Submitted:
25 September 2023
Posted:
26 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Ménière’s Disease
3. Mushroom Nutrition in Neurodegenerative Diseases
5. Targeting Neurogenisis with Mushroom Neutraceuticals
6. Mushroom Nutrition on Ménière’s Disease
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shahidi, F. Nutraceuticals and Functional Foods: Whole versus Processed Foods. Trends Food Sci. Technol. 2009, 20, 376–387. [Google Scholar] [CrossRef]
- Galanakis, C.M. Functionality of Food Components and Emerging Technologies. Foods 2021, 10, 128. [Google Scholar] [CrossRef]
- Coppens, P.; Da Silva, M.F.; Pettman, S. European Regulations on Nutraceuticals, Dietary Supplements and Functional Foods: A Framework Based on Safety. Toxicology 2006, 221, 59–74. [Google Scholar] [CrossRef]
- Kumar, K.; Mehra, R.; Guiné, R.P.F.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible Mushrooms: A Comprehensive Review on Bioactive Compounds with Health Benefits and Processing Aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef]
- Bell, V.; Silva, C.R.P.G.; Guina, J.; Fernandes, T.H. Mushrooms as Future Generation Healthy Foods. Front. Nutr. 2022, 9, 1050099. [Google Scholar] [CrossRef]
- Yadav, D.; Negi, P.S. Bioactive Components of Mushrooms: Processing Effects and Health Benefits. Food Res. Int. 2021, 148, 110599. [Google Scholar] [CrossRef]
- Chugh, R.M.; Mittal, P.; MP, N.; Arora, T.; Bhattacharya, T.; Chopra, H.; Cavalu, S.; Gautam, R.K. Fungal Mushrooms: A Natural Compound With Therapeutic Applications. Front. Pharmacol. 2022, 13, 925387. [Google Scholar] [CrossRef]
- Dávila Giraldo, L.R.; Pérez Jaramillo, C.C.; Méndez Arteaga, J.J.; Murillo-Arango, W. Nutritional Value and Antioxidant, Antimicrobial and Cytotoxic Activity of Wild Macrofungi. Microorganisms 2023, 11, 1158. [Google Scholar] [CrossRef]
