Submitted:
18 September 2023
Posted:
20 September 2023
You are already at the latest version
Abstract
Keywords:
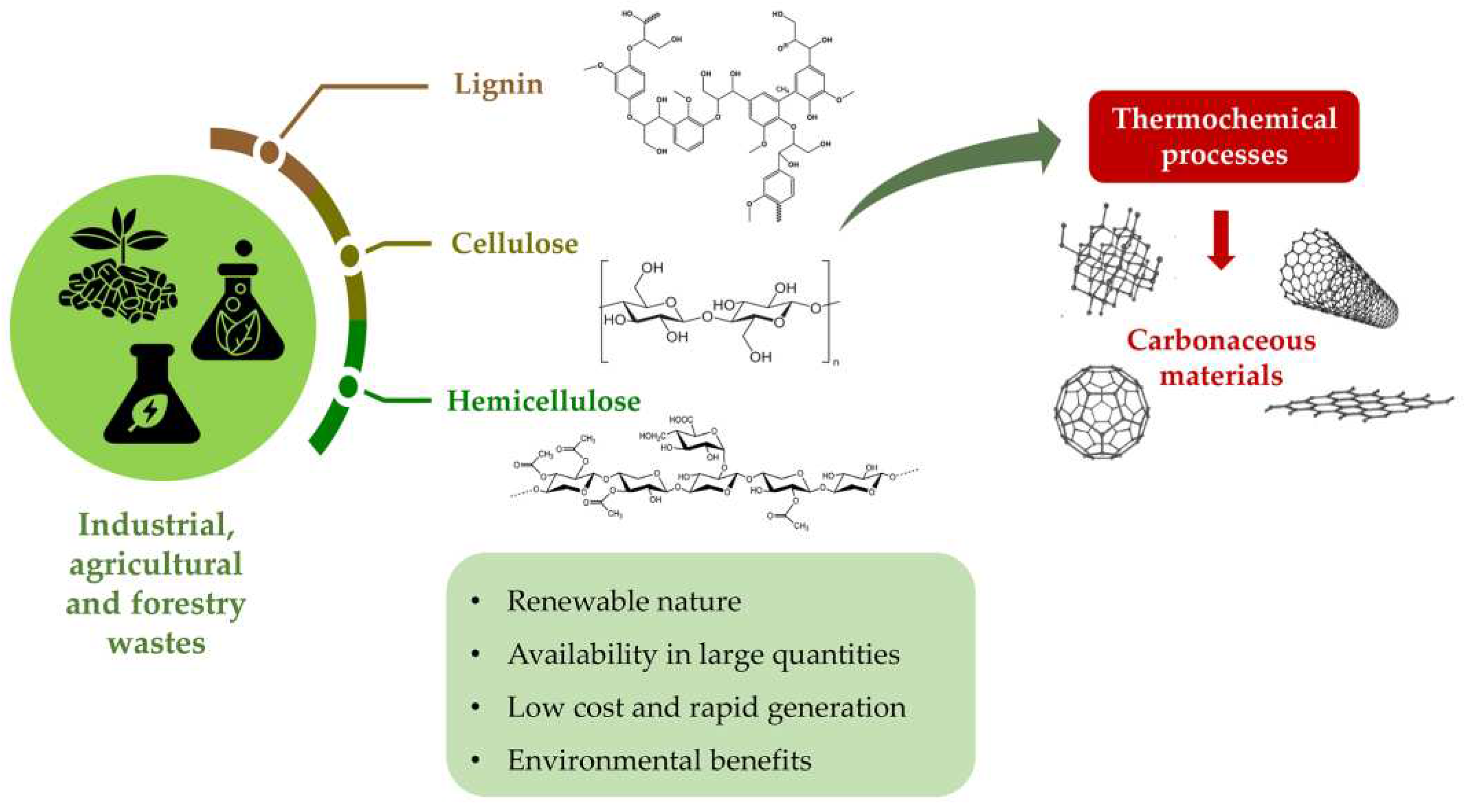
1. Introduction
2. Plant-based biomass
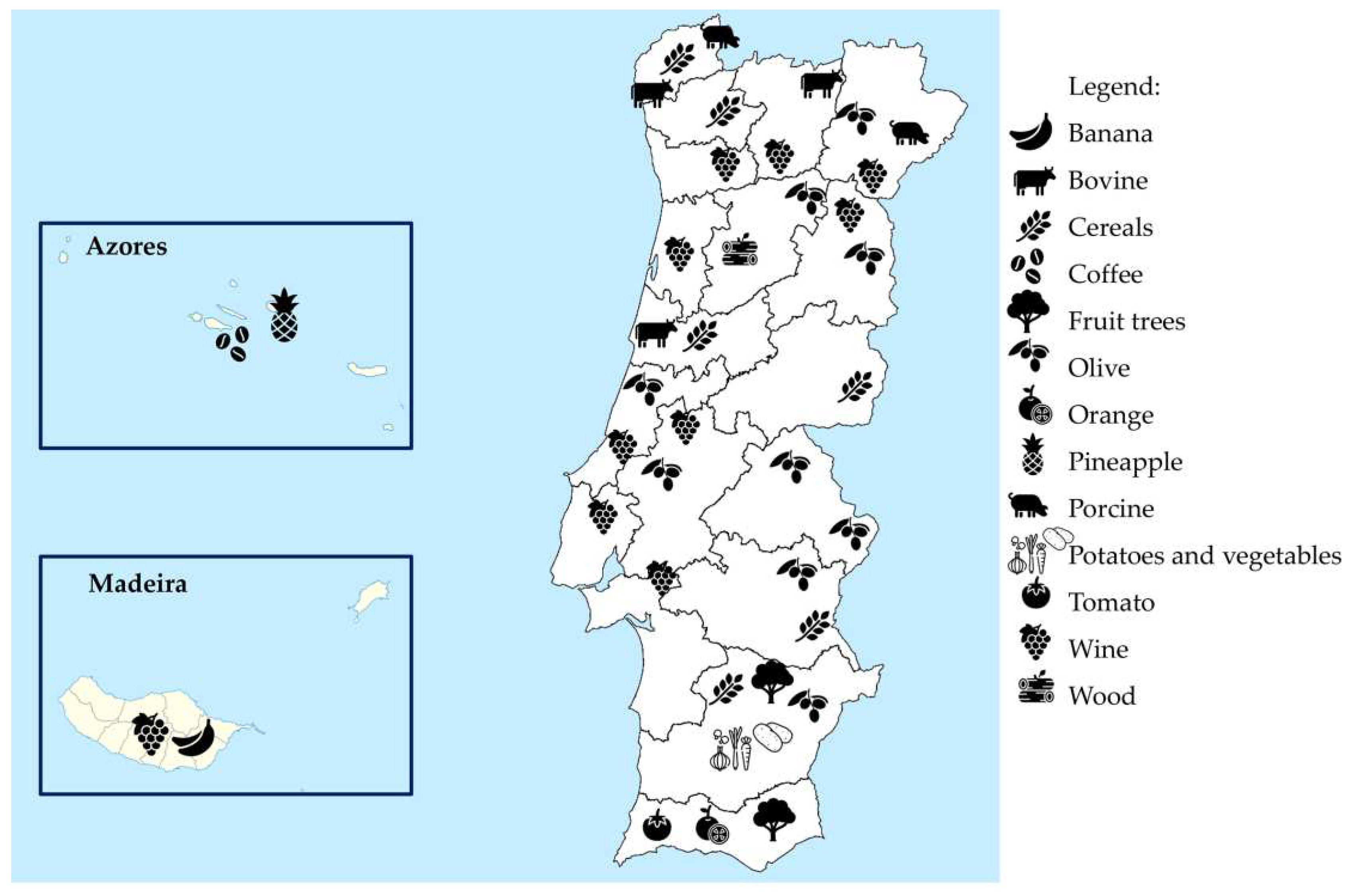
2.1. The Portuguese case study
3. Thermochemical conversion of agri-food wastes from the Portuguese industry
| Agri-food waste | Experimental parameters | Conclusions | Ref. |
|---|---|---|---|
| Pyrolysis | |||
| Banana pseudo-stem (BPS) | 5 to 10 pieces of BPS, 500 °C, 1.02 s, 200 mL/min N2 flow rate | BPS bio-oil was produced at a rate of 5.35 MJ/kg, which is relatively low when compared to petroleum fuel and other biomass pyrolysis fluids | [50] |
| Banana peel | 1 g sample, 720 °C, 10 min, 300 mL/min CO2 flow rate | Pyrolysis of the banana peel with CO2 enhanced the aromaticity of biocrude, accelerating liquid pyrolysate dehydrogenation without the use of any catalysts | [51] |
| Bagasse | Ba/Mg molar of 1:1, CA-to-CB ratio of 4:1, 300 °C, 20 s | BaMg-MMO demonstrated a promising catalytic performance on the synthesis of 4-vinylphenol during the rapid pyrolysis of bagasse | [52] |
| Grape bagasse | 100 g sample, 700 °C, a heating rate of 5 °C/ms, 60 min, 200 mL/min N2 flow rate | > 95 % of Cu(II) was removed from aqueous media using chars produced through the thermochemical conversion of grape bagasse | [53] |
| Olive oil pomace | 1 mg sample, 500 °C, heating rate of 20 °C/ms, 15 s | The ashes could serve as a catalyst to create bio-oil of higher grades | [54] |
| Orange peels | 4.5 g sample, 9 g of metal oxide, 500 °C, 25 °C/min, 1h | 5.69 and 4.82 times more 3-furaldehyde were produced by pyrolysis with Cu2O and Fe2O3, respectively | [55] |
| Peach seeds | 1 g sample, 300 kPa, 15 min, 100 mL/min N2 flow rate | In the range of 10-20, 37-50, and 10-20 % wt. of the pyrolyzed feedstock, respectively, pyrolysis gas, oil, and char were produced | [56] |
| Potato peels | 30 g sample, 500 °C, 30 °C/min, 30 min, 100 mL/min of N2 flow rate | The bio-oil and bio-char yield was 23.6 and 29.5 %, respectively | [57] |
| Sugarcane bagasse | 100 μm particle size, heating rate of 50 °C/min, 15.5 min, 493 °C, 225 mL/min N2 flow rate | 46.7 wt % of bio-oil yield was achieved at optimal pyrolysis conditions | [58] |
| Sugarcane bagasse | 10 % wt raw mixture, 1-3 kW, 400 °C, 25–10 kPa, 30 – 50 min, 500-600 mL/min N2 flow rate | The microwave pyrolysis by-products suggested a CO2 reduction potential of 47.9 CO2 eq/kg | [59] |
| Hydrothermal carbonization | |||
| Apple bagasse | 500 g sample, 3 L H2O, 180 °C, 2 h | The process provided stable carbonaceous solids that may be used as a CO2 neutral fuel (30 MJ/kg) and soil enhancer, in which 80-93 % of carbon was recovered | [60] |
| Banana peels | 5 g sample, 50 mL H2O, 300 °C, 1–2 h | The carbonized banana peel removed 99 % of Cd2+, whereas the raw peel removed 75 % | [61] |
| Banana stalks | 5 g sample, 50 mL H2O, 160–200 °C, 1 – 3 h | Higher heating value ranged from 18.1 to 18.9 MJ/kg, whereas the hydrochar yield ranged from 57.8 to 75.3 % | [62] |
