRef]
- Gower, B.A.; Chandler-Laney, P.C.; Ovalle, F.; Goree, L.L.; Azziz, R.; Desmond, R.A.; Granger, W.M.; Goss, A.M.; Bates, G.W. Favourable Metabolic Effects of a Eucaloric Lower-Carbohydrate Diet in Women with PCOS. Clin Endocrinol 2013, 79, 550–557. [CrossRef]
- Khan, S.S.; Ning, H.; Wilkins, J.T.; Allen, N.; Carnethon, M.; Berry, J.D.; Sweis, R.N.; Lloyd-Jones, D.M. Association of Body Mass Index With Lifetime Risk of Cardiovascular Disease and Compression of Morbidity. JAMA Cardiol 2018, 3, 280. [CrossRef]
- Palgi, A.; Read, J.L.; Greenberg, I.; Hoefer, M.A.; Bistrian, B.R.; Blackburn, G.L. Multidisciplinary Treatment of Obesity with a Protein-Sparing Modified Fast: Results in 668 Outpatients. Am J Public Health 1985, 75, 1190–1194. [CrossRef]
- Ferrannini, E.; Baldi, S.; Frascerra, S.; Astiarraga, B.; Heise, T.; Bizzotto, R.; Mari, A.; Pieber, T.R.; Muscelli, E. Shift to Fatty Substrate Utilization in Response to Sodium–Glucose Cotransporter 2 Inhibition in Subjects Without Diabetes and Patients With Type 2 Diabetes. Diabetes 2016, 65, 1190–1195. [CrossRef]
- Monda, V.; Polito, R.; Lovino, A.; Finaldi, A.; Valenzano, A.; Nigro, E.; Corso, G.; Sessa, F.; Asmundo, A.; Di Nunno, N.; et al. Short-Term Physiological Effects of a Very Low-Calorie Ketogenic Diet: Effects on Adiponectin Levels and Inflammatory States. IJMS 2020, 21, 3228. [CrossRef]
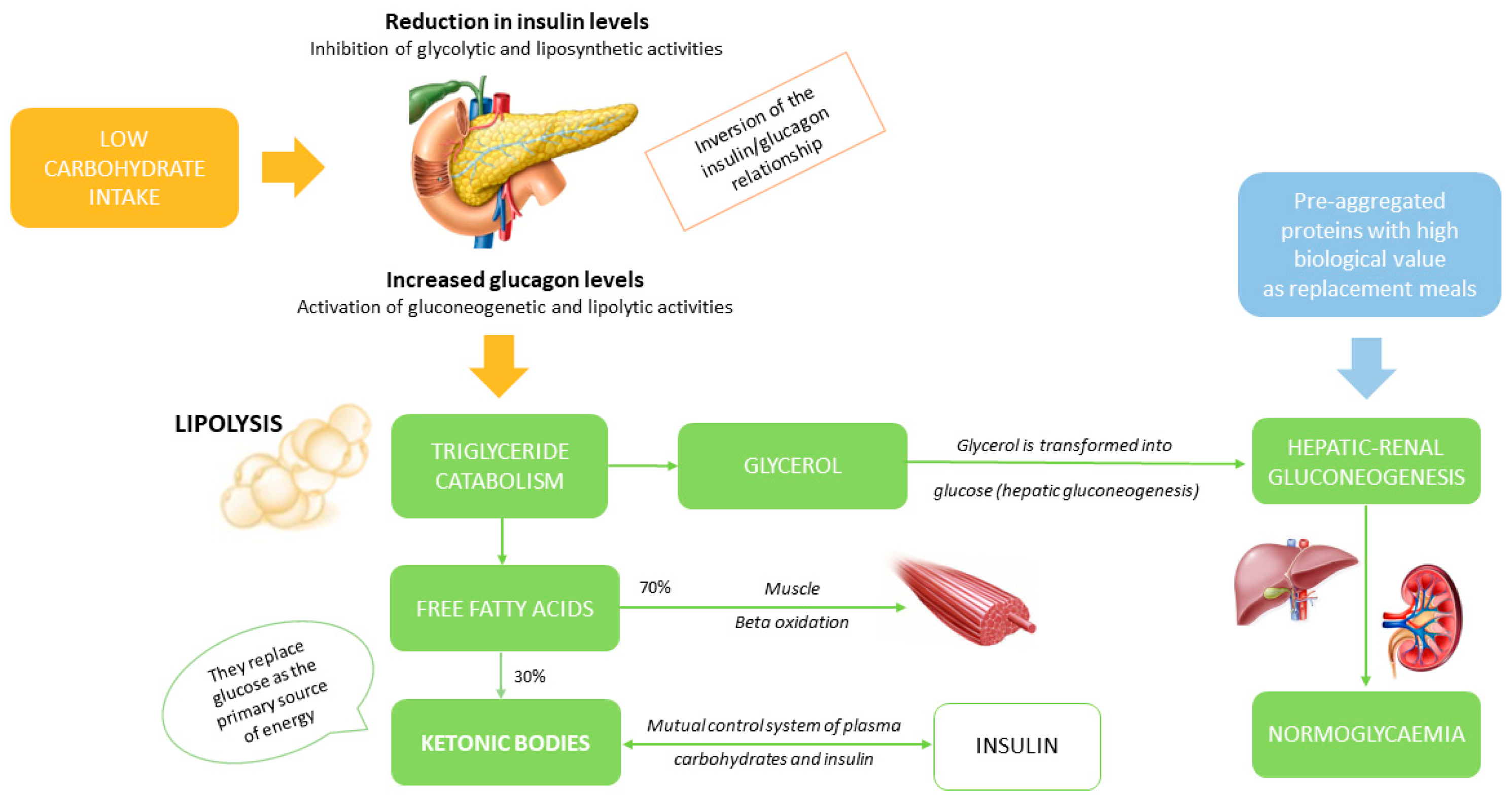

| Patients with Cushing’s disease (N=15) |
Controls (N=15) |
p |
|
| Mean ± SD | Mean ± SD | ||
| Clinical parameters | |||
| Age (years) | 47.2 ± 10.6 | 50.8 ± 11.7 | 0.535 |
| BMI (Kg/m2) | 35.7 ± 4.5 | 32.8 ± 2.14 | 0.175 |
| Waist circumference (cm) | 114.4 ± 12.7 | 96.6 ± 14.2 | 0.035 |
