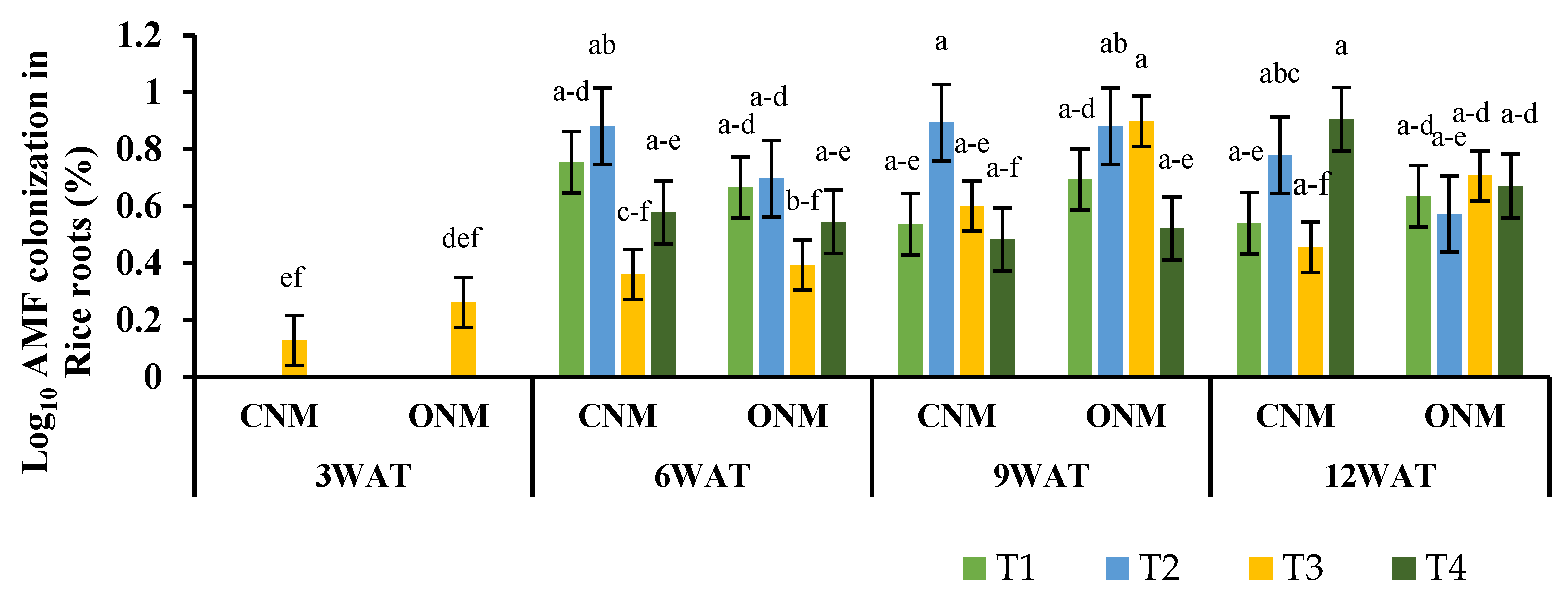1. Introduction
Rice (
Oryza sativa L.) is considered a staple food in many countries in the world, including Sri Lanka. Today, the overuse of agrochemicals in rice cultivation has become a global issue that is concerning for both environmental and human health, although commonly practiced in Sri Lanka [
1]. The use of bio-fertilizer is a desirable alternative to synthetic fertilizer due to its eco-friendly nature [
2]. Arbuscular mycorrhizal fungi (AMF) are important candidates for use as bio-fertilizers [
3]. These fungi form a symbiotic association with the root system in 72% of all vascular plants [
4] and has been found to provide a range of benefits to rice plants [
5]. AMF can enhance rice plant growth and development mainly by improving the uptake of several macro- and micro-nutrients such as nitrogen (N), phosphorus (P), potassium (K), copper (Cu), and zinc (Zn) [
5] while also enhancing soil properties, which supports healthy plants [
6]. In addition, by improving plant tolerance, AMF can increase rice production under unfavorable abiotic conditions such as drought and waterlogged conditions [
7]. Moreover, AMF enhance the resistance of rice plants to diseases (e.g., rice sheath blight [
8] and rice blast [
9]) caused by
Rhizoctonia solani and
Magnaporthe oryzae, respectively. Nonetheless, AMF rarely colonize under lowland conditions due to the anoxic environment, although they readily form associations with upland rice plants [
10].
According to previous studies, such reduced colonization can be improved through the incorporation of AMF inoculum into the soil [
11,
12] and by using different field management practices that promote AMF symbiosis such as water management [
13] and crop rotation [
14]. Intercropping highly mycorrhizal plants would also be a possible field practice to increase AMF abundance in soil. In this aspect, many studies have been carried out on the potential use of mycorrhizal plants along with upland rice [
15,
16,
17,
18,
19], though thus far, no such studies have been reported for lowland rice cultivation.
In the present study, we attempted to enhance the abundance of AMF in flooded rice soil through different farming practices, intercropping an efficient mycorrhizal symbiont and field inoculation of farm-produced AMF. We further assessed their effects on lowland rice growth and yield under conventional and organic nutrient management. For this, we selected vetiver grass (
Chrysopogon zizanioides) to intercrop with lowland rice due to its highly mycorrhizal potential [
20] and ability to withstand water-logged conditions [
21]. It should be noted that vetiver grass would be compatible with the agronomic practices of rice farming since both plants belong to the same family, the Poaceae [
22]. Additionally, vetiver plant has high economic value [
23].