- Galappaththi, M.C.A.; Patabendige, N.M.; Premarathne, B.M.; Hapuarachchi, K.K.; Tibpromma, S.; Dai, D.Q.; Suwannarach, N.; Rapior, S.; Karunarathna, S.C. A Review of Ganoderma Triterpenoids and Their Bioactivities. Biomol. 2023, Vol. 13, Page 24 2022, 13, 24. [Google Scholar] [CrossRef]
- Bhambri, A.; Srivastava, M.; Mahale, V.G.; Mahale, S.; Karn, S.K. Mushrooms as Potential Sources of Active Metabolites and Medicines. Front. Microbiol. 2022, 13, 837266. [Google Scholar] [CrossRef]
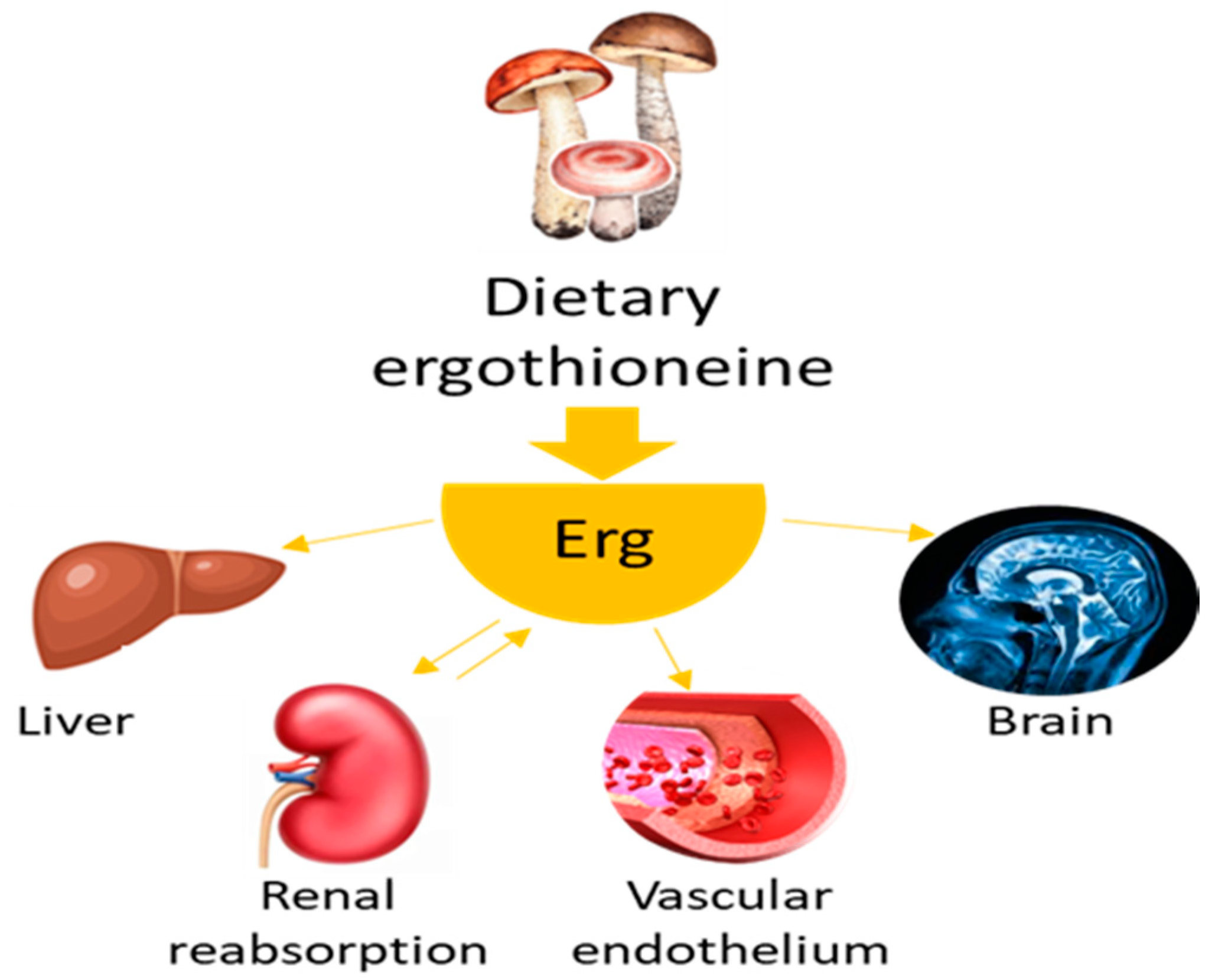
- Tian, X.; Thorne, J.L.; Moore, J.B. Ergothioneine: An Underrecognised Dietary Micronutrient Required for Healthy Ageing? Br. J. Nutr. 2023, 129, 104–114. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Synthetic L-ergothioneine (Ergoneine®) as a Novel Food Pursuant to Regulation (EC) No 258/97. EFSA J. 2016, 14. [Google Scholar] [CrossRef]
- Ayimbila, F.; Keawsompong, S. Nutritional Quality and Biological Application of Mushroom Protein as a Novel Protein Alternative. Curr. Nutr. Rep. 2023, 12, 290–307. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Cruz, M.; Losoya, C.; Nobre, C.; Loredo, A.; Rodríguez, R.; Contreras, J.; Belmares, R. Edible Mushrooms as a Novel Protein Source for Functional Foods. Food Funct. 2020, 11, 7400–7414. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Joshi, P.B.; Ahmad, Z.; Hemeg, H.A.; Olatunde, A.; Naz, S.; Hafeez, N.; Simal-Gandara, J. Edible Mushrooms as Potential Functional Foods in Amelioration of Hypertension. Phyther. Res. 2023, 37, 2644–2660. [Google Scholar] [CrossRef]
- Singh, R.S.; Walia, A.K.; Kennedy, J.F. Mushroom Lectins in Biomedical Research and Development. Int. J. Biol. Macromol. 2020, 151, 1340–1350. [Google Scholar] [CrossRef]
