1. Introduction
Cancer is a group of diseases that refer to abnormal cell division leading to uncontrolled cell growth and proliferation, and when occurring in the colon or rectum, the disease is defined as colorectal cancer (CRC) [
1]. In 2022, the World Health Organization (WHO) and the International Agency for Research on Cancer (IARC) have estimated that CRC was the third most common cancer worldwide with approximately 1.9 million cases annually and the second leading cause of death for oncological reasons with globally 900 000 deaths [
2,
3]. CRC mainly originates from a benign tumor or adenomatous polyp that evolves into a dangerous malignant tumor [
4]. This transformation is characterized by the capacity of the cells to infiltrate the different histological layers of the organs [
5]. The most dangerous stage is the metastatic phase, when the cancer cells have acquired the ability to detach from the initial tumor and invade other organs through the blood or lymph, and create secondary tumors [
6]. Today, CRC treatment is at a crossroad, strategies involve many conventional and advanced scientific methods. These therapies incorporate surgery/polypectomy, radiation therapy, chemotherapy, combination therapy, immunotherapy and targeted therapy [
7,
8,
9]. Unfortunately, these methods are still insufficient for the complete cure of advanced CRC. Researchers have tried to provide modern alternative approaches to fight CRC resistance mechanisms [
10]. Over the past decade, significant progress in CRC treatments has been achieved through the development of novel drugs and elaborate treatment protocols. However, the increasing resistance of tumor cells toward these new drugs and persistent side effects due to their toxicity on healthy tissues make it imperative to add other options to CRC treatment regimens.
Photodynamic therapy (PDT) has attracted widespread attention in recent years as a non-invasive and highly selective approach for cancer treatment [
11,
12]. The molecular mechanism of PDT involves the photoactivation of a photosensitizer (PS) at an appropriate wavelength in the presence of oxygen molecules [
13,
14,
15]. In effect, PDT exploits the potency of visible light to generate cytotoxic agents in a spatially and temporally controlled manner to directly damage the targeted tumor cells and tissues [
16,
17]. PDT is mainly associated to the production of reactive oxygen species (ROS), which are involved in cell death [
18,
19]. To happen, the PS must absorb at least one photon to be promoted to a sufficiently long-lived excited state, and then to induce photodynamic reactions in an oxygenated environment [
20]. Under the effect of light irradiation, the PS is activated and goes from a ground to an excited state [
21,
22]. At this stage, the PS is very unstable and loses its excess energy either directly or via an excited triplet state intermediate [
23]. The excited triplet state will slowly return to the ground state by photochemical reactions of type I or II. Both reactions may take place simultaneously, their kinetics being strongly correlated to the presence of oxygen, the substrate concentration, and the nature of the PS. In type I reactions, the free radicals may further react with oxygen to produce ROS [
24,
25]. Superoxide anion initially produced via type I pathway by monovalent reduction does not cause oxidative damage but reacts with itself to generate oxygen and hydrogen peroxide. However, in type II reactions, the excited PS transfers its energy directly to molecular oxygen to form singlet oxygen. These highly cytotoxic ROS can oxidize a variety of biomolecules, inducing an acute stress response and triggering a series of redox signaling pathways, generally leading to cell death [
26,
27,
28]. Currently, the most widely used PS in PDT are tetrapyrrole derivatives such as porphyrins, chlorins, bacteriochlorins, and phthalocyanines. Nevertheless, the main inconveniences of these planar aromatic PS are their low water solubility, which limits intravenous administration, their poor photophysical properties due to PS aggregation, and their low tumor selectivity, thus overall limiting their use in the clinic [
29].
Chemotherapy is essential to cancer treatments. Indeed, platinum-based drugs like oxaliplatin and carboplatin, which remain at the forefront of the CRC regimens, are used on an everyday basis [
30]. Unfortunately, this type of metal-based drugs has shown significant side effects, which has led to the search for new and less toxic anticancer metal-based agents [
31]. Among other metal-based drugs, ruthenium (Ru) derivatives have received much attention due to their interesting properties [
32]. And some Ru-based chemotherapeutics have already entered clinical trials [
33]. Moreover, about 15 years ago, combining PDT and chemotherapy with Ru-based complexes was introduced [
34,
35], and today such a Ru complex, TLD1433, is in clinical trial [
36]. TLD1433 is a cationic bis(4,4’-dimethyl-2,2’-bipyridine) (dmbp) and 2-(2,2':5',2''-terthiophene-5-yl)-1,3,7,8-tetraaza-1H-cyclopenta[l]phenanthrene (ip-3t) Ru(II) complex with the general formula [Ru(dmbp)
2(ip-3t)]
2+. This complex exploits the rich photophysical properties of polypyridyl Ru-based complexes. Upon photoactivation of the Ru-center, an intra-ligand charge transfer (ILCT) is taking place, which generates
1O
2. This ruthenium complex combines efficiently photochemotherapy and PDT in a single molecule.
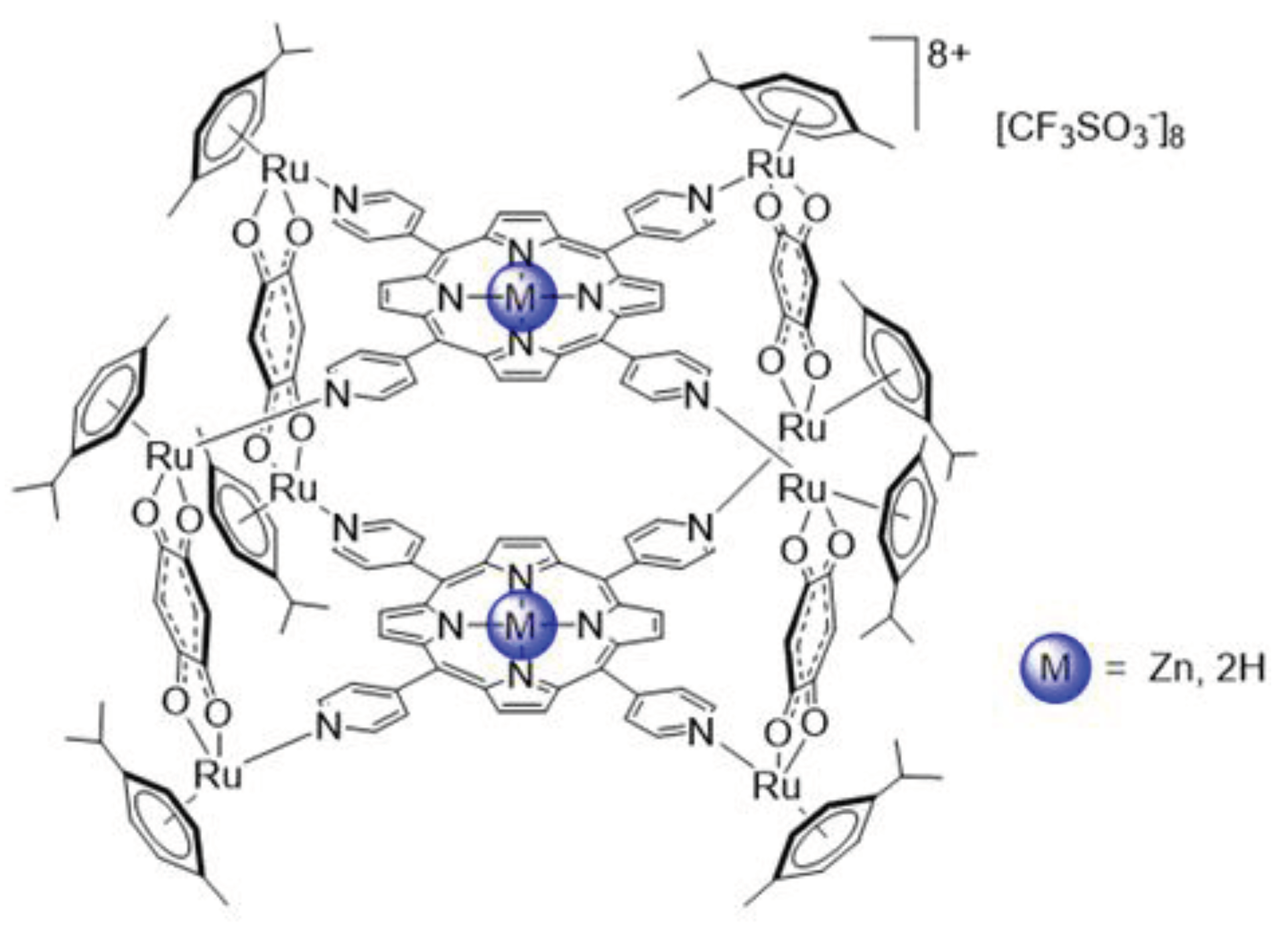
Recently, we have proposed another strategy to associate PS and Ru-based complexes in a single molecule, using a coordination self-assembly process [
37]. In these systems, two tetrapyridylporphyrin units are linked by four arene-Ru clips (
Figure 1). Such octanuclear complexes allow internalization of the PS to cells, and they show moderate dark cytotoxicity on ovarian cancer cell lines (≈ 8 μM) [
38]. Herein, we are looking at the possibility of using these metalla-assemblies to treat CRC by a combination of PDT and chemotherapy. Therefore, we have verified whether the photoactivity of the PS has been modified or not when it is part of a metalla-assembly by investigating the anticancer effect of the functionalized 2H-TPyP and Zn-TPyP with arene-Ru complexes on human HCT116 and HT-29 colorectal cancer cell lines. Then, for better understanding the cell death process involved, we examined the cell cycle distribution, phosphatidylserines externalization, as well as caspase-3 activation, poly-ADP ribose polymerase (PARP) cleavage and DNA fragmentation. Subsequently, consistent with other PDT studies, our results demonstrated that once photoactivated, 2H-TPyP-arene-Ru and Zn-TPyP-arene-Ru assemblies generate cellular ROS production and achieve their anticancer effects through an apoptotic process.
3. Discussion
PDT is an innovative cancer therapy that offers advantages over conventional treatments. Despite its potential advantages, only a small number of PS have been approved in the clinic, mainly porphyrin-type compounds [
39]. This type of PS is often limited due to low solubility in biological media, but when incorporated into delivery vectors, they can be internalized into cells. Based on this assumption, the conjugation of porphyrin with metals has received much attention [
40]. Recent scientific studies showed that ruthenium complexes are one of the most interesting metal-based drugs used in the treatment of several cancers such as colon cancer [
32,
33]. The interesting properties of Ru complexes have led to their potential use in various fields such as PS and photoactive DNA cleavage agents for therapeutic purposes [
41]. Several studies reported that porphyrin-Ru complexes had significant anticancer effects. Bogoeva et al. reported Ru porphyrin-induced photodamage in bladder cancer cells [
42]. In addition, Schmitt et al. demonstrated that 5,10,15,20-tetra(4-pyridyl)porphyrin (TPP) arene Ru (II) derivatives exhibited excellent phototoxicities toward melanoma cells when exposed to light at 652 nm [
34]. Cellular uptake and localization microscopy studies of [Ru
4(η
6-C
6H
5CH
3)
4(TPP)Cl
8] revealed that they accumulated in the cytoplasm of melanoma cells. Another study provided by Rani-Beeram et al. established that fluorinated Ru porphyrin presented a strong DNA interaction that leads to its cleavage in melanoma cells [
35]. More recently, we reported that cubic or prismatic cages can serve as an ideal carrier for PS to treat rheumatoid arthritis [
37,
43].
