Submitted:
12 September 2023
Posted:
14 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
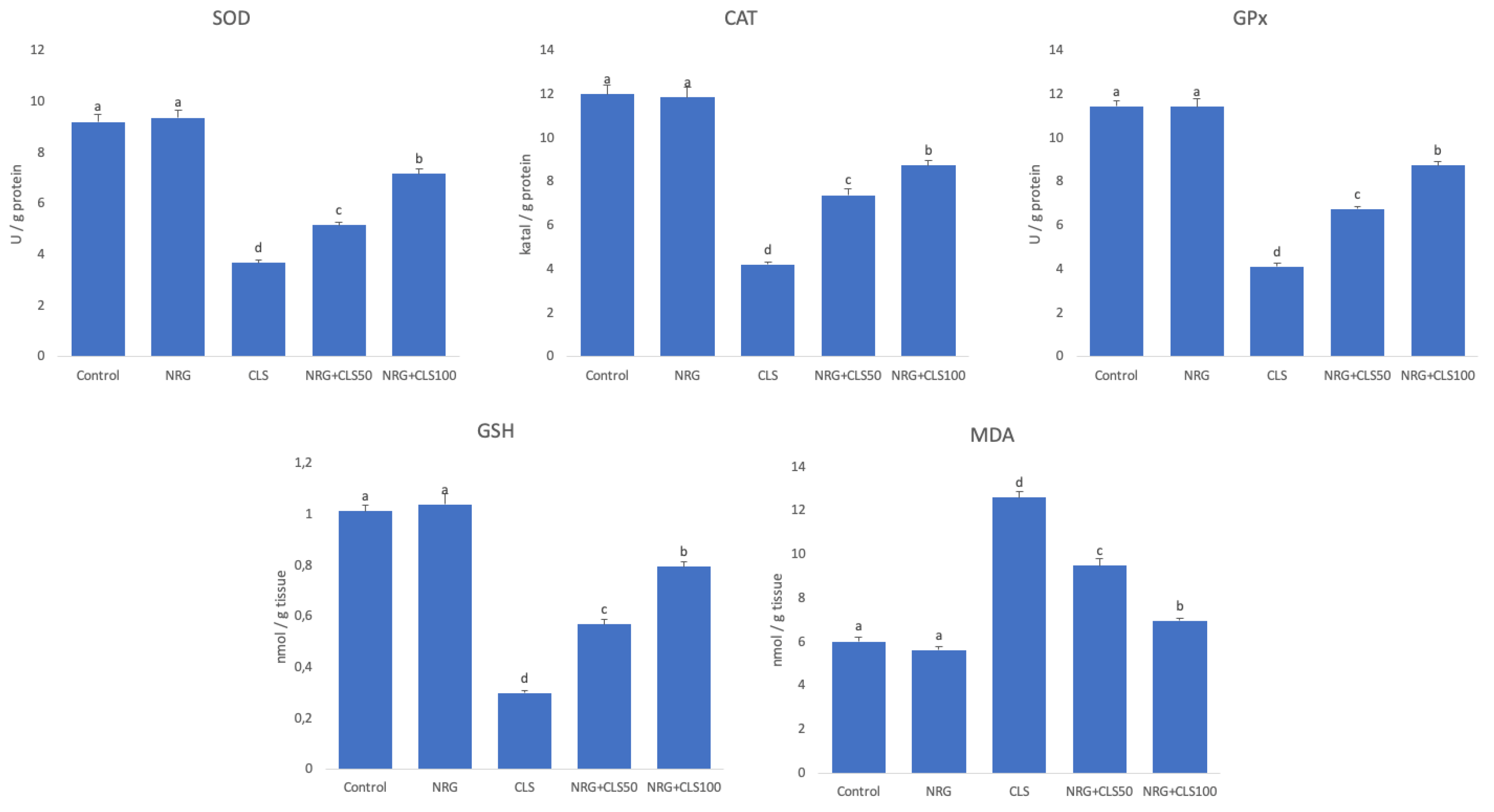
2.1. Oxidant and Antioxidant Status Findings
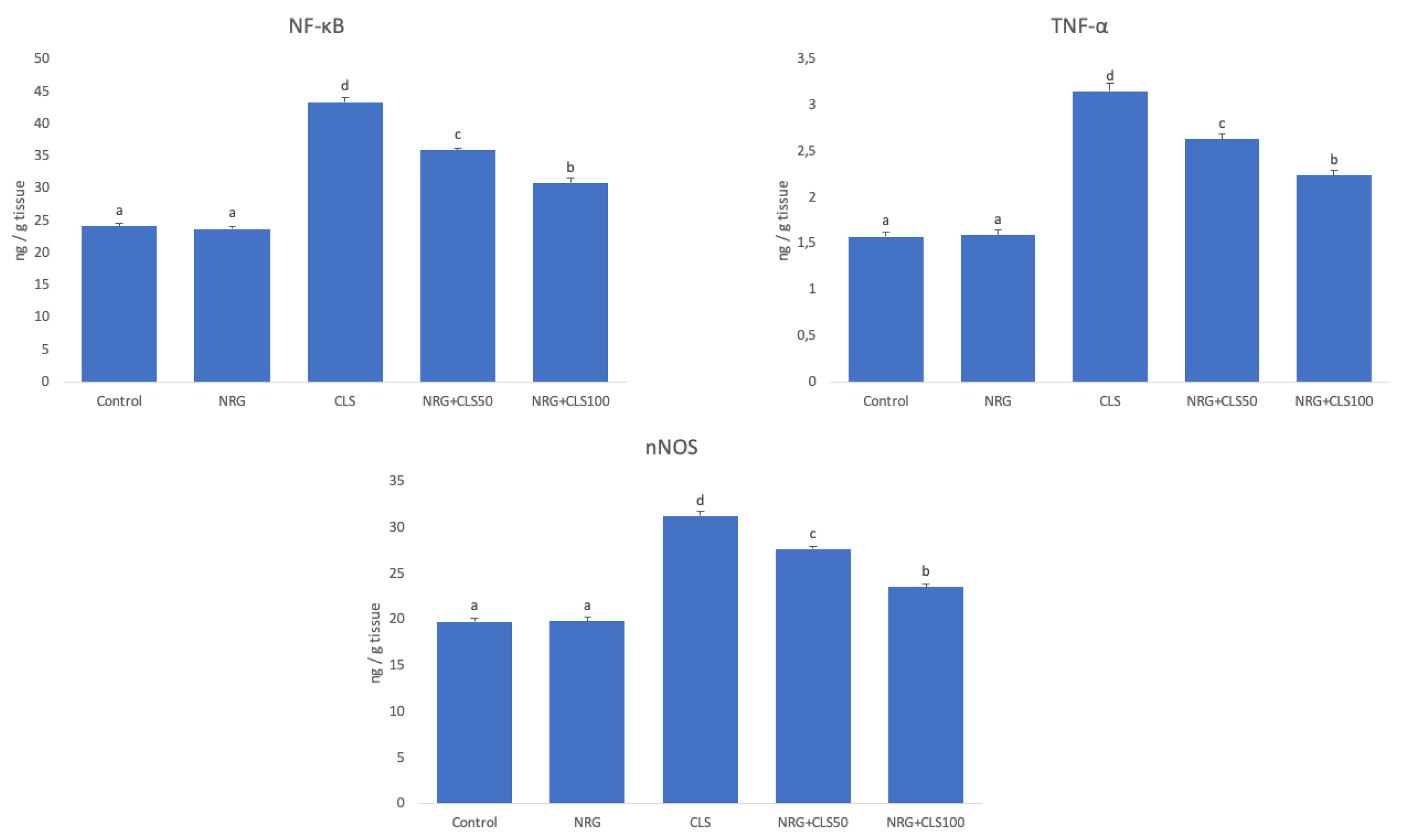
2.2. Inflammation Markers findings
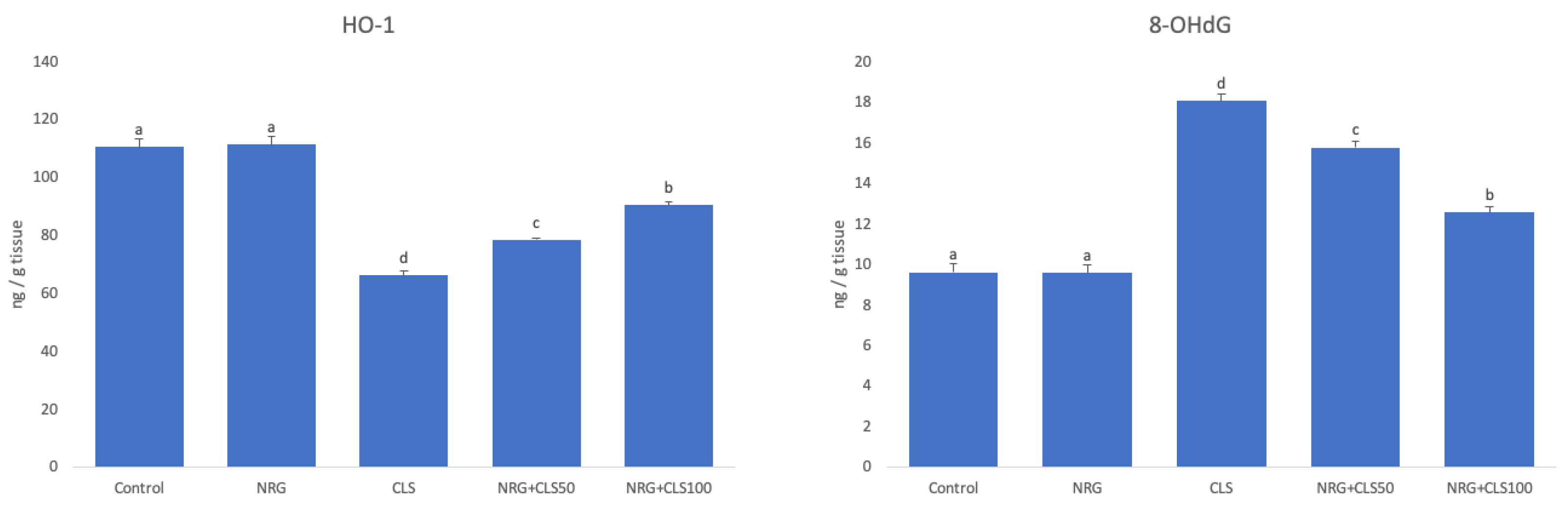
2.3. Findings of HO-1 and 8−OHdG Levels
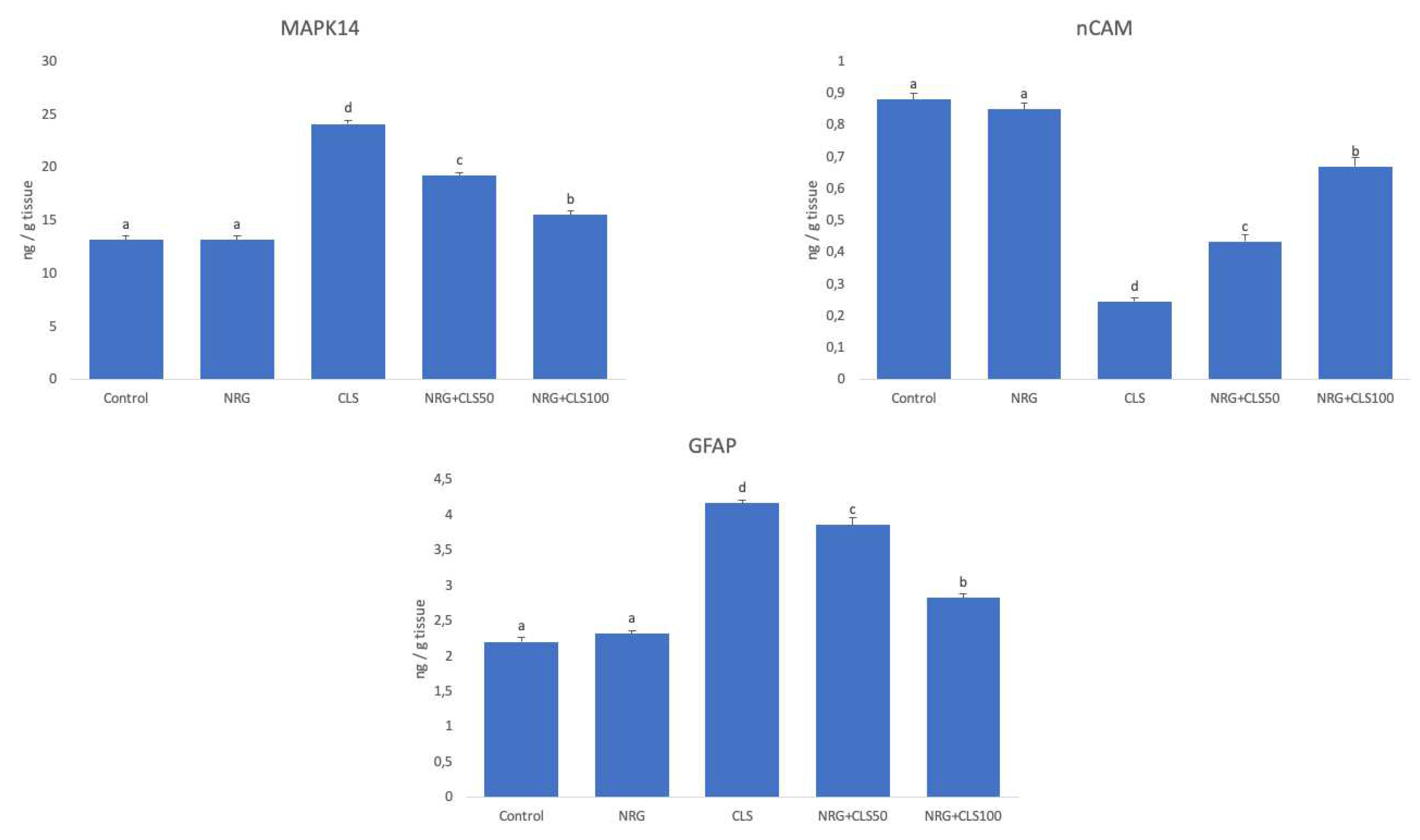
2.4. Findings of MAPK14, nCAM and GFAP Levels
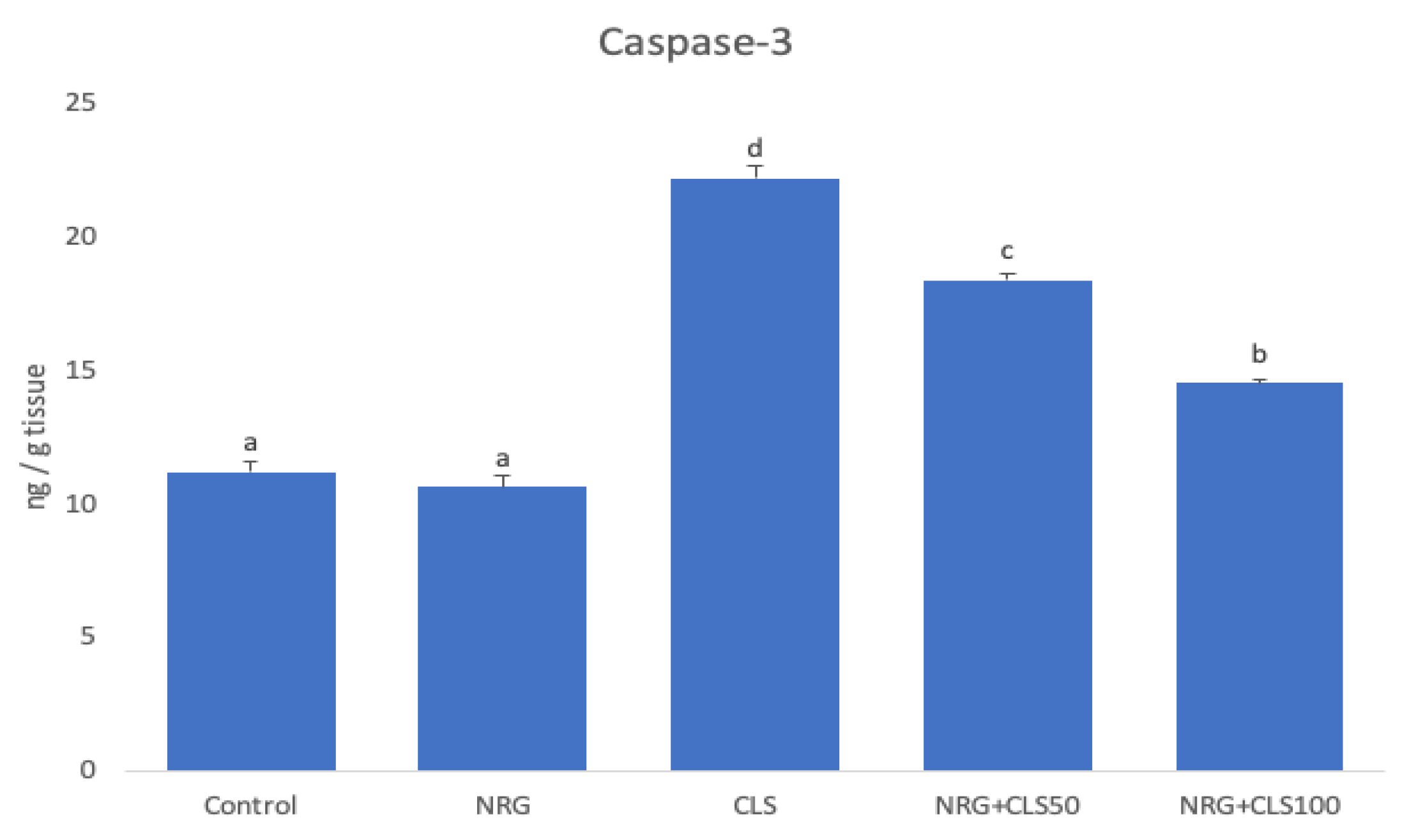
2.5. Apoptotic Markers Findings
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Collection of samples
4.3. Lipid peroxidation analysis
4.4. Antioxidant analysis
4.5. Analysis of Inflammatory Markers
4.6. Analysis of MAPK14, nCAM and GFAP Levels
4.7. Analysis of HO-1 and 8−OHdG Levels
4.8. Analysis of Apoptotic Marker
4.9. Statistical analysis
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aksu EH, Kandemir FM, Küçükler S, Mahamadu A. Improvement in colistin-induced reproductive damage, apoptosis, and autophagy in testes via reducing oxidative stress by chrysin. J Biochem Mol Toxicol 2018 ; 32(11) : e22201. [CrossRef]