| Systolic blood pressure (mmHg) | 125.8 ± 15.6 | 122.3 ± 8.6 | 0.453 |
| Diastolic blood pressure (mmHg) | 88.2 ± 7.8 | 83.2 ± 4.8 | 0.057 |
| Hormonal parameters | |||
| Fasting serum cortisol (mcg/dL) | 13.2 ± 4.11 | 11.2 ± 3.44 | 0.443 |
| Fasting serum ACTH (ng/L) | 77.8 ± 40.8 | 27.1 ± 19.5 | 0.043 |
| Urinary free cortisol (mcg/24h) | 56.8 ± 30.9 | 33.4 ± 10.7 | <0.001 |
| Salivary cortisol | 6.6 ± 2.76 | 0.76 ± 0.49 | <0.001 |
| Cortisol after low dose suppression test (mcg/dL) | 4.14 ± 3.55 | 0.99 ± 0.66 | 0.002 |
| Androstenedione (mcg/L) | 2.86 ± 2.31 | 0.71 ± 0.34 | <0.001 |
| DHEAS (mcg/dL) | 1743 ± 1110 | 624.5 ± 324.5 | 0.008 |
| Cortisone (mcg/L) | 15.1 ± 3.9 | 18.6 ± 6.5 | 0.624 |
| 17OHP (mcg/L) | 0.53 ± 0.22 | 0.32 ± 0.21 | 0.339 |
| Metabolic parameters | |||
| Glycaemia (mmol/L) | 4.91 ± 0.83 | 4.44 ± 0.34 | 0.239 |
| Insulinemia (microU/mL) | 9.48 ± 8.44 | 7.46 ± 3.83 | 0.639 |
| HbA1c (%) | 5.96 ± 0.96 | 5.14 ± 0.32 | 0.045 |
| Total cholesterol (mmol/L) | 4.96 ± 0.6 | 4.97 ± 0.6 | 0.637 |
| HDL cholesterol (mmol/L) | 1.36 ± 0.28 | 1.38 ± 0.53 | 0.958 |
| Triglycerides (mmol/L) | 1.2 ± 0.5 | 0.82 ± 0.37 | 0.116 |
| LDL cholesterol (mmol/L) | 2.63 ± 0.58 | 2.8 ± 0.44 | 0.931 |
| GOT (U/L) | 17.5 ± 5.06 | 22.2 ± 10.8 | 0.131 |
| GPT (U/L) | 22.2 ± 10.8 | 22.8 ± 6.96 | 0.919 |
| Alkaline phosphatase (U/L) | 71.7 ± 21.6 | 72.2 ± 19.6 | 0.975 |
| GammaGT (U/L) | 15.1 ± 8.01 | 15 ± 12.1 | 0.445 |
| Creatinine (mg/dL) | 0.72 ± 0.08 | 0.71 ± 0.13 | 0.854 |
| Na (mmol/L) | 140.2 ± 2.16 | 139.8 ± 1.78 | 0.758 |
| K (mmol/L) | 4.4 ± 0.42 | 4.2 ± 0.44 | 0.408 |
| Calcium (mg/dL) | 9.36 ± 0.25 | 9.31 ± 0.26 | 0.717 |
| Phosphorus (mg/dL) | 3.28 ± 0.29 | 3.56 ± 0.23 | 0.395 |
