1. Introduction
Silicon oxide is a versatile compound that has been used in microelectronic devices and as protective films against corrosion of metallic surfaces [
1]. Owing to that, there is a constant search for economically viable deposition routes resulting in high quality SiOx films. In this scenario, the atomic layer deposition (ALD) technique is widely used as it allows the deposition of dense films with nanometric thicknesses. However, the routes used for the growth of SiOx films by ALD are thermal routes requiring, therefore, high temperatures [
2]. Since glow discharge plasmas contain large amounts of oxidative species, Plasma Enhanced-ALD, PEALD, stands out as a very promising alternative as it allows, in the oxidation step [
3], the removal of organics by at lower temperatures in comparison to those conventionally used in ALD.
Precursors based on aminosilanes (SiN
xH
y) are extremely reactive and promote the first stage of the reaction in a self-limiting manner, without the need of high temperatures [
4]. Even so, only a few authors have investigated the use of tris(dimethylamino)silane, TDMAS, as a precursor. That is a consequence of the fact that the third dimethyl group in the precursor molecule may hinder the formation of pure SiOx films, due to the high steric hindrance and, consequently, the high energy required to break the bonds involving such carbon-containing groups [
1].
In this context, the aim of this work is to evaluate the possibility of removing the third methyl group from the structure of films prepared from TDMAS, adjusting the parameters of the PEALD process, and developing, therefore, a low-energy route to produce SiOx films. More specifically, it has been evaluated the effect of the plasma oxidation time and composition on the chemical structure of the samples.
3. Results and Discussion
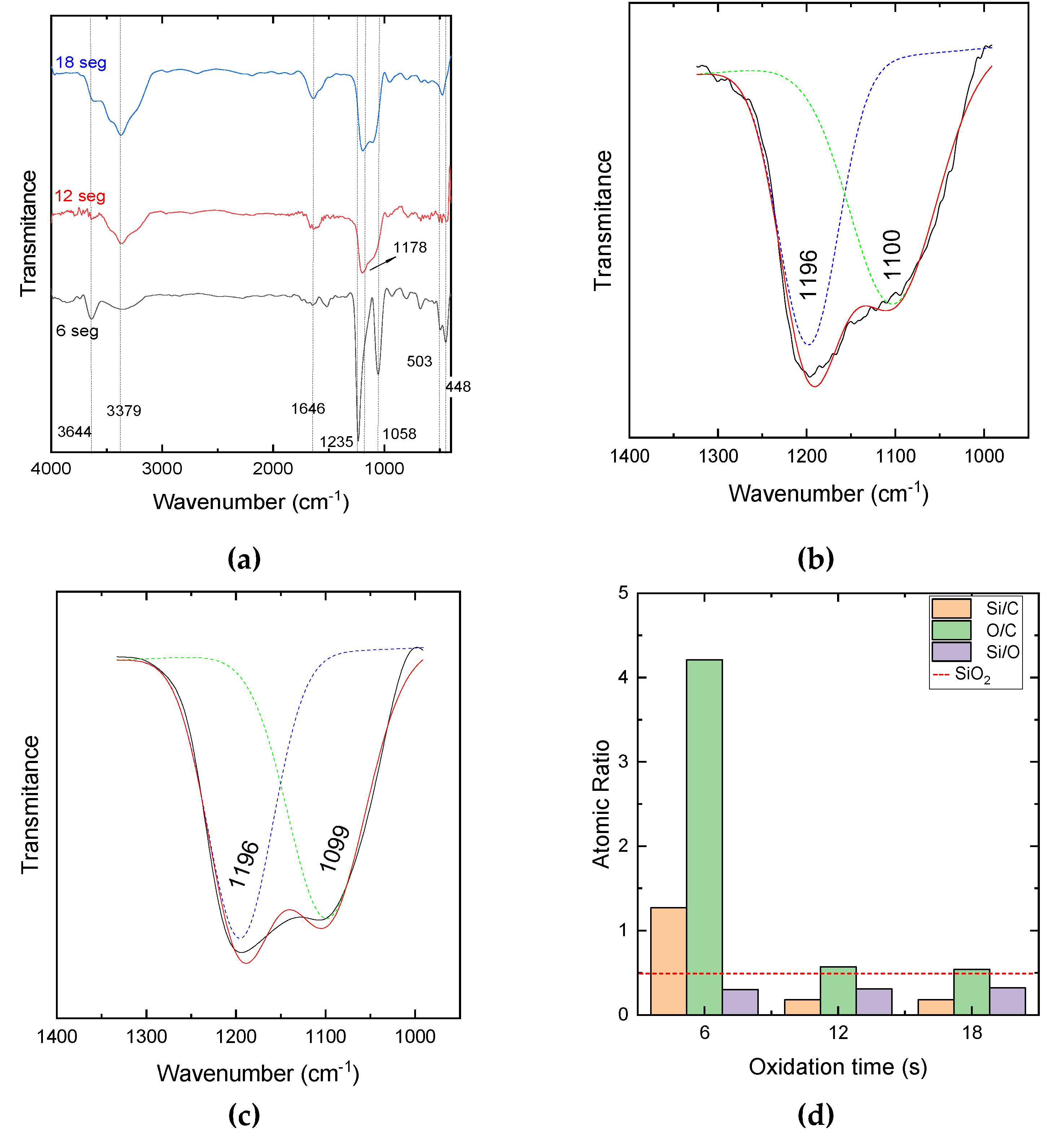
The effect of oxidation time, in pure oxygen plasma,
tox, on the chemical structure of the films can be inferred with the help of
Figure 1 (a), which presents the infrared absorption spectra of the films exposed for 6, 12 and, 18 s to the oxidation plasma. In general, the infrared spectra of the samples prepared with higher
tox (12 and 18 s) present wider peaks. The peak at 3379 cm
-1 is assigned to the stretching of N-H bonds, which is confirmed by the appearance of the contribution at 1646 cm