- Aksu EH, Kandemir FM, Küçükler S. The effects of hesperidin on colistin-induced reproductive damage, autophagy, and apoptosis by reducing oxidative stress. Andrologia 2021 ; 53(2) : e13900. [CrossRef]
- Çelik H, Kandemir FM, Caglayan C, Özdemir S, Çomaklı S, Kucukler S, et al. Neuroprotective effect of rutin against colistin-induced oxidative stress, inflammation and apoptosis in rat brain associated with the CREB/BDNF expressions. Mol Biol Rep 2020 ; 47(3) : 2023-2034. [CrossRef]
- Dai C, Li J, Lin W, Li G, Sun M, Wang F, et al. Electrophysiology and ultrastructural changes in mouse sciatic nerve associated with colistin sulfate exposure. Toxicol Mech Methods 2012 ; 22(8) : 592-6. [CrossRef]
- Dai C, Li J, Li J. New insight in colistin induced neurotoxicity with the mitochondrial dysfunction in mice central nervous tissues. Exp Toxicol Pathol 2013 ; 65(6) : 941-8. [CrossRef]
- Dai C, Tang S, Biao X, Xiao X, Chen C, Li J. Colistin induced peripheral neurotoxicity involves mitochondrial dysfunction and oxidative stress in mice. Mol Biol Rep 2019 ; 46(2) : 1963-72. [CrossRef]
- Caglayan C, Kandemir FM, Ayna A, Gür C, Küçükler S, Darendelioğlu E. Neuroprotective effects of 18β-glycyrrhetinic acid against bisphenol A-induced neurotoxicity in rats: involvement of neuronal apoptosis, endoplasmic reticulum stress and JAK1/STAT1 signaling pathway. Metab Brain Dis 2022 ; 37(6) : 1931-40. [CrossRef]
- Varışlı B, Caglayan C, Kandemir FM, Gür C, Ayna A, Genç A, et al. Chrysin mitigates diclofenac-induced hepatotoxicity by modulating oxidative stress, apoptosis, autophagy and endoplasmic reticulum stress in rats. Mol Biol Rep 2023 ; 50(1) : 433-42. [CrossRef]
- Ghanbari-Movahed M, Jackson G, Farzaei MH, Bishayee A. A Systematic Review of the Preventive and Therapeutic Effects of Naringin Against Human Malignancies. Front Pharmacol 2021 ; 12: 639840. [CrossRef]