- Guillamón, E.; García-Lafuente, A.; Lozano, M.; D́arrigo, M.; Rostagno, M.A.; Villares, A.; Martínez, J.A. Edible Mushrooms: Role in the Prevention of Cardiovascular Diseases. Fitoterapia 2010, 81, 715–723. [Google Scholar] [CrossRef]
- Sousa, A.S.; Araújo-Rodrigues, H.; Pintado, M.E. The Health-Promoting Potential of Edible Mushroom Proteins. Curr. Pharm. Des. 2022, 29. [Google Scholar] [CrossRef]
- El-Maradny, Y.A.; El-Fakharany, E.M.; Abu-Serie, M.M.; Hashish, M.H.; Selim, H.S. Lectins Purified from Medicinal and Edible Mushrooms: Insights into Their Antiviral Activity against Pathogenic Viruses. Int. J. Biol. Macromol. 2021, 179, 239–258. [Google Scholar] [CrossRef]
- Zhou, R.; Liu, Z.K.; Zhang, Y.N.; Wong, J.H.; Ng, T.B.; Liu, F. Research Progress of Bioactive Proteins from the Edible and Medicinal Mushrooms. Curr. Protein Pept. Sci. 2018, 20, 196–219. [Google Scholar] [CrossRef]
- Fu, T.T.; Shen, L. Ergothioneine as a Natural Antioxidant Against Oxidative Stress-Related Diseases. Front. Pharmacol. 2022, 13, 850813. [Google Scholar] [CrossRef] [PubMed]
- Katsube, M.; Watanabe, H.; Suzuki, K.; Ishimoto, T.; Tatebayashi, Y.; Kato, Y.; Murayama, N. Food-Derived Antioxidant Ergothioneine Improves Sleep Difficulties in Humans. J. Funct. Foods 2022, 95, 105165. [Google Scholar] [CrossRef]
- Pang, L.; Wang, T.; Liao, Q.; Cheng, Y.; Wang, D.; Li, J.; Fu, C.; Zhang, C.; Zhang, J. Protective Role of Ergothioneine Isolated from Pleurotus Ostreatus against Dextran Sulfate Sodium-Induced Ulcerative Colitis in Rat Model. J. Food Sci. 2022, 87, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Törős, G.; El-Ramady, H.; Prokisch, J.; Velasco, F.; Llanaj, X.; Nguyen, D.H.H.; Peles, F. Modulation of the Gut Microbiota with Prebiotics and Antimicrobial Agents from Pleurotus Ostreatus Mushroom. Foods 2023, 12, 2010. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Nair, A.; Kulkarni, N.; Todewale, N.; Jobby, R. Exploring Mushroom Polysaccharides for the Development of Novel Prebiotics: A Review. Int. J. Med. Mushrooms 2023, 25, 1–10. [Google Scholar] [CrossRef]