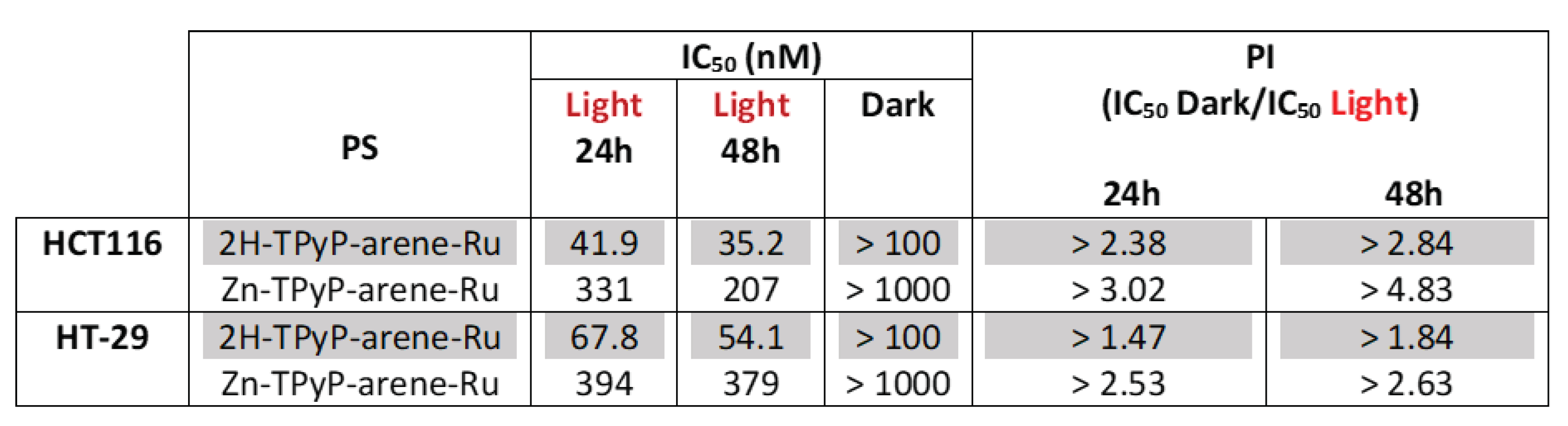
In the current study, we have determined the biological activity of cationic TPyP-arene-Ru metalla-assemblies (
Figure 1) on CRC cells. We wanted to evaluate the potential of such octanuclear assemblies as anticancer agents on CRC. For this purpose, we determined the effect of 2H-TPyP-arene-Ru and Zn-TPyP-arene-Ru complexes associated with PDT on two human CRC cell lines (HCT116 and HT-29). First of all, we evaluated the cytotoxicity and photocytotoxicity of 2H-TPyP-arene-Ru and Zn-TPyP-arene-Ru, and we demonstrated that both structures have a significant photo-cytotoxic effect on the two CRC cell lines studied, IC
50 values being in the nanomolar range. However, in the dark, the concentrations required to observe a cytotoxic effect on those cell lines are much higher (
Table 1), suggesting that PDT is the dominant therapy over chemotherapy. This result was not necessarely surprising considering that the structure of the assemblies contains two units of tetrapyridylporphin as PS, which has the effect of strengthening the effectiveness of the PDT treatment and consequently providing a better therapeutic effect under light. The IC
50 values of 2H-TPyP-arene-Ru coupled with PDT on both cell lines are 5 to 8-times higher when compared to the Zn-tetrapyridylporphin analog. This can be linked to the stronger fluorescence of PS with a metallic center. Fluorescence is a consequence of the energetic decay from the excited state of the PS to the minimum energy state. Therefore, high fluorescence quantum yield (Ø
F) suggests that most of the energy in the singlet excited state of the PS returns to the ground state without passing through the triplet excited state. Consequently, generating more fluorescence, but leaving behind less energy in the triplet state to interact with O
2 and to produce ROS. However, in all cases, we systematically obtained under PDT a significant cytotoxic effect for which the IC
50 values are in the nanomolar range.
Inhibition of cancer cell proliferation by cytotoxic drugs could be the result of induction of apoptosis or cell cycle arrest or a combination of both processes. Therefore, we investigated the cell growth mechanism inhibition by flow cytometry analysis. We demonstrated that 2H-TPyP-arene-Ru and Zn-TPyP-arene-Ru coupled with PDT led to a significant increase in the proportion of apoptotic cells, as reflected by the sub-G1 peaks. Whereas, only 2H-TPyP-arene-Ru at 48h post-irradiation showed accumulation of S-phase cells, while Zn-TPyP-arene-Ru-PDT showed no significant effect on cell cycle distribution. In order to evaluate the induction of apoptosis mechanism leading to cell death, we investigated by flow cytometry the percentage of phosphatidylserines externalization of apoptotic cells by annexin-V-FITC/PI dual staining assay. We established that photoactivation of 2H-TPyP-arene-Ru and Zn-TPyP-arene-Ru increased dramatically the cumulative rate of early and late apoptosis, which can confirm cell death via apoptosis. These results are in agreement with a study held by Silva
et al. reporting the apoptotic cell death in human colon carcinoma HCT116 cells treated with Ru(II)-thymine complexes [
44]. Furthermore, to validate the apoptotic mechanism, we evaluated the last stages in the death mechanism up to DNA fragmentation, we demonstrated that 2H-TPyP-arene-Ru and Zn-TPyP-arene-Ru coupled with PDT induced caspase-3 activation, significant PARP cleavage and DNA fragmentation. This result can be related to the strong potential of Ru complexes’ photophysical and photochemical properties that allow them to bind to DNA and induces its cleavage by photoactivation [
45]. These results agree with Lu
et al. study, which reports the anticancer effect of Ru complexes on hepatocellular carcinoma [
46].
4. Materials and Methods
4.1. Materials
DMEM medium, DMEM red-phenol-free medium, RPMI 1640 medium, RPMI 1640 red-phenol-free medium, fetal bovine serum (FBS), L-glutamine, and penicillin-streptomycin were purchased from Gibco BRL (Cergy-Pontoise, France). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), cell death detection enzyme-linked immunosorbent assay PLUS (ELISA) and human anti-β-actin antibody were obtained from Sigma-Aldrich (Saint-Quentin-Fallavier, France). Poly-ADP-ribose polymerase (PARP) antibody and goat anti-rabbit IgG secondary antibody conjugated to horseradish peroxidase (HRP) were acquired from Cell Signaling Technology-Ozyme (Saint-Quentin-en-Yvelines, France). Rabbit anti-mouse IgG-IgM H&L HRP secondary antibody, Annexin V-FITC, and propidium iodide (PI) were obtained from Invitrogen-Thermo Fisher Scientific (Villebon-Sur-Yvette, France). Immobilon Western Chemiluminescent HRP Substrate was acquired from Merck (Lyon, France).
2H-TPyP-arene-Ru and Zn-TPyP-arene-Ru complexes were prepared as previously described [
47]. Stock solutions of TPyP-arene-Ru complexes were dissolved at 1 mM concentration in DMSO, then were diluted in culture medium to obtain the appropriate final concentrations just before use. The concentration of DMSO in culture medium was lower than 0.1% in all cases, which is considered to be non-toxic.
4.2. Cell culture and treatment
Human CRC cell lines HT-29 and HCT116 were purchased from the American Type Culture Collection (ATCC-LGC Standards, Mosheim, France). Cells were grown in DMEM medium for HT-29 cells and RPMI 1640 medium for HCT116 cells. Culture mediums were supplemented with 10% FBS, 1% L-glutamine and 100 U/mL penicillin, and 100 µg/mL streptomycin. Cultures were maintained in a humidified atmosphere containing 5% CO2 at 37°C. For all experiments, cells were seeded at 2.1×104, 1.2×104 cells/cm2 for HT-29 and HCT116 cells respectively. Cells were washed and the culture medium was replaced by a red phenol-free appropriate culture medium before PDT.
4.3. Cytotoxicity and phototoxicity
Cytotoxicity and phototoxicity was determined using an MTT assay. Briefly, cells were seeded in 96-well culture plates and grown for 36h in an appropriate culture medium before exposure or not to 2H-TPyP-arene-Ru or Zn-TPyP-arene-Ru complexes. After 24h incubation, cells were washed and irradiated or not with a 630-660 nm CURElight lamp at 75 J/cm
2 (PhotoCure ASA, Oslo, Norway). The emission spectrum of this light source is shown in
Figure S1 (Supporting Information). MTT assays were performed 24 and 48h post-irradiation and cell cytotoxicity was expressed as a percentage of each treatment condition by normalizing to untreated cells.
4.4. Intracellular ROS production
Cellular ROS production was quantified using the 2',7'-dichlorofluorescein diacetate cellular reactive oxygen species detection assay kit. Cells were seeded in 25 cm2 culture flasks at the determined density and cultured for 36h. Cells were then treated or not with 2H-TPyP-arene-Ru or Zn-TPyP-arene-Ru at the determined IC50 values. After 24h incubation, cells were labeled with DCFDA for 30 min at 37°C. The cells were then washed, the medium replaced by the corresponding medium without phenol red, and irradiated or not. Cellular ROS generation was then quantified 1h after irradiation by flow cytometry analysis. Hydrogen peroxide (H2O2) was used as a positive control at 800 μM.
4.5. Cellular internalization
4.5.1. Flow cytometry with AMNIS imaging
Cells were seeded in 25 cm2 culture flasks at the determined density and cultured for 36h. Cells were then treated with 2H-TPyP-arene-Ru or Zn-TPyP-arene-Ru at the determined IC50 values. After 1-4h incubation, the natural fluorescence of 2H-TPyP-arene-Ru (excitation: 405 nm; emission: 650 nm) or Zn-TPyP-arene-Ru (excitation: 561 nm; emission: 655 nm) was analyzed by flow cytometry coupled with AMNIS® image analysis, and the data were analyzed with IDEAS® software (Merck).
4.5.2. Confocal microscopy
Cells were seeded for 36h in an incubation chamber (ibidi) coated with a gel containing acetic acid (20 mM) and type I collagen (3 mg/mL). Cells were then treated at the determined IC50 values. Photographs were taken using a Carl Zeiss LSM 510 confocal laser microscope. Meta - x1000 (Marly-le-Roi, France). Co-localization was analyzed using the ImageJ software.