2. Materials and Methods
2.1. Experimental site and design
The field experiment was carried out in the research unit of the Faculty of Agriculture, Rajarata University of Sri Lanka at Puliyankulama, Anuradhapura, North Central Province of the island during the 2019/2020 Maha season (wet season, November-March), where the mean seasonal temperature is 29.2 °C and rainfall is 962 mm. A conventional rice field which has clay loam Low Humic Glay soil and followed over three years was selected as the experimental site.
The experiments used the split-plot randomized complete block design (RCBD) with three blocks. Two nutrient management systems (conventional and organic) were distributed randomly in the main plots. The sub-plots were allocated to an untreated control (T0) and other three treatments—soil inoculation of AMF (T1), intercropping of vetiver (T2), and a combination of AMF and vetiver (T3). The main plots were surrounded by bunds with a height of 0.3 m height and a width of 0.45 m. Drainage canals were prepared in between the bunds of the main plots to prevent cross-contamination of added organic and inorganic fertilizer. The sub-plot area was approximately 34 m2, and each was separated by a bund 0.3 high and 0.3 m wide.
Fifteen-day-old seedlings of rice (Bg 300 variety-three months duration) were selected for this experiment. The seedlings were raised in a dapog nursery and were transplanted with two plants per hill, at a 0.2 × 0.2 m spacing, on the puddled and leveled plots. On the same day, uniformly aged vetiver grass (C. zizanioides L.) slips that had been trimmed at 15 cm lengths from the root system were intercropped without changing the rice plant density in the desired sub-plots (in two rows; four plants per one row at 1 × 1.8 m spacing). Inorganic fertilizers (Urea 0.0225 kg/m2, Triple Super Phosphate (TSP) 0.0055 kg/m2, muriate of potash (MOP) 0.006 kg/m2, and zinc sulphate (ZnSO4) 0.0005 kg/m2), and commercially available compost (0.25 kg/m2) were applied to the main plots, according to the manufacturers recommended dosage and guidelines. Application of mycorrhizal inoculum to the desired sub-plots was done with 2 kg of prepared inoculum before transplantation and 1 kg of inoculum at the 3rd, 5th, and 7th weeks after transplanting.
2.2. Preparation of native AMF inoculum
AMF inoculum was prepared by establishing trap cultures. Soil samples from the upper layer (0–15 cm) with fine root fragments of herbaceous plants [
24] were collected from undisturbed water-logged sites in a dry zone environment. AMF spores were then thoroughly homogenized with sterilized fine sand and decomposed cow dung in a ratio of 1:3:1 (v/v) and then added to sterilized plastic pots. Untreated sorghum (
Sorghum bicolor) seeds were surface sterilized with 70% ethanol for two minutes and 1% sodium hypochlorite (NaOCl) for three minutes, followed by washing with sterile distilled water (7–10 times) and soaked in sterile distilled water for 12 hours [
25]. Then the seeds were sown in pots (60‒80 seeds per pot) and kept in the plant house for six weeks. Pots were irrigated (manual watering) regularly as per the requirement of the plants and no synthetic fertilizers were added. A rhizospheric sand-soil mixture containing colonized root fragments that had been separated from the trap plant culture was used as the AMF inoculum. Before soil application, representative roots samples were stained and checked for their AMF colonization potential as described below.
2.3. Sampling
After transplanting, randomly selected healthy nine hills were carefully uprooted from 1 m2 area of each sub-plot at the tillering stage (3 weeks after transplanting; WAT), panicle initiation stage (6 WAT), heading stage (9 WAT), and harvesting stage (12 WAT). Plants were thoroughly washed (without damaging the roots) to remove any adherent soil particles before determining the growth parameters of plant height and dry weights of shoots and roots. At the harvesting stage, yield components (e.g., panicles/m2, spikelet/panicle, filled-grain percent, and weight of 1,000 grains) were measured to obtain the grain yield (kg/m2). At each sampling, 5–10 fine rice roots per plant were separated, washed with tap water, and stored in 70% ethanol solution until the percentage of mycorrhizal colonization was determined.
2.4. Mycorrhizal colonization
The root colonization percentage of AMF was determined after staining with trypan blue, adopting the procedure described by Phillips and Hayman [
26]. The rice plant roots stored in 70% ethanol were washed with distilled water, cut into 1 cm lengths, transferred into test tubes containing 10% (w/v) potassium hydroxide (KOH), and incubated at 90 °C for 15–30 min. Thereafter, roots were washed with running tap water and acidified with 1% hydrochloric acid (HCl) for 15–20 minutes. The root segments were stained with trypan blue (0.05% of trypan blue in lactoglycerol) at 90 °C for five minutes. Stained root fragments were washed with running tap water and de-stained by lactoglycerol.
The modified grid transect method [
27] was used for AMF quantification. Ten root segments were randomly selected from each stained sample and mounted in glycerine on a microscopic slide. The roots were then gently squeezed under a cover slip and viewed using a compound microscope (Labomed-Sigma, U.S.A.). The presence of AMF structures such as arbuscules, vesicles, or inter-cellular aseptic hyphae were scored for 100 intersections of roots per sample. Then the percentage of AMF colonized roots intersects over the total number of roots intersects was calculated.