- Kerezoudi, E.N.; Mitsou, E.K.; Gioti, K.; Terzi, E.; Avgousti, I.; Panagiotou, A.; Koutrotsios, G.; Zervakis, G.I.; Mountzouris, K.C.; Tenta, R.; et al. Fermentation of Pleurotus Ostreatus and Ganoderma Lucidum Mushrooms and Their Extracts by the Gut Microbiota of Healthy and Osteopenic Women: Potential Prebiotic Effect and Impact of Mushroom Fermentation Products on Human Osteoblasts. Food Funct. 2021, 12, 1529–1546. [Google Scholar] [CrossRef]
- Moshtaghi, O.; Sahyouni, R.; Lin, H.W.; Ghavami, Y.; Djalilian, H.R. A Historical Recount: Discovering Menière’s Disease and Its Association with Migraine Headaches. Otol. Neurotol. 2016, 37, 1199–1203. [Google Scholar] [CrossRef]
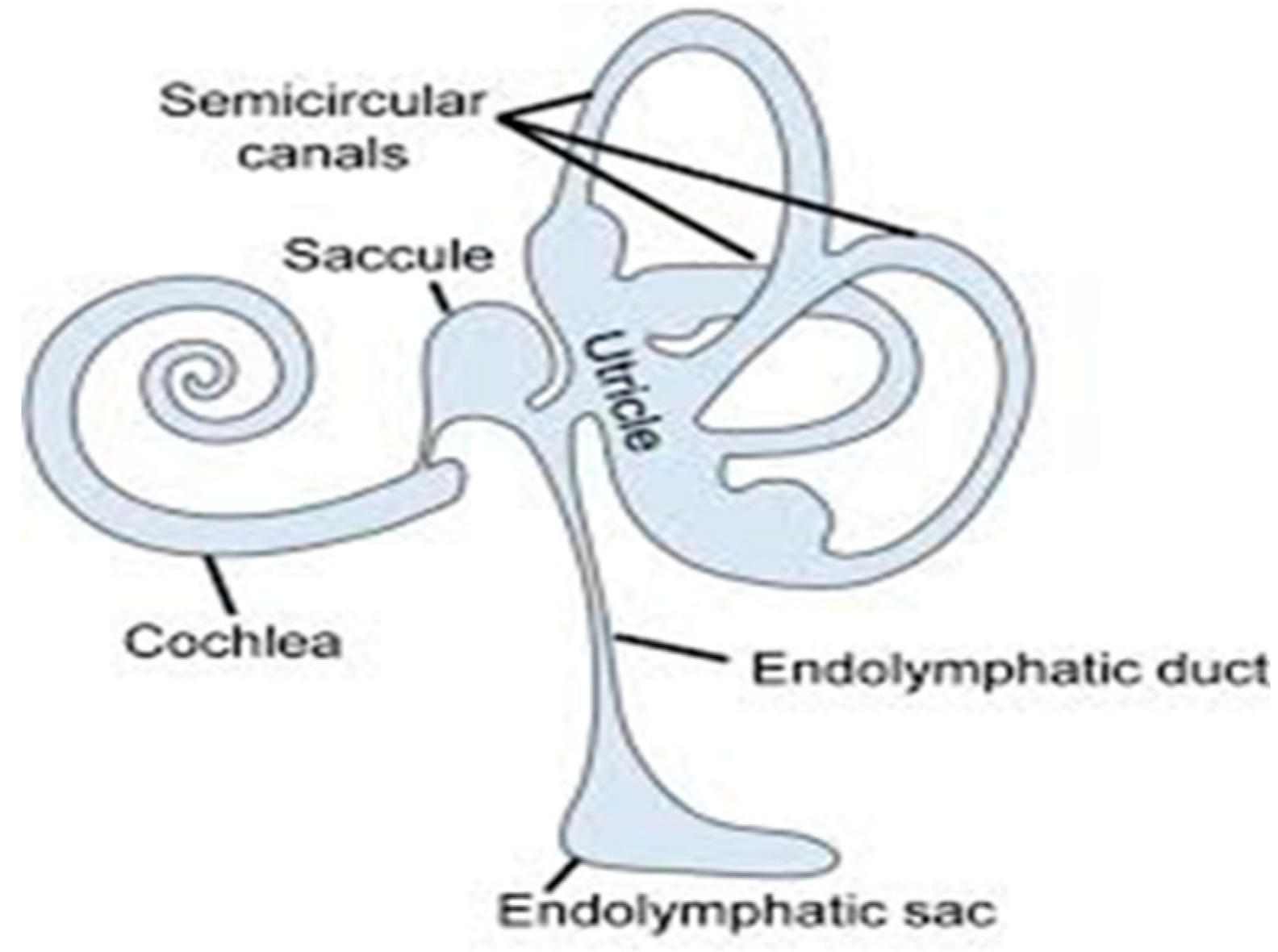
- Nakashima, T.; Pyykkö, I.; Arroll, M.A.; Casselbrant, M.L.; Foster, C.A.; Manzoor, N.F.; Megerian, C.A.; Naganawa, S.; Young, Y.H. Meniere’s Disease. Nat. Rev. Dis. Prim. 2016, 2, 1–18. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Q.; Pan, X.; Li, W.; Liu, W.; Jiang, W.; Huang, L.; Peng, A.; Zhang, Z. Up-Regulated Expression of Interferon-Gamma, Interleukin-6 and Tumor Necrosis Factor-Alpha in the Endolymphatic Sac of Meniere’s Disease Suggesting the Local Inflammatory Response Underlies the Mechanism of This Disease. Front. Neurol. 2022, 13, 781031. [Google Scholar] [CrossRef]
- Koç, A. Benign Paroxysmal Positional Vertigo: Is It Really an Otolith Disease? J. Int. Adv. Otol. 2022, 18, 62–70. [Google Scholar] [CrossRef]
- Koenen, L.; Andaloro, C. Meniere Disease; StatPearls Publishing, 2023; ISBN 9781496318442.