-1, also related to N-H groups. However, on the spectrum of the film produced with oxidation during 18 s may be overlapped with the band ascribed to ν-O-H (3644 cm
-1) [
6]. Another very prominent band around 1180 cm
-1, can be deconvoluted in two peaks, as depicted in
Figure 1(b) and (c) for the bands in the spectra of samples oxidized for 12 and 18 s, respectively. Such contributions, centered at 1196 and 1100 cm
-1 (
tox = 12 s) and on 1196 and 1099 cm
-1 (
tox = 18 s), can be attributed to SiO (1230 and 1080 cm
-1) [
7]; Si-N (1125, 1190 and 1220 cm
-1 )[
7,
8]; and Si-CHx (1230 cm
-1 ) [
9].
Different features can be observed in the spectrum of the film oxidized for 6 s. The drop in peak intensity at 3379 cm
-1, the disappearance of the peak at 1646 cm
-1 and the separation of the band initially centered around 1178 cm
-1 indicate that the chemical neighborhood of the Si-O functionals becomes more restricted. The reduction in the concentration of Si-N, C-H, N-H contaminants makes the peaks sharper, indicating that the formation of SiOx films is favored by the decrease in plasma oxidation time. This hypothesis is supported by the presence of peaks at 430 to 470 cm
-1, related to Si-O bonds [
10]. Although the most intense peaks of the spectrum indicate the formation of a SiO
x film, the presence of smaller intensity contributions at 450 cm
-1 (Si-O-Si) [
11], 500 cm
-1 (Si-N) [
12] and 3379 cm
-1 (N-H) [
6] are consistent with the presence of contaminants. Interestingly, the increase in
tox does not favor the removal of the third dimethylamino group, but rather the reincorporation of residual groups from the plasma oxidation atmosphere.
The atomic ratios obtained from EDS analysis are shown in
Figure 1(d). According to Renlund et al. [
13], the Si/C ratio in silicon suboxides tends to be higher as the C contamination decreases. A decrease in the Si/C ratio is observed with the increase of
tox for 12 and 18 s, suggesting an increment in the proportion of C retained in the film. A similar behavior is observed for the O/C ratio whereas the Si/O ratio is roughly independent of
tox . These results also corroborate the hypothesis that the shortest oxidation time promotes the removal of organic moieties without favoring the re-incorporation of residual groups.
