Submitted:
29 September 2023
Posted:
29 September 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Statistical analysis
4. Results
Discussion
Patents
Funding
Acknowledgments
Conflicts of Interest
References
- Konieczka, K.; Flammer, A.J.; Todorova, M.; Meyer, P.; Flammer, J. Retinitis pigmentosa and ocular blood flow. EPMA J. 2012, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.F.; Tillman, A.; et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet (London, England). 2017, 390, 849–860. [Google Scholar] [CrossRef]
- Langham, M.E.; Kramer, T. Decreased choroidal blood flow associated with retinitis pigmentosa. Eye (London, England). 1990, 4 Pt 2, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Falsini, B.; Anselmi, G.M.; Marangoni, D.; D'Esposito, F.; Fadda, A.; Di Renzo, A.; et al. Subfoveal choroidal blood flow and central retinal function in retinitis pigmentosa. Investigative ophthalmology & visual science. 2011, 52, 1064–1069. [Google Scholar]
- Grunwald, J.E.; Maguire, A.M.; Dupont, J. Retinal hemodynamics in retinitis pigmentosa. American journal of ophthalmology. 1996, 122, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Cellini, M.; Strobbe, E.; Gizzi, C.; Campos, E.C. ET-1 plasma levels and ocular blood flow in retinitis pigmentosa. Canadian journal of physiology and pharmacology. 2010, 88, 630–635. [Google Scholar] [CrossRef]
- Wolf, S.; Pöstgens, H.; Bertram, B.; Schulte, K.; Teping, C.; Reim, M. [Hemodynamic findings in patients with retinitis pigmentosa]. Klinische Monatsblatter fur Augenheilkunde. 1991, 199, 325–329. [Google Scholar] [CrossRef]
- Reiner, A.; Fitzgerald, M.E.C.; Del Mar, N.; Li, C. Neural control of choroidal blood flow. Progress in retinal and eye research. 2018, 64, 96–130. [Google Scholar] [CrossRef]
- McDougal, D.H.; Gamlin, P.D. Autonomic control of the eye. Comprehensive Physiology. 2015, 5, 439–473. [Google Scholar]
- Goadsby, P.J.; Edvinsson, L.; Ekman, R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Annals of neurology. 1988, 23, 193–196. [Google Scholar] [CrossRef]
- Suzuki, N.; Hardebo, J.E.; Owman, C. Trigeminal fibre collaterals storing substance P and calcitonin gene-related peptide associate with ganglion cells containing choline acetyltransferase and vasoactive intestinal polypeptide in the sphenopalatine ganglion of the rat. An axon reflex modulating parasympathetic ganglionic activity? Neuroscience. 1989, 30, 595–604. [Google Scholar] [PubMed]
- Hosaka, F.; Yamamoto, M.; Cho, K.H.; Jang, H.S.; Murakami, G.; Abe, S. Human nasociliary nerve with special reference to its unique parasympathetic cutaneous innervation. Anatomy & cell biology. 2016, 49, 132–137. [Google Scholar]
- Suzuki, N.; Hardebo, J.E.; Owman, C. Origins and pathways of cerebrovascular nerves storing substance P and calcitonin gene-related peptide in rat. Neuroscience. 1989, 31, 427–438. [Google Scholar] [CrossRef]
- Atalay, B.; Bolay, H.; Dalkara, T.; Soylemezoglu, F.; Oge, K.; Ozcan, O.E. Transcorneal stimulation of trigeminal nerve afferents to increase cerebral blood flow in rats with cerebral vasospasm: a noninvasive method to activate the trigeminovascular reflex. Journal of neurosurgery. 2002, 97, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G.A.; Bogduk, N.; Goadsby, P.J.; Duckworth, J.W.; Lance, J.W. Decreased carotid arterial resistance in cats in response to trigeminal stimulation. Journal of neurosurgery. 1984, 61, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Edvinsson, L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Annals of neurology. 1993, 33, 48–56. [Google Scholar] [CrossRef]
- Stjernschantz, J.; Geijer, C.; Bill, A. Electrical stimulation of the fifth cranial nerve in rabbits: effects on ocular blood flow, extravascular albumin content and intraocular pressure. Experimental eye research. 1979, 28, 229–238. [Google Scholar] [CrossRef]
- Bill, A.; Nilsson, S.F. Control of ocular blood flow. Journal of cardiovascular pharmacology. 1985, 7 (Suppl. 3), S96–102. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Iftikhar, M.; Hafiz, G.; Akhlaq, A.; Tsai, G.; Wehling, D.; et al. Oral N-acetylcysteine improves cone function in retinitis pigmentosa patients in phase I trial. The Journal of clinical investigation. 2020, 130, 1527–1541. [Google Scholar] [CrossRef]
- Oudemans-van Straaten, H.M.; Spoelstra-de Man, A.M.; de Waard, M.C. Vitamin C revisited. Critical care (London, England). 2014, 18, 460. [Google Scholar] [CrossRef]