4.6. Cell cycle analysis
The cell cycle distributions in colorectal HT-29 and HCT116 cell lines were analyzed by flow cytometry using propidium iodide (PI) staining. For each cell line, cells were treated or not with the determined IC50 values of 2H-TPyP-arene-Ru and Zn-TPyP-arene-Ru for 24 and 48h, then harvested with trypsin. For flow cytometry analysis, 1.5×106 cells of each condition were collected, washed with PBS, and fixed by adding 1 mL of chilled 70% ethanol in PBS and stored at -20°C. Following fixation, cells were pelleted, washed in cold PBS, resuspended in 500 µL of cold PBS containing 30 µL of RNase A (10 mg/mL), and incubated for 30 min at room temperature. After staining with 25 µL of PI, the percentage of cells in each stage of the cell cycle was determined using the FACS system (BD Biosciences, San Jose, CA). All the experiments were performed on three samples.
4.7. Mechanisms of apoptosis
4.7.1. Annexin V-FITC/PI dual staining assay
The annexin V-FITC/PI dual staining assay was used to determine the percentage of apoptotic cells. For each cell line, cells were treated or not with the determined IC50 values of 2H-TPyP-arene-Ru and Zn-TPyP-arene-Ru for 24 and 48h and then harvested with trypsin. 2.5×105 cells of each condition were collected, washed in PBS, centrifuged, and resuspended in 300 µL binding buffer (1X) containing 5 µL of annexin V-FITC and 1 µL of PI (0.1 mg/mL) at room temperature in the dark. After 15 min incubation, cells were analyzed for the percentage undergoing apoptosis using the FACS system (BD Biosciences).
4.7.2. Quantitative analysis of activated caspases-3/-7
Cells were seeded in 96-well plates at the determined density and cultured for 36h. Cells were then treated or not with 2H-TPyP-arene-Ru or Zn-TPyP-arene-Ru at the determined IC50 values. After 24h incubation, the culture medium was replaced by the corresponding phenol red-free medium, and the cells were then irradiated or not. Cells were then treated with caspases-3/-7 green reagent (5 μM) and placed in the IncuCyte® S3 live cell analysis system (Sartorius). Every 2h, cells were imaged at a rate of 4 images per well in phase contrast and green fluorescence using x20 magnification. The number of cells in apoptosis was quantified with IncuCyte® software (Sartorius) using the ratio of the number of fluorescent cells.
4.7.3. Protein extraction and western blot analysis
For each cell line, cells were treated or not with the determined IC
50 values of 2H-TPyP-arene-Ru and Zn-TPyP-arene-Ru for 24 and 48h and then harvested with trypsin. For total protein extraction, collected samples of each condition were washed in PBS. Then, the total cell pool was centrifuged at 200 g for 5 min at 4°C and homogenized in RIPA lysis buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 1% sodium deoxycholate, 1% NP-40, 0.1% SDS, 20 mg/mL of aprotinin) containing protease inhibitors according to the manufacturer's instructions as previously described [
48]. The protein level was determined using the Bradford method. Proteins (60 µg) were separated on 12.5% SDS-PAGE gels and transferred to PVDF membranes (Amersham Pharmacia Biotech, Saclay, France). Membranes were probed with respective human antibodies against caspase-3, cleaved caspase-3, PARP and β-actin used as a loading control, according to the manufacturer's instructions. After incubation with appropriate secondary antibodies, blots were developed using the "Immobilon Western" substrate following the manufacturer's protocol and G: BOX system (Syngene, Ozyme).
4.7.4. DNA fragmentation
For each cell line, cells were treated or not at the determined IC
50 concentrations of 2H-TPyP-arene-Ru and Zn-TPyP-arene-Ru for 24 and 48h and then harvested with trypsin. Histone release from the nucleus during apoptosis was analyzed using the Cell Death Detection ELISA
PLUS as previously described [
49]. 2×10
5 cells of each condition were obtained and DNA fragmentation was measured according to the manufacturer's protocol.
4.8. Statistical Analysis
All quantitative results are expressed as the mean ± standard error of the mean (SEM) of separate experiments. Statistical significance was evaluated by the two-tailed unpaired Student’s t-test and expressed as: *p < 0.05; **p < 0.01 and ***p < 0.001.
Author Contributions
Conceptualization, J.M, B.T., and B.L.; Methodology, J.M., A.P., M.G.-V., L.P., C.O. and C.C.; Validation, S.A., M.D.-A. and B.L., Writing-original draft preparation, J.M., and A.P.; Writing—review and editing, B.T., and B.L. All authors have read and agreed to the published version of the manuscript.
Figure 1.
Molecular structures of the metalla-assemblies. 2H-TPyP-arene-Ru (M = 2H) or Zn-TPyP-arene-Ru (M = Zn).
Figure 1.
Molecular structures of the metalla-assemblies. 2H-TPyP-arene-Ru (M = 2H) or Zn-TPyP-arene-Ru (M = Zn).
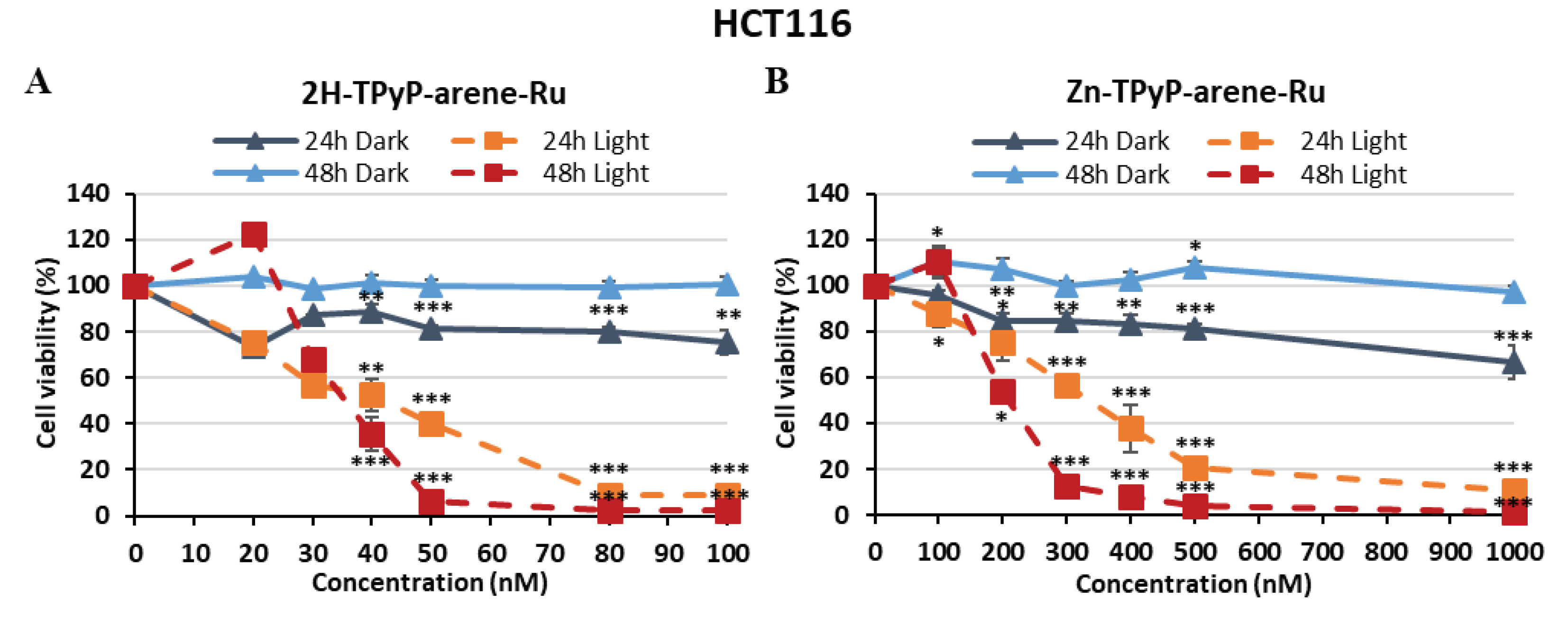
Figure 2.
Photocytotoxic effect of 2H-TPyP-arene-Ru (A) and Zn-TPyP-arene-Ru (B) on HCT116 cells. Cells were seeded in 96-well plates and cultured for 36h before being treated or not with 2H-TPyP-arene-Ru or Zn-TPyP-arene-Ru. After 24h incubation, cells were irradiated or not with a 630-660 nm CURElight lamp at 75 J/cm2 (PhotoCure ASA, Oslo, Norway). MTT assays were performed at 24 and 48h after irradiation, and cell cytotoxicity was expressed as a percentage of each treatment condition compared with untreated cells. Data are presented as mean ± SEM (n = 3). * p < 0.05; ** p < 0.01 and *** p < 0.001.
Figure 2.
Photocytotoxic effect of 2H-TPyP-arene-Ru (A) and Zn-TPyP-arene-Ru (B) on HCT116 cells. Cells were seeded in 96-well plates and cultured for 36h before being treated or not with 2H-TPyP-arene-Ru or Zn-TPyP-arene-Ru. After 24h incubation, cells were irradiated or not with a 630-660 nm CURElight lamp at 75 J/cm2 (PhotoCure ASA, Oslo, Norway). MTT assays were performed at 24 and 48h after irradiation, and cell cytotoxicity was expressed as a percentage of each treatment condition compared with untreated cells. Data are presented as mean ± SEM (n = 3). * p < 0.05; ** p < 0.01 and *** p < 0.001.
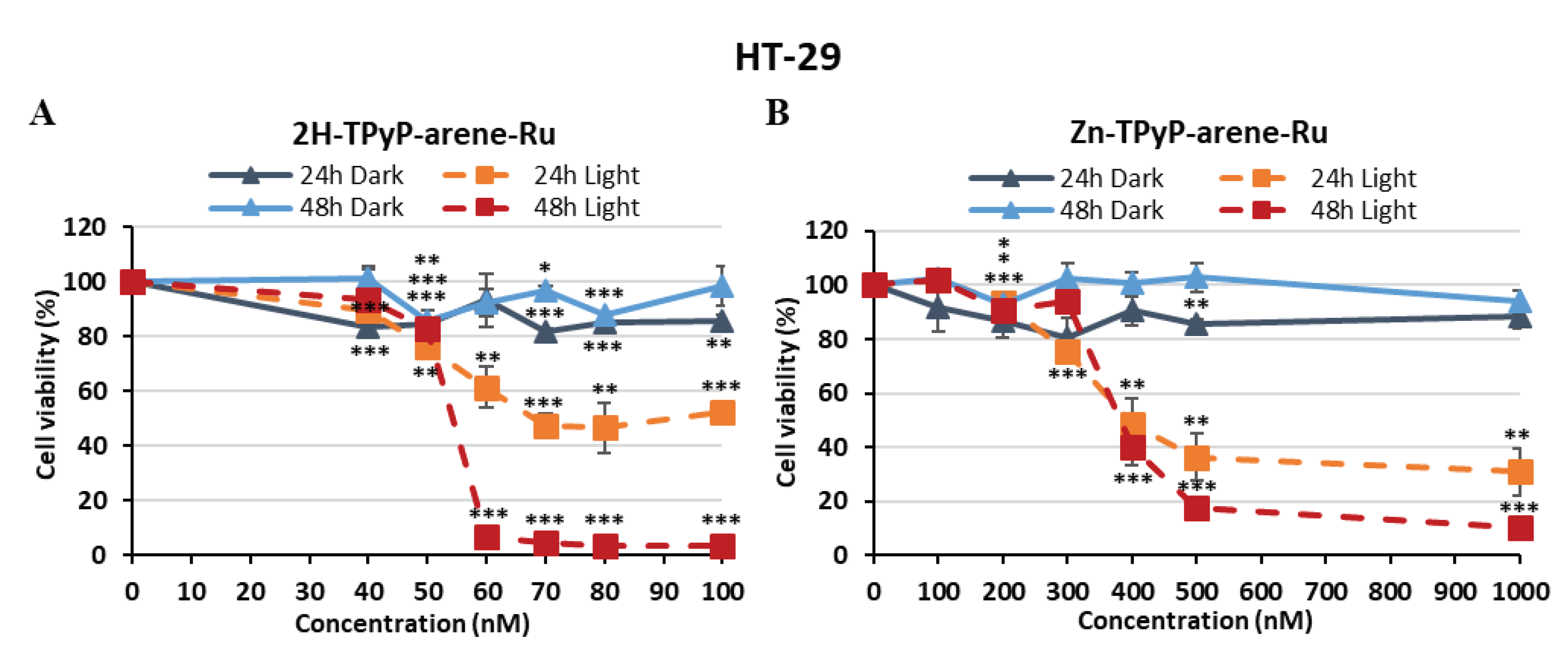
Figure 3.