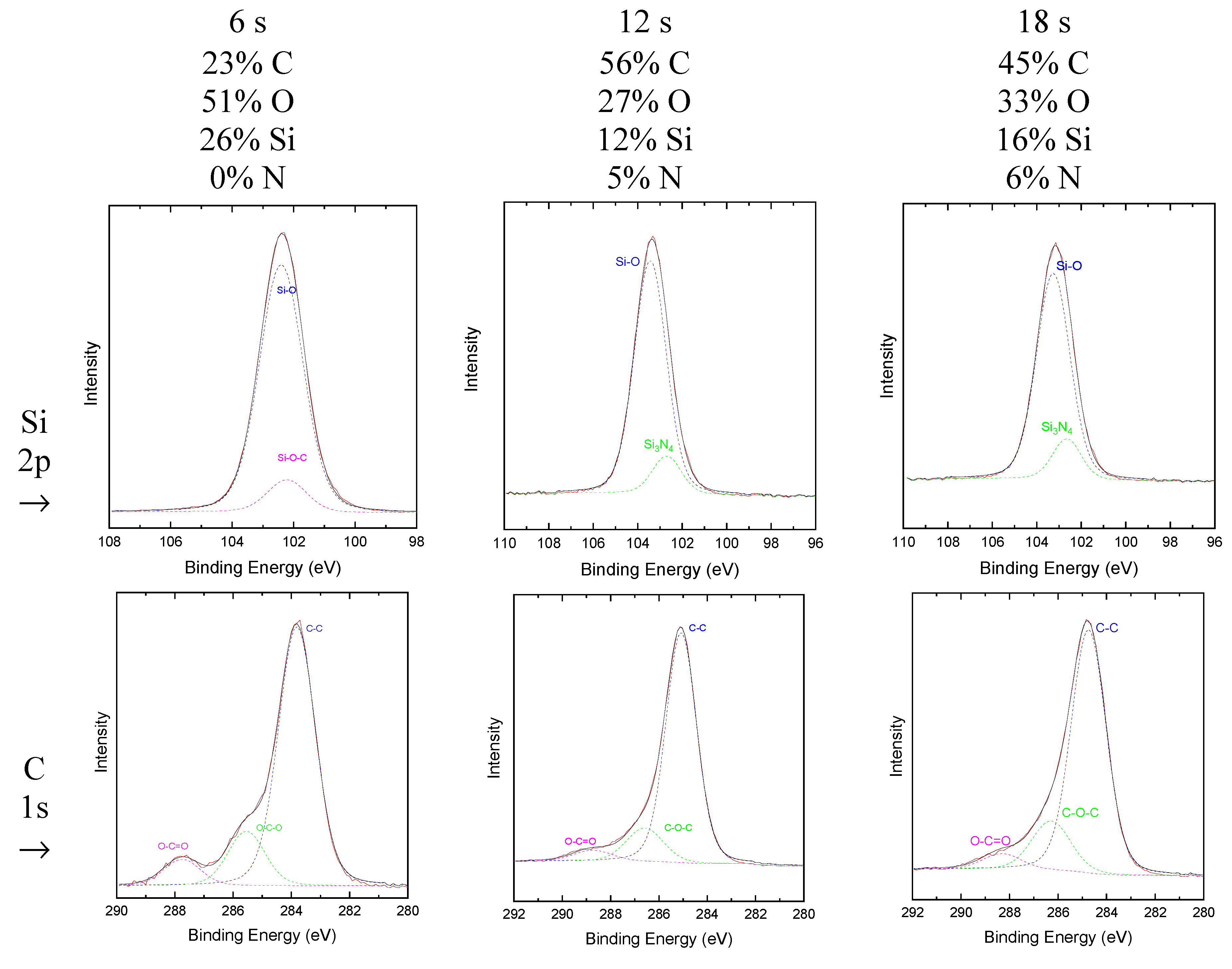
Figure 2 shows the high-resolution spectra of Si 2p and C 1s, obtained by XPS, for the samples investigated here. Carbon, oxygen, and silicon, elements present in the precursor molecule, were observed in all the samples while N was detected only in the samples oxidized for 12 s and 18 s. For fitting the Si 2p peaks of these two last samples, a component related to Si
3N
4 was used instead of Si-CH, since the detection range of this last bond would appear at a higher energy edge. This indicates the absence of such groups in the films. However, Si-O and Si-O-C groups were included. These results are in good agreement with the infrared results that suggested the deposition of SiO
x-type films with reduced organic contamination.
The atomic proportions of the elements derived from the XPS spectra are also presented in
Figure 2. As it can be noticed the shorter the plasma oxidation time, the smaller the proportions of C (23%) and N (0%) in the film surface. Consistently with the previous results, the proportion of C increases and that of silicon decreases with
tox larger than 6 s. For the latter, the structure is composed of 26% Si, 51% O and 23% C, with no identification of a significant amount of N. As C-containing groups were not necessary for the fitting of the Si high resolution peak of this sample, the contribution of C to the elemental composition of this film is assumed to be due to the adsorption of atmospheric C. Indeed, when the sample was plasma sputtered with Ar ions prior to the acquisition of the spectrum, only 6% of C was detected.