| Grape pomace | 250 g sample, 1250 mL H2O, 180 °C, 1h | 97.08 % of hydrochar yield was attained, supporting the potential application of grape pomace for solid biofuel | [63] |
| Olive pomace | 1:1 sample: H2O ratio, 300 °C, 24 h | For energy purposes, the hydrochar obtained from olive pomace showed several advantages due to its lower ash content | [64] |
| Orange peels | 6 g sample, 1 mL H2O, 210 °C, 180 min | Upgrading of orange peels into value-added chemicals, such as 5-hydroxymethylfurfural, furfural, levulinic acid, and alkyl levulinates | [65] |
| Pineapple and watermelon peels | 85 g sample, 4 L H2O, 180 °C, 90 min | The yields and energy content of the hydrochars generated ranged from 25 to 69 % and 17 to 22 MJ/kg, respectively | [66] |
| Potato peels | 50 g sample, 50 mL H2O, 200 °C, 25 h | Potato peel hydrochar adsorption capacity for Congo red | [67] |
| Sugarcane bagasse | 3 g sample, 50 mL H2O, 200 °C, 18-20 h | The biochar obtained from sugarcane bagasse might be used as a sorbent to remove pollutants from water | [68] |
| Wine sludge | 6 – 12 mL sample, 200 °C, 24 h | The hydrochar products' higher heating value increased from 19.5 MJ/kg for a reactor filled to 24 % of its capacity to 21.36 MJ/kg for a reactor filled to 40 % | [69] |
| Ionothermal carbonization (ITC) | |||
| Coca bean shells | 3 g of sample, 10.8 g [Bmim][FeCl4], 240 °C, 20 h | The positive effects of the ITC method on mass yield, carbon yield, and specific surface area in [Bmim][FeCl4] were well demonstrated | [70] |
| Sugarcane bagasse | 1 g sample, 40.2 mmol imidazolium tetrachloroferrate, 240 °C, 20 h | High-specific surface area ionochars with tunable CO2 uptake/retention, tuneable pore volume, and unique nanostructures were produced | [71] |
| Torrefaction | |||
| Agri-food industry waste | 50 g sample, 200–300 °C, 1 h | The hydrophobic characteristics of agri-food waste improved as the torrefaction process temperature increased | [72] |
| Banana leaf | 260 g sample, 220–280 °C, 1 min | Torrefaction of banana leaves increased bioenergy-related qualities, showed better combustion efficiency, and decreased emissions potential | [73] |
| Grape pomace | 60 % w/w sample, 225–275 °C, 30 min | At 275 °C, the carbon content increased by 4.29 wt %, and the calorific value reached 25.84 MJ/kg | [74] |
| Grape pomace | 10 % w/w sample, 225 °C, 10 min | Using the torrefaction process, most of the phenolic compounds were not volatilized and remained in biochar | [75] |
| Olive pomace | 16 g, 200–290 °C, 30 min | The findings showed that when the torrefaction temperature improved, the yield of mass and energy declined but the production of greater heating value rose | [76] |
| Orange peels | 60 g sample, 200 °C, 60 min | Orange peels showed excellent odour adsorption ability | [77] |
| Sugarcane bagasse | DT, 5 g sample, 280 °C, 20 minWT, 5 g sample, 180 °C, 20 min | Both WT and DT processes promoted the heating value of sugarcane bagasse by around 5.0-17.9 % | [78] |
| Steam explosion (SE) | |||