- Akintunde JK, Akintola TE, Adenuga GO, Odugbemi ZA, Adetoye RO, Akintunde OG. Naringin attenuates Bisphenol-A mediated neurotoxicity in hypertensive rats by abrogation of cerebral nucleotide depletion, oxidative damage and neuroinflammation. Neurotoxicology 2020 ; 81: 18-33. [CrossRef]
- Kaur G, Prakash A. Involvement of the nitric oxide signaling in modulation of naringin against intranasal manganese and intracerbroventricular β-amyloid induced neurotoxicity in rats. J Nutr Biochem 2020 ; 76 : 108255. [CrossRef]
- Garabadu D, Agrawal N. Naringin Exhibits Neuroprotection Against Rotenone-Induced Neurotoxicity in Experimental Rodents. Neuromolecular Med. 2020 ; 22(2) : 314-30. [CrossRef]
- Semis HS, Kandemir FM, Caglayan C, Kaynar O, Genc A, Arıkan SM. Protective effect of naringin against oxaliplatin-induced peripheral neuropathy in rats: A behavioral and molecular study. J Biochem Mol Toxicol 2022 ; 36(9) : e23121. [CrossRef]
- Hanedan B, Ozkaraca M, Kirbas A, Kandemir FM, Aktas MS, Kilic K, et al. Investigation of the effects of hesperidin and chrysin on renal injury induced by colistin in rats. Biomed Pharmacother 2018 ; 108 : 1607-16. [CrossRef]
- Akaras N, Abuc OO, Koc K, Bal T, Geyikoglu F, Atilay H, et al. (1 → 3)-β-d-glucan enhances the toxicity induced by Bortezomib in rat testis. J Food Biochem 2020 ; 44(3) : e13155. [CrossRef]
- Thayumanavan G, Jeyabalan S, Fuloria S, Sekar M, Ravi M, Selvaraj LK, et al. Silibinin and naringenin against bisphenol a-ınduced neurotoxicity in Zebrafish model-potential flavonoid molecules for new drug design, development, and therapy for neurological disorders. Molecules 2022 ; 27(8) : 2572. [CrossRef]
- Kandemir FM, Kucukler S, Caglayan C, Gur C, Batil AA, Gülçin İ. Therapeutic effects of silymarin and naringin on methotrexate-induced nephrotoxicity in rats: Biochemical evaluation of anti-inflammatory, antiapoptotic, and antiautophagic properties. J. Food Biochem 2017; 41(5) , e12398. [CrossRef]
- Sagliyan A, Benzer F, Kandemir FM, Gunay C, Han MC, Ozkaraca M. Beneficial effects of oral administrations of grape seed extract on healing of surgically induced skin wounds in rabbits. Revue Méd. Vét 2012 ; 163(1) : 11-17.
- Ömür AD, Kandemir FM, Yıldırım BA, Akman O, Şenocak EA, Eldutar E, et al. Protective effect of dandelion (Taraxacum officinale) extract Against gentamicin-induced reproductive damage in male rats. Kafkas Univ. Vet. Fak 2016 ; 22 : 929-36.
- Ileriturk M, Kandemir O, Akaras N, Simsek H, Genc A, Kandemir FM. Hesperidin has a protective effect on paclitaxel-induced testicular toxicity through regulating oxidative stress, apoptosis, inflammation and endoplasmic reticulum stress. Reprod Toxicol 2023 ; 118 : 108369. [CrossRef]
- Ileritürk M., Kandemir Ö. Protective Effect of Rutin on Malathion-induced Gastric Toxicity: Evaluation of Oxidative Stress, Inflammation and Apoptosis. F.U. Vet. J. Health Sci 2023 ; 37(2) : 139-45.