Photocytotoxic effect of 2H-TPyP-arene-Ru (A) and Zn-TPyP-arene-Ru (B) on HT-29 cells. Cells were seeded in 96-well plates and cultured for 36h before being treated or not with 2H-TPyP-arene-Ru or Zn-TPyP-arene-Ru. After 24h incubation, the cells were washed and irradiated or not with a CURElight lamp from 630-660 nm at 75 J/cm2 (PhotoCure ASA, Oslo, Norway). MTT assays were performed at 24 and 48h after irradiation, and cell cytotoxicity was expressed as a percentage of each treatment condition compared with untreated cells. Data are presented as mean ± SEM (n = 3). * p < 0.05; ** p < 0.01 and *** p < 0.001.
Figure 3.
Photocytotoxic effect of 2H-TPyP-arene-Ru (A) and Zn-TPyP-arene-Ru (B) on HT-29 cells. Cells were seeded in 96-well plates and cultured for 36h before being treated or not with 2H-TPyP-arene-Ru or Zn-TPyP-arene-Ru. After 24h incubation, the cells were washed and irradiated or not with a CURElight lamp from 630-660 nm at 75 J/cm2 (PhotoCure ASA, Oslo, Norway). MTT assays were performed at 24 and 48h after irradiation, and cell cytotoxicity was expressed as a percentage of each treatment condition compared with untreated cells. Data are presented as mean ± SEM (n = 3). * p < 0.05; ** p < 0.01 and *** p < 0.001.
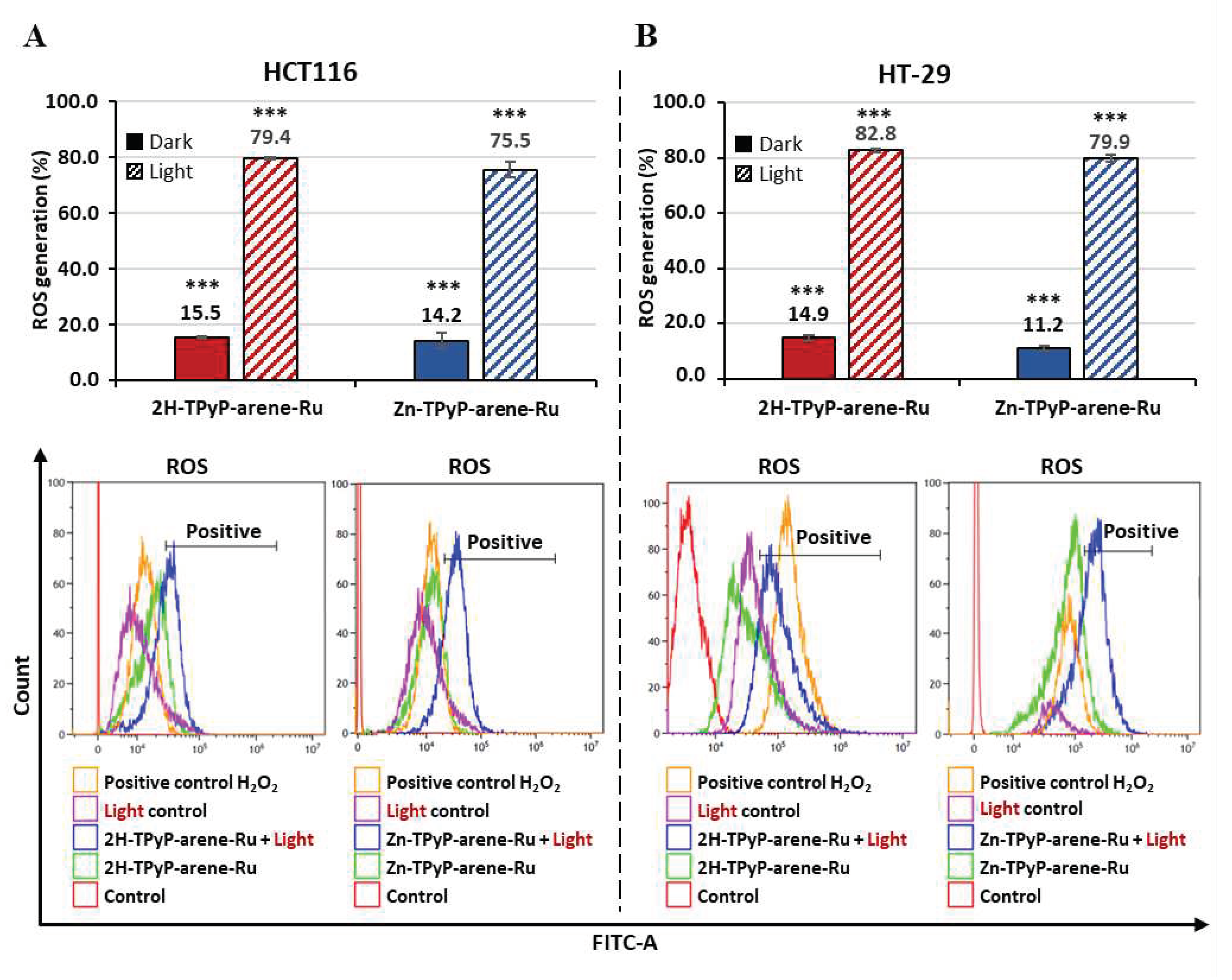
Figure 4.
ROS generation by TPyP-arene-Ru on HCT116 (A) and HT-29 (B) cell lines. Cells were treated or not with 2H-TPyP-arene-Ru or Zn-TPyP-arene-Ru at IC50 concentrations. The cells were labeled with DCFDA and irradiated or not. ROS production was then quantified by flow cytometry and interpreted using the % positive fluorescence values given in the tables. *** p < 0.001.
Figure 4.
ROS generation by TPyP-arene-Ru on HCT116 (A) and HT-29 (B) cell lines. Cells were treated or not with 2H-TPyP-arene-Ru or Zn-TPyP-arene-Ru at IC50 concentrations. The cells were labeled with DCFDA and irradiated or not. ROS production was then quantified by flow cytometry and interpreted using the % positive fluorescence values given in the tables. *** p < 0.001.
Figure 5.
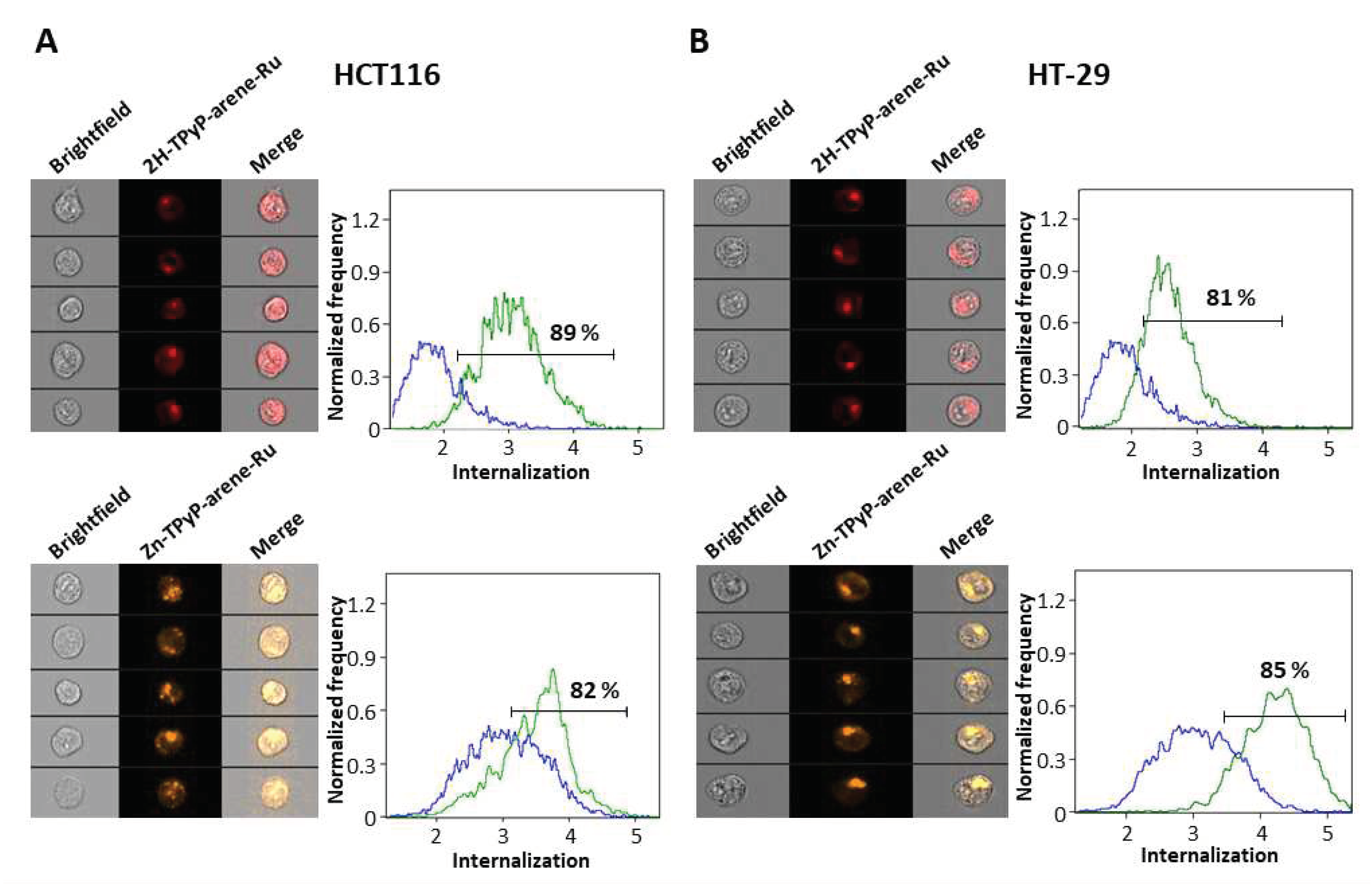
Cellular internalization of TPyP-arene-Ru in HCT116 (A) and HT-29 (B) cells. Cells were treated with 2H-TPyP-arene-Ru and Zn-TPyP-arene-Ru at IC50 concentrations. After 24h incubation, the fluorescence of 2H-TPyP-arene-Ru (excitation: 405 nm; emission: 650 nm) and Zn-TPyP-arene-Ru (excitation: 561 nm; emission: 655 nm) were analyzed by flow cytometry coupled to AMNIS® image analysis.