| Apple pomace | 500 g sample, 151.9 °C, 5 min | 21.58 % of soluble dietary fibre yield was achieved, and its physicochemical properties were improved | [79] |
| Grape pomace | 100 g sample, 170 °C, 3 min | Free extracts' antioxidant activity was increased by SE whereas the activity of bound extracts was diminished | [80] |
| Grape seeds | 100 g sample, 0–15 MPa, 30–60 s | The SE reduced the mean degree of procyanidin polymerization and made grape seeds more lose and porous | [81] |
| Pineapple leaves | 150 g sample, 204 °C, 5 min | Without the use of any chemicals, the SE treatment may raise the cellulose fraction while decreasing the partial concentrations of hemicellulose and lignin | [82] |
| Pineapple peel | 20 g, 1.5 MPa, 30 s | SE treatment can break the bulk volume of dietary fibres and increase the surface area | [83] |
| Potatoes peels | 300 g sample, 0.35 MPa, 121 s | The water-holding capacity, oil-holding capacity, and swelling capacity values for potato peels significantly increased | [84] |
| Olive oil bagasse | 300 g, 160–200 °C, 5 min | 54–76 % of the bound oil and 18–32 % of the bound β-sitosterol were recovered | [85] |
| Sugarcane bagasse | 20 kg sample, 190 °C, 5 min | After pulping and bleaching, the procedure was quite effective and removed around 97 % of the lignin | [86] |
| Sugarcane bagasse | 60 g sample, 205 °C, 10 min | The cellulose nanofibers were successfully prepared by SE and could be applied in several fields (e.g., food packaging, and electronic device) | [87] |
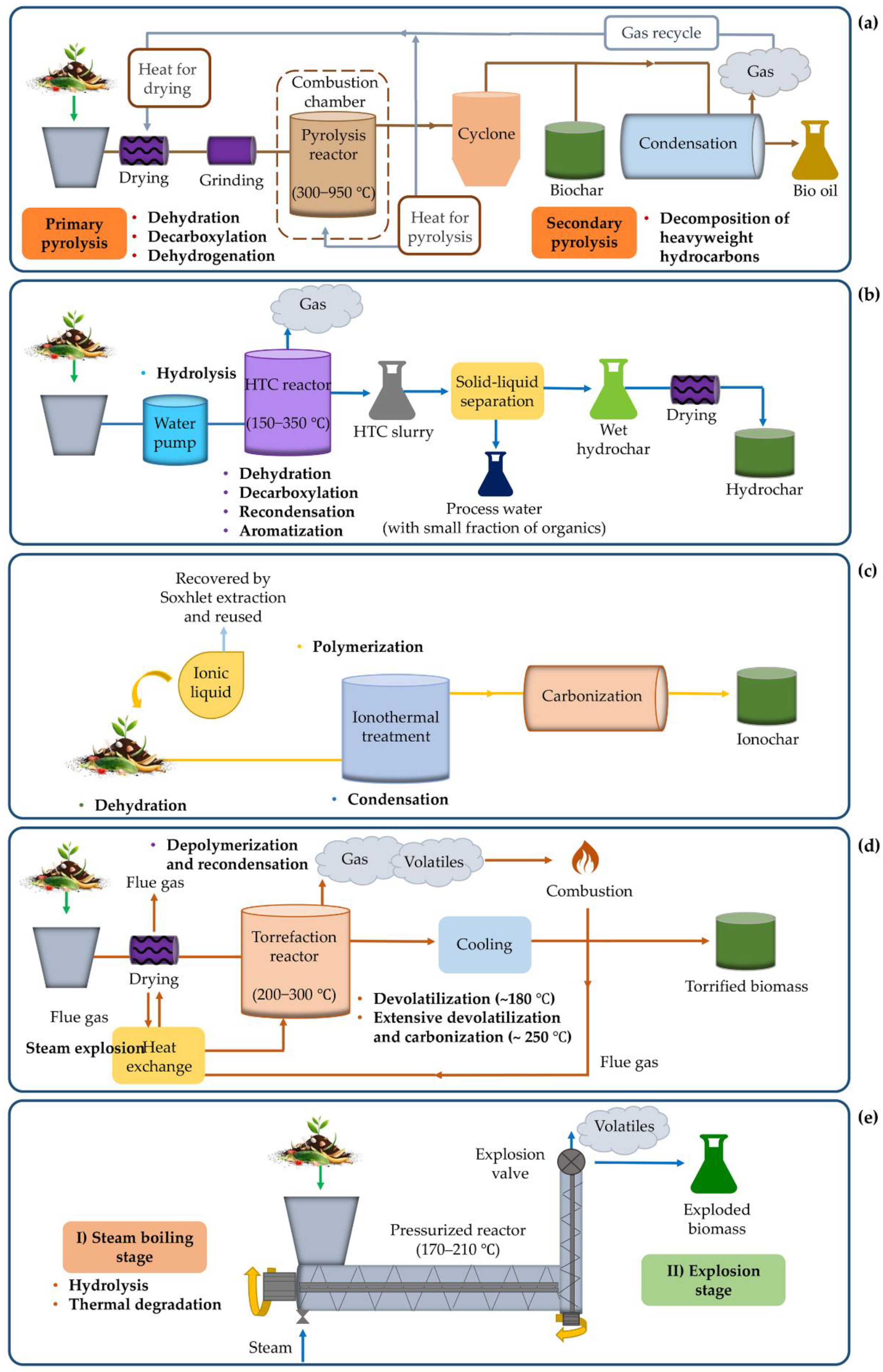
3.1. Pyrolysis
3.2. Hydrothermal carbonization
3.3. Ionothermal carbonization
3.4. Torrefaction
3.5. Steam explosion
4. Applications of carbonaceous materials
4.1. Environmental applications
4.2. Catalytic applications
4.3. Energy conversion and storage applications
4.4. Biological applications
5. Concluding remarks
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Xu, C.; Champagne, P. Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers. Manage. 2010, 51, 969–982. [Google Scholar] [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Chemistry and Specialty Industrial Applications of Lignocellulosic Biomass. Waste and Biomass Valorization 2020, 12, 2145–2169. [Google Scholar] [CrossRef]
- Yang, D.-P.; Li, Z.; Liu, M.; Zhang, X.; Chen, Y.; Xue, H.; Ye, E.; Luque, R. Biomass-Derived Carbonaceous Materials: Recent Progress in Synthetic Approaches, Advantages, and Applications. ACS Sustainable Chemistry & Engineering 2019, 7, 4564–4585. [Google Scholar] [CrossRef]
- Varma, R.S. Biomass-Derived Renewable Carbonaceous Materials for Sustainable Chemical and Environmental Applications. ACS Sustainable Chemistry & Engineering 2019, 7, 6458–6470. [Google Scholar] [CrossRef]
- Hu, B.; Yu, S.H.; Wang, K.; Liu, L.; Xu, X.W. Functional carbonaceous materials from hydrothermal carbonization of biomass: An effective chemical process. Dalton Trans. 2008, 5414–5423. [Google Scholar] [CrossRef]