2.5. Statistical analysis
Statistical analyses were performed using the SAS (version 9.0) statistical tool. Analysis of variance (two-way ANOVA) was carried out using the repeated measure MIXED model. The growth stage was considered as the repeated factor. Before the analysis, AMF colonization percentages were tested for normality and log-transformed. The mean separation was done using the Least Significant Difference (LSD) method at a 5% probability level.
3. Results and Discussion
3.1. AMF colonization of rice roots under different treatments
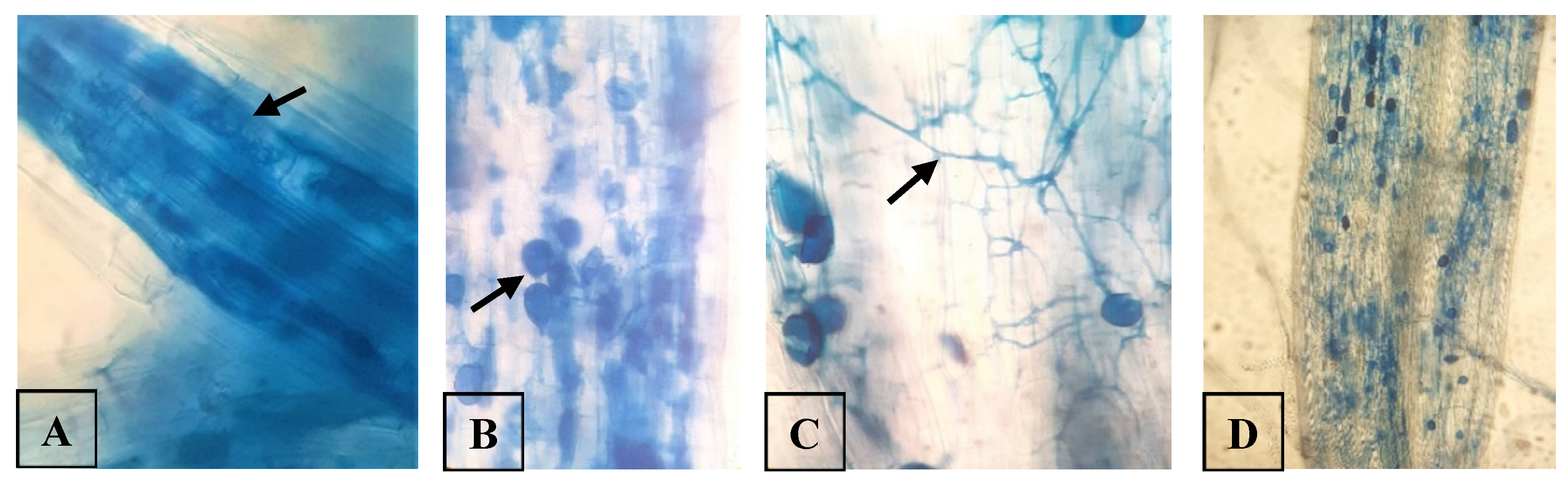
It should be noted that AMF are typically characterized by the presence of finely branched hyphal structures (arbuscules) within the cortical cells of plant roots [
28]. In addition, AMF consist of intra-radical hyphae, extra-radical mycelia, large spores, and terminal globular structures referred to as vesicles in some species [
29]. In the present study, we observed arbuscules, vesicles, and intra-radical hyphae within stained rice roots (
Figure 1). Among them, fungal hyphae and vesicles were observed to be prominent, compared to arbuscules. Bernaola et al. [
30] reported similar observations in naturally colonized rice roots and suggested that it was due to the tendency of arbuscules to degrade quickly.
The mean root colonization percentages of AMF at different growth stages of rice plants are presented in
Figure 2. These data do not show a predominant increase in rice root colonization in response to added AMF inoculum compared to the non-inoculated treatments. Root colonization ranged between 0–15.8%. Nevertheless, previous studies have shown that significantly higher root colonization rates can be achieved by adding AMF inoculum. For example, Secilia and Bagyaraj [
11] and Zhang et al. [
31] reported significantly higher root colonization (12–19% and 31–33%, respectively) by the AMF inoculation, under field level and greenhouse-plot experiment, respectively.
In the present study, a considerable amount of AMF colonization was reported (0–4.98%) in the non-inoculated controls (
Figure 2), which is in agreement with Purakayastha and Chhonkar [
32], Wangiyana et al. [
33], and Chareesri et al. [
12], who reported 2.6%, 3–5% and 7% of indigenous AMF colonization in non-inoculated rice plants, respectively. Similarly, Kalamulla et al. [
34] and Chen et al. [
35] have reported 36.40% and 19.5 ± 7.2% of natural AMF colonization rates from the rice farmer fields in Sri Lanka and China, respectively.
Our experiment indicated that AMF colonization in different treatments was very low or did not seem to occur at all in the rice plant’s initial stage (3WAT). Higher colonization was then observed in later stages, panicle initiation (6WAT) to harvesting stage (12WAT) (
Figure 2). The lowest initial colonization rates could be due to anoxic conditions caused by continuous inundation to manage weeds at the beginning of cultivation. Also, the development of aerenchyma tissues in rice plants would also be a possible reason for this observation. Aerenchyma tissues supply oxygen to rice roots under flooded conditions and are poorly developed in seedlings [
36]. At later stages of the plant, those tissues are well developed, and AMF is well-established with sufficient oxygen [
37]. Solaiman and Hirata [
38] observed a similar pattern in non-inoculated treatments, which resulted from colonization of native AMF inhabiting the soil. However, since they inoculated AMF at the nursery stage, higher initial root colonization was reported in AMF-inoculated plants even under initial submergence conditions.