Comparing the atomic proportions obtained by EDS and XPS, it is promptly observed that the two analyzes indicate that a longer oxidation time decreased the amount of Si and increases the amount of C incorporated in the film structure. This shows that SiOx-like structures, free of the third methylamine bond, are obtained only for the shortest oxidation time (6 s) in pure O2 plasma, which also represents the treatment with the lowest energy cost. In this structure, hydrogen atoms share the oxygen of the siloxane network, forming hydroxyls (O-H), normally observed in silicon oxides. The organic groups (C-H, C-C and C-O), originated by atmospheric contamination, are present only on the surface of the film.
In the second set of experiments, it has been investigated the effect of the addition of argon in the oxidation plasma on the chemical structure of the films. To do that, the deposition step was conducted with the same conditions used in the previous experiments (TDMAS + Ar, 150°C, 260-270 mTorr) but purging for only 4 s. Furthermore, the oxidation was conducted in atmospheres containing pure O
2 (100%) or O
2 + Ar (81% O
2 + 19% Ar) mixtures preserving the other parameters (150°C, 260 - 270 mTorr, 6 s) previously used in the oxidation process.
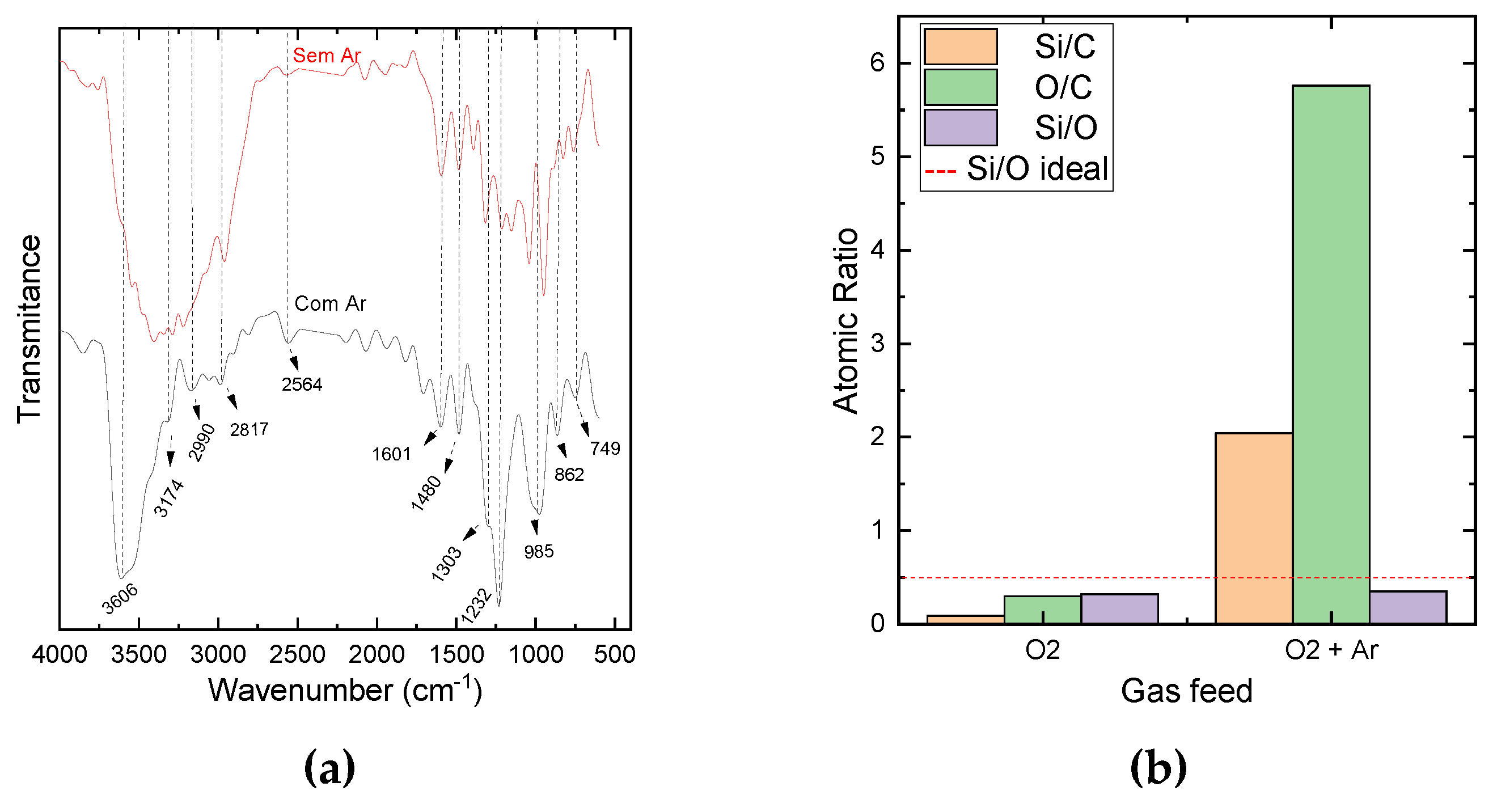
Figure 3 (a) shows the infrared spectra of the samples deposited with and without the addition of Ar to the oxidation atmosphere.