- Bruno, R.M.; Daghini, E.; Ghiadoni, L.; Sudano, I.; Rugani, I.; Varanini, M.; et al. Effect of acute administration of vitamin C on muscle sympathetic activity, cardiac sympathovagal balance, and baroreflex sensitivity in hypertensive patients. The American journal of clinical nutrition. 2012, 96, 302–308. [Google Scholar] [CrossRef]
- Owsley, C.; McGwin, G.; Jr Scilley, K.; Kallies, K. Development of a questionnaire to assess vision problems under low luminance in age-related maculopathy. Investigative ophthalmology & visual science. 2006, 47, 528–535. [Google Scholar]
- Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health and quality of life outcomes. 2006, 4, 79. [CrossRef]
- Heneghan, C.; Goldacre, B.; Mahtani, K.R. Why clinical trial outcomes fail to translate into benefits for patients. Trials. 2017, 18, 122. [Google Scholar] [CrossRef] [PubMed]
- Weldring, T.; Smith, S.M. Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs). Health services insights. 2013, 6, 61–68. [Google Scholar] [CrossRef]
- Li, Z.Y.; Kljavin, I.J.; Milam, A.H. Rod photoreceptor neurite sprouting in retinitis pigmentosa. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995, 15, 5429–5438. [Google Scholar] [CrossRef]
- Wang, W.; Lee, S.J.; Scott, P.A.; Lu, X.; Emery, D.; Liu, Y.; et al. Two-Step Reactivation of Dormant Cones in Retinitis Pigmentosa. Cell reports. 2016, 15, 372–385. [Google Scholar] [CrossRef]
- Wang, W.; Kini, A.; Wang, Y.; Liu, T.; Chen, Y.; Vukmanic, E.; et al. Metabolic Deregulation of the Blood-Outer Retinal Barrier in Retinitis Pigmentosa. Cell reports. 2019, 28, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Toto, L.; Borrelli, E.; Mastropasqua, R.; Senatore, A.; Di Antonio, L.; Di Nicola, M.; et al. Macular Features in Retinitis Pigmentosa: Correlations Among Ganglion Cell Complex Thickness, Capillary Density, and Macular Function. Investigative ophthalmology & visual science. 2016, 57, 6360–6366. [Google Scholar]
- Hayreh, S.S. In vivo choroidal circulation and its watershed zones. Eye (London, England). 1990, 4 Pt 2, 273–289. [Google Scholar] [CrossRef]
- Punzo, C.; Kornacker, K.; Cepko, C.L. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nature neuroscience. 2009, 12, 44–52. [Google Scholar] [CrossRef]
- Ferrara, N. Vascular endothelial growth factor: molecular and biological aspects. Current topics in microbiology and immunology. 1999, 237, 1–30. [Google Scholar] [PubMed]
- Chader, G.J. PEDF: Raising both hopes and questions in controlling angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2001, 98, 2122–2124. [Google Scholar] [CrossRef]
- Talks, K.L.; Harris, A.L. Current status of antiangiogenic factors. British journal of haematology. 2000, 109, 477–489. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature. 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Salom, D.; Diaz-Llopis, M.; García-Delpech, S.; Udaondo, P.; Sancho-Tello, M.; Romero, F.J. Aqueous humor levels of vascular endothelial growth factor in retinitis pigmentosa. Investigative ophthalmology & visual science. 2008, 49, 3499–3502. [Google Scholar]
- Cellini, M.; Santiago, L.; Versura, P.; Caramazza, R. Plasma levels of endothelin-1 in retinitis pigmentosa. Ophthalmologica Journal international d'ophtalmologie International journal of ophthalmology Zeitschrift fur Augenheilkunde. 2002, 216, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.Q.; Zhang, M.; Matis, K.I.; Kim, C.; Rosenblatt, M.I. Vascular endothelial growth factor mediates corneal nerve repair. Investigative ophthalmology & visual science. 2008, 49, 3870–3878. [Google Scholar]
- Lv, Y.X.; Zhong, S.; Tang, H.; Luo, B.; Chen, S.J.; Chen, L.; et al. VEGF-A and VEGF-B Coordinate the Arteriogenesis to Repair the Infarcted Heart with Vagus Nerve Stimulation. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2018, 48, 433–449. [Google Scholar] [CrossRef]
- Luo, B.; Wu, Y.; Liu, S.L.; Li, X.Y.; Zhu, H.R.; Zhang, L.; et al. Vagus nerve stimulation optimized cardiomyocyte phenotype, sarcomere organization and energy metabolism in infarcted heart through FoxO3A-VEGF signaling. Cell death & disease. 2020, 11, 971. [Google Scholar]
- Rogers, R.C.; Kita, H.; Butcher, L.L.; Novin, D. Afferent projections to the dorsal motor nucleus of the vagus. Brain research bulletin. 1980, 5, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000, 405, 458–462. [Google Scholar] [CrossRef]