- Weinreich, H.M.; Agrawal, Y. The Link Between Allergy and Menière’s Disease. Curr. Opin. Otolaryngol. Head Neck Surg. 2014, 22, 227. [Google Scholar] [CrossRef]
- Pan, T.; Zhao, Y.; Ding, Y.J.; Lu, Z.Y.; Ma, F.R. [The Pilot Study of Type Ⅰ Allergic Reaction in Meniere’s Disease Patients]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2017, 52, 89–92. [Google Scholar] [CrossRef]
- Dai, Q.; Wang, D.; Zheng, H. The Polymorphic Analysis of the Human Potassium Channel Kcne Gene Family in Meniere’s Disease-a Preliminary Study. J. Int. Adv. Otol. 2019, 15, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sierra, C.; Gallego-Martinez, A.; Requena, T.; Frejo, L.; Batuecas-Caletrió, A.; Lopez-Escamez, J.A. Variable Expressivity and Genetic Heterogeneity Involving DPT and SEMA3D Genes in Autosomal Dominant Familial Meniere’s Disease. Eur. J. Hum. Genet. 2016, 25, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Lopes, K.D.C.; Sartorato, E.L.; Da Silva-Costa, S.M.; De Macedo Adamov, N.S.; Ganança, F.F. Ménière’s Disease: Molecular Analysis of Aquaporins 2, 3 and Potassium Channel KCNE1 Genes in Brazilian Patients. Otol. Neurotol. 2016, 37, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Sato, T.; Kuramasu, T.; Hibino, H.; Kitahara, T.; Horii, A.; Matsushiro, N.; Fuse, Y.; Kubo, T. Ménière’s Disease Is Associated with Single Nucleotide Polymorphisms in the Human Potassium Channel Genes, KCNE1 and KCNE3. ORL 2005, 67, 289–293. [Google Scholar] [CrossRef]
- Brandalise, F.; Roda, E.; Ratto, D.; Goppa, L.; Gargano, M.L.; Cirlincione, F.; Priori, E.C.; Venuti, M.T.; Pastorelli, E.; Savino, E.; et al. Hericium Erinaceus in Neurodegenerative Diseases: From Bench to Bedside and Beyond, How Far from the Shoreline? J. Fungi 2023, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Atayik, M.C.; Çakatay, U. Redox Signaling in Impaired Cascades of Wound Healing: Promising Approach. Mol. Biol. Rep. 2023, 1–10. [Google Scholar] [CrossRef]
- Poon, H.F.; Calabrese, V.; Scapagnini, G.; Butterfield, D.A. Free Radicals: Key to Brain Aging and Heme Oxygenase as a Cellular Response to Oxidative Stress. Journals Gerontol. Ser. A 2004, 59, M478–M493. [Google Scholar] [CrossRef]