- Berenguer, C.V.; Andrade, C.; Pereira, J.A.M.; Perestrelo, R.; Câmara, J.S. Current Challenges in the Sustainable Valorisation of Agri-Food Wastes: A Review. Processes 2023, 11, 20. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, Y.; Balasubramanian, R. Synthesis, formation mechanisms and applications of biomass-derived carbonaceous materials: A critical review. Journal of Materials Chemistry A 2021, 9, 24759–24802. [Google Scholar] [CrossRef]
- Geoghegan-Quinn, M. Innovating for sustainable growth: A Bioeconomy for Europe. 2012.
- Daioglou, V.; Doelman, J.C.; Wicke, B.; Faaij, A.; van Vuuren, D.P. Integrated assessment of biomass supply and demand in climate change mitigation scenarios. Global Environ. Change 2019, 54, 88–101. [Google Scholar] [CrossRef]
- European Commission. A sustainable bioeconomy for Europe: Strengthening the connection between economy, society and the environment; European Commission, 2018. [Google Scholar]
- Stegmann, P.; Londo, M.; Junginger, M. The circular bioeconomy: Its elements and role in European bioeconomy clusters. Resources, Conservation & Recycling: X 2020, 6, 100029. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Maniatis, K. Security of supply, strategic storage and Covid19: Which lessons learnt for renewable and recycled carbon fuels, and their future role in decarbonizing transport? Appl Energy 2020, 271, 115216. [Google Scholar] [CrossRef]
- Ferreira, S.; Monteiro, E.; Brito, P.; Vilarinho, C. Biomass resources in Portugal: Current status and prospects. Renewable and Sustainable Energy Reviews 2017, 78, 1221–1235. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Loureiro, L.M.E.F.; Sá, L.C.R.; Silva, H.F.C. Waste Recovery through Thermochemical Conversion Technologies: A Case Study with Several Portuguese Agroforestry By-Products. Clean Technologies 2020, 2, 377–391. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, D.; Wu, C.; Gu, S. State-of-the-art on the production and application of carbon nanomaterials from biomass. Green Chemistry 2018, 20, 5031–5057. [Google Scholar] [CrossRef]
- Hamelinck, C.N.; Hooijdonk, G.v.; Faaij, A.P.C. Ethanol from lignocellulosic biomass: Techno-economic performance in short-, middle- and long-term. Biomass Bioenergy 2005, 28, 384–410. [Google Scholar] [CrossRef]
- Rahimi, Z.; Anand, A.; Gautam, S. An overview on thermochemical conversion and potential evaluation of biofuels derived from agricultural wastes. Energy Nexus 2022, 7, 100125. [Google Scholar] [CrossRef]
- Meng, L.Y.; Wang, B.; Ma, M.G.; Zhu, J.F. Cellulose-based Nanocarriers as Platforms for Cancer Therapy. Curr. Pharm. Des. 2017, 23, 5292–5300. [Google Scholar] [CrossRef]
- Esa, F.; Tasirin, S.M.; Rahman, N.A. Overview of Bacterial Cellulose Production and Application. Agriculture and Agricultural Science Procedia 2014, 2, 113–119. [Google Scholar] [CrossRef]
- Jang, W.D.; Hwang, J.H.; Kim, H.U.; Ryu, J.Y.; Lee, S.Y. Bacterial cellulose as an example product for sustainable production and consumption. Microb Biotechnol 2017, 10, 1181–1185. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, Z. Lignin-based Functional Nanocomposites. In Functional Materials from Lignin, WORLD SCIENTIFIC (EUROPE): 2017; Volume 3, pp. 49–80.