Figure 5.
Cellular internalization of TPyP-arene-Ru in HCT116 (A) and HT-29 (B) cells. Cells were treated with 2H-TPyP-arene-Ru and Zn-TPyP-arene-Ru at IC50 concentrations. After 24h incubation, the fluorescence of 2H-TPyP-arene-Ru (excitation: 405 nm; emission: 650 nm) and Zn-TPyP-arene-Ru (excitation: 561 nm; emission: 655 nm) were analyzed by flow cytometry coupled to AMNIS® image analysis.
Figure 6.
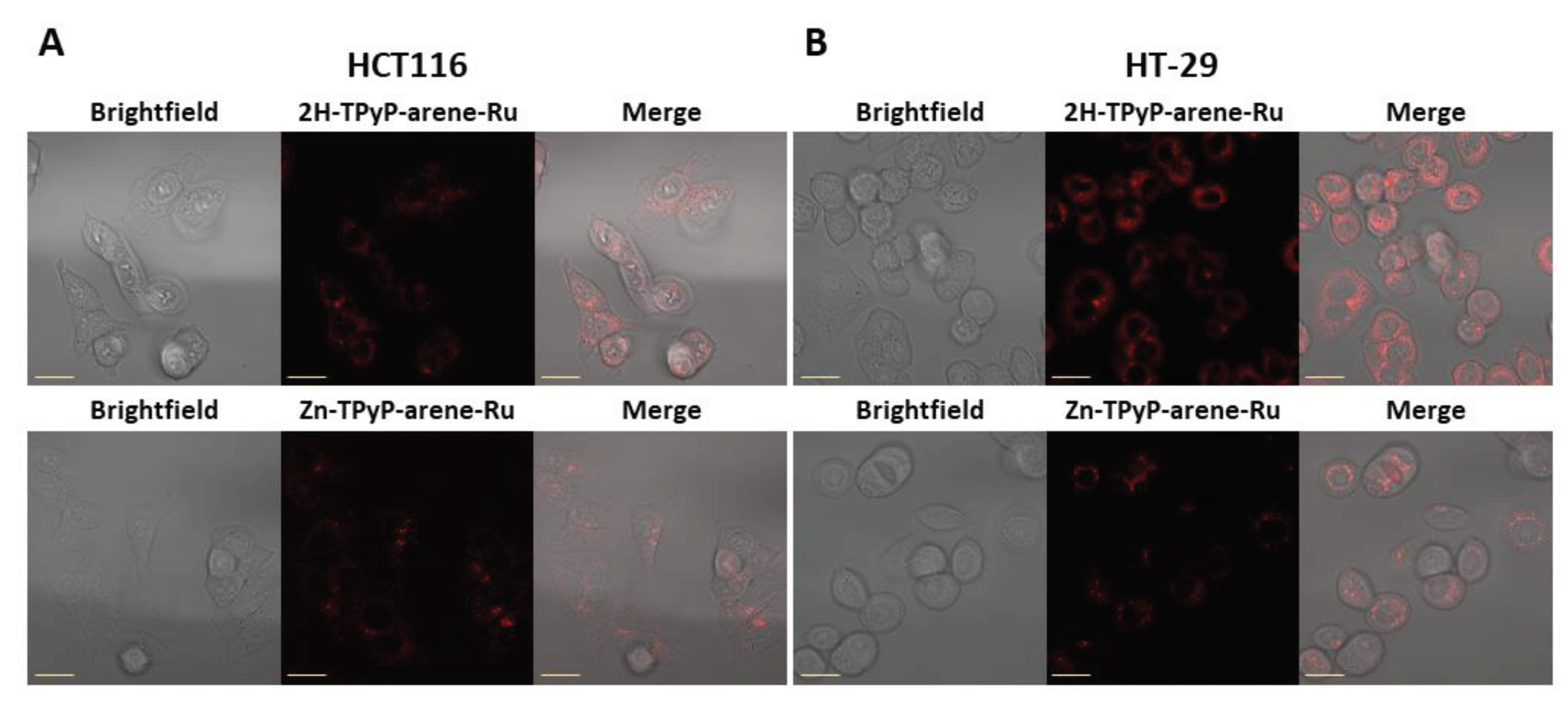
Cellular internalization of TPyP-arene-Ru by confocal microscopy on HCT116 (A) and HT-29 (B) cells. Cells were seeded at the appropriate density and cultured for 36h in an incubation chamber with a coating of type I collagen and acetic acid. Cells were then treated with 2H-TPyP-arene-Ru (excitation: 405 nm; emission: 650 nm) and Zn-TPyP-arene-Ru (excitation: 561 nm; emission: 655 nm) at IC50 concentrations. Fluorescence of both compounds was determined by confocal microscopy and cell internalization was determined using the Image J image-processing software. Images show the different treatment conditions. Yellow scale bar = 20 µm.
Figure 6.
Cellular internalization of TPyP-arene-Ru by confocal microscopy on HCT116 (A) and HT-29 (B) cells. Cells were seeded at the appropriate density and cultured for 36h in an incubation chamber with a coating of type I collagen and acetic acid. Cells were then treated with 2H-TPyP-arene-Ru (excitation: 405 nm; emission: 650 nm) and Zn-TPyP-arene-Ru (excitation: 561 nm; emission: 655 nm) at IC50 concentrations. Fluorescence of both compounds was determined by confocal microscopy and cell internalization was determined using the Image J image-processing software. Images show the different treatment conditions. Yellow scale bar = 20 µm.
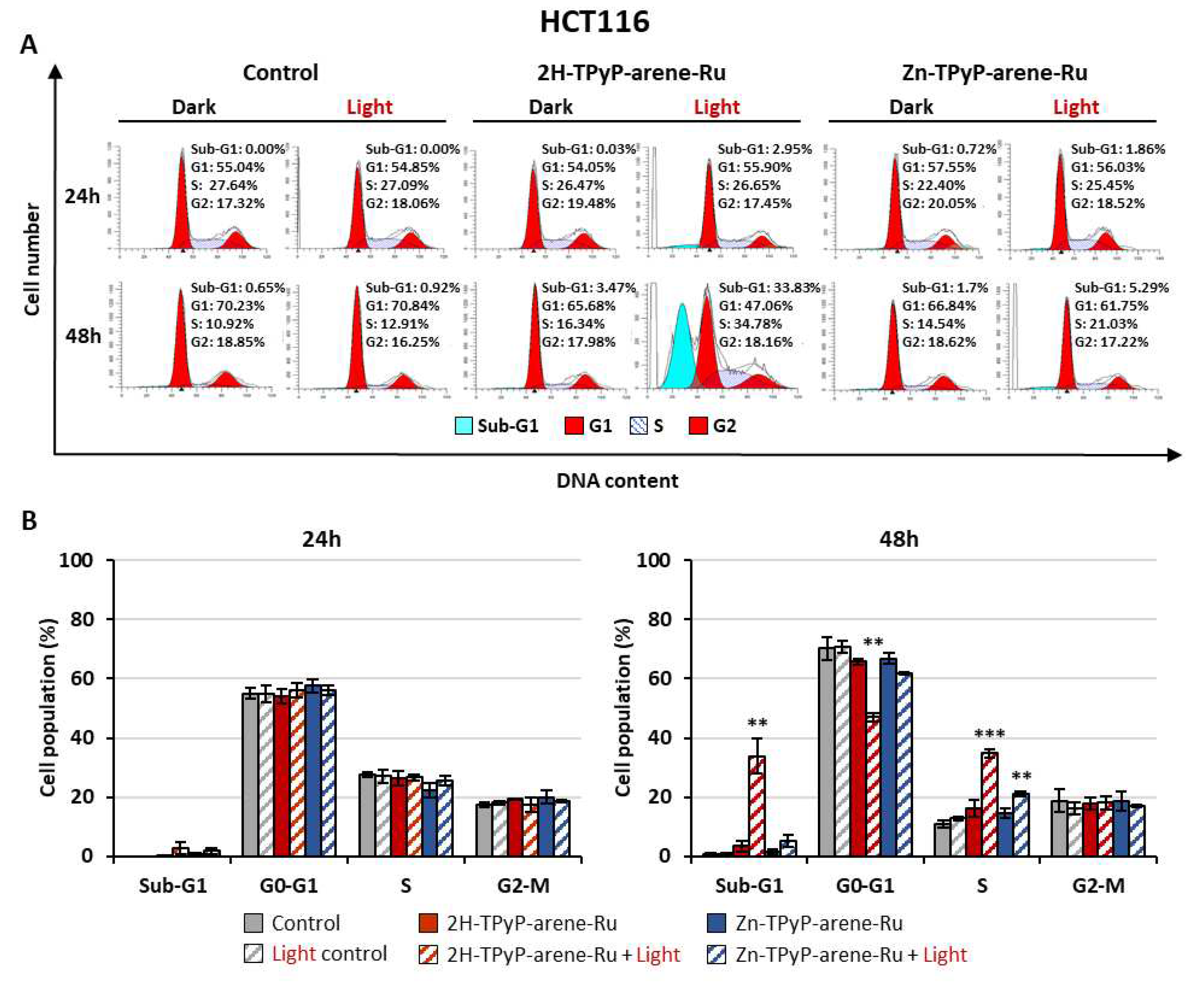
Figure 7.
Effects of photoactivation of TPyP-arene-Ru on the cell cycle distribution in HCT116 cells. Cells were grown for 36h in an appropriate culture medium before exposure or not to 2H-TPyP-arene-Ru or Zn-TPyP-arene-Ru complexes at IC50 concentrations. After 24h incubation, cells were irradiated or not with a 630-660 nm CURElight lamp at 75 J/cm2 (PhotoCure ASA, Oslo, Norway). Then subjected to flow cytometry analysis using PI staining after PDT. Images of cell cycle analysis (A) were representative of three separate experiments. Results of flow cytometry analysis are represented by histograms (B) that display the percentage of cells in each cell cycle phase. Data are shown as mean ± SEM (n = 3). ** p < 0.01 and *** p < 0.001.
Figure 7.
