Submitted:
02 September 2023
Posted:
05 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
2.1. Study design and data source
2.2. Data extraction
2.3. Descriptive and Statistical analyses
2.4. Ethics
3. Results
3.1. Descriptive results from SINFONIA
3.2. Descriptive results from RNF
3.3. Statistical results
4. Discussion
Supplementary Materials
References
- Stokel-Walker Chris. What do we know about covid vaccines and preventing transmission? BMJ 2022;376. [CrossRef]
- EMA. COVID-19 vaccines | European Medicines Agency. Available at https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-vaccines. Accessed January 12, 2023, 2023.
- Burn Edward, Roel Elena, Pistillo Andrea, Fernández-Bertolín Sergio, Aragón Maria, Raventós Berta, et al. Thrombosis and thrombocytopenia after vaccination against and infection with SARS-CoV-2 in Catalonia, Spain. Nat Commun 2022 131 2022;13(1):1–11. [CrossRef]
- Wise Jacqui. Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ 2021;372:n699. [CrossRef]
- EMA. COVID-19 Safety update Vaxzevria vaccine - 14 April 2021. Available at https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-vaxzevria-previously-covid-19-vaccine-astrazeneca-14-april-2021_en.pdf. Accessed January 12, 2023, 2021.
- Shay David K., Gee Julianne, Su John R., Myers Tanya R., Marquez Paige, Liu Ruiling, et al. Safety Monitoring of the Janssen (Johnson & Johnson) COVID-19 Vaccine — United States, March–April 2021. MMWR Morb Mortal Wkly Rep 2021;70(18):680–4. [CrossRef]
- EMA. Signal assessment report on embolic and thrombotic events (SMQ) with COVID-19 Vaccine (ChAdOx1-S [recombinant]) – Vaxzevria (previously COVID-19 Vaccine AstraZeneca) (Other viral vaccines) EPITT no:19683. Available at https://www.ema.europa.eu/en/documents/prac-recommendation/signal-assessment-report-embolic-thrombotic-events-smq-covid-19-vaccine-chadox1-s-recombinant_en.pdf. Accessed January 12, 2023, 2021.
- EMA. COVID-19 Vaccine Janssen: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets | European Medicines Agency. Available at https://www.ema.europa.eu/en/news/covid-19-vaccine-janssen-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood. Accessed January 12, 2023, 2020.
- EMA. COVID-19 vaccine safety update VAXZEVRIA AstraZeneca AB. Available at https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-vaxzevria-previously-covid-19-vaccine-astrazeneca-21-may-2021_en.pdf. Accessed January 12, 2023, 2021.
- EMA. COVID-19 vaccine safety update VAXZEVRIA AstraZeneca AB. Available at https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-vaxzevria-previously-covid-19-vaccine-astrazeneca-11-november-2021_en.pdf. Accessed January 12, 2023, 2021.
- Pottegård Anton, Lund Lars Christian, Karlstad Øystein, Dahl Jesper, Andersen Morten, Hallas Jesper, et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ 2021;373. [CrossRef]
- Simpson C. R., Shi T., Vasileiou E., Katikireddi S. V., Kerr S., Moore E., et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med 2021 277 2021;27(7):1290–7. [CrossRef]
- Lee Eun Ju, Cines Douglas B., Gernsheimer Terry, Kessler Craig, Michel Marc, Tarantino Michael D., et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol 2021;96(5):534–7. [CrossRef]
- 14 Perrella A, Mucherino S, Guarino I, Nerilli M, Maraolo AE, Capoluongo N, Coscioni E, Trama U, Menditto E, Orlando V. Postvaccination SARS-CoV-2 Infections among Healthcare Professionals: A Real World Evidence Study. Vaccines (Basel). 2022 Mar 25;10(4):51.