- Wikberg, H.; Ohra-aho, T.; Pileidis, F.; Titirici, M.-M. Structural and Morphological Changes in Kraft Lignin during Hydrothermal Carbonization. ACS Sustainable Chemistry & Engineering 2015, 3, 2737–2745. [Google Scholar] [CrossRef]
- Azadi, P.; Inderwildi, O.R.; Farnood, R.; King, D.A. Liquid fuels, hydrogen and chemicals from lignin: A critical review. Renewable and Sustainable Energy Reviews 2013, 21, 506–523. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Galkin, M.V.; Samec, J.S. Lignin Valorization through Catalytic Lignocellulose Fractionation: A Fundamental Platform for the Future Biorefinery. ChemSusChem 2016, 9, 1544–1558. [Google Scholar] [CrossRef] [PubMed]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Sorek, N.; Yeats, T.H.; Szemenyei, H.; Youngs, H.; Somerville, C.R. The Implications of Lignocellulosic Biomass Chemical Composition for the Production of Advanced Biofuels. Bioscience 2014, 64, 192–201. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polymer Chemistry 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Agarwal, B.; Ahluwalia, V.; Pandey, A.; Sangwan, R.S.; Elumalai, S. Sustainable Production of Chemicals and Energy Fuel Precursors from Lignocellulosic Fractions. In Biofuels: Technology, Challenges and Prospects; Agarwal, A.K., Agarwal, R.A., Gupta, T., Gurjar, B.R., Eds.; Springer Singapore: Singapore, 2017; pp. 7–33. [Google Scholar] [CrossRef]
- Vincenti, B.; Paris, E.; Carnevale, M.; Palma, A.; Guerriero, E.; Borello, D.; Paolini, V.; Gallucci, F. Saccharides as Particulate Matter Tracers of Biomass Burning: A Review. Int. J. Environ. Res. Public. Health 2022, 19. [Google Scholar] [CrossRef]
- Lu, Y.; Thomas, L.; Schmidt, S. Differences in the thermal behavior of beet and cane sucrose sources. J. Food Eng. 2017, 201, 57–70. [Google Scholar] [CrossRef]
- He, K.; Sun, J.; Wang, X.; Zhang, B.; Zhang, Y.; Zhang, R.; Shen, Z. Saccharides Emissions from Biomass and Coal Burning in Northwest China and Their Application in Source Contribution Estimation. Atmosphere 2021, 12, 821. [Google Scholar] [CrossRef]
- Flórez Pardo, L.M.; López Galán, J.E.; Lozano Ramírez, T. Saccharide Biomass for Biofuels, Biomaterials, and Chemicals. In Biomass and Green Chemistry: Building a Renewable Pathway; Vaz, S., Jr., Ed.; Springer International Publishing: Cham, 2018; pp. 11–30. [Google Scholar] [CrossRef]
- Caetano, M.; Igreja, C.; Marcelino, F.; Costa, H. Estatísticas e dinâmicas territoriais multiescala de Portugal Continental 1995-2007-2010 com base na Carta de Uso e Ocupação do Solo (COS); Direção-Geral do Território, Relatório técnico: Lisbon, Portugal, 2017. [Google Scholar]
- Nunes, L.J.R.; Casau, M.; Matias, J.C.O.; Dias, M.F. Assessment of Woody Residual Biomass Generation Capacity in the Central Region of Portugal: Analysis of the Power Production Potential. Land 2022, 11, 1722. [Google Scholar] [CrossRef]
- European, C.; Joint Research, C. Brief on agricultural biomass production; Publications Office, 2018. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Matias, J.C.O.; Catalão, J.P.S. Biomass in the generation of electricity in Portugal: A review. Renewable and Sustainable Energy Reviews 2017, 71, 373–378. [Google Scholar] [CrossRef]
- Sikkema, R.; Steiner, M.; Junginger, M.; Hiegl, W.; Hansen, M.T.; Faaij, A. The European wood pellet markets: Current status and prospects for 2020. Biofuels, Bioproducts and Biorefining 2011, 5, 250–278. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Matias, J.C.O.; Catalão, J.P.S. Wood pellets as a sustainable energy alternative in Portugal. Renewable Energy 2016, 85, 1011–1016. [Google Scholar] [CrossRef]
- Enes, T.; Aranha, J.; Fonseca, T.; Lopes, D.; Alves, A.; Lousada, J. Thermal Properties of Residual Agroforestry Biomass of Northern Portugal. Energies 2019, 12, 1418. [Google Scholar] [CrossRef]
- Nunes, L.J.; Meireles, C.I.; Pinto Gomes, C.J.; de Almeida Ribeiro, N.M. Socioeconomic Aspects of the Forests in Portugal: Recent Evolution and Perspectives of Sustainability of the Resource. Forests 2019, 10, 361. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Raposo, M.A.M.; Meireles, C.I.R.; Pinto Gomes, C.J.; Ribeiro, N.M.C.A. Control of Invasive Forest Species through the Creation of a Value Chain: Acacia dealbata Biomass Recovery. Environments 2020, 7, 39. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Raposo, M.A.M.; Meireles, C.I.R.; Pinto Gomes, C.J.; Ribeiro, N.M.C.A. Control of Invasive Forest Species through the Creation of a Value Chain: Acacia dealbata Biomass Recovery. Environments 2020, 7, 39. [Google Scholar] [CrossRef]
- Ferreira, S.; Monteiro, E.; Brito, P.; Vilarinho, C. Biomass resources in Portugal: Current status and prospects. Renewable & Sustainable Energy Reviews 2017, 78, 1221–1235. [Google Scholar] [CrossRef]
- Fontes, A.V.P.; João, I.M.; Silva, J.M. Multicriteria evaluation of biomass residues in Portugal to second generation bioethanol production. Production 2021, 31. [Google Scholar] [CrossRef]
- Parliament, E. Directive (EU) 2015/1513 of the European Parliament and of the Council of 9 September 2015 amending Directive 98/70/EC relating to the quality of petrol and diesel fuels and amending Directive 2009/28/EC on the promotion of the use of energy from renewable sources. CotEU European Parliament, Editor 2015.
- Abreu, M.; Moura, P.; Reis, A.; Eusébio, A.; Oliveira, A.; Pinto, F.; Alexandre, J.; Silva, L.; Trancoso, M.; Gírio, F. The CONVERTE project: Biomass potential for energy; 2017. [Google Scholar]
- Abreu, M.; Reis, A.; Moura, P.; Fernando, A.L.; Luís, A.; Quental, L.; Patinha, P.; Gírio, F. Evaluation of the Potential of Biomass to Energy in Portugal—Conclusions from the CONVERTE Project. Energies 2020, 13, 937. [Google Scholar] [CrossRef]
- Perera, S.M.H.D.; Wickramasinghe, C.; Samarasiri, B.K.T.; Narayana, M. Modeling of thermochemical conversion of waste biomass – a comprehensive review. Biofuel Research Journal 2021, 8, 1481–1528. [Google Scholar] [CrossRef]