Effects of photoactivation of TPyP-arene-Ru on the cell cycle distribution in HCT116 cells. Cells were grown for 36h in an appropriate culture medium before exposure or not to 2H-TPyP-arene-Ru or Zn-TPyP-arene-Ru complexes at IC50 concentrations. After 24h incubation, cells were irradiated or not with a 630-660 nm CURElight lamp at 75 J/cm2 (PhotoCure ASA, Oslo, Norway). Then subjected to flow cytometry analysis using PI staining after PDT. Images of cell cycle analysis (A) were representative of three separate experiments. Results of flow cytometry analysis are represented by histograms (B) that display the percentage of cells in each cell cycle phase. Data are shown as mean ± SEM (n = 3). ** p < 0.01 and *** p < 0.001.
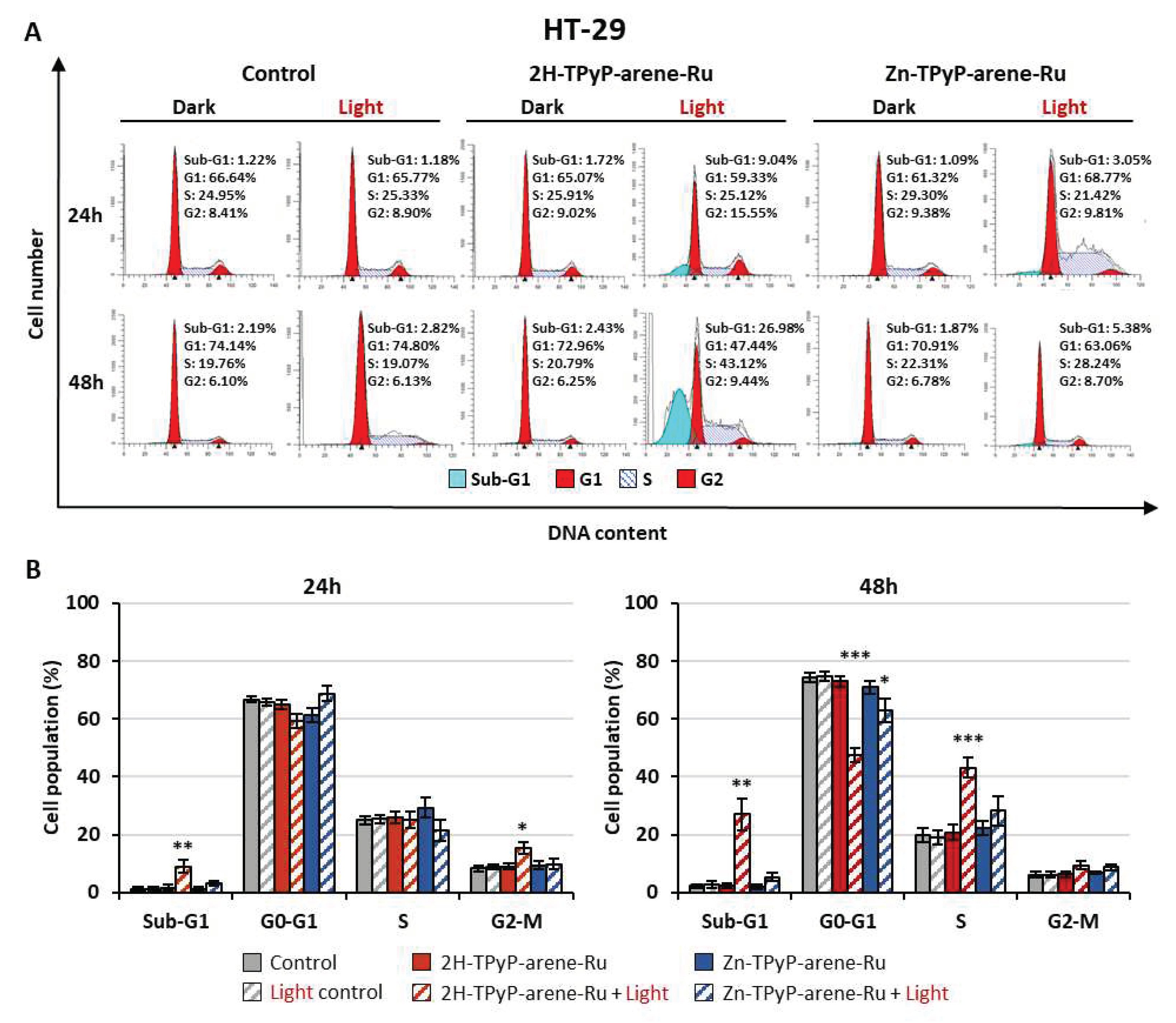
Figure 8.
Effects of photoactivation of TPyP-arene-Ru on the cell cycle distribution in HT-29 cells. Cells were grown for 36h in an appropriate culture medium before exposure or not to 2H-TPyP-arene-Ru or Zn-TPyP-arene-Ru complexes at IC50 concentrations. After 24h incubation, cells were irradiated or not with a 630-660 nm CURElight lamp at 75 J/cm2 (PhotoCure ASA, Oslo, Norway). Then subjected to flow cytometry analysis using PI staining after PDT. Images of cell cycle analysis (A) were representative of three separate experiments. Results of flow cytometry analysis are represented by histograms (B) that display the percentage of cells in each cell cycle phase. Data are shown as mean ± SEM (n = 3). * p < 0.05; ** p < 0.01 and *** p < 0.001.
Figure 8.
Effects of photoactivation of TPyP-arene-Ru on the cell cycle distribution in HT-29 cells. Cells were grown for 36h in an appropriate culture medium before exposure or not to 2H-TPyP-arene-Ru or Zn-TPyP-arene-Ru complexes at IC50 concentrations. After 24h incubation, cells were irradiated or not with a 630-660 nm CURElight lamp at 75 J/cm2 (PhotoCure ASA, Oslo, Norway). Then subjected to flow cytometry analysis using PI staining after PDT. Images of cell cycle analysis (A) were representative of three separate experiments. Results of flow cytometry analysis are represented by histograms (B) that display the percentage of cells in each cell cycle phase. Data are shown as mean ± SEM (n = 3). * p < 0.05; ** p < 0.01 and *** p < 0.001.
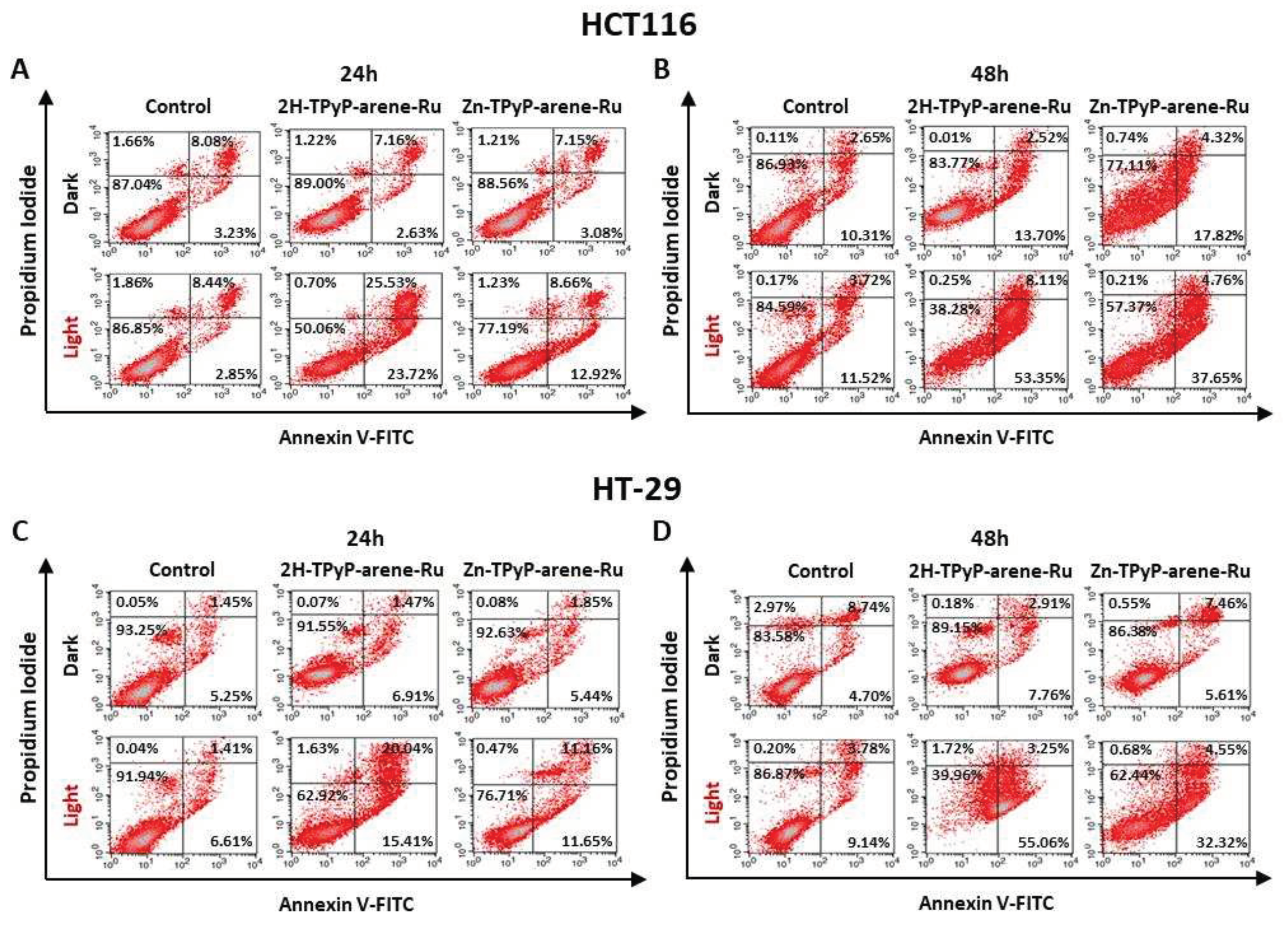
Figure 9.
Apoptosis effects of photoactivation of TPyP-arene-Ru on HCT116 (A-B) and HT-29 (C-D) cell lines. Cells were grown for 36h in an appropriate culture medium before exposure or not to 2H-TPyP-arene-Ru or Zn-TPyP-arene-Ru complexes at IC50 concentrations. After 24h incubation, cells were irradiated or not with a 630-660 nm CURElight lamp at 75 J/cm2 (PhotoCure ASA, Oslo, Norway). HCT116 and HT-29 cells were also stained, 24h post-PDT (A-C) and 48h post-PDT (B-D) with Annexin V-FITC and PI, and apoptosis was analyzed by flow cytometry. The upper right quadrant represents the percentage of late apoptosis, and the lower right quadrant represents early apoptosis. Images shown were representative of three separate experiments.
Figure 9.