- Liuzzi, G.M.; Petraglia, T.; Latronico, T.; Crescenzi, A.; Rossano, R. Antioxidant Compounds from Edible Mushrooms as Potential Candidates for Treating Age-Related Neurodegenerative Diseases. Nutrients 2023, 15, 1913. [Google Scholar] [CrossRef]
- Fekete, M.; Szarvas, Z.; Fazekas-Pongor, V.; Feher, A.; Csipo, T.; Forrai, J.; Dosa, N.; Peterfi, A.; Lehoczki, A.; Tarantini, S.; et al. Nutrition Strategies Promoting Healthy Aging: From Improvement of Cardiovascular and Brain Health to Prevention of Age-Associated Diseases. Nutrients 2022, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.N.; Mishra, D.; Singh, P.; Vamanu, E.; Singh, M.P. Therapeutic Applications of Mushrooms and Their Biomolecules along with a Glimpse of in Silico Approach in Neurodegenerative Diseases. Biomed. Pharmacother. 2021, 137, 111377. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Li, S.; Feng, X.; Li, L.; Hao, J.; Wang, D.; Wang, Q. Mushroom Polysaccharides as Potential Candidates for Alleviating Neurodegenerative Diseases. Nutrients 2022, 14, 4833. [Google Scholar] [CrossRef] [PubMed]
- Al-Qudah, M.A.; Al-Dwairi, A. Mechanisms and Regulation of Neurotrophin Synthesis and Secretion. Neurosci. J. 2016, 21, 306–313. [Google Scholar] [CrossRef]
- Iskusnykh, I.Y.; Zakharova, A.A.; Pathak, D. Glutathione in Brain Disorders and Aging. Molecules 2022, 27, 324. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Calabrese, E.J. Vitagenes, Cellular Stress Response, and Acetylcarnitine: Relevance to Hormesis. BioFactors 2009, 35, 146–160. [Google Scholar] [CrossRef]
- Calabrese, V.; Ontario, M. Mushroom Nutrition In Neurodegenerative Diseases. Clin. J. Mycol. 2022, 6. [Google Scholar] [CrossRef]
- Trovato Salinaro, A.; Pennisi, M.; Di Paola, R.; Scuto, M.; Crupi, R.; Cambria, M.T.; Ontario, M.L.; Tomasello, M.; Uva, M.; Maiolino, L.; et al. Neuroinflammation and Neurohormesis in the Pathogenesis of Alzheimer’s Disease and Alzheimer-Linked Pathologies: Modulation by Nutritional Mushrooms. Immun. Ageing 2018, 15, 1–8. [Google Scholar] [CrossRef]