| Vitamin D (mcg/L) | 21.4 ± 5.22 | 25.6 ± 5.09 | 0.033 |
| PTH (ng/L) | 52.7 ± 17.5 | 43 ± 11.8 | 0.351 |
| C-reactive protein (mg/L) | 2.44 ± 1.95 | 0.79 ± 0.75 | 0.145 |
| Dio | 0.97 ± 0.66 | 2.91 ± 2.77 | <0.001 |
| AUC 2h insulinemia (uU/ml 120 min) | 11370 ± 5592 | 4977 ± 1636.5 | 0.040 |
| AUC 2h glycaemia (mmol/l 120 min) | 1004.1 ± 330.3 | 700.6 ± 111.9 | 0.022 |
| HOMA-IR | 2.06 ± 1.95 | 1.44 ± 0.71 | 0.521 |
| ISI-Matsuda | 5.08 ± 3.66 | 7.85 ± 3.73 | 0.049 |
| Controls (N=15) |
|||||
|---|---|---|---|---|---|
| Baseline Mean (± SD) |
Week 1 Mean (± SD) |
Week 3 Mean (± SD) |
Week 5 Mean (± SD) |
p |
|
| Clinical parameters | |||||
| BMI (Kg/m2) | 32.8 ± 2.14 | 27.4 ± 3.14 | 26.3 ± 2.91 | 25.5 ± 2.33 | 0.003 |
| Waist circumference (cm) | 96.6 ± 14.2 | 95.1 ± 14.8 | 93.2 ± 13.5 | 92 ± 13.3 | 0.002 |
| Systolic blood pressure (mmHg) | 122.3 ± 8.6 | 119.4 ± 6.7 | 115.3 ± 5.6 | 114.7 ± 6.3 | 0.025 |
| Diastolic blood pressure (mmHg) | 83.2 ± 4.8 | 81.5 ± 3.6 | 80.7 ± 4.2 | 78.2 ± 4.6 | 0.007 |
| Hormonal parameters | |||||
| Fasting serum cortisol (mcg/dL) | 11.2 ± 3.44 | 18.8 ± 1.83 | 17.9 ± 2.11 | 15.7 ± 2.05 | 0.180 |
| Urinary free cortisol (mcg/24h) | 33.4 ± 10.7 | 29.5 ± 15.7 | 32.6 ± 17.8 | 27.9 ± 14.3 | 0.552 |
| Salivary cortisol | 0.76 ± 0.49 | 0.85 ± 0.59 | 0.48 ± 0.15 | 0.52 ± 0.27 | 0.818 |
| 17OHP (mcg/L) | 0.32 ± 0.21 | 0.34 ± 0.28 | 0.45 ± 0.27 | 0.41 ± 0.22 | 0.768 |
| Androstenedione (mcg/dL) | 0.71 ± 0.34 | 1.21 ± 0.75 | 1.35 ± 1.05 | 1.15 ± 0.95 | 0.567 |
| DHEAS (mcg/L) | 624.5 ± 324.5 | 557.1 ± 194.5 | 587.9 ± 234.5 | 598.4 ± 205.5 | 0.768 |
| Cortisone (mcg/L) | 18.6 ± 6.5 | 20.1 ± 5.6 | 19.5 ± 4.9 | 18.6 ± 7.3 | 0.645 |