- Mani Avinash, Ojha Vineeta. Thromboembolism after COVID-19 Vaccination: A Systematic Review of Such Events in 286 Patients. Ann Vasc Surg 2022;84:12. [CrossRef]
- Lai Daoyuan, Zhang Yan Dora, Lu Junfeng. Venous Thromboembolism following Two Doses of COVID-19 mRNA Vaccines in the US Population, 2020-2022. Vaccines 2022;10(8). [CrossRef]
- Hviid Anders, Hansen Jørgen Vinsløv, Thiesson Emilia Myrup, Wohlfahrt Jan. Association of AZD1222 and BNT162b2 COVID-19 Vaccination With Thromboembolic and Thrombocytopenic Events in Frontline Personnel : A Retrospective Cohort Study. Ann Intern Med 2022;175(4):541–6. [CrossRef]
- Pawlowski Colin, Rincón-Hekking John, Awasthi Samir, Pandey Viral, Lenehan Patrick, Venkatakrishnan A. J., et al. Cerebral Venous Sinus Thrombosis is not Significantly Linked to COVID-19 Vaccines or Non-COVID Vaccines in a Large Multi-State Health System. J Stroke Cerebrovasc Dis 2021;30(10). [CrossRef]
- Li Xintong, Burn Edward, Duarte-Salles Talita, Yin Can, Reich Christian, Delmestri Antonella, et al. Comparative risk of thrombosis with thrombocytopenia syndrome or thromboembolic events associated with different covid-19 vaccines: international network cohort study from five European countries and the US. BMJ 2022;379:e071594. [CrossRef]
- Hippisley-Cox Julia, Patone Martina, Mei Xue W., Saatci Defne, Dixon Sharon, Khunti Kamlesh, et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ 2021;374. [CrossRef]
- Burn Edward, Li Xintong, Delmestri Antonella, Jones Nathan, Duarte-Salles Talita, Reyes Carlen, et al. Thrombosis and thrombocytopenia after vaccination against and infection with SARS-CoV-2: a population-based cohort analysis. MedRxiv 2021:2021.07.29.21261348. [CrossRef]
- Schultz Nina H., Sørvoll Ingvild H., Michelsen Annika E., Munthe Ludvig A., Lund-Johansen Fridtjof, Ahlen Maria T., et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N Engl J Med 2021;384(22):2124–30. [CrossRef]
- Greinacher Andreas, Thiele Thomas, Warkentin Theodore E., Weisser Karin, Kyrle Paul A., Eichinger Sabine. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med 2021;384(22):2092–101. [CrossRef]
- Greinacher Andreas, Selleng Kathleen, Mayerle Julia, Palankar Raghavendra, Wesche Jan, Reiche Sven, et al. Anti-platelet factor 4 antibodies causing VITT do not cross-react with SARS-CoV-2 spike protein. Blood 2021;138(14):1269–77. [CrossRef]
- Dotan Arad, Shoenfeld Yehuda. Perspectives on vaccine induced thrombotic thrombocytopenia. J Autoimmun 2021;121. [CrossRef]
- Perricone Carlo, Ceccarelli Fulvia, Nesher Gideon, Borella Elisabetta, Odeh Qasim, Conti Fabrizio, et al. Immune thrombocytopenic purpura (ITP) associated with vaccinations: a review of reported cases. Immunol Res 2014;60(2–3):226–35. [CrossRef]
- Cecinati Valerio, Principi Nicola, Brescia Letizia, Giordano Paola, Esposito Susanna. Vaccine administration and the development of immune thrombocytopenic purpura in children. Https://DoiOrg/104161/Hv23601 2013;9(5):1158–62. [CrossRef]
- Stone Daniel, Liu Ying, Shayakhmetov Dmitry, Li Zong-Yi, Ni Shaoheng, Lieber André. Adenovirus-platelet interaction in blood causes virus sequestration to the reticuloendothelial system of the liver. J Virol 2007;81(9):4866–71. [CrossRef]
- Jin Ying Yu, Yu Xiu Nan, Qu Zhang Yi, Zhang Ai Ai, Xing Yu Ling, Jiang Li Xin, et al. Adenovirus type 3 induces platelet activation in vitro. Mol Med Rep 2014;9(1):370–4. [CrossRef]
- Chiang Kate Chander, Gupta Ajay. Mechanism of Thrombosis with AstraZeneca and J & J Vaccines: Expert Opinion by Kate Chander Chiang & Ajay Gupta, MD | Leaders in Pharmaceutical Business Intelligence (LPBI) Group. Available at https://pharmaceuticalintelligence.com/2021/04/14/mechanism-of-thrombosis-with-astrazeneca-and-j-j-vaccines-expert-opinion-by-kate-chander-chiang-ajay-gupta-md/. Accessed January 17, 2023, 2021.
- Kima Soo Yeon, Kwon Whi An, Shina Seung Pil, Seo Ho Kyung, Lim Soo Jeong, Jung Yuh Seog, et al. Electrostatic interaction of tumor-targeting adenoviruses with aminoclay acquires enhanced infectivity to tumor cells inside the bladder and has better cytotoxic activity. Drug Deliv 2018;25(1):49–58. [CrossRef]
- Leng Xing Hong, Hong Song Yun, Larrucea Susana, Zhang Wei, Li Tong Tong, López José A., et al. Platelets of female mice are intrinsically more sensitive to agonists than are platelets of males. Arterioscler Thromb Vasc Biol 2004;24(2):376–81. [CrossRef]
- Perrella A, Bisogno M, D’Argenzio A, Trama U, Coscioni E, Orlando V. Risk of SARS-CoV-2 Infection Breakthrough among the Non-Vaccinated and Vaccinated Population in Italy: A Real-World Evidence Study Based on Big Data. Healthcare (Basel). 2022 Jun 10;10(6):1085.