The spectrum of the film grown when only oxygen was used in the oxidation process reveals the presence of peaks characteristic of the Si-O bond at 1219, 1146, 1038, 944 and a doublet at 825-755 cm
-1. In addition, absorptions ascribed to Si-CH bond (1300-1500 cm
-1), N-H and O-H bonds (1600 cm
-1) [
6,
7,
14,
15] are also observed. Furthermore, a broad band observed in the range from 3713 to 2948 cm
-1, suggests the presence of peaks assigned to vibrations of N-H (3400 cm
-1), O-H and H
2O (3700 cm
-1) and C-H (2957 cm
-1) bonds. It is interesting to mention the substantial difference between the infrared spectrum of this sample and that of the sample prepared with the same oxidation step (150°C, 260 – 270 mTorr, 6 s) but with different purging time and oxygen flow (
Figure 1(d)). The higher contribution of C-containing groups in the spectrum of the film prepared with 4 s of purging time, shows that the deposition and oxidation kinetics are also sensitive to the purge time.
Different features are observed in the spectrum of the film grown with the addition of argon in the oxidation plasma. Whereas the peak attributed to methylsilyl groups at 1390 cm
-1 practically disappears, the one at 1303 cm
-1 becomes only a shoulder of a prominent band at 1232 cm
-1. These modifications indicate a reduction in the contribution of methylsilyl groups together with an enrichment of the density of SiO
x connections. Furthermore, the broad band in the highest wavenumber region, ascribed to various contributions (N-H, C-H and H
2O) becomes thinner and more prominent and the intensities of the bands related to Si-O (1232, 985, 862 and 749 cm
-1) increase. Taken together, these results suggest that the addition of Ar increases TDMAS fragmentation in the plasma favoring the formation of volatile carbon-containing species (CO and CO
2, for instance). In this way, carbon can be pumped out from the discharge by the vacuum system and, consequently, less carbon is available to be incorporated in the film structure. In addition, the addition of Ar to the plasma, within a certain range, tends to increase the average energy and concentration of reactive species [
16]. Consequently, the oxygen reactivity may also increase, making the etching of deposited carbon – containing species more effective. This hypothesis is corroborated by the atomic ratios results obtained from EDS analysis presented in
Figure 3 (b). It is clearly noticed the enhancement of Si/C and O/C ratios with the addition of Ar to the oxidation step, reinforcing the supposition of the reduction of the proportion of carbon. It is worthy to verify that C content detected in the sample produced under the best oxidation condition evaluated here was only 15%, but when the sample was plasma sputtered with Ar ions this value decrease to less than 10%. This phenomenon is the same observed in the previous experiments, that were conducted in different conditions. This coincidence of results suggests that, in fact, the carbon detected in both cases results from post-plasma reactions when the sample is exposed to atmosphere and not from the oxidation process.