- Wasilczuk, K.M.; Bayer, K.C.; Somann, J.P.; Albors, G.O.; Sturgis, J.; Lyle, L.T.; et al. Modulating the Inflammatory Reflex in Rats Using Low-Intensity Focused Ultrasound Stimulation of the Vagus Nerve. Ultrasound in medicine & biology. 2019, 45, 481–489. [Google Scholar]
- Liu, L.; Dana, R.; Yin, J. Sensory neurons directly promote angiogenesis in response to inflammation via substance P signaling. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2020, 34, 6229–6243. [Google Scholar] [CrossRef] [PubMed]
- Ziche, M.; Morbidelli, L.; Pacini, M.; Geppetti, P.; Alessandri, G.; Maggi, C.A. Substance P stimulates neovascularization in vivo and proliferation of cultured endothelial cells. Microvascular research. 1990, 40, 264–278. [Google Scholar] [CrossRef]
- Kim, J.H.; Jung, Y.; Kim, B.S.; Kim, S.H. Stem cell recruitment and angiogenesis of neuropeptide substance P coupled with self-assembling peptide nanofiber in a mouse hind limb ischemia model. Biomaterials. 2013, 34, 1657–1668. [Google Scholar] [CrossRef]
- Kohara, H.; Tajima, S.; Yamamoto, M.; Tabata, Y. Angiogenesis induced by controlled release of neuropeptide substance P. Biomaterials. 2010, 31, 8617–8625. [Google Scholar] [CrossRef]
- Wei, F.Y.; Chow, S.K.; Leung, K.S.; Qin, J.; Guo, A.; Yu, O.L.; et al. Low-magnitude high-frequency vibration enhanced mesenchymal stem cell recruitment in osteoporotic fracture healing through the SDF-1/CXCR4 pathway. European cells & materials. 2016, 31, 341–354. [Google Scholar]
- Shinagawa, K.; Mitsuhara, T.; Okazaki, T.; Takeda, M.; Yamaguchi, S.; Magaki, T.; et al. The characteristics of human cranial bone marrow mesenchymal stem cells. Neuroscience letters. 2015, 606, 161–166. [Google Scholar] [CrossRef]
- Yoshida, N.; Ikeda, Y.; Notomi, S.; Ishikawa, K.; Murakami, Y.; Hisatomi, T.; et al. Laboratory evidence of sustained chronic inflammatory reaction in retinitis pigmentosa. Ophthalmology. 2013, 120, e5–e12. [Google Scholar] [CrossRef]
- Xue, K.; Wang, M.; Chen, J.; Huang, X.; Xu, G. Retinal nerve fiber layer analysis with scanning laser polarimetry and RTVue-OCT in patients of retinitis pigmentosa. Ophthalmologica Journal international d'ophtalmologie International journal of ophthalmology Zeitschrift fur Augenheilkunde. 2013, 229, 38–42. [Google Scholar] [CrossRef] [PubMed]
- García-Ayuso, D.; Di Pierdomenico, J.; Vidal-Sanz, M.; Villegas-Pérez, M.P. Retinal Ganglion Cell Death as a Late Remodeling Effect of Photoreceptor Degeneration. International journal of molecular sciences. 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chiluwal, A.; Afridi, A.; Chaung, W.; Powell, K.; Yang, W.L.; et al. Trigeminal Nerve Stimulation: A Novel Method of Resuscitation for Hemorrhagic Shock. Critical care medicine. 2019, 47, e478–e84. [Google Scholar] [CrossRef] [PubMed]
- Komeima, K.; Rogers, B.S.; Lu, L.; Campochiaro, P.A. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proceedings of the National Academy of Sciences of the United States of America. 2006, 103, 11300–11305. [Google Scholar] [CrossRef]
- Rose, R.C.; Bode, A.M. Ocular ascorbate transport and metabolism. Comparative biochemistry and physiology A, Comparative physiology. 1991, 100, 273–285. [Google Scholar] [CrossRef]
- Hosoya, K.; Minamizono, A.; Katayama, K.; Terasaki, T.; Tomi, M. Vitamin C transport in oxidized form across the rat blood-retinal barrier. Investigative ophthalmology & visual science. 2004, 45, 1232–1239. [Google Scholar]
- Taddei, S.; Virdis, A.; Ghiadoni, L.; Magagna, A.; Salvetti, A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation. 1998, 97, 2222–2229. [Google Scholar] [CrossRef]
- Jolly, J.K.; Wagner, S.K.; Martus, P.; MacLaren, R.E.; Wilhelm, B.; Webster, A.R.; et al. Transcorneal Electrical Stimulation for the Treatment of Retinitis Pigmentosa: A Multicenter Safety Study of the OkuStim® System (TESOLA-Study). Ophthalmic research. 2020, 63, 234–243. [Google Scholar] [CrossRef]
- Miura, G.; Sugawara, T.; Kawasaki, Y.; Tatsumi, T.; Nizawa, T.; Baba, T.; et al. Clinical Trial to Evaluate Safety and Efficacy of Transdermal Electrical Stimulation on Visual Functions of Patients with Retinitis Pigmentosa. Scientific reports. 2019, 9, 11668. [Google Scholar] [CrossRef]
- de Rossi, F.; Guidobaldi, M.; Turco, S.; Amore, F. Transorbital electrical stimulation in retinitis pigmentosa. Better results joining visual pattern stimulation? Brain stimulation. 2020, 13, 1173–1174. [Google Scholar] [CrossRef]
- Reiner, A.; Fitzgerald, M.C. CL Neural Control of Ocular Blood Flow. Editors. Ocular Blood Flow. Berlin Heidelberge:springer; 2012.