Apoptosis effects of photoactivation of TPyP-arene-Ru on HCT116 (A-B) and HT-29 (C-D) cell lines. Cells were grown for 36h in an appropriate culture medium before exposure or not to 2H-TPyP-arene-Ru or Zn-TPyP-arene-Ru complexes at IC50 concentrations. After 24h incubation, cells were irradiated or not with a 630-660 nm CURElight lamp at 75 J/cm2 (PhotoCure ASA, Oslo, Norway). HCT116 and HT-29 cells were also stained, 24h post-PDT (A-C) and 48h post-PDT (B-D) with Annexin V-FITC and PI, and apoptosis was analyzed by flow cytometry. The upper right quadrant represents the percentage of late apoptosis, and the lower right quadrant represents early apoptosis. Images shown were representative of three separate experiments.
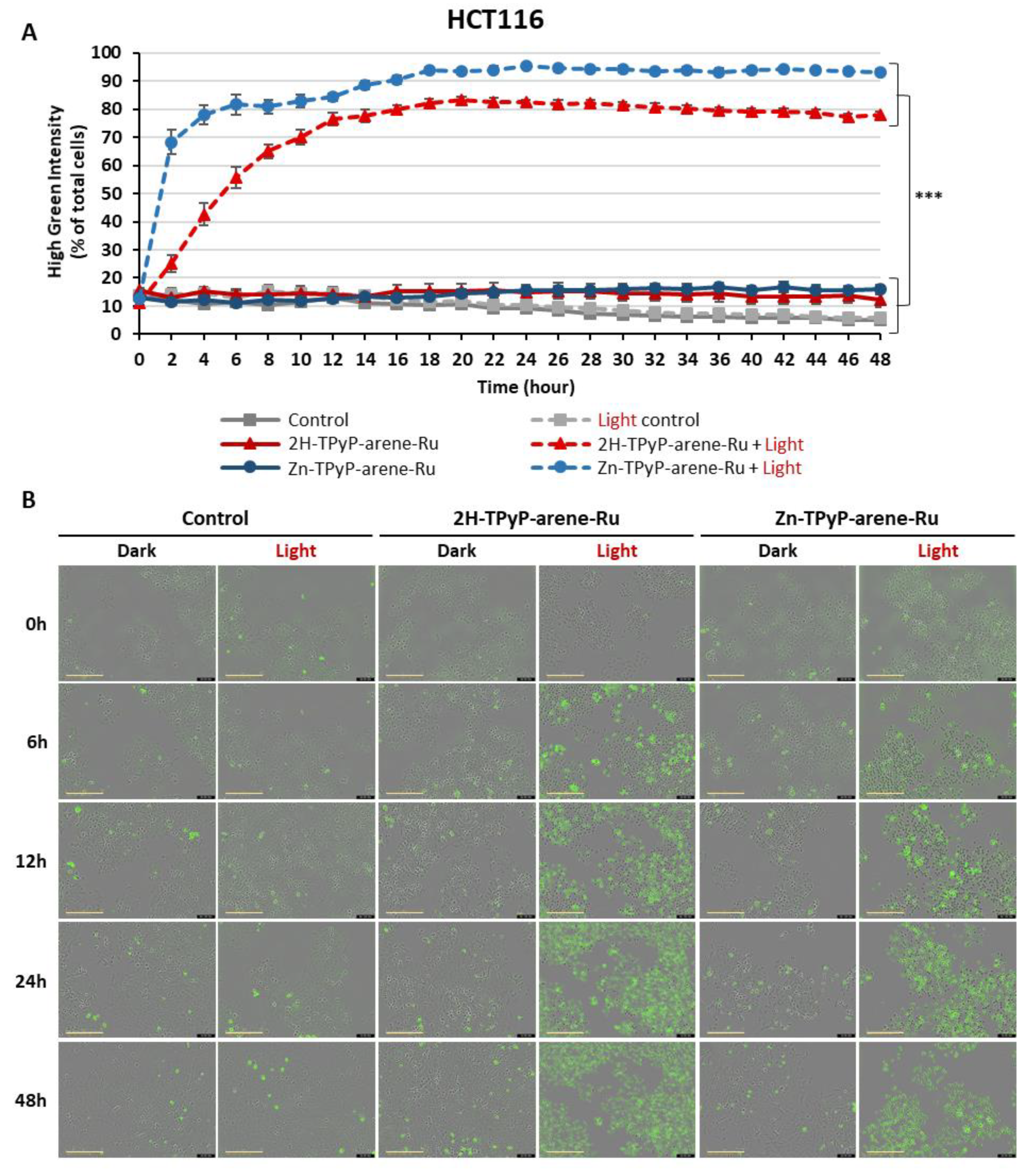
Figure 10.
Quantitative analysis of activated caspases-3/-7 in HCT116 cells over 48h. HCT116 cells were seeded and cultured for 36h and then treated with 2H-TPyP-arene-Ru or Zn-TPyP-arene-Ru at IC50 concentrations. Cells were then irradiated, co-treated with caspases-3/-7 green reagent and placed in the IncuCyte® S3 live cell analysis system. Every 2h, cells were imaged at a rate of 4 images per well in phase contrast and green fluorescence using the x20 objective. A: The number of cells undergoing apoptosis was quantified using IncuCyte® software using the ratio of the percentage of green fluorescent cells normalized by the percentage of total cells in each well over 48h. Data are shown as mean ± SEM (n = 3). *** p < 0.001. B: Representative images are shown for each condition at 0, 6, 12, 24 and 48h. Yellow scale bar = 200 µm.
Figure 10.
Quantitative analysis of activated caspases-3/-7 in HCT116 cells over 48h. HCT116 cells were seeded and cultured for 36h and then treated with 2H-TPyP-arene-Ru or Zn-TPyP-arene-Ru at IC50 concentrations. Cells were then irradiated, co-treated with caspases-3/-7 green reagent and placed in the IncuCyte® S3 live cell analysis system. Every 2h, cells were imaged at a rate of 4 images per well in phase contrast and green fluorescence using the x20 objective. A: The number of cells undergoing apoptosis was quantified using IncuCyte® software using the ratio of the percentage of green fluorescent cells normalized by the percentage of total cells in each well over 48h. Data are shown as mean ± SEM (n = 3). *** p < 0.001. B: Representative images are shown for each condition at 0, 6, 12, 24 and 48h. Yellow scale bar = 200 µm.
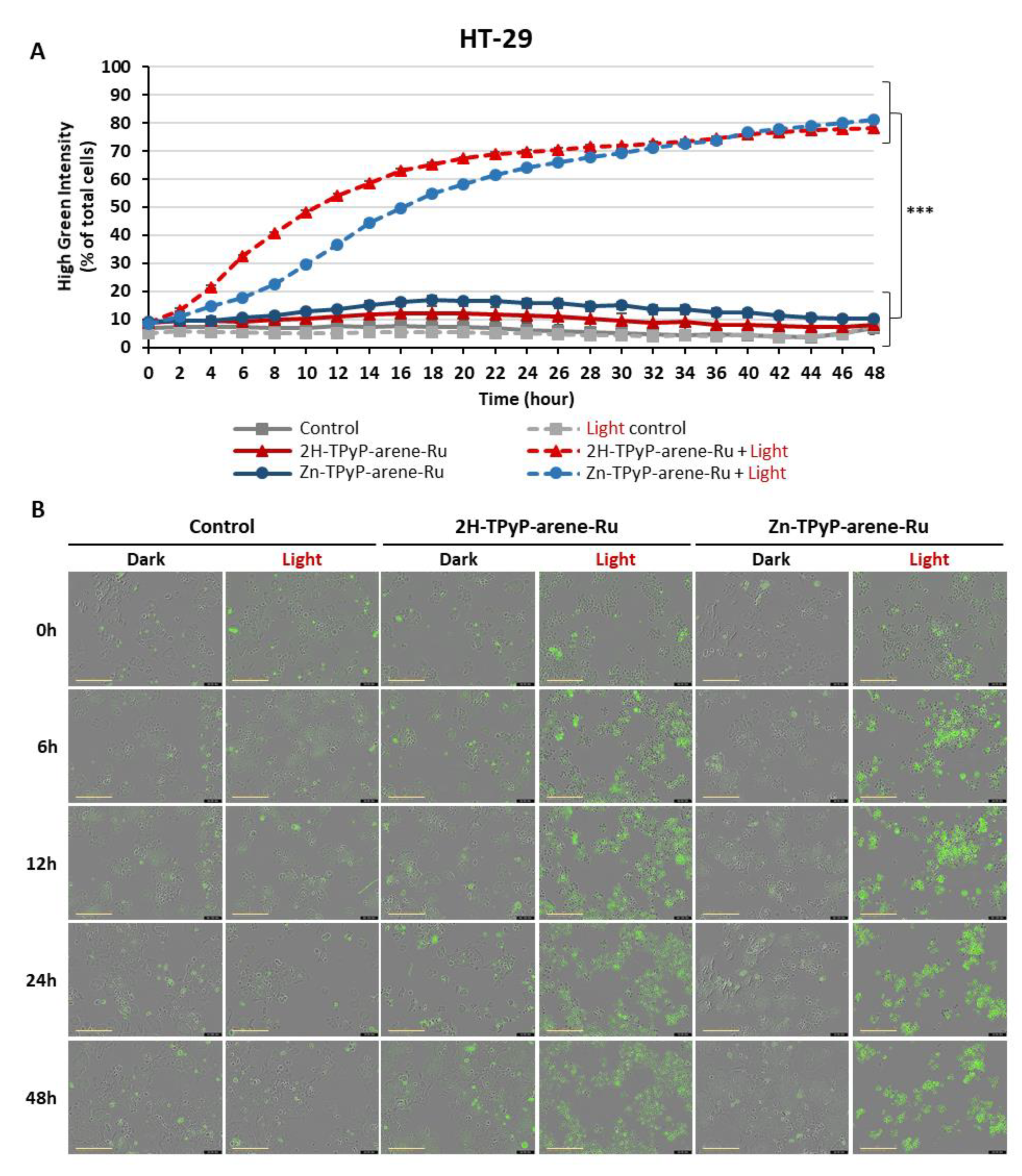
Figure 11.
Quantitative analysis of activated caspases-3/-7 in HT-29 cells over 48h. HT-29 cells were seeded and cultured for 36h and then treated with 2H-TPyP-arene-Ru or Zn-TPyP-arene-Ru at IC50 concentrations. Cells were then irradiated, co-treated with caspases-3/-7 green reagent and placed in the IncuCyte® S3 live cell analysis system. Every 2h, cells were imaged at a rate of 4 images per well in phase contrast and green fluorescence using the x20 objective. A: The number of cells undergoing apoptosis was quantified using IncuCyte® software using the ratio of the percentage of green fluorescent cells normalized by the percentage of total cells in each well over 48h. Data are shown as mean ± SEM (n = 3). ***p < 0.001. B: Representative images are shown for each condition at 0, 6, 12, 24 and 48h. Yellow scale bar = 200 µm.
Figure 11.