| Variable | Level |
Vaccine doses (N=12,692,852) |
|
| N | % | ||
| Gender | Female | 6,509,475 | 51.28 |
| Male | 6,183,377 | 48.72 | |
| Age | 5 – 11 years | 242,449 | 1.91 |
| 12 – 19 years | 1,086,703 | 8.56 | |
| 20 – 29 years | 1,548,365 | 12.20 | |
| 30 – 39 years | 1,595,933 | 12.57 | |
| 40 – 49 years | 1,957,496 | 15.42 | |
| 50 – 59 years | 2,232,236 | 17.59 | |
| 60 – 69 years | 1,827,060 | 14.39 | |
| 70 – 79 years | 1,374,019 | 10.83 | |
| 80 – 89 years | 708,684 | 5.58 | |
| > 90 years | 119,901 | 0.94 | |
| Not available | 6 | 0.00 | |
| Doses | First | 4,718,844 | 37.18 |
| Second | 4,239,383 | 33.40 | |
| Third | 3,572,382 | 28.14 | |
| Fourth | 162,243 | 1.28 | |
| Type of vaccine | Pfizer-BioNtech | 8,257,218 | 65.05 |
| Moderna | 3,085,673 | 24.31 | |
| Oxford–AstraZeneca | 1,232,608 | 9.71 | |
| Janssen | 116,061 | 0.91 | |
| Novavax | 1,292 | 0.02 | |
| Variable | Level | Pfizer-BioNtech (N=398) | Oxford–AstraZeneca (N=148) |
Moderna (N=82) |
Janssen (N=13) |
| Number of vascular events per ICSR | Mean | 3.7 | 3.8 | 4.7 | 2.6 |
| Gender | Female (%) | 275 (69.10) | 117 (79.05) | 55 (67.07) | 6 (46.15) |
| Male (%) | 122 (30.65) | 28 (18.92) | 27 (32.93) | 7 (53.85) | |
| Unknown (%) | 1 (0.25) | 3 (2.03) | - | - | |
| Age | Median (IQR) |
47.97 (57.35-35.54) |
56.22 (65-87-45.55) |
45.00 (56.87-33.03) |
27.00 (39.53-18.99) |
| Seriousness | Serious (%) | 147 (36.93) | 66 (44.59) | 45 (54.88) | 3 (23.08) |
| Not serious (%) | 251 (63.07) | 82 (55.41) | 37 (45.12) | 10 (76.92) | |
| Doses | First dose (%) | 180 (45.23) | 105 (70.95) | 38 (46.34) | 6 (46.15) |
| Second dose (%) | 131 (32.91) | 9 (6.08) | 19 (23.17) | - | |
| Third dose (%) | 41 (10.30) | - | 11 (13.42) | - | |
| Not available (%) | 46 (11.56) | 34 (22.97) | 14 (17.07) | 7 (53.85) |
|
Thrombotic Events (Preferred terms of MedDRA) |
Oxford–AstraZeneca Vaccine |
Janssen Vaccine |
Moderna Vaccine |
Pfizer-BioNtech Vaccine |
Total |
| Thrombosis | 8 | 1 | 2 | 6 | 17 |
| Venous Thrombosis | 3 | 0 | 1 | 6 | 10 |
| Deep vein thrombosis | 4 | 0 | 2 | 3 | 9 |
| D-dimer of fibrin increased | 5 | 0 | 2 | 2 | 9 |
| Thrombophlebitis of the leg | 4 | 0 | 0 | 3 | 7 |
| Thrombophlebitis | 1 | 1 | 1 | 4 | 7 |
| Phlebitis | 2 | 0 | 2 | 3 | 7 |
| Infarction | 1 | 0 | 0 | 5 | 6 |