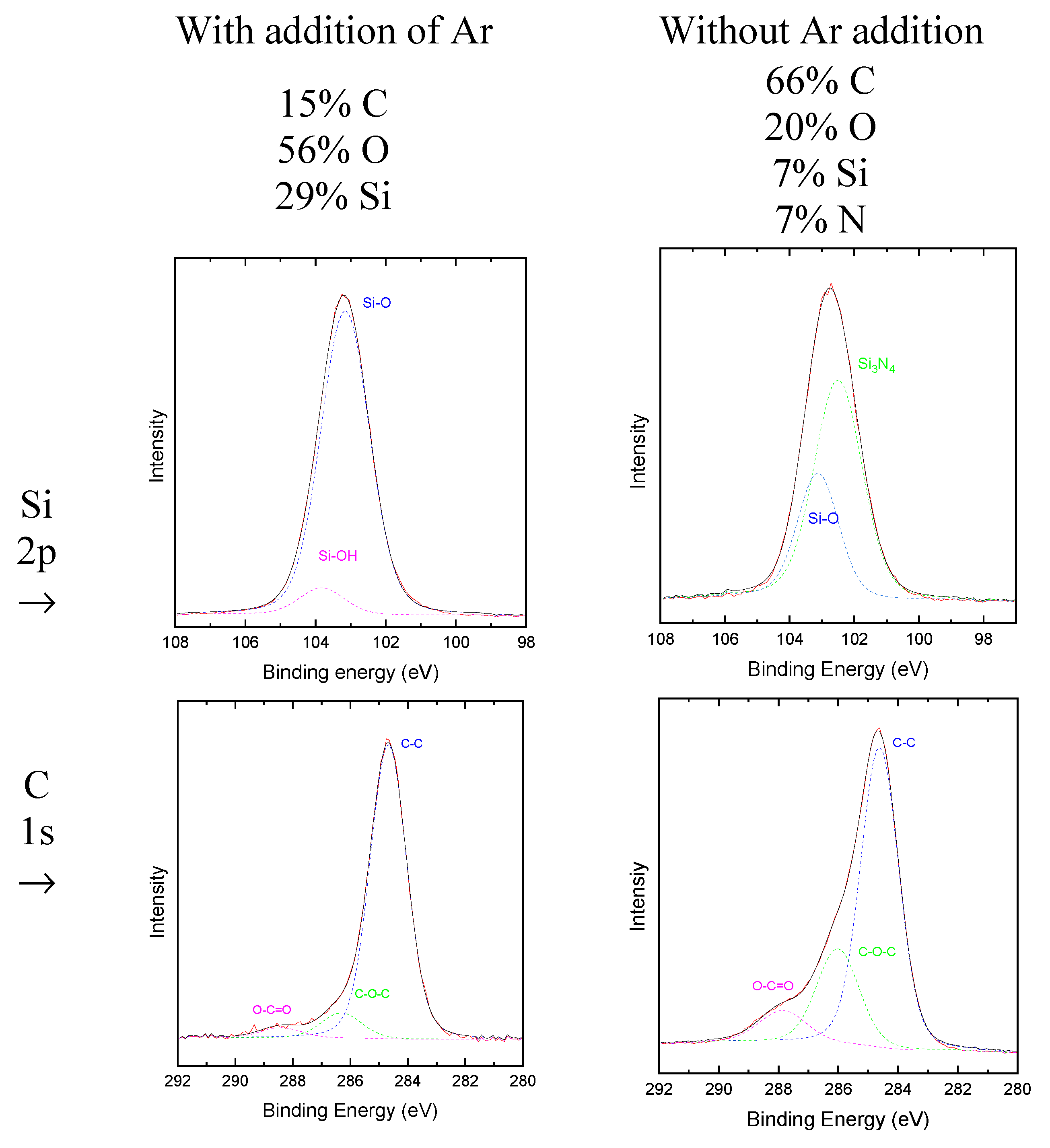
Figure 4 presents high resolution XPS spectra of Si 2p and C 1s of films deposited with and without the addition or argon to the oxidation plasma gas feed. Si 2p peaks was fitted with Si-O and Si(OH) components when oxidation process was conducted in Ar-containing plasmas and Si-O and Si
3N
4 when pure oxygen plasmas was used. It means that, for films oxidized in pure oxygen plasma, radicals incorporated in the SiO
x structure are saturated by N. On the other hand, when oxidation is conducted in Ar-containing plasmas, radicals are passivated by OH groups. Considering the high-resolution C peak, it is observed that C is bonded to O and other C atom. In good agreement with previous results, no N groups were detected in the film that was oxidized in the Ar-containing process. The Si/O ratios for this material were 0.4 for EDS and 0.52 for XPS results, that is around the stoichiometric ratio (0.5). Thus, the films prepared in the presence of Ar in the oxidative stage can be classified as a silicon suboxide. Such results shows that Ar has a synergistic effect in the oxidation step. Finally, the third methylamine connection of the precursor was not identified in the chemical structure of the film when this oxidation route was used.