AMF can form a common fungal network in intercropping systems by connecting plants arranged in rows [
39]. However, in our experiment, intercropping of vetiver grass with or without AMF inoculation did not result in a statistically significant (P < 0.05) increase in mycorrhizal colonization in rice roots. Although vetiver is known to be a highly mycorrhizal plant, AMF colonization was found to be very low under waterlogged conditions (data not presented). Similarly, AMF colonization did not differ between the rice plants grown under both nutrient management systems, suggesting that AMF can survive even in the presence of agrochemicals. In contrast, our findings also received contrasting results from the work done by Dhillion and Ampornpan [
40], who reported a significantly higher colonization level in the non-chemical treatments than all other chemical fertilizer treatments. Similarly, Watanarojanaporn et al. [
13] reported higher AMF colonization in rice roots in compost-applied treatments rather than mineral fertilizer.
3.2. Rice plant growth and yield under different treatments
Generally, it is expected that the effects of AMF on plant growth will be enhanced when the fungal colonization rates are high [
41]. However, Ruíz-Sánchez et al. [
7] highlighted that the plant growth stimulation by the AMF symbiosis does not always depend upon the degree of root colonization; instead, it mainly relies on the fungal and plant species involved along with the environmental conditions. Also, there is no reported threshold value of root colonization necessary to enhance plant growth [
7], and inconsistent results could be recorded.
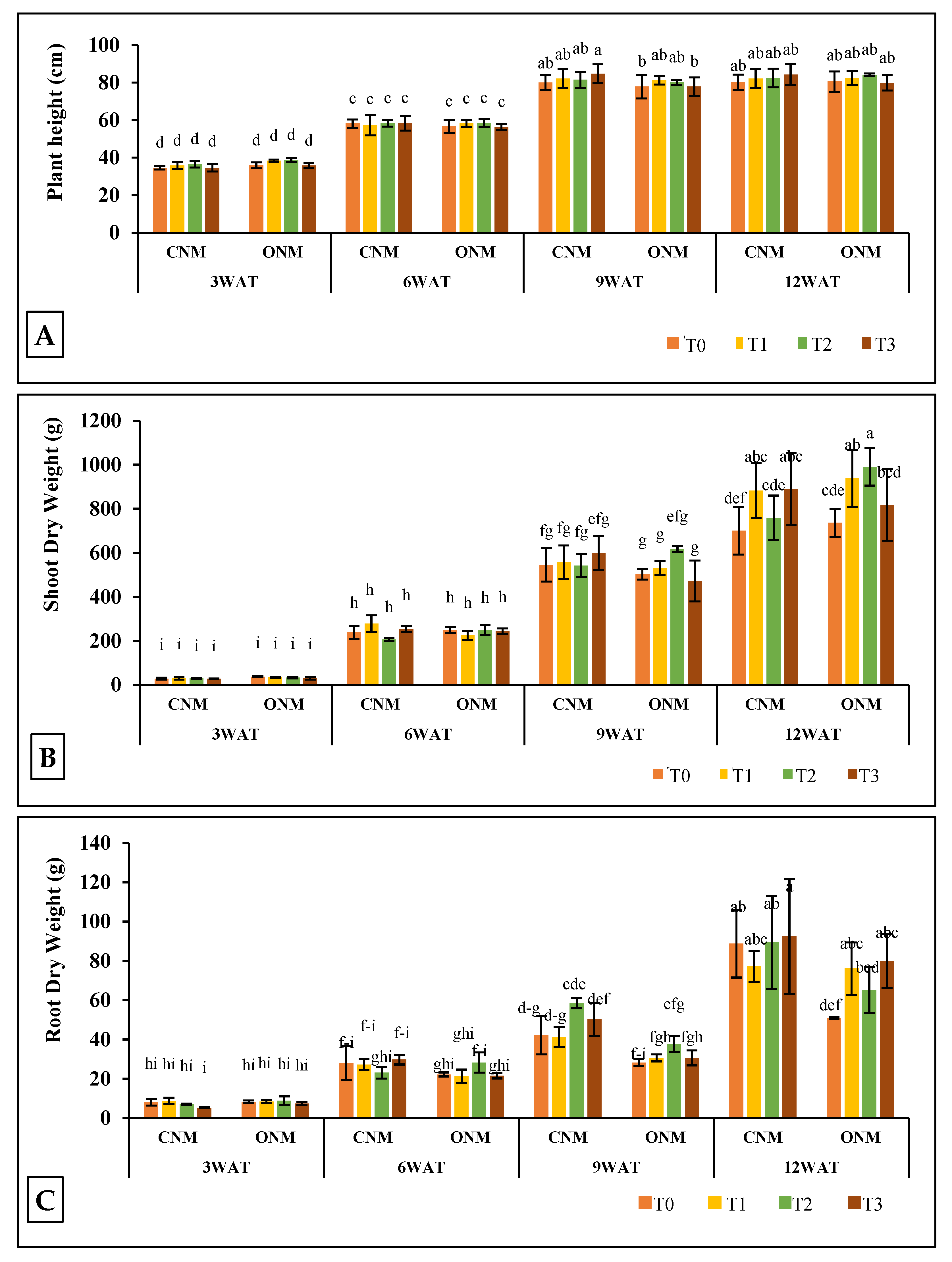
In our study, the effect of AMF on rice plant heights remained non-significant except at the heading stage (9WAT), which showed significantly higher (P < 0.05) plant heights in T1 of ONM and T3 of CNM compared to the respective controls (