- Hiraba, H.; Inoue, M.; Gora, K.; Sato, T.; Nishimura, S.; Yamaoka, M.; et al. Facial vibrotactile stimulation activates the parasympathetic nervous system: study of salivary secretion, heart rate, pupillary reflex, and functional near-infrared spectroscopy activity. BioMed research international. 2014, 2014, 910812. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.Y.; Kim, J.H.; Cho, Y.S.; Mah, W.; Bae, Y.C. Quantitative analysis of afferents expressing substance P, calcitonin gene-related peptide, isolectin B4, neurofilament 200, and Peripherin in the sensory root of the rat trigeminal ganglion. The Journal of comparative neurology. 2015, 523, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.S.; Kim, S.; Nam, S.; Um, J.; Kim, Y.H.; Son, Y. Effect of substance P on recovery from laser-induced retinal degeneration. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2015, 23, 268–277. [Google Scholar] [CrossRef]
- Backman, L.J.; Eriksson, D.E.; Danielson, P. Substance P reduces TNF-α-induced apoptosis in human tenocytes through NK-1 receptor stimulation. British journal of sports medicine. 2014, 48, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.; Son, B.K.; Kim, S.; Son, Y.; Yu, S.-Y.; Hong, H.S. Substance P prevents development of proliferative vitreoretinopathy in mice by modulating TNF-α. Mol Vis. 2017, 23, 933–943. [Google Scholar]