- Tiwari, S.; Thakur, R.; Shankar, J. Role of Heat-Shock Proteins in Cellular Function and in the Biology of Fungi. Biotechnol. Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Zhao, X.; Yin, K.; Feng, R.; Miao, R.; Lin, J.; Cao, L.; Ni, Y.; Li, W.; Zhang, Q. Genome-Wide Identification and Analysis of the Heat-Shock Protein Gene in L. Edodes and Expression Pattern Analysis under Heat Shock. Curr. Issues Mol. Biol. 2023, 45, 614–627. [Google Scholar] [CrossRef]
- Trovato, A.; Siracusa, R.; Di Paola, R.; Scuto, M.; Ontario, M.L.; Bua, O.; Di Mauro, P.; Toscano, M.A.; Petralia, C.C.T.; Maiolino, L.; et al. Redox Modulation of Cellular Stress Response and Lipoxin A4 Expression by Hericium Erinaceus in Rat Brain: Relevance to Alzheimer’s Disease Pathogenesis. Immun. Ageing 2016, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Lipoxins as Biomarkers of Lupus and Other Inflammatory Conditions. Lipids Health Dis. 2011, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
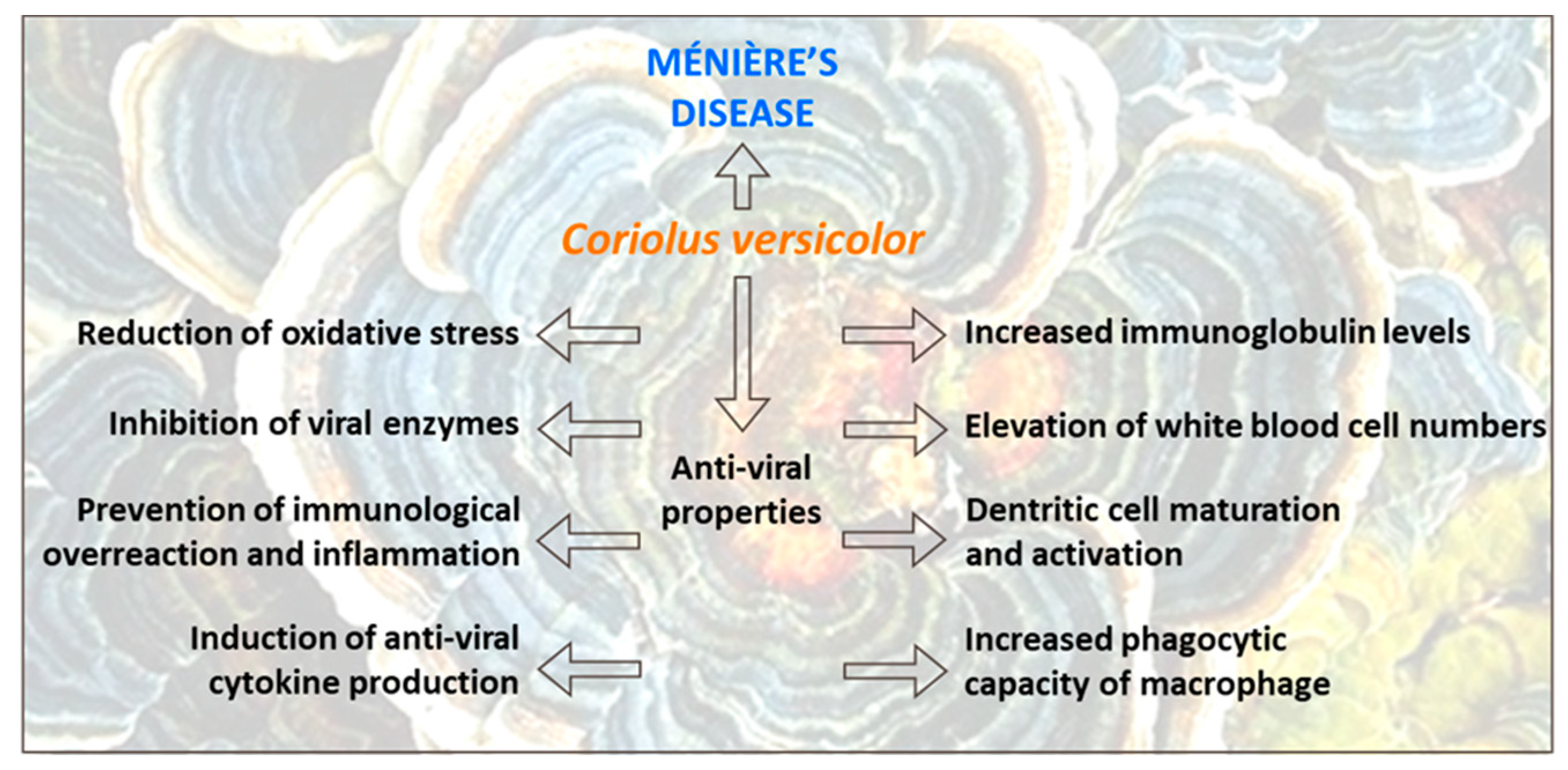
- Cordaro, M.; Modafferi, S.; D’Amico, R.; Fusco, R.; Genovese, T.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Interdonato, L.; Di Paola, D.; et al. Natural Compounds Such as Hericium Erinaceus and Coriolus Versicolor Modulate Neuroinflammation, Oxidative Stress and Lipoxin A4 Expression in Rotenone-Induced Parkinson’s Disease in Mice. Biomedicines 2022, 10, 2505. [Google Scholar] [CrossRef]
- Ólafsdóttir, H.F.; Bush, D.; Barry, C. The Role of Hippocampal Replay in Memory and Planning. Curr. Biol. 2018, 28, R37–R50. [Google Scholar] [CrossRef]
- Wheeler, D.W.; White, C.M.; Rees, C.L.; Komendantov, A.O.; Hamilton, D.J.; Ascoli, G.A. Hippocampome.Org: A Knowledge Base of Neuron Types in the Rodent Hippocampus. Elife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Ferreiro, E.; Fernandes, T. Targeting Neurogenesis with Mushroom Nutrition: A Mini Review. Clin. J. Mycol. 2022, 6. [Google Scholar] [CrossRef]
- Audesse, A.J.; Webb, A.E. Mechanisms of Enhanced Quiescence in Neural Stem Cell Aging. Mech. Ageing Dev. 2020, 191, 111323. [Google Scholar] [CrossRef]
- Barros, A.B.; Ferrão, J.; Fernandes, T. A Safety Assessment of <em>Coriolus Versicolor</Em> Biomass as a Food Supplement. Food Nutr. Res. 2016, 60. [Google Scholar] [CrossRef]