|
Metabolic parameters |
|||||
| Glycaemia (mmol/L) | 4.44 ± 0.34 | 4.03 ± 0.98 | 4.22 ± 0.71 | 3.83 ± 0.16 | 0.074 |
| Insulinemia (microU/mL) | 7.46 ± 3.83 | 10.1 ± 9.51 | 4.15 ± 3.32 | 5.65 ± 5.05 | 0.241 |
| HbA1c (%) | 5.14 ± 0.32 | 5.25 ± 0.63 | 5.25 ± 0.49 | 5.11 ± 0.71 | 0.290 |
| Total cholesterol (mmol/L) | 4.97 ± 0.6 | 4.48 ± 0.2 | 4.07 ± 0.67 | 4.07 ± 0.75 | 0.026 |
| HDL cholesterol (mmol/L) | 1.38 ± 0.53 | 1.47 ± 0.62 | 1.5 ± 0.69 | 1.6 ± 0.32 | 0.001 |
| Triglycerides (mmol/L) | 0.82 ± 0.37 | 0.89 ± 0.27 | 0.69 ± 0.1 | 0.71 ± 0.22 | 0.145 |
| LDL cholesterol (mmol/L) | 2.8 ± 0.44 | 2.69 ± 0.2 | 2.28 ± 0.1 | 2.25 ± 0.14 | 0.132 |
| GOT (U/L) | 22.2 ± 10.8 | 22.5 ± 3.53 | 22.5 ± 0.71 | 21.5 ± 2.12 | 0.212 |
| GPT (U/L) | 22.8 ± 6.96 | 16 ± 2.82 | 18.5 ± 2.12 | 18 ± 1.97 | 0.267 |
| Alkaline phosphatase (U/L) | 72.2 ± 19.6 | 78.5 ± 27.5 | 69.5 ± 26.1 | 69 ± 25.4 | 0.120 |
| GammaGT (U/L) | 15 ± 12.1 | 12.5 ± 7.07 | 9.5 ± 4.94 | 9.5 ± 3.53 | 0.290 |
| Creatinine (mg/dL) | 0.71 ± 0.13 | 0.74 ± 0.11 | 0.77 ± 0.13 | 0.76 ± 0.15 | 0.501 |
| Na (mmol/L) | 139.8 ± 1.78 | 139.5 ± 2.12 | 138.5 ± 2.09 | 139 ± 2.02 | 0.572 |
| K (mmol/L) | 4.2 ± 0.44 | 4.05 ± 0.21 | 4.15 ± 0.35 | 3.91 ± 0.28 | 0.129 |
| Calcium (mg/dL) | 9.31 ± 0.26 | 9.29 ± 0.12 | 9.45 ± 0.21 | 9.3 ± 0.27 | 0.244 |
| Phosphorus (mg/dL) | 3.56 ± 0.23 | 3.55 ± 0.21 | 3.35 ± 0.51 | 3.81 ± 0.14 | 0.753 |
| Vitamin D (mcg/L) | 24.6 ± 9.09 | 34 ± 8.09 | 37.5 ± 7.78 | 42.5 ± 17.6 | 0.514 |
| PTH (ng/L) | 43 ± 11.8 | 32 ± 2.82 | 37.5 ± 2.12 | 37 ± 1.41 | 0.267 |
| C-reactive protein (mg/L) | 0.79 ± 0.75 | 1.42 ± 1.1 | 1.07 ± 0.65 | 0.76 ± 0.14 | 0.443 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