Figure 3A). Ruíz-Sánchez et al. [
42] also reported significantly higher plant heights due to AMF inoculation. Shoot dry weights were significantly higher at 12WAT when AMF were applied alone (T1) and together with vetiver (T3) under both NMSs (
Figure 3B). Root dry weight however significantly low in T1 of CNM, while it was significantly higher in T3 at 12 WAT. Also, it was significantly higher in both T1 and T3 of ONM (
Figure 3C). Secilia and Bagyaraj [
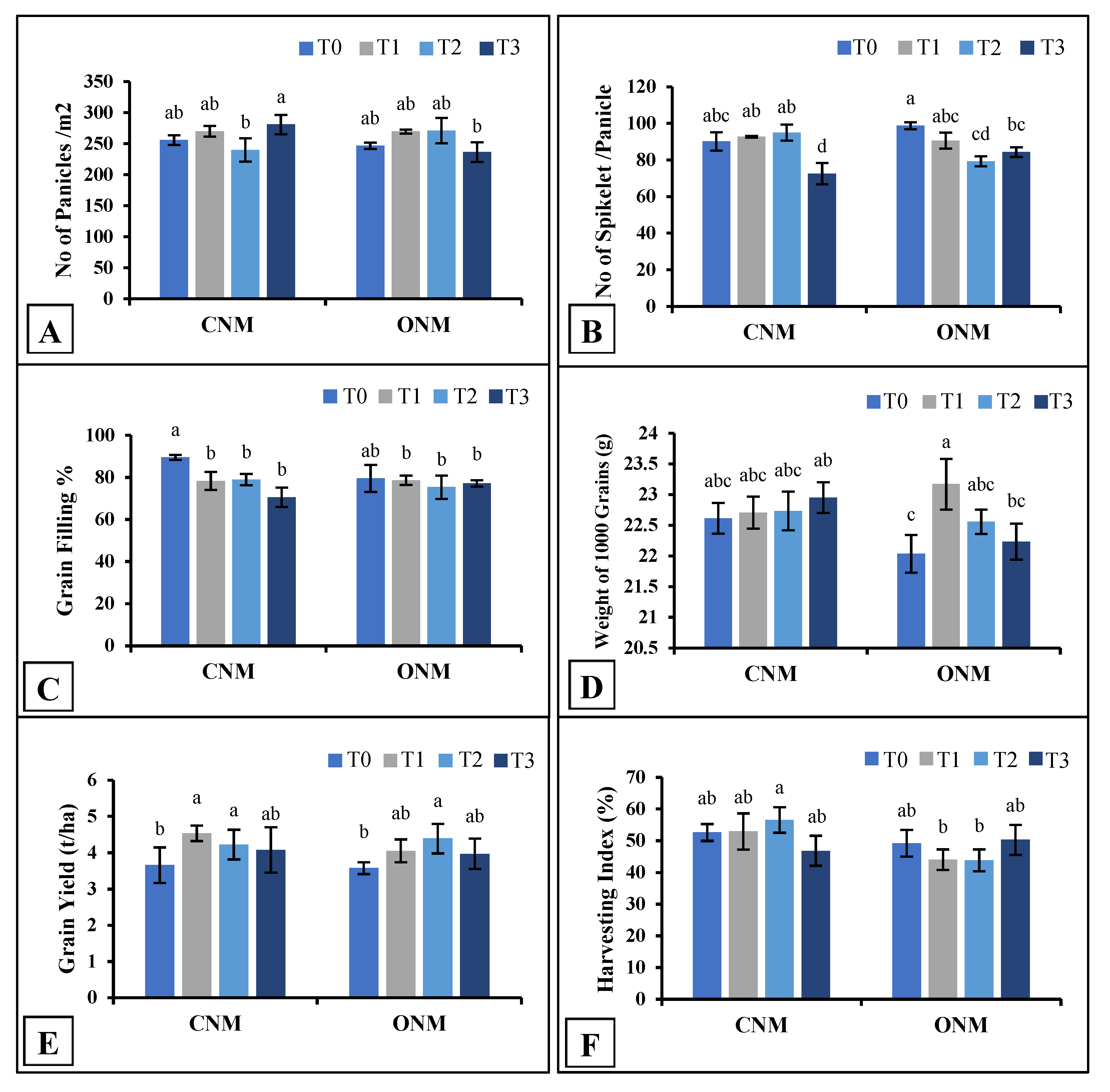
11] reported that field application of AMF significantly increases shoot biomass but not root biomass. Results on rice yield components also showed inconsistent variations against the application of AMF. However, grain yields were significantly higher in each AMF-added treatment (either T1 or T3) than their respective controls (
Figure 4E). Chareesri et al. [
12] also observed significantly higher rice yield with no such effect on rice growth derived by AMF and suggested this could have happened owing to the increased N and P allocation to the panicles during the grain-filling stage in AMF colonized plants.
In the present study, intercropping of vetiver (T2) did not produce a remarkable negative or positive impact that is enough to validate any conclusions relating to rice plant growth. Interestingly, the rice yield significantly increased in the vetiver-intercropped plots both with and without AMF inoculation. Furthermore, we observed no negative effects caused by the management practices of rice cultivation (e. g., irrigation, nutrient management, and pest control) on the growth of vetiver grass. Therefore, we suggest that vetiver can be successfully intercropped with lowland rice. Further, we implemented T3 to obtain a synergistic effect as a result of the combined benefits of AMF and vetiver; however, no such effect was observed in most of the measured parameters.