- Taib, R.M.; Abdullah, N.; Aziz, N.S.M. Bio-oil derived from banana pseudo-stem via fast pyrolysis process. Biomass Bioenergy 2021, 148, 106034. [Google Scholar] [CrossRef]
- Kwon, D.; Lee, S.S.; Jung, S.; Park, Y.-K.; Tsang, Y.F.; Kwon, E.E. CO2 to fuel via pyrolysis of banana peel. Chem. Eng. J. 2020, 392, 123774. [Google Scholar] [CrossRef]
- Li, Y.; Hu, B.; Fu, H.; Zhang, Z.-x.; Guo, Z.-t.; Zhou, G.-z.; Zhu, L.-j.; Liu, J.; Lu, Q. Fast pyrolysis of bagasse catalyzed by mixed alkaline-earth metal oxides for the selective production of 4-vinylphenol. J. Anal. Appl. Pyrolysis 2022, 164, 105531. [Google Scholar] [CrossRef]
- da Silva, C.M.S.; da Boit Martinello, K.; Lutke, S.F.; Godinho, M.; Perondi, D.; Silva, L.F.O.; Dotto, G.L. Pyrolysis of grape bagasse to produce char for Cu(II) adsorption: A circular economy perspective. Biomass Convers Biorefin 2022, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Alcazar-Ruiz, A.; Garcia-Carpintero, R.; Dorado, F.; Sanchez- Silva, L. Valorization of olive oil industry subproducts: Ash and olive pomace fast pyrolysis. Food Bioprod. Process. 2021, 125, 37–45. [Google Scholar] [CrossRef]
- Zhang, W.; Xia, H.; Deng, Y.; Zhang, Q.; Xin, C. Slow pyrolysis of waste navel orange peels with metal oxide catalysts to produce high-grade bio-oil. Green Processing and Synthesis 2022, 11, 218–228. [Google Scholar] [CrossRef]
- Altantzis, A.-I.; Kallistridis, N.-C.; Stavropoulos, G.; Zabaniotou, A. Peach Seeds Pyrolysis Integrated into a Zero Waste Biorefinery: An Experimental Study. Circular Economy and Sustainability 2021, 2, 351–382. [Google Scholar] [CrossRef]
- Daimary, N.; Eldiehy, K.S.H.; Boruah, P.; Deka, D.; Bora, U.; Kakati, B.K. Potato peels as a sustainable source for biochar, bio-oil and a green heterogeneous catalyst for biodiesel production. Journal of Environmental Chemical Engineering 2022, 10, 107108. [Google Scholar] [CrossRef]
- Onokwai, A.O.; Okokpujie, I.P.; Ajisegiri, E.S.A.; Nnodim, C.T.; Kayode, J.F.; Tartibu, L.K. Application of response surface methodology for the modelling and optimisation of bio-oil yield via intermediate pyrolysis process of sugarcane bagasse. Advances in Materials and Processing Technologies 2023, 1–19. [Google Scholar] [CrossRef]
- Allende, S.; Brodie, G.; Jacob, M.V. Energy recovery from sugarcane bagasse under varying microwave-assisted pyrolysis conditions. Bioresource Technology Reports 2022, 20, 101283. [Google Scholar] [CrossRef]
- Suarez, L.; Benavente-Ferraces, I.; Plaza, C.; de Pascual-Teresa, S.; Suarez-Ruiz, I.; Centeno, T.A. Hydrothermal carbonization as a sustainable strategy for integral valorisation of apple waste. Bioresour. Technol. 2020, 309, 123395. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, I.; Flagiello, F.; Ward, N.I.; Arellano-García, H.; Avignone-Rossa, C.; Felipe-Sotelo, M. Valorisation of banana peels by hydrothermal carbonisation: Potential use of the hydrochar and liquid by-product for water purification and energy conversion. Bioresource Technology Reports 2020, 12, 100582. [Google Scholar] [CrossRef]
- Islam, M.A.; Akber, M.A.; Limon, S.H.; Akbor, M.A.; Islam, M.A. Characterization of solid biofuel produced from banana stalk via hydrothermal carbonization. Biomass Conversion and Biorefinery 2019, 9, 651–658. [Google Scholar] [CrossRef]
- PetroviĿ, J.; PerišiĿ, N.; MaksimoviĿ, J.D.; MaksimoviĿ, V.; KragoviĿ, M.; StojanoviĿ, M.; LauševiĿ, M.; MihajloviĿ, M. Hydrothermal conversion of grape pomace: Detailed characterization of obtained hydrochar and liquid phase. J. Anal. Appl. Pyrolysis 2016, 118, 267–277. [Google Scholar] [CrossRef]
- Erses Yay, A.S.; Birinci, B.; Açıkalın, S.; Yay, K. Hydrothermal carbonization of olive pomace and determining the environmental impacts of post-process products. Journal of Cleaner Production 2021, 315, 128087. [Google Scholar] [CrossRef]
- Satira, A.; Paone, E.; Bressi, V.; Iannazzo, D.; Marra, F.; Calabrò, P.S.; Mauriello, F.; Espro, C. Hydrothermal Carbonization as Sustainable Process for the Complete Upgrading of Orange Peel Waste into Value-Added Chemicals and Bio-Carbon Materials. Applied Sciences 2021, 11, 10983. [Google Scholar] [CrossRef]
- Azaare, L.; Commeh, M.K.; Smith, A.M.; Kemausuor, F. Co-hydrothermal carbonization of pineapple and watermelon peels: Effects of process parameters on hydrochar yield and energy content. Bioresource Technology Reports 2021, 15, 100720. [Google Scholar] [CrossRef]
- Goyi, A.A.; Sher Mohammad, N.M.; Omer, K.M. Preparation and characterization of potato peel derived hydrochar and its application for removal of Congo red: A comparative study with potato peel powder. International Journal of Environmental Science and Technology 2023. [Google Scholar] [CrossRef]
- Prasannamedha, G.; Kumar, P.S.; Mehala, R.; Sharumitha, T.J.; Surendhar, D. Enhanced adsorptive removal of sulfamethoxazole from water using biochar derived from hydrothermal carbonization of sugarcane bagasse. J. Hazard. Mater. 2021, 407, 124825. [Google Scholar] [CrossRef]
- Vasileiadou, M.A.; Altiparmaki, G.; Moustakas, K.; Vakalis, S. Quality of Hydrochar from Wine Sludge under Variable Conditions of Hydrothermal Carbonization: The Case of Lesvos Island. Energies 2022, 15, 3574. [Google Scholar] [CrossRef]
- Cibien, L.; Parot, M.; Fotsing, P.N.; Gaveau, P.; Woumfo, E.D.; Vieillard, J.; Napoli, A.; Brun, N. Ionothermal carbonization in [Bmim][FeCl4]: An opportunity for the valorization of raw lignocellulosic agrowastes into advanced porous carbons for CO2 capture. Green Chemistry 2020, 22, 5423–5436. [Google Scholar] [CrossRef]
- Aldroubi, S.; El-Sakhawy, M.; Kamel, S.; Hesemann, P.; Mehdi, A.; Brun, N. Ionothermal carbonization of sugarcane bagasse in imidazolium tetrachloroferrate ionic liquids: Effect of the cation on textural and morphological properties. Green Chemistry 2023, 25, 3533–3542. [Google Scholar] [CrossRef]
- Dyjakon, A.; Noszczyk, T.; Smędzik, M. The Influence of Torrefaction Temperature on Hydrophobic Properties of Waste Biomass from Food Processing. Energies 2019, 12, 4609. [Google Scholar] [CrossRef]