- Scheibe, M.; van Thriel, C.; Hummel, T. Responses to trigeminal irritants at different locations of the human nasal mucosa. The Laryngoscope. 2008, 118, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Kalloniatis, M.; Tomisich, G. Amino acid neurochemistry of the vertebrate retina. Progress in retinal and eye research. 1999, 18, 811–866. [Google Scholar] [CrossRef]
- Zilberter, Y.; Harkany, T.; Holmgren, C.D. Dendritic release of retrograde messengers controls synaptic transmission in local neocortical networks. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2005, 11, 334–344. [Google Scholar] [CrossRef]
- Rountree, C.M.; Inayat, S.; Troy, J.B.; Saggere, L. Differential stimulation of the retina with subretinally injected exogenous neurotransmitter: A biomimetic alternative to electrical stimulation. Scientific reports. 2016, 6, 38505. [Google Scholar] [CrossRef]
- Mattson, M.P. Glutamate and neurotrophic factors in neuronal plasticity and disease. Annals of the New York Academy of Sciences. 2008, 1144, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Aït-Ali, N.; Fridlich, R.; Millet-Puel, G.; Clérin, E.; Delalande, F.; Jaillard, C.; et al. Rod-derived cone viability factor promotes cone survival by stimulating aerobic glycolysis. Cell. 2015, 161, 817–832. [Google Scholar] [CrossRef] [PubMed]
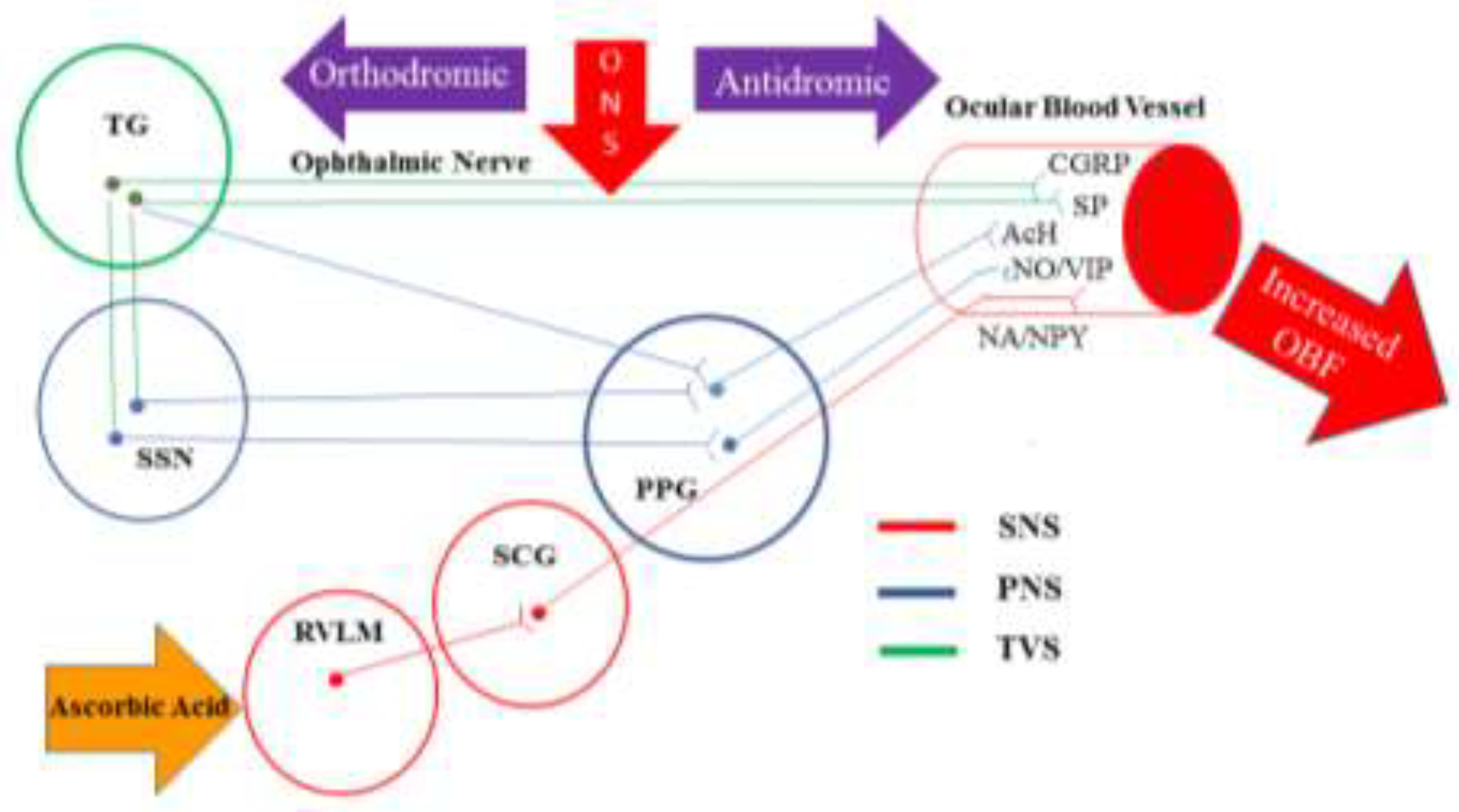
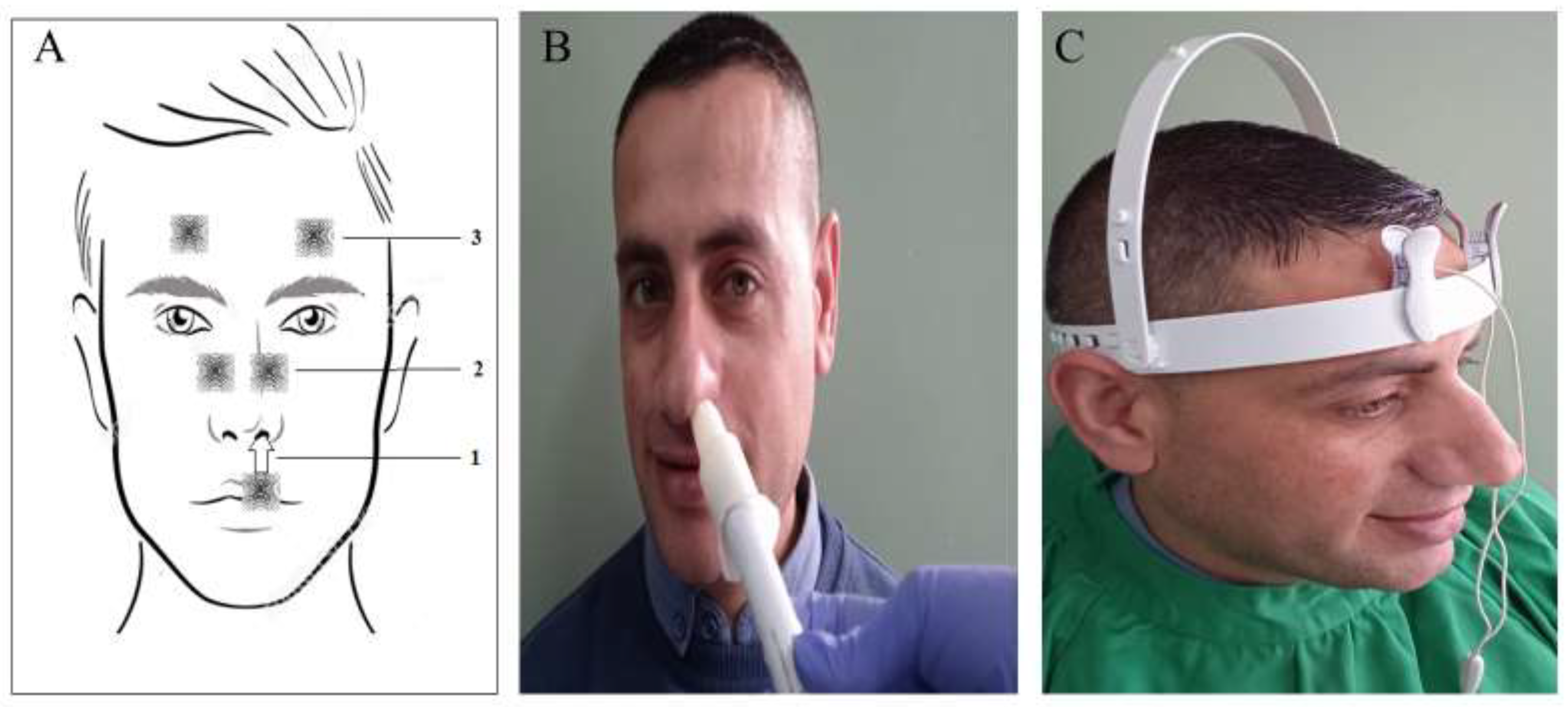
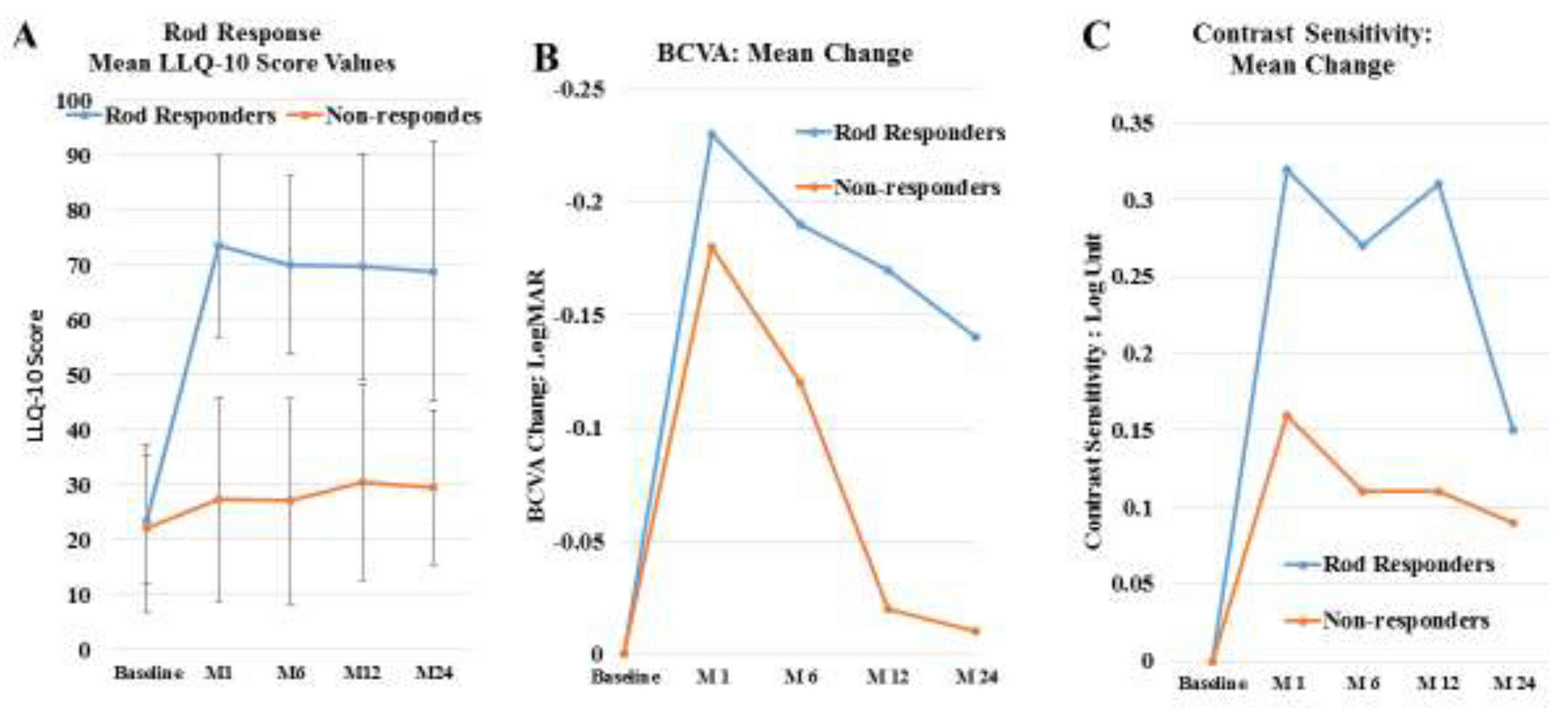
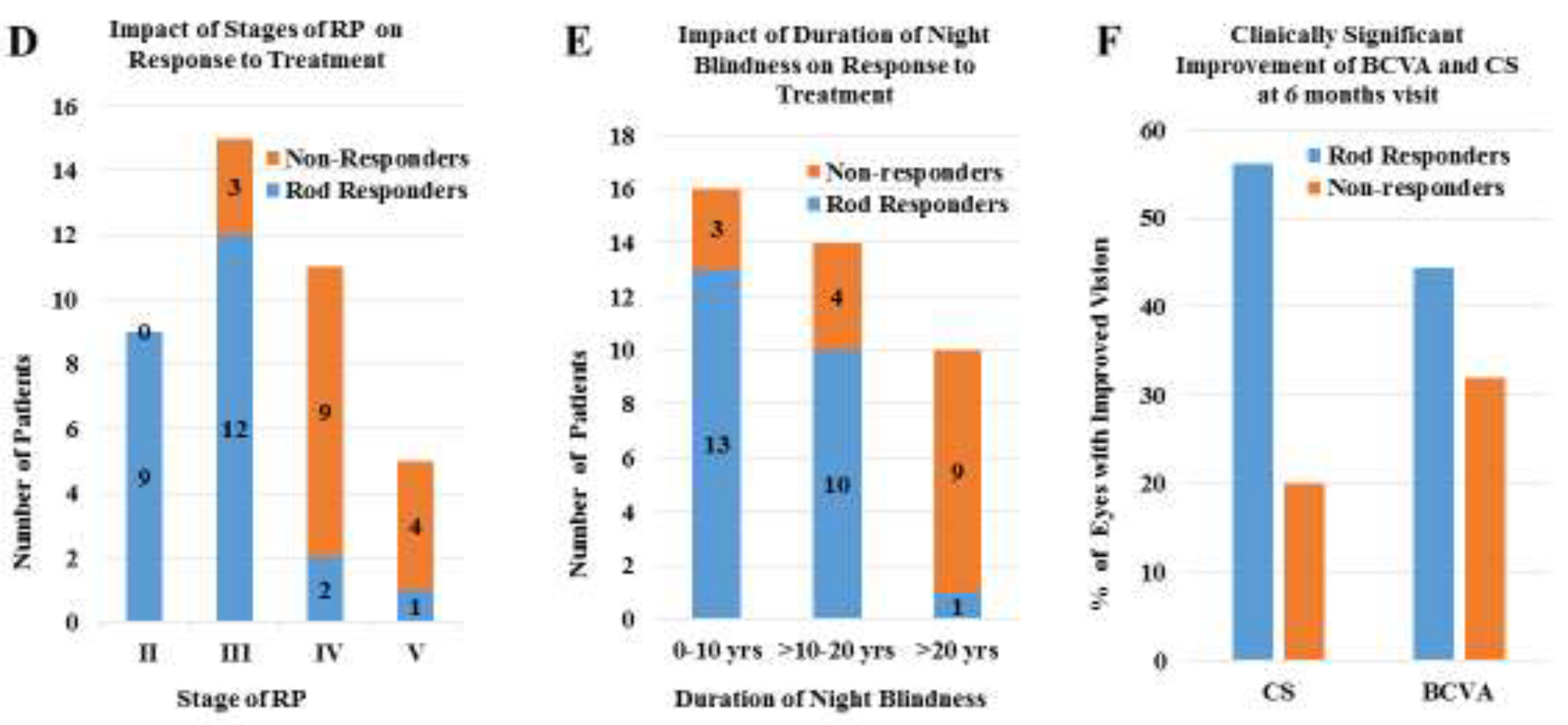
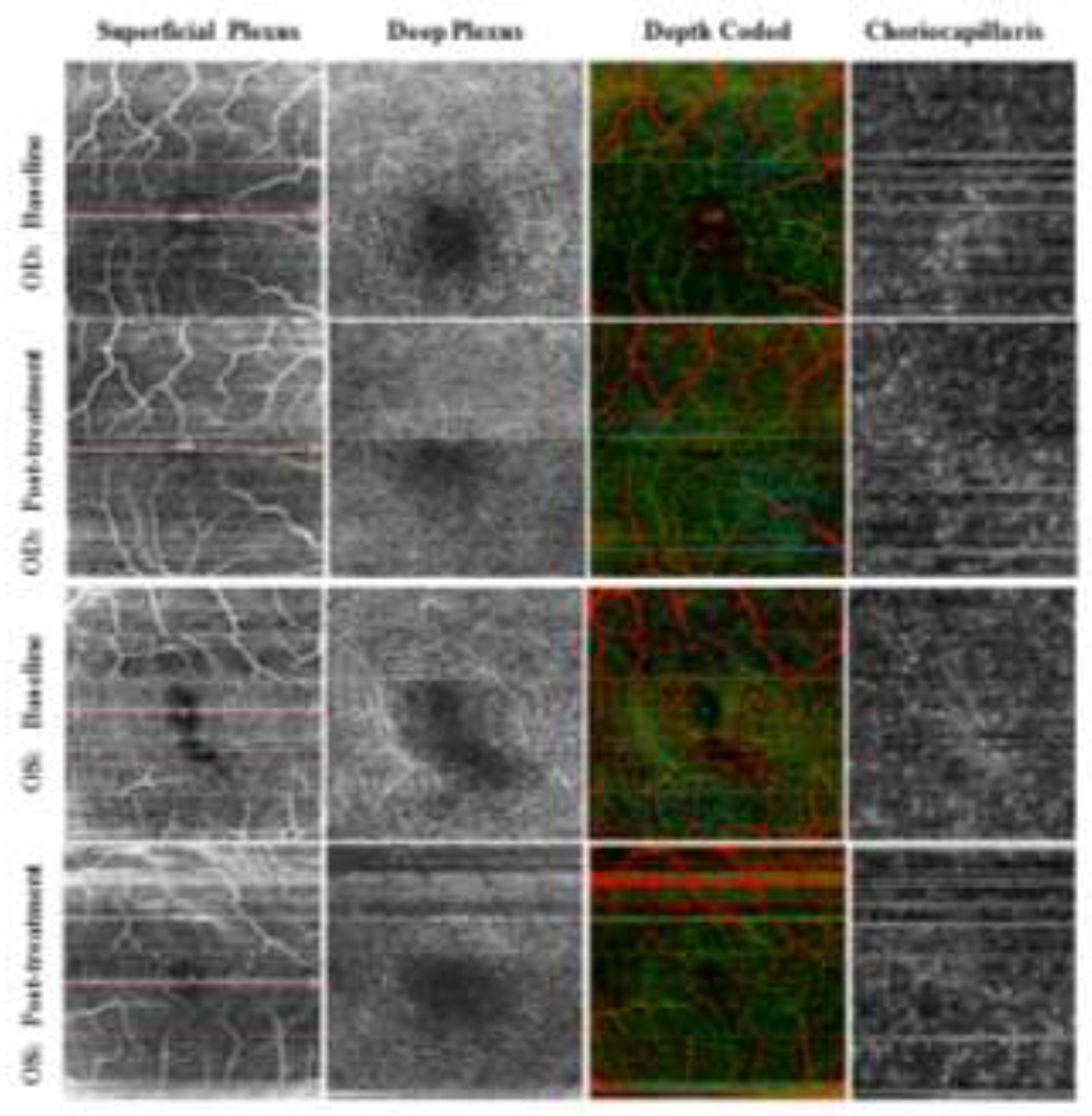
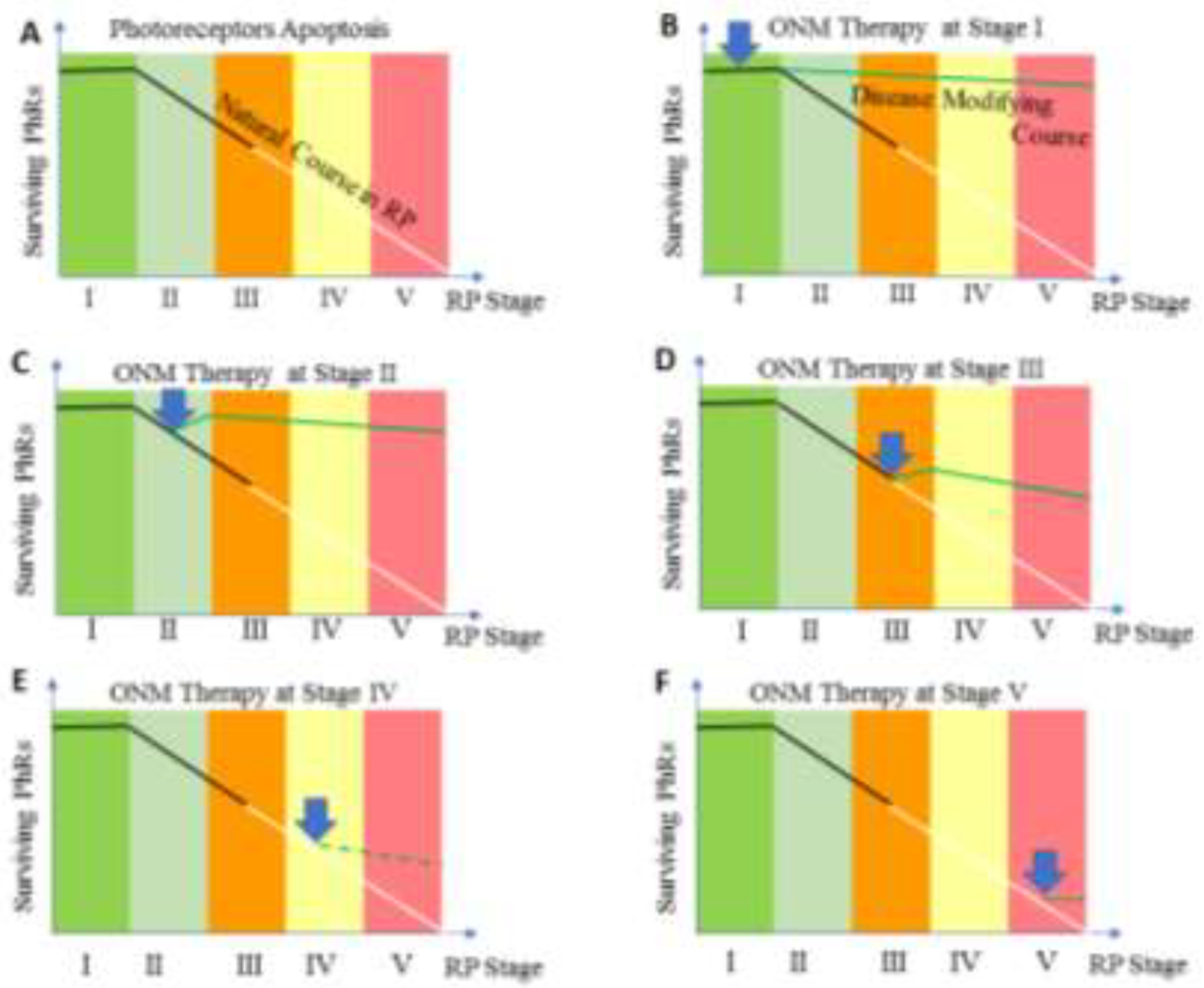
| Stage | Clinical Description |
|---|---|
| Stage 1 | Patients may experience mild night vision disturbance. Fundus examination reveals normally looking retina with no pigmentary changes, no visible retinal blood vessels (RBVs) changes, or optic nerve pallor. Positive ERG findings are detected |
| Stage II | Mild to moderate attenuation of RBVs that extend beyond the mid periphery of retina but not reaching ora serrata, no or mild optic disc pallor, bone spicule pigmentary changes are limited to mid-periphery of the retina. |
| Stage III | Moderate to severe attenuation of RBVs that extend up to midperiphery with mild optic nerve pallor. Pigmentary changes are located at mid-periphery and extend centrally reaching the vascular arcade. |