The two NMSs statistically non-significant results showed plant growth except in plant heights at 3WAT and 9WAT and root dry weight at 9WAT. Plant height was significantly higher in ONMS compared to CNMS at 3WAT, and both plant height and root dry weight were significantly higher in CNMS at 9WAT (data not shown). Also, none of the yield parameters did not significantly differ between the two NMSs. This observation suggests that ONM is also as effective as CNM in rice cultivation. Sarker et al. [
43] mentioned that chemical fertilizers provide readily available nutrients to plants; however, compost releases nutrients slowly through microbial activity and, in addition, improves the soil physical conditions that would be favorable for healthy plant growth. Therefore, organic manure could be used as a better alternative to synthetic chemical fertilizer, as they are environmentally friendly.
4. Conclusions
In this study, it was shown that field inoculation with native AMF, intercropping of vetiver, and AMF plus vetiver can improve the productivity of rice plants and lead to higher grain yields. However, there was no noticeable growth improvement with the addition of the treatments. Moreover, rice plant growth, yield, and AMF colonization did not differ significantly between the organic and conventional nutrient management systems. Interestingly, no negative impact was observed for the colonization of AMF under the CNMS. Therefore, overall it can be suggested AMF inoculums can be utilized to obtain a higher yield (as resulted; 0.45 kg/m2) in CNMS. Vetiver alone showed the best results (over the AMF inoculum) in ONMS (0.44 kg/m2); thus, it could be better to use this in such a farming system.
Author Contributions
Conceptualization, K.W.A. Madhushan and P.N. Yapa; Data curation, Dissanayake Mudiyanselage Dharmasiri Dissanayake, Tikka Devage Chamarika Priyadarshani and Xiao-Yan Wang; Formal analysis, K.W.A. Madhushan, Dissanayake Mudiyanselage Dharmasiri Dissanayake, Tikka Devage Chamarika Priyadarshani and P.N. Yapa; Funding acquisition, Abdallah Elgorban and Turki Dawoud; Investigation, Samantha C. Karunarathna, Steven Stephenson, Abdallah Elgorban, Turki Dawoud, Alviti Kankanamalage Hasith Priyashantha, P.N. Yapa and Xiao-Yan Wang; Methodology, K.W.A. Madhushan, Dissanayake Mudiyanselage Dharmasiri Dissanayake, Alviti Kankanamalage Hasith Priyashantha and Xiao-Yan Wang; Project administration, Samantha C. Karunarathna; Resources, Abdallah Elgorban and Samantha C. Karunarathna; Software, Tikka Devage Chamarika Priyadarshani; Supervision, Samantha C. Karunarathna; Validation, Steven Stephenson and P.N. Yapa; Visualization, Steven Stephenson, Alviti Kankanamalage Hasith Priyashantha and Xiao-Yan Wang; Writing—original draft, K.W.A. Madhushan, Samantha C. Karunarathna and P.N. Yapa; Writing—review & editing, Samantha C. Karunarathna, Tikka Devage Chamarika Priyadarshani, Steven Stephenson, Abdallah Elgorban, Turki Dawoud, Alviti Kankanamalage Hasith Priyashantha, Samantha C. Karunarathna, P.N. Yapa and Xiao-Yan Wang. All authors have read and agreed to the published version of the manuscript.
Funding
Financial assistance given by the World Bank Group through the project Accelerating Higher Education Expansion and Development Operation (AHEAD), DOR, Grant No. 79, Rajarata University of Sri Lanka, is highly appreciated. This research was partially supported by the National Natural Science Foundation of China (NSFC 32260004).
Acknowledgments
This work was supported by the “Yunnan Revitalization Talents Support Plan” (“Young Talents” Program and “High-End Foreign Experts” Program) for support. Xiao-Yan Wang thanks the Academician (Expert) Workstation of Yunnan Province Program (No. 202105AF150014) for financial support. The authors extend their appreciation to the Researchers Supporting (Project numbers RSP2023R197 and RSP2023R120), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Magamage, C.; Waidyaratna, W.H.M.C.U.; Dhanapala, W.P.A.P.; Panampitiya, D.M. Determination of heavy metals in rice available in Kandy district, Sri Lanka. Annals Sri Lanka Department of Agriculture 2017, 19, 351–368. [Google Scholar]
- Yapa, N.; Lakmali, D.; Zoysa, D.; Silva, K.S.; Manawadu, C.; Herath, B.M.; Madhushan, A.; Perera, G.; Ratnayakae, O.; Kapilan, R.; Rathnayake, A. Biofertilizers: An Emerging Trend in Agricultural Sustainability. Chiang Mai J. Sci. 2022, 49, 608–640. [Google Scholar] [CrossRef]
- Madhushan, K.W.A.; Herath, B.M.M.D.; Karunarathna, S.C.; Yapa, P.N. Role of arbuscular mycorrhizal fungi in sustainable crop production and forestry in Sri Lanka-A review. Stud. Fungi. 2021, 6, 437–449. [Google Scholar] [CrossRef]