- Alves, J.L.F.; da Silva, J.C.G.; Sellin, N.; Pra, F.B.; Sapelini, C.; Souza, O.; Marangoni, C. Upgrading of banana leaf waste to produce solid biofuel by torrefaction: Physicochemical properties, combustion behaviors, and potential emissions. Environ Sci Pollut Res Int 2022, 29, 25733–25747. [Google Scholar] [CrossRef]
- Tamelová, B.; Malaťák, J.; Velebil, J.; Gendek, A.; Aniszewska, M. Energy Utilization of Torrefied Residue from Wine Production. Materials 2021, 14, 1610. [Google Scholar] [CrossRef]
- del Pozo, C.; Bartrolí, J.; Alier, S.; Puy, N.; Fàbregas, E. Production, identification, and quantification of antioxidants from torrefaction and pyrolysis of grape pomace. Fuel Process. Technol. 2021, 211, 106602. [Google Scholar] [CrossRef]
- Allouzi, M.M.A.; Lee, L.Y.; Gan, S.; Thangalazhy-Gopakumar, S. Torrefaction of olive pomace with low-density polyethylene (LDPE) plastic and its interactive effects. Thermochim. Acta 2023, 724, 179495. [Google Scholar] [CrossRef]
- Sobol, Ł.; Łyczko, J.; Dyjakon, A.; Sroczyński, R. Relationship between Odor Adsorption Ability and Physical–Hydraulic Properties of Torrefied Biomass: Initial Study. Energies 2023, 16, 1780. [Google Scholar] [CrossRef]
- Jarunglumlert, T.; Bampenrat, A.; Sukkathanyawat, H.; Pavasant, P.; Prommuak, C. Enhancing the potential of sugarcane bagasse for the production of ENplus quality fuel pellets by torrefaction: An economic feasibility study. Biofuel Research Journal 2022, 9, 1707–1720. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, K.; Tian, X.; Sui, W.; Wu, T.; Wang, S.; Jin, Y.; Zhu, Q.; Meng, J.; Zhang, M. Combined Modification of Soluble Dietary Fibers from Apple Pomace by Steam Explosion and Enzymatic Hydrolysis to Improve its Structural, Physicochemical and Functional Properties. Waste and Biomass Valorization 2022, 13, 4869–4879. [Google Scholar] [CrossRef]
- Cui, W.; Wang, Y.; Sun, Z.; Cui, C.; Li, H.; Luo, K.; Cheng, A. Effects of steam explosion on phenolic compounds and dietary fiber of grape pomace. Lwt 2023, 173, 114350. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Wang, A.; Cheng, L.; Wang, W.; Liu, Y.; Ullah, S.; Yuan, Q. Production of oligomeric procyanidins by mild steam explosion treatment of grape seeds. Bioresources and Bioprocessing 2021, 8, 23. [Google Scholar] [CrossRef]
- Tanpichai, S.; Witayakran, S.; Boonmahitthisud, A. Study on structural and thermal properties of cellulose microfibers isolated from pineapple leaves using steam explosion. Journal of Environmental Chemical Engineering 2019, 7, 102836. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, Y.; Zhang, L.; Wang, X.; Lin, L. Effect of Steam Explosion Modification and in Vitro Simulated Digestion on Antioxidant Capacity of Dietary Fiber of Pineapple Peel. IOP Conference Series: Earth and Environmental Science 2019, 330, 042055. [Google Scholar] [CrossRef]
- Wang, T.; Liang, X.; Ran, J.; Sun, J.; Jiao, Z.; Mo, H. Response surface methodology for optimisation of soluble dietary fibre extraction from sweet potato residue modified by steam explosion. Int. J. Food Sci. Tech. 2017, 52, 741–747. [Google Scholar] [CrossRef]
- Secmeler, O.; Guclu Ustundag, O.; Fernandez-Bolanos, J.; Rodriguez-Gutierrez, G. Effect of subcritical water and steam explosion pretreatments on the recovery of sterols, phenols and oil from olive pomace. Food Chem. 2018, 265, 298–307. [Google Scholar] [CrossRef]
- Rocha, G.J.M.; Andrade, L.P.; Martín, C.; Araujo, G.T.; Mouchrek Filho, V.E.; Curvelo, A.A.S. Simultaneous obtaining of oxidized lignin and cellulosic pulp from steam-exploded sugarcane bagasse. Ind. Crops Prod. 2020, 147, 112227. [Google Scholar] [CrossRef]
- Hongrattanavichit, I.; Aht-Ong, D. Nanofibrillation and characterization of sugarcane bagasse agro-waste using water-based steam explosion and high-pressure homogenization. Journal of Cleaner Production 2020, 277, 123471. [Google Scholar] [CrossRef]
- Nanda, S.; Dalai, A.K.; Berruti, F.; Kozinski, J.A. Biochar as an Exceptional Bioresource for Energy, Agronomy, Carbon Sequestration, Activated Carbon and Specialty Materials. Waste and Biomass Valorization 2015, 7, 201–235. [Google Scholar] [CrossRef]
- Collard, F.-X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renewable and Sustainable Energy Reviews 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renewable and Sustainable Energy Reviews 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels, Bioproducts and Biorefining 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Lynam, J.G.; Mumme, J. Hydrothermal Carbonization of Biomass for Energy and Crop Production. Applied Bioenergy 2014, 1. [Google Scholar] [CrossRef]
- Fang, J.; Zhan, L.; Ok, Y.S.; Gao, B. Minireview of potential applications of hydrochar derived from hydrothermal carbonization of biomass. Journal of Industrial and Engineering Chemistry 2018, 57, 15–21. [Google Scholar] [CrossRef]
- Chen, C.; Liang, W.; Fan, F.; Wang, C. The Effect of Temperature on the Properties of Hydrochars Obtained by Hydrothermal Carbonization of Waste Camellia oleifera Shells. ACS Omega 2021, 6, 16546–16552. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Baloch, H.A.; Griffin, G.J.; Mubarak, N.M.; Bhutto, A.W.; Abro, R.; Mazari, S.A.; Ali, B.S. An overview of effect of process parameters on hydrothermal carbonization of biomass. Renewable and Sustainable Energy Reviews 2017, 73, 1289–1299. [Google Scholar] [CrossRef]
- Isemin, R.; Muratova, N.; Kuzmin, S.; Klimov, D.; Kokh-Tatarenko, V.; Mikhalev, A.; Milovanov, O.; Dalibard, A.; Ibitowa, O.A.; Nowotny, M.; et al. Characteristics of Hydrochar and Liquid Products Obtained by Hydrothermal Carbonization and Wet Torrefaction of Poultry Litter in Mixture with Wood Sawdust. Processes 2021, 9, 2082. [Google Scholar] [CrossRef]
- Falco, C.; Marco-Lozar, J.P.; Salinas-Torres, D.; Morallón, E.; Cazorla-Amorós, D.; Titirici, M.M.; Lozano-Castelló, D. Tailoring the porosity of chemically activated hydrothermal carbons: Influence of the precursor and hydrothermal carbonization temperature. Carbon 2013, 62, 346–355. [Google Scholar] [CrossRef]