| Stage IV | Severe attenuation of RBVs that ended with formation of a complete vascular arcade, combined with moderate optic nerve pallor. Pigmentary changes are becoming more diffuse and extend centrally to the inside of vascular arcade, but sparing the macula. Central retinal thickness is less than 150 µ as measured by OCTA |
| Stage V | Severe attenuation or complete obliteration of RBVs that ended at a short distance from optic nerve. Optic pallor is remarkable and the macula is involved by pigmentary changes. Stage V is also coined to any retina with central retinal thickness of 100 µ or less as measured by OCTA |
| Q | Subject | Question Text |
|---|---|---|
| 1 | Night Vision | Do you have difficulty seeing at night? |
| 2 | Peripheral vision | Do you have difficulty with your peripheral vision at night? |
| 3 | Reading at dim light | Do you have difficulty reading menus in dimly lit restaurants or reading the newspaper/book without good lighting? |
| 4 | Depth of Perception | Do you have difficulty with depth perception at night? |
| 5 | Color Vision | Do you have difficulty seeing colors at night? |
| 6 | Home life | Do you have difficulty seeing furniture in dimly lit rooms? |
| 7 | Social life | Because of your vision, do you have difficulty going out to nighttime social events such as sport events, the theater, friend’s homes, church, mosque or restaurants? |
| 8 | Parties at dim light | Do you have difficulty seeing in candlelight? |
| 9 | Dependency on others | Do you depend on others to help you because of your vision at night or under poor lighting? |
| 10 | Psychological Stress | Do you worry or are you concerned that you might fall at night because of your vision? |
| Total cohort | |
|---|---|
| (40 patients; 76 eye) | |
| Age, Mean ±SD | 28.4 ± 14.1 yrs. |
| 4-52 yrs. | |
| M/F (male %) | 23/17 (57.5%) |
| Inheritance | |
| AR | 21 (52.5%) |
| AD | 2 (5%) |
| Simplex | 17 (42.5%) |
| Rang/ | 2-46 yrs. |
| Mean Duration of night blindness | 16.2 ± 12.3 yrs. |
| Duration | |
| 0-10 yrs. | 16 (40%) |
| >10-20 yrs. | 14 (35%) |
| >20-40 yrs | 10 (25%) |
| Stage of the Disease * | |
| II, no. of patients (%) | 9 (22.5%) |
| III, no. of patients/ (%) | 15 (37.5%) |
| IV, no. of patients (%) | 11 (27.5%) |
| V, no. of patients/ (%) | 5 (12.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