- Brundrett, M. C.; Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, P.; Kumar, U.; Sugitha, T.C.K.; Parameswaran, C.; Sahoo, S.; Binodh, A.K.; Jahan, A.; Anandan, A. Arbuscular mycorrhizal fungi (AMF) for sustainable rice production. In: Advances in Soil Microbiology: Recent Trends and Future Prospects. Adhya, T.K., Lal, B., Mohapatra, B., Paul, D., Das, S. Eds.; Springer, Singapore, 2017; 2, pp. 99‒126. [CrossRef]
- Okonji, C.J.; Sakariyawo, O.S.; Okeleye, K.A.; Osunbiyi, A.G.; Ajayi, E.O. Effects of arbuscular mycorrhizal fungal inoculation on soil properties and yield of selected rice varieties. J. Agric. Sci. (Belgr.) 2018, 63, 153–170. [Google Scholar] [CrossRef]
- Ruíz-Sánchez, M.; Aroca, R.; Muñoz, Y.; Polón, R.; Ruiz-Lozano, J.M. The arbuscular mycorrhizal symbiosis enhances the photosynthetic efficiency and the antioxidative response of rice plants subjected to drought stress. J. Plant Physiol. 2010, 167, 862–869. [Google Scholar] [CrossRef]
- Baby, U.I.; Manibhushanrao, K. Influence of organic amendments on arbuscular mycorrhizal fungi in relation to rice sheath blight disease. Mycorrhiza. 1996, 6, 201–206. [Google Scholar] [CrossRef]
- Campos-Soriano, L.I.D.I.A.; García-Martínez, J.; Segundo, B.S. The arbuscular mycorrhizal symbiosis promotes the systemic induction of regulatory defence-related genes in rice leaves and confers resistance to pathogen infection. Mol. Plant Pathol. 2012, 13, 579–592. [Google Scholar] [CrossRef]
- Ilag, L.L.; Rosales, A.M.; Elazegui, F.A.; Mew, T.W. Changes in the population of infective endomycorrhizal fungi in a rice-based cropping system. Plant Soil. 1987, 103, 67–73. [Google Scholar] [CrossRef]
- Secilia, J.; Bagyaraj, D.J. Evaluation and first-year field testing of efficient vesicular arbuscular mycorrhizal fungi for inoculation of wetland rice seedlings. World J. Microbiol. Biotechnol. 1994, 10, 381–384. [Google Scholar] [CrossRef]
- Chareesri, A.; De Deyn, G.B.; Sergeeva, L.; Polthanee, A.; Kuyper, T.W. Increased arbuscular mycorrhizal fungal colonization reduces yield loss of rice (Oryza sativa L. ) under drought. Mycorrhiza. 2020, 30, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Watanarojanaporn, N.; Boonkerd, N.; Tittabutr, P.; Longtonglang, A.; Young, J.P.W.; Teaumroong, N. Effect of rice cultivation systems on indigenous arbuscular mycorrhizal fungal community structure. Environ. Microbiol. 2013, 28, 316–324. [Google Scholar] [CrossRef]
- Paranavithana, T.M.; Marasinghe, S.; Perera, G.A.D.; Ratnayake, R.R. Effects of crop rotation on enhanced occurrence of arbuscular mycorrhizal fungi and soil carbon stocks of lowland paddy fields in seasonaly dry tropics. Paddy Water Environ. 2021, 19, 217–226. [Google Scholar] [CrossRef]
- Pasolon, Y.B.; Hirata, H. Effect of white clover (Trifolium repens L.) intercropping on growth and nutrient uptake of upland rice (Oryza sativa L.) in relation to VA-mycorrhizae and soil fertility, In: Plant Nutrition — from Genetic Engineering to Field Practice. Developments in Plant and Soil Sciences. Barrow, N.J., Eds.; Springer, Dordrecht, 1993; 54, pp. 331‒334. [CrossRef]
- Shen, Q.; Chu, G. Bi-directional nitrogen transfer in an intercropping system of peanut with rice cultivated in aerobic soil. Biol. Fertil. Soils. 2004, 40, 81–87. [Google Scholar] [CrossRef]
- Maiti, D.; Barnwal, M.K.; Rana, S.K.; Variar, M.; Singh, R.K. Enhancing native arbuscular mycorrhizal association to improve phosphorus nutrition of rainfed upland rice (Oryza sativa L. ) through cropping systems. Indian Phytopathol. 2006, 59, 432–438. [Google Scholar]
- Li, Y.; Ran, W.; Zhang, R.; Sun, S.; Xu, G. Facilitated legume nodulation, phosphate uptake and nitrogen transfer by arbuscular inoculation in an upland rice and mung bean intercropping system. Plant Soil. 2009, 315, 285–296. [Google Scholar] [CrossRef]
- Ren, L.; Lou, Y.; Zhang, N.; Zhu, X.; Hao, W.; Sun, S.; Shen, Q.; Xu, G. Role of arbuscular mycorrhizal network in carbon and phosphorus transfer between plants. Biol. Fertil. Soils. 2013, 49, 3–11. [Google Scholar] [CrossRef]
- Siripin, S. Microbiology associated with the vetiver plant. In Proceedings of the second international conference on vetiver (ICV-2). Bangkok: Office of the Royal Development Projects Board, 18‒22 January 2000. [Google Scholar]
- Piriyaprin S, Sunanthapongsuk V, Limtong P, Leaungvutiviroj C, Pasda N. Study on soil microbial biodiversity in rhizosphere of vetiver grass in degradating soil. In 17th WCSS, Thailand, 14‒21 August 2002. 21 August.