Quantitative analysis of activated caspases-3/-7 in HT-29 cells over 48h. HT-29 cells were seeded and cultured for 36h and then treated with 2H-TPyP-arene-Ru or Zn-TPyP-arene-Ru at IC50 concentrations. Cells were then irradiated, co-treated with caspases-3/-7 green reagent and placed in the IncuCyte® S3 live cell analysis system. Every 2h, cells were imaged at a rate of 4 images per well in phase contrast and green fluorescence using the x20 objective. A: The number of cells undergoing apoptosis was quantified using IncuCyte® software using the ratio of the percentage of green fluorescent cells normalized by the percentage of total cells in each well over 48h. Data are shown as mean ± SEM (n = 3). ***p < 0.001. B: Representative images are shown for each condition at 0, 6, 12, 24 and 48h. Yellow scale bar = 200 µm.
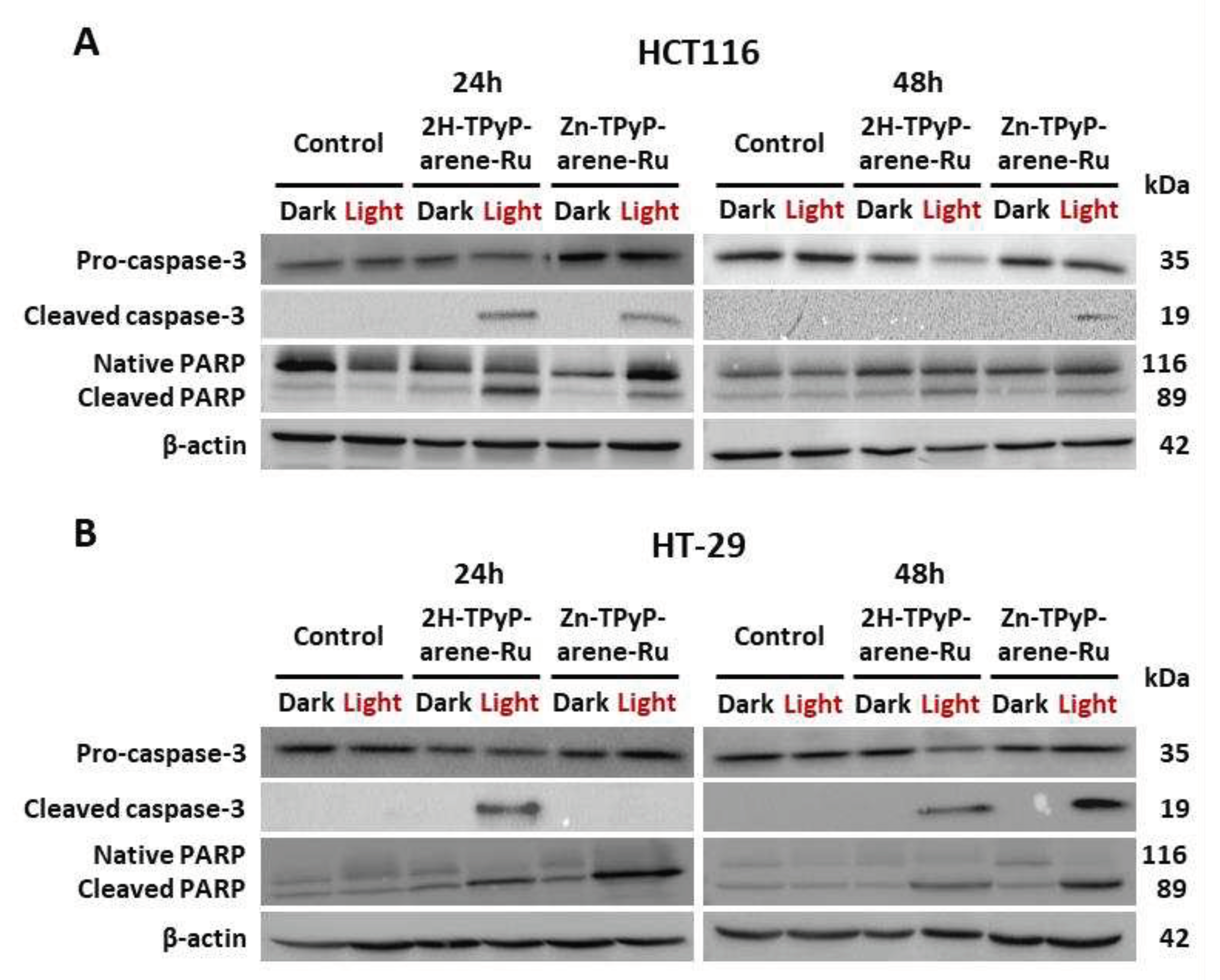
Figure 12.
Effects of photoactivation of TPyP-arene-Ru on protein expression of apoptotic markers in HCT116 (A) and HT-29 (B) cells. Cells were seeded at the determined density and cultured for 36h, then treated or not with 2H-TPyP-arene-Ru or Zn-TPyP-arene-Ru at IC50 concentrations. After 24h incubation, the culture medium was replaced and cells were irradiated or not. At 24 and 48h post-irradiation, the cells were then recovered and protein expression determined by WB. β-actin was used as the reference protein.
Figure 12.
Effects of photoactivation of TPyP-arene-Ru on protein expression of apoptotic markers in HCT116 (A) and HT-29 (B) cells. Cells were seeded at the determined density and cultured for 36h, then treated or not with 2H-TPyP-arene-Ru or Zn-TPyP-arene-Ru at IC50 concentrations. After 24h incubation, the culture medium was replaced and cells were irradiated or not. At 24 and 48h post-irradiation, the cells were then recovered and protein expression determined by WB. β-actin was used as the reference protein.
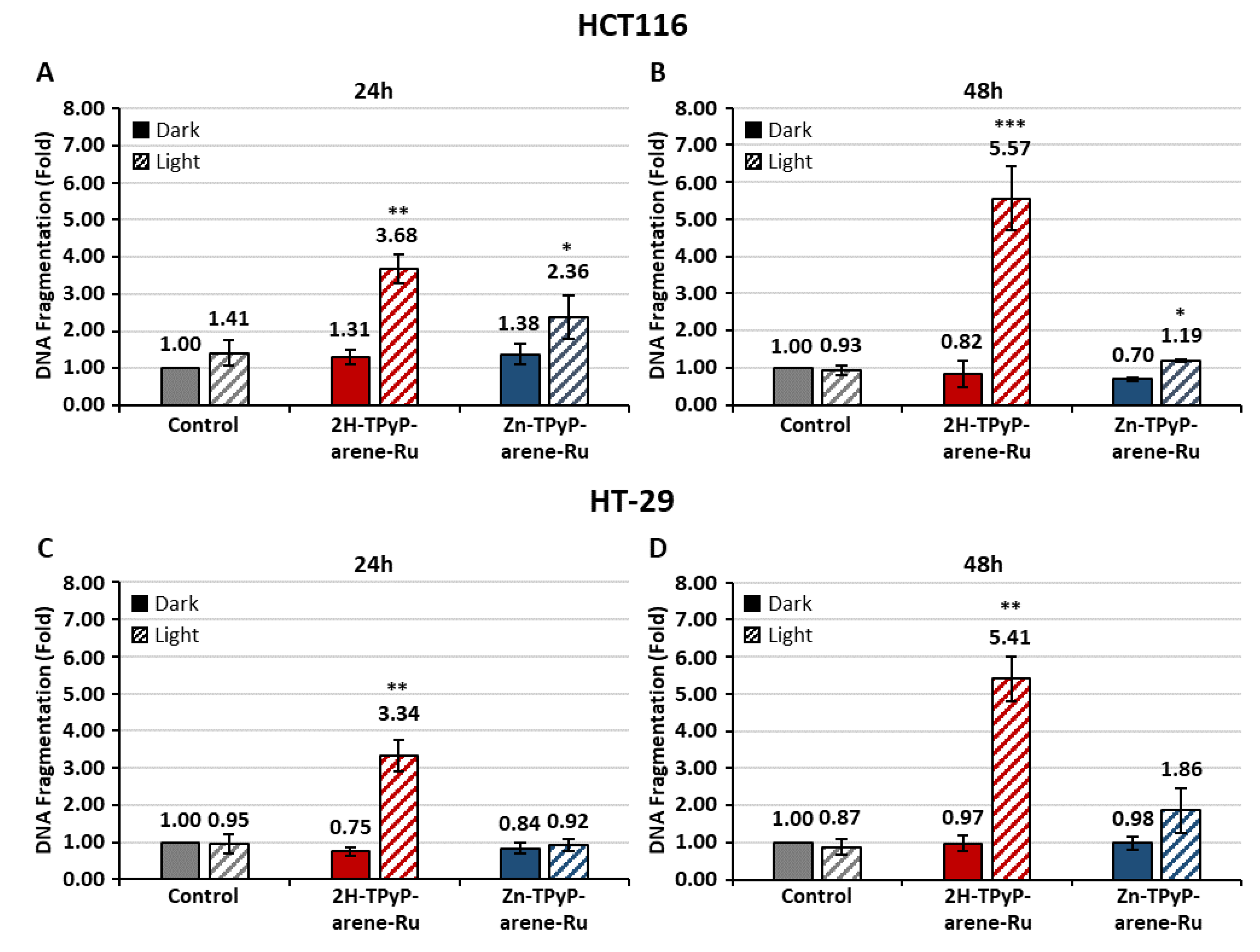
Figure 13.
Effects of photoactivation of TPyP-arene-Ru on DNA fragmentation HCT116 (A-B) and HT-29 (C-D) cells. Cells were grown for 36h in an appropriate culture medium before exposure or not to 2H-TPyP or Zn-TPyP-arene-Ru complexes at IC50 concentrations. After 24h incubation, cells were irradiated or not with a 630-660 nm CURElight lamp at 75 J/cm2 (PhotoCure ASA, Oslo, Norway). DNA fragmentation in both cell lines 24h post-PDT (A-C) and 48h post-PDT (B-D) was quantified from cytosol extracts by ELISA. Results are reported as n-fold compared to control. Data are shown as mean ± SEM (n= 3). * p < 0.05, ** p < 0.01 and *** p < 0.001.
Figure 13.
Effects of photoactivation of TPyP-arene-Ru on DNA fragmentation HCT116 (A-B) and HT-29 (C-D) cells. Cells were grown for 36h in an appropriate culture medium before exposure or not to 2H-TPyP or Zn-TPyP-arene-Ru complexes at IC50 concentrations. After 24h incubation, cells were irradiated or not with a 630-660 nm CURElight lamp at 75 J/cm2 (PhotoCure ASA, Oslo, Norway). DNA fragmentation in both cell lines 24h post-PDT (A-C) and 48h post-PDT (B-D) was quantified from cytosol extracts by ELISA. Results are reported as n-fold compared to control. Data are shown as mean ± SEM (n= 3). * p < 0.05, ** p < 0.01 and *** p < 0.001.
Table 1.
IC50 values (nM) determined with MTT assays on HCT116 and HT-29 cells. PI = phototoxic index.
Table 1.
IC50 values (nM) determined with MTT assays on HCT116 and HT-29 cells. PI = phototoxic index.