- Fulvio, P.F.; Lee, J.S.; Mayes, R.T.; Wang, X.; Mahurin, S.M.; Dai, S. Boron and nitrogen-rich carbons from ionic liquid precursors with tailorable surface properties. Phys. Chem. Chem. Phys. 2011, 13, 13486–13491. [Google Scholar] [CrossRef]
- Kubo, S.; Demir-Cakan, R.; Zhao, L.; White, R.J.; Titirici, M.M. Porous carbohydrate-based materials via hard templating. ChemSusChem 2010, 3, 188–194. [Google Scholar] [CrossRef]
- Xu, F.; Chen, Y.; Tang, M.; Wang, H.; Deng, J.; Wang, Y. Acid Induced Self-Assembly Strategy to Synthesize Ordered Mesoporous Carbons from Biomass. ACS Sustainable Chemistry & Engineering 2016, 4, 4473–4479. [Google Scholar] [CrossRef]
- Xiao, P.-W.; Guo, D.; Zhao, L.; Han, B.-H. Soft templating synthesis of nitrogen-doped porous hydrothermal carbons and their applications in carbon dioxide and hydrogen adsorption. Microporous Mesoporous Mater. 2016, 220, 129–135. [Google Scholar] [CrossRef]
- Huang, X.; Yamasaki, K.; Kudo, S.; Sperry, J.; Hayashi, J.-i. Influence of ionic liquid type on porous carbon formation during the ionothermal pyrolysis of cellulose. J. Anal. Appl. Pyrolysis 2020, 145, 104728. [Google Scholar] [CrossRef]
- Xie, Z.L.; Su, D.S. Ionic Liquid Based Approaches to Carbon Materials Synthesis. Eur. J. Inorg. Chem. 2014, 2015, 1137–1147. [Google Scholar] [CrossRef]
- Zhang, P.; Gong, Y.; Wei, Z.; Wang, J.; Zhang, Z.; Li, H.; Dai, S.; Wang, Y. Updating biomass into functional carbon material in ionothermal manner. ACS Appl Mater Interfaces 2014, 6, 12515–12522. [Google Scholar] [CrossRef]
- Chiou, B.-S.; Cao, T.; Valenzuela-Medina, D.; Bilbao-Sainz, C.; Avena-Bustillos, R.J.; Milczarek, R.R.; Du, W.-X.; Glenn, G.M.; Orts, W.J. Torrefaction kinetics of almond and walnut shells. J. Therm. Anal. Calorim. 2017, 131, 3065–3075. [Google Scholar] [CrossRef]
- Bates, R.B.; Ghoniem, A.F. Modeling kinetics-transport interactions during biomass torrefaction: The effects of temperature, particle size, and moisture content. Fuel 2014, 137, 216–229. [Google Scholar] [CrossRef]
- Barskov, S.; Zappi, M.; Buchireddy, P.; Dufreche, S.; Guillory, J.; Gang, D.; Hernandez, R.; Bajpai, R.; Baudier, J.; Cooper, R.; et al. Torrefaction of biomass: A review of production methods for biocoal from cultured and waste lignocellulosic feedstocks. Renewable Energy 2019, 142, 624–642. [Google Scholar] [CrossRef]
- Cahyanti, M.N.; Doddapaneni, T.; Kikas, T. Biomass torrefaction: An overview on process parameters, economic and environmental aspects and recent advancements. Bioresour. Technol. 2020, 301, 122737. [Google Scholar] [CrossRef]
- Gong, S.-H.; Im, H.-S.; Um, M.; Lee, H.-W.; Lee, J.-W. Enhancement of waste biomass fuel properties by sequential leaching and wet torrefaction. Fuel 2019, 239, 693–700. [Google Scholar] [CrossRef]
- Shankar Tumuluru, J.; Sokhansanj, S.; Hess, J.R.; Wright, C.T.; Boardman, R.D. REVIEW: A review on biomass torrefaction process and product properties for energy applications. Industrial Biotechnology 2011, 7, 384–401. [Google Scholar] [CrossRef]
- Ma, C.; Ni, L.; Guo, Z.; Zeng, H.; Wu, M.; Zhang, M.; Zheng, B. Principle and Application of Steam Explosion Technology in Modification of Food Fiber. Foods 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Devin, I.; Chrusciel, L.; Brosse, N. Steam Explosion Pretreatment of Lignocellulosic Biomass: A Mini-Review of Theorical and Experimental Approaches. Front Chem 2021, 9, 705358. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, J.; Ren, X.; Lau, A.; Rezaei, H.; Takada, M.; Bi, X.; Sokhansanj, S. Steam explosion of lignocellulosic biomass for multiple advanced bioenergy processes: A review. Renewable and Sustainable Energy Reviews 2022, 154, 111871. [Google Scholar] [CrossRef]
- Sui, W.; Xie, X.; Liu, R.; Wu, T.; Zhang, M. Effect of wheat bran modification by steam explosion on structural characteristics and rheological properties of wheat flour dough. Food Hydrocolloids 2018, 84, 571–580. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota – A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, P.; Zheng, L.; Shi, X.; Zheng, H. Carbon nanomaterials with sp or/and sp hybridization in energy conversion and storage applications: A review. Energy Storage Materials 2020, 26, 349–370. [Google Scholar] [CrossRef]
- Yang, Z.; Ren, J.; Zhang, Z.; Chen, X.; Guan, G.; Qiu, L.; Zhang, Y.; Peng, H. Recent advancement of nanostructured carbon for energy applications. Chem. Rev. 2015, 115, 5159–5223. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Kedia, D.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.-H. Carbon nanotubes: A potential material for energy conversion and storage. Prog. Energy Combust. Sci. 2018, 64, 219–253. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Vassilev, V.S. Advantages and disadvantages of composition and properties of biomass in comparison with coal: An overview. Fuel 2015, 158, 330–350. [Google Scholar] [CrossRef]
- Abdulyekeen, K.A.; Umar, A.A.; Patah, M.F.A.; Daud, W.M.A.W. Torrefaction of biomass: Production of enhanced solid biofuel from municipal solid waste and other types of biomass. Renewable and Sustainable Energy Reviews 2021, 150, 111436. [Google Scholar] [CrossRef]
- Shukla, M.K.; Dong, W.-L.; Azizov, S.; Singh, K.R.B.; Kumar, D.; Singh, R.P.; Singh, J. Trends of bioderived carbonaceous materials for futuristic biomedical applications. Mater. Lett. 2022, 311, 131606. [Google Scholar] [CrossRef]
- Parvin, N.; Mandal, T.K. Synthesis of a highly fluorescence nitrogen-doped carbon quantum dots bioimaging probe and its in vivo clearance and printing applications. RSC Advances 2016, 6, 18134–18140. [Google Scholar] [CrossRef]
- Wareing, T.C.; Gentile, P.; Phan, A.N. Biomass-Based Carbon Dots: Current Development and Future Perspectives. ACS Nano 2021, 15, 15471–15501. [Google Scholar] [CrossRef] [PubMed]
- Saikia, M.; Das, T.; Dihingia, N.; Fan, X.; Silva, L.F.O.; Saikia, B.K. Formation of carbon quantum dots and graphene nanosheets from different abundant carbonaceous materials. Diamond Relat. Mater. 2020, 106, 107813. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