- Gupta, P.; Roy, S.; Mahindrakar, A.B. Treatment of water using water hyacinth, water lettuce and vetiver grass-a review. Environ. Res. 2012, 2, 202–215. [Google Scholar] [CrossRef]
- Kumar, D.; Nikhil, K. Vetiver grass for manifold uses: a critical review. Int. J. Eng. Tech. Res. 2016, 4, 146–152. [Google Scholar]
- Chaurasia, B.; Pandey, A.; Palni, L. Distribution, colonization and diversity of arbuscular mycorrhizal fungi associated with central Himalayan rhododendrons. For. Ecol. Manag. 2005, 207, 315–324. [Google Scholar] [CrossRef]
- Selvakumar, G.; Kim, K.; Walitang, D.; Chanratana, M.; Kang, Y.; Chung, B.; Sa, T. Trap culture technique for propagation of arbuscular mycorrhizal fungi using different host plants. Korean J. Soil Sci. Fertili. 2016, 49, 608–613. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Douds, J.D.D.; Millner, P.D. Biodiversity of arbuscular mycorrhizal fungi in agroecosystems. Agric. Ecosys. Environ. 1999, 74, 77–93. [Google Scholar] [CrossRef]
- Peterson, R.L.; Massicotte, H.B.; Melville, L.H. Mycorrhizas: anatomy and cell biology. NRC Research Press, 2004.
- Bernaola, L.; Cange, G.; Way, M.O.; Gore, J.; Hardke, J.; Stout, M. Natural colonization of rice by arbuscular mycorrhizal fungi in different production areas. Rice Sci. 2018, 25, 169–174. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Ma, F.; Bloomfield, K.J.; Yang, J.; Atkin, O.K. Is resource allocation and grain yield of rice altered by inoculation with arbuscular mycorrhizal fungi? J. Plant Ecol. 2014, 8, 436–448. [Google Scholar] [CrossRef]
- Purakayastha, T.J.; Chhonkar, P.K. Influence of vesicular-arbuscular mycorrhizal fungi (Glomus etunicatum L. ) on mobilization of zinc in wetland rice (Oryza sativa L.). Biol. Fertil. Soils. 2001, 33, 323–327. [Google Scholar] [CrossRef]
- Wangiyana, W.; Cornish, P.S.; Morris, E.C. Arbuscular mycorrhizal fungi dynamics in contrasting cropping systems on vertisol and regosol soils of Lombok, Indonesia. Exp. Agric. 2006, 42, 427–439. [Google Scholar] [CrossRef]
- Kalamulla, R.; Sandaruwan, D.; Karunarathna, S.C.; Stephenson, S.L.; Tibpromma, S.; Elgorban, A.M.; Al-Rejaie, S.; Yapa, P.N.; Suwannarach, N. Assessment of Community Dynamics of Arbuscular Mycorrhizal Fungi in the Rice (Oryza sativa L. ) Rhizosphere and Potential Application as Biofertilizer. Sustainability. 2022, 14, 16537. [Google Scholar] [CrossRef]
- Chen, X.W.; Wu, F.Y.; Li, H.; Chan, W.F.; Wu, S.C.; Wong, M.H. Mycorrhizal colonization status of lowland rice (Oryza sativa L. ) in the southeastern region of China. Environ. Sci. Pollut. Res. 2017, 24, 5268–5276. [Google Scholar] [CrossRef]
- Wang, X.; Yao, H.; Wong, M.H.; Ye, Z. Dynamic changes in radial oxygen loss and iron plaque formation and their effects on Cd and As accumulation in rice (Oryza sativa L. ). Environ. Geochem. Health. 2013, 35, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, T.; Li, Y.; Björn, L.O.; Rosendahl, S.; Olsson, P.A.; Li, S.; Fu, X. Community dynamics of arbuscular mycorrhizal fungi in high-input and intensively irrigated rice cultivation systems. Appl. Environ. Microbiol. 2015, 81, 2958–2965. [Google Scholar] [CrossRef] [PubMed]
- Solaiman, M.Z.; Hirata, H. Effect of arbuscular mycorrhizal fungi inoculation of rice seedlings at the nursery stage upon performance in the paddy field and greenhouse. Plant Soil. 1997, 191, 1–12. [Google Scholar] [CrossRef]
- Qiao, X.; Bei, S.; Li, H.; Christie, P.; Zhang, F.; Zhang, J. Arbuscular mycorrhizal fungi contribute to over yielding by enhancing crop biomass while suppressing weed biomass in intercropping systems. Plant Soil. 2016, 406, 173–185. [Google Scholar] [CrossRef]
- Dhillion, S.S.; Ampornpan, L.A. The influence of inorganic nutrient fertilization on the growth, nutrient composition and vesicular arbuscular mycorrhizal colonization of pretransplant rice (Oryza sativa L. ) plants. Biol. Fertil. Soils. 1992, 13, 85–91. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal symbiosis. Academic press, 2010; pp. 800. [CrossRef]
- Ruíz-Sánchez, M.; Armada, E.; Muñoz, Y.; de Salamone, I.E.G.; Aroca, R.; Ruíz-Lozano, J.M.; Azcón, R. Azospirillum and arbuscular mycorrhizal colonization enhance rice growth and physiological traits under well-watered and drought conditions. J. Plant Physiol. 2011, 168, 1031–1037. [Google Scholar] [CrossRef]
- Sarker, M.A.R.; Pramanik, M.Y.A.; Faruk, G.M.; Ali, M.Y. Effect of green manures and levels of nitrogen on some growth attributes of transplant aman rice. Pak. J. Biol. Sci. 2004, 7, 739–742. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).