- Şimşek H, Akaras N, Gür C, Küçükler S, Kandemir FM. Beneficial effects of Chrysin on Cadmium-induced nephrotoxicity in rats: Modulating the levels of Nrf2/HO-1, RAGE/NLRP3, and Caspase-3/Bax/Bcl-2 signaling pathways. Gene 2023 ; 875 : 147502. [CrossRef]
- Gur C, Akarsu SA, Akaras N, Tuncer SC, Kandemir FM. Carvacrol reduces abnormal and dead sperm counts by attenuating sodium arsenite-induced oxidative stress, inflammation, apoptosis, and autophagy in the testicular tissues of rats. Environ Toxicol 2023; 38(6) : 1265-76. [CrossRef]
- Simsek H, Akaras N. Acacetin ameliorates acetylsalicylic acid-induced gastric ulcer in rats by interfering with oxidative stress, inflammation, and apoptosis. Int J Med Biochem 2023; 6(2) : 96-103. [CrossRef]
- Akaras N, Kandemir FM, Şimşek H, Gür C, Aygörmez S. Antioxidant, Antiinflammatory, and Antiapoptotic Effects of Rutin in Spleen Toxicity Induced by Sodium Valproate in Rats. TJNS 2023 ; 12(2) : 138-44.
- Yardım A, Kucukler S, Özdemir S, Çomaklı S, Caglayan C, Kandemir FM, et al. Silymarin alleviates docetaxel-induced central and peripheral neurotoxicity by reducing oxidative stress, inflammation and apoptosis in rats. Gene 2021 ; 769 : 145239. [CrossRef]
- Celik H, Kucukler S, Ozdemir S, Comakli S, Gur C, Kandemir FM, et al. Lycopene protects against central and peripheral neuropathy by inhibiting oxaliplatin-induced ATF-6 pathway, apoptosis, inflammation and oxidative stress in brains and sciatic tissues of rats. Neurotoxicology 2020 ; 80 : 29-40. [CrossRef]
- Yardım A, Kandemir FM, Çomaklı S, Özdemir S, Caglayan C, Kucukler S, et al. Protective Effects of Curcumin Against Paclitaxel-Induced Spinal Cord and Sciatic Nerve Injuries in Rats. Neurochem Res 2021 ; 46(2) : 379-95. [CrossRef]
- Gur C, Kandemir FM, Caglayan C, Satıcı E. Chemopreventive effects of hesperidin against paclitaxel-induced hepatotoxicity and nephrotoxicity via amendment of Nrf2/HO-1 and caspase-3/Bax/Bcl-2 signaling pathways. Chem Biol Interact 2022 ; 365 : 110073. [CrossRef]
- Akcılar R, Akcılar A, Koçak C, Koçak FE, Bayat Z, Şimşek H, et al. Effects of Ukrain on intestinal apoptosis caused by ischemia-reperfusion injury in rats. Int J Clin Exp Med 2015 ; 8(12) : 22158-66.
- Akaras N, Bal T, Atilay H, Selli J, Halici MB. Protective effects of agomelatine on testicular damage caused by bortezomib. Biotech Histochem 2017 ; 92(8) : 552-59. [CrossRef]
- Şimşek H, Demiryürek Ş, Demir T, Atabay HD, Çeribasi AO, Bayraktar R, et al. Assessment of expressions of Bcl-XL, b-FGF, Bmp-2, Caspase-3, PDGFR-α, Smad1 and TGF-β1 genes in a rat model of lung ischemia/reperfusion. Iran J Basic Med Sci 2016 ; 19(2) : 209-14.
- Temel Y, Çağlayan C, Ahmed BM, Kandemir FM, Çiftci M. The effects of chrysin and naringin on cyclophosphamide-induced erythrocyte damage in rats: biochemical evaluation of some enzyme activities in vivo and in vitro. Naunyn Schmiedebergs Arch Pharmacol 2021 ; 394(4) : 645-54. [CrossRef]
- Semis HS, Kandemir FM, Kaynar O, Dogan T, Arikan SM. The protective effects of hesperidin against paclitaxel-induced peripheral neuropathy in rats. Life Sci 2021; 287 : 120104. [CrossRef]
- Placer ZA, Cushman LL, Johnson BC. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal Biochem 1966 ; 16(2) : 359-64. [CrossRef]
- Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem 1988 ; 34(3) : 497-500. [CrossRef]
- Aebi H. Catalase in vitro. Methods Enzymol 1984 ; 105 : 121-26.
- Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 1976 ; 71(4) : 952-58. [CrossRef]
- Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968; 25(1):192-205. [CrossRef]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193(1):265-75. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