| Pulmonary embolism | 1 | 0 | 4 | 1 | 6 |
| Thrombosis of the leg | 2 | 0 | 0 | 3 | 5 |
| Thrombosis of saphenous vein | 4 | 0 | 0 | 1 | 5 |
| Ischemia | 2 | 0 | 1 | 1 | 4 |
| Deep vein thrombosis of a limb | 1 | 1 | 1 | 1 | 4 |
| Blood clot | 3 | 0 | 0 | 0 | 3 |
| Thrombus | 1 | 0 | 0 | 2 | 3 |
| Deep vein thrombosis (limbs) | 1 | 0 | 0 | 2 | 3 |
| Fibrinogen increased | 2 | 0 | 1 | 0 | 3 |
| Thromboembolism | 0 | 1 | 0 | 1 | 2 |
| Arterial thrombosis of a limb | 1 | 0 | 0 | 1 | 2 |
| Arterial occlusion, not specified | 1 | 0 | 1 | 0 | 2 |
| Deep vein thrombosis of a leg | 1 | 0 | 0 | 1 | 2 |
| Femoral deep vein thrombosis | 0 | 0 | 1 | 1 | 2 |
| Transient ischemic attack | 0 | 0 | 0 | 1 | 1 |
| D-dimer of fibrin abnormal | 1 | 0 | 0 | 0 | 1 |
| Coagulation disorder | 0 | 0 | 0 | 1 | 1 |
| Phlebitis of the arm | 1 | 0 | 0 | 0 | 1 |
| Phlebitis of a lower limb | 0 | 0 | 0 | 1 | 1 |
| Phlebothrombosis | 0 | 0 | 0 | 1 | 1 |
| Phlebothrombosis of alower limb | 0 | 0 | 0 | 1 | 1 |
| Ischemic stroke | 0 | 0 | 0 | 1 | 1 |
| Infarction of spleen | 0 | 0 | 0 | 1 | 1 |
| Intestinal infarction | 0 | 0 | 0 | 1 | 1 |
| Myocardial infarction | 0 | 0 | 0 | 1 | 1 |
| Cerebral ischemia | 1 | 0 | 0 | 0 | 1 |
| Chronic cerebral ischemia | 0 | 0 | 0 | 1 | 1 |
| Ischemia not specified | 1 | 0 | 0 | 0 | 1 |
| Splenic ischemia | 0 | 0 | 0 | 1 | 1 |
| Micro-embolism | 0 | 0 | 0 | 1 | 1 |
| Cerebral arterial occlusion | 0 | 0 | 0 | 1 | 1 |
| Pulmonary thromboembolism | 0 | 0 | 1 | 0 | 1 |
| Venous thromboembolism | 0 | 0 | 0 | 1 | 1 |
| Deep thrombophlebitis | 1 | 0 | 0 | 0 | 1 |
| Thrombosis of the arm | 1 | 0 | 0 | 0 | 1 |
| Femoral Arterial Thrombosis | 0 | 0 | 1 | 0 | 1 |
| Thrombosis of the axillary vein | 0 | 0 | 0 | 1 | 1 |
| Thrombosis of varicose veins | 1 | 0 | 0 | 0 | 1 |
| Venous thrombosis (limbs) | 1 | 0 | 0 | 0 | 1 |
| Venous thrombosis of the arm | 1 | 0 | 0 | 0 | 1 |
| Deep vein thrombosis of the arm | 0 | 0 | 0 | 1 | 1 |
| Left deep vein thrombosis | 0 | 0 | 1 | 0 | 1 |
| Total | 57 | 4 | 22 | 63 | 146 |
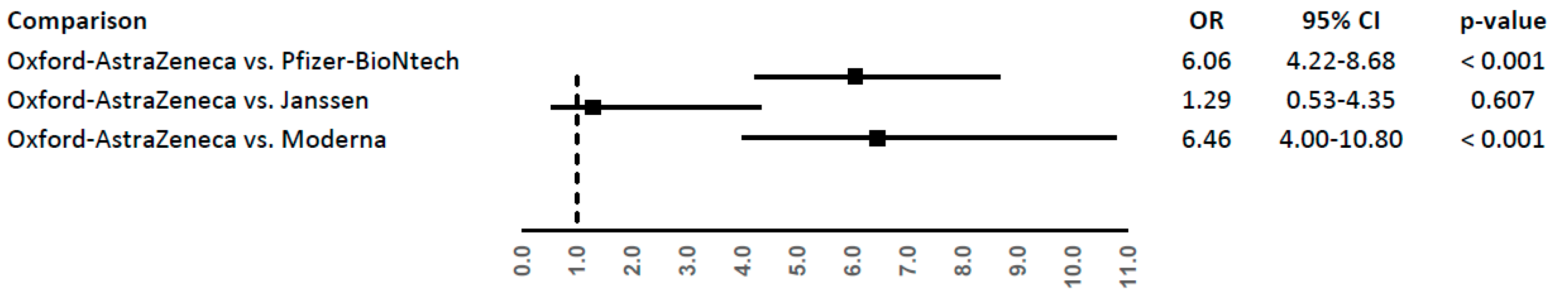
| COVID-19 vaccine | Reporting rate (95% CI) |
| Oxford–AstraZeneca | 4.62 (3.50-5.99) |
| Janssen | 3.45 (0.94-8.82) |
| Pfizer-BioNtech | 0.76 (0.59-0.98) |
| Moderna | 0.71 (0.45-1.08) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