- Piatti, V.C.; Ewe, L.A.; Leutgeb, J.K. Neurogenesis in the Dentate Gyrus: Carrying the Message or Dictating the Tone. Front. Neurosci. 2013, 7, 45461. [Google Scholar] [CrossRef]
- Uffelman, C.N.; Chan, N.I.; Davis, E.M.; Wang, Y.; McGowan, B.S.; Campbell, W.W. An Assessment of Mushroom Consumption on Cardiometabolic Disease Risk Factors and Morbidities in Humans: A Systematic Review. Nutrients 2023, 15, 1079. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, D.; Mehel, D.M.; Küçüköner, Ö.; Ağrı, İ.; Yemiş, T.; Akgül, G.; Özgür, A. Vestibular Evoked Myogenic Potentials in Patients With Low Vitamin B12 Levels. Ear. Nose. Throat J. 2021, 100, NP231–NP235. [Google Scholar] [CrossRef] [PubMed]
- Jorfi, M.; Maaser-Hecker, A.; Tanzi, R.E. The Neuroimmune Axis of Alzheimer’s Disease. Genome Med. 2023, 15, 1–25. [Google Scholar] [CrossRef]
- Kishimoto-Urata, M.; Urata, S.; Fujimoto, C.; Yamasoba, T. Role of Oxidative Stress and Antioxidants in Acquired Inner Ear Disorders. Antioxidants 2022, 11, 1469. [Google Scholar] [CrossRef]
- Scuto, M.; Di Mauro, P.; Ontario, M.L.; Amato, C.; Modafferi, S.; Ciavardelli, D.; Salinaro, A.T.; Maiolino, L.; Calabrese, V. Nutritional Mushroom Treatment in Meniere’s Disease with Coriolus Versicolor: A Rationale for Therapeutic Intervention in Neuroinflammation and Antineurodegeneration. Int. J. Mol. Sci. 2019, 21, 284. [Google Scholar] [CrossRef]
- Owen, J.B.; Allan Butterfiel, D. Measurement of Oxidized/Reduced Glutathione Ratio. Methods Mol. Biol. 2010, 648, 269–277. [Google Scholar] [CrossRef]
- Habtemariam, S. Trametes Versicolor (Synn. Coriolus Versicolor) Polysaccharides in Cancer Therapy: Targets and Efficacy. Biomedicines 2020, 8, 135. [Google Scholar] [CrossRef]
- Alberio, T.; Brughera, M.; Lualdi, M. Current Insights on Neurodegeneration by the Italian Proteomics Community. Biomedicines 2022, 10, 2297. [Google Scholar] [CrossRef]
- Reddy, D.S.; Abeygunaratne, H.N. Experimental and Clinical Biomarkers for Progressive Evaluation of Neuropathology and Therapeutic Interventions for Acute and Chronic Neurological Disorders. Int. J. Mol. Sci. 2022, 23, 11734. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




